Evolution of Metakaolin Thermal and Chemical Activation from Natural Kaolin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
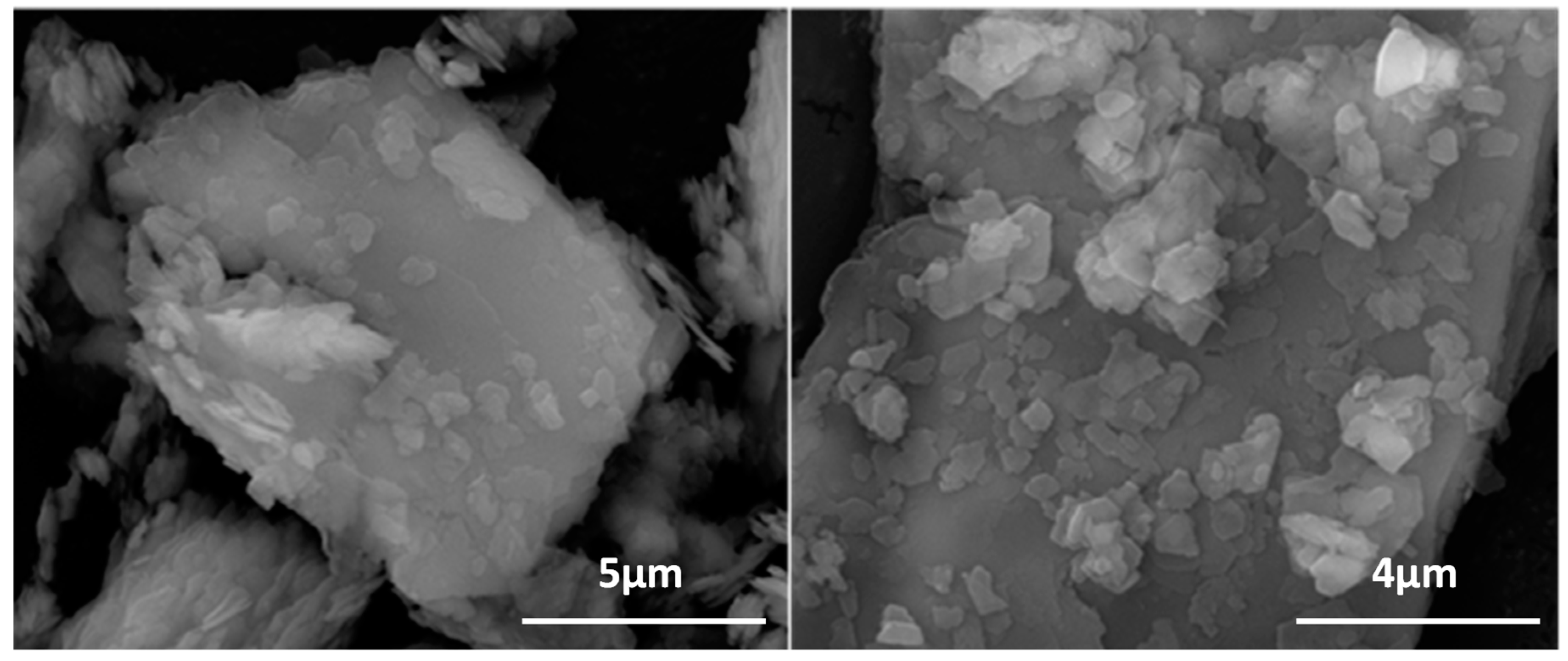
3.1. Natural Kaolin
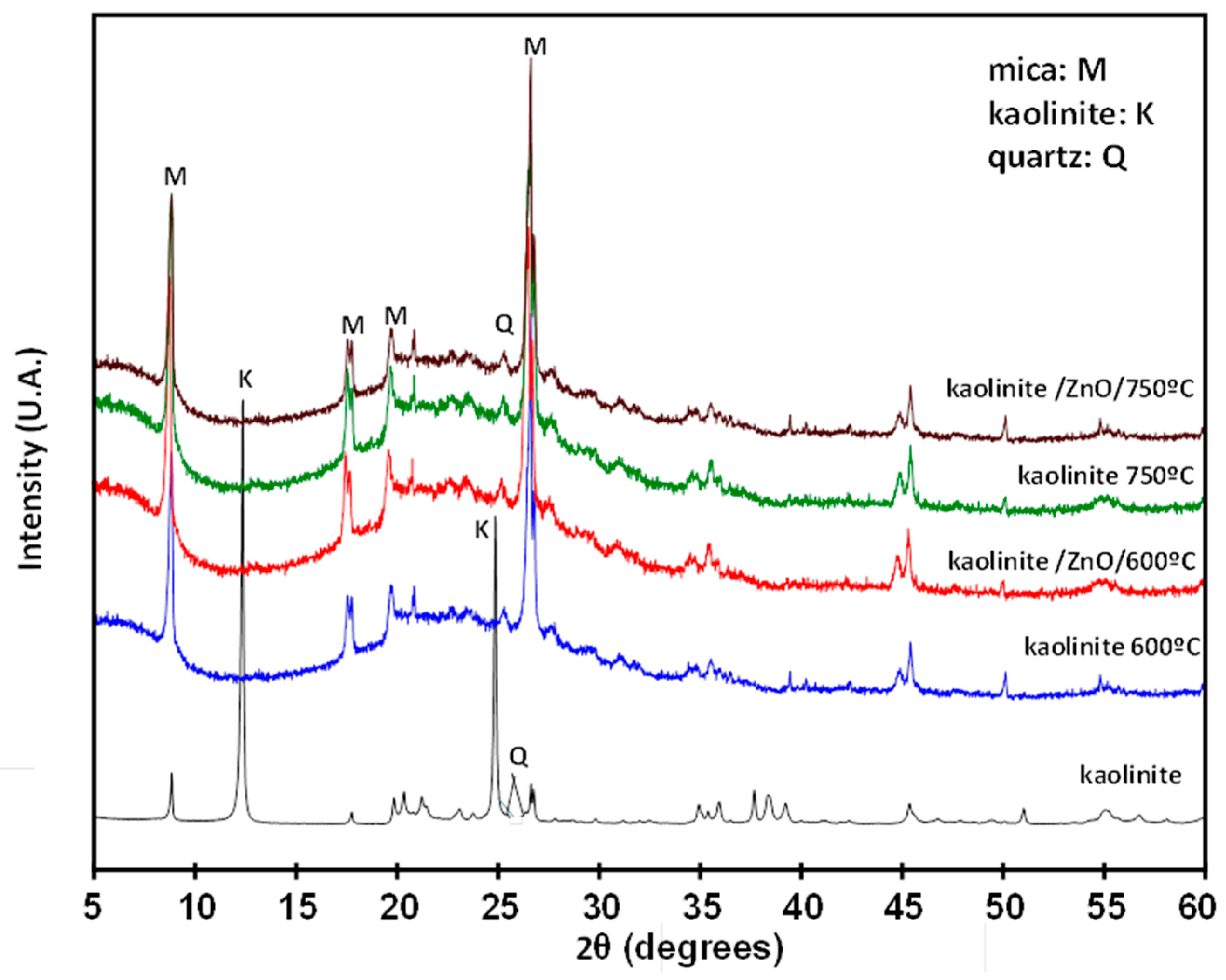
3.2. Chemical and Thermal Analysis by Activated Kaolin
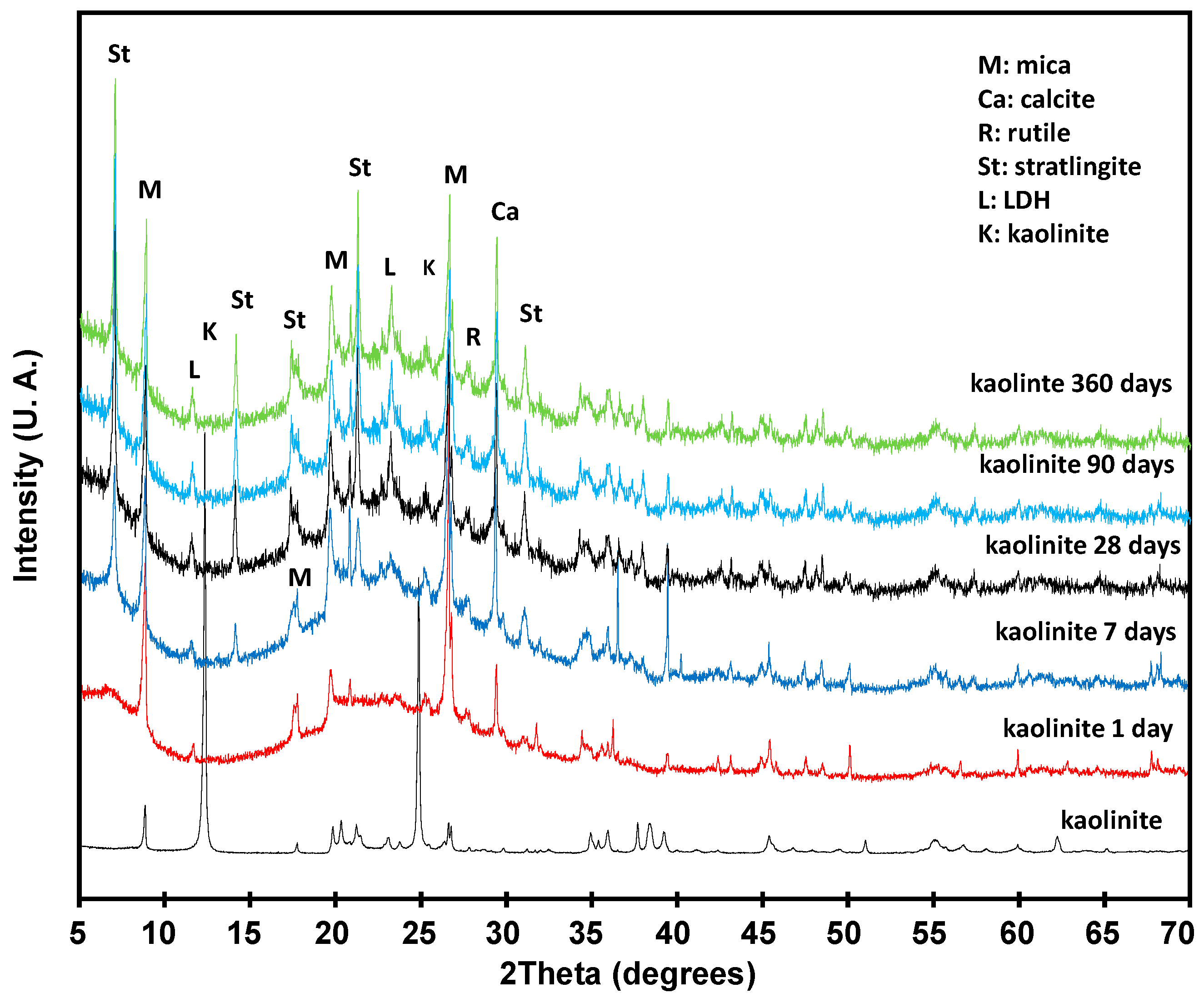
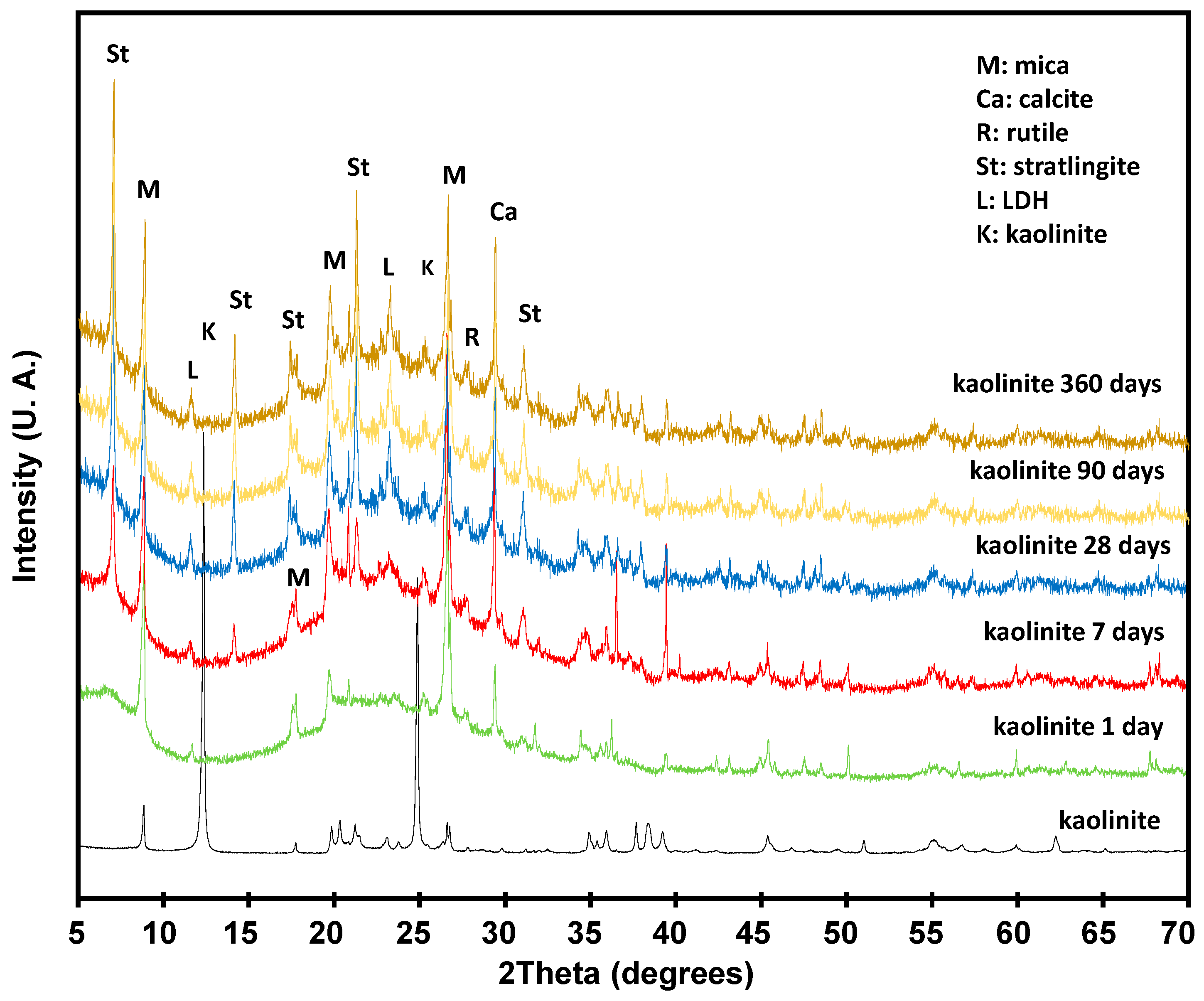
3.3. Pozzolanic Reaction
3.3.1. Solid Phase
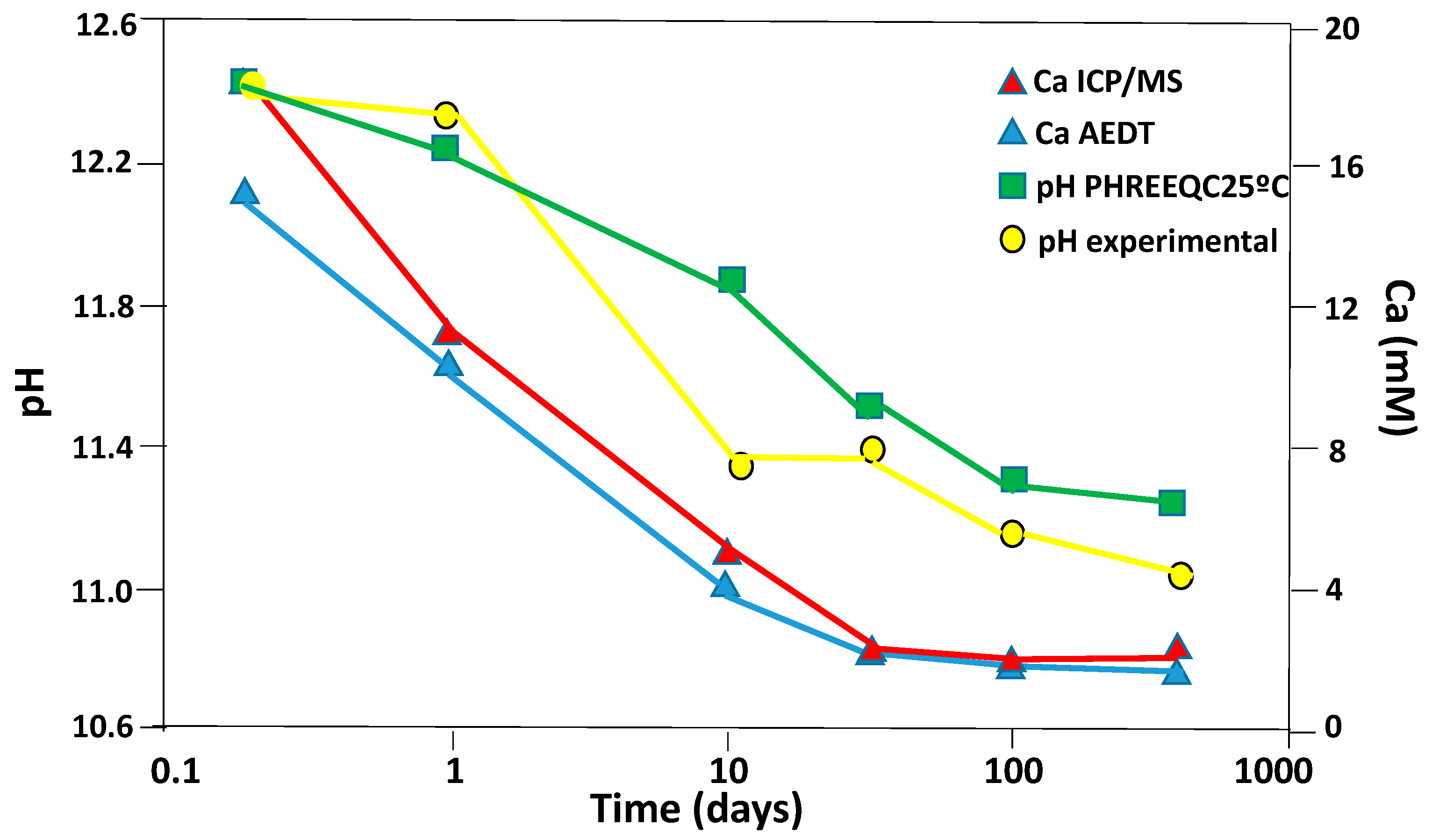
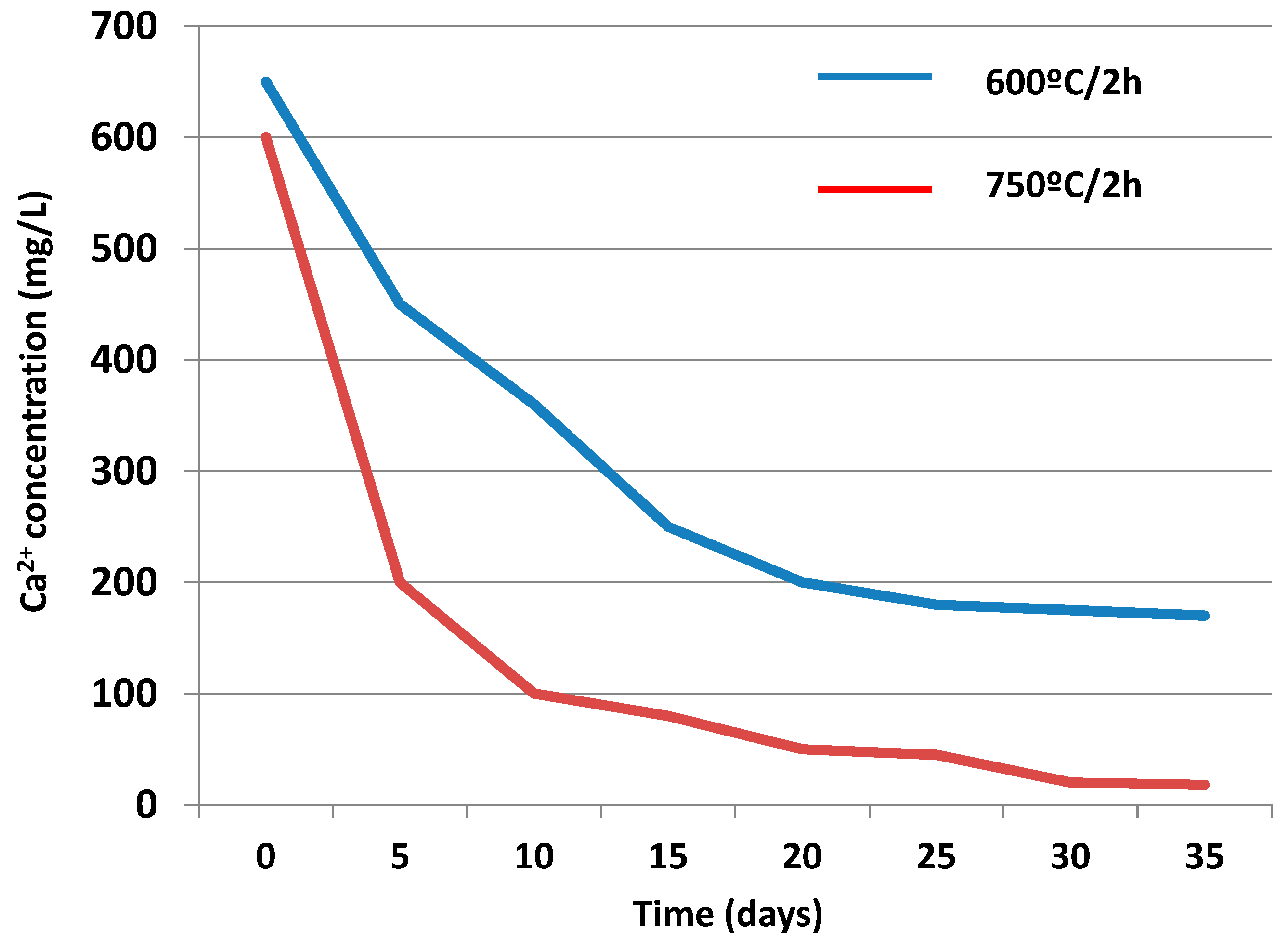
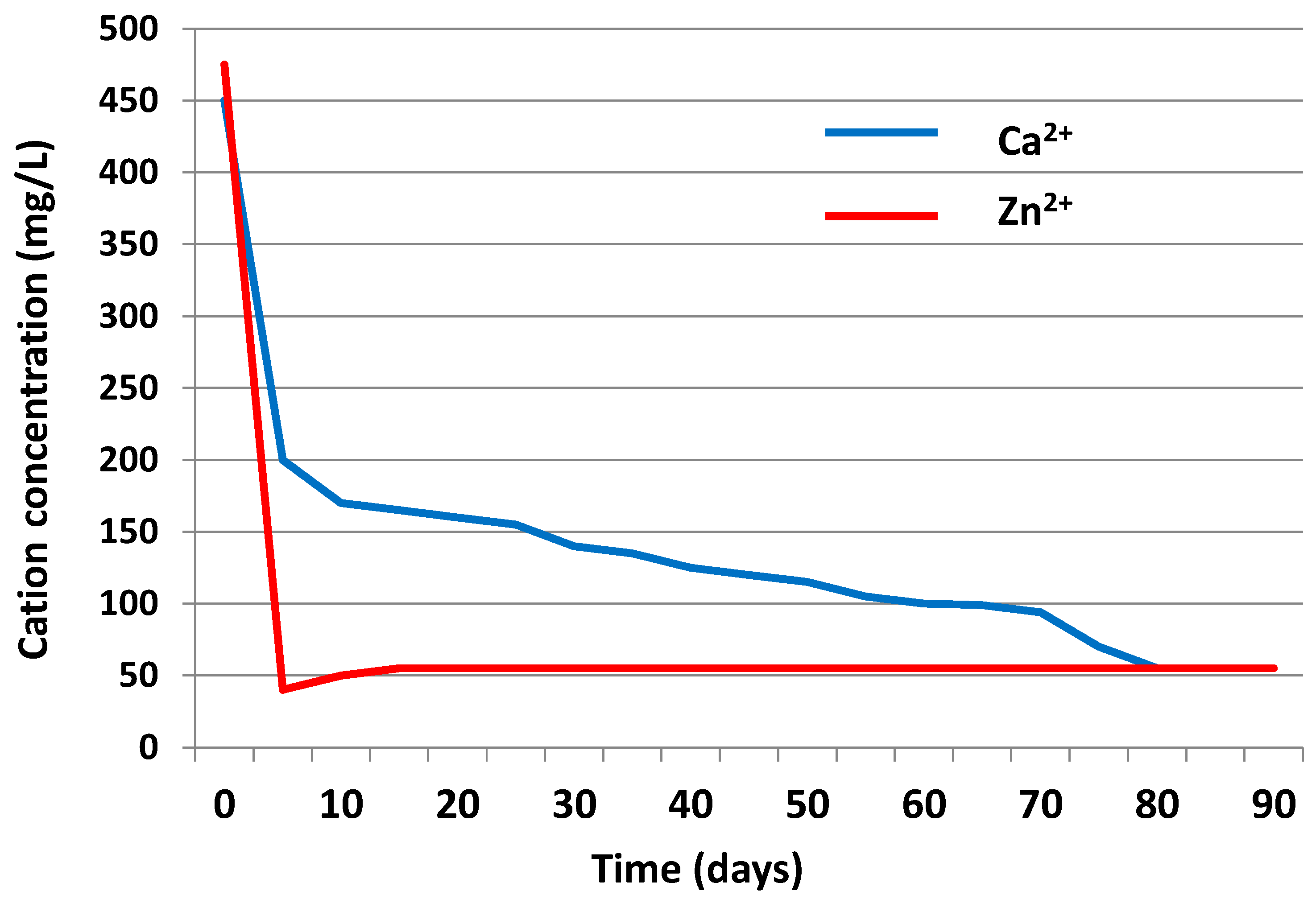
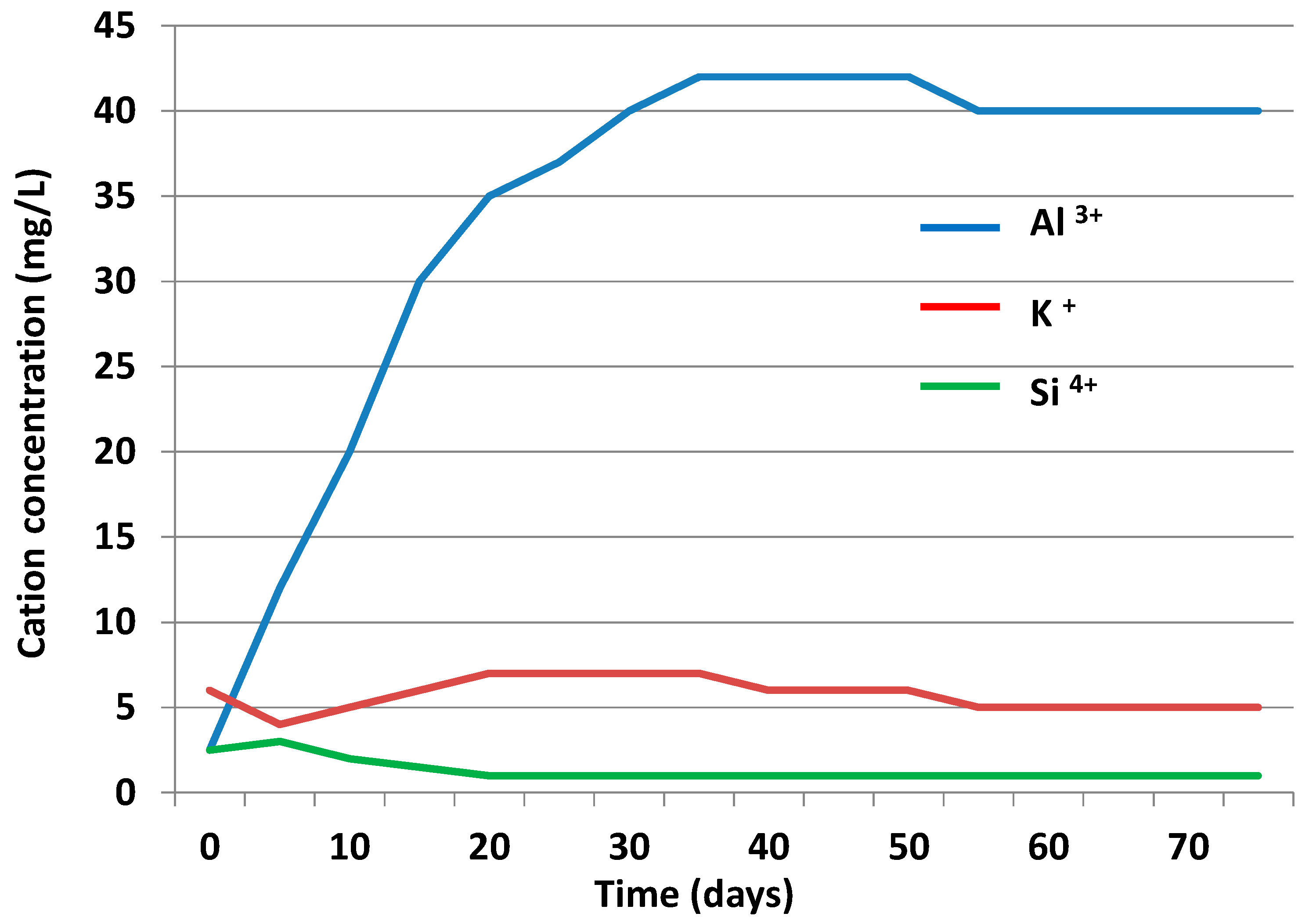
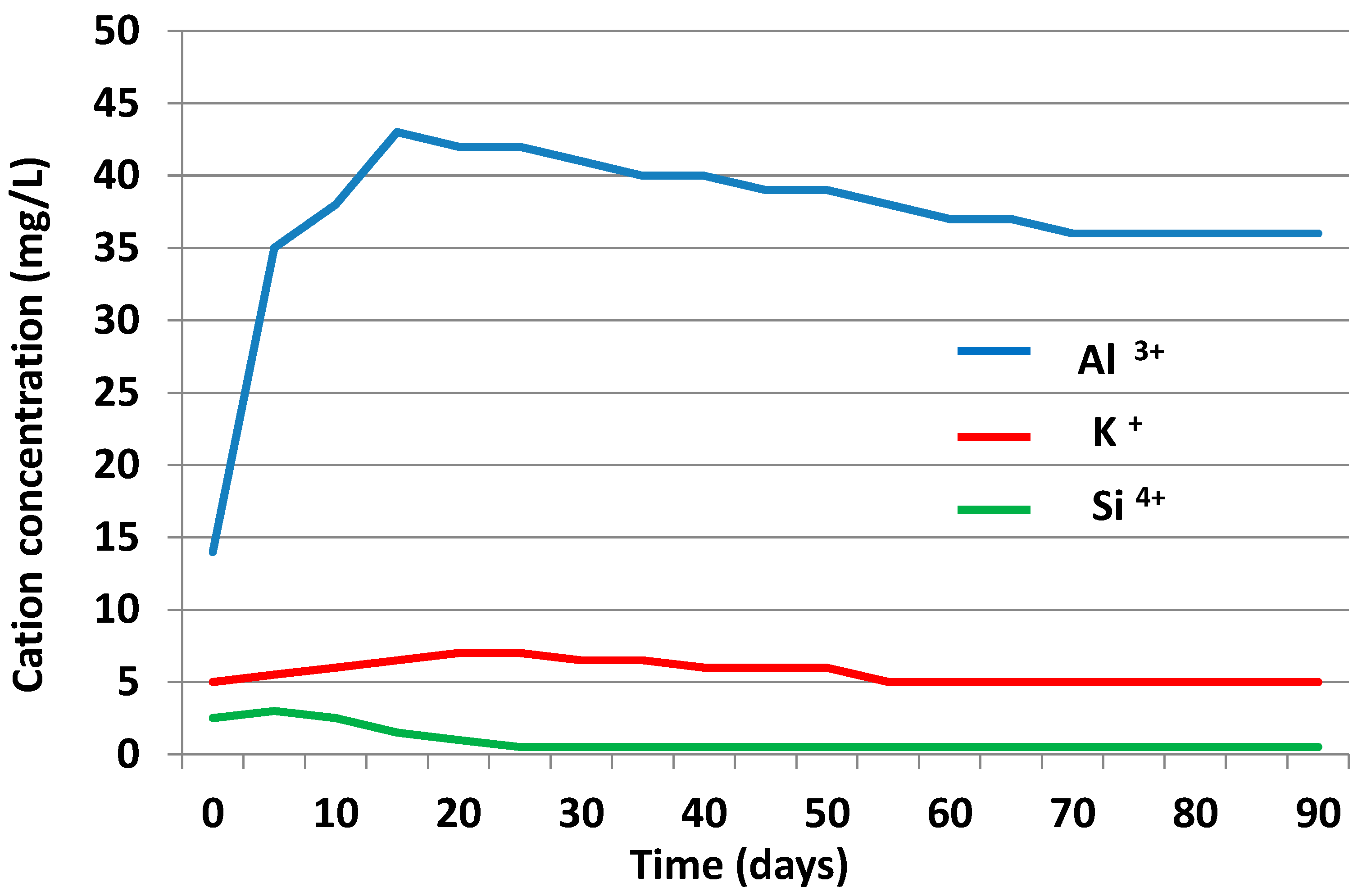
3.3.2. Liquid Phase
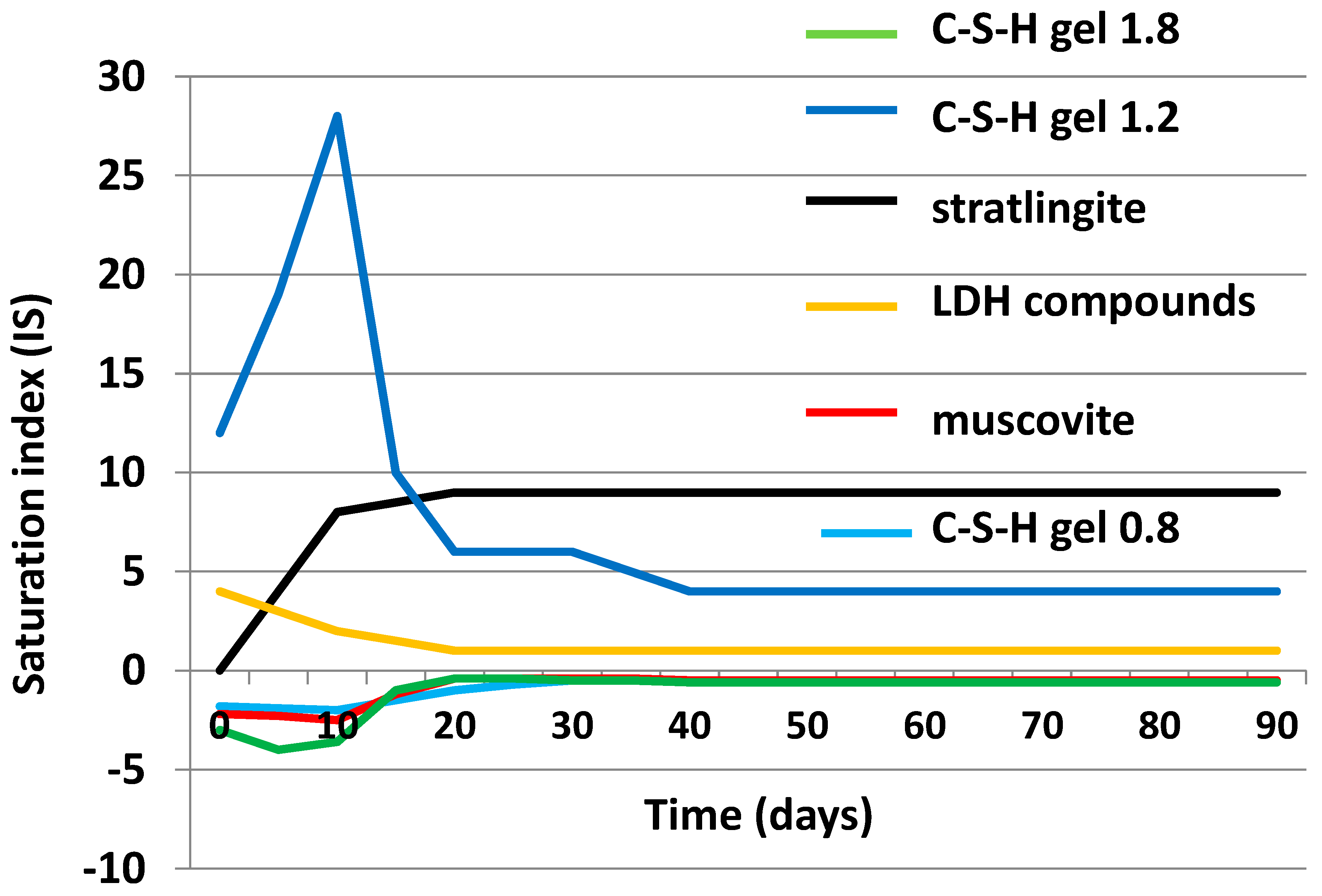
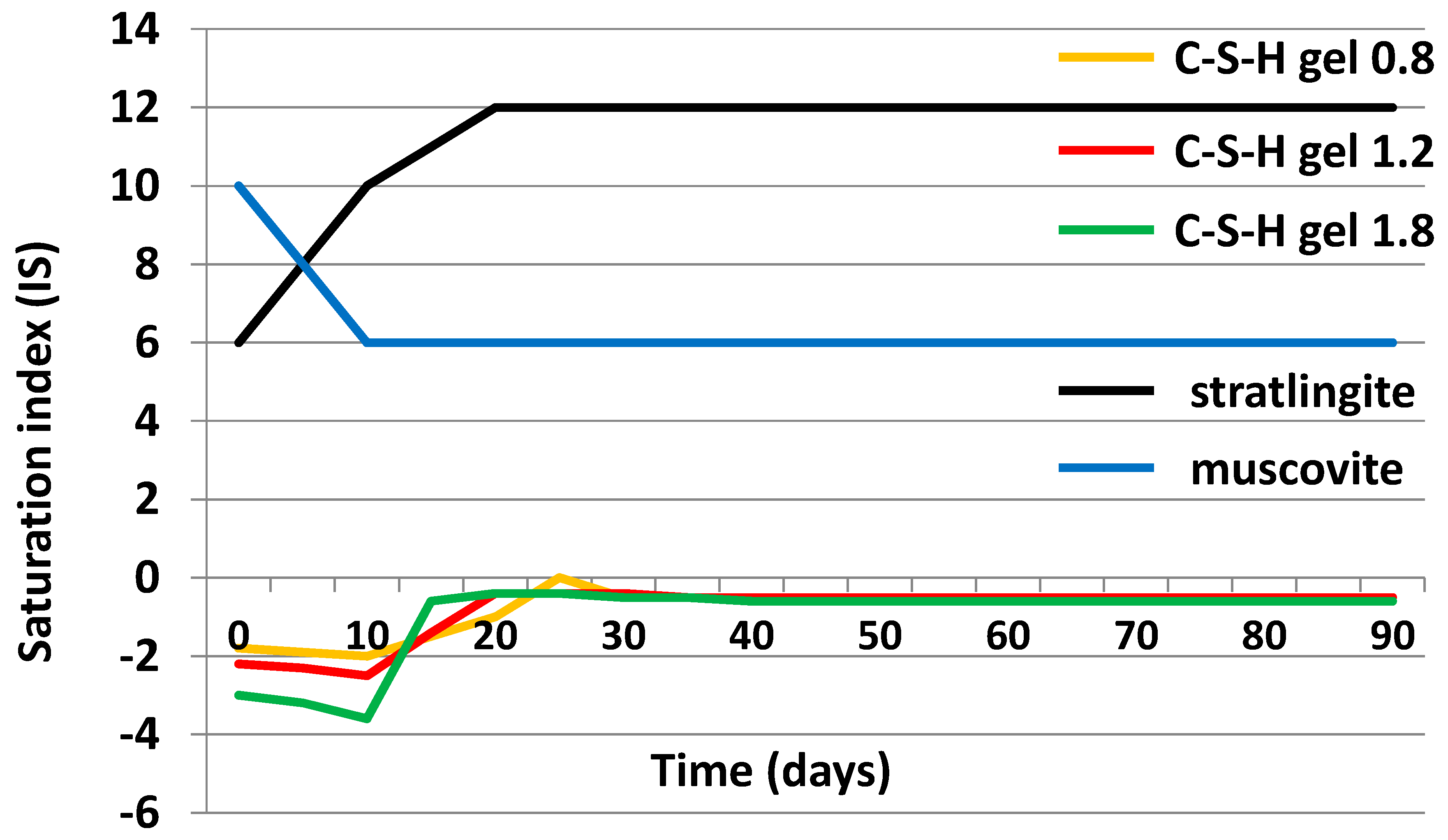
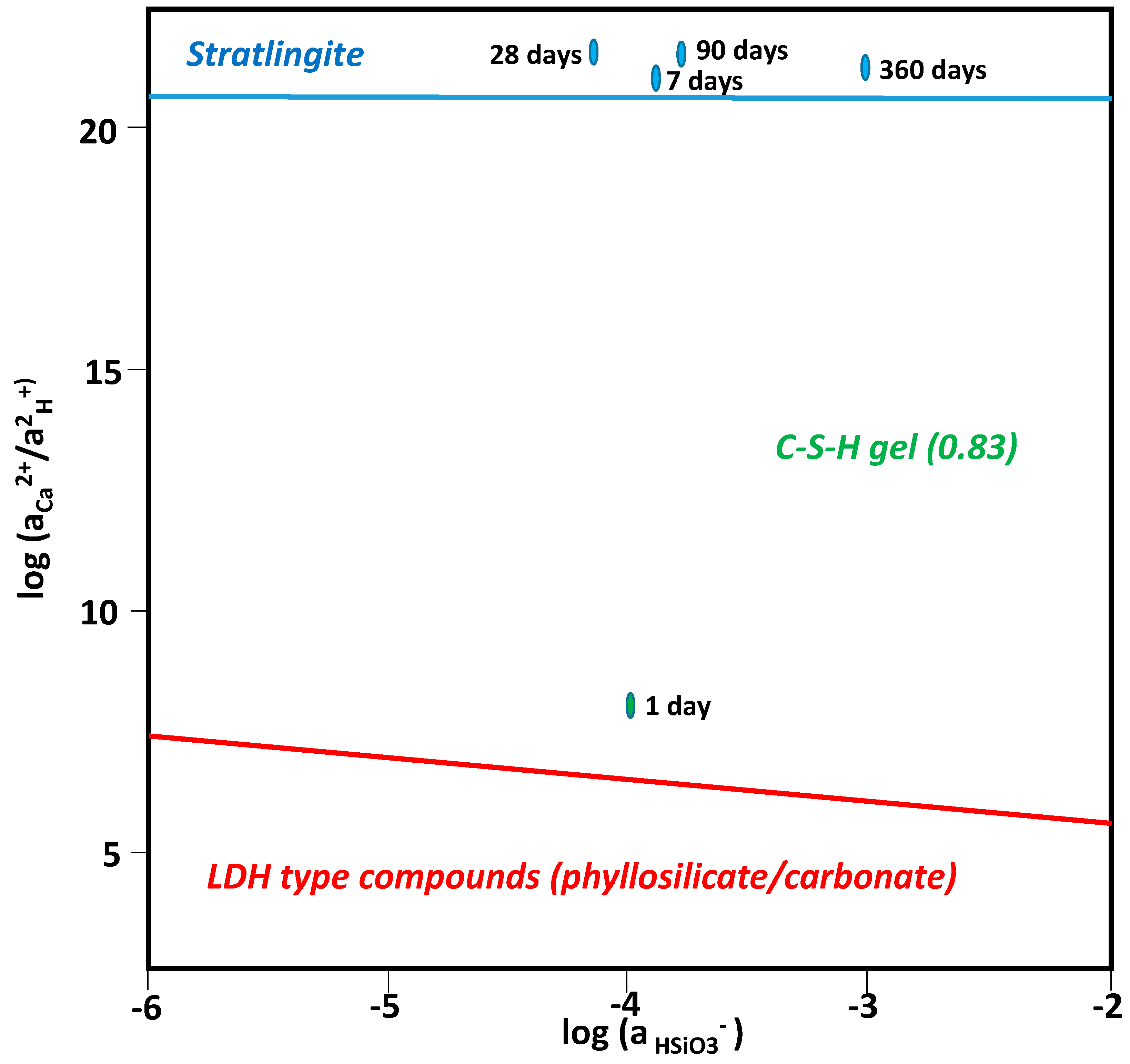
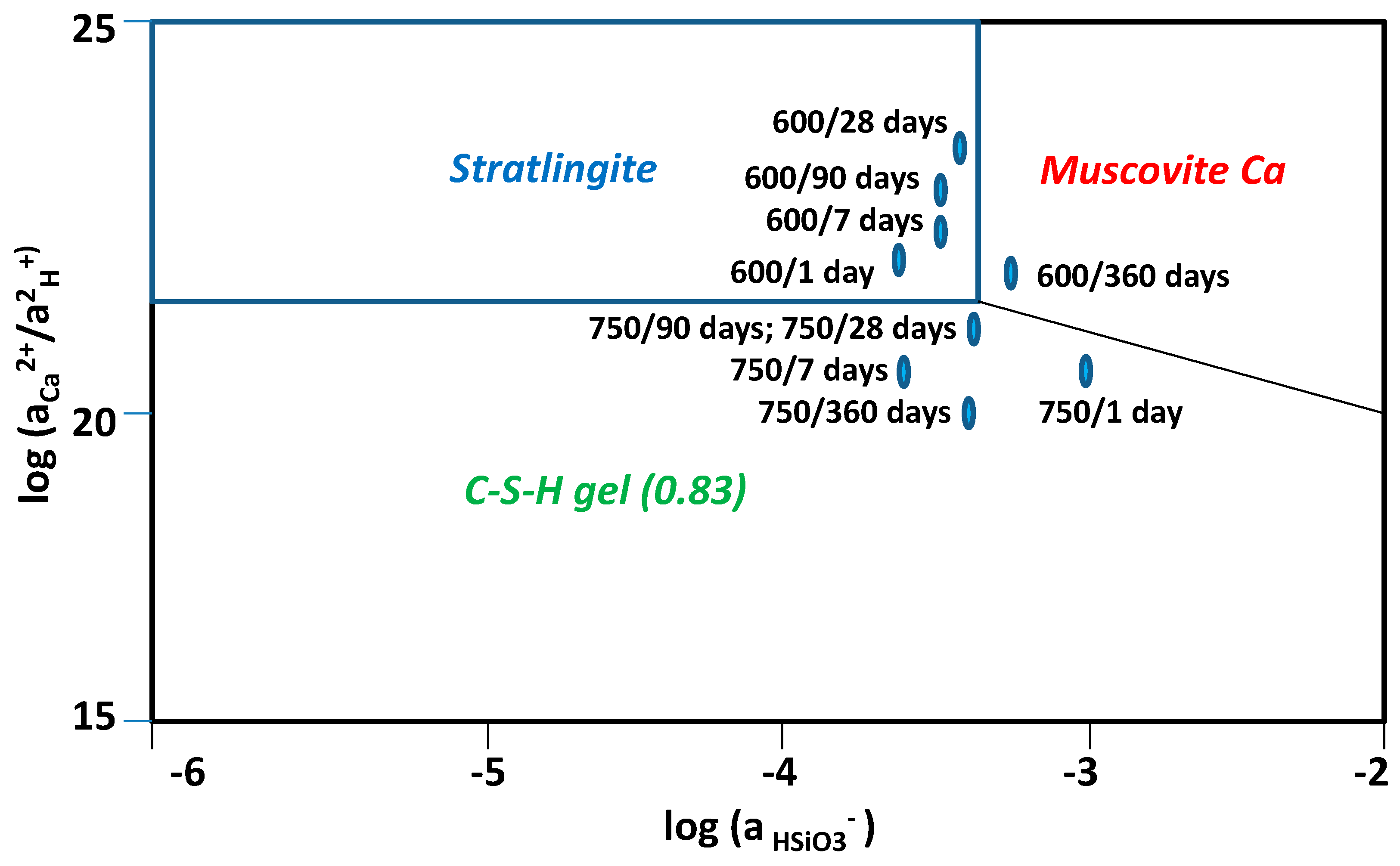
3.4. Chemical Speciation of the Aqueous Phases: Use of the PHREEQC Code
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Frias, M.; Vigil, R.; Garcia, R.; de Soto, I.; Medina, C.; Sánchez de Rojas, M.I. Scientific and technical aspects of blended cement matrices containing activated slate wastes. Cem. Concr. Comp. 2014, 48, 19–25. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Siddique, R. Recent advances in understanding the role of supplementary cementitious materials in concrete. Cem. Concr. Res. 2015, 78, 71–80. [Google Scholar] [CrossRef]
- Stark, A. Recent advances in the field of cement hydration and microstructure analysis. Cem. Concr. Res. 2011, 41, 666–678. [Google Scholar] [CrossRef]
- Frias, M.; Sánchez de Rojas, M.I.; Rodríguez, O. Paper sludge, an environmentally sound alternative source of MK-based cementitious materials: A review. Constr. Build. Mat. 2015, 74, 37–48. [Google Scholar] [CrossRef]
- Hewlett, P.C. Lea´s Chemistry of Cement and Concrete, 4th ed.; Elsevier Ltd.: Oxford, UK, 1992. [Google Scholar]
- Rashad, S.A.M. Metakaolin as cementitious material: History, scours, production and composition—A comprehensive overview. Constr. Build. Mat. 2013, 41, 303–318. [Google Scholar] [CrossRef]
- Van Damme, H. Concrete material science: Past, present, and future innovations. Cem. Concr. Res. 2018, 112, 5–24. [Google Scholar] [CrossRef]
- Roussel, N. Rheological requirements for printable concretes. Cem. Concr. Res. 2018, 112, 76–85. [Google Scholar] [CrossRef]
- Pera, J.; Ambroise, J.; Chabannet, M.; Chabannet, M. Transformation of wastes into complementary cementing materials. In Proceedings of the Seventh CANMET/ACI/JCI International Conference on Fly Ash, Silica Fume, Slag and Natural Pozzolans in Concrete, Madras, India, 1 January 2001; pp. 459–475. [Google Scholar]
- Vegas, I.; Ureta, J.; Frias, M.; Rodríguez, O.; Garcia, R.; Vigil, R. Scientific and Technical Aspects on the Use of Thermally Treated Paper Sludges in Cement; Wascon: Lyon, France, 2006; pp. 519–530. [Google Scholar]
- Rodríguez, O. Valorización de un Residuo Industrial Procedente de la Industria Papelera Como Material Puzolánico. Ph.D. Thesis, UAM, Madrid, Spain, 2008; p. 217. [Google Scholar]
- Ferreiro, S. Activación Térmica del Lodo de Papel Estucado Para su Valorización Como Adición Puzolánica en la Industria Cementera. Ph.D. Thesis, Universidad de Castilla La Mancha, Ciudad Real, Spain, 2010; p. 262. [Google Scholar]
- Sánchez de Rojas, M.I.; Marin, F.; Rivera, J.; Frias, M. Morphology and properties in blended cement with ceramic wastes as a pozzolanic material. J. Am. Ceram. Soc. 2006, 89, 3701–3705. [Google Scholar] [CrossRef]
- Frias, M. Study of hydrated phases present in a MK-lime cured at 60 °C and 60 months of reaction. Cem. Concr. Res. 2006, 36, 827–831. [Google Scholar] [CrossRef]
- Villar-Cociña, E.; Frias, M.; Valencia Morales, E.; Sánchez de Rojas, M.I. An evaluation of different kinetic models for determining the kinetic coefficients in sugar cane Straw-clay ash/lime system. Adv. Cem. Res. 2006, 18, 26–27. [Google Scholar] [CrossRef]
- Organismo de Normalización España. Métodos de Ensayo de Cementos. Parte V: Ensayo de Puzolanicidad para los Cementos Puzolánicos; AENOR: Madrid, Spain, 2006. [Google Scholar]
- Moore, M.; Reynolds, R.C. X-ray Diffraction and the Identification and Analysis of Clay Minerals, 2nd ed.; Oxford University Press: Oxford, UK, 1997. [Google Scholar]
- Pera, J.; Amrouz, A. Development of highly reactive metakaolin from paper sludge. Adv. Cem. Based Mat. 1998, 7, 49–56. [Google Scholar] [CrossRef]
- Vigil, R.; Frias, M.; Sánchez de Rojas, M.I.; Vegas, I.; Garcia, R. Mineralogical and morphological changes of calcined paper sludge at different temperatures and retention in furnace. Appl. Clay Sci. 2007, 36, 279–286. [Google Scholar] [CrossRef]
- Vigil de la Villa, R.; Rodriguez, O.; Garcia, R.; Frias, M. Mineral phases in an activated kaolinitic waste blended cement system. Appl. Clay Sci. 2010, 50, 137–142. [Google Scholar] [CrossRef]
- Garcia, R.; de la Villa, R.V.; Rodríguez, O.; Frias, M. Study of hydrated phases present in calcined paper sludge (metakaolinite)/saturated CaO dissolution system cured at 40 °C and 28 days of reaction. Mat. Sci. Eng. A 2010, 527, 3936–3941. [Google Scholar] [CrossRef]
- Vigil de la Villa, R.; Frias, M.; Garcia Giménez, R.; Martinez Ramirez, S.; Fernández-Carrasco, L. Chemical and mineral transformations that occur in mine waste and washery rejects during pre-utilization calcination. Int. J. Coal Geol. 2014, 132, 123–130. [Google Scholar] [CrossRef]
- García, R.; Vigil de la Villa, R.; Frías, M.; Rodríguez, O.; Martínez-Ramírez, S.; Fernández-Carrasco, L.; de Soto, I.S.; Villar Cociña, E. Mineralogical study of calcined coal waste in a pozzolan/Ca (OH)2 system. Appl. Clay Sci. 2015, 108, 45–54. [Google Scholar] [CrossRef]
- Ambroise, J.; Murat, M.; Pera, J. Investigations on synthetic binders obtained by middle-temperature thermal dissociation of clay minerals. Silic. Indust. 1986, 7, 99–107. [Google Scholar]
- Sayanam, R.A.; Kalsotra, A.K.; Mehta, S.K.; Sing, R.S.; Mandal, G. Studies on thermal transformations and pozzolanic activities of clay from Jammu region (India). J. Thermal Anal. 1989, 35, 9–106. [Google Scholar] [CrossRef]
- O´Farrell, M.; Sabir, B.B.; Wild, S. Strength and chemical resistance of mortars containing brick manufacturing clays subjected to different treatments. Cem. Concr. Comp. 2006, 28, 790–799. [Google Scholar] [CrossRef]
- Frías, M.; Vigil de la Villa, R.; García, R.; Sánchez de Rojas, M.I.; Juan Valdés, A. The influence of slate waste activation conditions on mineralogical changes and pozzolanic behavior. J. Am. Ceram. Soc. 2013, 96, 1–7. [Google Scholar] [CrossRef]
- Brindley, G.W.; Brown, G. Crystal Structures of Clay Minerals and their X-ray Identification; Mineralogical Society Monograph: London, UK, 1980. [Google Scholar]
- Santos Silva, A.; Gameiro, A.; Grillo, J.; Veiga, R.; Velosa, A. Long-term behaviour of lime-metakaolin pastes at ambient temperature and humic curing condition. Appl. Clay Sci. 2014, 88–89, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Shi, C.; Grattan-Bellew, P.E.; Stegemann, J.A. Conversion of a waste mud into a pozzolanic material. Constr. Build. Mat. 1999, 13, 279–284. [Google Scholar] [CrossRef]
- Favier, A.; Habert, G.; D’Espinose de Lacaillerie, J.B.; Roussel, N. Mechanical properties and compositional heterogeneities of fresh geopolymer pastes. Cem. Concr. Res. 2013, 48, 9–16. [Google Scholar] [CrossRef]
- Favier, A.; Habert, G.; Roussel, N.; D’Espinose de Lacaillerie, J.B. A multinuclear static NMR study of geopolymerisation. Cem. Concr. Res. 2015, 75, 104–109. [Google Scholar] [CrossRef]
- Abdolhosseini Qomi, M.; Krakowiak, K.; Bauchy, M.; Stewars, K.L.; Shahsavari, R.; Jagannathan, D.; Brommer, D.B.; Baronnet, A.; Buehler, M.J.; Yip, S.; et al. Combinatorial molecular optimization of cement hydrates. Combinatorial molecular optimization of cement hydrates. Nat. Commun. 2014, 5, 4960. [Google Scholar] [CrossRef] [PubMed]
- García, R.; Vigil de la Villa, R.; Vegas, I.; Frías, M.; Sánchez de Rojas, M.I. The pozzolanic properties of paper sludge waste. Constr. Build. Mat. 2008, 22, 1484–1490. [Google Scholar] [CrossRef]
- Frias, M.; Rodríguez, O.; Nebreda, B.; Garcia, R.; Villar-Cociña, E. Influence of activation temperature of kaolinite-based clay wastes on the pozzolanic activity and kinetic parameters. Adv. Cem. Res. 2010, 22, 135–142. [Google Scholar] [CrossRef]
- Ioannidou, K.; Pellenq, R.J.M.; del Gado, E. Controlling local packing and growth in calcium–silicate–hydrate gels. Soft Matter 2014, 8, 1121–1130. [Google Scholar] [CrossRef]
- Ioannidou, K.; Krakowiak, K.J.; Bauchy, M.; Hoover, C.G.; Masoero, E.; Yip, S.; Ulm, F.J.; Levitz, P.; Pellenq, R.J.M.; del Gado, E. Mesoscale texture of cement hydrates. Proc. Natl. Acad. Sci. USA 2016, 113, 2029–2034. [Google Scholar] [CrossRef] [Green Version]
- Ioannidou, K.; Kanduc, M.; Li, L.; Frenkel, D.; Dobnikar, J.; Flenkel, D.; Del Gado, E. The crucial effect of early-stage gelation on the mechanical properties of cement hydrates. Nat. Commun. 2016, 7, 12106. [Google Scholar] [CrossRef] [Green Version]
- De Silva, P.S.; Glasser, F.P. Phase relations in the system CaO-Al2O3-SiO2-H2O relevant to MK-Calcium Hydroxide. Cem. Concr. Res. 1993, 23, 627–639. [Google Scholar] [CrossRef]
- Nicoleau, L.; Nonat, A. A new view on the kinetics of tricalcium silicate hydration. Cem. Concr. Res. 2016, 86, 1–11. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C.A.J. User´s guide to PHREEQC (version 2). In A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; US Geological Survey: Reston, VA, USA, 1999; p. 312. [Google Scholar]
- Blanc, P.; Lassin, A.; Piantone, P.; Burnol, A. Thermoddem a Database Devoted to Waste Minerals; BRGM: Orleans, France, 2007; p. 6009. Available online: http://thermoddem.brgm.fr (accessed on 10 June 2020).
- Johnson, J.W.; Oelkers, E.H.; Helgeson, H.C. SUPCRT92: A software package for calculating the standard molal thermodynamic properties of minerals, gases, aqueous species, and reactions from 1 to 5000 bar and 0 to 1000 °C. Comput. Geosci. 1992, 18, 899–947. [Google Scholar] [CrossRef]
- Lothenbach, B.; Matschei, T.; Möschner, G.; Glasser, F.P. Thermodynamic modelling of the effect of temperature on the hydration and porosity of Portland cement. Cem. Concr. Res. 2008, 38, 1–18. [Google Scholar] [CrossRef] [Green Version]
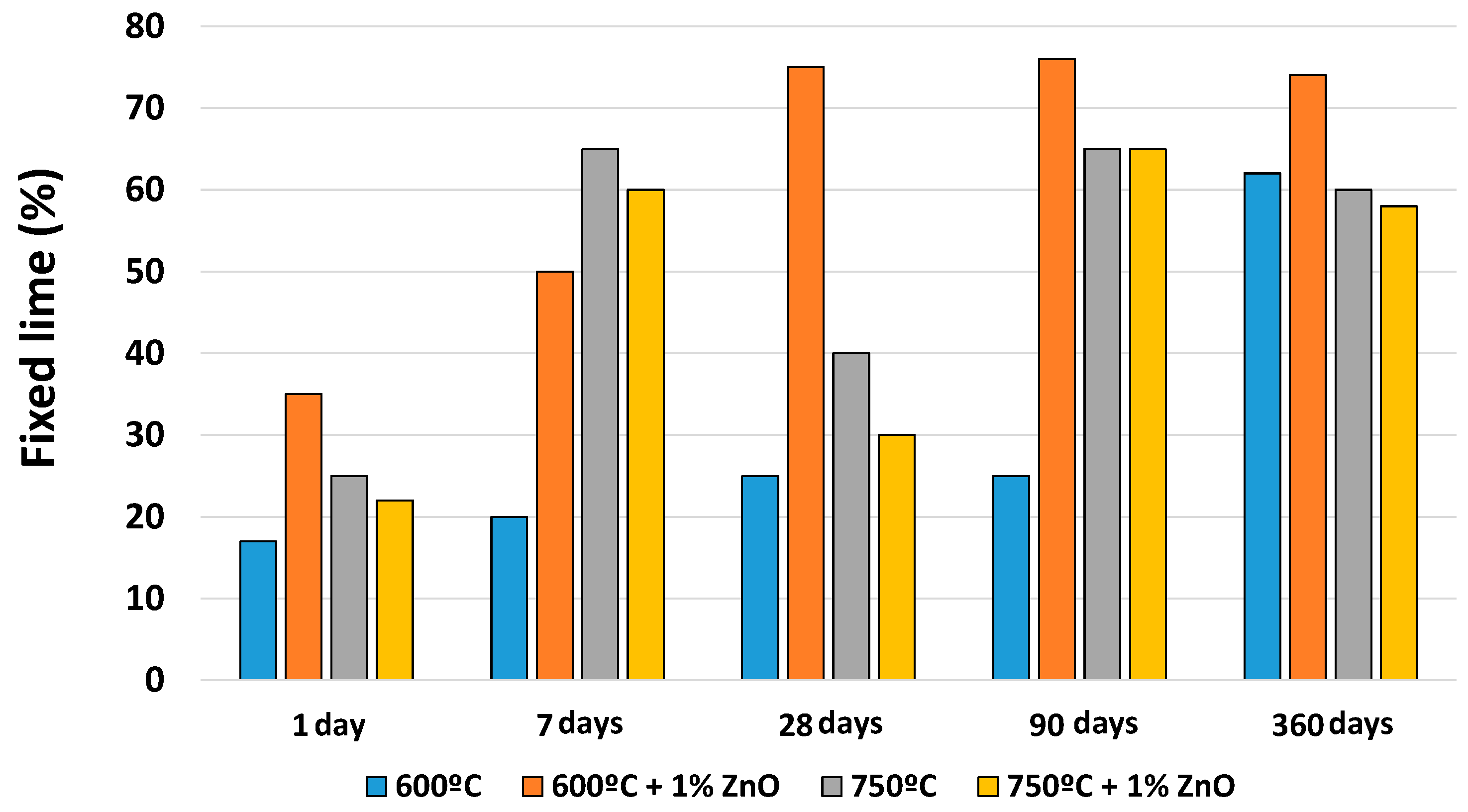
| Oxides | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | TiO2 | P2O5 | MnO2 | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (%) | 45.04 | 39.35 | 0.79 | 0.05 | 0.22 | 1.02 | 0.10 | 0.16 | 0.04 | 0.01 | 13.13 |
| Oxides (%) | Muscovite | Kaolinite | Quartz |
|---|---|---|---|
| MgO | 0.78 ± 0.06 | n.d. | n.d. |
| Al2O3 | 36.11 ± 1.27 | 41.86 ± 1.23 | n.d. |
| SiO2 | 49.07 ± 1.38 | 58.14 ± 2.41 | 100 |
| K2O | 11.02 ± 1.11 | n.d. | n.d. |
| TiO2 | 0.45 ± 0.,11 | n.d. | n.d. |
| Fe2O3 | 2.57 ± 0.94 | n.d. | n.d. |
| RB | χ2 | Kaolinite (%) | Mica (%) | Quartz (%) | Amorphous Material (%) | |
|---|---|---|---|---|---|---|
| Natural kaolin | 12.3 | 4.9 | 45 | 30 | 18 | 7 |
| Samples | Temperature (°C) | RB | X2 | Kaolinite (%) | Muscovite (%) | Quartz (%) | Amorphous Material (%) |
|---|---|---|---|---|---|---|---|
| Kaolin | 600 | 6.3 | 5.8 | n.d. | 30 | 18 | 52 |
| Kaolin + 1% ZnO | 600 | 5.9 | 5.2 | n.d. | 30 | 18 | 52 |
| Kaolin | 750 | 7.4 | 6.4 | n.d. | 35 | 18 | 47 |
| Kaolin + 1% ZnO | 750 | 6.8 | 5.9 | n.d. | 35 | 18 | 47 |
| Time (days) | RB | X2 | Calcite (%) | LDH (%) | Muscovite 3T (%) | Quartz (%) | Stratlingite (%) | Amorphous Material (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 8.25 | 6.4 | 12 | 8 | 39 | 9 | n.d. | 32 |
| 7 | 6.47 | 4.9 | 10 | 5 | 22 | 8 | 28 | 27 |
| 28 | 6.12 | 3.5 | 21 | 4 | 19 | 7 | 30 | 19 |
| 90 | 6.31 | 4.2 | 20 | 4 | 17 | 5 | 34 | 20 |
| 360 | 7.24 | 5.6 | 18 | 4 | 16 | 3 | 35 | 24 |
| Time (days) | RB | X2 | Calcite (%) | LDH (%) | Muscovite 3T (%) | Quartz (%) | Stratlingite (%) | Amorphous Material (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 8.62 | 5.7 | 22 | 18 | 29 | 7 | n.d. | 24 |
| 7 | 7.35 | 6.2 | 18 | 15 | 25 | 4 | 31 | 7 |
| 28 | 6.47 | 5.9 | 12 | 13 | 21 | n.d. | 33 | 21 |
| 90 | 8.25 | 5.8 | 10 | 8 | 15 | n.d. | 34 | 33 |
| 360 | 5.98 | 6.3 | 9 | 6 | 11 | n.d. | 36 | 38 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez, I.; de Soto, I.S.; Casas, M.; Vigil de la Villa, R.; García-Giménez, R. Evolution of Metakaolin Thermal and Chemical Activation from Natural Kaolin. Minerals 2020, 10, 534. https://doi.org/10.3390/min10060534
Sánchez I, de Soto IS, Casas M, Vigil de la Villa R, García-Giménez R. Evolution of Metakaolin Thermal and Chemical Activation from Natural Kaolin. Minerals. 2020; 10(6):534. https://doi.org/10.3390/min10060534
Chicago/Turabian StyleSánchez, Isabel, Isabel Sonsoles de Soto, Marina Casas, Raquel Vigil de la Villa, and Rosario García-Giménez. 2020. "Evolution of Metakaolin Thermal and Chemical Activation from Natural Kaolin" Minerals 10, no. 6: 534. https://doi.org/10.3390/min10060534
APA StyleSánchez, I., de Soto, I. S., Casas, M., Vigil de la Villa, R., & García-Giménez, R. (2020). Evolution of Metakaolin Thermal and Chemical Activation from Natural Kaolin. Minerals, 10(6), 534. https://doi.org/10.3390/min10060534





