Precipitation of Mn Oxides in Quaternary Microbially Induced Sedimentary Structures (MISS), Cape Vani Paleo-Hydrothermal Vent Field, Milos, Greece
Abstract
1. Introduction
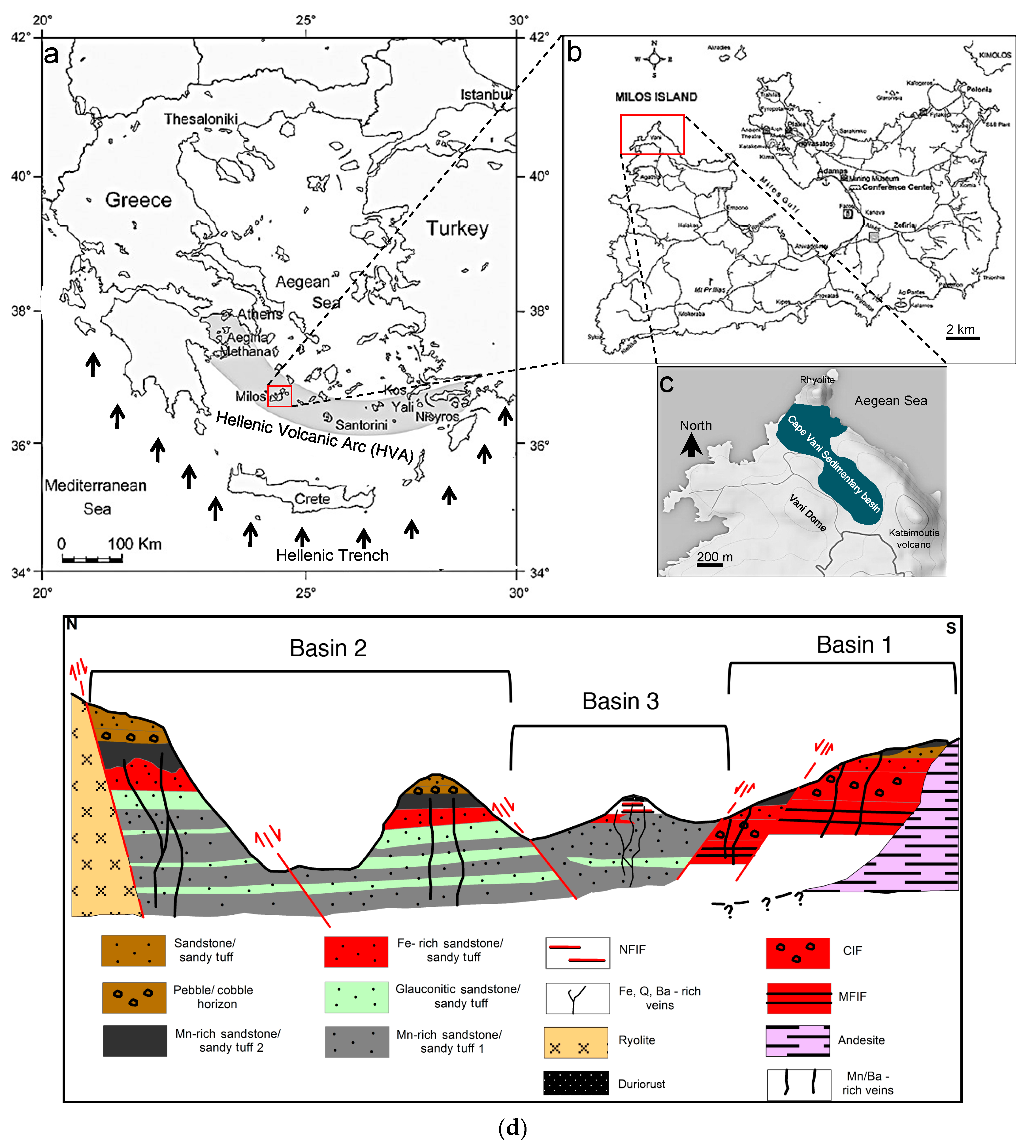
Geology, Mn Mineralization and “Microbially Induced Sedimentary Structures” (MISS) of the Cape Vani Mine
2. Materials and Methods
2.1. Sampling
2.2. Petrographic, Chemical and Mineralogical (Field-Emission Gun Scanning Electron Microscope (FEG-SEM), Field-Emission Gun Transmission Electron Microscope (FEG-TEM)) Analysis
2.3. Electron Paramagnetic Resonance (EPR)
2.4. Organic Carbon Analysis
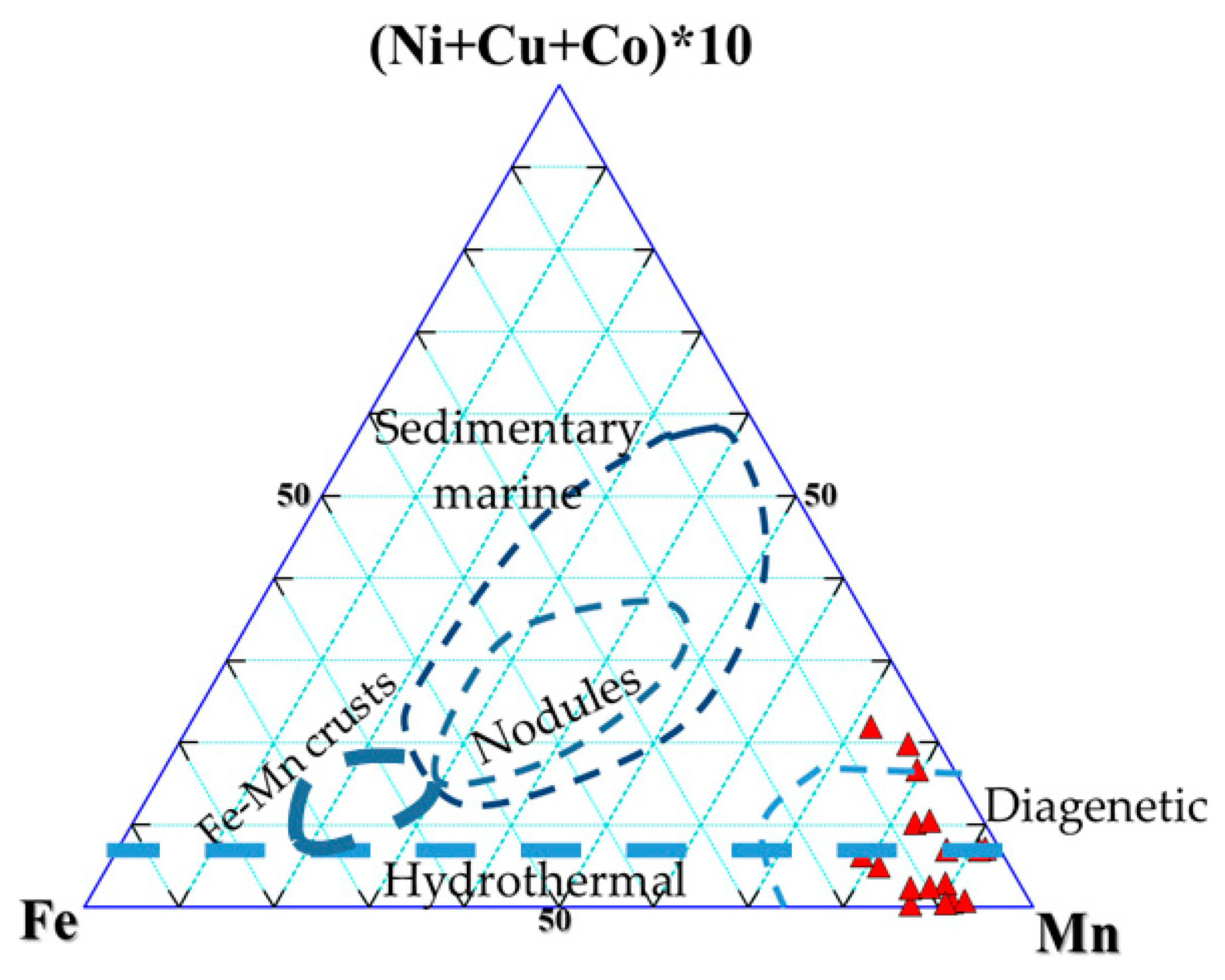
3. Results
3.1. Whole-Rock Geochemistry
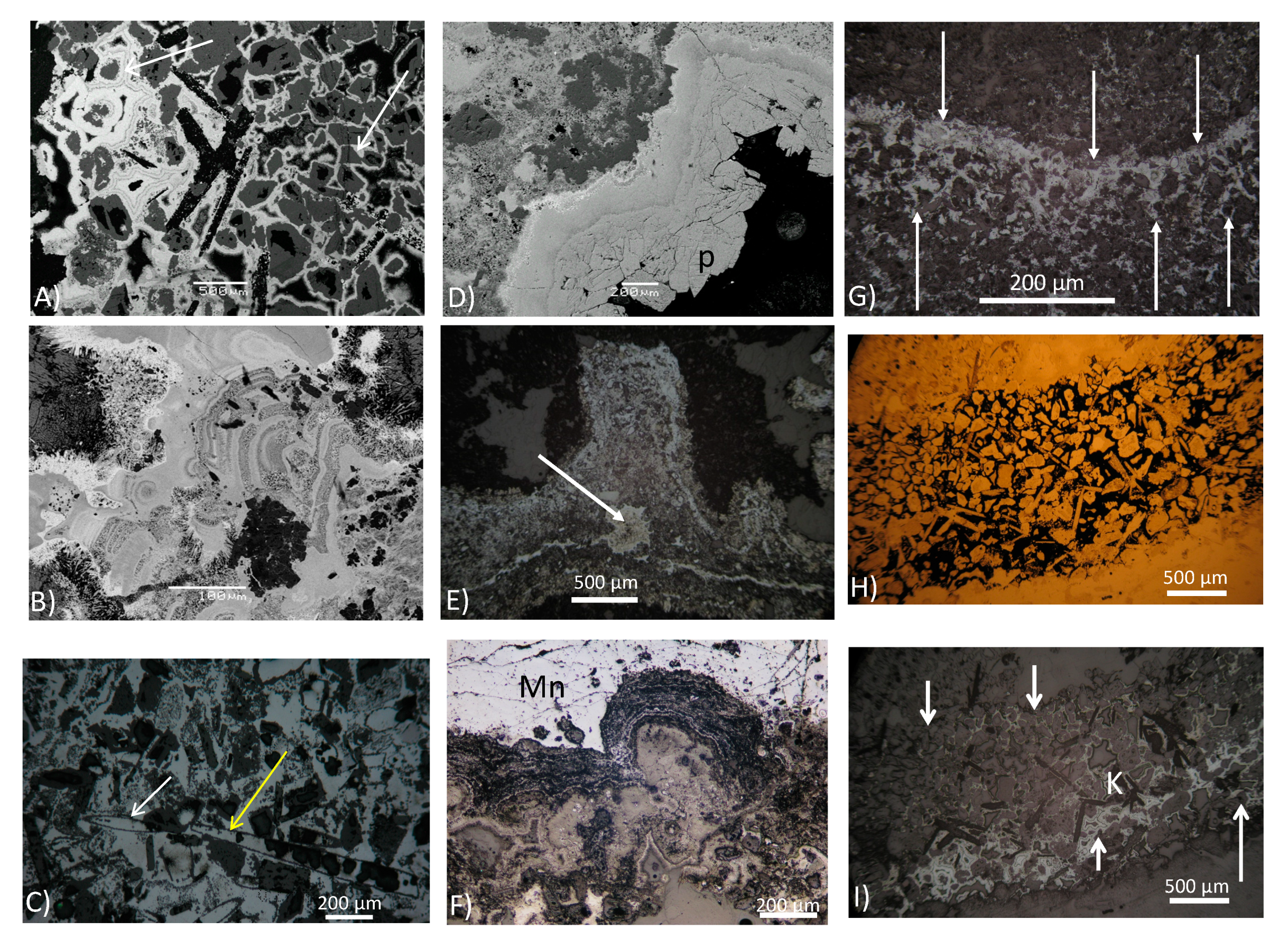
3.2. The Mn Oxide Cement: Microtextures and Mineralogy
3.2.1. Microscopic Textural Diversity
3.2.2. Mineralogical and Textural Characterization of Todorokite
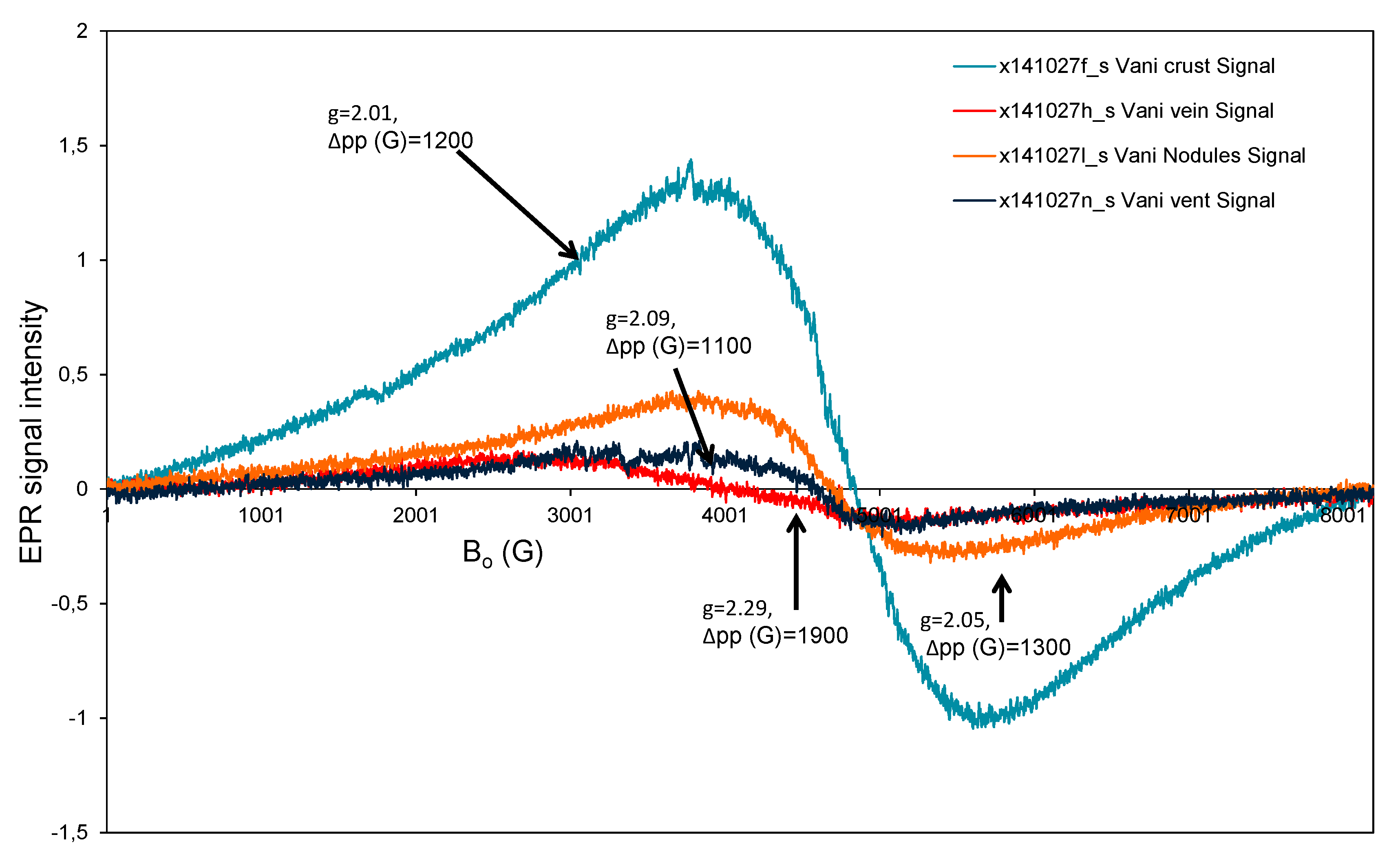
3.3. Electron Paramagnetic Resonance (EPR) Study
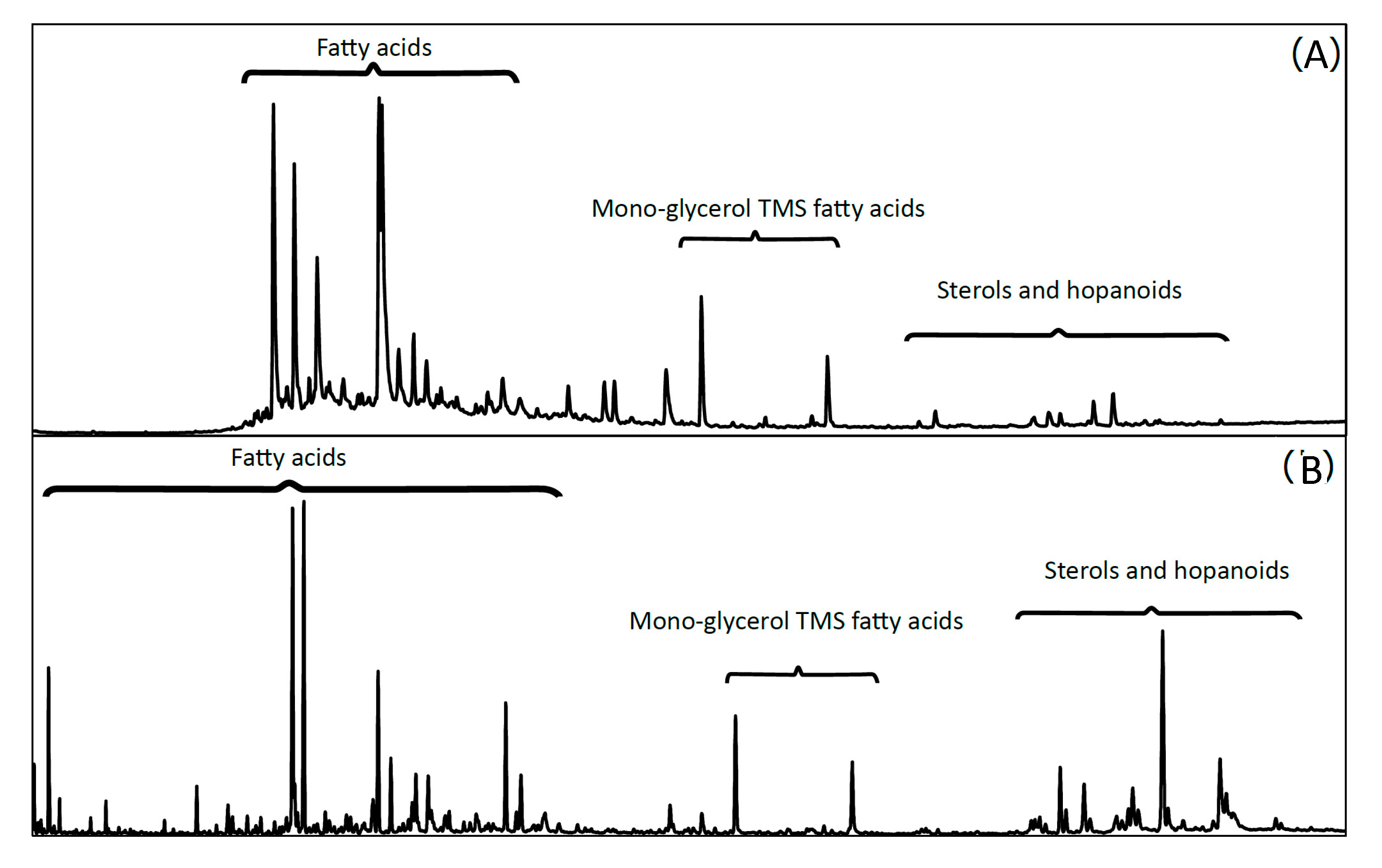
3.4. Total Organic Carbon [TOCorg (%)] and Lipid Biomarker Analysis
3.5. Carbon Isotopes [Bulk δ13Corg (‰), and Lipid Specific δ13Corg (‰)]
4. Discussion
4.1. Biogenicity of the Cape Vani Microbially Induced Sedimentary Structures (MISS) Revisited
4.1.1. Similarity of Form to Modern MISS
- Structures generated from a change in growth direction, such as tufted microstructures (Figure 3E), macroscopic reticulated surface patterns resembling ‘elephant skin’ (see Figure 6 in Kilias (2012) [46]), and microscopic pseudocolumnar structures and microstromatolite-like fabrics of Mn oxides (Figure 3F). The tufted microstructures (Figure 3E) compare strikingly well in size and shape with tufted biofilm structures left by modern cyanobacterial mats in their host sediments [90,91,92,93], as well as experimental tufted cyanobacteria biofilms [94]. The tufted morphology suggests coordinated growth commonly known from cyanobacterial mats in modern environments [93]. Consequently, tufted microstructures may be interpreted as a result of vertical growth of phototactic cyanobacteria stabilized by EPS. This is supported by the Mn oxide-filled bubble-like structure (white arrow in Figure 3E) similar to oxygen-rich bubbles trapped within modern oxygenic cyanobacterial mat fabrics [93].
- Features and fabrics related to trapping and binding of sediment, such as microbial lamina-bound volcaniclastic detrital grains (Figure 3H,I).
- Features derived from physical mat destruction, such as desiccation and cracks with upturned and curled margins, and jelly rolls and cracks (see Figure 7 in Kilias (2012) [46]), gas bubbles aerating the jelly mat below supporting mat detachment (see Figure 9D in Kilias (2012) [46]), and erosional edges, chip production (see Figure 10 in Kilias (2012) [46]).
- Features derived from mat decay and diagenesis, such as punctured gas domes, indicating sealing by mats interacting with diagenetic gas production (see Figure 9A,B in Kilias (2012) [46]).
4.1.2. Distribution Reflecting the Hydrodynamic Conditions of the Depositional Environment
4.1.3. Preserved Organic (Lipid) Material and Bulk and Lipid-Specific Corg Isotope Biomarkers
4.1.4. Textural Evidence of Sediment (Bio) Stabilization
4.2. Biogenicity and Syngenicity of Mn Oxides in Mn–Mineralized Microbially Induced Sedimentary Structures (MnMISS)
4.2.1. Biogenicity
4.2.2. Syngenicity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Southam, G.; Saunders, J.A. The geomicrobiology of ore deposits. Econ. Geol. 2005, 100, 1067–1084. [Google Scholar] [CrossRef]
- Scott, C.; Kelley, K.D.; Slack, J.F. The geobiology of sediment-hosted mineral deposits. Soc. Econ. Geol. Spec. Publ. 2014, 18, 17–35. [Google Scholar]
- Polgári, M.; Gyollai, I.; Fintor, K.; Horváth, H.; Pál-Molnár, E.; Biondi, J.C. Microbially mediated ore-forming processes and cell mineralization. Front. Microbiol. 2019, 10, 2731. [Google Scholar] [CrossRef] [PubMed]
- Tebo, B.M.; Bargar, J.R.; Clement, B.G.; Dick, G.J.; Murray, K.J.; Parker, D.; Verity, R.; Webb, S.M. Biogenic manganese oxides: Properties and Mechanisms of Formation. Annu. Rev. Earth Planet. Sci. 2004, 32, 287–328. [Google Scholar] [CrossRef]
- Tebo, B.M.; Johnson, H.A.; McCarthy, J.K.; Templeton, A.S. Geomicrobiology of manganese (II) oxidation. Trends Microbiol. 2005, 13, 421–428. [Google Scholar] [CrossRef]
- Johnson, J.E.; Webb, S.M.; Ma, C.; Fischer, W.W. Manganese mineralogy and diagenesis in the sedimentary rock record. Geochim. Cosmochim. Acta 2016, 173, 210–231. [Google Scholar] [CrossRef]
- Spiro, T.G.; Bargar, J.R.; Sposito, G.; Tebo, B.M. Bacteriogenic manganese oxides. Acc. Chem. Res. 2010, 43, 2–9. [Google Scholar] [CrossRef]
- Jung, H.; Chadha, T.S.; Kim, D.; Biswas, P.; Jun, Y.S. Photochemically assisted fast abiotic oxidation of manganese and formation of δ-MnO2 nanosheets in nitrate solution. Chem. Commun. 2017, 53, 4445–4448. [Google Scholar] [CrossRef]
- Johnson, K.; Purvis, G.; Lopez-Capel, E.; Peacock, C.; Gray, N.; Wagner, T.; März, C.; Bowen, L.; Ojeda, J.; Finlay, N.; et al. Towards a mechanistic understanding of carbon stabilization in manganese oxides. Nat. Commun. 2015, 6, 1–11. [Google Scholar] [CrossRef]
- Junta, J.L.; Hochella, M.F. Manganese (II) oxidation at mineral surfaces: A microscopic and spectroscopic study. Geochim. Cosmochim. Acta 1994, 58, 4985–4999. [Google Scholar] [CrossRef]
- Madden, A.S.; Hochella, M.F. A test of geochemical reactivity as a function of mineral size: Manganese oxidation promoted by hematite nanoparticles. Geochim. Cosmochim. Acta 2005, 69, 389–398. [Google Scholar] [CrossRef]
- Learman, D.R.; Wankel, S.D.; Webb, S.M.; Martinez, N.; Madden, A.S.; Hansel, C.M. Coupled biotic-abiotic Mn (II) oxidation pathway mediates the formation and structural evolution of biogenic Mn oxides. Geochim. Cosmochim. Acta 2011, 75, 6048–6063. [Google Scholar] [CrossRef]
- Johnson, J.E.; Web, S.M.; Thomas, K.; Ono, S.; Kirschvink, J.L.; Fischer, W.W. Manganese-oxidizing photosynthesis before the rise of cyanobacteria. Proc. Natl. Acad. Sci. USA 2013, 110, 11238–11243. [Google Scholar] [CrossRef] [PubMed]
- Saratovsky, I.; Gurr, S.J.; Hayward, M.A. The structure of manganese oxide formed by the fungus Acremonium sp. strain KR21-2. Geochim. Cosmochim. Acta 2009, 73, 3291–3300. [Google Scholar] [CrossRef]
- Hansel, C.M.; Zeiner, C.A.; Santelli, C.M.; Webb, S.M. Mn (II) oxidation by an ascomycete fungus is linked to superoxide production during asexual reproduction. Proc. Natl. Acad. Sci. USA 2012, 109, 12621–12625. [Google Scholar] [CrossRef]
- Chaput, D.L.; Fowler, A.J.; Seo, O.; Duhn, K.; Hansel, C.M.; Santelli, C.M. Mn oxide formation by phototrophs: Spatial and temporal patterns, with evidence of an enzymatic superoxide-mediated pathway. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Soldatova, A.V.; Butterfield, C.; Oyerinde, O.F.; Tebo, B.M.; Spiro, T.G. Multicopper oxidase involvement in both Mn (II) and Mn (III) oxidation during bacterial formation of MnO2. J. Biol. Inorg. Chem. 2012, 17, 1151–1158. [Google Scholar] [CrossRef]
- Butterfield, C.N.; Soldatova, A.V.; Lee, S.W.; Spiro, T.G.; Tebo, B.M. Mn (II,III) oxidation and MnO2 mineralization by an expressed bacterial multicopper oxidase. Proc. Natl. Acad. Sci. USA 2013, 110, 11731–11735. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Z.; Chen, H.; Liu, J.; Liu, C.; Ni, H.; Zhao, C.; Ali, M.; Liu, F.; Li, L. Surface Mn (II) oxidation actuated by a multicopper oxidase in a soil bacterium leads to the formation of manganese oxide minerals. Sci. Rep. 2015, 5, 10895. [Google Scholar] [CrossRef]
- Romano, C.A.; Zhou, M.; Song, Y.; Wysocki, V.H.; Dohnalkova, A.C.; Kovarik, L.; Paša-Tolić, L.; Tebo, B.M. Biogenic manganese oxide nanoparticle formation by a multimeric multicopper oxidase Mnx. Nat. Commun. 2017, 8, 1–8. [Google Scholar] [CrossRef]
- Anderson, C.R.; Johnson, H.A.; Caputo, N.; Davis, R.E.; Torpey, J.W.; Tebo, B.M. Mn (II) oxidation is catalyzed by heme peroxidases in “Aurantimonas manganoxydans” strain SI85-9A1 and Erythrobacter sp. strain SD-21. Appl. Environ. Microbiol. 2009, 75, 4130–4138. [Google Scholar] [CrossRef] [PubMed]
- Glenn, J.K.; Akileswaran, L.; Gold, M.H. Mn (II) oxidation is the principal function of the extracellular Mn-peroxidase from Phanerochaete chrysosporium. Arch. Biochem. Biophys. 1986, 251, 688–696. [Google Scholar] [CrossRef]
- Barboza, N.R.; Guerra-Sá, R.; Leão, V.A. Mechanisms of manganese bioremediation by microbes: An overview. J. Chem. Technol. Biotechnol. 2016, 91, 2733–2739. [Google Scholar] [CrossRef]
- Learman, D.R.; Voelker, B.M.; Vazquez-Rodriguez, A.I.; Hansel, C.M. Formation of manganese oxides by bacterially generated superoxide. Nat. Geosci. 2011, 4, 95–98. [Google Scholar] [CrossRef]
- Learman, D.R.; Voelker, B.M.; Madden, A.S.; Hansel, C.M. Constraints on superoxide mediated formation of manganese oxides. Front. Microbiol. 2013, 4, 262. [Google Scholar] [CrossRef]
- Daye, M.; Klepac-Ceraj, V.; Pajusalu, M.; Rowland, S.; Farrell-Sherman, A.; Beukes, N.; Tamura, N.; Fournier, G.; Bosak, T. Light-driven anaerobic microbial oxidation of manganese. Nature 2019, 576, 311–314. [Google Scholar] [CrossRef]
- Post, J.E. Manganese oxide minerals: Crystal structures and economic and environmental significance. Proc. Natl. Acad. Sci. USA 1999, 96, 3447–3454. [Google Scholar] [CrossRef]
- Hein, J.R.; Koschinsky, A. Deep-Ocean Ferromanganese Crusts and Nodules. In Treatise on Geochemistry, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 273–291. [Google Scholar] [CrossRef]
- Maynard, J.B. The chemistry of manganese ores through time: A signal of increasing diversity of earth-surface environments. Econ. Geol. 2010, 105, 535–552. [Google Scholar] [CrossRef]
- Rajabzadeh, M.A.; Haddad, F.; Polgári, M.; Fintor, K.; Walter, H.; Molnár, Z.; Gyollai, I. Investigation on the role of microorganisms in manganese mineralization from Abadeh-Tashk area, Fars Province, southwestern Iran by using petrographic and geochemical data. Ore Geol. Rev. 2017, 80, 229–249. [Google Scholar] [CrossRef]
- Yu, W.; Polgári, M.; Gyollai, I.; Fintor, K.; Szabó, M.; Kovács, I.; Fekete, J.; Du, Y.; Zhou, Q. Microbial metallogenesis of Cryogenian manganese ore deposits in South China. Precambrian Res. 2019, 322, 122–135. [Google Scholar] [CrossRef]
- Hein, J.R.; Koski, R.A. Bacterially mediated diagenetic origin for chert-hosted manganese deposits in the Franciscan Complex, California Coast Ranges (USA). Geology 1987, 15, 722–726. [Google Scholar] [CrossRef]
- Gutzmer, J.; Schaefer, M.O.; Beukes, N.J. Red bed-hosted oncolitic manganese ore of the Paleoproterozoic Soutpansberg group, Bronkhorstfontein, South Africa. Econ. Geol. 2002, 97, 1151–1166. [Google Scholar] [CrossRef]
- Roy, S. Sedimentary manganese metallogenesis in response to the evolution of the Earth system. Earth Sci. Rev. 2006, 77, 273–305. [Google Scholar] [CrossRef]
- Tsikos, H.; Matthews, A.; Erel, Y.; Moore, J.M. Iron isotopes constrain biogeochemical redox cycling of iron and manganese in a Palaeoproterozoic stratified basin. Earth Planet. Sci. Lett. 2010, 298, 125–134. [Google Scholar] [CrossRef]
- Planavsky, N.J.; Asael, D.; Hofmann, A.; Reinhard, C.T.; Lalonde, S.V.; Knudsen, A.; Wang, X.; Ossa Ossa, F.; Pecoits, E.; Smith, A.J.B.; et al. Evidence for oxygenic photosynthesis half a billion years before the Great Oxidation Event. Nat. Geosci. 2014, 7, 283–286. [Google Scholar] [CrossRef]
- Polgári, M.; Hein, J.R.; Vigh, T.; Szabó-Drubina, M.; Fórizs, I.; Bíró, L.; Müller, A.; Tóth, A.L. Microbial processes and the origin of the Úrkút manganese deposit, Hungary. Ore Geol. Rev. 2012, 47, 87–109. [Google Scholar] [CrossRef]
- Biondi, J.C.; Lopez, M. Urucum Neoproterozoic–Cambrian manganese deposits (MS, Brazil): Biogenic participation in the ore genesis, geology, geochemistry, and depositional environment. Ore Geol. Rev. 2017, 91, 335–386. [Google Scholar] [CrossRef]
- Ostwald, J. Evidence for a biogeochemical origin of the Groote Eylandt manganese ores. Econ. Geol. 1981, 76, 556–567. [Google Scholar] [CrossRef]
- Ostwald, J. The biogeochemical origin of the Groote Eylandt manganese oxide pisoliths and ooliths, northern Australia. Ore Geol. Rev. 1990, 5, 469–490. [Google Scholar] [CrossRef]
- Hein, J.R.; Stamatakis, M.G.; Dowling, J.S. Trace metal-rich Quaternary hydrothermal manganese oxide and barite deposit, Milos Island, Greece. In Transactions of the Institutions of Mining and Metallurgy, Section B: Applied Earth Science; Taylor and Francis Ltd.: Didcot, UK, 2000; Volume 109, pp. 67–76. [Google Scholar]
- Liakopoulos, A.; Glasby, G.P.; Papavassiliou, C.T.; Boulegue, J. Nature and origin of the Vani manganese deposit, Milos, Greece: An overview. Ore Geol. Rev. 2001, 18, 181–209. [Google Scholar] [CrossRef]
- Ivarsson, M.; Kilias, S.P.; Broman, C.; Neubeck, A.; Drake, H.; Chi Fru, E.; Bengtson, S.; Naden, J.; Detsi, K.; Whitehouse, M.J. Exceptional preservation of fungi as H2-bearing fluid inclusions in an early Quaternary paleohydrothermal system at Cape Vani, Milos, Greece. Minerals 2019, 9, 749. [Google Scholar] [CrossRef]
- Glasby, G.P.; Papavassiliou, C.T.; Mitsis, J.; Valsami-Jones, E.; Liakopoulos, A.; Renner, R.M. The Vani manganese deposit, Milos island, Greece: A fossil stratabound Mn-Ba-Pb-Zn-As-Sb-W-rich hydrothermal deposit. In Developments in Volcanology; Elsevier: Amsterdam, The Netherlands, 2005; Volume 7, pp. 255–291. [Google Scholar]
- Stewart, A.L.; McPhie, J. Facies architecture and Late Pliocene - Pleistocene evolution of a felsic volcanic island, Milos, Greece. Bull. Volcanol. 2006, 68, 703–726. [Google Scholar] [CrossRef]
- Kilias, S.P. Microbial Mat–Related Structures in the Quaternary Cape Vani Manganese-Oxide (-Barite) Deposit, NW Milos Island, Greece. In Microbial Mats in Siliciclastic Depositional Systems through Time; SEPM Special Publication: Tulsa, OK, USA, 2012; Volume 101, pp. 97–110. [Google Scholar]
- Alfieris, D.; Voudouris, P.; Spry, P.G. Shallow submarine epithermal Pb-Zn-Cu-Au-Ag-Te mineralization on western Milos Island, Aegean Volcanic Arc, Greece: Mineralogical, geological and geochemical constraints. Ore Geol. Rev. 2013, 53, 159–180. [Google Scholar] [CrossRef]
- Papavassiliou, K.; Voudouris, P.; Kanellopoulos, C.; Glasby, G.; Alfieris, D.; Mitsis, I. New geochemical and mineralogical constraints on the genesis of the Vani hydrothermal manganese deposit at NW Milos island, Greece: Comparison with the Aspro Gialoudi deposit and implications for the formation of the Milos manganese mineralization. Ore Geol. Rev. 2017, 80, 594–611. [Google Scholar] [CrossRef]
- Chi Fru, E.; Ivarsson, M.; Kilias, S.P.; Bengtson, S.; Belivanova, V.; Marone, F.; Fortin, D.; Broman, C.; Stampanoni, M. Fossilized iron bacteria reveal a pathway to the biological origin of banded iron formation. Nat. Commun. 2013, 4, 1–7. [Google Scholar] [CrossRef]
- Chi Fru, E.; Ivarsson, M.; Kilias, S.P.; Frings, P.J.; Hemmingsson, C.; Broman, C.; Bengtson, S.; Chatzitheodoridis, E. Biogenicity of an early quaternary iron formation, Milos Island, Greece. Geobiology 2015, 13, 225–244. [Google Scholar] [CrossRef]
- Chi Fru, E.; Kilias, S.; Ivarsson, M.; Rattray, J.E.; Gkika, K.; McDonald, I.; He, Q.; Broman, C. Sedimentary mechanisms of a modern banded iron formation on Milos Island, Greece. Solid Earth 2018, 9, 573–598. [Google Scholar] [CrossRef]
- Kilias, S.P.; Nomikou, P.; Papanikolaou, D.; Polymenakou, P.N.; Godelitsas, A.; Argyraki, A.; Carey, S.; Gamaletsos, P.; Mertzimekis, T.J.; Stathopoulou, E.; et al. New insights into hydrothermal vent processes in the unique shallow-submarine arc-volcano, Kolumbo (Santorini), Greece. Sci. Rep. 2013, 3, 2421. [Google Scholar] [CrossRef]
- Fytikas, M.; Innocenti, F.; Kolios, N.; Manetti, P.; Mazzuoli, R.; Poli, G.; Rita, F.; Villari, L. Volcanology and petrology of volcanic products from the island of Milos and neighbouring islets. J. Volcanol. Geotherm. Res. 1986, 28, 297–317. [Google Scholar] [CrossRef]
- Papanikolaou, D.; Lekkas, E.; Syskakis, D. Tectonic analysis of the geothermal field of Milos Island. Bull. Geol. Soc. Greece 1990, 24, 27–46. [Google Scholar]
- Dando, P.R.; Aliani, S.; Arab, H.; Bianchi, C.N.; Brehmer, M.; Cocito, S.; Fowler, S.W.; Gundersen, J.; Hooper, L.E.; Kölbl, R.; et al. Hydrothermal studies in the Aegean sea. Phys. Chem. Earth Part B Hydrol. Ocean. Atmos. 2000, 25, 1–8. [Google Scholar] [CrossRef]
- Valsami-Jones, E.; Baltatzis, E.; Bailey, E.H.; Boyce, A.J.; Alexander, J.L.; Magganas, A.; Anderson, L.; Waldron, S.; Ragnarsdottir, K.V. The geochemistry of fluids from an active shallow submarine hydrothermal system: Milos island, Hellenic Volcanic Arc. J. Volcanol. Geotherm. Res. 2005, 148, 130–151. [Google Scholar] [CrossRef]
- Wu, S.F.; You, C.F.; Lin, Y.P.; Valsami-Jones, E.; Baltatzis, E. New boron isotopic evidence for sedimentary and magmatic fluid influence in the shallow hydrothermal vent system of Milos Island (Aegean Sea, Greece). J. Volcanol. Geotherm. Res. 2016, 310, 58–71. [Google Scholar] [CrossRef]
- Kilias, S.P.; Naden, J.; Cheliotis, I.; Shepherd, T.J.; Constandinidou, H.; Crossing, J.; Simos, I. Epithermal gold mineralisation in the active Aegen volcanic arc: The Profitis Ilias deposits, Milos Island, Greece. Miner. Depos. 2001, 36, 32–44. [Google Scholar] [CrossRef]
- Naden, J.; Kilias, S.P.; Darbyshire, D.P.F. Active geothermal systems with entrained seawater as modern analogs for transitional volcanic-hosted massive sulfide and continental magmato-hydrothermal mineralization: The example of Milos Island, Greece. Geology 2005, 33, 541–544. [Google Scholar] [CrossRef]
- Smith, D.J.; Naden, J.; Miles, A.J.; Bennett, H.; Bicknell, S.H. Mass wasting events and their impact on the formation and preservation of submarine ore deposits. Ore Geol. Rev. 2018, 97, 143–151. [Google Scholar] [CrossRef]
- Plimer, I. Milos Geologic History; Koan Publishing House: Athens, Greece, 2000; ISBN 9607586438. [Google Scholar]
- Kilias, S.P.; Detsi, K.; Godelitsas, A.; Typas, M.; Naden, J.; Marantos, Y. Evidence of Mn-oxide biomineralization, Vani Mn deposit, Milos, Greece. In Proceedings of the Ninth Bienniela SGA Meeting, Dublin, UK, 20–23 August 2007; Andrew, C.J., Ed.; Digging Deeper: Dublin, UK, 2007; pp. 1069–1072. [Google Scholar]
- Noffke, N.; Gerdes, G.; Klenke, T.; Krumbein, W.E. Microbially Induced Sedimentary Structures: A New Category within the Classification of Primary Sedimentary Structures. J. Sediment. Res. 2001, 71, 649–656. [Google Scholar] [CrossRef]
- Noffke, N. Geobiology: Microbial Mats in Sandy Deposits from the Archean Era to Today; Springer: Berlin, Germany, 2010; p. 194. ISBN 9783642127717. [Google Scholar]
- Stoll, S.; Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef]
- Ichihara, K.; Fukubayashi, Y. Preparation of fatty acid methyl esters for gas-liquid chromatography. J. Lipid Res. 2010, 51, 635–640. [Google Scholar] [CrossRef]
- Gallegos, E.J. Identification of New Steranes, Terpanes, and Branched Paraffins in Green River Shale by Combined Capillary Gas Chromatography and Mass Spectrometry. Anal. Chem. 1971, 43, 1151–1160. [Google Scholar] [CrossRef]
- Bonatti, E.; Kraemer, T.; Rydell, H. Classification and genesis of submarine iron-manganese deposits. In Ferromanganese Deposits of the Ocean Floor; Horn, D.R., Ed.; National Science Foundation: Washington, DC, USA, 1972; pp. 149–165. [Google Scholar]
- Conly, A.G.; Scott, S.D.; Bellon, H. Metalliferous manganese oxide mineralization associated with the Boléo Cu-Co-Zn district, Mexico. Econ. Geol. 2011, 106, 1173–1196. [Google Scholar] [CrossRef]
- Hein, J.R.; Koschinsky, A.; Halbach, P.; Manheim, F.T.; Bau, M.; Kang, J.K.; Lubick, N. Iron and manganese oxide mineralization in the Pacific. Geol. Soc. Spec. Publ. 1997, 119, 123–138. [Google Scholar] [CrossRef]
- Hein, J.R.; Schulz, M.S.; Dunham, R.E.; Stern, R.J.; Bloomer, S.H. Diffuse flow hydrothermal manganese mineralization along the active Mariana and southern Izu-Bonin arc system, western Pacific. J. Geophys. Res. Solid Earth 2008, 113, B08S14. [Google Scholar] [CrossRef]
- Noffke, N.; Christian, D.; Wacey, D.; Hazen, R.M. Microbially induced sedimentary structures recording an ancient ecosystem in the ca. 3.48 Billion-year-old dresser formation, pilbara, Western Australia. Astrobiology 2013, 13, 1103–1124. [Google Scholar] [CrossRef] [PubMed]
- Akai, J.; Akiyama, S.; Tsuchiyama, A.; Akai, K. Ocean manganese nodules as stromatolite with a fractal like-signature. Phys. Chem. Earth 2013, 58, 42–48. [Google Scholar] [CrossRef]
- Feng, X.H.; Zhu, M.; Ginder-Vogel, M.; Ni, C.; Parikh, S.J.; Sparks, D.L. Formation of nano-crystalline todorokite from biogenic Mn oxides. Geochim. Cosmochim. Acta 2010, 74, 3232–3245. [Google Scholar] [CrossRef]
- Atkins, A.L.; Shaw, S.; Peacock, C.L. Nucleation and growth of todorokite from birnessite: Implications for trace-metal cycling in marine sediments. Geochim. Cosmochim. Acta 2014, 144, 109–125. [Google Scholar] [CrossRef]
- Atkins, A.L.; Shaw, S.; Peacock, C.L. Release of Ni from birnessite during transformation of birnessite to todorokite: Implications for Ni cycling in marine sediments. Geochim. Cosmochim. Acta 2016, 189, 158–183. [Google Scholar] [CrossRef]
- Wu, Z.; Peacock, C.L.; Lanson, B.; Yin, H.; Zheng, L.; Chen, Z.; Tan, W.; Qiu, G.; Liu, F.; Feng, X. Transformation of Co-containing birnessite to todorokite: Effect of Co on the transformation and implications for Co mobility. Geochim. Cosmochim. Acta 2019, 246, 21–40. [Google Scholar] [CrossRef]
- Jung, H.; Taillefert, M.; Sun, J.; Wang, Q.; Borkiewicz, O.J.; Liu, P.; Yang, L.; Chen, S.; Chen, H.; Tang, Y. Redox Cycling Driven Transformation of Layered Manganese Oxides to Tunnel Structures. J. Am. Chem. Soc. 2020, 142, 2506–2513. [Google Scholar] [CrossRef]
- Buatier, M.D.; Guillaume, D.; Wheat, C.G.; Hervé, L.; Adatte, T. Mineralogical characterization and genesis of hydrothermal Mn oxides from the flank of the Juan the Fuca Ridge. Am. Mineral. 2004, 89, 1807–1815. [Google Scholar] [CrossRef]
- Gutzmer, J.; Beukes, N.J. Asbestiform manjiroite and todorokite from the Kalahari manganese field, South Africa. South Afr. J. Geol. 2000, 103, 163–174. [Google Scholar] [CrossRef]
- Michailidis, K.M.; Nicholson, K.; Nimfopoulos, M.K.; Pattrick, R.A.D. An EPMA and SEM study of the Mn-oxide mineralization of Kato Nevrokopi, Macedonia, northern Greece: Controls on formation of the Mn4+ oxides. Geol. Soc. Spec. Publ. 1997, 119, 265–280. [Google Scholar] [CrossRef]
- Penn, R.L.; Banfield, J.F. Imperfect oriented attachment: Dislocation generation in defect-free nanocrystals. Science 1998, 281, 969–971. [Google Scholar] [CrossRef]
- Bodeï, S.; Manceau, A.; Geoffroy, N.; Baronnet, A.; Buatier, M. Formation of todorokite from vernadite in Ni-rich hemipelagic sediments. Geochim. Cosmochim. Acta 2007, 71, 5698–5716. [Google Scholar] [CrossRef]
- Xu, H.; Chen, T.; Konishi, H. HRTEM investigation of trilling todorokite and nano-phase Mn-oxides in manganese dendrites. Am. Mineral. 2010, 95, 556–562. [Google Scholar] [CrossRef]
- Kaneda, T. Valine as a source of the branched short chain precursor in the biosynthesis of iso-C14, iso-C15, iso-C16 and iso-C17 fatty acids by Bacillus, subtilis. Biochem. Biophys. Res. Commun. 1963, 10, 283–287. [Google Scholar] [CrossRef]
- Callac, N.; Posth, N.R.; Rattray, J.E.; Yamoah, K.K.Y.; Wiech, A.; Ivarsson, M.; Hemmingsson, C.; Kilias, S.P.; Argyraki, A.; Broman, C.; et al. Modes of carbon fixation in an arsenic and CO2-rich shallow hydrothermal ecosystem. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Davies, N.S.; Liu, A.G.; Gibling, M.R.; Miller, R.F. Resolving MISS conceptions and misconceptions: A geological approach to sedimentary surface textures generated by microbial and abiotic processes. Earth Sci. Rev. 2016, 154, 210–246. [Google Scholar] [CrossRef]
- Perry, R.S.; Mcloughlin, N.; Lynne, B.Y.; Sephton, M.A.; Oliver, J.D.; Perry, C.C.; Campbell, K.; Engel, M.H.; Farmer, J.D.; Brasier, M.D.; et al. Defining biominerals and organominerals: Direct and indirect indicators of life. Sediment. Geol. 2007, 201, 157–179. [Google Scholar] [CrossRef]
- Schieber, J. Mat features in sandstones-Diagenetic features related to mat metabolism and decay. In Atlas of Microbial Mat Features Preserved within the Siliciclastic Rock Record; Schieber, J., Bose, P.K., Eriksson, P.G., Banerjee, S., Sarkar, S., Altermann, W., Catuneanu, O., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 72–75. ISBN 978-0-444-52859-9. [Google Scholar]
- Gerdes, G.; Klenke, T.; Noffke, N. Microbial signatures in peritidal siliciclastic sediments: A catalogue. Sedimentology 2000, 47, 279–308. [Google Scholar] [CrossRef]
- Gerdes, G. Structures left by modern microbial mats in their host sediments. In Atlas of Microbial Mat Features Preserved within the Siliciclastic Rock Record; Schieber, J., Bose, P.K., Eriksson, P.G., Banerjee, S., Sarkar, S., Altermann, W., Catuneanu, O., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 5–38. ISBN 978-0-444-52859-9. [Google Scholar]
- Westall, F.; Steele, A.; Toporski, J.; Walsh, M.; Allen, C.; Guidry, S.; McKay, D.; Gibson, E.; Chafetz, H. Polymeric substances and biofilms as biomarkers in terrestrial materials: Implications for extraterrestrial samples. J. Geophys. Res. E Planets 2000, 105, 24511–24527. [Google Scholar] [CrossRef]
- Homann, M.; Heubeck, C.; Airo, A.; Tice, M.M. Morphological adaptations of 3.22 Ga-old tufted microbial mats to Archean coastal habitats (Moodies Group, Barberton Greenstone Belt, South Africa). Precambrian Res. 2015, 266, 47–64. [Google Scholar] [CrossRef]
- Bosak, T.; Bush, J.W.M.; Flynn, M.R.; Liang, B.; Ono, S.; Petroff, A.P.; Sim, M.S. Formation and stability of oxygen-rich bubbles that shape photosynthetic mats. Geobiology 2010, 8, 45–55. [Google Scholar] [CrossRef]
- Hill, C.; Corcoran, P.L.; Aranha, R.; Longstaffe, F.J. Microbially induced sedimentary structures in the Paleoproterozoic, upper Huronian Supergroup, Canada. Precambrian Res. 2016, 281, 155–165. [Google Scholar] [CrossRef]
- Noffke, N.; Eriksson, K.A.; Hazen, R.M.; Simpson, E.L. A new window into Early Archean life: Microbial mats in Earth’s oldest siliciclastic tidal deposits (3.2 Ga Moodies Group, South Africa). Geology 2006, 34, 253–256. [Google Scholar] [CrossRef]
- Harazim, D.; Callow, R.H.T.; Mcilroy, D. Microbial mats implicated in the generation of intrastratal shrinkage (‘synaeresis’) cracks. Sedimentology 2013, 60, 1621–1638. [Google Scholar] [CrossRef]
- Aubineau, J.; El Albani, A.; Chi Fru, E.; Gingras, M.; Batonneau, Y.; Buatois, L.A.; Geffroy, C.; Labanowski, J.; Laforest, C.; Lemée, L.; et al. Unusual microbial mat-related structural diversity 2.1 billion years ago and implications for the Francevillian biota. Geobiology 2018, 16, 476–497. [Google Scholar] [CrossRef]
- Dick, G.J.; Clement, B.G.; Webb, S.M.; Fodrie, F.J.; Bargar, J.R.; Tebo, B.M. Enzymatic microbial Mn (II) oxidation and Mn biooxide production in the Guaymas Basin deep-sea hydrothermal plume. Geochim. Cosmochim. Acta 2009, 73, 6517–6530. [Google Scholar] [CrossRef]
- Templeton, A.S.; Staudigel, H.; Tebo, B.M. Diverse Mn (II)-oxidizing bacteria isolated from submarine basalts at Loihi Seamount. Geomicrobiol. J. 2005, 22, 127–139. [Google Scholar] [CrossRef]
- Jahnke, L.L.; Summons, R.E.; Hope, J.M.; Des Marais, D.J. Carbon isotopic fractionation in lipids from methanotrophic bacteria II: The effects of physiology and environmental parameters on the biosynthesis and isotopic signatures of biomarkers. Geochim. Cosmochim. Acta 1999, 63, 79–93. [Google Scholar] [CrossRef]
- Schouten, S.; Rijpstra, W.I.C.; Kok, M.; Hopmans, E.C.; Summons, R.E.; Volkman, J.K.; Sinninghe Damsté, J.S. Molecular organic tracers of biogeochemical process in a saline meromictic lake. Geochim. Cosmochim. Acta 2001, 65, 1629–1640. [Google Scholar] [CrossRef]
- Summons, R.E.; Jahnke, L.L.; Roksandic, Z. Carbon isotopic fractionation in lipids from methanotrophic bacteria: Relevance for interpretation of the geochemical record of biomarkers. Geochim. Cosmochim. Acta 1994, 58, 2853–2863. [Google Scholar] [CrossRef]
- Jahnke, L.L.; Summons, R.E.; Dowling, L.M.; Zahiralis, K.D. Identification of methanotrophic lipid biomarkers in cold-seep mussel gills: Chemical and isotopic analysis. Appl. Environ. Microbiol. 1995, 61, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Valentine, D.L. Methanotrophic bacteria occupy benthic microbial mats in shallow marine hydrocarbon seeps, Coal Oil Point, California. J. Geophys. Res. Biogeosci. 2008, 113, G01015. [Google Scholar] [CrossRef]
- Winters, K.; Parker, P.L.; Van Baalen, C. Hydrocarbons of blue-green algae: Geochemical significance. Science 1969, 163, 467–468. [Google Scholar] [CrossRef] [PubMed]
- Volkman, J.K.; Eglinton, G.; Corner, E.D.S.; Forsberg, T.E.V. Long-chain alkenes and alkenones in the marine coccolithophorid Emiliania huxleyi. Phytochemistry 1980, 19, 2619–2622. [Google Scholar] [CrossRef]
- Thiel, V.; Merz-Preiß, M.; Reitner, J.; Michaelis, W. Biomarker studies on microbial carbonates: Extractable lipids of a calcifying cyanobacterial mat (Everglades, USA). Facies 1997, 36, 163–172. [Google Scholar] [CrossRef]
- Nitti, A.; Daniels, C.A.; Siefert, J.; Souza, V.; Hollander, D.; Breitbart, M. Spatially resolved genomic, stable isotopic, and lipid analyses of a modern freshwater microbialite from Cuatro Ciénegas, Mexico. Astrobiology 2012, 12, 685–698. [Google Scholar] [CrossRef]
- Bižić, M.; Klintzsch, T.; Ionescu, D.; Hindiyeh, M.Y.; Günthel, M.; Muro-Pastor, A.M.; Eckert, W.; Urich, T.; Keppler, F.; Grossart, H.P. Aquatic and terrestrial cyanobacteria produce methane. Sci. Adv. 2020, 6, eaax5343. [Google Scholar] [CrossRef]
- Treude, T.; Orphan, V.; Knittel, K.; Gieseke, A.; House, C.H.; Boetius, A. Consumption of methane and CO2 by methanotrophic microbial mats from gas seeps of the anoxic Black Sea. Appl. Environ. Microbiol. 2007, 73, 2271–2283. [Google Scholar] [CrossRef]
- Paul, B.G.; Ding, H.; Bagby, S.C.; Kellermann, M.Y.; Redmond, M.C.; Andersen, G.L.; Valentine, D.L. Methane-oxidizing bacteria shunt carbon to microbial mats at a marine hydrocarbon seep. Front. Microbiol. 2017, 8, 186. [Google Scholar] [CrossRef]
- Noffke, N.; Gerdes, G.; Klenke, T.; Krumbein, W.E. A microscopic sedimentary succession of graded sand and microbial mats in modern siliciclastic tidal flats. Sediment. Geol. 1997, 110, 1–6. [Google Scholar] [CrossRef]
- Kim, S.S.; Bargar, J.R.; Nealson, K.H.; Flood, B.E.; Kirschvink, J.L.; Raub, T.D.; Tebo, B.M.; Villalobos, M. Searching for biosignatures using electron paramagnetic resonance (EPR) analysis of manganese oxides. Astrobiology 2011, 11, 775–786. [Google Scholar] [CrossRef]
- Ivarsson, M.; Broman, C.; Gustafsson, H.; Holm, N.G. Biogenic Mn-oxides in subseafloor basalts. PLoS ONE 2015, 10, e0128863. [Google Scholar] [CrossRef]
- Eriksson, P.G.; Schieber, J.; Bouougri, E.; Gerdes, G.; Porada, H.; Banerjee, S.; Bose, P.K. Classification of structures left by microbial mats in their host sediments. In Atlas of Microbial Mat Features Preserved within the Siliciclastic Rock Record; Schieber, J., Bose, P.K., Eriksson, P.G., Banerjee, S., Sarkar, S., Altermann, W., Catuneanu, O., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 39–52. ISBN 978-0-444-52859-9. [Google Scholar]
- Bell, J.B.; Woulds, C.; van Oevelen, D. Hydrothermal activity, functional diversity and chemoautotrophy are major drivers of seafloor carbon cycling. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]

| Sample | MISS Type 1 | Fe | Ca | P | Mg | Ti | Al | K | Na | S | SiO2 | Mn | |
| wt.% | |||||||||||||
| MI-10–28 | upturned margins | 0.61 | 0.18 | 0.030 | 0.15 | 0.127 | 5.32 | 3.86 | 0.134 | <0.1 | 42.69 | 7.22 | |
| MI-10–37 | Mn nodules | 7.98 | 0.12 | 0.031 | 0.10 | 0.082 | 3.18 | 2.96 | 0.100 | <0.1 | 26.31 | 14.18 | |
| MI-10–20 | growth bedding | 1.11 | 0.19 | 0.005 | 0.06 | 0.201 | 4.93 | 4.00 | 0.257 | <0.1 | 44.26 | 14.88 | |
| MI-10–24 | fossil gas dome | 1.75 | 0.19 | 0.014 | 0.28 | 0.111 | 4.48 | 4.22 | 0.219 | 0.1 | 36.09 | 14.62 | |
| MI-10–26 | mat layer structure | 3.22 | 0.15 | 0.006 | 0.03 | 0.043 | 2.07 | 3.17 | 0.060 | <0.1 | 29.12 | 10.71 | |
| MI-10–29 | Mn nodules | 1.58 | 0.13 | 0.009 | 0.11 | 0.163 | 4.11 | 2.47 | 0.094 | <0.1 | 35.86 | 14.49 | |
| MI-10–12 | roll-up structure | 3.89 | 0.34 | 0.014 | 0.24 | 0.051 | 1.94 | 2.57 | 0.286 | <0.1 | 18.41 | 24.86 | |
| MI-10–15 | roll-up structure | 2.25 | 0.43 | 0.023 | 0.29 | 0.038 | 2.28 | 3.58 | 0.442 | <0.1 | 30.12 | 29.58 | |
| MI-10–22 | mat fragments/chips | 1.71 | 0.09 | 0.005 | 0.09 | 0.056 | 4.24 | 3.73 | 0.161 | <0.1 | 33.47 | 13.29 | |
| MI-10–27 | upturned margins | 0.67 | 0.10 | 0.032 | 0.06 | 0.132 | 4.96 | 3.10 | 0.097 | <0.1 | 38.9 | 9.80 | |
| MI-10–26S 2 | sandstone/sandy tuff | 2.52 | 0.11 | 0.004 | 0.05 | 0.101 | 3.92 | 5.22 | 0.087 | 0.2 | 62.05 | 0.92 | |
| MI-10–29S 2 | sandstone/sandy tuff | 1.14 | 0.06 | 0.003 | 0.25 | 0.252 | 6.70 | 6.41 | 0.089 | 0.2 | 64.7 | 0.95 | |
| MI-27S 2 | sandstone/sandy tuff | 0.67 | 0.11 | 0.031 | 0.06 | 0.183 | 6.56 | 5.52 | 0.097 | 0.1 | 64.13 | 0.66 | |
| MI-34S 2 | sandstone/sandy tuff | 0.68 | 0.07 | 0.006 | 0.02 | 0.064 | 2.42 | 3.62 | 0.062 | <0.1 | 72.33 | 3.07 | |
| MI-34 | wrinkle structures | 2.66 | 0.15 | 0.015 | 0.06 | 0.055 | 2.78 | 3.88 | 0.082 | <0.1 | 33.03 | 9.63 | |
| MI-33 | wrinkle structures | 0.76 | 0.08 | 0.006 | 0.09 | 0.110 | 2.90 | 4.14 | 0.212 | <0.1 | 66.71 | 6.41 | |
| Sample | MISS Type 1 | Zn | Ag | Ni | Co | As | U | Th | Sr | Cd | Sb | V | La |
| ppm | |||||||||||||
| MI-10–28 | upturned margins | 1802 | 7.2 | 4.2 | 8.1 | 736 | 4.0 | 7.1 | 592 | 1.6 | 43.0 | 11 | 11.7 |
| MI-10–37 | Mn nodules | 6881 | 7.2 | 9.1 | 78.6 | 3551 | 6.5 | 3.1 | 449 | 13.0 | 126.8 | 21 | 16.2 |
| MI-10–20 | growth bedding | 5216 | 35.8 | 9.9 | 35.6 | 2020 | 7.6 | 4.1 | 1652 | 5.3 | 293.1 | 91 | 23.2 |
| MI-10–24 | fossil gas dome | 6884 | 9.4 | 4.5 | 4.2 | 511 | 2.5 | 5.1 | 638 | 23.8 | 45.7 | 11 | 10.9 |
| MI-10–26 | mat layer structure | 3679 | 12.8 | 3.4 | 9.4 | 2031 | 4.5 | 1.4 | 2232 | 2.1 | 235.5 | 30 | 16.9 |
| MI-10–29 | Mn nodules | 2632 | 24.6 | 8.6 | 15.8 | 1457 | 7.4 | 5.3 | 1336 | 4.0 | 161.6 | 56 | 16.5 |
| MI-10–12 | roll-up structure | 4225 | 26.0 | 13.6 | 12.0 | 302 | 5.4 | 2.2 | 2125 | 40.9 | 8.1 | 22 | 27.0 |
| MI-10–15 | roll-up structure | 4183 | 14.1 | 7.1 | 20.7 | 317 | 9.0 | 2.1 | 1782 | 37.0 | 43.1 | 1 | 22.2 |
| MI-10–22 | mat fragments/chips | 4936 | 16.3 | 7.9 | 13.0 | 2003 | 4.5 | 3.0 | 495 | 2.1 | 415.9 | 68 | 21.2 |
| MI-10–27 | upturned margins | 1458 | 9.5 | 3.6 | 8.7 | 1445 | 4.0 | 5.3 | 646 | 2.6 | 30.6 | 1 | 12.7 |
| MI-10–26S 2 | sandstone/sandy tuff | 562 | 34.4 | 2.7 | 1.6 | 618 | 2.6 | 2.5 | 2463 | 0.6 | 109.0 | 29 | 3.9 |
| MI-10–29S 2 | sandstone/sandy tuff | 495 | 43.9 | 3.4 | 2.5 | 74 | 2.9 | 5.8 | 938 | 0.2 | 33.8 | 30 | 9.1 |
| MI-27S 2 | sandstone/sandy tuff | 446 | 10.8 | 2.6 | 1.4 | 144 | 3.3 | 3.9 | 594 | 0.2 | 17.2 | 31 | 4.8 |
| MI-34S 2 | sandstone/sandy tuff | 396 | 16.8 | 1.8 | 2.6 | 170 | 1.6 | 2.2 | 1968 | 1.1 | 37.7 | 10 | 4.6 |
| MI-34 | wrinkle structures | 2449 | 13.9 | 3.7 | 20.3 | 1246 | 3.0 | 2.8 | 1957 | 5.3 | 86.2 | <1 | 12.2 |
| MI-33 | wrinkle structures | 589 | 43.6 | 5.3 | 2.5 | 211 | 2.5 | 3 | 636 | 0.5 | 68.3 | 16 | 4.7 |
| Sample | MISS Type 1 | Cr | Ba | W | Zr | Ce | Sn | Th | Y | Nb | Ta | Be | Sc |
| ppm | |||||||||||||
| MI-10–28 | upturned and curled margins | 16 | 4328 | 22.3 | 56.7 | 30 | 1.3 | 7.1 | 18.8 | 4.7 | 0.4 | 7 | 9 |
| MI-10–37 | Mn nodules | 19 | >10,000 | 55.6 | 24.9 | 34 | 1.1 | 3.1 | 20.8 | 2.3 | 0.2 | 20 | 6 |
| MI-10–20 | growth bedding | 25 | >10,000 | 103.9 | 44.6 | 47 | 1.5 | 4.1 | 16.2 | 3.5 | 0.2 | 10 | 4 |
| MI-10–24 | fossil gas dome | 15 | >10,000 | 28.0 | 37.5 | 18 | 0.7 | 5.1 | 12.3 | 3.4 | 0.3 | 6 | 5 |
| MI-10–26 | mat layer structure | 13 | >10,000 | 66.7 | 12.0 | 25 | 0.8 | 1.4 | 8.0 | 1.1 | <0.1 | 12 | 2 |
| MI-10–29 | Mn nodules | 24 | >10,000 | 34.4 | 37.6 | 22 | 1.8 | 5.3 | 9.4 | 4.8 | 0.4 | 2 | 3 |
| MI-10–12 | roll-up structure | 17 | >10,000 | 4.2 | 14.2 | 45 | 0.6 | 2.2 | 59.7 | 1.5 | 0.1 | 2 | 5 |
| MI-10–15 | roll-up structure | 19 | >10,000 | 5.7 | 11.3 | 29 | 0.6 | 2.1 | 33.2 | 1.3 | <0.1 | 5 | 2 |
| MI-10–22 | mat fragments/chips | 22 | >10,000 | 157.2 | 20.8 | 18 | 1.1 | 3.0 | 9.0 | 2.0 | 0.2 | 23 | 3 |
| MI-10–27 | upturned and curled margins | 14 | >10,000 | 16.1 | 40.1 | 19 | 1.3 | 5.3 | 15.9 | 4.2 | 0.3 | 6 | 5 |
| MI-10–26S 2 | sandstone/sandy tuff | 10 | 1128 | 24.4 | 26.0 | 7 | 0.9 | 2.5 | 5.3 | 2.4 | 0.2 | 3 | 3 |
| MI-10–29S 2 | sandstone/sandy tuff | 13 | 2912 | 12.4 | 60.5 | 15 | 1.9 | 5.8 | 6.6 | 8.6 | 0.6 | 2 | 5 |
| MI-27S 2 | sandstone/sandy tuff | 13 | 4653 | 12.4 | 42.8 | 10 | 1.8 | 3.9 | 8.1 | 5.6 | 0.3 | 1 | 2 |
| MI-34S 2 | sandstone/sandy tuff | 9 | 3172 | 4.3 | 22.8 | 9 | 0.7 | 2.2 | 3.6 | 2.1 | 0.2 | 1 | >1 |
| MI-34 | wrinkle structures | 16 | >10,000 | 11.1 | 20.1 | 15 | 0.9 | 2.8 | 11.6 | 1.6 | 0.2 | 5 | 3 |
| MI-33 | wrinkle structures | 30 | >10,000 | 4.3 | 22.6 | 8 | 0.9 | 3.0 | 4.2 | 2.6 | 0.2 | 2 | 2 |
| Sample | MISS Type 1 | Li | Rb | Hf | Mo | Cu | Pb | ||||||
| ppm | |||||||||||||
| MI-10–28 | Upturned margins | 15.8 | 100.8 | 1.8 | 25.8 | 12.17 | >10,000 | ||||||
| MI-10–37 | Mn nodules | 7.0 | 88.0 | 0.7 | 188.8 | 2423 | >10,000 | ||||||
| MI-10–20 | growth bedding | 6.3 | 108.6 | 1.4 | 13.1 | 787.0 | >10,000 | ||||||
| MI-10–24 | fossil gas dome | 4.2 | 124.1 | 1.1 | 11.0 | 35.8 | 949.8 | ||||||
| MI-10–26 | mat layer structure | 6.1 | 93.0 | 0.4 | 17.7 | 484.6 | >10,000 | ||||||
| MI-10–29 | Mn nodules | 10.7 | 67.7 | 1.2 | 54.7 | 1351 | >10,000 | ||||||
| MI-10–12 | roll-up structure | 9.5 | 81.8 | 0.5 | 25.1 | 94.1 | 4561 | ||||||
| MI-10–15 | roll-up structure | 28.7 | 102.8 | 0.3 | 44.6 | 81.8 | 561.5 | ||||||
| MI-10–22 | mat fragments/chips | 6.0 | 107.3 | 0.6 | 38.3 | 828.7 | >10,000 | ||||||
| MI-10–27 | upturned and curled margins | 10.3 | 81.8 | 1.3 | 12.9 | 1244 | >10,000 | ||||||
| MI-10–26S 2 | sandstone/sandy tuff | 2.8 | 186.2 | 0.7 | 1.3 | 58.5 | 1292 | ||||||
| MI-10–29S 2 | sandstone/sandy tuff | 6.6 | 191.1 | 2.0 | 4.1 | 30.5 | 107.6 | ||||||
| MI-27S2 | sandstone/sandy tuff | 11.3 | 183.8 | 1.3 | 1.0 | 51.0 | 2495 | ||||||
| MI-34S2 | sandstone/sandy tuff | 22.7 | 129.5 | 0.7 | 6.0 | 72.8 | 555.3 | ||||||
| MI-34 | wrinkle structures | 23.4 | 112.2 | 0.7 | 38.2 | 357.5 | 3149 | ||||||
| MI-33 | wrinkle structures | 52.0 | 127.4 | 0.7 | 10.8 | 158.0 | 90.7 | ||||||
| Sample No (MISS Type) 1 | Bulk δ13Corg vs. PDB (‰) | Corg (%) | FAME range | iso/anti iso FAME | Alkanes | Hopanoids | Terpenoid Alcohol/Sterols/Sterane | Lipid Origin |
|---|---|---|---|---|---|---|---|---|
| MI-04–27 (roll-up structure) | iC12:0, iC15:0, aiC15:0 | |||||||
| MI-10–15 (Roll-up structure) | −22.72 | 0.022 | C35-C37 | C29-C35 | Amyrin | Prokaryote/Eukaryote | ||
| MI-10–22 (mat fragments and chips) | −23.57 | 0.015 | C16:0-C24:0 (−32.0 to −28.0 ‰) | C29-C34 | Prokaryote/Eukaryote | |||
| MI-10–24 (fossil gas dome) | −22.91 | 0.015 | C16:0-C18:0 | C29-C35 | Prokaryote | |||
| MI-10–27 (upturned/curled margins) | −21.51 | 0.017 | C16:0-C18:0 | C29-C32 | Cholesterol TMS, Neoergosterol, Campesterol, β-cholesterol TMS | Prokaryote/Eukaryote | ||
| MI-10–27a (upturned/curled margins) | −22.0 | 0.021 | C14:0-C18:0 (−29.9 to −29.0 ‰) | C29-C34 (−47.2 to −51.00 ‰) | Prokaryote | |||
| MI-10–29 (Mn nodules) | −24.9 | 0.014 | C16:0-C18:0 | C29-C35 | Prokaryote | |||
| MI-10–31 (wrinkle structures) | −25.53 | 0.011 | C16:0-C18:0 | iC17:0 | C29-C35 | Prokaryote | ||
| MI-10–33 (wrinkle structures) | −25.0 | 0.015 | C15:0-C19:0 | iC19:0 | C29-C35 | Prokaryote | ||
| MI-10–34 (wrinkle structures) | −25.4 | 0.017 | C16:0-C18:0 (−30.2 to −30.7‰) | C29-C35 (−39.2 to −37.4‰) | 5-α-Cholestane | Prokaryote/Eukaryote | ||
| SMO-05–10 (mat fragments and chips) | −23.43 | 0.018 | C16:0-C18:0 | C29-C31 | Prokaryote | |||
| VA-05–10 (growth bedding) | −27.34 | 0.011 | C16:0-C18:0 (−27.1 to −26.3‰) | iC15:0, aiC19:0 | C29-C31 (−29.0 to −24.3‰) | C29-C31 (−48.2 to −38.6‰) | Prokaryote/Eukaryote | |
| VA-05–16 (Mn nodules) | −25.57 | 0.012 | C16:0-C18:0 | C30, Diploptene | Cholest-5-en3β-ol, 23-methyl cholesta-5,22-dien3β-ol, 24-methyl cholesta-7,22-dien3β-ol, 24-methyl cholesta-en3β-ol, 24-ethyl cholesta-5,22E-dien3β-ol | Prokaryote/Eukaryote | ||
| VA-05–18 (growth bedding) | −27.19 | 0.011 | C16:0-C18:0 | Unknown | ||||
| VA-05–20 (growth bedding) | −27–19 | 0.011 | C12:0-C18:0 (−31.9 to 29.4‰) | Eukaryote | ||||
| SMO-05–09 (growth bedding) | −26.38 | 0.046 | C14:0-C18:0 | iC15:0 | Prokaryote |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kilias, S.P.; Ivarsson, M.; Chi Fru, E.; Rattray, J.E.; Gustafsson, H.; Naden, J.; Detsi, K. Precipitation of Mn Oxides in Quaternary Microbially Induced Sedimentary Structures (MISS), Cape Vani Paleo-Hydrothermal Vent Field, Milos, Greece. Minerals 2020, 10, 536. https://doi.org/10.3390/min10060536
Kilias SP, Ivarsson M, Chi Fru E, Rattray JE, Gustafsson H, Naden J, Detsi K. Precipitation of Mn Oxides in Quaternary Microbially Induced Sedimentary Structures (MISS), Cape Vani Paleo-Hydrothermal Vent Field, Milos, Greece. Minerals. 2020; 10(6):536. https://doi.org/10.3390/min10060536
Chicago/Turabian StyleKilias, Stephanos P., Magnus Ivarsson, Ernest Chi Fru, Jayne E. Rattray, Håkan Gustafsson, Jonathan Naden, and Kleopatra Detsi. 2020. "Precipitation of Mn Oxides in Quaternary Microbially Induced Sedimentary Structures (MISS), Cape Vani Paleo-Hydrothermal Vent Field, Milos, Greece" Minerals 10, no. 6: 536. https://doi.org/10.3390/min10060536
APA StyleKilias, S. P., Ivarsson, M., Chi Fru, E., Rattray, J. E., Gustafsson, H., Naden, J., & Detsi, K. (2020). Precipitation of Mn Oxides in Quaternary Microbially Induced Sedimentary Structures (MISS), Cape Vani Paleo-Hydrothermal Vent Field, Milos, Greece. Minerals, 10(6), 536. https://doi.org/10.3390/min10060536





