Mineralogical and Geochemical Characterization of Talc from Two Mexican Ore Deposits (Oaxaca and Puebla) and Nine Talcs Marketed in Mexico: Evaluation of Its Cosmetic Uses
Abstract
1. Introduction
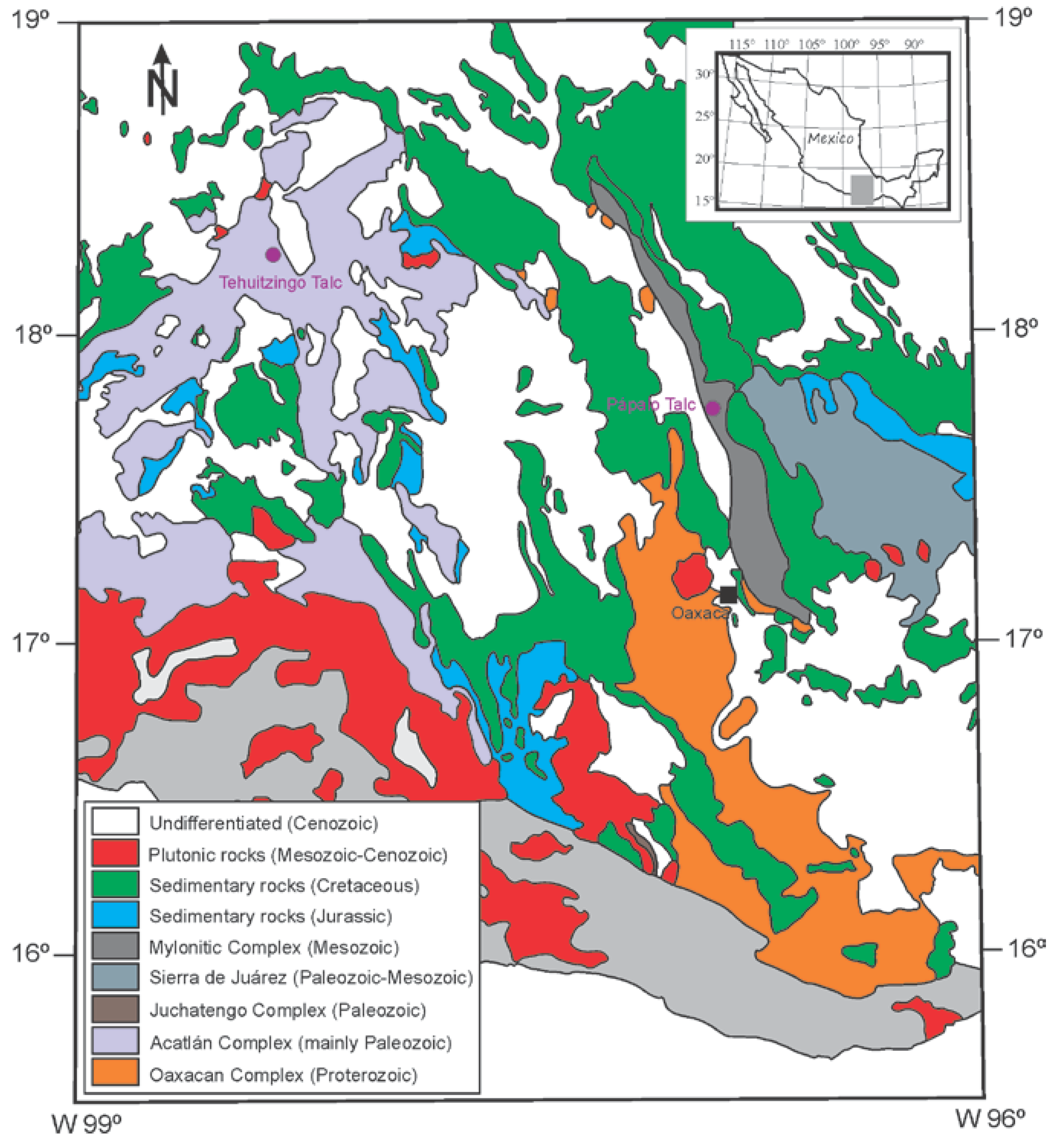
Geology and Genesis of Talc Ore Deposits
2. Materials and Methods
2.1. Selection and Preparation of Samples
2.2. Colorimetry
2.3. X-Ray Diffraction (XRD)
2.4. Short Wave Infrared Spectroscopy (SWIR)
2.5. Scanning Electron Microscopy (SEM)
2.6. Granulometry
2.7. Chemistry of Whole Rock and Trace Elements by Inductively Coupled Plasma
2.8. Thermogravimetric Analysis
3. Results and Discussion
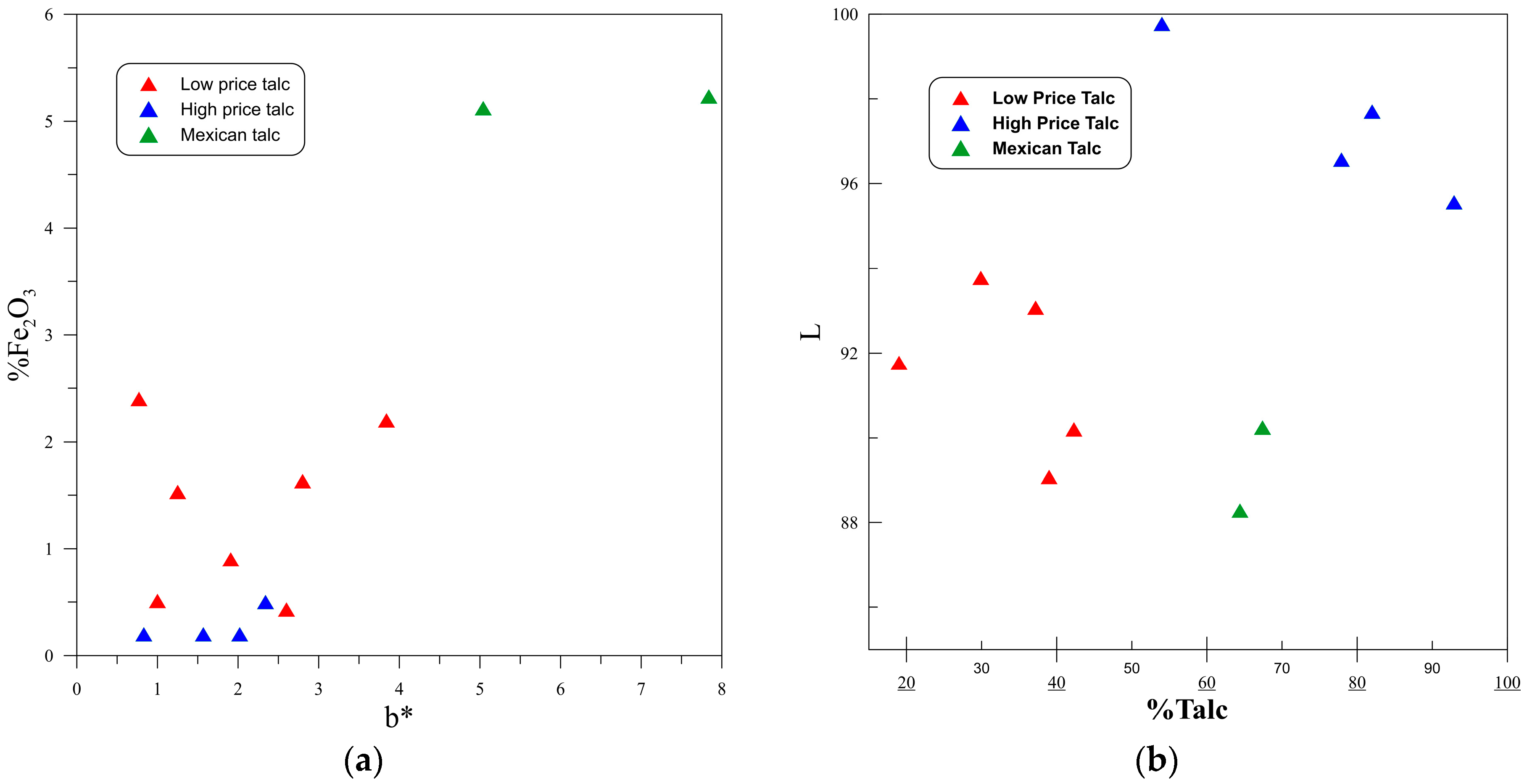
3.1. Color
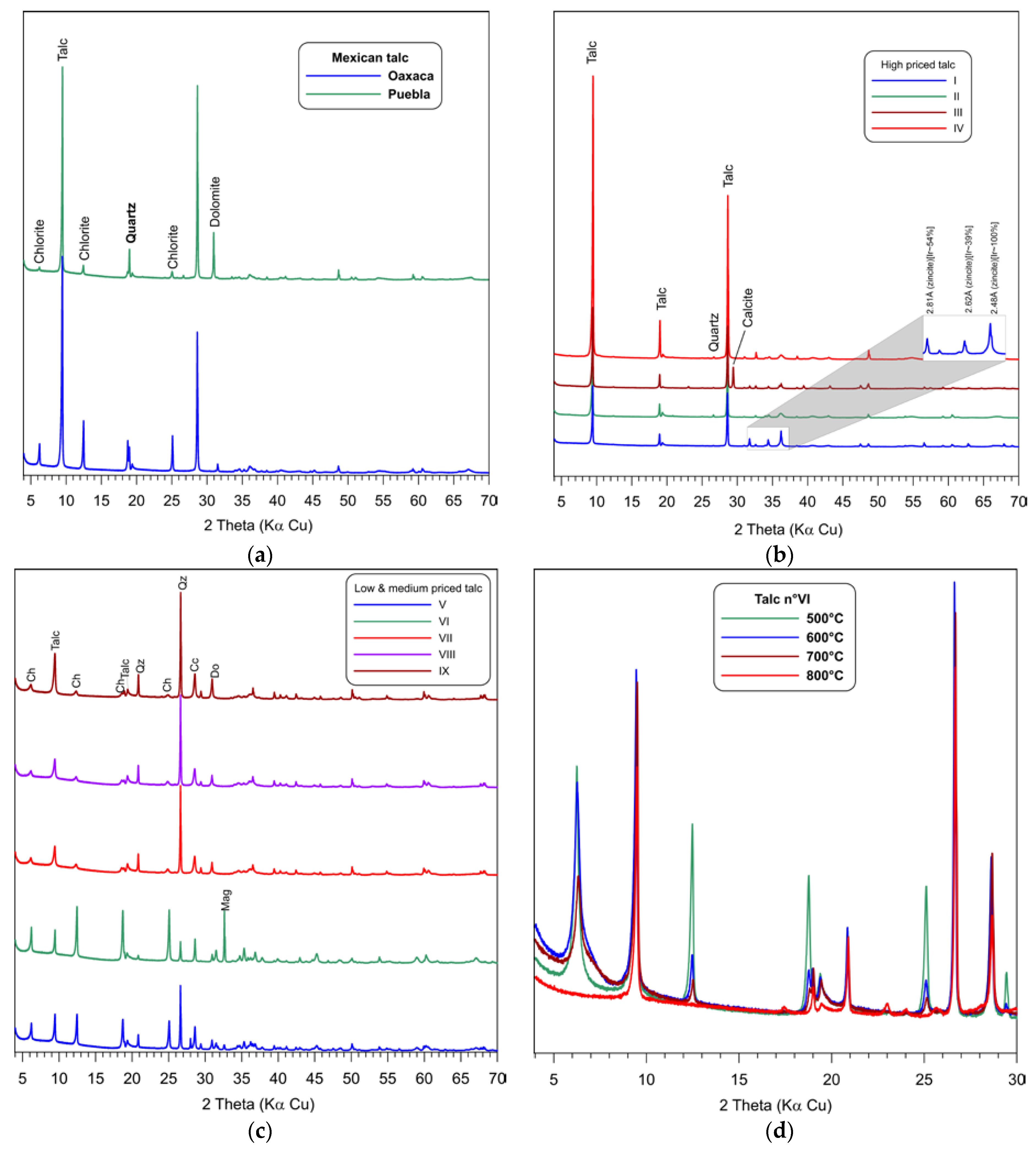
3.2. Mineralogy by X-Ray Diffraction (XRD)
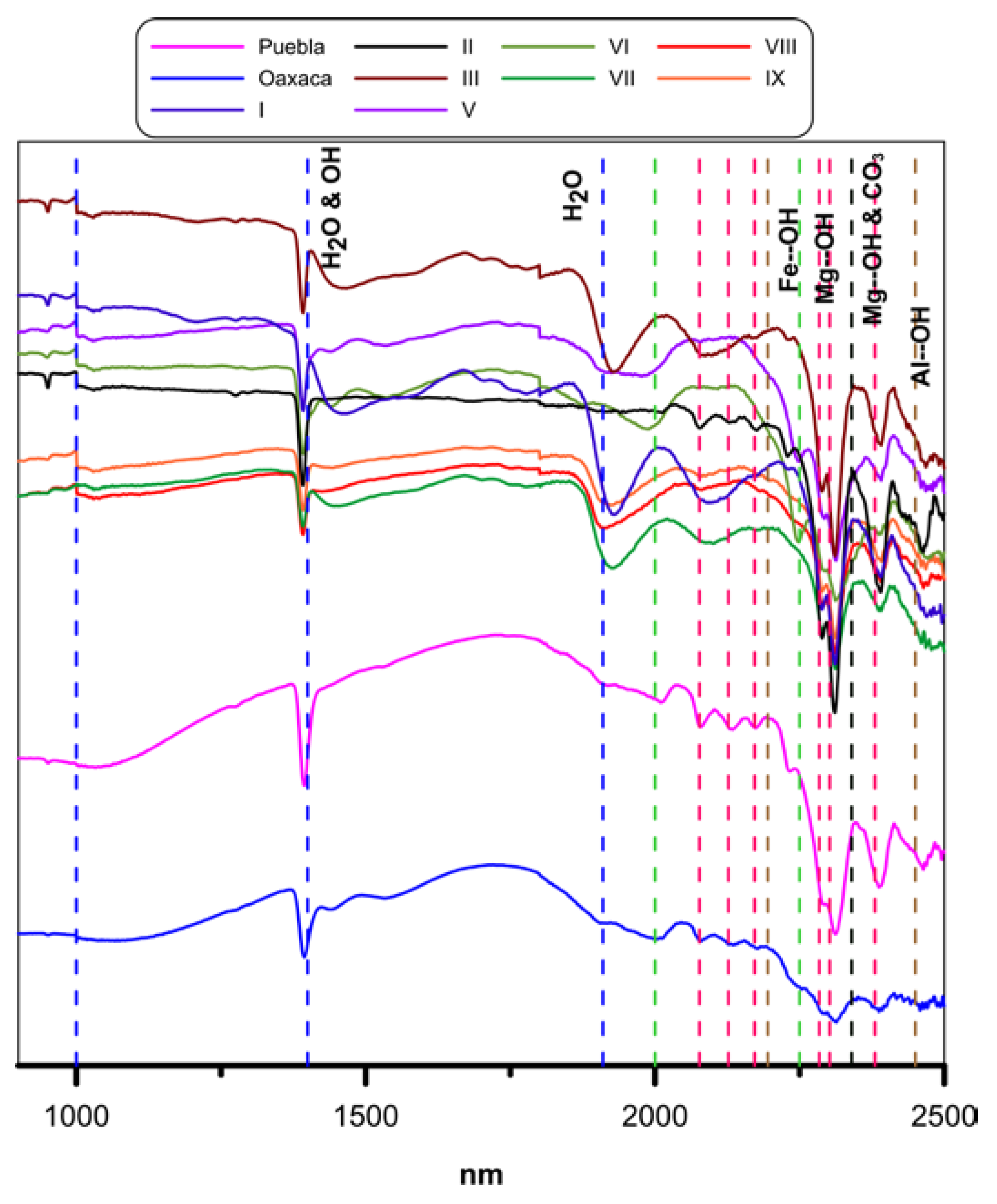
3.3. Mineralogy by Short Wave Infrared Spectroscopy (SWIR)
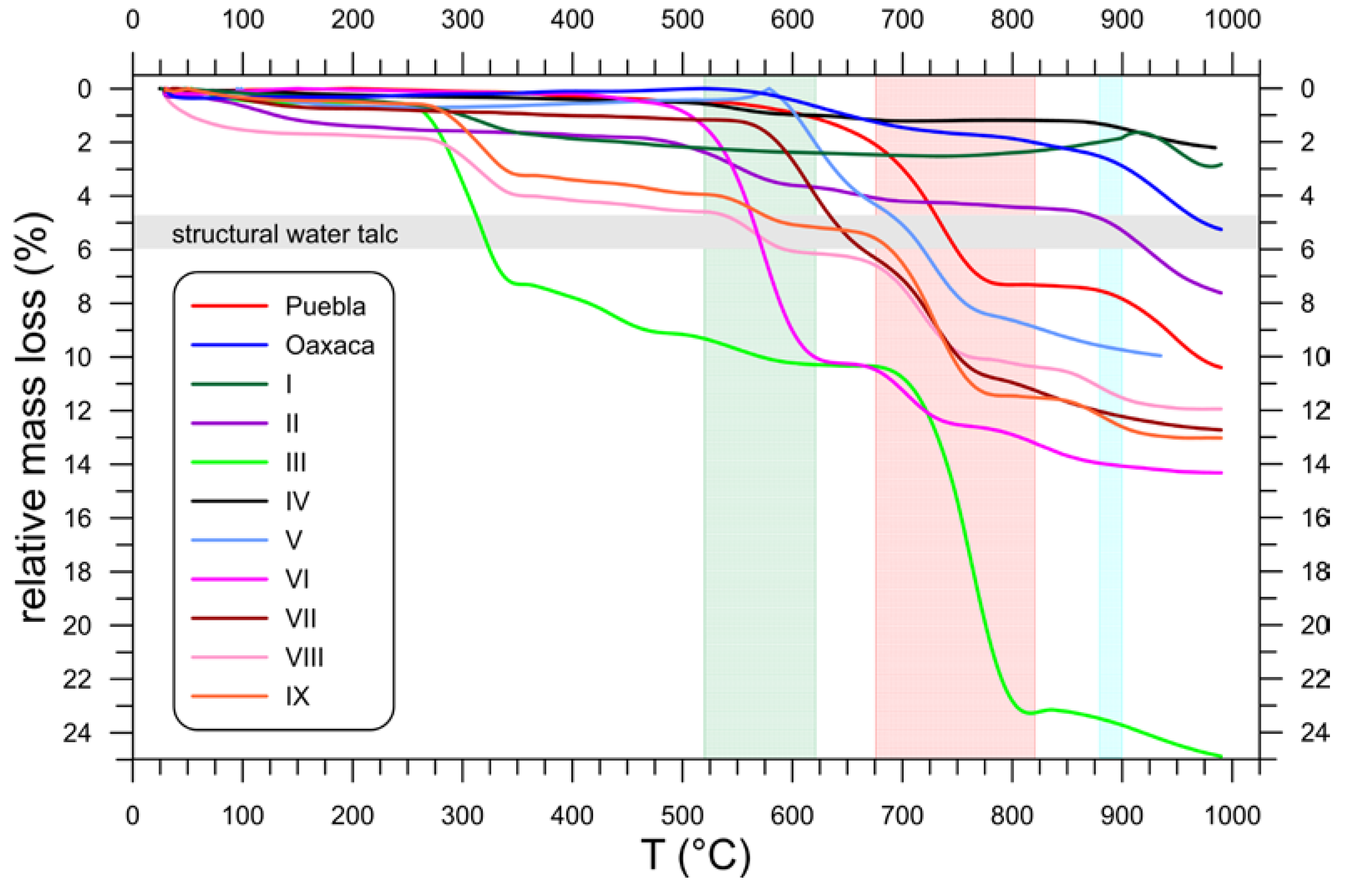
3.4. Thermal Behavior (TG)
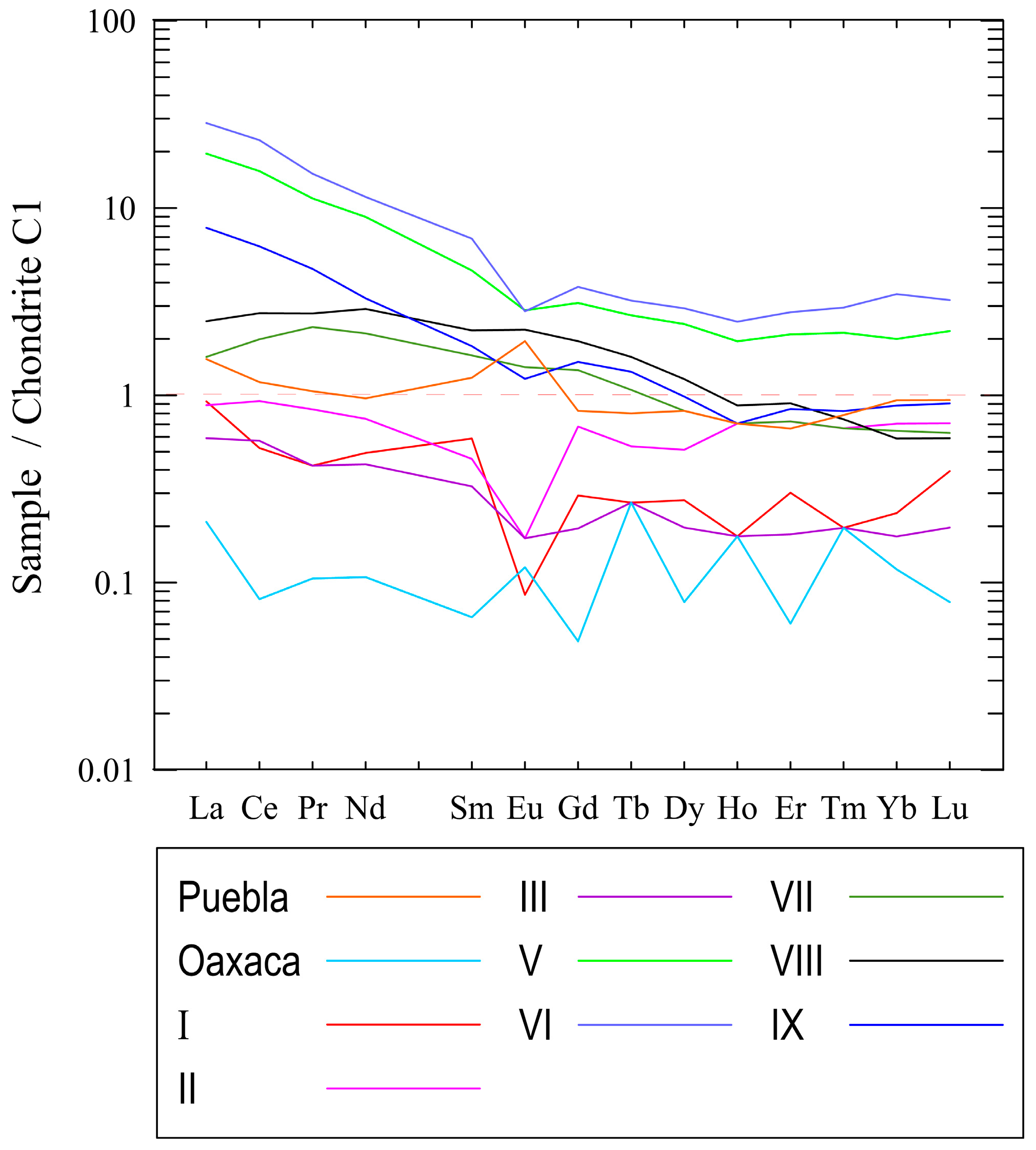
3.5. Geochemistry of Major, Trace and Lanthanide Elements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Galán Huertos, E. Mineralogía Aplicada; Síntesis: Madrid, Spain, 2003; ISBN 84-9756-114-7. [Google Scholar]
- Van Olphen, H. An Introduction to Clay Colloid Chemistry; Wiley: New York, NY, USA, 1977. [Google Scholar]
- Gámiz, E.; Caballero, E.; Delgado Rodríguez, M.; Delgado Calvo-Flores, R. Étude de talcs espagnols à usage pharmaceutique. Composition minéralogique, chimique, propriétés physico-chimiques. Ann. Pharm. Françaises 1989, 47, 53–61. [Google Scholar]
- Michot, L.J.; Villiéras, F.; François, M.; Yvon, J.; LeDred, R.; Cases, J.M. The structural microscopic hydrophobicity of talc. Langmuir 1994, 10, 3765–3773. [Google Scholar] [CrossRef]
- López-Galindo, A.; Viseras, C.; Cerezo, P. Compositional, technical, and safety specifications of clays to be used as pharmaceuticals and cosmetic products. App. Clay Sci. 2007, 36, 51–63. [Google Scholar] [CrossRef]
- Wiewióra, A.; Sánchez-Soto, P.J.; Avilés, M.A.; Justo, A.; Pérez-Maqueda, L.A.; Pérez-Rodríguez, J.L.; Bylina, P. Talc from Puebla de Lillo. Spain. I. XRD study. Appl. Clay Sci. 1997, 12, 233–245. [Google Scholar]
- Nkoumbou, C.; Villiéras, F.; Njopwouo, D.; Ngoune, C.Y.; Barres, O.; Pelletier, M.; Razafitianamaharavo, A.; Yvon, J. Physicochemical properties of talc ore from three deposits of Lamal Pougue area (Yaounde Pan-African Belt, Cameroon), in relation to industrial uses. Appl. Clay Sci. 2008, 41, 113–132. [Google Scholar] [CrossRef]
- Virta, R.L. Talc and pyrophyllite. In Mineral Commodity Summaries 2004; USGS: Reston, VA, USA, 2004. [Google Scholar]
- Pialy, P.; Nkoumbou, C.; Villiéras, F.; Razafitianamaharavo, A.; Barres, O.; Pelletier, M.; Ollivier, G.; Bihannic, I.; Njopwouo, D.; Yvon, J.; et al. Characterization for industrial applications of clays from Lembo deposit, Mount Bana (Cameroon). Clay Min. 2008, 43, 415–435. [Google Scholar] [CrossRef]
- Ersoy, B.; Dikmen, S.; Yildiz, A.; Gören, R.; Elitok, O. Mineralogical and physicochemical properties of talc from Emirdağ, Afyonkarahisar, Turkey, Turk. J. Earth Sci. 2013, 22, 632–644. [Google Scholar] [CrossRef]
- McCarthy, E.F.; Genco, N.A.; Reade, E.H., Jr. Industrial Minerals & Rocks: Commodities, Markets, and Uses; Kogel, J.E., Trivedi, N.C., Barker, J.M., Krukowski, S.T., Eds.; Society for Mining, Metallurgy and Exploration, Inc.: Englewood, CO, USA, 2006. [Google Scholar]
- Hu, F.; Gong, N.; Zhang, L.; Lu, Y.; Zhang, P.; Xiao, X.; Liao, L. Quantitative analysis of trace level asbestos in pharmaceutical talc by powder X-ray diffraction. Anal. Methods 2014, 6, 1862–1867. [Google Scholar] [CrossRef]
- Fiume, M.M.; Boyer, I.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; et al. Safety Assessment of Talc as Used in Cosmetics. Int. J. Toxicol. 2015, 34, 66S–129S. [Google Scholar] [CrossRef]
- McCarthy, E.F.; Genco, N.A.; Reade, E.H., Jr. Industrial Minerals and Rocks; Society for Mining, Metallurgy & Exploration: Englewood, CO, USA, 2006; pp. 971–986. [Google Scholar]
- Delgado-Argote, L.A.; López-Martínez, M.; York, D.; Hall, C.M. Geologic framework and geochronology of ultramafic complexes of southern México. Can. J. Earth Sci. 1992, 29, 1590–1604. [Google Scholar] [CrossRef]
- Ortega-Gutiérrez, F.; Mitre-Salazar, L.M.; Roldán-Quintana, J.; Aranda-Gómez, J.; Morán-Zenteno, D.; Alaniz-Alvarez, S.; Nieto-Samaniego, A. Carta Geológica de la República Mexicana. Escala 1:2,000,000, 5th ed.; Instituto de Geología: Ciudad de Mexico, Mexico, 1992. [Google Scholar]
- Carballido-Sánchez, E.A.; Delgado-Argote, L.A. Geología del cuerpo serpentinítico de Tehuitzingo, Estado de Puebla-interpretación preliminar de su emplazamiento. Rev. Inst. Geol. UNAM 1989, 2, 134–148. [Google Scholar]
- Ortega-Gutiérrez, F. Estratigrafía del Complejo Acatlán en la Mixteca Baja, Estados de Puebla y Oaxaca. Rev. Mex. Cienc. Geol. 1978, 2, 112–131. [Google Scholar]
- González-Mancera, G.; Ortega-Gutiérrez, F.; Proenza, J.A.; Atudorei, V. Petrology and geochemistry of Tehuitzingo Serpentinites (Acatlán complex, SW Mexico). Bol. Soc. Geol. Mex. 2009, 61, 419–435. [Google Scholar] [CrossRef]
- Delgado-Argote, L.A. Regional implications of the Jurassic-Cretaceous volcanosedimentary Cuicateco Terrane, Oaxaca, Mexico. Geof. Internac. 1989, 28, 939–973. [Google Scholar]
- Mexicano, S.G. Anuario Estadístico de la Minería Mexicana 2018; Servicio Geológico Mexicano: Ciudad de México, Mexico, 2018. [Google Scholar]
- Hunter, R.S. New Reflectometer and Its Use for Whiteness Measurement. J. Opt. Soc. Amer. 1960, 50, 44–48. [Google Scholar] [CrossRef]
- Stensby, P.S. Optical Brighteners and Their Evaluation. Soap Chem. Spec. 1967, 97, 41. [Google Scholar]
- Moore, D.M.; Reynolds, R.C., Jr. X-Ray Diffraction and the Identification and Analysis of Clay Minerals, 2nd ed.; Oxford University Press: Oxford, UK; New York, NY, USA, 1997. [Google Scholar]
- Nkoumbou, C.; Njopwouo, D.; Villiéras, F.; Njoya, A.; Yonta Ngouné, C.; Ngo Ndjock, L.; Tchoua, M.F.; Yvon, J. Talc indices from Boumnyebel (Central Cameroon), physicochemistry and geochemistry. J. Afr. Earth Sci. 2006, 45, 61–73. [Google Scholar] [CrossRef]
- Holland, H.J.; Murtagh, M.J. An XRD morphology index for talcs: The effect of particle size and morphology on the specific surface area. Adv. X-Ray Anal. 2000, 42, 421. [Google Scholar]
- Soriano, M.; Melgosa, M.; Sánchez-Marañón, M.; Delgado, G.; Gámiz, E.; Delgado, R. Whiteness of Talcum Powders as a Quality Index for Pharmaceutical Uses. Color Res. Appl. 1998, 23, 178–185. [Google Scholar]
- Krause, J.B. Mineralogical characterization of cosmetic talc products. J. Toxicol. Env. Health 1977, 2, 1223–1226. [Google Scholar] [CrossRef]
- Bishop, J.; Lane, M.; Dyar, M.; Brown, A. Reflectance and emission spectroscopy study of four groups of phyllosilicates: Smectites, kaolinite-serpentines, chlorites and micas. Clay Min. 2008, 43, 35–54. [Google Scholar] [CrossRef]
- Herrmann, W.; Blake, M.; Doyle, M.; Huston, D.; Kamprad, J.; Merry, N.; Pontual, S. Short Wavelength Infrared (SWIR) Spectral Analysis of Hydrothermal Alteration Zones Associated with Base Metal Sulfide Deposits at Rosebery and Western Tharsis, Tasmania, and Highway-Reward, Queensland. Econ. Geol. 2001, 96, 939–955. [Google Scholar] [CrossRef]
- Gámiz, E.; Soriano, M.; Delgado, G.; Párraga, J.; Delgado, R. A morphological study of talcs with scanning electron microscopy (SEM): Pharmaceutical applications. ARS Pharm. 2002, 43, 173–185. [Google Scholar]
- Ewell, R.H.; Bunting, E.N.; Geller, R.F. Thermal decomposition of talc. J. Res. Natl. Bur. Stand. 1935, 15, 551–556. [Google Scholar] [CrossRef]
- Balek, V.; Šubrt, J.; Pérez-Maqueda, L.A.; Beneš, M.; Bountseva, I.M.; Beckman, I.N.; Pérez-Rodríguez, J.L. Thermal behavior of ground talc mineral. J. Min. Metall. 2008, 44B, 7–17. [Google Scholar] [CrossRef]
- Földvári, M. Handbook of thermogravimetric system of minerals and its use in geological practice. Occas. Pap. Geol. Inst. Hung. 2011, 213, 1–180. [Google Scholar]
- Sánchez-Soto, P.J.; Wiewióra, A.; Avilés, M.A.; Justo, A.; Pérez-Maqueda, L.A.; Pérez Rodríguez, J.L.; Bylina, P. Talc from Puebla de Lillo, Spain. II. Effect of dry grinding on particle size and shape. Appl. Clay Sci. 1997, 12, 297–312. [Google Scholar] [CrossRef]
- McDonough, W.F.; Sun, S.S. The Composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]


| Sample | L (%) | a* | b* | Fe2O3 | WI (%) |
|---|---|---|---|---|---|
| Oaxaca | 90.24 | −0.05 | 5.04 | 5.12 | 75.3 |
| Puebla | 88.28 | 0.66 | 7.84 | 5.23 | 62.8 |
| I | 96.57 | −0.07 | 1.57 | 0.20 | 92.1 |
| II | 97.70 | −0.08 | 2.02 | 0.20 | 91.9 |
| III | 99.77 | −0.47 | 2.34 | 0.50 | 94.2 |
| IV | 95.56 | 0.04 | 0.83 | 0.20 | 93.0 |
| V | 93.77 | −0.05 | 1.00 | 0.51 | 90.9 |
| VI | 91.77 | −0.03 | 1.91 | 0.90 | 86.1 |
| VII | 93.07 | −0.14 | 1.25 | 1.53 | 89.7 |
| VIII | 89.07 | 0.20 | 2.80 | 1.63 | 80.1 |
| IX | 90.20 | −0.02 | 2.60 | 0.43 | 82.5 |
| Sample | 1A Talc | 2M Talc | Cl | Q | Cc | Ma | Do | Clay | Zin | GOF * | Type |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Oaxaca | 40.0 | 31.4 | 26.1 | 2.6 | - | - | - | - | - | 1.83 | Green-white |
| Puebla | 20.4 | 47.0 | 8.3 | 5.3 | - | - | 19.0 | - | - | 1.69 | Green-white |
| I | 15.3 | 63.4 | - | 5.5 | - | - | 5.1 | 3.5 | 7.2 | 1.44 | White |
| II | 14.6 | 68.6 | 5.5 | 4.1 | - | 5.9 | - | - | 1.3 | 1.93 | White |
| III | 32.8 | 16.7 | 5.7 | 9.4 | 28.0 | 2.1 | 1.3 | - | 4.0 | 2.28 | White |
| IV | 39.6 | 50.5 | - | 6.9 | - | 0.9 | 1.7 | - | 0.4 | 1.09 | White |
| V | 28.6 | 2.7 | 38.2 | 13.6 | 3.1 | 1.6 | 6.8 | 4.1 | 1.3 | 1.89 | Green |
| VI | 15.6 | 7.6 | 49.7 | 4.4 | 0.2 | 15.2 | 6.5 | - | 0.8 | 2.23 | Green |
| VII | 27.9 | 9.3 | 34.4 | 20.4 | 13.9 | - | 3.7 | - | 0.4 | 1.47 | Green |
| VIII | 28.2 | 10.8 | 29.4 | 23.6 | 1.7 | - | 6.3 | - | - | 1.53 | Green |
| IX | 29.4 | 10.3 | 19.8 | 20.5 | 6.7 | - | 12.7 | - | - | 0.6 | Green |
| Sample | FWHM | I(004) | I(020) | (I004 + 2I020) | Imorphological |
|---|---|---|---|---|---|
| Oaxaca | 0.1568 | 4233 | 1207 | 6647 | 0.6368 |
| Puebla | 0.1146 | 7028 | 1058 | 9144 | 0.7686 |
| I | 0.2886 | 8026 | 1685 | 11396 | 0.7043 |
| II | 0.1448 | 8727 | 2175 | 13077 | 0.6677 |
| III | 0.1886 | 9501 | 565 | 10631 | 0.8937 |
| IV | 0.1734 | 9321 | 669 | 10659 | 0.8744 |
| V | 0.1488 | 2024 | 4889 | 11802 | 0.1715 |
| VI | 0.1536 | 1948 | 1800 | 5548 | 0.3156 |
| VII | 03262 | 626 | 526 | 1678 | 0.3731 |
| VIII | 0.2759 | 610 | 1604 | 3818 | 0.1598 |
| IX | 0.2331 | 813 | 1499 | 3811 | 0.2133 |
| Sample | Sand | Silt | Clay |
|---|---|---|---|
| (62–2000 µm) | (4–62 µm) | (<4 µm) | |
| Oaxaca | 1.4 | 86.7 | 11.9 |
| Puebla | 2.8 | 87.7 | 9.5 |
| I | 3.5 | 86.9 | 9.6 |
| II | 3.0 | 88.2 | 8.7 |
| III | 1.0 | 88.4 | 10.6 |
| IV | 3.8 | 85.0 | 11.2 |
| V | 0.15 | 82.4 | 17.5 |
| VI | 0.17 | 82.2 | 17.7 |
| VII | 0.18 | 78.8 | 21.0 |
| VIII | - | 76.2 | 23.8 |
| IX | 0.72 | 87.2 | 12.1 |
| Sample | SiO2 | Al2O3 | MgO | MnO | Fe2O3 | CaO | NaO | K2O | TiO2 | P2O5 | LOI | TOTAL |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Australia (Seabrook) | 61.9 | 0.28 | 31.4 | - | - | 0.33 | 0.01 | <0.01 | - | - | 6.05 | 100 |
| China (Hiachen) | 63.5 | 0.06 | 31.5 | - | - | 0.25 | 0.01 | <0.01 | - | - | 4.91 | 100.5 |
| Oaxaca | 54.79 | 3.66 | 29.15 | 0.03 | 5.12 | 0.04 | 0.02 | <0.01 | 0.01 | 0.01 | 6.01 | 98.84 |
| Puebla | 49.31 | 0.95 | 26.16 | 0.10 | 5.23 | 4.97 | <0.01 | <0.01 | 0.01 | 0.01 | 11.3 | 98.04 |
| I | 61.66 | 1.87 | 25.02 | <0.01 | 0.31 | 0.44 | 0.06 | <0.01 | <0.01 | 0.08 | 8.93 | 98.37 |
| II | 59.92 | 0.44 | 31.15 | <0.01 | 0.08 | 0.49 | 0.01 | <0.01 | 0.01 | 0.11 | 7.28 | 99.49 |
| III | 44.91 | 0.45 | 19.45 | <0.01 | 0.06 | 15.64 | 0.03 | <0.01 | <0.01 | 0.07 | 16.2 | 96.81 |
| IV | 64.96 | 0.08 | 29.32 | <0.01 | 0.17 | 0.21 | 0.01 | <0.01 | <0.01 | 0.04 | 5.13 | 99.92 |
| V | 46.99 | 8.05 | 25.36 | 0.03 | 0.92 | 3.13 | 0.02 | 0.09 | 0.11 | 0.06 | 13.3 | 98.06 |
| VI | 35.82 | 14.01 | 31.43 | 0.01 | 0.51 | 1.22 | 0.01 | 0.18 | 0.17 | 0.03 | 15.9 | 99.29 |
| VII | 51.35 | 2.48 | 17.03 | 0.05 | 1.33 | 4.08 | 0.04 | 0.01 | 0.01 | 0.08 | 20.7 | 97.16 |
| VIII | 57.72 | 3.16 | 21.72 | 0.05 | 1.62 | 3.3 | 0.03 | 0.01 | <0.01 | 0.09 | 11.9 | 99.60 |
| IX | 48.25 | 4.03 | 20.09 | 0.01 | 0.43 | 1.19 | 0.03 | 0.04 | 0.06 | 0.07 | 24.3 | 98.50 |
| Sample | Cr | Ni | Cu | As | Mo | Co | Sn | Sb | Ta | W | Pb | Bi | Th | U | V | Be | Zr | Ba | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oaxaca | 1360 | 1440 | <10 | <5 | <2 | 40 | <1 | 0.7 | <0.01 | <0.5 | <5 | <0.1 | <0.05 | 0.04 | 37 | <1 | 4 | 12 | - |
| Puebla | 2280 | 1480 | 20 | 121 | <2 | 46 | <1 | 0.8 | 0.14 | <0.5 | 11 | <0.1 | <0.05 | 0.16 | 26 | <1 | 4 | 554 | - |
| I | <20 | <1 | <10 | <5 | <2 | <1 | <1 | 0.2 | <0.01 | <0.5 | <5 | <0.1 | 0.15 | 3.22 | 7 | <1 | 10 | 4 | >10,000 |
| II | <20 | <1 | <10 | <5 | <2 | <1 | <1 | 0.2 | 0.07 | <0.5 | 6 | <0.1 | 0.25 | 5.29 | 10 | <1 | 10 | 3 | 3793 |
| III | <20 | <1 | <10 | <5 | <2 | <1 | <1 | 1.3 | <0.01 | <0.5 | <5 | <0.1 | 0.09 | 1.32 | 7 | <1 | 9 | 10 | >10,000 |
| IV | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| V | <20 | <20 | 10 | <5 | 12 | 1 | <1 | 0.3 | 0.15 | 0.6 | 67 | 0.4 | 1.72 | 1.46 | 28 | <1 | 65 | 630 | 9000 |
| VI | 20 | <20 | <10 | <5 | <2 | 1 | <1 | <0.2 | 0.27 | 1.3 | 5 | <0.1 | 2.7 | 1.12 | 22 | <1 | 105 | 9 | 4480 |
| VII | <20 | <20 | 10 | <5 | 43 | <1 | <1 | 0.5 | <0.01 | 18.5 | 172 | 0.4 | <0.05 | 1.54 | 40 | <1 | 8 | 1183 | >10,000 |
| VII | <20 | <20 | <10 | <5 | 26 | <1 | <1 | 0.3 | <0.01 | <0.5 | 144 | <0.1 | <0.05 | 1.57 | 47 | <1 | 5 | 1790 | 860 |
| VIII | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| IX | <20 | <20 | <10 | <5 | 4 | <1 | <1 | <0.2 | <0.01 | <0.5 | 12 | <0.1 | 0.79 | 0.74 | 15 | <1 | 31 | 187 | 90 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pi-Puig, T.; Animas-Torices, D.Y.; Solé, J. Mineralogical and Geochemical Characterization of Talc from Two Mexican Ore Deposits (Oaxaca and Puebla) and Nine Talcs Marketed in Mexico: Evaluation of Its Cosmetic Uses. Minerals 2020, 10, 388. https://doi.org/10.3390/min10050388
Pi-Puig T, Animas-Torices DY, Solé J. Mineralogical and Geochemical Characterization of Talc from Two Mexican Ore Deposits (Oaxaca and Puebla) and Nine Talcs Marketed in Mexico: Evaluation of Its Cosmetic Uses. Minerals. 2020; 10(5):388. https://doi.org/10.3390/min10050388
Chicago/Turabian StylePi-Puig, Teresa, Dante Yosafat Animas-Torices, and Jesús Solé. 2020. "Mineralogical and Geochemical Characterization of Talc from Two Mexican Ore Deposits (Oaxaca and Puebla) and Nine Talcs Marketed in Mexico: Evaluation of Its Cosmetic Uses" Minerals 10, no. 5: 388. https://doi.org/10.3390/min10050388
APA StylePi-Puig, T., Animas-Torices, D. Y., & Solé, J. (2020). Mineralogical and Geochemical Characterization of Talc from Two Mexican Ore Deposits (Oaxaca and Puebla) and Nine Talcs Marketed in Mexico: Evaluation of Its Cosmetic Uses. Minerals, 10(5), 388. https://doi.org/10.3390/min10050388





