Effect of Mineralogy on the Beneficiation of REE from Heavy Mineral Sands: The Case of Nea Peramos, Kavala, Northern Greece
Abstract
1. Introduction
Geological Setting
2. Samples and Methods
2.1. Sampling
2.2. Sample Characterization
2.2.1. XRD Analysis
2.2.2. SEM/EDS Analysis
2.2.3. Bulk Chemical Analysis
2.3. Beneficiation Tests
2.3.1. Particle Size Analysis
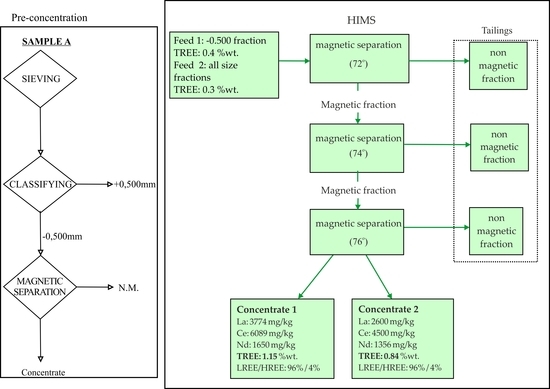
2.3.2. Magnetic Separation
- (i)
- The whole sample passing through −0.500 mm was driven to magnetic separation.
- (ii)
- Each size fraction separately (down to +0.212 mm) was tested for magnetic separation.
2.4. Evaluation of Beneficiation Tests
3. Results
3.1. Mineralogy of the Sands
SEM Analysis of REE Minerals
3.2. REE Geochemistry
3.3. Particle Size Analysis and REE Distribution
3.4. REE Distribution in the Magnetic Fractions
4. Discussion
Effects of Mineral Magnetic Susceptibility on the HIMS Treatment
5. Conclusions
- Allanite-(Ce) is the major host mineral for light REE (LREE), whereas monazite, zircon, and thorite constitute trace amounts. Titanite displays low concentration in LREE <0.1–0.2%. Metamict allanite is common and is thorium-enriched relative to the nonmetamict allanite.
- A simple screening can achieve a satisfactory prebenefication.
- A stepwise magnetic separation improves the recovery of REE.
- Magnetic separation for each particle size fraction separately improves the recovery of REE and reaches recoveries of 75–90% in just the 20% of feed material.
- The grades of magnetic concentrate for the two processes (all particle size fractions and undersize 0.500 mm) were 0.84% and 1.15% TREE, respectively, at the recoveries of 87–92% and 79–96%.
- The increase in REE content is associated with the increase of thorium content in concentrates regarding placer sands from Nea Peramos, Greece.
- The use of gravimetric methods (such as Wilfley shaking table) did not contribute much to the beneficiation of the ores.
- Radioactive wastes from a potential REE processing operation of Nea Peramos HM sands should be carefully encountered for the risk to the environment and humans.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mudd, G.M.; Jowitt, S.M. Rare earth elements from heavy mineral sands: Assessing the potential of a forgotten resource. Appl. Earth Sci. (Trans. Inst. Min. Metall. B) 2016, 125, 107–113. [Google Scholar] [CrossRef]
- EU Commission. Study on the Review of the List of Critical Raw Materials; European Commission: Brussels, Belgium, 2017. [Google Scholar]
- McLennan, B.; Gorder, G.D.; Ali, S.H. Sustainability of rare earths—An overview of the state of knowledge. Minerals 2013, 3, 304–317. [Google Scholar] [CrossRef]
- Balomenos, E.; Davris, P.; Deady, E.; Yang, J.; Panias, D.; Friedrich, B.; Binnemans, K.; Seisenbaeva, G.; Dittrich, C.; Kalvig, P.; et al. The EURARE Project: Development of a sustainable exploitation scheme for Europe’s Rare Earth Ore deposits. Johns. Matthey Technol. Rev. 2017, 61, 142–153. [Google Scholar] [CrossRef]
- Moss, R.; Tzimas, E.; Willis, P.; Arendorf, J.; Thompson, P.; Chapman, A.; Morley, N.; Sims, E.; Bryson, R.; Peason, J.; et al. Critical metals in the path towards the decarbonization of the EU energy sector. In Assessing Rare Metals as Supply-Chain Bottlenecks in Low-Carbon Energy Technologies; JRC Report EUR 25994 EN; Publications Office of the European Union: Brussels, Belgium, 2013; p. 242. [Google Scholar]
- Goodenough, K.; Schilling, J.; Jonsson, E.; Kalvig, P.; Charles, N.; Tuduri, J.; Deady, E.A.; Sadeghi, M.; Schiellerup, H.; Muller, A.; et al. Europe’s rare earth element resource potential: An overview of REE metallogenetic provinces and their geodynamic setting. Ore Geol. Rev. 2016, 72 Pt 1, 838–856. [Google Scholar] [CrossRef]
- Guyonnet, D.; Planchon, M.; Rollat, A.; Escalon, V.; Vaxelaire, S.; Tuduri, J. Primary and secondary sources of rare earths in the EU-28: Results of the ASTER project. In Proceedings of the ERES 2014—1st Conference on European Rare Earth Resources, Milos, Greece, 4–7 September 2014; pp. 66–72. [Google Scholar]
- Deady, E.; Lacinska, A.; Goodenough, K.M.; Shaw, R.A.; Roberts, N.M.W. Volcanic-Derived Placers as a Potential Resource of Rare Earth Elements: The Aksu Diamas Case Study, Turkey. Minerals 2019, 9, 208. [Google Scholar] [CrossRef]
- Deady, É.; Mouchos, E.; Goodenough, K.; Williamson, B.; Wall, F. A review of the potential for rare-earth element resources from European red muds: Examples from Seydişehir, Turkey and Parnassus-Giona, Greece. Mineral. Mag. 2016, 80, 43–61. [Google Scholar] [CrossRef]
- Pergamalis, F.; Karageorgiou, D.E.; Koukoulis, A. The location of Tl, REE, Th, U, Au deposits in the seafront zones of Nea Peramos-Loutra Eleftheron area, Kavala (N. Greece) using γ radiation. Bull. Geol. Soc. Greece 2001, 34, 1023–1029. [Google Scholar]
- Pergamalis, F.; Karageorgiou, D.E.; Koukoulis, A.; Katsikis, I. Mineralogical and chemical composition of sand ore deposits in the seashore zone N. Peramos-L. Eleftheron (N. Greece). Bull. Geol. Soc. Greece 2001, 34, 845–850. [Google Scholar] [CrossRef]
- Institute of Geological and Mineral Exploration (IGME). Geological Map of Greece, Nikisiani-Loutra Eleftheron Sheet, 1:50000; Kronberg, P., Schenk, P.F., Eds.; IGME: Madrid, Spain, 1974. [Google Scholar]
- Institute of Geological and Mineral Exploration (IGME). Geological Map of Greece, Kavala Sheet, 1:50000; Kronberg, P., Ed.; IGME: Madrid, Spain, 1974. [Google Scholar]
- Eliopoulos, D.; Economou, G.; Tzifas, I.; Papatrechas, C. The potential of Rare Earth elements in Greece. In Proceedings of the ERES2014: First European Rare Earth Resources Conference, Milos, Greece, 4–7 September 2014; pp. 308–316. [Google Scholar]
- Papadopoulos, A.; Koroneos, A.; Christofides, G.; Stoulos, S. Natural radioactivity distribution and gamma radiation exposure of beach sands close to Kavala pluton, Greece. Open Geosci. 2015, 7, 64. [Google Scholar] [CrossRef]
- Papadopoulos, A.; Koroneos, A.; Christofides, G.; Papadopoulou, L. Geochemistry of beach sands from Kavala, Northern Greece. Ital. J. Geosci. 2016, 135, 526–539. [Google Scholar] [CrossRef]
- Angelatou, V.; Papamanoli, S.; Stouraiti, C.; Papavasiliou, K. REE distribution in the Black Sands in the Area of Loutra Eleftheron, Kavala, Northern Greece: Mineralogical and Geochemical Characterization of Fractions from Grain Size and Magnetic Separation Analysis (doi:10.3390/IECMS2018-05455). Available online: https://sciforum.net/paper/view/conference/5455 (accessed on 25 April 2020).
- Tzifas, I.; Papadopoulos, A.; Misaelides, P.; Godelitsas, A.; Göttlicher, J.; Tsikos, H.; Gamaletsos, P.N.; Luvizotto, G.; Karydas, A.G.; Petrelli, M.; et al. New insights into mineralogy and geochemistry of allanite-bearing Mediterranean coastal sands from Northern Greece. Geochemistry 2019, 79, 247–267. [Google Scholar] [CrossRef]
- Papadopoulos, A.; Christofides, G.; Koroneos, A.; Stoulos, S. Natural radioactivity distribution and gamma radiation exposure of beach sands from Sithonia Peninsula. Cent. Eur. J. Geosci. 2014, 6, 229–242. [Google Scholar] [CrossRef]
- Papadopoulos, A.; Christofides, G.; Koroneos, A.; Hauzenberger, C. U Th and REE content of heavy minerals from beach sand samples of Sithonia Peninsula (northern Greece). J. Mineral. Geochem. 2015, 192, 107–116. [Google Scholar] [CrossRef]
- Filippidis, A.; Misaelides, P.; Clouvas, A.; Godelitsas, A.; Barbayiannis, N.; Anousis, I. Mineral, chemical and radiological investigation of a black sand at Touzla Cape, near Thessaloniki, Greece. Environ. Geochem. Health 1997, 19, 83–88. [Google Scholar] [CrossRef]
- Papadopoulos, A.; Koroneos, A.; Christofides, G.; Stoulos, S. Natural Radioactivity Distribution and Gamma Radiation exposure of Beach sands close to Maronia and Samothraki Plutons, NE Greece. Geol. Balc. 2015, 43, 1–3. [Google Scholar]
- Papadopoulos, A.; Tzifas, I.; Tsikos, H. The Potential for REE and Associated Critical Metals in Coastal Sand (Placer) Deposits of Greece: A Review. Minerals 2019, 9, 469. [Google Scholar] [CrossRef]
- Eliopoulos, D.; Aggelatou, V.; Oikonomou, G.; Tzifas, I. REE in black sands: The case of Nea Peramos and Strymonikos gulf. In Proceedings of the ERES, Santorini, Greece, 28–31 May 2017; pp. 49–50. [Google Scholar]
- Sengupta, D.; Van Gosen, B.S. Placer-type rare earth element deposits. Rev. Econ. Geol. 2016, 18, 81–100. [Google Scholar]
- Jordens, A.; Cheng, Y.P.; Waters, K.E. A review of the beneficiation of rare earth element bearing minerals. Miner. Eng. 2013, 41, 97–114. [Google Scholar] [CrossRef]
- Jordens, A.; Sheridan, R.S.; Rowson, N.A.; Waters, K.E. Processing a rare earth mineral deposit using gravity and magnetic separation. Miner. Eng. 2014, 62, 9–18. [Google Scholar] [CrossRef]
- Jordens, A.; Marion, C.; Langlois, R.; Grammatikopoulos, T.; Sheridan, R.; Teng, C.; Demers, H.; Gauvin, R.; Rowson, N.; Waters, N. Beneficiation of the Nechalacho rare-earth deposit. Part 2: Characterization of products from gravity and magnetic separation. Miner. Eng. 2016. [Google Scholar] [CrossRef]
- Yang, X.; Makkonen, H.T.; Pakkanen, L. Rare Earth Occurrences in Streams of Processing a Phosphate Ore. Minerals 2019, 9, 262. [Google Scholar] [CrossRef]
- Caster, S.B.; Hendrick, J.B. Rare Earth Elements. In Industrial Minerals and Rocks: Commodities, Markets, and Uses, 7th ed.; Kogel, J.E., Trivedi, N.C., Barker, J.M., Krudowski, S.T., Eds.; SME: Dearborn, MI, USA, 2006; p. 1568. [Google Scholar]
- Burg, J.-P.; Ricou, L.-E.; Ivanov, Z.; Godfriaux, I.; Dimov, D.; Klain, L. Syn-metamorphic nappe complex in the Rhodope Massif. Structure and kinematics. Terra Nova 1996, 8, 6–15. [Google Scholar] [CrossRef]
- Ricou, L.-E.; Burg, J.-P.; Godfriaux, I.; Ivanov, Z. Rhodope and Vardar: The metamorphic and the olistostromic paired belts related to the Cretaceous subduction under Europe. Geodin. Acta 1998, 11, 285–309. [Google Scholar] [CrossRef]
- Mposkos, E.; Kostopoulos, D. Diamond, former coesite and supersilicic garnet in metasedimentary rocks from the Greek Rhodope: A new ultrahigh-pressure metamorphic province established. Earth Planet. Sci. Lett. 2001, 192, 497–506. [Google Scholar] [CrossRef]
- Perraki, M.; Proyer, A.; Mposkos, E.; Kaindl, R.; Hoinkes, G. Raman micro-spectroscopy on diamond, graphite and other carbon polymorphs from the ultrahigh-pressure metamorphic Kimi Complex of the Rhodope Metamorphic Province, NE Greece. Earth Planet. Sci. Lett. 2006, 241, 672–685. [Google Scholar] [CrossRef]
- Brun, J.P.; Sokoutis, D. Kinematics of the Southern Rhodope Core Complex (North Greece). Int. J. Earth Sci. 2007, 96, 1079–1099. [Google Scholar] [CrossRef]
- Liati, A.; Gebauer, D.; Fanning, C.M. Geochronology of the Alpine UHP Rhodope Zone: A review of isotopic ages and constraints on the geodynamic evolution. In Ultrahigh–Pressure Metamorphism: 25 Years after the Discovery of Coesite and Diamond; Dobrzhinetskaya, L., Faryad, S.W., Wallis, S., Cuthbert, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 295–324. [Google Scholar]
- Burg, J.P. Rhodope: From Mesozoic convergence to Cenozoic extension. Review of petro-structural data in the geochronological frame. J. Virtual Explor. 2012, 42, 1–44. [Google Scholar] [CrossRef]
- Tranos, M.D. Slip preference analysis of faulting driven by strike-slip Andersonian stress regimes: An alternative explanation of the Rhodope metamorphic core complex (northern Greece). J. Geol. Soc. 2017, 174, 129–141. [Google Scholar] [CrossRef]
- Dinter, D.A.; Royden, L. Late Cenozoic extension in northeastern Greece: Strymon Valley detachment system and Rhodope metamorphic core complex. Geology 1993, 21, 45–48. [Google Scholar] [CrossRef]
- Papanikolaou, D.; Panagopoulos, A. On the structural style of southern Rhodope, Greece. Geol. Balc. 1981, 11, 13–22. [Google Scholar]
- Dinter, D.A.; MacFarlane, A.; Hames, W.; Isachsen, C.; Bowring, S.; Royden, L. U-Pb and 40Ar/39Ar geochronology of the Symvolon granodiorite: Implications for the thermal and structural evolution of the Rhodope metamorphic core complex, northeastern Greece. Tectonics 1995, 14, 886–908. [Google Scholar] [CrossRef]
- Pe-Piper, G.; Piper, D.J.; Lentz, D.R. The Igneous Rocks of Greece: The Anatomy of an Orogeny; Gebruder Borntraeger: Berlin, Germany, 2002; 573p. [Google Scholar]
- Hubbard, C.; Snyder, R. RIR—Measurement and Use in Quantitative XRD. Powder Diffr. 1988, 3, 74–77. [Google Scholar] [CrossRef]
- Anthony, J.W.; Bideaux, R.A.; Bladh, K.W.; Nichols, M.C. Handbook of mineralogy. In Mineralogical Society of America; Mineral Data Publishing: Chantilly, VA, USA, 2001. [Google Scholar]
- Rosenblum, S.; Brownfield, I.K. Magnetic Susceptibilities of Minerals—Report for US Geological Survey; U.S. Geological Survey: Reston, VA, USA, 1999; pp. 1–33.
- Gupta, C.K.; Krishnamurthy, N. Extractive Metallurgy of Rare Earths; CRC Press: Boca Raton, FL, USA, 2005; p. 484. [Google Scholar]
- Rudnick, R.L.; Gao, S. Composition of the continental crust. Crust 2003, 3, 1–64. [Google Scholar]
- McDonough, W.F.; Sun, S.S. The Composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Reisman, D.; Weber, R.; McKernan, J.; Northeim, C. Rare Earth Elements: A Review of Production, Processing, Recycling, and Associated Environmental Issues; EPA Report EPA/600/R-12/572; U.S. Environmental Protection Agency (EPA): Washington, DC, USA, 2013. Available online: https://nepis.epa.gov/Adobe/PDF/P100EUBC.pdf (accessed on 30 August 2019).
- Weng, Z.H.; Jowitt, S.M.; Mudd, G.M.; Haque, N. Assessing rare earth element mineral deposit types and links to environmental impacts. Appl. Earth Sci. 2013, 122, 83–96. [Google Scholar] [CrossRef]
- Dutta, T.; Kim, K.-H.; Uchimiya, M.; Kwonc, E.E.; Jeon, B.-H.; Deep, A.; Yun, S.-T. Global demand for rare-earth resources and strategies for green mining. Environ. Res. 2016, 150, 182–190. [Google Scholar] [CrossRef]
- Wall, F.; Rollat, A.; Pell, R.S. Responsible sourcing of critical metals. Elements 2017, 13, 313–318. [Google Scholar] [CrossRef]
- Grammatikopoulos, T.; Mercer, W.; Gunning, C. Mineralogical characterisation using QEMSCAN of the Nechalacho heavy rare earth metal deposit, Northwest Territories, Canada. Can. Metall. Q. 2013, 52, 265–277. [Google Scholar] [CrossRef]
- British Geological Survey. Rare Earth elements profile. In Mineral Profile Series; BGS NERC: Keyworth, UK, 2011; p. 53. [Google Scholar]
- Yang, X.; Satur, J.V.; Sanematsu, K.; Laukkanen, J.; Saastamoinen, T. Beneficiation studies of a complex REE ore. Miner. Eng. 2015, 71, 55–64. [Google Scholar] [CrossRef]
- Jordens, A.; Marion, C.; Kuzmina, O.; Waters, K.E. Physicochemical aspects of allanite flotation. J. Rare Earths 2014, 32, 476–486. [Google Scholar] [CrossRef]
- Angelatou, V.; Drossos, E. Beneficiation of green black sands for REE recovery. In Proceedings of the ERES, Santorini, Greece, 28 June–1 July 2017; pp. 49–50. [Google Scholar]








| REE-Mineral | Chemical Formula | Density (g/cm3) | Magnetic Properties | Weight % REO | ThO2 | UO2 |
|---|---|---|---|---|---|---|
| Silicates | ||||||
| Allanite (Ce) | (Ce,Ca,Y)2(Al,Fe2+,Fe3+)3(SiO4)3(OH) | 3.50–4.20 | paramagnetic | 3–51 | 0–3 | - |
| Allanite (Y) | (Y,Ce,Ca)2(Al,Fe3+)3(SiO4)3(OH) | n/a | paramagnetic | 3–51 | 0–3 | - |
| Cheralite (Ce) | (Ca,Ce,Th)(P,Si)O4 | 5.28 | n/a | - | <30 | - |
| Sphene (titanite) | (Ca,REE)TiSiO5 | 3.48–3.60 | paramagnetic | <3 | - | - |
| Thorite | (Th,U)SiO4 | 6.63–7.20 | paramagnetic | <3 | 70–80 | 10–16 |
| Zircon | (Zr,REE)SiO4 | 4.60–4.70 | diamagnetic | - | 0.1–0.8 | - |
| Phosphates | ||||||
| Apatite | Ca5(PO4)3(F,Cl,OH) | 3.17 | n/a | ~19 | - | - |
| Fluorapatite | (Ca,Ce)5(PO4) | 3.10–3.25 | n/a | - | - | - |
| Monazite (Ce) | (Ce,La,Nd,Th)PO4 | 4.98–5.43 | paramagnetic | 35–71 | 0–20 | 0–16 |
| Monazite (La) | (La,Ce,Nd,Th)PO4 | 5.17–5.27 | paramagnetic | 35–71 | 0–20 | 0–16 |
| Monazite (Nd) | (Nd,Ce,La,Th)PO4 | 5.43 | paramagnetic | 35–71 | 0–20 | 0–16 |
| Rhabdophane (Ce) | (Ce,La)PO4.H2O | 3.77–4.01 | n/a | - | - | - |
| Xenotime (Y) | YPO4 | 4.40–5.10 | paramagnetic | 52–67 | - | 0–5 |
| Carbonates | ||||||
| Bastnäsite (Ce) | (Ce,La)(CO3)F | 4.9–5.2 | paramagnetic | 70–74 | 0–0.3 | 0.09 |
| Bastnäsite (La) | (La,Ce)(CO3)F | n/a | paramagnetic | 70–74 | 0–0.3 | 0.09 |
| Bastnäsite (Y) | Y(CO3)F | 3.90–4.00 | paramagnetic | 70–74 | 0–0.3 | 0.09 |
| Synchysite (Nd) | Ca(Nd,La)(CO3)2F | 4.11 (calc) | n/a | - | - | - |
| Mineral | Weight % | Nominal Specific Gravity | Magnetic Property |
|---|---|---|---|
| Albite | 39 | 2.68 | Diamagnetic |
| Quartz | 31 | 2.63 | Diamagnetic |
| K-feldspar | 11 | 2.57 | Diamagnetic |
| Titanite | 8 | 3.4–3.6 | Paramagnetic |
| Mg-hornblende | 6 | 3.24 | Paramagnetic |
| Allanite | 3 | 3.75 | Paramagnetic |
| Hematite | 2 | 5.30 | Ferromagnetic |
| Magnetite | 1 | 5.20 | Ferromagnetic |
| Trace minerals | <0.5 | - | - |
| Total | 100 |
| Sample | NP 115 | NP 116 | NP 117 | NP 117A | NP 118 | NP 119 | NP 120 | NP 121 | NP 122 | NP 123 | NP 124 | NP 125 | NP 126 | NP 127 | NP 128 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LREE | 600 | 826 | 1407 | 315 | 707 | 3504 | 118 | 2024 | 2225 | 7645 | 6646 | 4552 | 98 | 2297 | 787 |
| HREE | 47 | 61 | 98 | 27 | 51 | 204 | 18 | 147 | 160 | 465 | 409 | 295 | 15 | 76 | 47 |
| TREE | 654 | 894 | 1509 | 344 | 762 | 3716 | 143 | 2181 | 2393 | 8124 | 7068 | 4857 | 116 | 2381 | 842 |
| Particle Size (mm) | Mass | Weight | La | Ce | Nd | Th | La | Ce | Nd | Th |
|---|---|---|---|---|---|---|---|---|---|---|
| (g) | (%) | Concentration (mg/kg) | Mass (mg) | |||||||
| +1.70 | 55.6 | 2.42 | 16 | 25 | 10 | 7 | 0.89 | 1.39 | 0.56 | 0.39 |
| −1.70 + 0.850 | 230.7 | 10.02 | 54 | 106 | 38 | 25 | 12.46 | 24.45 | 8.77 | 5.77 |
| −0.850 + 0.500 | 640.48 | 27.82 | 416 | 777 | 247 | 190 | 266.44 | 497.65 | 158.20 | 121.69 |
| −0.500 + 0.425 | 324.62 | 14.10 | 1044 | 1983 | 607 | 455 | 338.90 | 643.72 | 197.04 | 147.70 |
| −0.425 + 0.355 | 307.32 | 13.35 | 1445 | 2719 | 821 | 641 | 444.08 | 835.60 | 252.31 | 196.99 |
| −0.355 + 0.300 | 286.26 | 12.43 | 1598 | 3018 | 891 | 723 | 457.44 | 863.93 | 255.06 | 206.97 |
| −0.300 + 0.212 | 353.02 | 15.33 | 852 | 1643 | 487 | 390 | 300.77 | 580.01 | 171.92 | 137.68 |
| −0.212 + 0.150 | 69.12 | 3.00 | 507 | 968 | 305 | 240 | 35.04 | 66.91 | 21.08 | 16.59 |
| −0.150 | 35.05 | 1.52 | 2031 | 3840 | 1162 | 945 | 71.19 | 134.59 | 40.73 | 33.12 |
| Total | 2302.17 | 1927.21 | 3648.27 | 1105.66 | 866.90 | |||||
| Feed: Sample A (All Fractions) | La | Ce | Nd | Th | La | Ce | Nd | Th | |
| Mass (g) | Concentration (mg/kg) | Mass (g) | |||||||
| 1995 | 942 | 1647 | 512 | 365 | 1.87 | 3.29 | 1.021 | 0.728 | |
| Particle size | magnetic fractions (mg/kg) | Mass (g) | |||||||
| −1.70 + 0.850 | 42.55 | 261 | 447 | 137 | 93 | 0.0111 | 0.0190 | 0.0058 | 0.0040 |
| −0.850 + 0.500 | 162.23 | 1301 | 2309 | 717 | 392 | 0.2111 | 0.3746 | 0.1163 | 0.0636 |
| −0.500 + 0.425 | 101.65 | 2764 | 4802 | 1444 | 1099 | 0.2810 | 0.4881 | 0.1468 | 0.1117 |
| −0.425 + 0.355 | 109.81 | 3627 | 6321 | 1865 | 1472 | 0.3983 | 0.6941 | 0.2048 | 0.1616 |
| −0.355 + 0.300 | 100.6 | 3838 | 6649 | 1968 | 1595 | 0.3861 | 0.6689 | 0.1980 | 0.1605 |
| −0.300 + 0.212 | 142.83 | 3044 | 5280 | 1563 | 1302 | 0.4348 | 0.7541 | 0.2232 | 0.1860 |
| Total | 659.67 | 1.7223 | 2.9989 | 0.8950 | 0.6873 | ||||
| Recovery (wt.%) | 92.1 | 91.1 | 87.6 | 94.4 | |||||
| Feed: −0.500 mm | La | Ce | Nd | Th | La | Ce | Nd | Th | |
| Mass (g) | Concentration (mg/kg) | Mass (g) | |||||||
| 530 | 1260 | 2223 | 670 | 460 | 1.9278 | 3.4012 | 1.0251 | 0.7038 | |
| Magnetic fraction | 490 | 3774 | 6089 | 1650 | 1300 | 1.8493 | 2.9836 | 0.8085 | |
| Recovery (wt.%) | 95.9 | 89.7 | 78.87 | 90.5 | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stouraiti, C.; Angelatou, V.; Petushok, S.; Soukis, K.; Eliopoulos, D. Effect of Mineralogy on the Beneficiation of REE from Heavy Mineral Sands: The Case of Nea Peramos, Kavala, Northern Greece. Minerals 2020, 10, 387. https://doi.org/10.3390/min10050387
Stouraiti C, Angelatou V, Petushok S, Soukis K, Eliopoulos D. Effect of Mineralogy on the Beneficiation of REE from Heavy Mineral Sands: The Case of Nea Peramos, Kavala, Northern Greece. Minerals. 2020; 10(5):387. https://doi.org/10.3390/min10050387
Chicago/Turabian StyleStouraiti, Christina, Vassiliki Angelatou, Sofia Petushok, Konstantinos Soukis, and Demetrios Eliopoulos. 2020. "Effect of Mineralogy on the Beneficiation of REE from Heavy Mineral Sands: The Case of Nea Peramos, Kavala, Northern Greece" Minerals 10, no. 5: 387. https://doi.org/10.3390/min10050387
APA StyleStouraiti, C., Angelatou, V., Petushok, S., Soukis, K., & Eliopoulos, D. (2020). Effect of Mineralogy on the Beneficiation of REE from Heavy Mineral Sands: The Case of Nea Peramos, Kavala, Northern Greece. Minerals, 10(5), 387. https://doi.org/10.3390/min10050387