Invisible Gold Paragenesis and Geochemistry in Pyrite from Orogenic and Sediment-Hosted Gold Deposits
Abstract
1. Introduction
2. Analytical Methods
3. Siting of Invisible Gold in Pyritic Ores and Its Significance
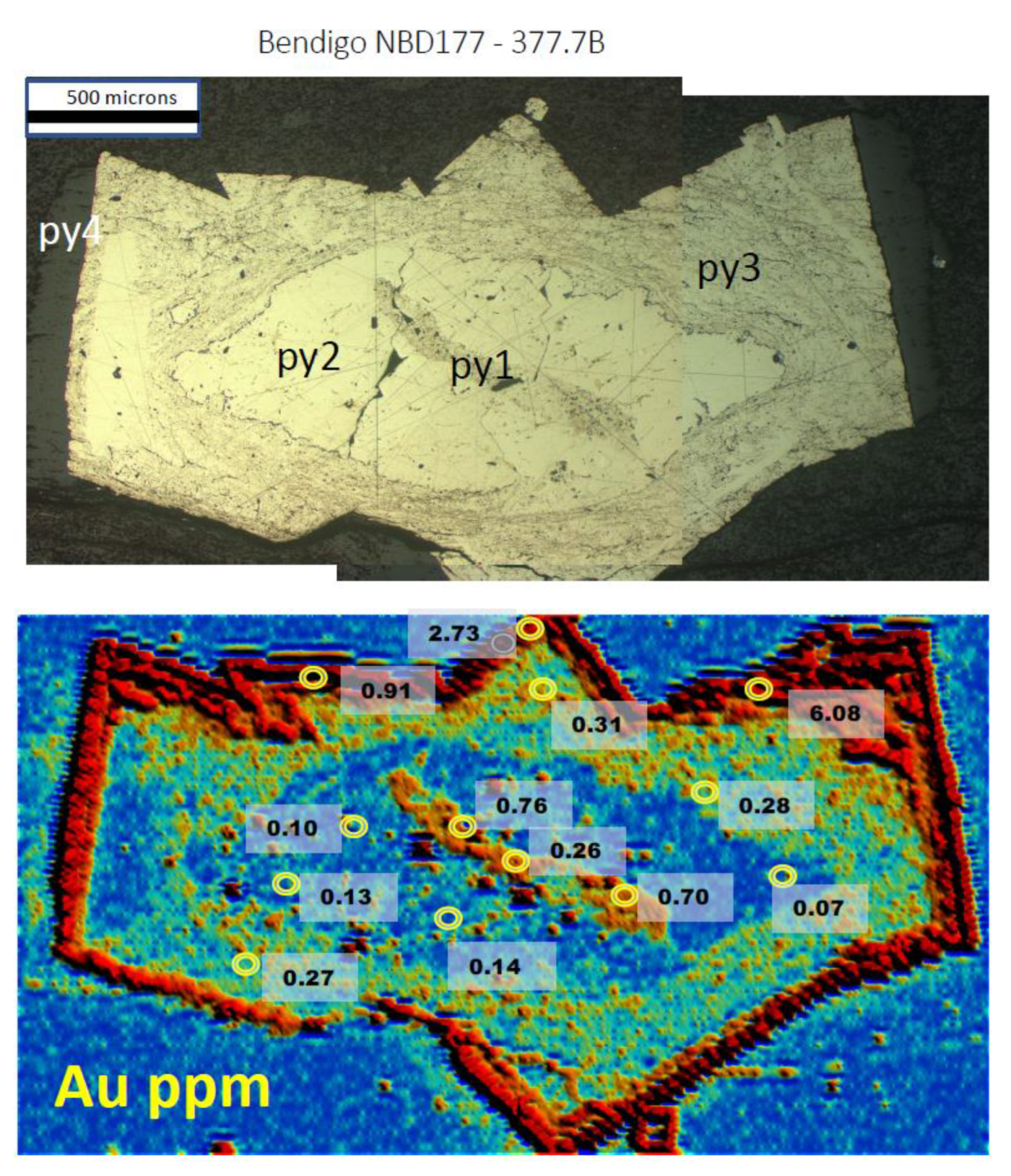
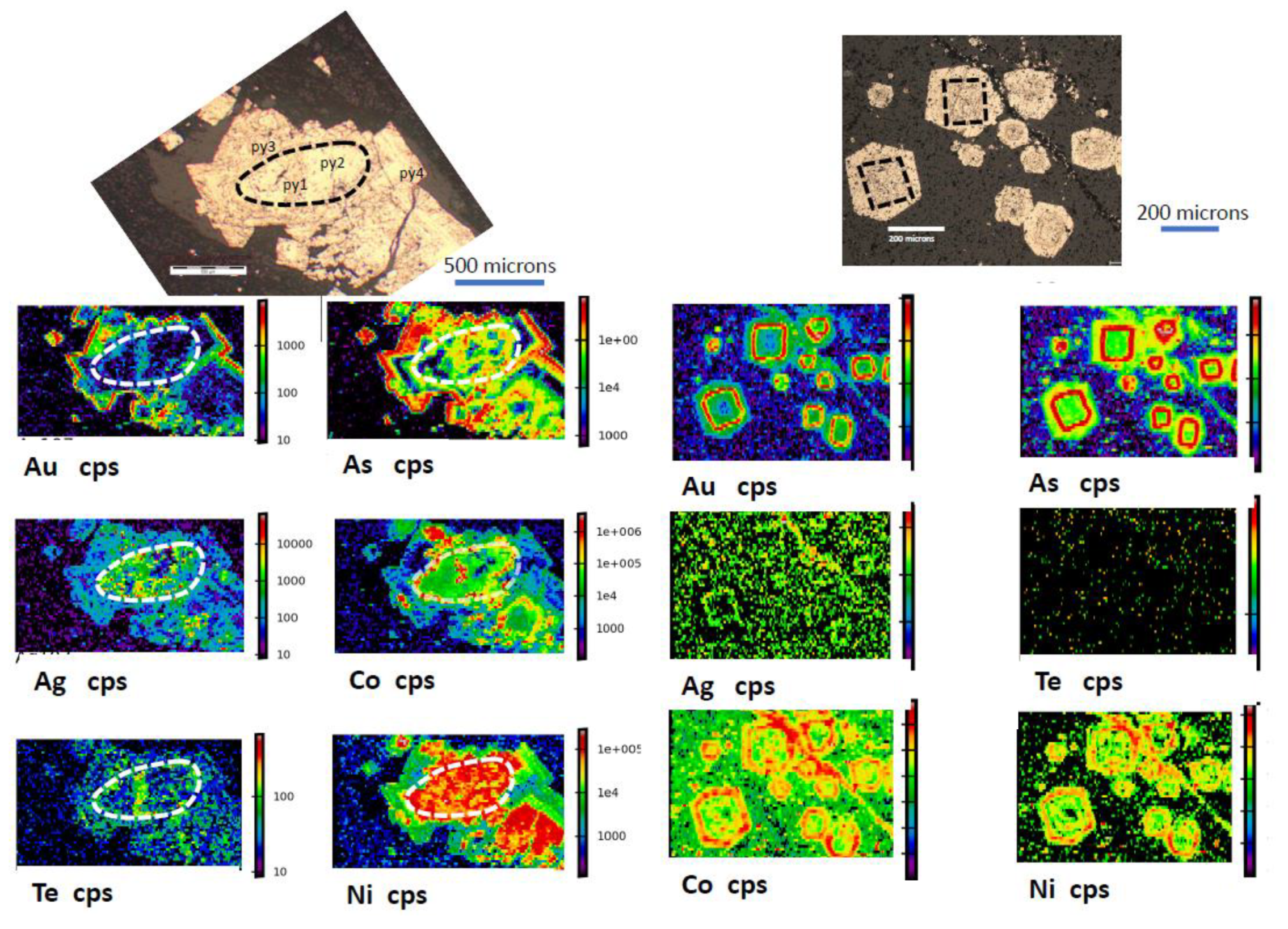
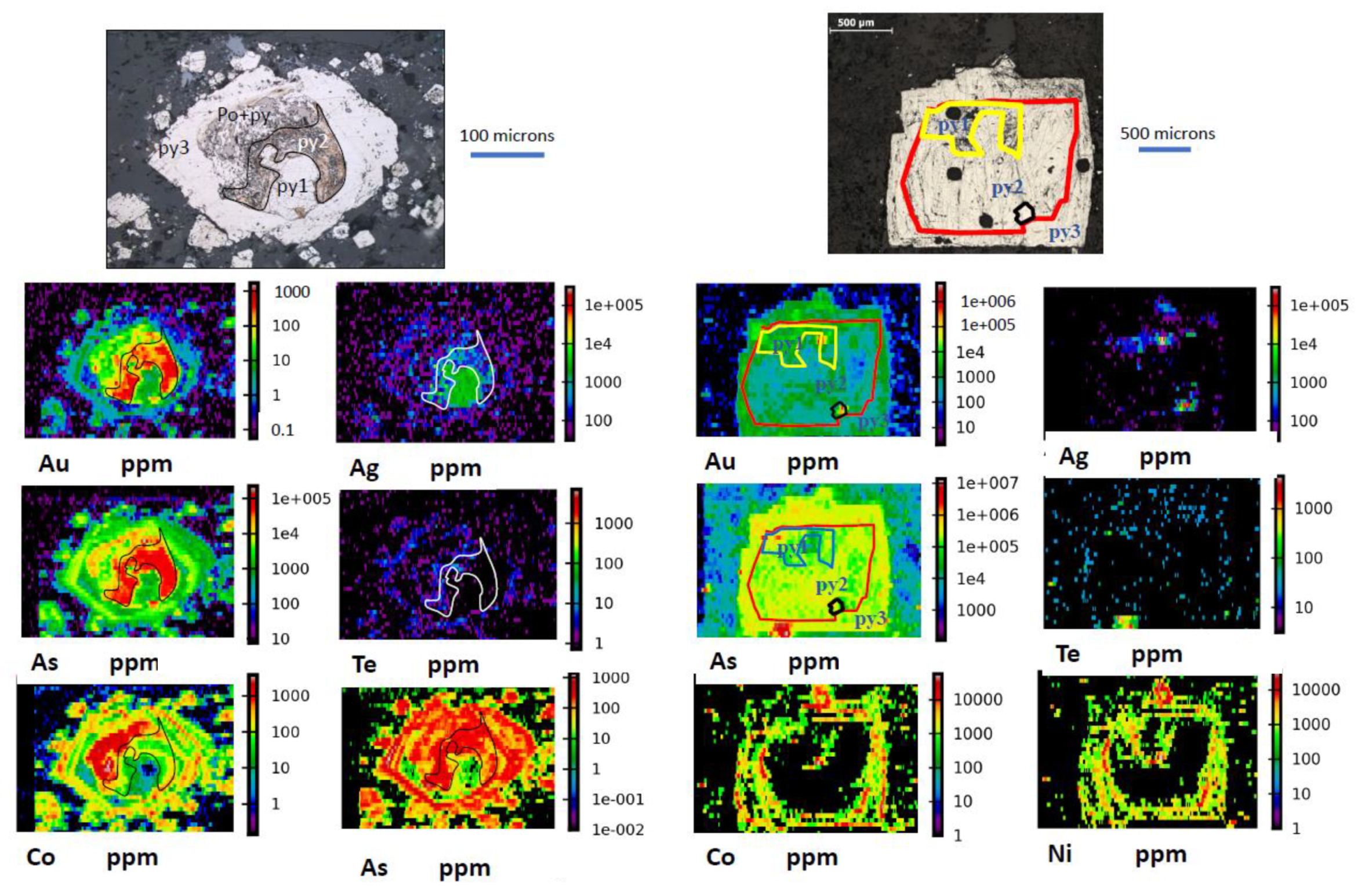
3.1. Gold Predominantly in the Core of Pyrite (py1)
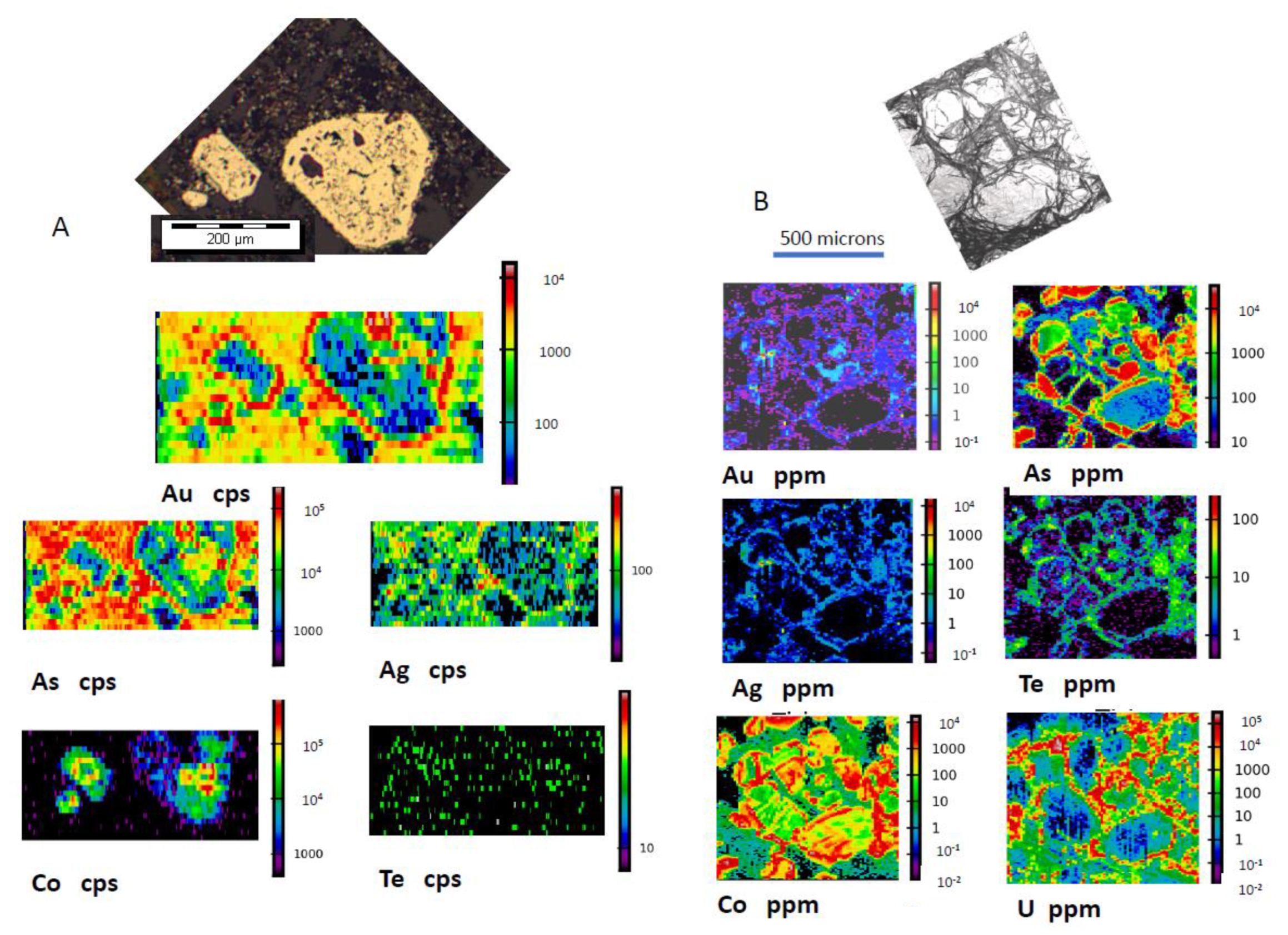
3.2. Gold Concentrated on the Rim of Pyrite
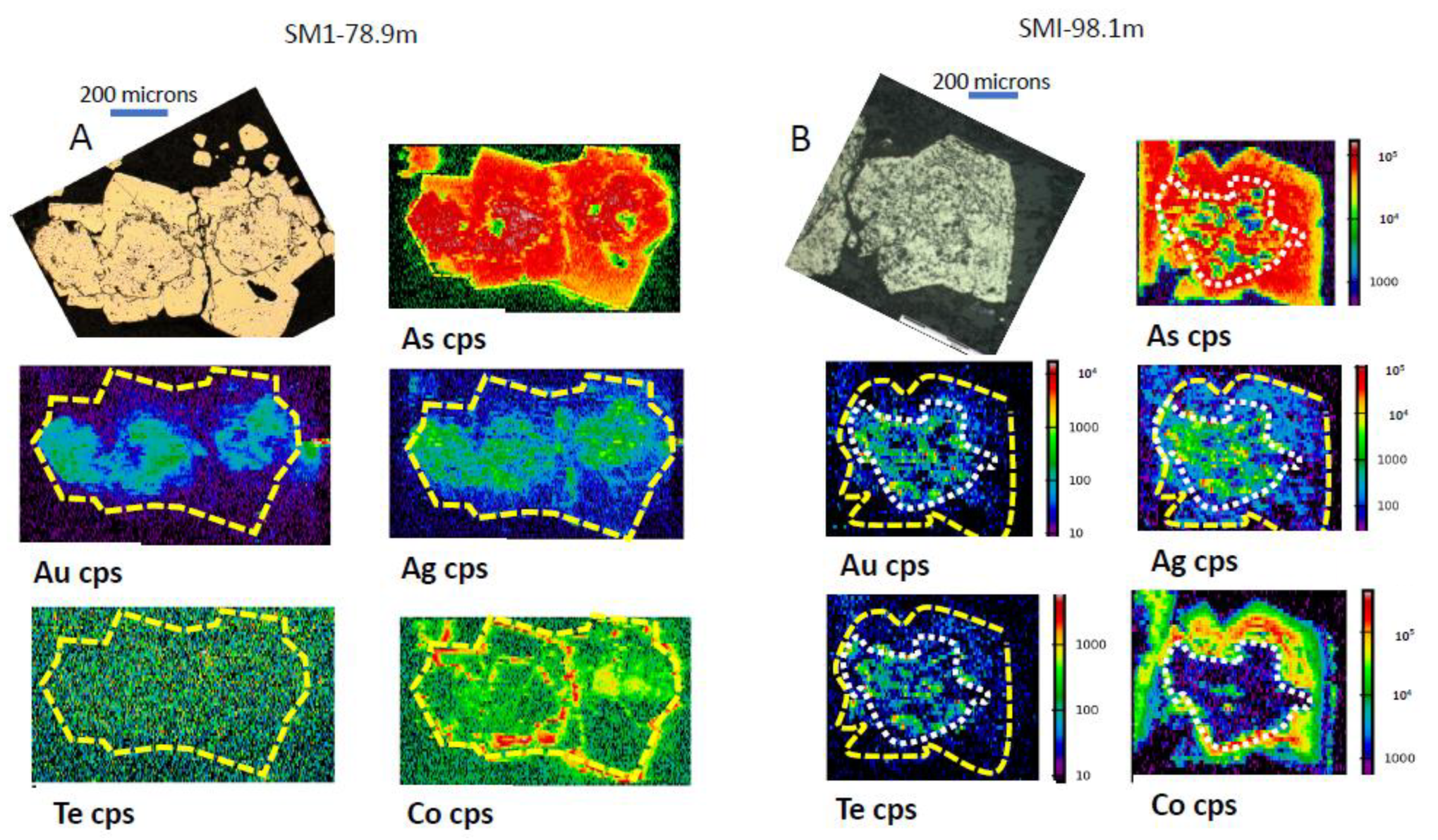
3.3. Gold Concentrated in Internal Zones of Pyrite
| Core Hg, Te, Ag, Pb, Sb, Tl, Cu → | Au-As-Ni → | Ni, Se, Co Rim |
| py1 | py2 | py3 |
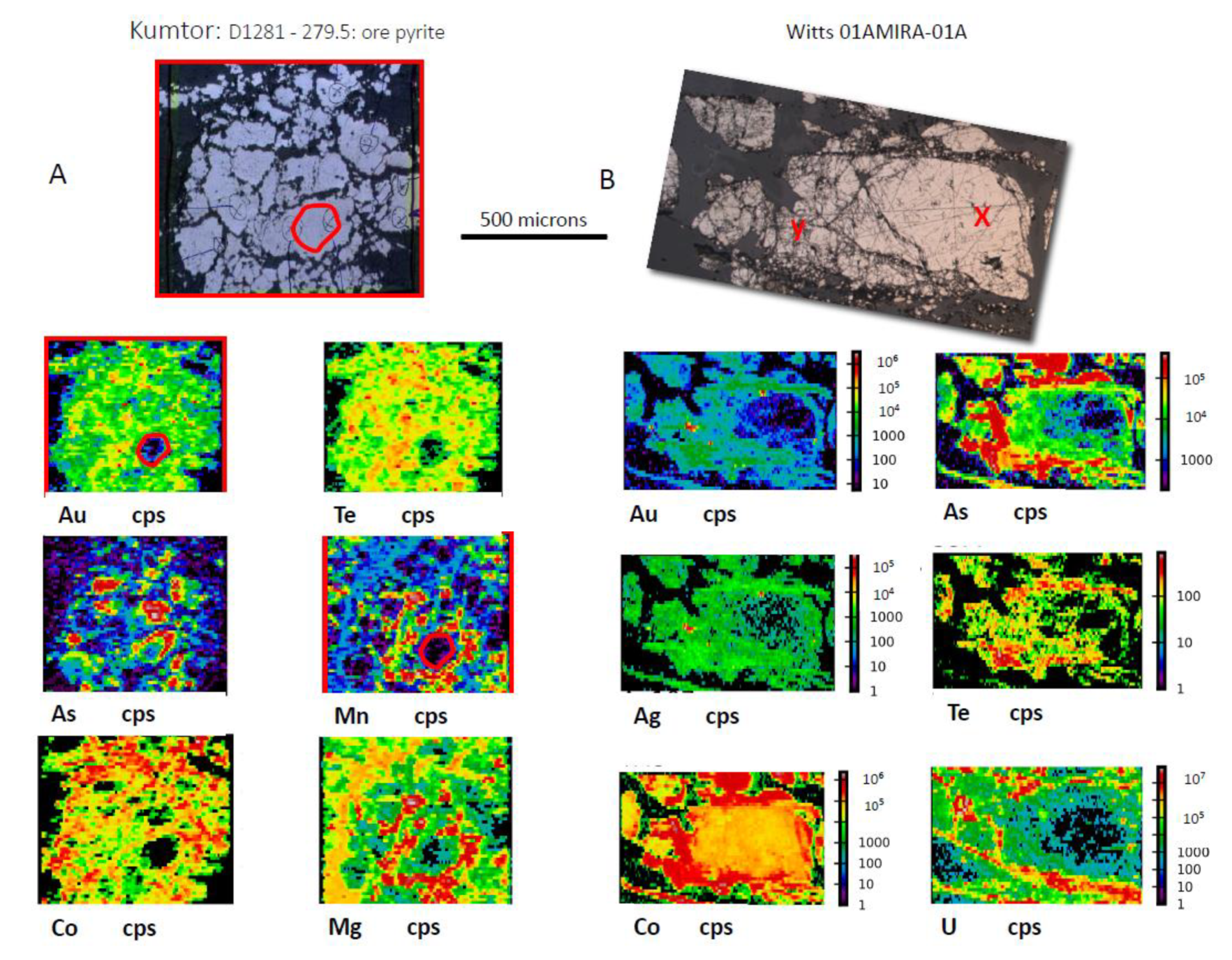
3.4. Gold Concentrated in Fractured Pyrite
- Early diagenetic py1 enriched in Au, Te, Pb, Sb, Bi, Mo, As, Ag, V and U;
- Overgrowth late diagenetic to early metamorphic pyrite enriched in Co, Ni, As, and Se;
- Early orogenic pyrite enriched in Au, As, Te, Ag, Pb, and Sb;
- Late orogenic pyrite overgrowths and pyrite fracturing carry Co, Ni, W, Au, and Te. This late orogenic event introduced the maximum pulse of gold.
4. Chemical Associations of Invisible Gold
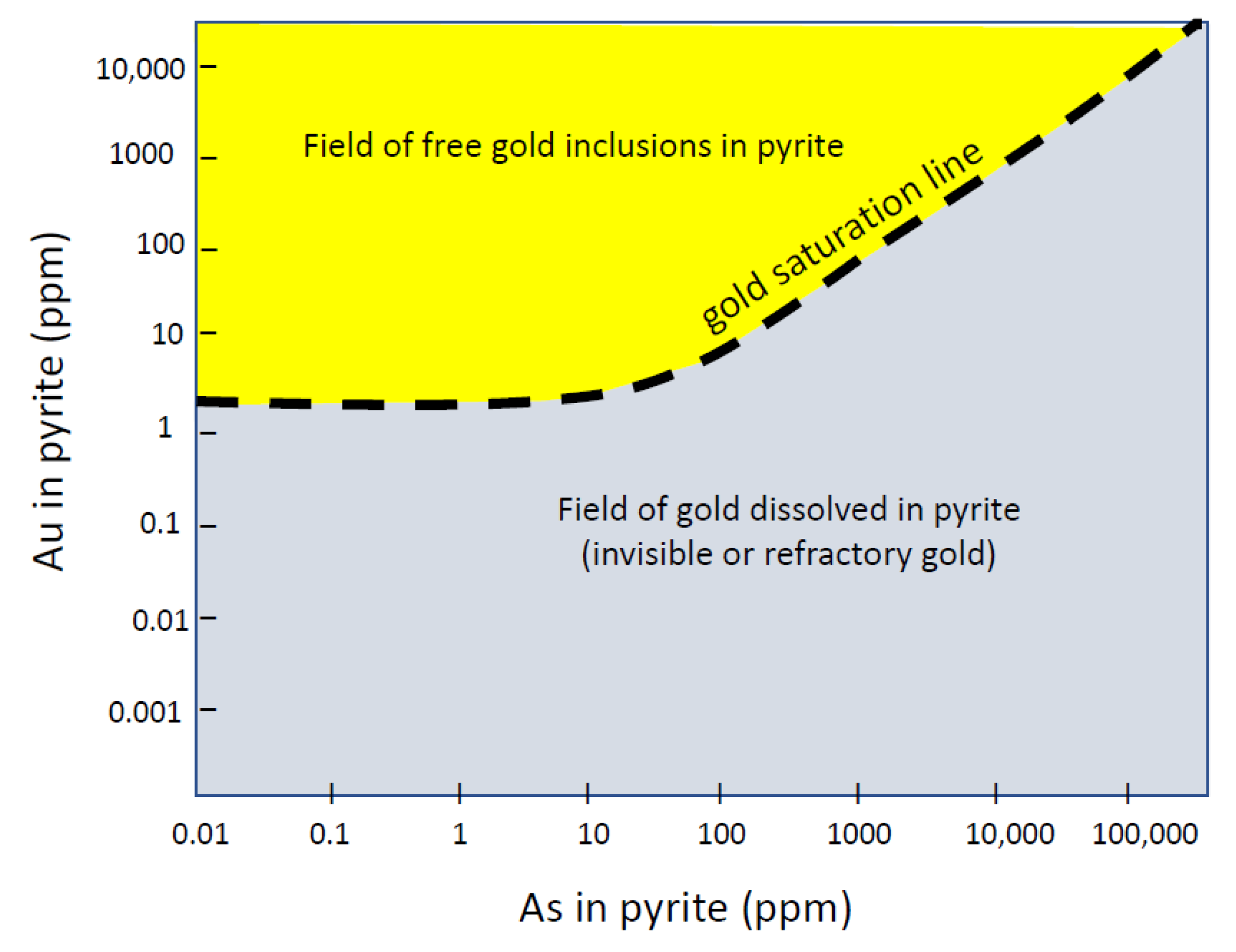
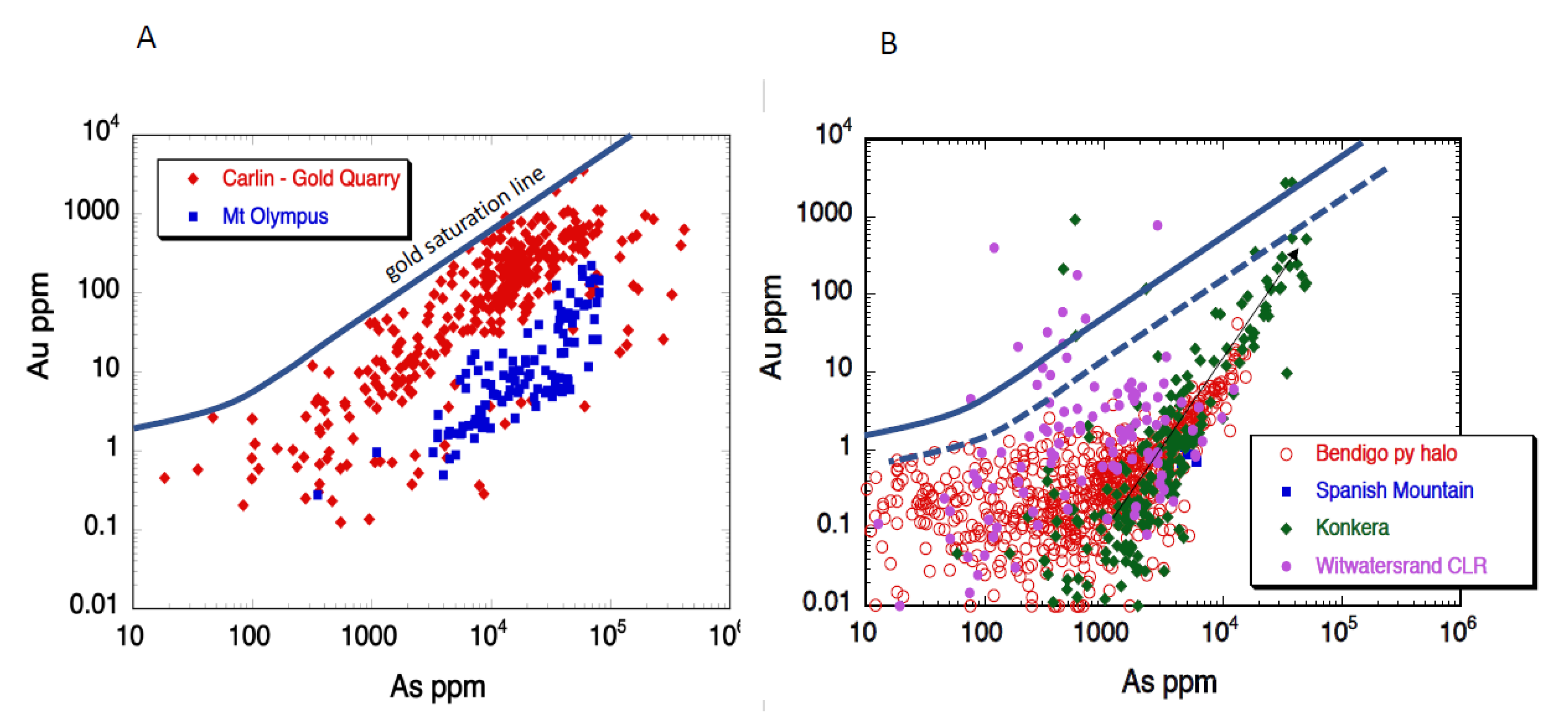
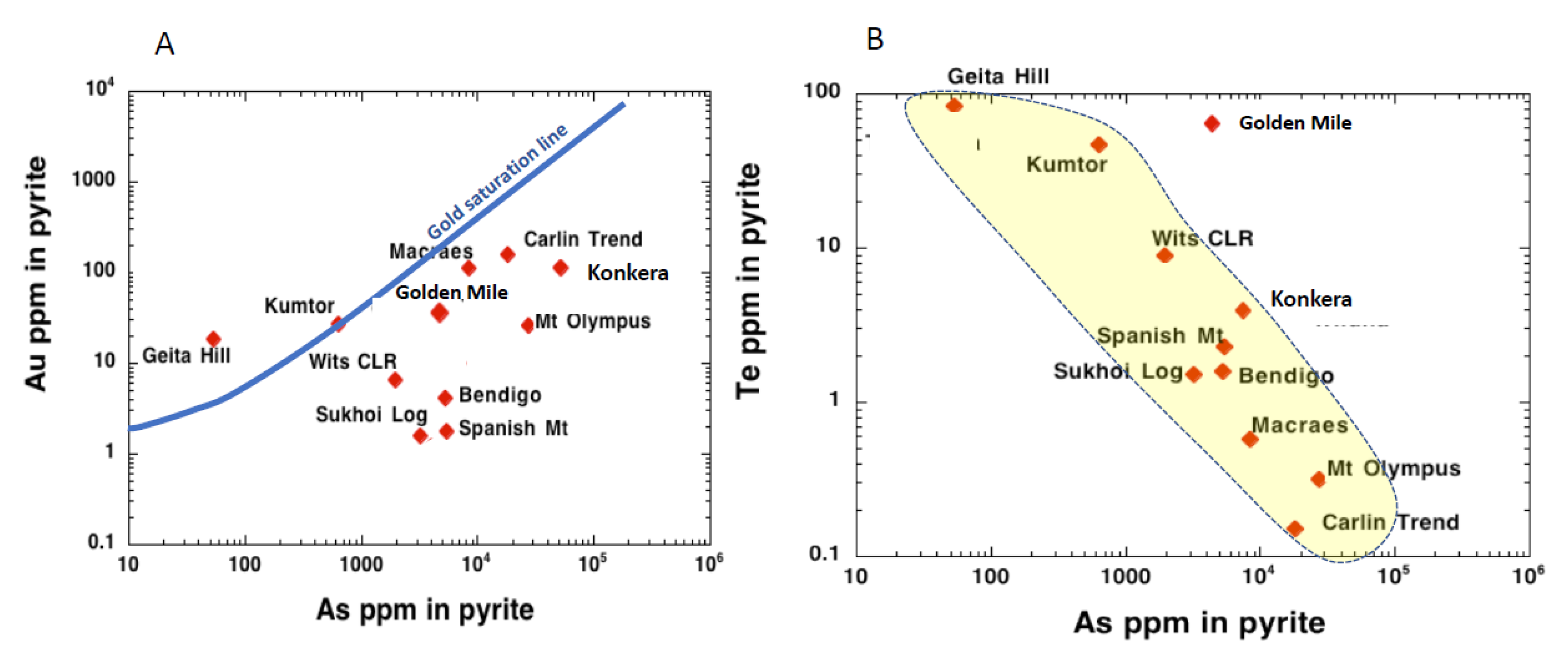
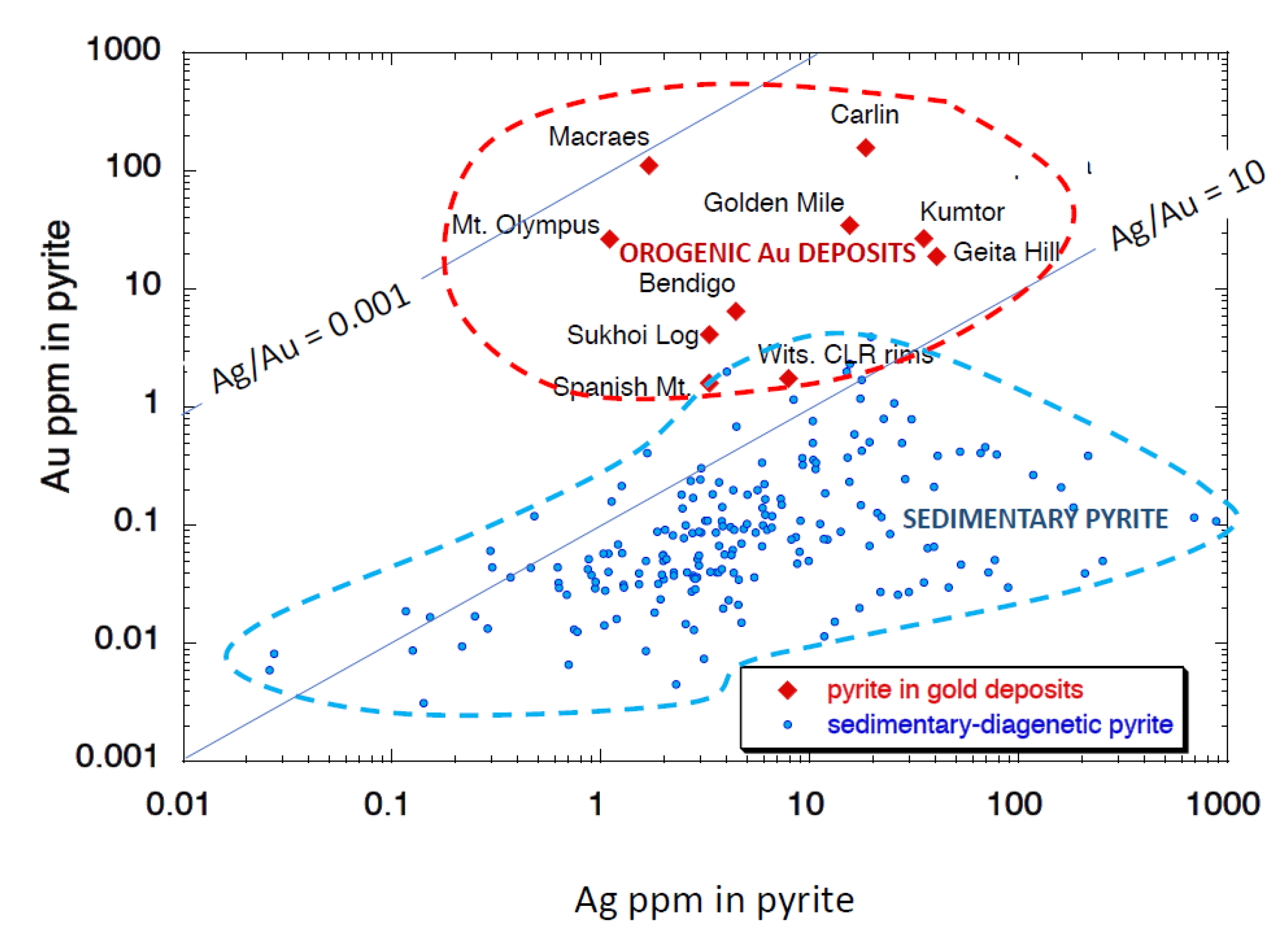
4.1. Gold-Arsenic Association
4.2. Gold-Tellurium Association
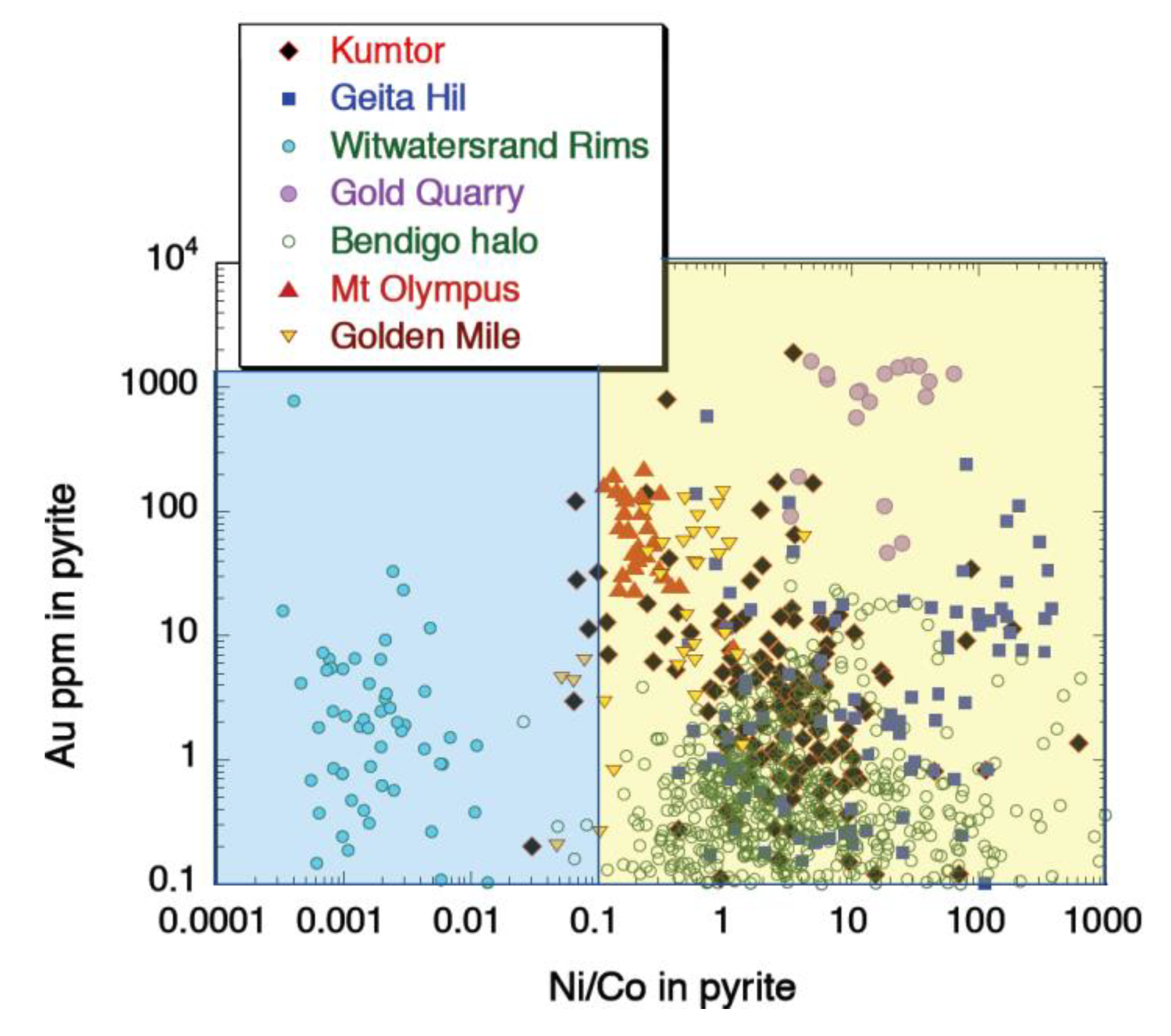
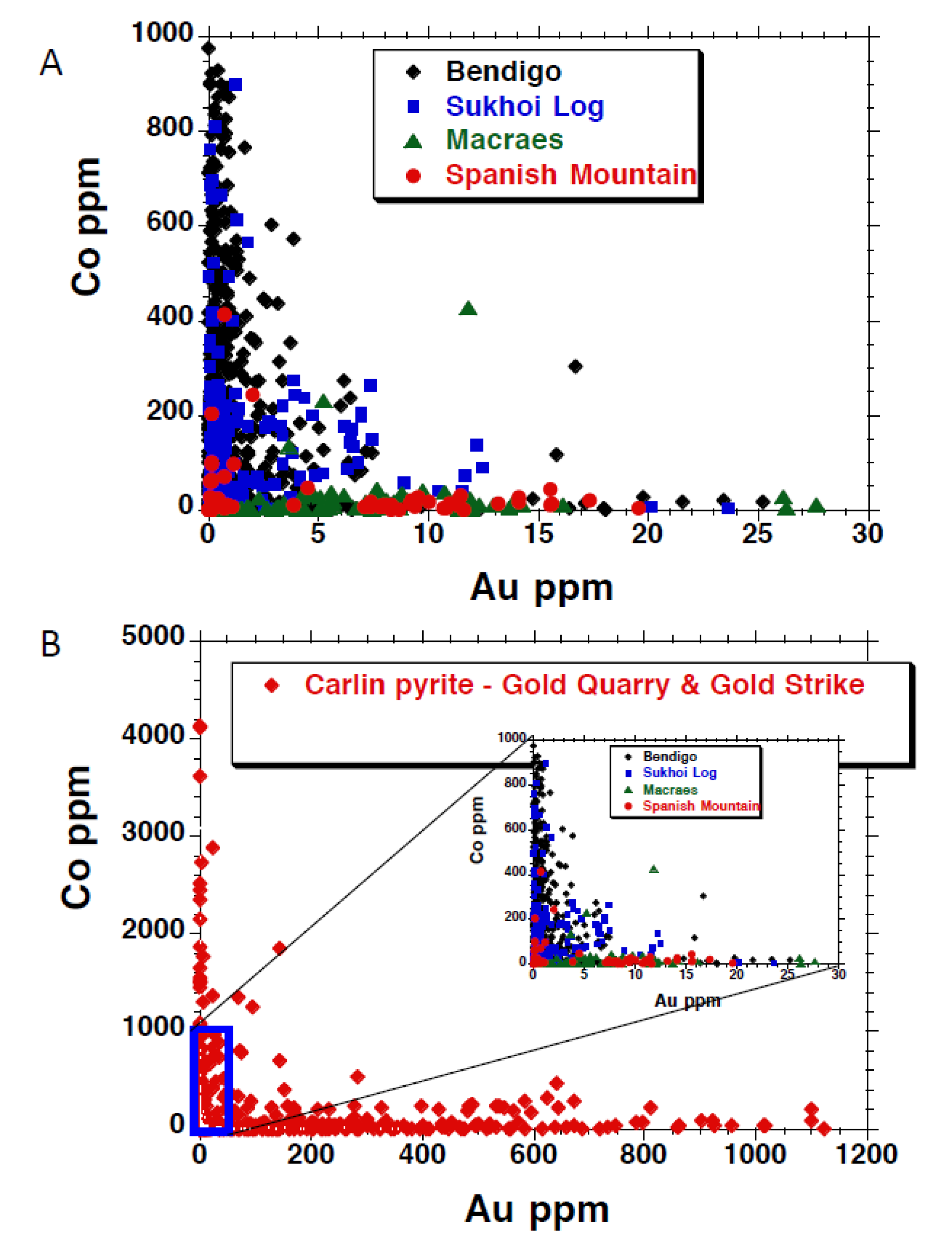
4.3. Gold-Cobalt-Nickel Relationships in Pyrite
5. Importance of Gold-Rich Rims on Pyrite
6. Comments on Transport of Au and As in Orogenic Fluids
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chryssoulis, S.L.; McMullen, J. Mineralogical investigation of gold ores. Dev. Miner. Process. 2005, 15, 21–71. [Google Scholar]
- Chryssoulis, S.; Dunne, R.; Coetzee, A. Diagnostic microbeam technology in gold ore processing. JOM 2004, 56, 53–57. [Google Scholar] [CrossRef]
- Spry, P.G.; Chryssoulis, S.; Ryan, C.G. Process mineralogy of gold: Gold from telluride-bearing ores. JOM 2004, 56, 60–62. [Google Scholar] [CrossRef]
- Reich, M.; Kesler, S.E.; Utsunomiya, S.; Palenik, C.S.; Chryssoulis, S.L.; Ewing, R. Solubility of gold in arsenian pyrite. Geochem. Cosmochim. Acta 2005, 69, 2781–2796. [Google Scholar] [CrossRef]
- Deditius, A.P.; Reich, M.; Kesler, S.E.; Utsunomiya, S.; Chryssoulis, S.L.; Walshe, J.; Ewing, R.C. The coupled geochemistry of Au and As in pyrite from hydrothermal ore deposits. Geochim. Cosmochim. Acta 2014, 140, 644–670. [Google Scholar] [CrossRef]
- Bortnikov, N.S.; Cabri, L.J.; Vikentiev, I.V.; Tagirov, B.R.; Mc Mahon, G.; Bogdanov, Y.A.; Stavrova, O.O. Invisible gold in sulfides from seafloor massive sulfide edifices. Geol. Ore Depos. 2003, 45, 201–212. [Google Scholar]
- Maslennikov, V.V.; Maslennikova, S.P.; Large, R.R.; Danyushevsky, L.V. Study of trace element zonation in vent chimneys from the Silurian Yaman-Kasy volcanic-hosted massive sulfide deposit (Southern Urals, Russia) using laser ablation-inductively coupled plasma mass spectrometry (LA-ICPMS). Econ. Geol. 2009, 104, 1111–1141. [Google Scholar] [CrossRef]
- Vikentyev, I.V. Invisible and microscopic gold in pyrite: methods and new data for massive sulfide ores of the Urals. Geol. Ore Depos. 2015, 57, 237–265. [Google Scholar] [CrossRef]
- Cook, N.J.; Chryssouilis, S.L. Concentrations of invisible gold in the common sulfides. Can. Mineral. 1990, 28, 1–16. [Google Scholar]
- Mumin, A.H.; Fleet, M.E.; Chryssoulis, S.L. Gold mineralisation in As-rich mesothermal gold ores of the Bogosu-Prestea mining district of the Ashanti Gold Belt, Ghana: remobalization of “invisible” gold. Miner. Depos. 1994, 29, 445–460. [Google Scholar] [CrossRef]
- Deditius, A.P.; Utsunomiya, S.; Ewing, R.C.; Kesler, S.E. Nanoscale “liquid” inclusions of As-Fe-S in arsenian pyrite. Am. Mineral. 2009, 94, 391–394. [Google Scholar] [CrossRef]
- Abraitis, P.K.; Pattrick, R.A.D.; Vaughan, D.J. Variations in the compositional, textural and electrical properties of natural pyrite: A review. Int. J. Miner. Process. 2004, 74, 41–59. [Google Scholar] [CrossRef]
- Cline, J.S. Timing of gold and arsenic sulphide mineral deposition at the Getchell Carlin-type gold deposit, north central Nevada. Econ. Geol. 2001, 96, 75–89. [Google Scholar] [CrossRef]
- Kusebauch, C.; Gleeson, S.A.; Oelze, M. Coupled partitioning of Au and As into pyrite controls formation of giant Au deposits. Sci. Adv. 2019, 5, eaav5891. [Google Scholar] [CrossRef] [PubMed]
- Cabri, L.J. Phase relations in the Au-Ag-Te systems and their mineralogical significance. Econ. Geol. 1965, 60, 1569–1606. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Cook, N.J.; Spry, P.G. Preface-Special Issue: Telluride and selenide minerals in gold deposits-how and why? Mineral. Petrol. 2006, 87, 163–169. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Spry, P.G.; Voudouris, P. Understanding gold-(silver)-telluride-(selenide) mineral deposits. Episodes 2009, 32, 249–263. [Google Scholar] [CrossRef]
- Zhao, J.; Pring, A. Mineral Transformations in Gold–(Silver) Tellurides in the Presence of Fluids: Nature and Experiment. Minerals 2019, 9, 167. [Google Scholar] [CrossRef]
- Danyushevsky, L.V.; Robinson, P.; Gilbert, S.; Norman, M.; Large, R.R.; Mc-Goldrick, P.; Shelley, J.M. A technique for routine quantitative multi-element analysis of sulphide minerals by laser ablation ICPMS. Geochem. Explor. Environ. Anal. 2011, 11, 51–60. [Google Scholar] [CrossRef]
- Large, R.R.; Danyushevsky, L.; Hollit, C.; Maslennikov, V.; Meffre, S.; Gilbert, S.; Bull, S.; Scott, R.; Emsbo, P.; Thomas, H.; et al. Gold and trace element zonation in pyrite using a laser imaging technique: Implications for the timing of gold in orogenic and Carlin-style sediment-hosted deposits. Econ. Geol. 2009, 104, 635–668. [Google Scholar] [CrossRef]
- Large, R.R.; Meffre, S.; Burnett, R.; Guy, B.; Bull, S.; Gilbert, S.; Goemann, K.; Danyushevsky, L. Evidence for an intrabasinal source and multiple concentration processes in the formation of the Carbon Leader Reef, Witwatersrand Supergroup, South Africa. Econ. Geol. 2013, 108, 1215–1241. [Google Scholar] [CrossRef]
- Longerich, H.P.; Jackson, S.E.; Gunther, D. Laser ablation inductively coupled plasma mass spectrometric transient signal data acquisition and analyte concentration calculation. J. Anal. At. Spectrom. 1996, 11, 899–904. [Google Scholar] [CrossRef]
- Large, R.R.; Maslennikov, V.; Robert, F.; Danyushevsky, L.V.; Chang, Z. Multistage sedimentary and metamorphic origin of pyrite and gold in the giant Sukhoi Log deposit, Lena gold province, Russia. Econ. Geol. 2007, 102, 1233–1267. [Google Scholar] [CrossRef]
- Thomas, H.V.; Large, R.R.; Bull, S.W.; Maslennikov, V.; Berry, R.F.; Fraser, R.; Froud, S.; Moye, R. Pyrite and pyrrhotite textures and composition in sediments, laminated quartz veins, and reefs at Bendigo gold mine, Australia: Insights for ore genesis. Econ. Geol. 2011, 106, 1–31. [Google Scholar] [CrossRef]
- Meffre, S.; Large, R.R.; Steadman, J.A.; Gregory, D.D.; Stepanov, A.S.; Kamenetsky, V.S.; Ehrig, K.; Scott, R.J. Multi-stage enrichment processes for large gold-bearing ore deposits. Ore Geol. Rev. 2016, 76, 268–279. [Google Scholar] [CrossRef]
- Safina, N.P.; Maslennikov, V.V. Sequence of mineral formation in clastic ores of the Saf’yanovka volcanic-hosted copper massive sulfide deposit, the Central Urals. Geol. Ore Depos. 2009, 51, 633–634. [Google Scholar] [CrossRef]
- Panteleyev, A.; Bailey, D.G.; Bloodgood, M.A.; Hancock, K.D. Geology and mineral deposits of the Quesnel River–Horsefly map area, central Quesnel Trough, British Columbia (NTS map sheets 93A/5, 6, 7, 11, 12, 13; 93B/9, 16; 93G/1; 93H/4): BC Ministry of Energy, Mines and Petroleum Resources. Geol. Surv. Branch Bull. 1996, 97, 156. [Google Scholar]
- Gregory, D.D.; Large, R.R.; Halpin, J.A.; Baturina, E.L.; Lyons, T.W.; Wu, S.; Danyushevsky, L.; Sack, P.J.; Chappaz, A.; Maslennikov, V.V.; et al. Trace element content of sedimentary pyrite in black shales. Econ. Geol. 2015, 110, 1389–1410. [Google Scholar] [CrossRef]
- Phillips, G.N.; Groves, D.I.; Martyn, J.E. An epigenetic origin for Archean banded iron-formation-hosted gold deposits. Econ. Geol. 1984, 79, 162–171. [Google Scholar] [CrossRef]
- Arehart, G.B.; Foland, K.A.; Naeser, C.W.; Kesler, S.E. 40Ar/39Ar, K/Ar, and fission track geochronology of sediment-hosted disseminated gold deposits at Post-Betze, Carlin Trend, northeastern Nevada. Econ. Geol. 1993, 88, 622–646. [Google Scholar] [CrossRef]
- Gopon, P.; Douglas, J.O.; Auger, M.A.; Hansen, L.; Wade, J.; Cline, J.S.; Robb, L.J.; Moody, M.P. A Nanoscale Investigation of Carlin-Type Gold Deposits: An Atom-Scale Elemental and Isotopic Perspective. Econ. Geol. 2019, 114, 1123–1133. [Google Scholar] [CrossRef]
- Law, J.D.; Phillips, G.N. Hydrothermal replacement model for Witwatersrand gold. In 100th Anniversary Volume; Society of Economic Geologists: Littleton, CO, USA, 2005; pp. 799–812. [Google Scholar]
- Tan, Q.; Xia, Y.; Xie, Z.; Wang, Z.; Wei, D.; Zhao, Y.; Yan, J.; Li, S. Two Hydrothermal Events at the Shuiyindong Carlin-Type Gold Deposit in Southwestern China: Insight from Sm–Nd Dating of Fluorite and Calcite. Minerals 2019, 9, 230. [Google Scholar] [CrossRef]
- Frimmel, H.E.; Groves, D.I.; Kirk, J.; Ruiz, J.; Chesley, J.; Minter, W.E. The formation and preservation of the Witwatersrand goldfields, the world’s largest gold province. Econ. Geol. 2005, 100, 769–797. [Google Scholar]
- Cox, S.F.; Sun, S.S.; Etheridge, M.A.; Wall, V.J.; Potter, T.F. Structural and geochemical controls on the development of turbidite-hosted gold quartz vein deposits, Wattle Gully Mine, central Victoria, Australia. Econ. Geol. 1995, 90, 1722–1746. [Google Scholar] [CrossRef]
- Steadman, J.A.; Large, R.R.; Meffre, S. Pyrite textures and chemical zoning in magmatic hydrothermal ores. Minerals 2020. (in preparation). [Google Scholar]
- Sener, A.K.; Young, C.; Groves, D.I.; Krapez, B.; Fletcher, I.R. Major orogenic gold episode associated with Cordilleran-style tectonics related to the assembly of Paleoproterozoic Australia? Geology 2005, 33, 225–228. [Google Scholar] [CrossRef]
- Fielding, I.O.; Johnson, S.P.; Meffre, S.; Zi, J.W.; Sheppard, S.; Large, R.R.; Rasmussen, B. Linking gold mineralization to regional-scale drivers of mineral systems using in situ U–Pb geochronology and pyrite LA-ICP-MS. Geosci. Front. 2018, 30, 89–105. [Google Scholar] [CrossRef]
- Maclean. NI 43101 Technical Report on the Konkera Gold Project for Ampella Mining Limitwd; Ravensgate Mining Industry Consultants: West Perth, Australia, 2014; 165p. [Google Scholar]
- Kitto, P.; Shepherd, R.; Rudd, T.; Ouedraogo, S. Konkera gold discovery: Unearthing a new gold province at Batie West in southern Burkina Faso. In Proceedings of the NewGen Gold 2011 Conference, Case Histories of Discovery, Perth, Australia, 12–13 November 2011. [Google Scholar]
- Large, R.R. Pyrite Textures and LA-ICPMS Trace Element Zonation; Defining the Main Gold Event at Konkera Gold Deposit, Burkina Faso; Consultant report to Ampella; University of Tasmania: Hobart, Australia, 2011; 70p. [Google Scholar]
- Craw, D.; Windle, S.J.; Angus, P.V. Gold mineralization without quartz veins in a ductile-brittle shear zone, Macraes Mine, Otago Schist, New Zealand. Miner. Depos. 1999, 34, 382–394. [Google Scholar] [CrossRef]
- Pitcairn, I.K.; Teagle, D.A.; Craw, D.; Olivo, G.R.; Kerrich, R.; Brewer, T.S. Sources of metals and fluids in orogenic gold deposits: Insights from the Otago and Alpine Schists, New Zealand. Econ. Geol. 2006, 101, 1525–1546. [Google Scholar] [CrossRef]
- Henne, A.; Craw, D. Synmetamorphic carbon mobility and graphite enrichment in metaturbidites as a precursor to orogenic gold mineralisation, Otago Schist, New Zealand. Miner. Depos. 2012, 47, 781–797. [Google Scholar] [CrossRef]
- Large, R.; Thomas, H.; Craw, D.; Henne, A.; Henderson, S. Diagenetic pyrite as a source for metals in orogenic gold deposits, Otago Schist, New Zealand. New Zealand J. Geol. Geophys. 2012, 55, 137–149. [Google Scholar] [CrossRef]
- Van Ryt, M.R.; Sanislav, I.V.; Dirks, P.H.; Huizenga, J.M.; Mturi, M.I.; Kolling, S.L. Alteration paragenesis and the timing of mineralised quartz veins at the world-class Geita Hill gold deposit, Geita Greenstone Belt, Tanzania. Ore Geol. Rev. 2017, 91, 765–779. [Google Scholar] [CrossRef]
- Seltmann, R.; Jenchuraeva, R. Paleozoic Geodynamics and Gold Deposits in the Kyrgyz Tien Shan Excursion Guidebook; International Geological Correlation Programme: Montreal, QC, Canada, 2001. [Google Scholar]
- Yakubchuk, A.S. Gold and base metal metallogeny of the Central Asian orogenic supercollage. Econ. Geol. 2005, 100, 1035–1068. [Google Scholar]
- Huston, D.L.; Sie, S.H.; Suter, G.F.; Cooke, D.C.; Both, R.A. Trace elements in sulfide minerals from eastern Australian volcanic-hosted massive sulfide deposits: Part 1 Proton microprobe analyses of pyrite, chalcopyrite and sphalerite. Econ. Geol. 1995, 90, 1167–1196. [Google Scholar] [CrossRef]
- Pals, D.W.; Spry, P.G. Telluride mineralogy of the low-sulfidation epithermal Emperor gold deposit, Vatukoula, Fiji. Mineral. Petrol. 2003, 79, 285–307. [Google Scholar] [CrossRef]
- Simon, G.; Kesler, S.E.; Chryssoulis, S. Geochemistry and textures of gold-bearing arsenian pyrite, Twin Creeks, Nevada; implications for deposition of gold in Carlin-type deposits. Econ. Geol. 1999, 94, 405–421. [Google Scholar] [CrossRef]
- Luo, S.; Nie, X.; Yang, M.; Fu, Y.; Zeng, P.; Wan, Q. Sorption of Differently Charged Gold Nanoparticles on Synthetic Pyrite. Minerals 2018, 8, 428. [Google Scholar] [CrossRef]
- Pridmore, D.F.; Shuey, R.T. The electrical resistivity of galena, pyrite, and chalcopyrite. Am. Mineral. 1976, 61, 248–259. [Google Scholar]
- Savage, K.S.; Stefan, D.; Lehner, S.W. Impurities and heterogeneity in pyrite: Influences on electrical properties and oxidation products. Appl. Geochem. 2008, 23, 103–120. [Google Scholar] [CrossRef]
- Pokrovski, G.S.; Akinfiev, N.N.; Borisova, A.Y.; Zotov, A.V.; Kouzmanov, K. Gold speciation and transport in geological fluids: insights from experiments and physical-chemical modelling. Geol. Society Lond. Spec. Publ. 2014, 402, 9–70. [Google Scholar] [CrossRef]
- Boyle, R.W. Hydrothermal transport and deposition of gold. Econ. Geol. 1969, 64, 112–115. [Google Scholar] [CrossRef]
- Williams-Jones, A.E.; Migdisov, A.A.; Andrew, C.J. The solubility of gold in crude oil: Implications for ore genesis. In Proceedings of the 9th Biennal SGA Meeting, Millpress, Dublin, Ireland, 20–23 August 2007. [Google Scholar]
- Williams-Jones, A.E.; Bowell, R.J.; Migdisov, A.A. Gold in solution. Elements 2009, 5, 281–287. [Google Scholar] [CrossRef]



| Deposit | Country | Host Rocks | Number of Pyrites Analysed | Quartz Veins? | Free or Invisible Au in Pyrite | Metamorphic Grade | Au Association | Other Au Related Elements | Au-as Rims on Pyrite? | Au in Internal Pyrite? | Interpred Timing of Main Au Event(s) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Carlin (Gold Quarry) | USA | black mudstone | 139 | no | invisible Au | sub-greenschist | As-Au | As, Sb, W, Hg, Tl | Yes | no | short pulse latest orogenic (py2) |
| Macraes | New Zealand | schist | 67 | minor veinlets | Mainly free | upper greenschist | As-Au | Ni, W, Ti | No | throughout internal zones | syn-orogenic (py1 and py3) |
| Mt Olympus | Australia | black shale | 115 | no | both | greenschist | As-Au | Cu, Hg, Tl, Sb | rarely | one internal zone with Au | short pulse syn-orogenic (py2) |
| Konkera | Burkina faso | volcaniclastics | 644 | no | both | greenschist | As-Au | Ni | No | internal py2 | early orogenic |
| Bendigo | Australia | shale, siltstone | 231 | saddle reefs | mainly free | lower greenschist | As-Au (-Te) | Ni | common | diagenetic cores have Au | pre-orogenic & latest orogenic (py1 and py3) |
| Sukhoi Log | Russia | black siltstone | 264 | minor veinlets | both | lower greenschist | As-Au (-Te) | Ni, Cu, Sb | No | Au in pyrite core & internal zones | pre-orogenic & syn-orogenic (py1 and py3/4) |
| Witwatersrand CLR | South Africa | conglomerate | 958 | no | both | lower greenschist | As-Te-Au | U, Ag, Co, Ni, Sb, Pd, Bi, Hg | Yes | Au in fractures in pyrite | pre-orogenic and syn-orogenic (py1 and Py2) |
| Golden Mile | Australia | dolerite | 35 | yes | both | lower greenschist | As-Te-Au | Ag, Cu, Sb | No | no data | no data |
| Spanish Mountain | Canada | black shale | 28 | no | both | lower greenschist | As-Te-Au | Ag, Ni, Pb | No | Au in pyrite core | pre-orogenic (py1) |
| Kumtor | Kyrgyzstan | black shale | 180 | no | both | lower greenschist | Te-Au | Co, Ni, Ag, Sb, Pb, Bi, As | No | Au in fractures in pyrite | pre-orogenic and post-orogenic (py1 and py3) |
| Geita Hill | Tanzania | BIF and chert | 105 | no | both | greenschist | Te-Au | Ag, Bi, Pb, Sb, Cu | No | Au with fine magnetite in pyrite core | ? py2 replacing magnetite in BIF chert layers |
| Deposit | As ppm | Te ppm | Au ppm | Ag ppm |
|---|---|---|---|---|
| Carlin Trend | 18,050 | 0.15 | 158 | 18.6 |
| Macraes Otago | 8480 | 0.58 | 112 | 1.7 |
| Golden Mile | 4340 | 61 | 35 | 15.5 |
| Kumtor | 633 | 46.7 | 26.8 | 35.1 |
| Mt Olympus | 27,292 | 0.32 | 26.6 | 1.1 |
| Geita Hill | 53.15 | 83.9 | 18.9 | 40.4 |
| Witwatersrand CLR | 1932 | 8.9 | 6.51 | 4.43 |
| Bendigo py halo | 5220 | 1.58 | 4.13 | 3.3 |
| Spanish Mountain | 5380 | 2.27 | 1.76 | 7.9 |
| Sukhoi Log | 3175 | 1.53 | 1.61 | 3.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Large, R.R.; Maslennikov, V.V. Invisible Gold Paragenesis and Geochemistry in Pyrite from Orogenic and Sediment-Hosted Gold Deposits. Minerals 2020, 10, 339. https://doi.org/10.3390/min10040339
Large RR, Maslennikov VV. Invisible Gold Paragenesis and Geochemistry in Pyrite from Orogenic and Sediment-Hosted Gold Deposits. Minerals. 2020; 10(4):339. https://doi.org/10.3390/min10040339
Chicago/Turabian StyleLarge, Ross R., and Valeriy V. Maslennikov. 2020. "Invisible Gold Paragenesis and Geochemistry in Pyrite from Orogenic and Sediment-Hosted Gold Deposits" Minerals 10, no. 4: 339. https://doi.org/10.3390/min10040339
APA StyleLarge, R. R., & Maslennikov, V. V. (2020). Invisible Gold Paragenesis and Geochemistry in Pyrite from Orogenic and Sediment-Hosted Gold Deposits. Minerals, 10(4), 339. https://doi.org/10.3390/min10040339





