Ore Genesis of the Kuergasheng Pb–Zn Deposit, Xinjiang Province, Northwest China: Constraints from Geology, Fluid Inclusions, and H–O–C–S–Pb Isotopes
Abstract
1. Introduction
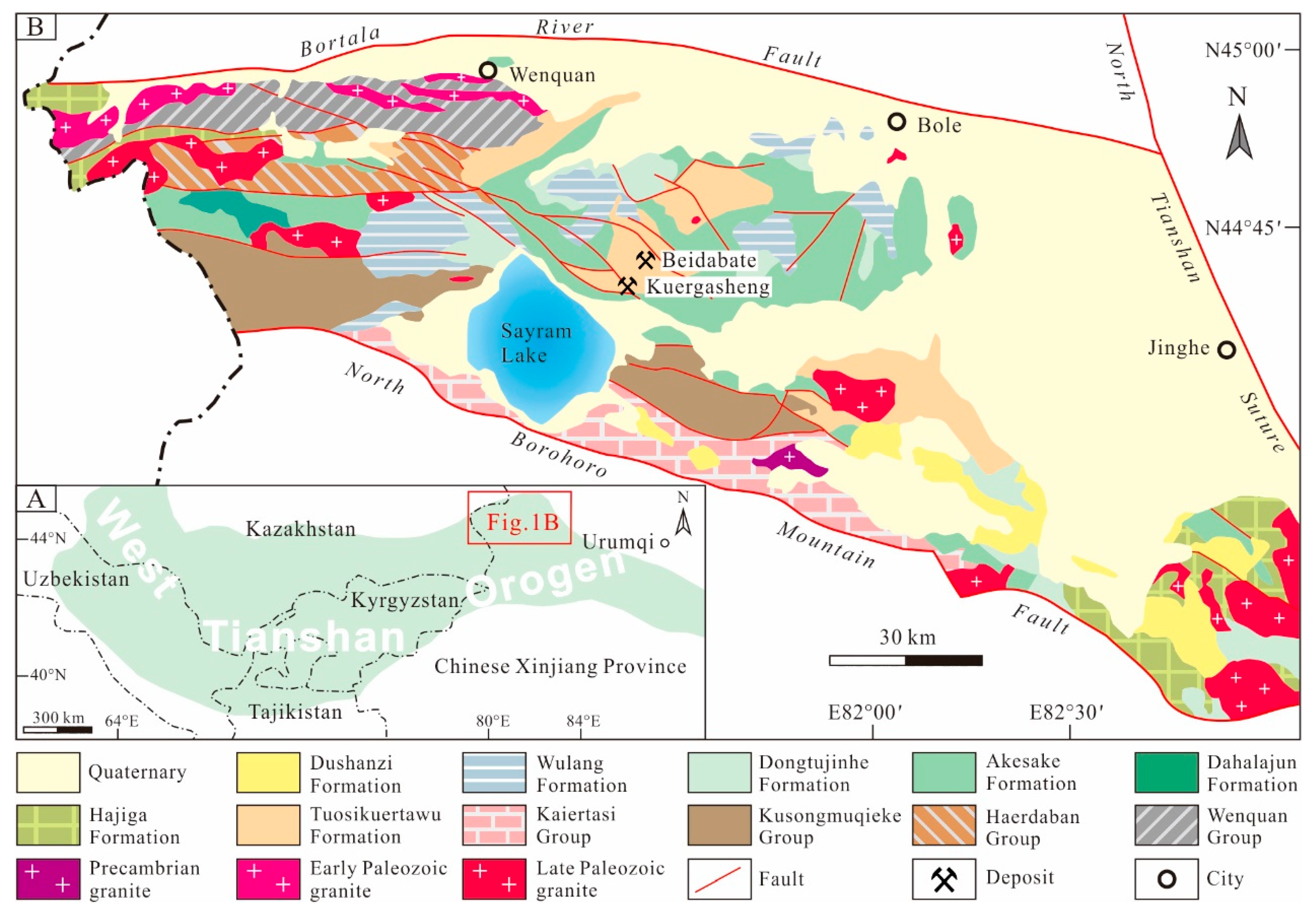
2. Regional and Deposit Geology
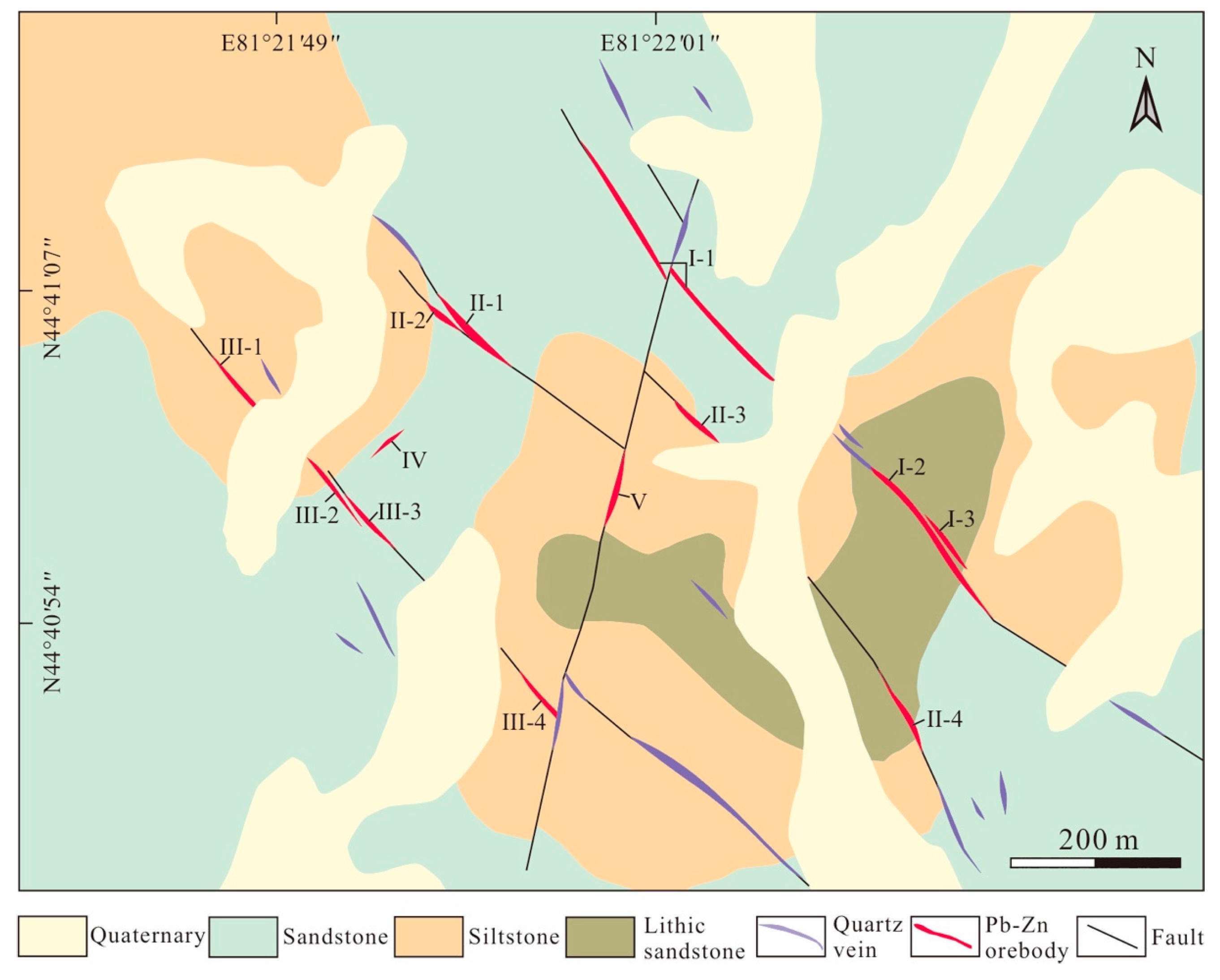
3. Mineralization Characteristics
4. Samples and Analytical Methods
4.1. Fluid Inclusions
4.2. H–O–C–S–Pb Isotopes
5. Results
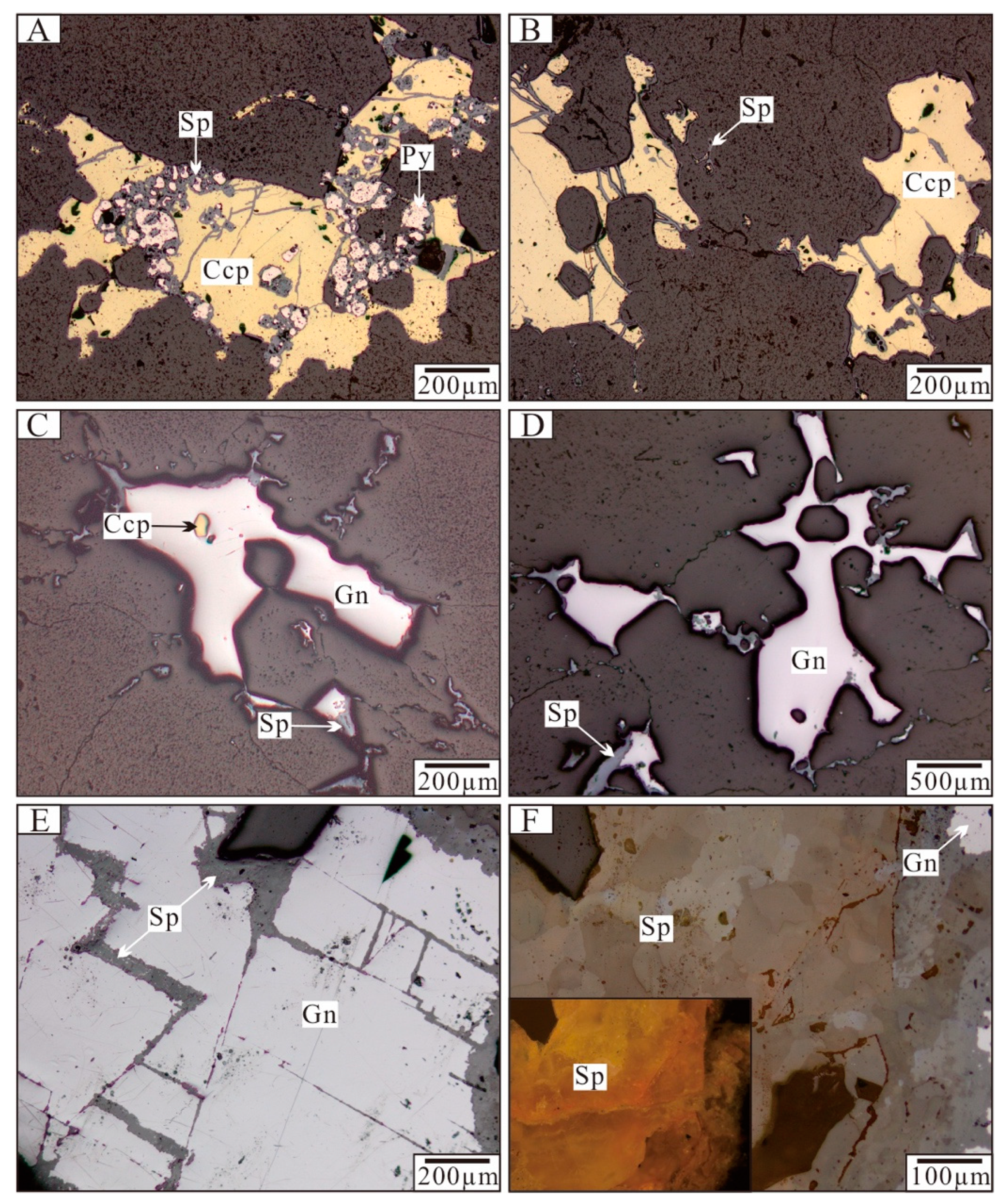
5.1. Fluid Inclusion Petrography
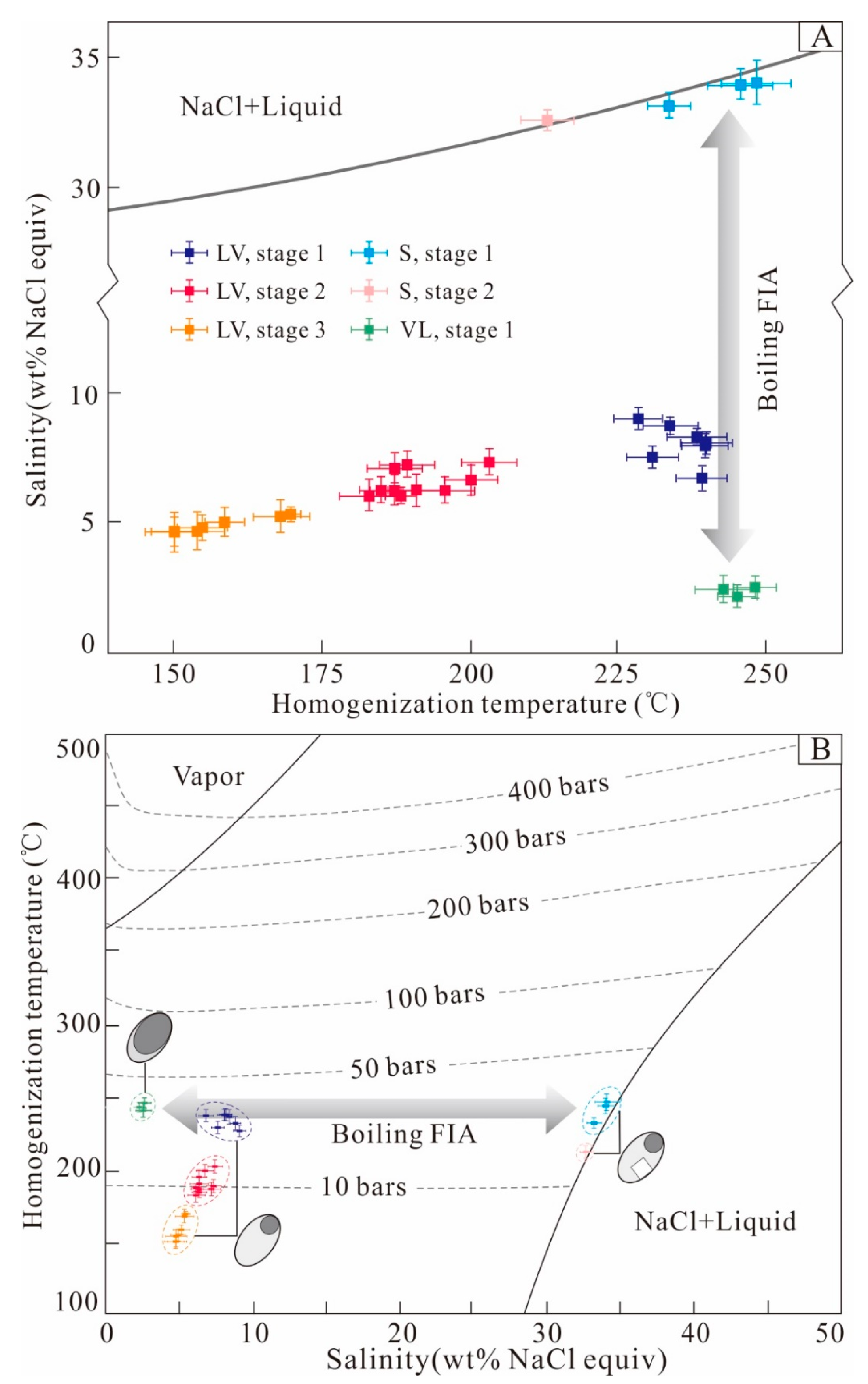
5.2. Fluid Inclusion Microthermometry
5.3. H–O–C–S–Pb Isotopes
6. Discussion
6.1. Fluid Boiling and Pressure Estimates
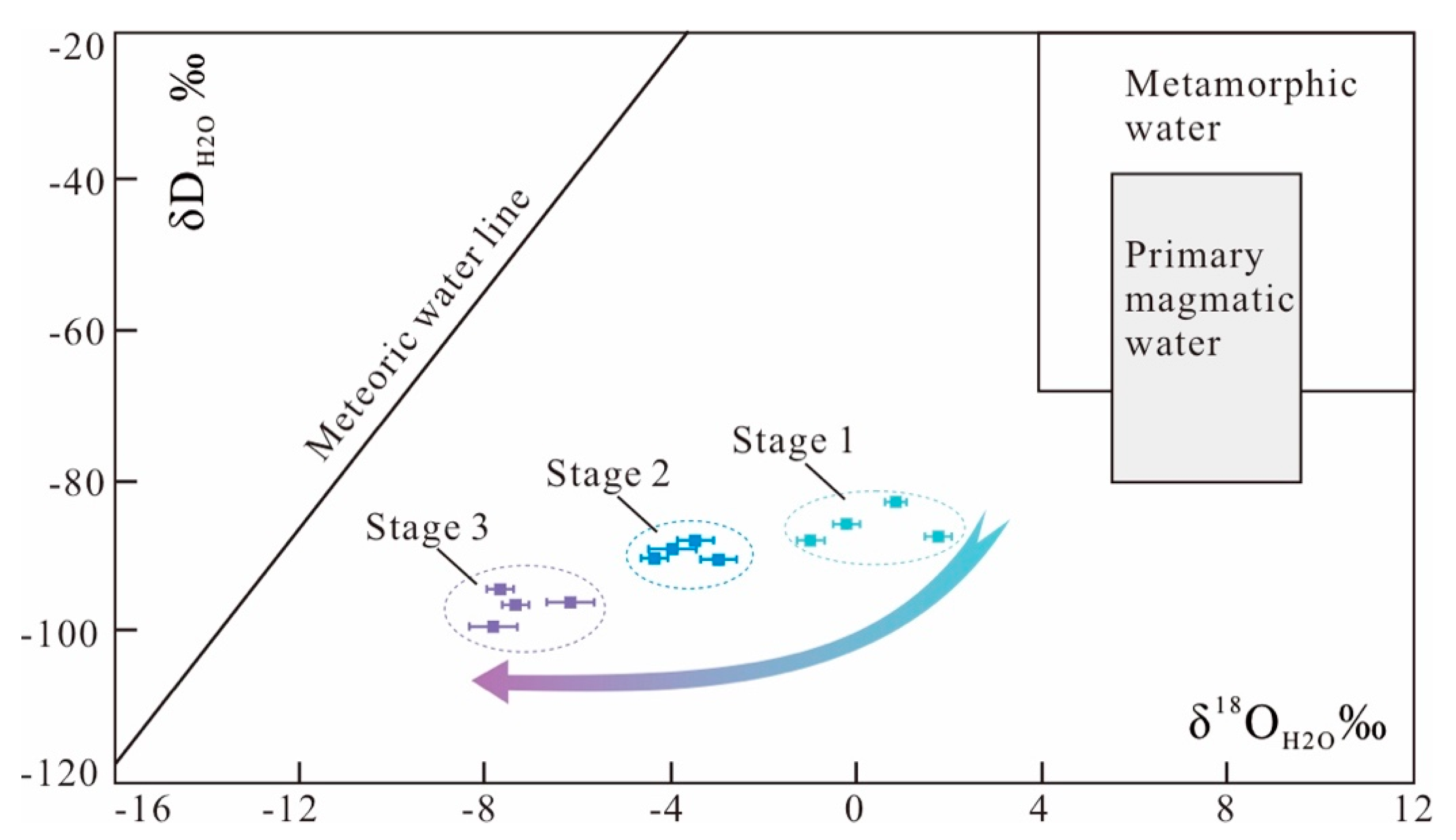
6.2. Fluid Sources and Evolution
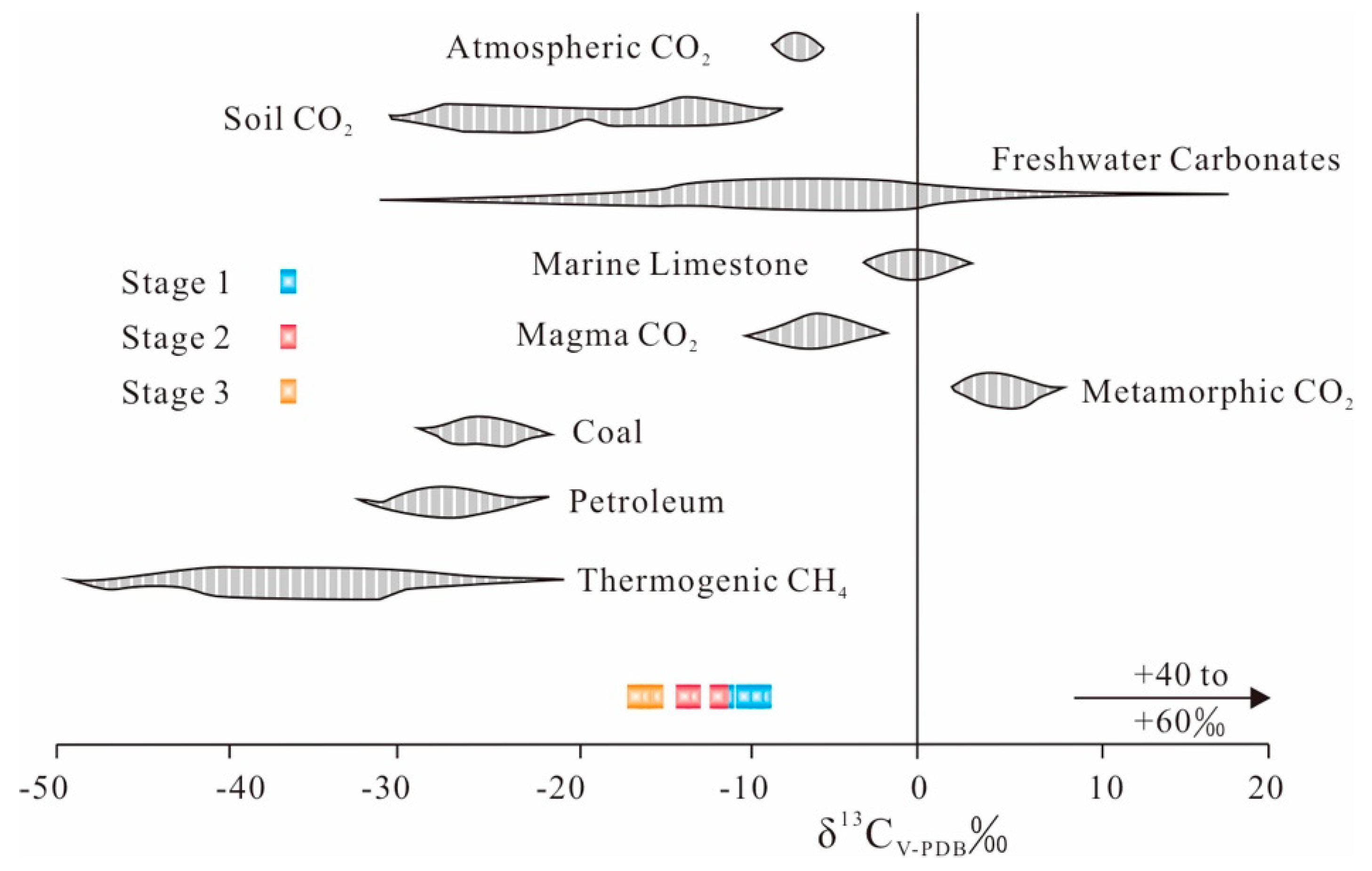
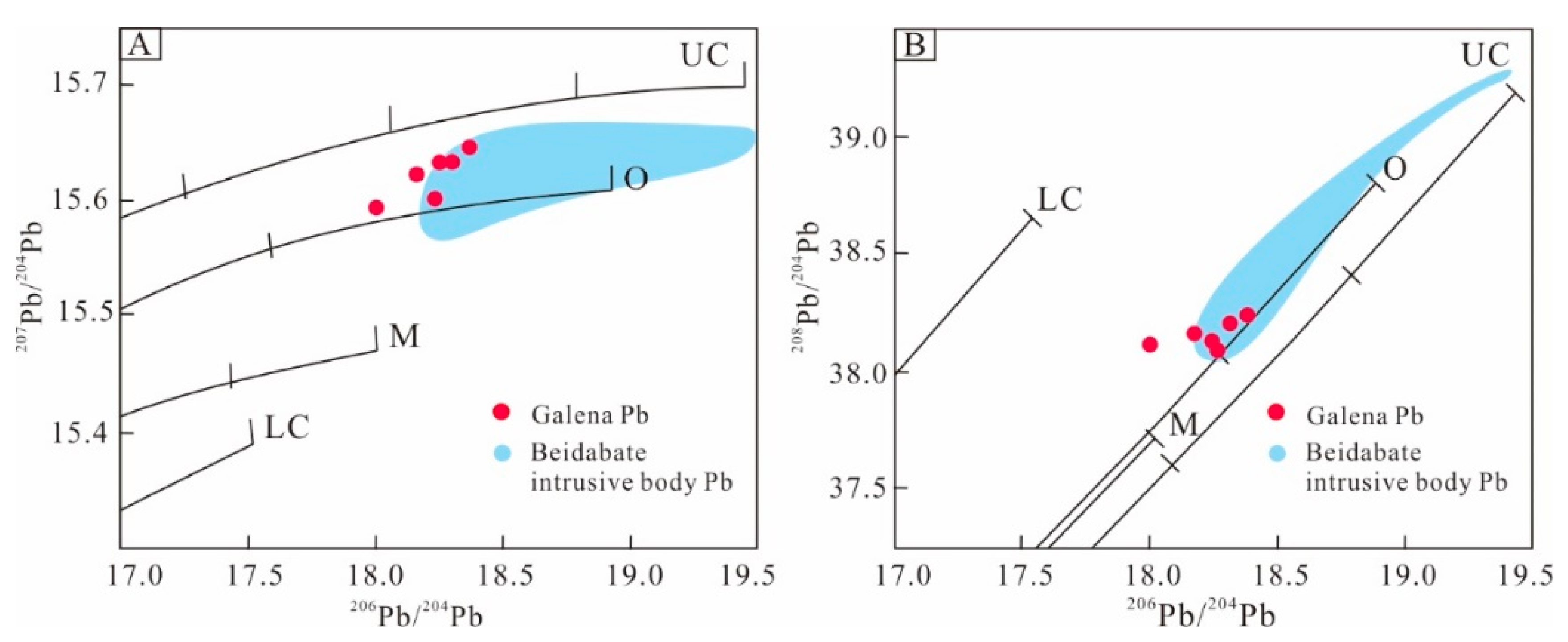
6.3. Sources of Ore Constituents
6.4. Genetic Model of the Kuergasheng Deposit
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Coleman, R.G. Continental growth of Northwest China. Tectonics 1989, 8, 621–635. [Google Scholar] [CrossRef]
- Şengör, A.C.; Natal’in, B.A.; Burtman, V.S. Evolution of the Altaid tectonic collage and Palaeozoic crustal growth in Eurasia. Nature 1993, 364, 299–306. [Google Scholar] [CrossRef]
- Jahn, B.M.; Wu, F.Y.; Chen, B. Massive granitoid generation in Central Asia: Nd isotope evidence and implication for continental growth in the Phanerozoic. Episodes 2000, 23, 82–92. [Google Scholar] [CrossRef]
- Kovalenko, V.I.; Yarmolyuk, V.V.; Kovach, V.P.; Kotov, A.B.; Kozakov, I.K.; Salnikova, E.B.; Larin, A.M. Isotopic provinces, mechanism of generation and sources of the continental curst in the Central Asian mobile belt: Geological and isotopic evidence. J. Asian Earth Sci. 2004, 23, 605–627. [Google Scholar] [CrossRef]
- Xiao, W.J.; Zhang, L.C.; Qin, K.Z. Paleozoic accretionary and collisional tectonics of the eastern Tianshan (China): Implications for the continental growth of central Asia. Am. J. Sci. 2004, 304, 370–395. [Google Scholar] [CrossRef]
- Abzalov, M. Zarmitan granitoid–hosted gold deposit, Tianshan belt Uzbekistan. Econ. Geol. 2007, 102, 519–532. [Google Scholar] [CrossRef]
- Biske, Y.S.; Seltmann, R. Paleozoic Tian–Shan as a transitional region between the Rheic and Urals–Turkestan oceans. Gondwana Res. 2010, 17, 602–613. [Google Scholar] [CrossRef]
- Gao, J.; Long, L.L.; Klemd, R.; Qian, Q.; Liu, D.Y.; Xiong, X.M.; Su, W.; Liu, W.; Wang, Y.T.; Yang, F.Q. Tectonic evolution of the South Tianshan orogen and adjacent regions, NW China: Geochemical and age constraints of granitoid rocks. Int. J. Earth Sci. 2009, 98, 1221–1238. [Google Scholar] [CrossRef]
- Lin, W.; Faure, M.; Shi, Y.H.; Wang, Q.C.; Li, Z. Palaeozoic tectonics of the south–western Chinese Tianshan: New insights from a structural study of the high–pressure/low–temperature metamorphic belt. Int. J. Earth Sci. 2009, 98, 1259–1274. [Google Scholar] [CrossRef]
- Kröner, A.; Alexeiev, D.V.; Hegner, E.; Rojas–Agrmonte, Y.; Corini, M.; Chao, Y.; Wong, J.; Windley, B.F.; Liu DTretyakov, A.A. Zircon and muscovite ages, geochemistry, and Nd–Hf isotopes for the Aktyuz metamorphic terrane: Evidence for an Early Ordovician collisional belt in the northern Tianshan of Kyrgyzstan. Gondwana Res. 2012, 21, 901–927. [Google Scholar] [CrossRef]
- Xiao, W.J.; Windley, B.F.; Allen, M.; Han, C.M. Paleozoic multiple accretionary and collisional tectonics of the Chinese Tianshan orogenic collage. Gandwana Res. 2013, 23, 1316–1341. [Google Scholar] [CrossRef]
- Xue, C.; Chi, G.; Li, Z.; Dong, X.F. Geology, geochemistry and genesis of the Cretaceous and Paleocene sandstone– and conglomerate–hosted Uragen Zn–Pb deposit, Xinjiang, China: A review. Ore Geol. Rev. 2014, 63, 328–342. [Google Scholar] [CrossRef]
- Xue, C.J.; Zhao, X.B.; Mo, X.X.; Chen, Y.C.; Dong, L.H.; Gu, X.X.; Zhang, Z.C.; Bakhtiar, N.; Nikolay, P.; Li, Z.D.; et al. Tectonic–metallogenic evolution of Western Tianshan giant Au–Cu–Zn–Pb metallogenic belt and prospecting orientation. Acta Geol. Sin. 2014, 88, 2490–2531, (In Chinese with English Abstract). [Google Scholar]
- Dai, Y.L. Geological characteristics and genesis of Kuergashengbulake Pb–Zn deposit. Miner. Resour. Geol. 1994, 8, 326–329, (In Chinese with English Abstract). [Google Scholar]
- Liu, F.M. Geological characteristics and genesis of Kuergasheng Pb–Zn deposit. Xinjiang Nonferrous Met. 2007, 3, 7–9, (In Chinese with English Abstract). [Google Scholar]
- Duan, S.X.; Xue, C.J.; Li, Y.; Shi, H.G.; Jia, Z.Y. Geology, fluid inclusions and isotopic geochemistry of Kuergasheng lead–zinc deposit in western Tianshan, Xinjiang. Miner. Depos. 2012, 31, 1014–1024, (In Chinese with English Abstract). [Google Scholar]
- Yi, Q.A.; Di, X.C.; Bian XMa, Y.Z. Geological features of Kuergasheng Pb–Zn deposit in Bole, Xinjiang. Xinjiang Nonferrous Met. 2013, 4, 30–33, (In Chinese with English Abstract). [Google Scholar]
- Liu, C.; Xue, C.J.; Chi, G.X.; Zhao, X.B.; Li, Y. Geology, geochronology and geochemistry of the Dabate Cu–Mo deposit, northern Chinese Tien Shan: Implications for spatial separation of copper and molybdenum mineralization. Ore Geol. Rev. 2018, 92, 542–557. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Wang, Z.L.; Zuo, G.C.; Liu, M.; Wang, L.S.; Wang, J.W. Ages and tectonic settings of the volcanic rocks in Dabate ore district in West Tianshan Mountains and their constraints on the porphyry-type mineralization. Acta Petrol. Sin. 2008, 82, 1494–1503, (In Chinese with English Abstract). [Google Scholar]
- Tang, G.J.; Wang, Q.; Wyman, D.A.; Sun, M.; Li, Z.X.; Zhao, Z.H.; Sun, W.D.; Jia, X.H.; Jiang, Z.Q. Geochronology and geochemistry of Late Paleozoic magmatic rocks in the Lamasu–Dabate area, northwestern Tianshan (west China): Evidence for a tectonic transition from arc to post–collisional setting. Lithos 2010, 119, 393–411. [Google Scholar] [CrossRef]
- Goldstein, R.H.; Reynolds, T.J. Systematics of fluid inclusions in diagenetic minerals. Soc. Sediment. Geol. Sepm Short Course 1994, 31, 1–199. [Google Scholar]
- Clayton, R.N.; Mayeda, T.K. The use of bromine pentafluoride in the extraction of oxygen from oxides and silicates for isotopic analysis. Geochim. Cosmochim. Acta 1963, 27, 43–52. [Google Scholar] [CrossRef]
- Robinson, B.W.; Kusakabe, M. Quantitative preparation of sulfur dioxide, for 34S/32S analyses, from sulfides by combustion with cuprous oxide. Anal. Chem. 1975, 47, 1179–1181. [Google Scholar] [CrossRef]
- Roedder, E. Fluid inclusions. Rev. Mineral. 1984, 12, 644. [Google Scholar]
- Hollister, L.S.; Burruss, R.C. Phase equilibria in fluid inclusions from the Khtada Lake metamorphic complex. Geochim. Cosmochim. Acta 1976, 40, 163–175. [Google Scholar] [CrossRef]
- Shepherd, T.J.; Rankin, A.H.; Alderton, D. A Practical Guide to Fluid Inclusion Studies; Blackie and Son: Glasgow, UK, 1985; p. 239. [Google Scholar]
- Goldstein, R.H. Petrographic analysis of fluid inclusions. In Fluid Inclusion: Analysis and Interpretation; Mineralogical Association of Canada: Ottawa, ON, Canada, 2003; Volume 32, pp. 9–53. [Google Scholar]
- Clayton, R.N.; O’Neil, J.R.; Mayeda, T.K. Oxygen isotope exchange between quartz and water. J. Geophys. Res. 1972, 77, 3057–3067. [Google Scholar] [CrossRef]
- Ramboz, C.; Pichavant, M.; Weisbrod, A. Fluid immiscibility in natural processes: Use and misuse of fluid inclusion data: II. Interpretation of fluid inclusion data in terms of immiscibility. Chem. Geol. 1982, 37, 1–27. [Google Scholar] [CrossRef]
- Li, S.D.; Zhang, X.B.; Gao, L.L. Ore genesis at the Jinchang gold–copper deposit in Heilongjiang Province, Northeastern China: Evidence from geology, fluid inclusions, and H–O–S isotopes. Minerals 2019, 9, 99. [Google Scholar] [CrossRef]
- Driesner, T.; Heinrich, C.A. The system H2O–NaCl. Part I: Correlation formulae for phase relations in temperature–pressure–composition space from 0 to 1000 °C, 0 to 5000 bar, and 0 to 1 XNaCl. Geochim. Cosmochim. Acta 2007, 71, 4880–4901. [Google Scholar] [CrossRef]
- Zhu, J.J.; Hu, R.; Richards, J.P.; Bi, X.; Zhong, H. Genesis and magmatic–hydrothermal evolution of the Yangla skarn Cu deposit, southwest China. Econ. Geol. 2015, 110, 631–652. [Google Scholar] [CrossRef]
- Hedenquist, J.W.; Richards, J.P. The influence of geochemical techniques on the development of genetic models for porphyry copper deposits. In Reviews in Economic Geology: Techniques in Hydrothermal Ore Deposits Geology; Richards, J.P., Larson, P.B., Eds.; Society of Economic Geologists, Inc.: Littleton, CO, USA, 1998; pp. 235–256. [Google Scholar]
- Siahcheshm, K.; Calagari, A.A.; Abedini, A. Hydrothermal evolution in the Maher–Abad porphyry Cu–Au deposit, SW Birjand, Eastern Iran: Evidence from fluid inclusions. Ore Geol. Rev. 2014, 58, 1–13. [Google Scholar] [CrossRef]
- Taylor, H. The application of oxygen and hydrogen isotope studies to problems of hydrothermal alteration and ore deposition. Econ. Geol. 1974, 69, 843–883. [Google Scholar] [CrossRef]
- Heinrich, C.A.; Driesner, T.; Stefánsson, A.; Seward, T.M. Magmatic vapor contraction and the transport of gold from the porphyry environment to epithermal ore deposits. Geology 2004, 32, 761–764. [Google Scholar] [CrossRef]
- Ulrich, T.; Gunther, D.; Heinrich, C.A. The evolution of a porphyry Cu–Au deposit, based on LA–ICP–MS analysis of fluid inclusions: Bajo de la Alumbrera, Argentina. Econ. Geol. 2002, 97, 1889–1920. [Google Scholar] [CrossRef]
- Clark, I.D.; Fritz, P. Environmental Isotopes in Hydrogeology; Lewis Publishers: New York, NY, USA, 1997; pp. 1–328. [Google Scholar]
- Rye, R.O.; Ohmoto, H. Sulfur and carbon isotopes and ore genesis: A review. Econ. Geol. 1974, 69, 826–842. [Google Scholar] [CrossRef]
- Ohmoto, H. Systematics of sulfur and carbon isotopes in hydrothermal ore deposits. Econ. Geol. 1972, 67, 551–578. [Google Scholar] [CrossRef]
- Ohmoto, H.; Goldhaber, M. Sulphur and carbon isotopes. In Geochemistry of Hydrothermal Ore Deposits; Barnes, H.L., Ed.; Wiley: New York, NY, USA, 1997; pp. 517–611. [Google Scholar]
- Zheng, Y.F. Stable Isotope Geochemistry; Science Press: Beijing, China, 2000; (In Chinese with English Abstract). [Google Scholar]
- Li, Y. Geological and Geochemical Characteristics and Genesis of Dabate Cu–Mo Deposit, Western Tianshan, Xinjiang. Master’s Thesis, China University of Geosciences, Beijing, China, 2012. (In Chinese with English Abstract). [Google Scholar]
- Yin, Y.Q.; Xie, X.; Wang, J.W.; Wang, Y.S.; Tian, W.; Liu, Y.H.; Liu, F.M. Geologic geochemistry characteristics of the Beidabate body in Wenquan County, Xinjiang. J. Guilin Univ. Technol. 2006, 26, 449–455, (In Chinese with English Abstract). [Google Scholar]
- Zartman, R.E.; Doe, B.R. Plumbotectonics–the Model. Tectonophysics 1981, 75, 135–162. [Google Scholar] [CrossRef]
- Bierlein, F.P.; McNaughton, N.J. Pb isotope fingerprinting of mesothermal gold deposits from central Victoria, Australia: Implications for ore genesis. Miner. Depos. 1998, 33, 633–638. [Google Scholar] [CrossRef]
- Qiu, Y.; McNaughton, N.J. Source of Pb in orogenic lode–gold mineralisation: Pb isotope constraints from deep crustal rocks from the southwestern Archaean Yilgarn Craton, Australia. Miner. Depos. 1999, 34, 366–381. [Google Scholar] [CrossRef]
- Stacey, J.S. Approximation of terrestrial lead isotope evolution by a two–stage model. Earth Planet. Sci. Lett. 1975, 26, 207–221. [Google Scholar] [CrossRef]
- Doe, B.R.; Zartman, R.E. Plumbotectonics, the Phanerozoic. In Geochemistry of Hydrothermal Ore Deposits, 2nd ed.; Barenes, H.L., Ed.; John Wiley: New York, NY, USA, 1979; pp. 509–567. [Google Scholar]
- Li, S.D.; Wang, K.Y.; Wang, Y.C.; Zhang, X.B.; Quan, H.Y. Genesis of the Bairendaba Ag–Zn–Pb deposit, Southern Great Xing’an Range, NE China: A fluid inclusion and stable isotope study. Geofluids 2017, 2017, 1206587. [Google Scholar] [CrossRef]




| Sample | Host Mineral | FIA | FI Type | Number | Gas–Liquid Ratio | Tm (ice) (°C) | Tm (NaCl) (°C) | Salinity (NaCl wt %) | Th (°C) |
|---|---|---|---|---|---|---|---|---|---|
| KR–1 | Quartz from pyrite– chalcopyrite– quartz veins (stage 1) | FIA–1 | LV | 8 | 10 | −6.3 to −5.3 | 8.3–9.6 (9.0 ± 0.4) | 221–235 (228 ± 4) | |
| FIA–2 | LV | 2 | 15 | −5.5 to −5.1 | 8.0–8.5 (8.3 ± 0.4) | 232–240 (234 ± 5) | |||
| KR–2 | FIA–3 | VL | 3 | 80 | −1.5 to −1.0 | 1.7–2.6 (2.2 ± 0.4) | 241–248 (245 ± 3) | ||
| FIA–4 | LV | 3 | 30 | −5.0 to −5.7 | 7.9–8.8 (2.5 ± 0.4) | 235–243 (239 ± 3) | |||
| KR–5 | FIA–5 | VL | 3 | 85 | −1.8 to −1.2 | 2.1–3.1 (9.0 ± 0.4) | 245–252 (248 ± 3) | ||
| S | 2 | 20 | 223–254 | 33.1–34.9 (34.0 ± 0.9) | 242–254 (248 ± 6) | ||||
| FIA–6 | LV | 7 | 15 | −4.7 to−3.8 | 6.2–7.4 (6.7 ± 0.4) | 235–246 (239 ± 4) | |||
| KR–7 | FIA–7 | VL | 4 | 90 | −1.8 to −1.0 | 1.7–3.1 (2.4 ± 0.5) | 238–249 (243 ± 4) | ||
| S | 3 | 15 | 226–249 | 33.3–34.6 (33.9 ± 0.5) | 239–251 (246 ± 5) | ||||
| KR–8 | FIA–8 | S | 6 | 20 | 215–238 | 32.7–33.9 (33.3 ± 0.4) | 229–238 (234 ± 3) | ||
| FIA–9 | LV | 9 | 15 | −5.0 to −4.2 | 6.7–7.9 (7.4 ± 0.4) | 225–235 (231 ± 3) | |||
| KR–9 | FIA–10 | LV | 6 | 10 | −4.9 to −5.3 | 7.7–8.3 (8.1 ± 0.2) | 235–247 (240 ± 4) | ||
| FIA–11 | LV | 3 | 15 | −5.8 to −5.5 | 8.5–8.9 (8.1 ± 0.2) | 229–239 (234 ± 4) | |||
| KR–12 | Quartz from galena– sphalerite– quartz (stage 2) | FIA–12 | LV | 5 | 10 | −4.8 to −3.9 | 6.3–7.6 (7.0 ± 0.5) | 182–194 (187 ± 4) | |
| KR–15 | FIA–13 | LV | 6 | 10 | −5.0 to−4.2 | 6.7–7.9 (7.3 ± 0.4) | 196–207 (203 ± 4) | ||
| FIA–14 | S | 3 | 15 | 200–205 | 31.9–32.1 (32.5 ± 0.3) | 209–220 (213 ± 5) | |||
| KR–16 | FIA–15 | LV | 6 | 15 | −4.5 to −3.1 | 5.1–7.2 (6.3 ± 0.7) | 183–191 (187 ± 2) | ||
| KR–19 | FIA–16 | LV | 8 | 20 | −4.9 to −4.1 | 6.6–7.7 (7.2 ± 0.3) | 182–196 (189 ± 5) | ||
| FIA–17 | LV | 4 | 15 | −4.3 to −3.5 | 5.7–6.9 (6.3 ± 0.4) | 179–189 (185 ± 3) | |||
| KR–20 | FIA–18 | LV | 5 | 15 | −4.9 to −3.6 | 5.9–7.7 (6.6 ± 0.5) | 194–206 (200 ± 4) | ||
| FIA–19 | LV | 4 | 10 | −4.2 to −3.5 | 5.7–6.7 (6.2 ± 0.4) | 189–203 (196 ± 5) | |||
| FIA–20 | LV | 2 | 10 | −3.8 to −3.6 | 5.9–6.2 (6.1 ± 0.2) | 185–190 (188 ± 3) | |||
| KR–22 | FIA–21 | LV | 8 | 15 | −4.7 to −3.5 | 5.7–7.4 (6.3 ± 0.6) | 183–199 (191 ± 5) | ||
| FIA–22 | LV | 9 | 10 | −4.2 to −3.2 | 5.3–6.7 (6.0 ± 0.5) | 173–189 (183 ± 5) | |||
| KR–23 | Quartz from sulfide-poor- calcite– quartz veins (stage 3) | FIA–23 | LV | 2 | 15 | −3.3 to −3.0 | 5.3–5.4 (5.4 ± 0.1) | 168–171 (170 ± 5) | |
| KR–25 | FIA–24 | LV | 10 | 10 | −3.5 to −2.3 | 3.9–5.7 (4.7 ± 0.6) | 146–158 (150 ± 3) | ||
| FIA–25 | L | 6 | |||||||
| KR–27 | FIA–26 | LV | 9 | 10 | −3.5 to −2.5 | 4.6–5.7 (4.8 ± 0.5) | 149–160 (155 ± 4) | ||
| FIA–27 | L | 8 | |||||||
| KR–29 | FIA–28 | LV | 6 | 10 | −3.6 to −2.8 | 4.6–5.9 (5.2 ± 0.6) | 161–172 (168 ± 4) | ||
| KR–30 | FIA–29 | LV | 2 | 10 | −3.2 to −2.8 | 4.6–5.3 (5.0 ± 0.4) | 155–160 (158 ± 3) | ||
| FIA–30 | LV | 4 | 5 | −3.6 to −2.2 | 3.7–5.9 (4.6 ± 0.8) | 149–162 (154 ± 5) | |||
| FIA–31 | LV | 5 | 5 | −3.2 to −2.0 | 3.4–5.3 (4.6 ± 0.7) | 145–157 (150 ± 5) | |||
| L | 2 |
| Sample | Stage | δ18Oqz (‰) SMOW | δDH2O (‰) SMOW | Th (°C) | δ18OH2O (‰) SMOW | δCH2O (‰) PDB |
|---|---|---|---|---|---|---|
| KR–2 | 1 | 10.1 | −83.1 | 242 ± 4 | 0.7 ± 0.2 | −9.1 |
| KR–5 | 1 | 9.5 | −86.9 | 243 ± 6 | 0.2 ± 0.3 | −11.5 |
| KR–7 | 1 | 10.9 | −85.6 | 244 ± 5 | 1.7 ± 0.3 | −9.4 |
| KR–9 | 1 | 8.5 | −87.4 | 238 ± 5 | −1.0 ± 0.3 | −10.5 |
| KR–15 | 2 | 8.3 | −90.3 | 206 ± 7 | −3.0 ± 0.4 | −13.9 |
| KR–19 | 2 | 8.1 | −90.1 | 188 ± 5 | −4.4 ± 0.3 | −11.8 |
| KR–20 | 2 | 8.5 | −87.6 | 196 ± 6 | −3.5 ± 0.4 | −13.2 |
| KR–22 | 2 | 8.6 | −88.5 | 187 ± 7 | −4.0 ± 0.5 | −12.0 |
| KR–25 | 3 | 7.9 | −94.7 | 150 ± 3 | −7.6 ± 0.3 | −16.0 |
| KR–27 | 3 | 7.8 | −96.5 | 155 ± 4 | −7.3 ± 0.3 | −16.2 |
| KR–29 | 3 | 8.1 | −96.2 | 168 ± 4 | −6.1 ± 0.5 | −15.5 |
| KR–30 | 3 | 7.6 | −99.2 | 158 ± 3 | −7.7 ± 0.5 | −16.6 |
| Sample | Mineral | Stage | δ34SGn (‰) CDT | δ34SH2S (‰) CDT | Th (°C) |
|---|---|---|---|---|---|
| KR–12 | Galena | 2 | 5.6 | 7.4 ± 0.1 | 187 ± 4 |
| KR–16 | Galena | 2 | 6.5 | 8.3 ± 0.1 | 187 ± 2 |
| KR–19 | Galena | 2 | 6 | 7.8 ± 0.1 | 189 ± 5 |
| KR–20 | Galena | 2 | 6.1 | 7.7 ± 0.1 | 200 ± 4 |
| KR–25 | Galena | 3 | 6.6 | 9.4 ± 0.1 | 150 ± 3 |
| KR–27 | Galena | 3 | 6.9 | 9.5 ± 0.1 | 155 ± 4 |
| Sample | Mineral | Stage | 206Pb/204Pb | 207Pb/204Pb | 208Pb/204Pb |
|---|---|---|---|---|---|
| KR–12 | Galena | 2 | 18.253 | 15.632 | 38.097 |
| KR–16 | Galena | 2 | 18.002 | 15.598 | 38.115 |
| KR–19 | Galena | 2 | 18.246 | 15.602 | 38.149 |
| KR–20 | Galena | 2 | 18.221 | 15.618 | 38.171 |
| KR–25 | Galena | 3 | 18.265 | 15.628 | 38.199 |
| KR–27 | Galena | 3 | 18.273 | 15.643 | 38.209 |
| Geological Unit | Lithology | Sample | Cu (ppm) | Pb (ppm) | Zn (ppm) | Cr (ppm) | Ni (ppm) | Co (ppm) | V (ppm) |
|---|---|---|---|---|---|---|---|---|---|
| Tuosikuertawu Formation | Medium –fine grained sandstone | KB–10 | 25.1 | 24.5 | 210.3 | 54.6 | 22.1 | 10.0 | 80.7 |
| KB–18 | 26.1 | 32.2 | 687.6 | 49.4 | 23.3 | 11.9 | 64.3 | ||
| KB–21 | 41.8 | 61.6 | 529.1 | 63.6 | 29.8 | 13.5 | 85.9 | ||
| Average | 31.0 | 39.4 | 475.6 | 55.9 | 25.1 | 11.8 | 77.0 | ||
| Siltstone | KB–4 | 25.2 | 20.5 | 90.1 | 49.0 | 25.3 | 10.4 | 66.6 | |
| KB–15 | 24.0 | 20.4 | 261.3 | 45.8 | 23.1 | 12.1 | 60.5 | ||
| KB–17 | 26.3 | 24.6 | 137.8 | 49.7 | 23.8 | 10.8 | 68.6 | ||
| KB–26 | 67.9 | 23.0 | 246.2 | 62.2 | 23.3 | 13.5 | 70.3 | ||
| Average | 35.9 | 22.1 | 183.8 | 51.7 | 23.8 | 11.7 | 66.5 | ||
| Medium –coarse grained sandstone | KB–2 | 32.7 | 30.7 | 133.2 | 69.8 | 38.9 | 13.4 | 117.7 | |
| KB–5 | 24.8 | 22.6 | 84.1 | 57.2 | 33.3 | 14.4 | 88.3 | ||
| KB–14 | 25.7 | 33.6 | 474.4 | 46.0 | 20.7 | 10.0 | 62.8 | ||
| KB–28 | 80.1 | 21.9 | 899.4 | 51.2 | 22.4 | 11.1 | 58.9 | ||
| Average | 40.8 | 27.2 | 397.8 | 56.1 | 28.8 | 12.2 | 81.9 | ||
| Beidabate intrusive body | C08–1 | 274.7 | 6.9 | 23.0 | 26.3 | 2.8 | 2.5 | 10.7 | |
| B25–1 | 40.3 | 6.2 | 31.0 | 22.0 | 2.5 | 2.5 | 10.9 | ||
| C7–2 | 202.5 | 15.2 | 32.0 | 24.0 | 2.8 | 2.5 | 9.2 | ||
| A2 | 371.7 | 7.2 | 28.0 | 34.2 | 4.2 | 2.6 | 16.9 | ||
| A12 | 357.9 | 20.7 | 47.0 | 27.4 | 2.8 | 2.4 | 9.2 | ||
| Average | 249.4 | 11.2 | 32.2 | 26.8 | 3.0 | 2.5 | 11.4 | ||
| Clarke | 30 | 15 | 60 | 70 | 44 | 12 | 95 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Chen, C.; Gao, L.; Xia, F.; Zhang, X.; Wang, K.; Arkin, K. Ore Genesis of the Kuergasheng Pb–Zn Deposit, Xinjiang Province, Northwest China: Constraints from Geology, Fluid Inclusions, and H–O–C–S–Pb Isotopes. Minerals 2020, 10, 592. https://doi.org/10.3390/min10070592
Li S, Chen C, Gao L, Xia F, Zhang X, Wang K, Arkin K. Ore Genesis of the Kuergasheng Pb–Zn Deposit, Xinjiang Province, Northwest China: Constraints from Geology, Fluid Inclusions, and H–O–C–S–Pb Isotopes. Minerals. 2020; 10(7):592. https://doi.org/10.3390/min10070592
Chicago/Turabian StyleLi, Shunda, Chuan Chen, Lingling Gao, Fang Xia, Xuebing Zhang, Keyong Wang, and Kurbanjan Arkin. 2020. "Ore Genesis of the Kuergasheng Pb–Zn Deposit, Xinjiang Province, Northwest China: Constraints from Geology, Fluid Inclusions, and H–O–C–S–Pb Isotopes" Minerals 10, no. 7: 592. https://doi.org/10.3390/min10070592
APA StyleLi, S., Chen, C., Gao, L., Xia, F., Zhang, X., Wang, K., & Arkin, K. (2020). Ore Genesis of the Kuergasheng Pb–Zn Deposit, Xinjiang Province, Northwest China: Constraints from Geology, Fluid Inclusions, and H–O–C–S–Pb Isotopes. Minerals, 10(7), 592. https://doi.org/10.3390/min10070592




