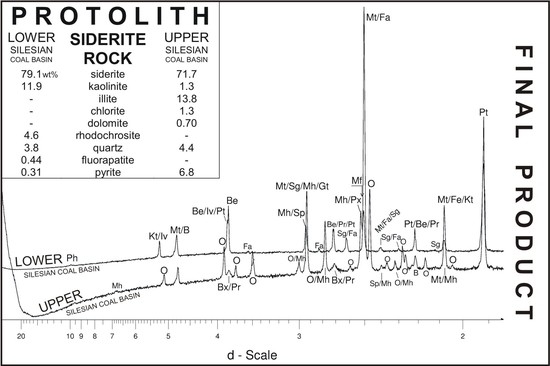
The Behaviour of Siderite Rocks in an Experimental Imitation of Pyrometamorphic Processes in Coal-Waste Fires: Upper and Lower Silesian Case, Poland
Abstract
1. Introduction
2. Methods
3. Materials
4. Results and Discussion
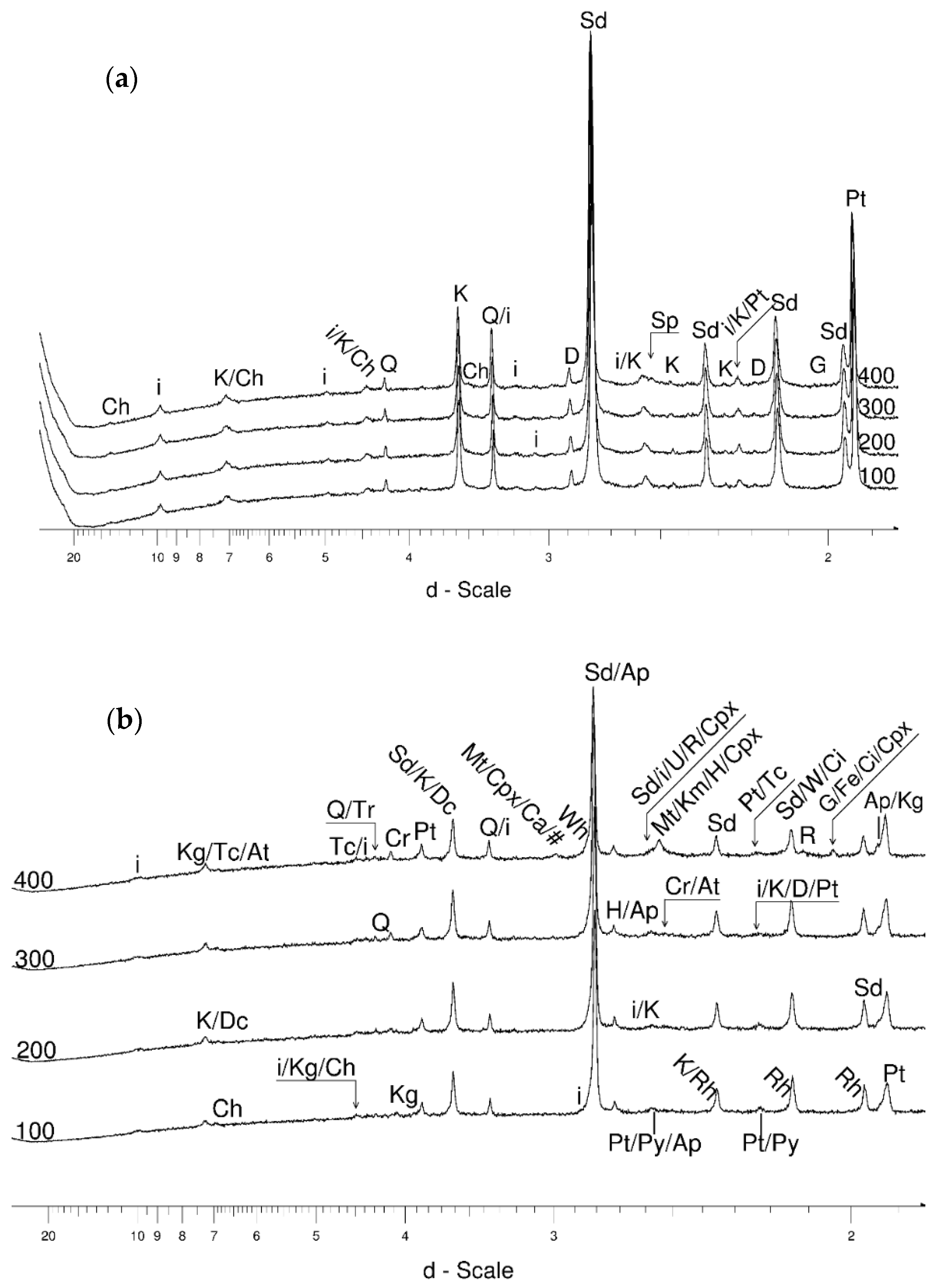
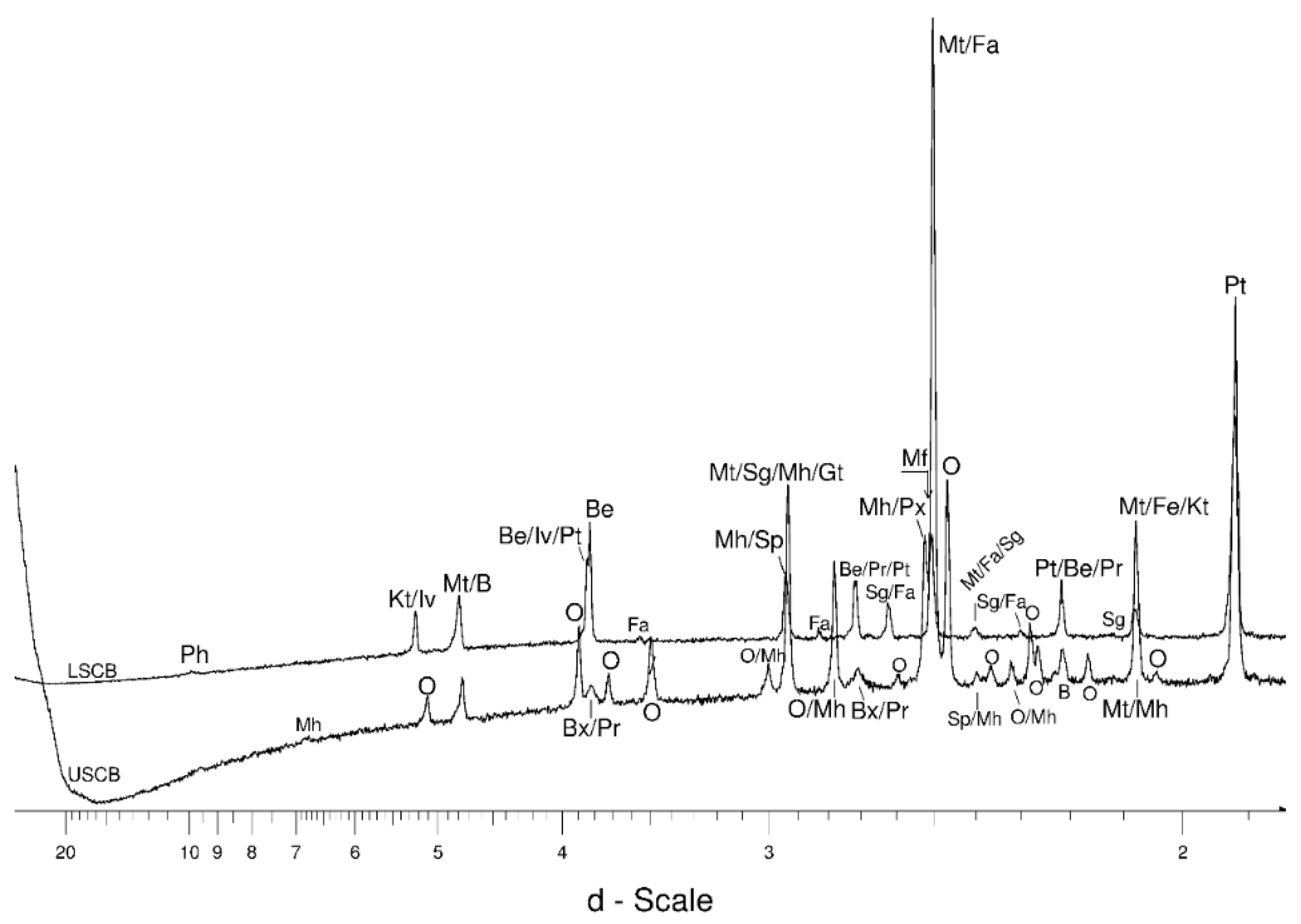
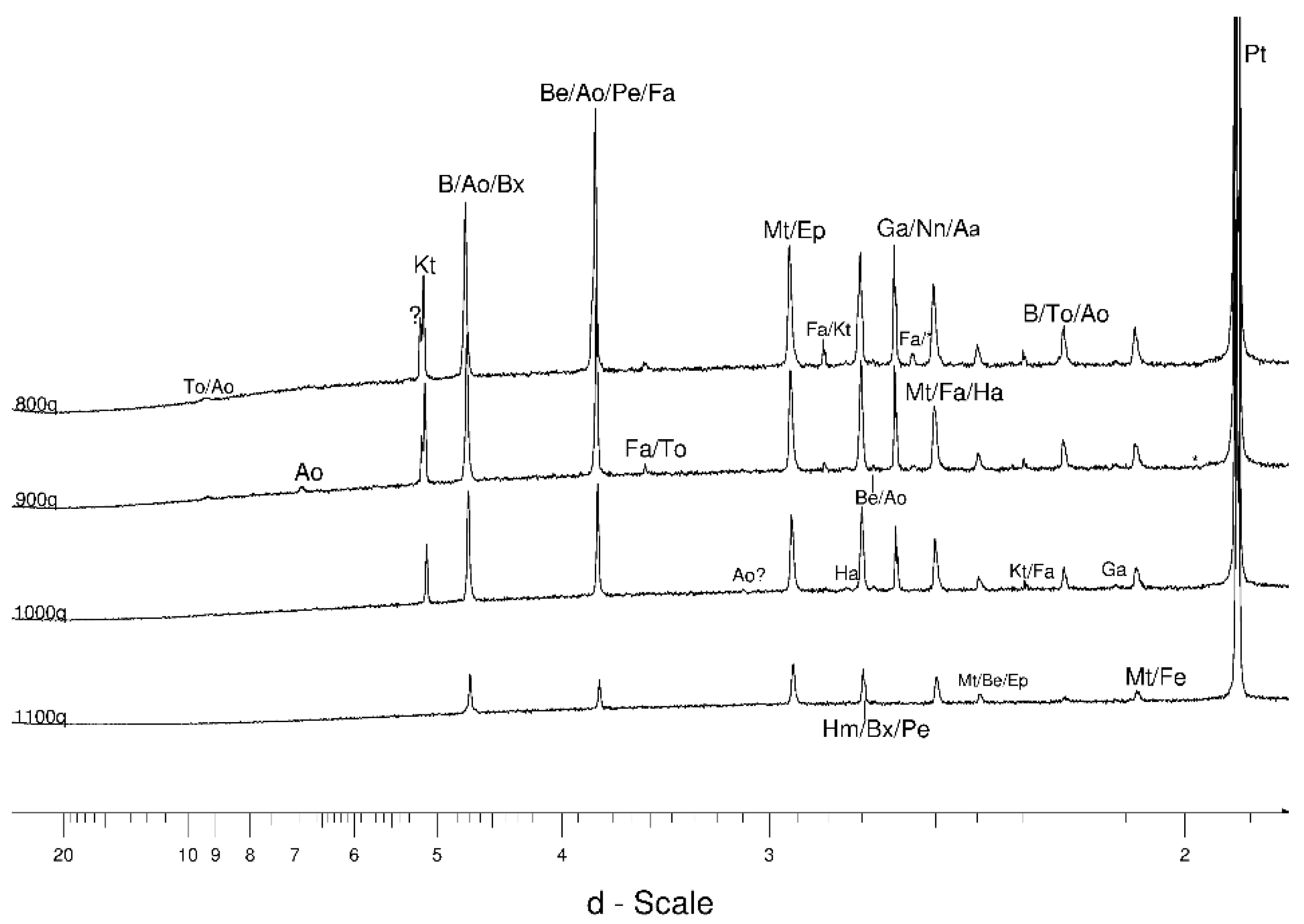
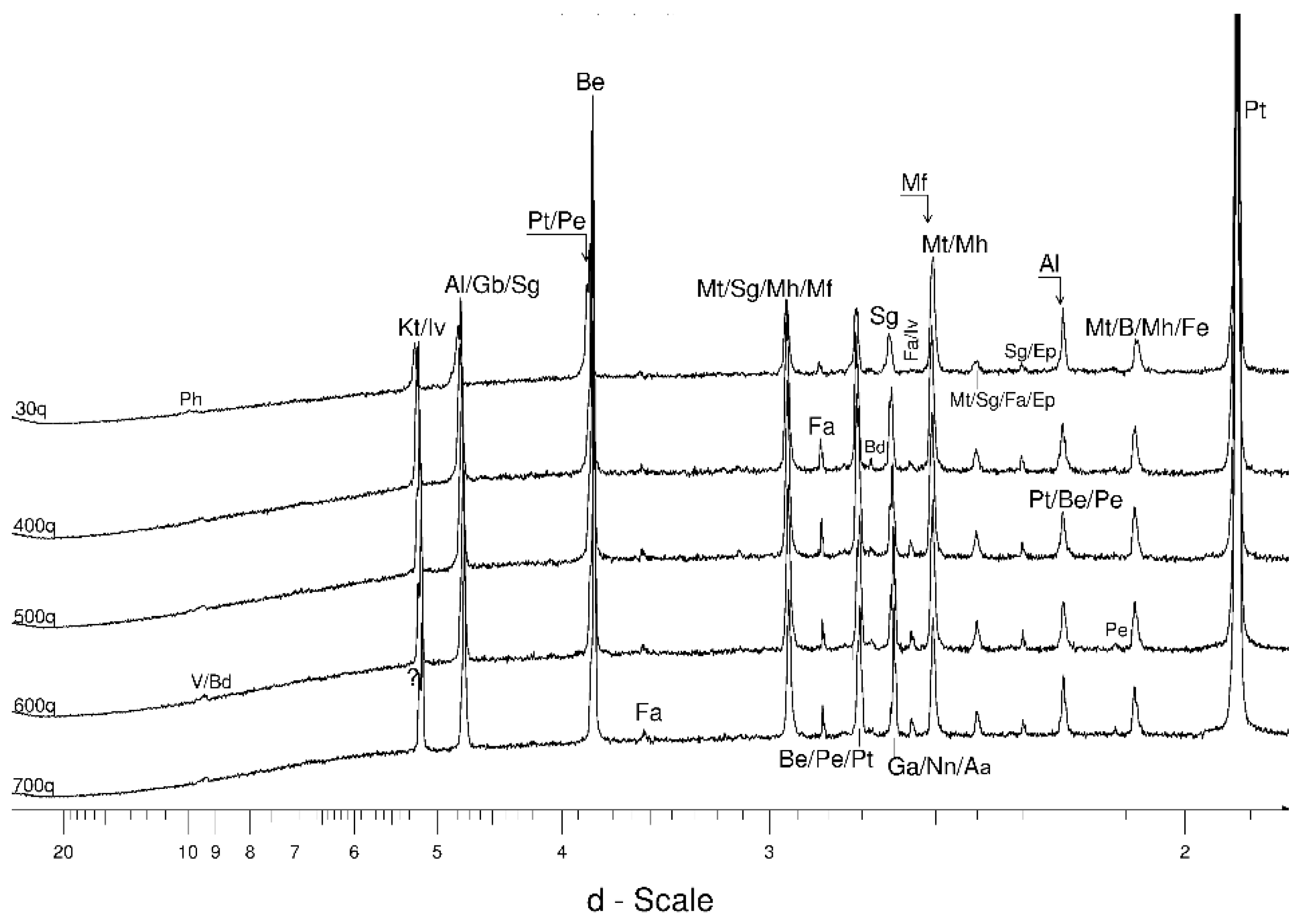
4.1. LSCB Siderite—Possible Phases Formed during the Heating—and Cooling Experiment
4.2. Comparative Characterization with USCB Siderite
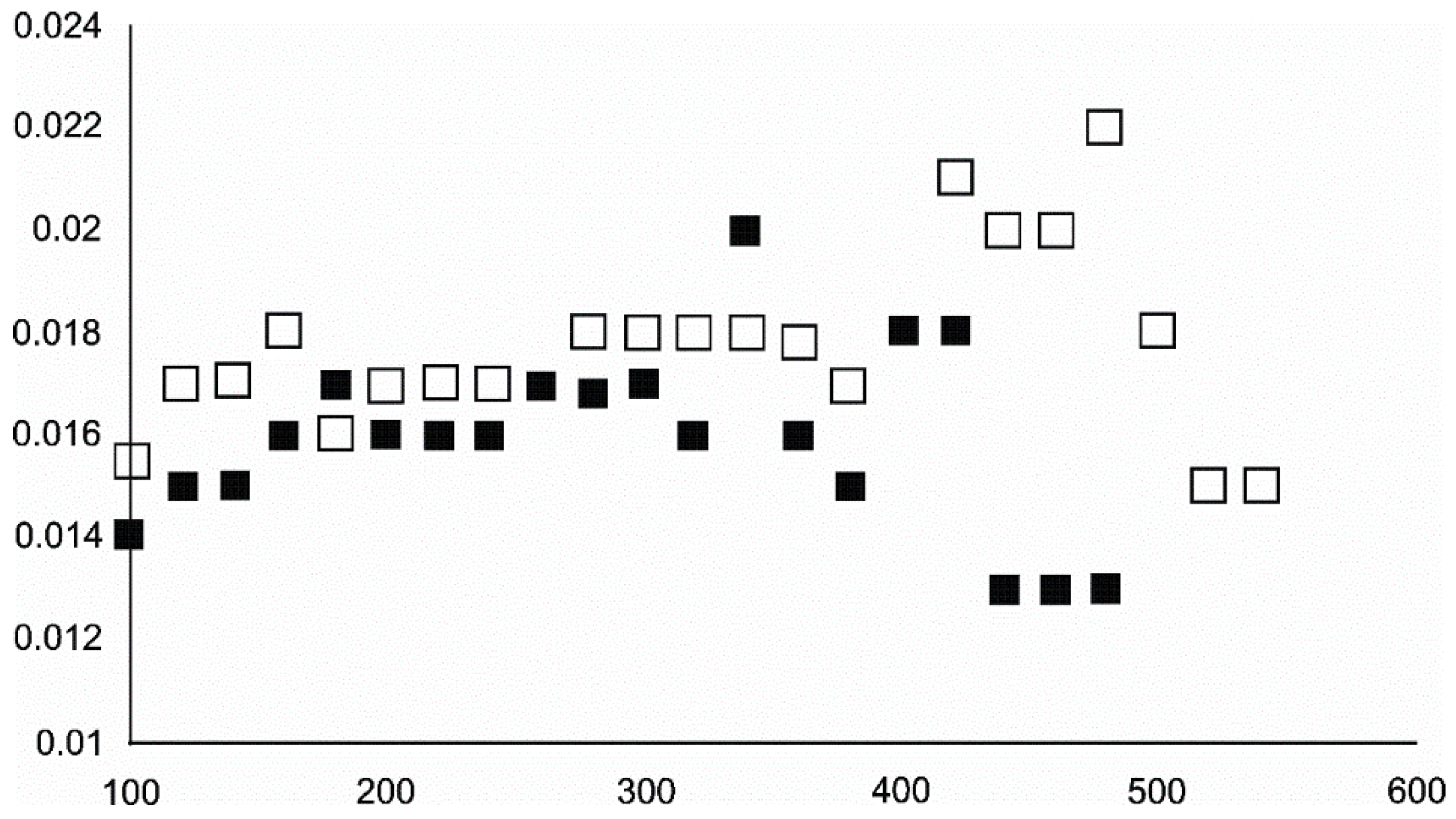
4.3. The Behaviour of the Main Mineral Phases during the Experiment
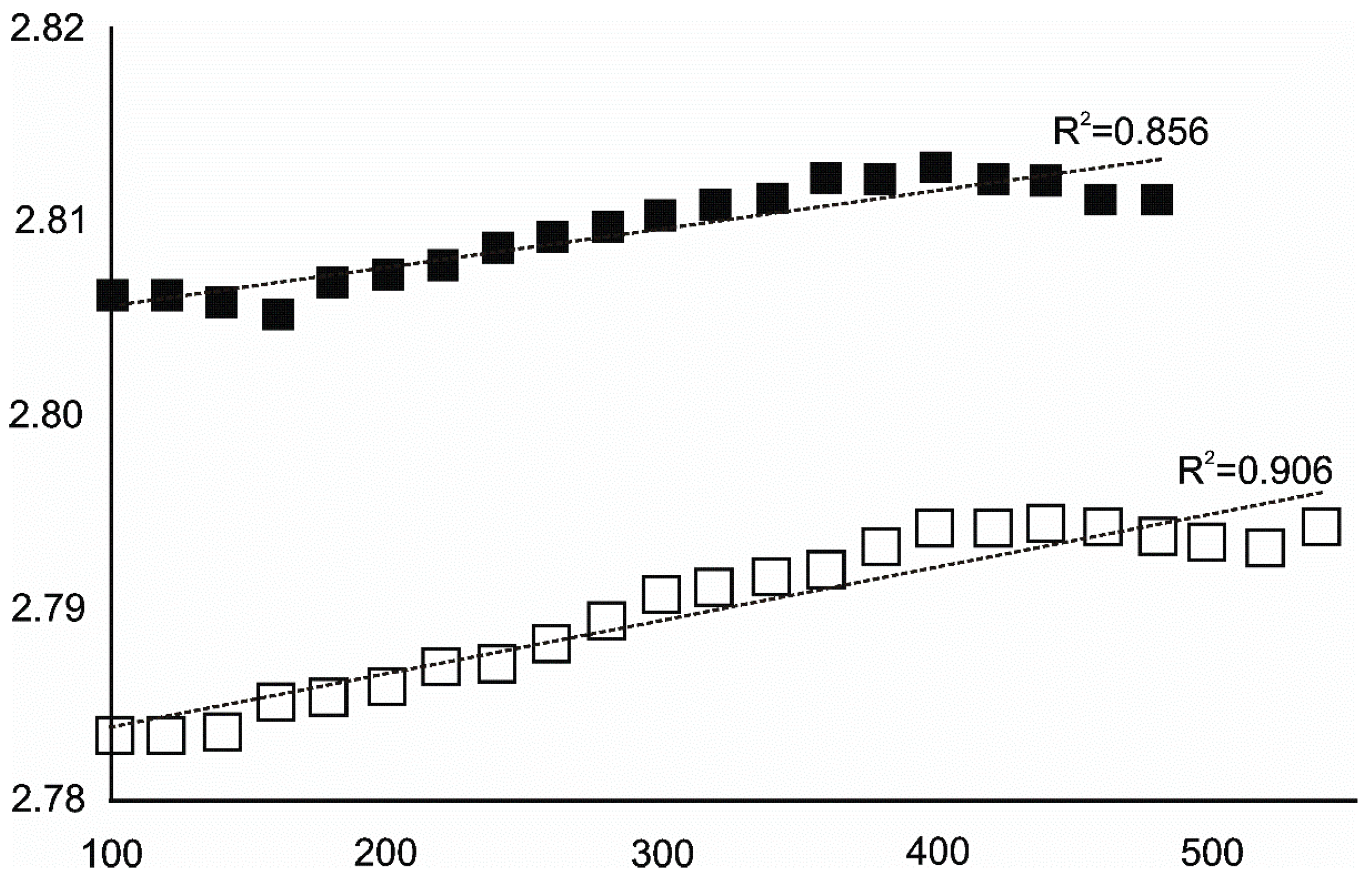
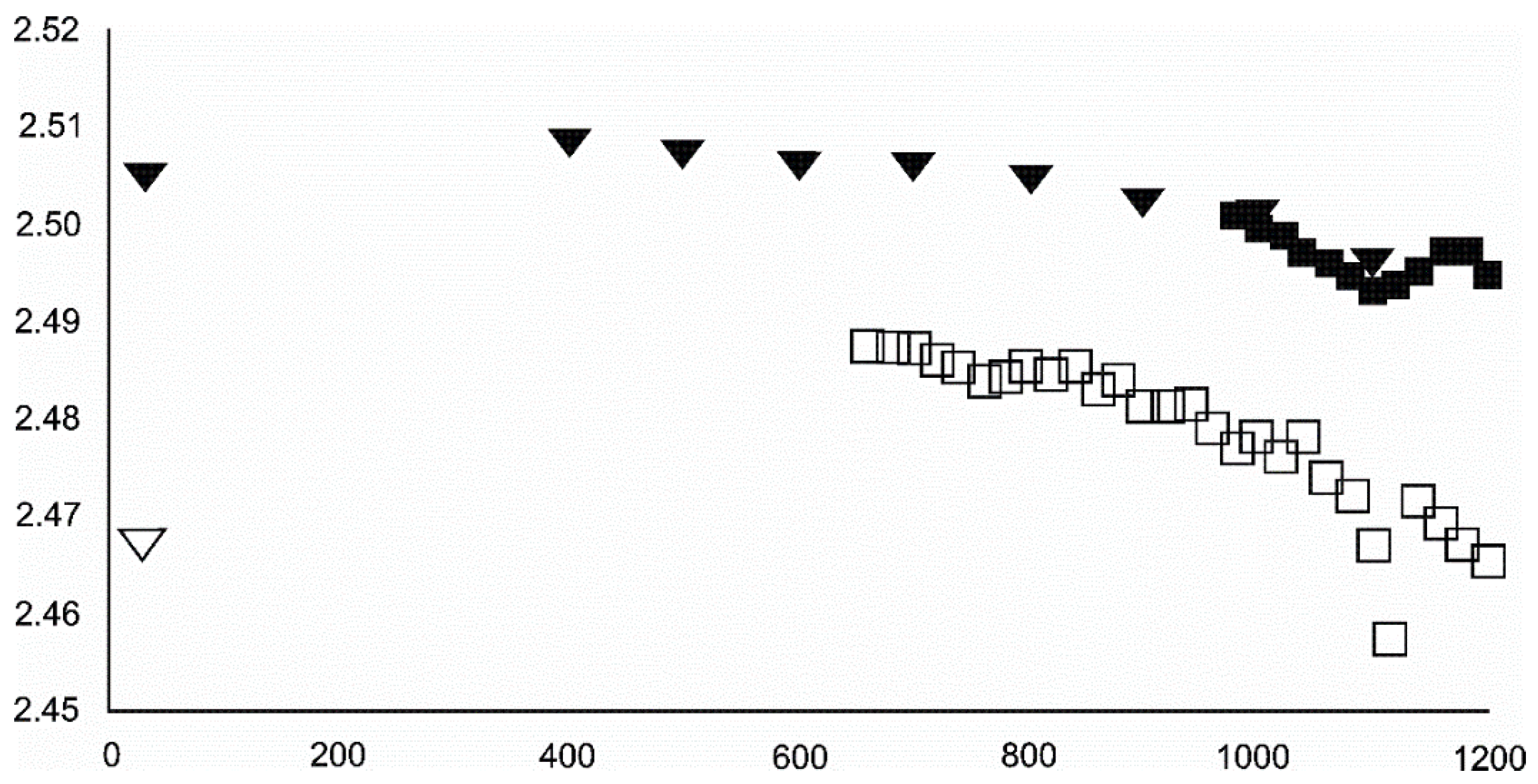
4.3.1. Siderite
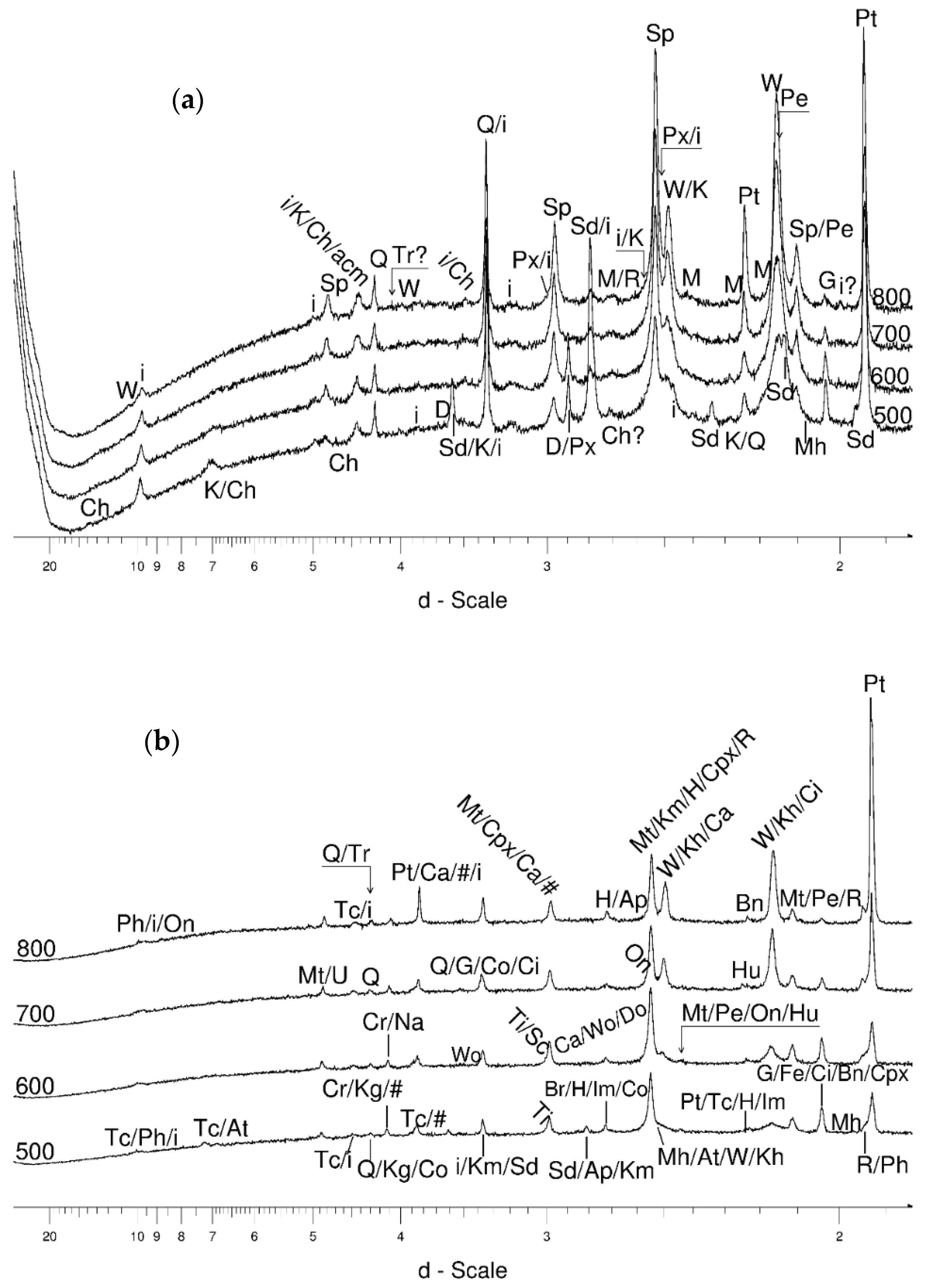
4.3.2. Magnetite and Wüstite
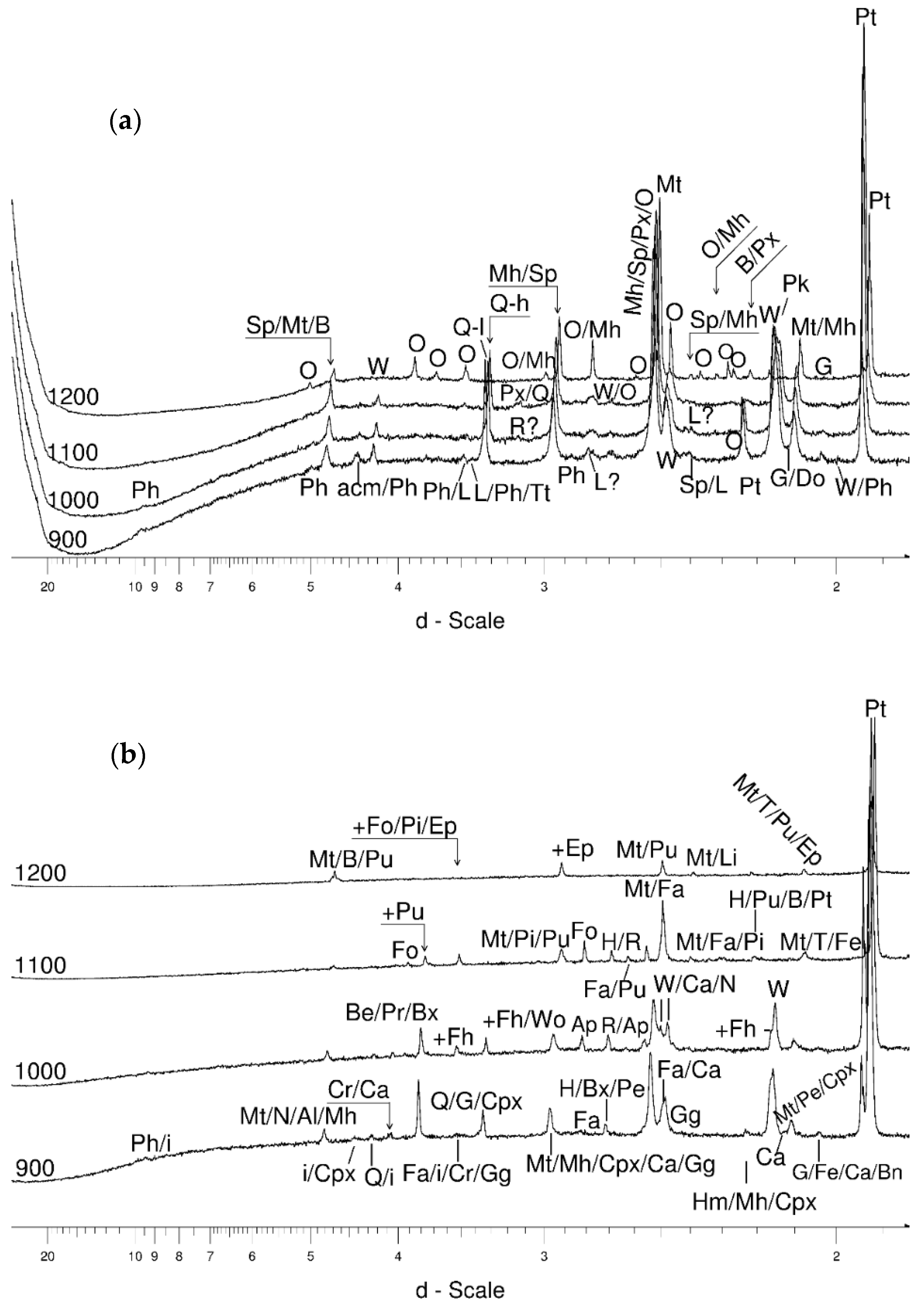
4.3.3. Olivine
4.3.4. Other Phases
5. Conclusions
- -
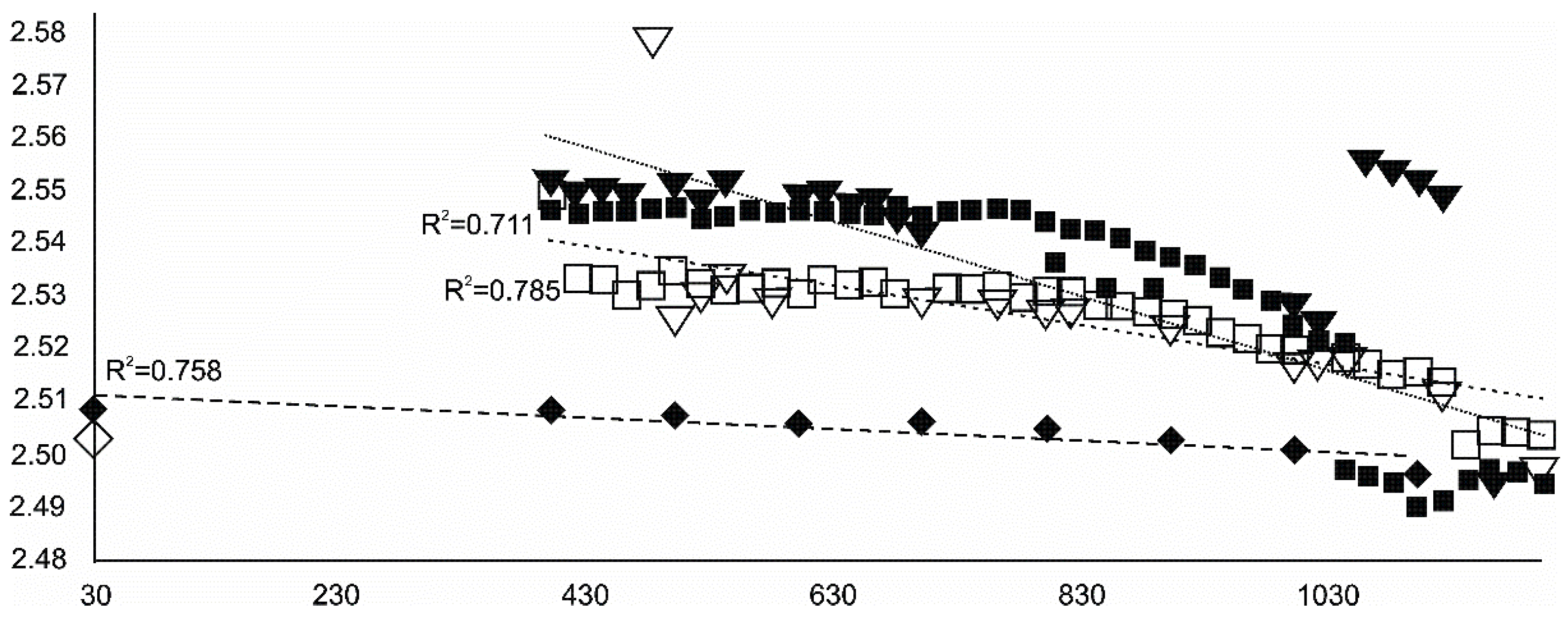
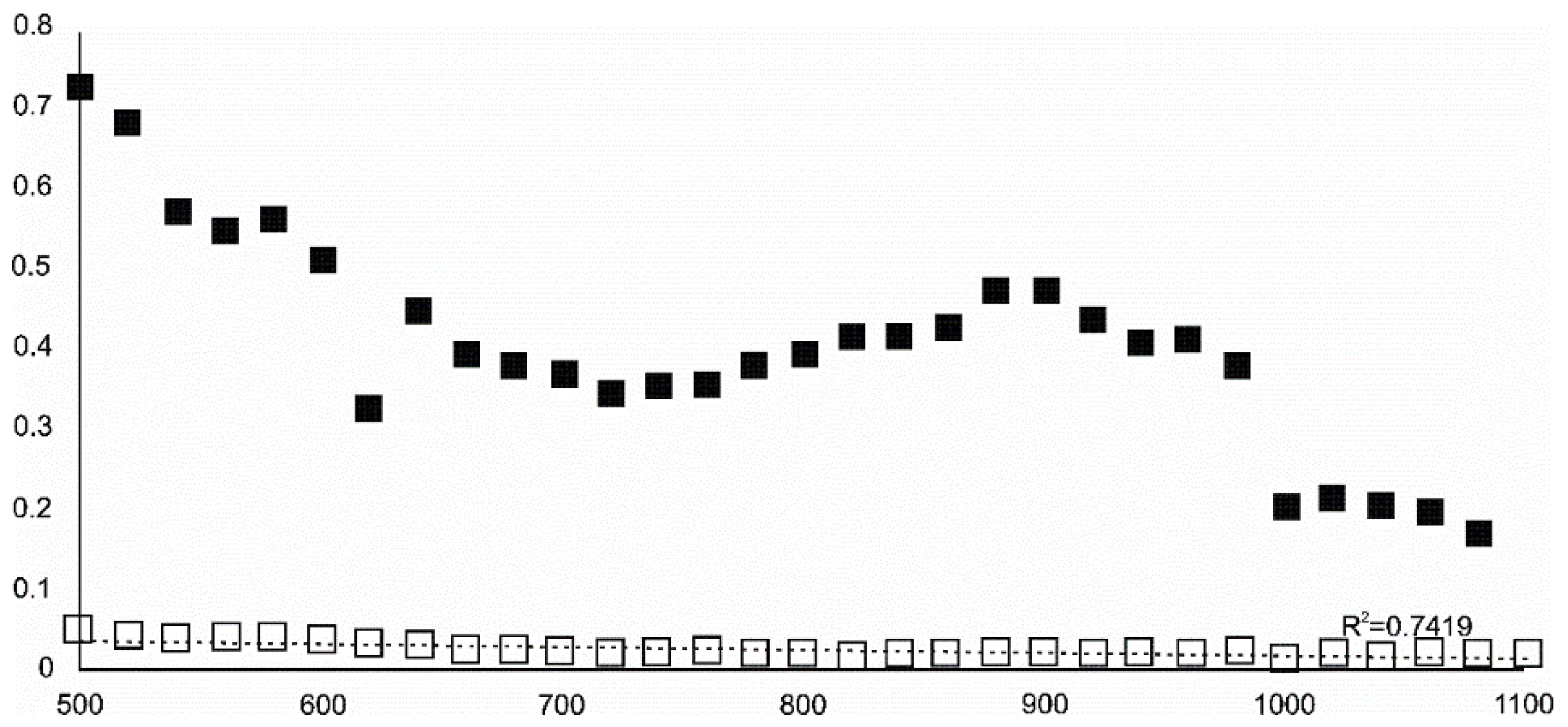
- Siderite was still present at 480 °C but diminished at 500 °C and, in the USCB sample, vanished at ~560 °C.
- -
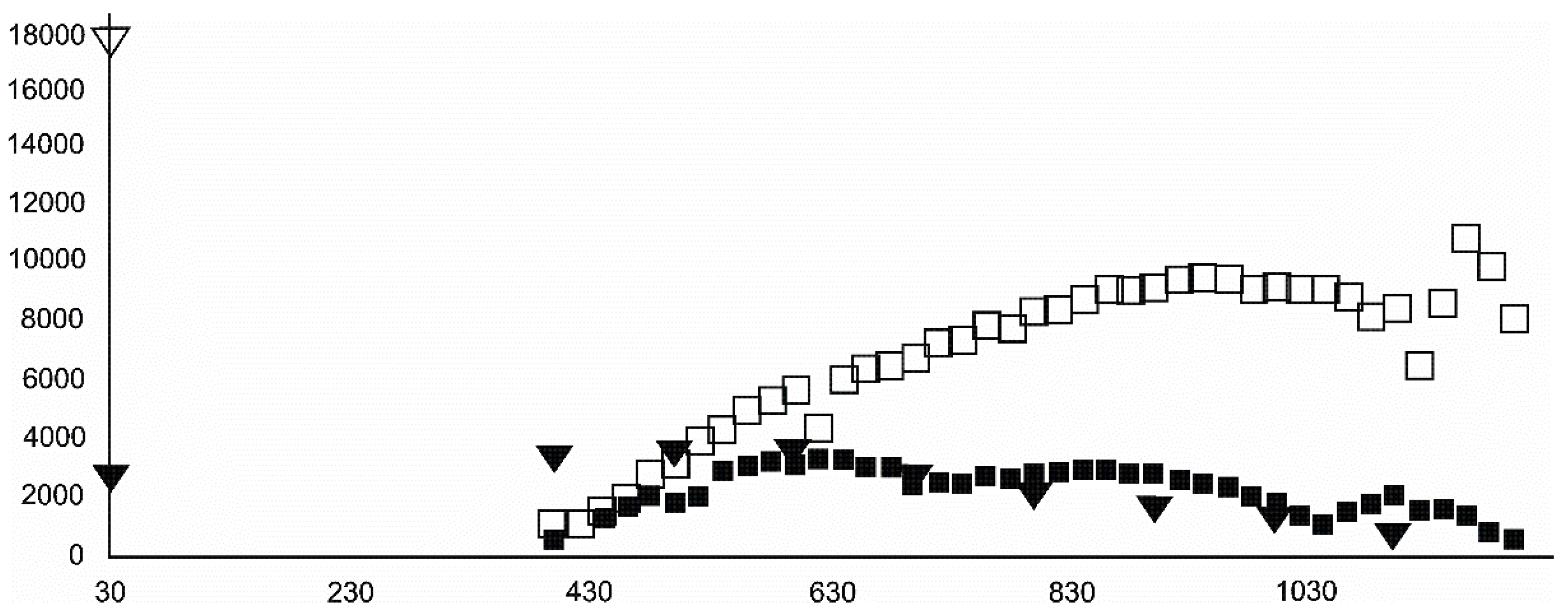
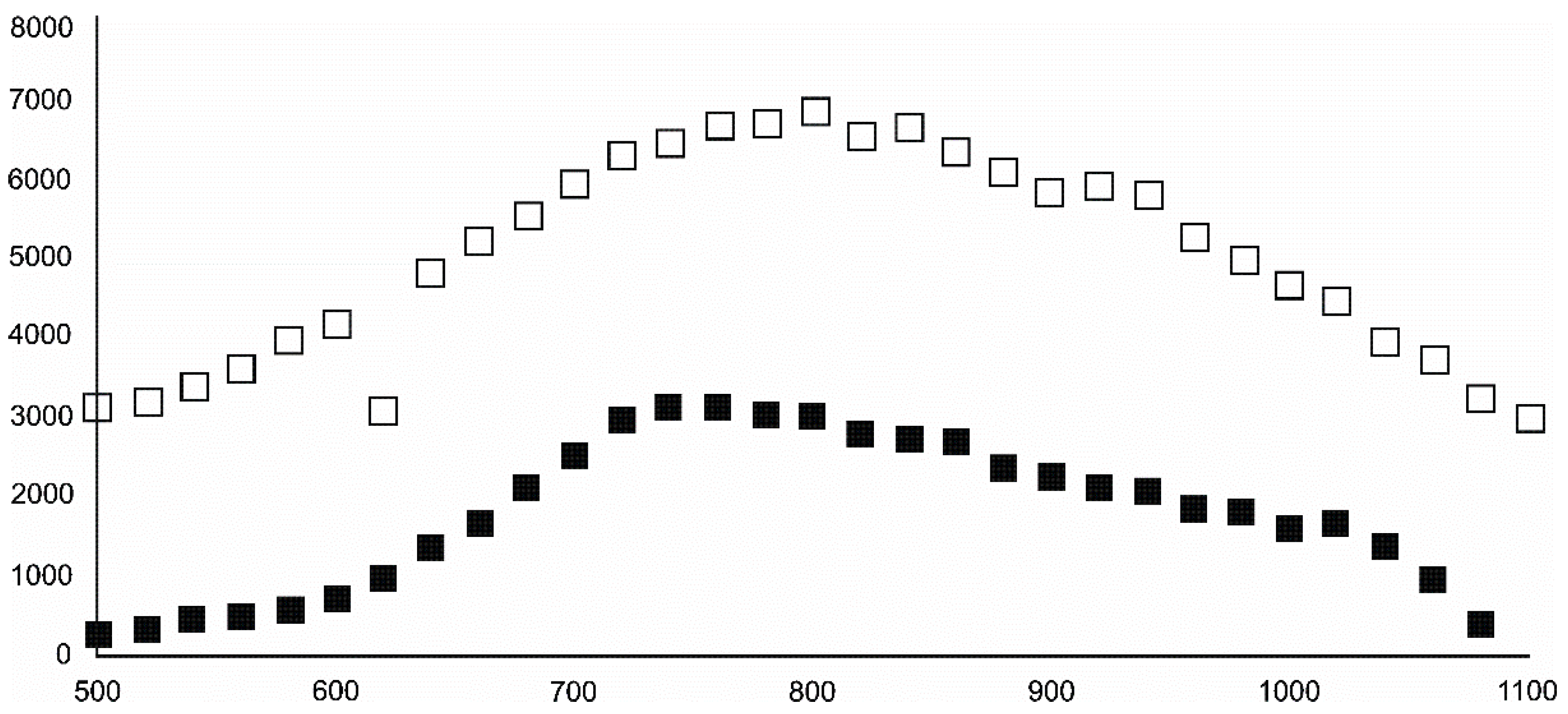
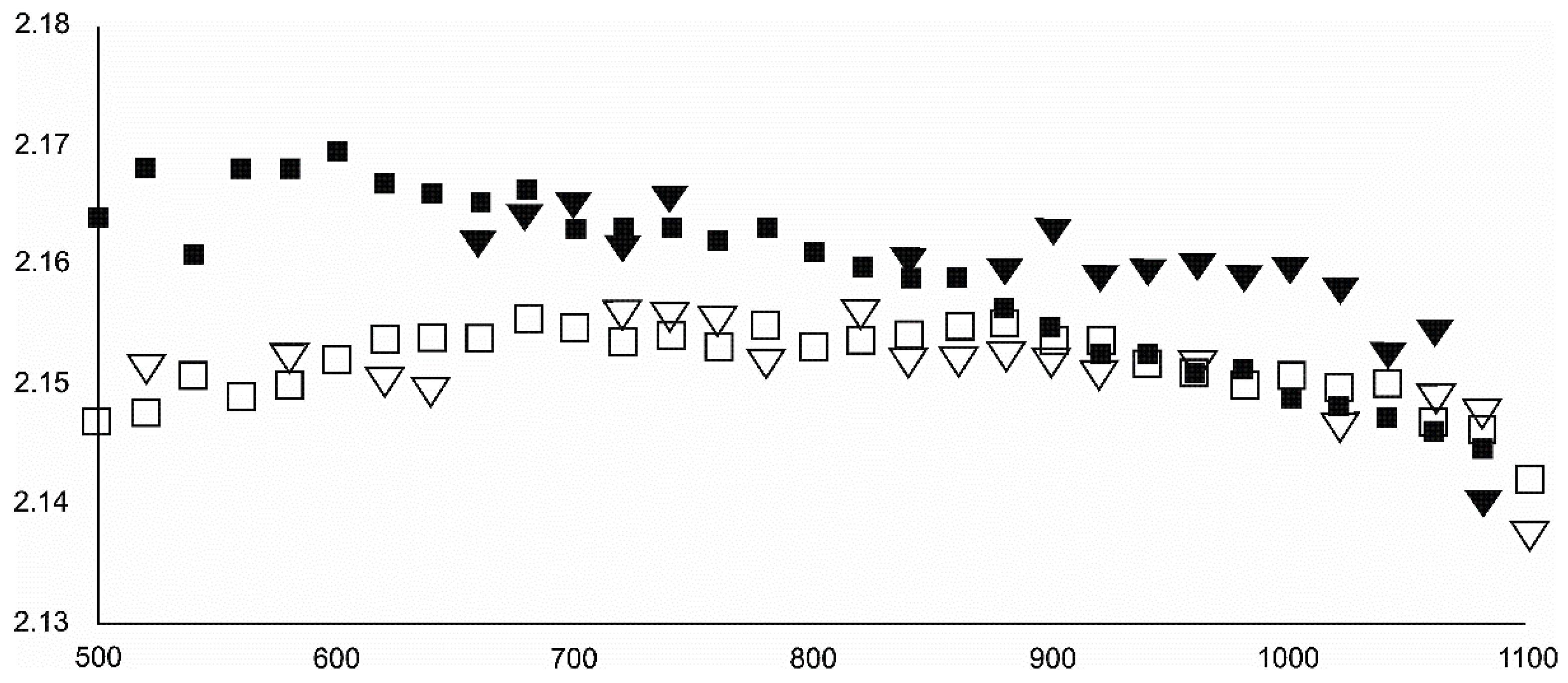
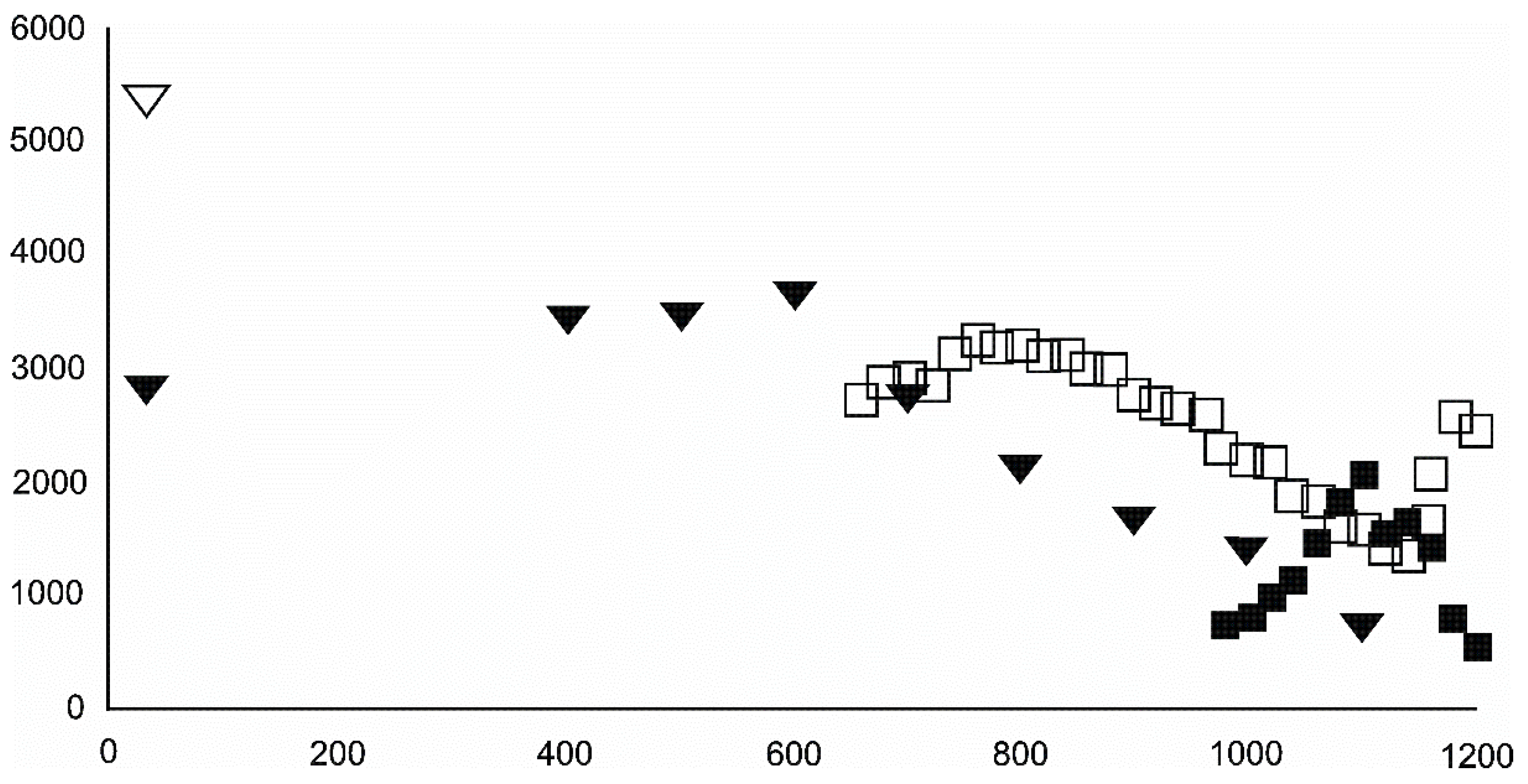
- Magnetite crystallized from 400 °C, with saturation expected at 660–680 °C. Magnetite crystallization was dynamic during the progressive process, as evidenced from the FWHM–temperature relationship, although it was still an important phase at the higher-temperature end of the process. The retrograde crystallization of still abundant magnetite in the cooling sample was likely calmer with saturation being reached at 600 °C—a temperature coinciding with that found for the progressive part of the experiment. Magnesioferrite admixture was especially possible in the 600–660 °C range with positions of magnetite reflections at further stages seeming to suggest Mg substitution in the magnetite itself.
- -
- Wüstite nucleation occurring in a semi-amorphous-like precursor at 500 °C was followed by a possible two-phase presence at 600 °C. A major crystallization event was expected between 620–640 °C. Saturation was reached at 780 °C as well as a second possible major nucleation event at ~880 °C. Traces of wüstite were expected to be present between 1080–1100 °C.
- -
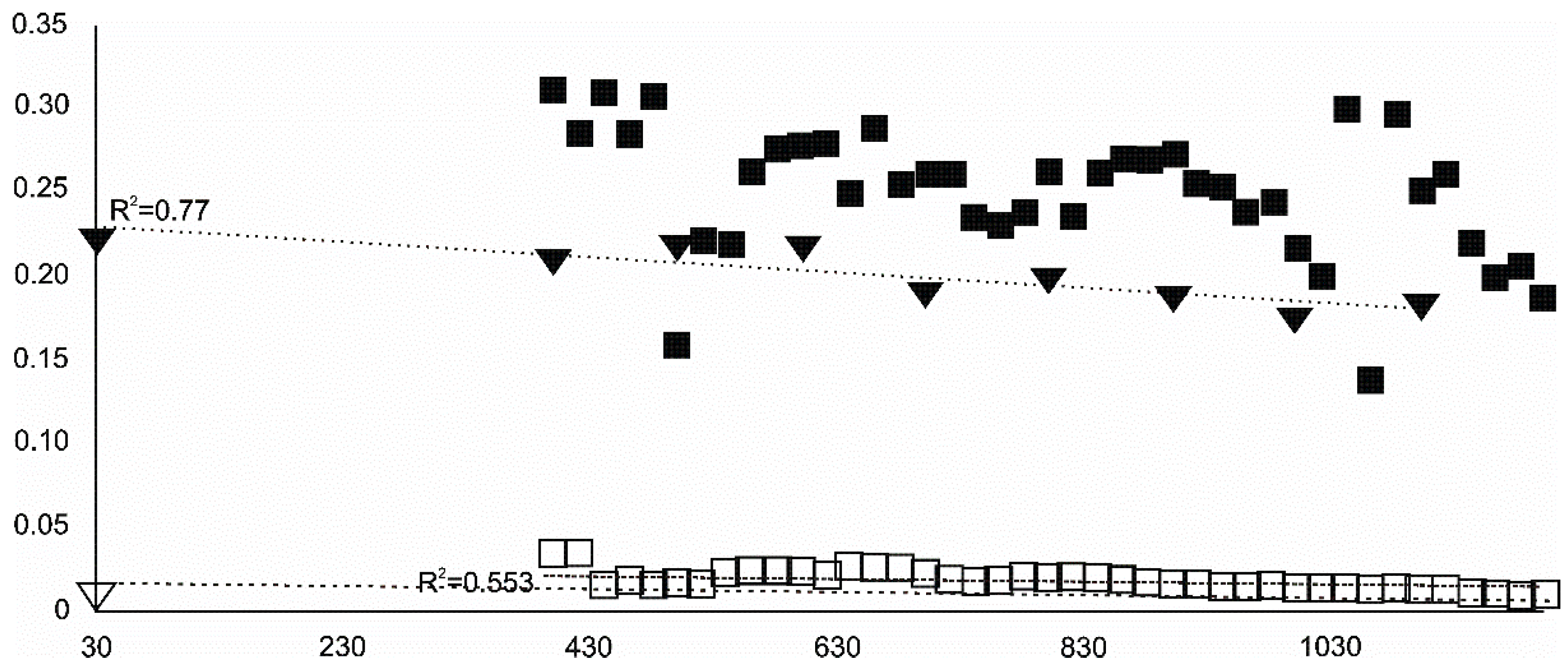
- Olivine became an important admixing species from ~900 °C or, more definitively, ~1100 °C. Saturation in the prograde part was reached at two steps in the USCB sample (~780–790 and ~1180 °C) and at ~1100 °C in the LSCB sample. Low- and mid-temperature counts-to-temperature function trajectories were similar but shifted towards higher temperatures in the LSCB siderite.
- -
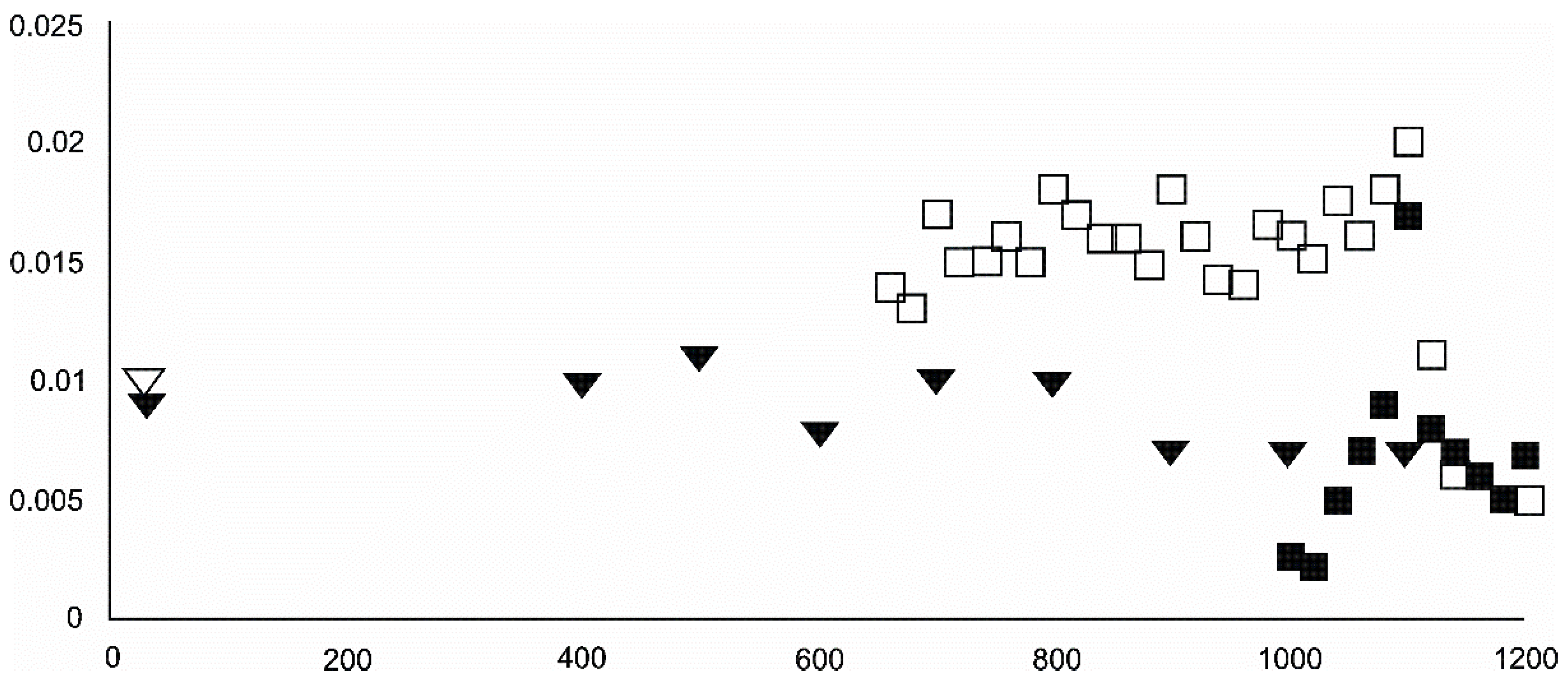
- Metallic iron and the garnet-structured skiagite were unconfirmed but possible additional final heating products. Iron seemed to grow up to 600 °C and then began to disappear. Its observed reflection seemed to change little in terms of its barycentrism throughout the entire process, suggesting a lack of diadochy substitutions.
- -
- Barringerite constituted an important reduced Fe phase formed during the experiment.
- -
- Cohenite was possible, especially in the 600–700 °C range. At 800 °C, the iron + cohenite pair seemed to be much more abundant than wüstite and especially graphite. On the other hand, all the reduced Fe species were clearly progressively removed starting at temperatures between 500–600 °C.
- -
- Graphite was especially prominent between 600–800 °C in the prograde phase of the experiment.
- -
- Clinopyroxene appeared to be most abundant at ~700 °C.
- -
- Muscovite mica developed from ~680 °C and was relatively abundant at 800 °C.
- -
- Cristobalite was important in the 500–600 °C range. Although still present at higher temperatures, typical tetragonal cristobalite may have been preceded by either C2221-structured SiO2 or a hexagonal polytype of tridymite.
- -
- Bayerite may have be an important admixing product that started to nucleate at ~1100 °C and continued growing through both prograde heating and retrograde cooling.
- -
- Graphite reflection at ~2.03 Å coincided with possible additional metallic iron.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heffern, E.L.; Coates, D.A. Geologic history of natural coal-bed fires, Powder River basin, USA. Int. J. Coal Geol. 2004, 59, 25–47. [Google Scholar] [CrossRef]
- Klika, Z.; Kozubek, T.; Martinec, P.; Kliková, C.; Dostál, Z. Mathematical modeling of bituminous coal seams burning contemporaneously with the formation of a variegated beds body. Int. J. Coal Geol. 2004, 59, 137–151. [Google Scholar] [CrossRef]
- Rădan, S.-C.; Rădan, S. Paleo-Coal Fires in the Western Dacic Basin, Romania. In Coal and Peat Fires: A Global Perspective; Stracher, G.B., Prakash, A., Sokol, E.V., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 339–349. [Google Scholar]
- Gürdal, G.; Hoşgörmez, H.; Özcan, D.; Li, X.; Liu, H.; Song, W. The pproperties of Çan Basin coals (Çanakkale-Turkey): Spontaneous combustion and combustion by-products. Int. J. Coal Geol. 2015, 138, 1–15. [Google Scholar] [CrossRef]
- Pone, J.D.N.; Hein, K.A.A.; Stracher, G.B.; Annegarn, H.J.; Finkleman, R.B.; Blake, D.R.; McCornack, J.K.; Schroeder, P. The spontaneous combustion of coal and its by-products in the Witbank and Sasolburg coalfields of South Africa. Int. J. Coal Geol. 2007, 72, 124–140. [Google Scholar] [CrossRef]
- Muszyński, M.; Skowroński, A.; Lipiarski, I. Red beds of the collapse-type breccia from the “Marcel” Coal Mine (Upper Silesian Coal Basin, Poland). Geologia 2006, 32, 345–367, (In Polish with English Abstract). [Google Scholar]
- Brooks, K.; Svanas, N.; Glasser, D. Evaluating the risk of spontaneous combustion in coal stockpiles. Fuel 1988, 67, 651–656. [Google Scholar] [CrossRef]
- Stracher, G.B.; Prakash, A.; Sokol, E.V. Coal and Peat Fires: A Global Perspective, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2011; 357p. [Google Scholar]
- Stracher, G.B.; Prakash, A.; Sokol, E.V. Coal and Peat Fires: A Global Perspective, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2013; 554p. [Google Scholar]
- Stracher, G.B.; Prakash, A.; Sokol, E.V. Coal and Peat Fires: A Global Perspective, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2015; 786p. [Google Scholar]
- Stracher, G.B.; Prakash, A.; Sokol, E.V. Coal and Peat Fires: A Global Perspective, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2019; 506p. [Google Scholar]
- Gorova, A.; Pavlychenko, A.; Kulyna, S. Environmental aspects of waste management on coal mining enterprises. In New Developments in Mining Engineering 2015, Theoretical and Practical Solutions of Mineral Resources Mining; CRC Press: Boca Raton, FL, USA, 2015; 600p. [Google Scholar]
- Nadłonek, W.; Cabała, J. Potentially toxic elements in soils and plants on a reclaimed coal-waste dump in southern Poland (preliminary study). Acta Geodyn. Geomater. 2016, 13, 271–279. [Google Scholar] [CrossRef][Green Version]
- Fabiańska, M.J.; Ciesielczuk, J.; Kruszewski, Ł.; Misz-Kennan, M.; Blake, D.R.; Stracher, G.; Moszumańska, I. Gaseous compounds and efflorescences generated in self-heating coal-waste dumps–a case study from the Upper- and Lower Silesian Coal Basins (Poland). Int. J. Coal Geol. 2013, 116–117, 247–261. [Google Scholar] [CrossRef]
- Křibek, B.; Sýkorová, I.; Veselovský, F.; Laufek, F.; Malec, J.; Knésl, I.; Majer, V. Trace element geochemistry of self-burning and weathering of a mineralized coal waste dump: The Novátor mine, Czech Republic. Int. J. Coal Geol. 2017, 173, 158–175. [Google Scholar] [CrossRef]
- Fabiańska, M.J.; Ciesielczuk, J.; Nádudvari, Á.; Misz-Kennan, M.; Kowalski, A.; Kruszewski, Ł. Environmental influence of gaseous emissions from self-heating coal waste dumps in Silesia, Poland. Environ. Geochem. Health 2019, 41, 575–601. [Google Scholar] [CrossRef]
- Kruszewski, Ł.; Fabiańska, M.; Ciesielczuk, J.; Segit, T.; Orłowski, R.; Motyliński, R.; Kusy, D.; Moszumańska, I. First multi-tool exploration of a gas-condensate-pyrolysate system from the environment of burning coal mine heaps: An in situ FTIR and laboratory GC and PXRD study based on Upper Silesian materials. Sci. Total Environ. 2018, 640–641, 1044–1071. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.; Da Silva, E.F.; Flores, D. Burning of coal waste piles from Douro Coalfield (Portugal): Petrological, geochemical and mineralogical characterization. Int. J. Coal Geol. 2010, 81, 359–372. [Google Scholar] [CrossRef]
- Ciesielczuk, J.; Kruszewski, Ł.; Majka, M. Comparative mineralogical study of thermally-altered coal-dump waste, natural rocks and the products of laboratory heating experiments. Int. J. Coal Geol. 2015, 139, 114–141. [Google Scholar] [CrossRef]
- Mendenhall, W.C. Shorter Contributions to General Geology; USGS Professional Paper; USGS: Washington, DC, USA, 1931.
- Bean, J.H. The Iron Ore Deposits of Ulu Rompin, Malaya. Ph.D. Thesis, Durham University, Durham, UK, 1975; 382p. Available online: http://etheses.dur.ac.uk/8144 (accessed on 23 February 2020).
- Zotov, A.; Mukhamet-Galeev, A.; Schott, J. An experimental study of kaolinite and dickite relative stability at 150–300 °C and the thermodynamic properties of dickite. Am. Min. 1998, 83, 516–524. [Google Scholar] [CrossRef]
- Uchida, E.; Iiyama, J.T. On Kamaishilite, Ca2Al2SiO6(OH)2, a New Mineral Dimorphous (Tetragonal) with Bicchulite from the Kamaishi Mine, Japan. Proc. Japan Acad. 1981, S7B, 239–243. [Google Scholar] [CrossRef][Green Version]
- Kruszewski, Ł.; Gatel, P.; Thiéry, V.; Moszumańska, I.; Kusy, D. Crystallochemical Behavior of Slag Minerals and the Occurrence of Potentially New Mineral Species from Lapanouse-de-Sévérac, France (Slag and Potentially New Minerals from Lapanouse-de-Sévérac, France). In Coal and Peat Fires: A Global Perspective; Stracher, G.B., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 243–300. [Google Scholar]
- Dick, H.J.B.; Erzinger, J.; Stokking, L.B. Shipboard Scientific Party, 1992. Site 504. In Proceedings of the Ocean Drilling Program: Initial Report; Ocean Drilling Program: College Station, TX, USA, 1992; Volume 140, pp. 37–200. [Google Scholar]
- Watanabe, T.; Kasami, H.; Ohshima, S. Compressional and shear wave velocities of serpentinized peridotites up to 200 MPa. Earth Planets Space 2007, 59, 233–244. [Google Scholar] [CrossRef]
- Potel, S.; Schmidt, S.T.; De Capitani, C. Composition of pumpellyite, epidote, and chlorite from New Caledonia—How important are metamorphic grade and whole-rock composition? Swiss J. Geos. Suppl. 2002, 82, 229–252. [Google Scholar]
- Pasero, M.; Reinecke, T. Crystal chemistry, HRTEM analysis, and polytypic behavior of ardennite. Eur. J. Mineral. 1991, 3, 819–830. [Google Scholar] [CrossRef]
- Orlandi, P.; Biagioni, C.; Pasero, M.; Mellini, M. Lavoisierite, Mn2+8[Al10(Mn3+Mg)][Si11P]O44(OH)2, a new mineral from Piedmont, Italy. Phys. Chem. Minerals 2013, 40, 239–249. [Google Scholar] [CrossRef]
- Jóźwiak, W.K.; Kaczmarek, E.; Maniecki, T.P.; Ignaczak, W.; Maniukiewicz, W. Reduction behavior of iron oxides in hydrogen and carbon monoxide atmospheres. Appl. Catal. A Gen. 2007, 326, 17–27. [Google Scholar] [CrossRef]
- Sokol, E.V.; Maksimova, N.V.; Nigmatulina, E.N.; Sharygin, V.V.; Kalugin, V.M. Combustion Metamorphism; Publishing House of the SB RAS: Novosibirsk, Russia, 2005; 312p, (In Russian, with some English Abstracts). [Google Scholar]
- Kruszewski, Ł.; Ciesielczuk, J.; Misz-Kennan, M.; Fabiańska, M. Chemical composition of glasses and associationg mineral species in various pyrometamorphic rocks from coal-mining dumps of the Lower Silesia. Min. Spec. Pap. 2014, 42, 70–71. [Google Scholar]
- Kruszewski, Ł.; Ciesielczuk, J.; Misz-Kennan, M. What have meteorites to do with coal fires? A case of Upper and Lower Silesian Basins. Min. Spec. Pap. 2012, 40, 28–29. [Google Scholar]
- Sharygin, V.V. Metal-phospide-sulphide assosiation in paralavas from dump of the Korkino open pit, Chelyabinsk coal basin. In Proceedings of the Mineralogy of the Urals, Miass, Russia, 22–26 August 2011; pp. 186–192. (In Russian). [Google Scholar]
- Vapnik, Y.; Galuskina, I.; Murashko, M.; Britvin, S.; Galuskin, E. The Hatrurim Complex—The New Unique Locality on World Mineral Map: The Review of Mineral Discoveries. In Proceedings of the Israel Geological Society, Annual Meeting, En Boqeq, Israel, 25–27 February 2014; pp. 143–144. [Google Scholar]
- Britvin, S.N.; Vapnik, Y.; Polekhovsky, Y.S.; Krivovichev, S.V.; Krzhizhanovskaya, M.G.; Gorelova, L.A.; Vereshchagin, O.S.; Shilovskikh, V.V.; Zaitsev, A.N. Murashkoite, FeP, a new terrestrial phosphide from pyrometamorphic rocks of the Hatrurim Formation, South Levant. Miner. Petrol. 2019, 113, 237–248. [Google Scholar] [CrossRef]
- Britvin, S.N.; Murashko, M.N.; Vapnik, Y.; Polekhovsky, Y.S.; Krivovichev, S.V.; Vereshchagin, O.S.; Shilovskikh, V.V.; Krzhizhanovskaya, M.G. Negevite, the pyrite-type NiP2, a new terrestrial phosphide. Am. Mineral. 2020, 105, 422–427. [Google Scholar]
- Kruszewski, Ł. Geochemical Behavior of Trace Elements in the Upper and Lower Silesian Basin Coal-Fire Gob Piles of Poland (Geochemical Behavior of Trace Elements in Silesian Coal-Fire Gob Piles). In Coal and Peat Fires: A Global Perspective; Stracher, G.B., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 407–449. [Google Scholar]
- Belgacem, K.; Llewellyn, P.L.; Kais, N.; Trabelski Ayadi, M. Thermal behaviour study of the talc. OAM RC 2008, 2, 332–336. [Google Scholar]
- Sokol, E.V.; Kokh, S.N.; Sharygin, V.V.; Danilovsky, V.A.; Seryotkin, Y.V.; Liferovich, R.; Deviatiiarova, A.S.; Nigmatulina, E.N.; Karmanov, N.S. Mineralogical diversity of Ca2SiO4-bearing combustion metamorphic rocks in the Hatrurim Basin: Implications for storage and partitioning of elements in oil shale clinkering. Minerals 2019, 9, 465. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruszewski, Ł.; Ciesielczuk, J. The Behaviour of Siderite Rocks in an Experimental Imitation of Pyrometamorphic Processes in Coal-Waste Fires: Upper and Lower Silesian Case, Poland. Minerals 2020, 10, 586. https://doi.org/10.3390/min10070586
Kruszewski Ł, Ciesielczuk J. The Behaviour of Siderite Rocks in an Experimental Imitation of Pyrometamorphic Processes in Coal-Waste Fires: Upper and Lower Silesian Case, Poland. Minerals. 2020; 10(7):586. https://doi.org/10.3390/min10070586
Chicago/Turabian StyleKruszewski, Łukasz, and Justyna Ciesielczuk. 2020. "The Behaviour of Siderite Rocks in an Experimental Imitation of Pyrometamorphic Processes in Coal-Waste Fires: Upper and Lower Silesian Case, Poland" Minerals 10, no. 7: 586. https://doi.org/10.3390/min10070586
APA StyleKruszewski, Ł., & Ciesielczuk, J. (2020). The Behaviour of Siderite Rocks in an Experimental Imitation of Pyrometamorphic Processes in Coal-Waste Fires: Upper and Lower Silesian Case, Poland. Minerals, 10(7), 586. https://doi.org/10.3390/min10070586