The Crystallinity of Apatite in Contact with Metamict Pyrochlore from the Silver Crater Mine, ON, Canada
Abstract
:1. Introduction
1.1. Apatite as a Nuclear Waste Storage Solution
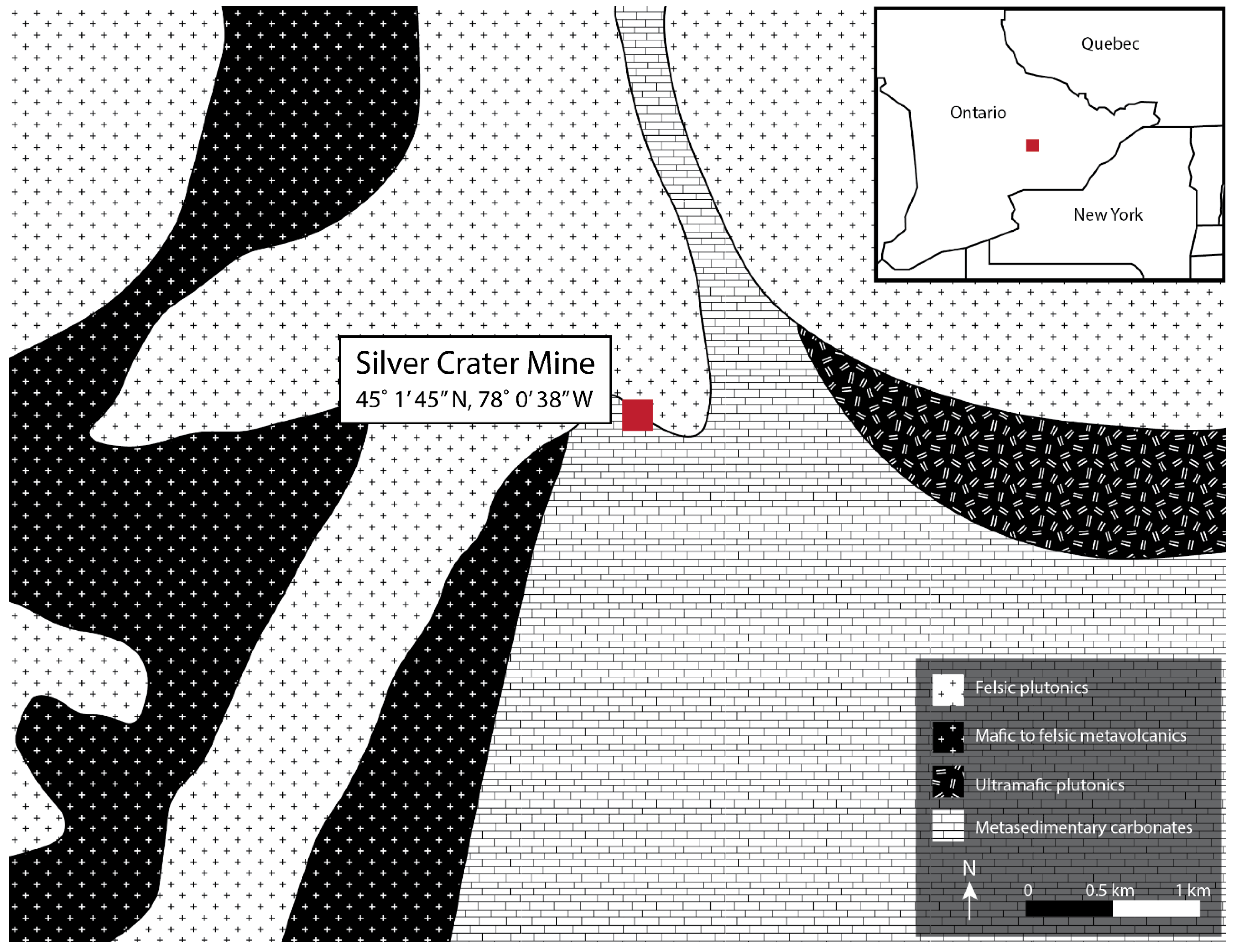
1.2. Geology of the Silver Crater Mine
2. Analytical Methods
2.1. X-ray Diffraction
2.2. Electron Microscopy
2.3. Mass Spectrometry
2.4. Stable Isotope Analysis
3. Results
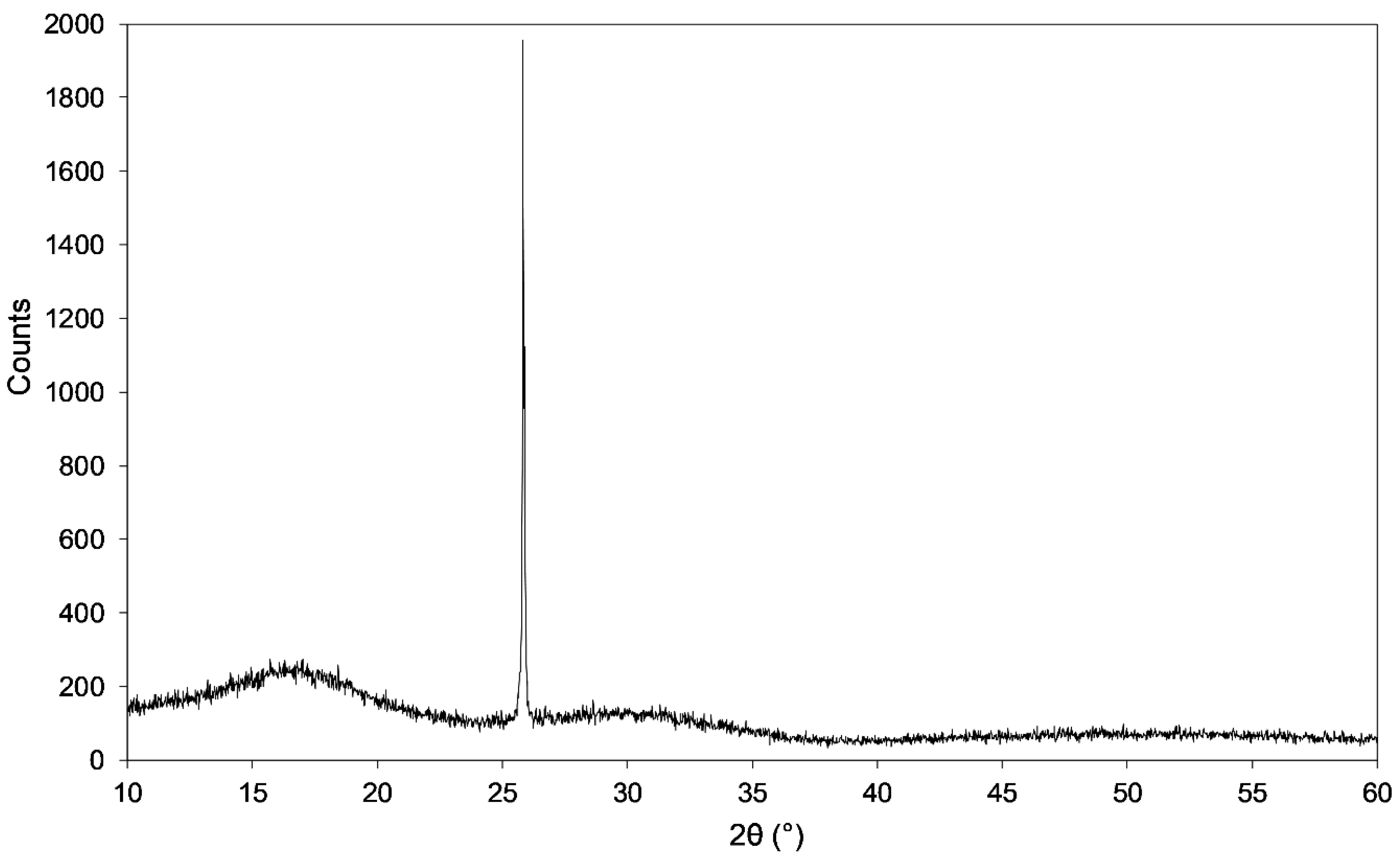
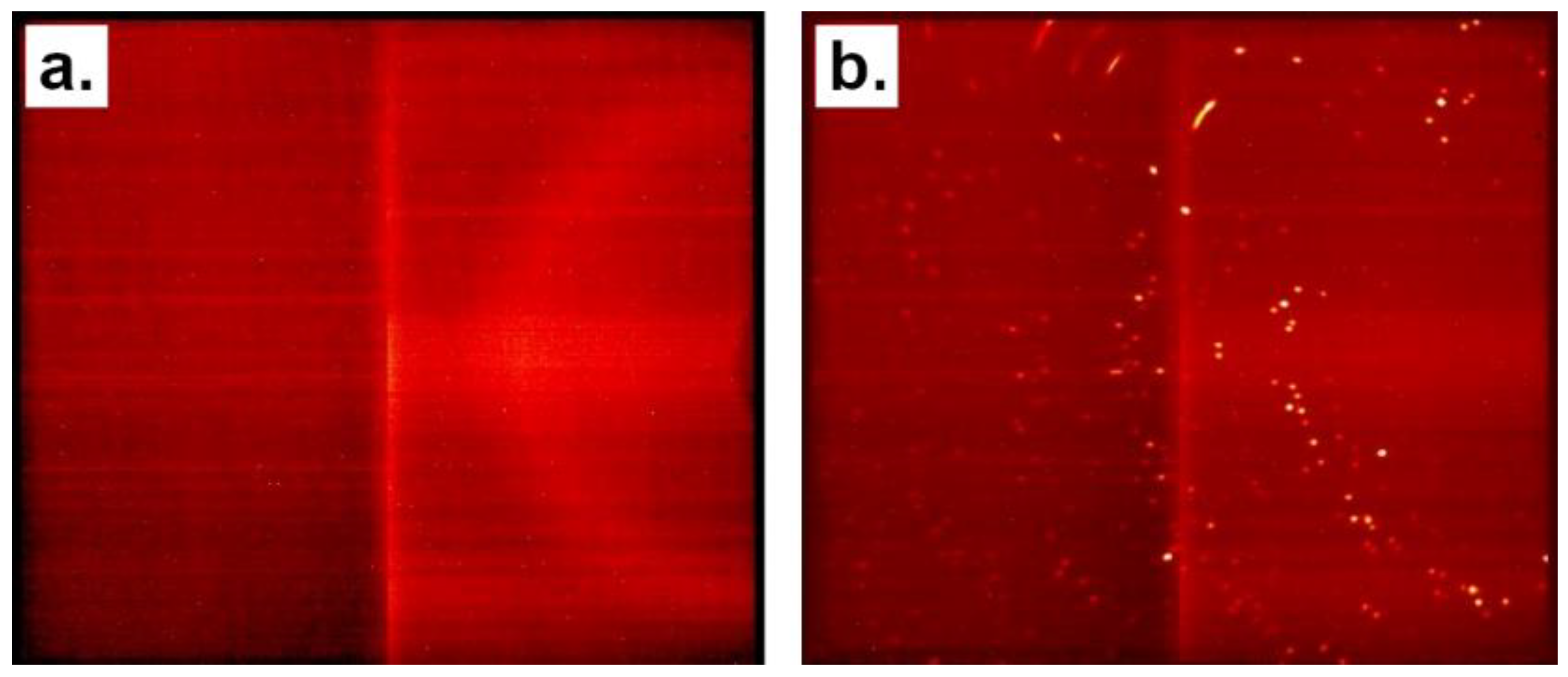
3.1. Broad X-ray Beam Analyses
3.2. Focused X-ray Beam Analyses
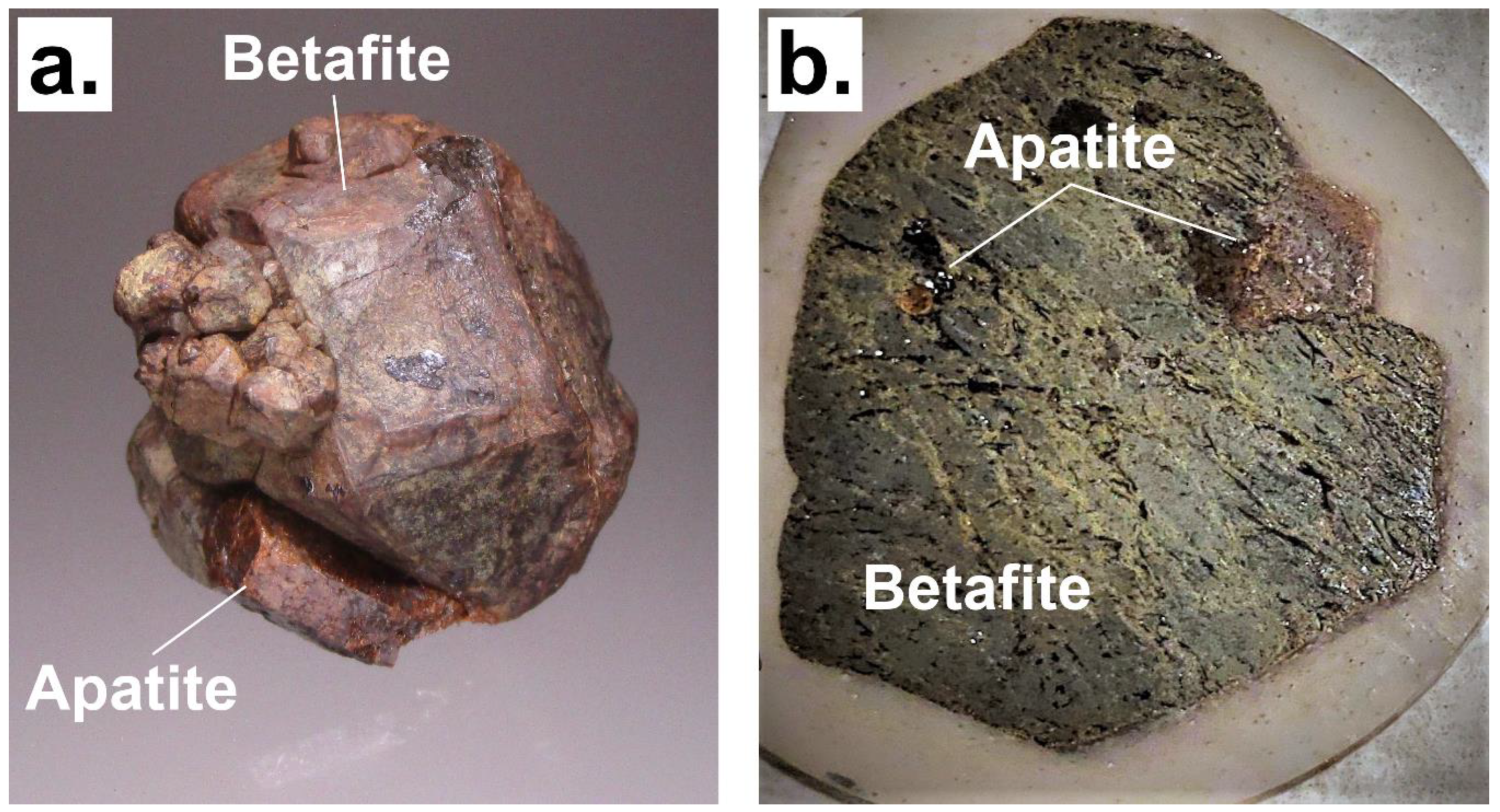
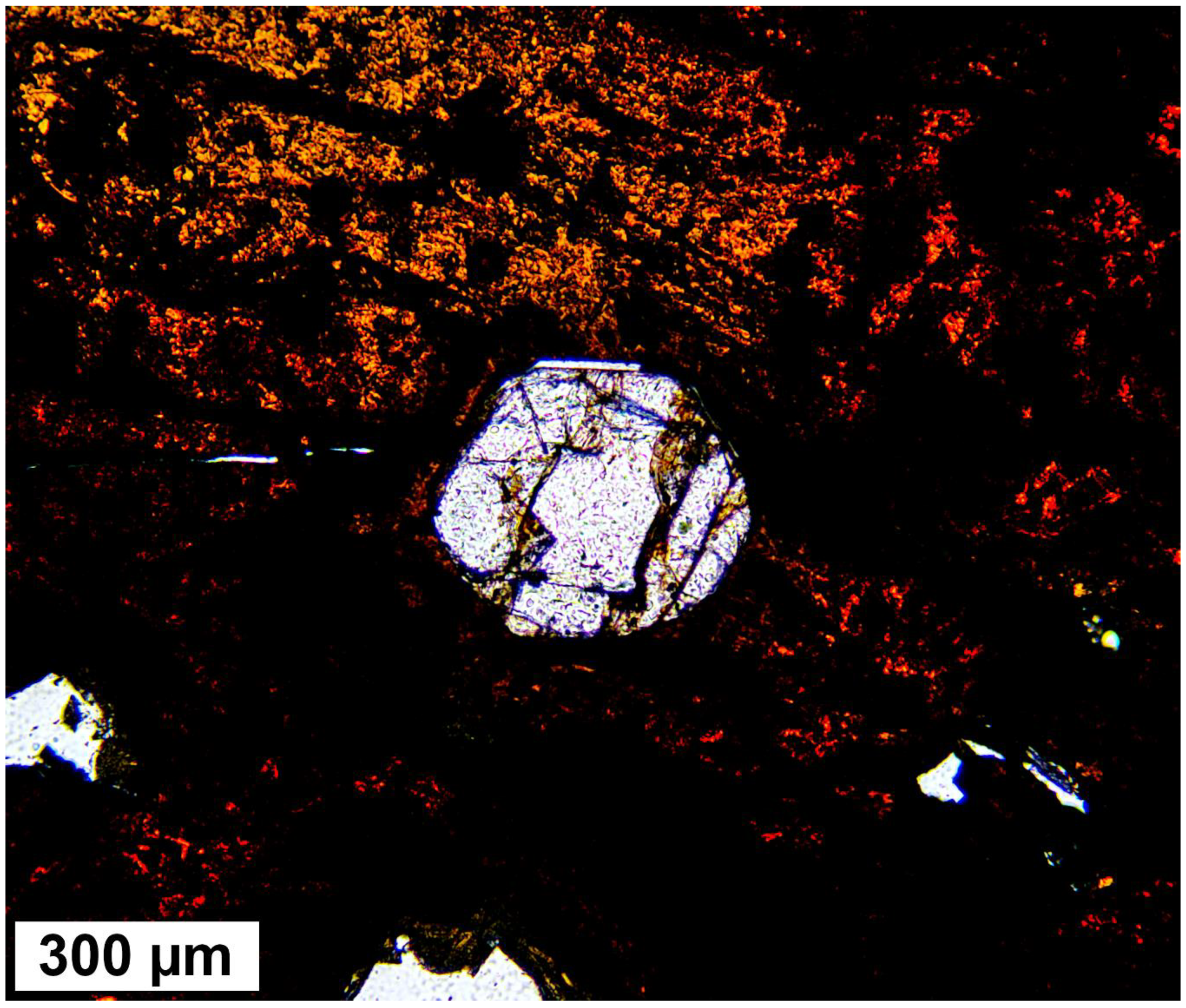
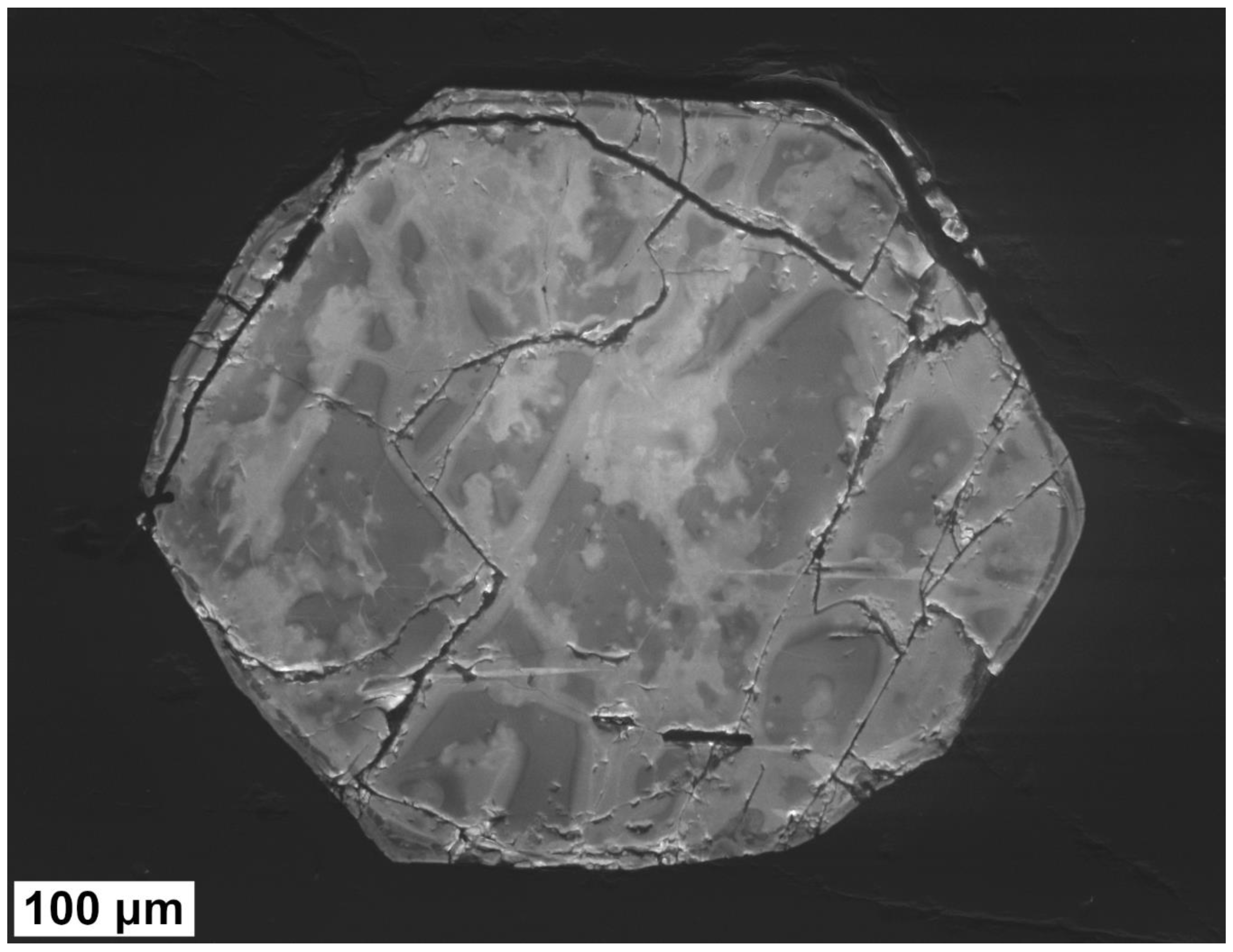
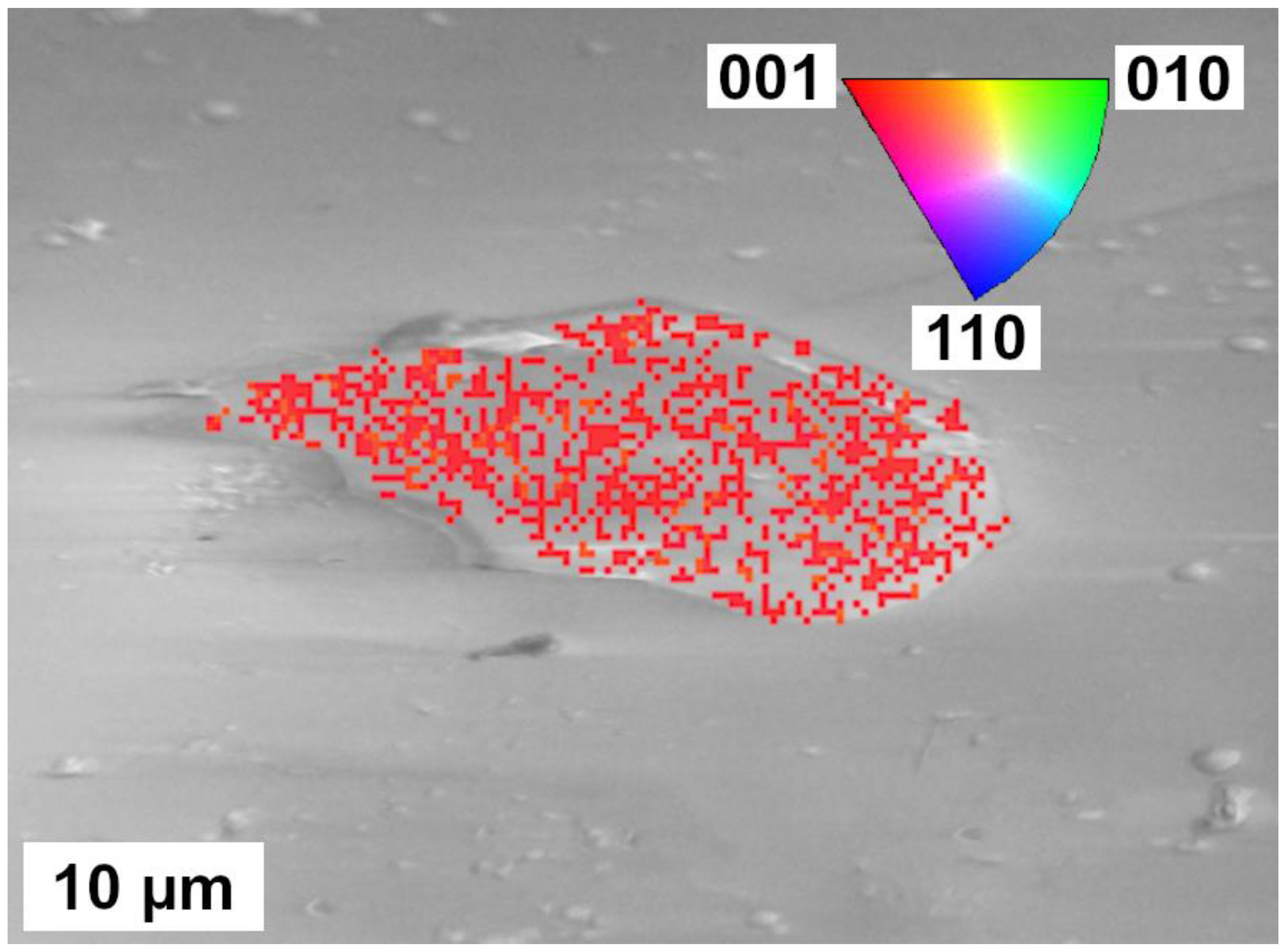
3.3. Electron Microscopy Analyses
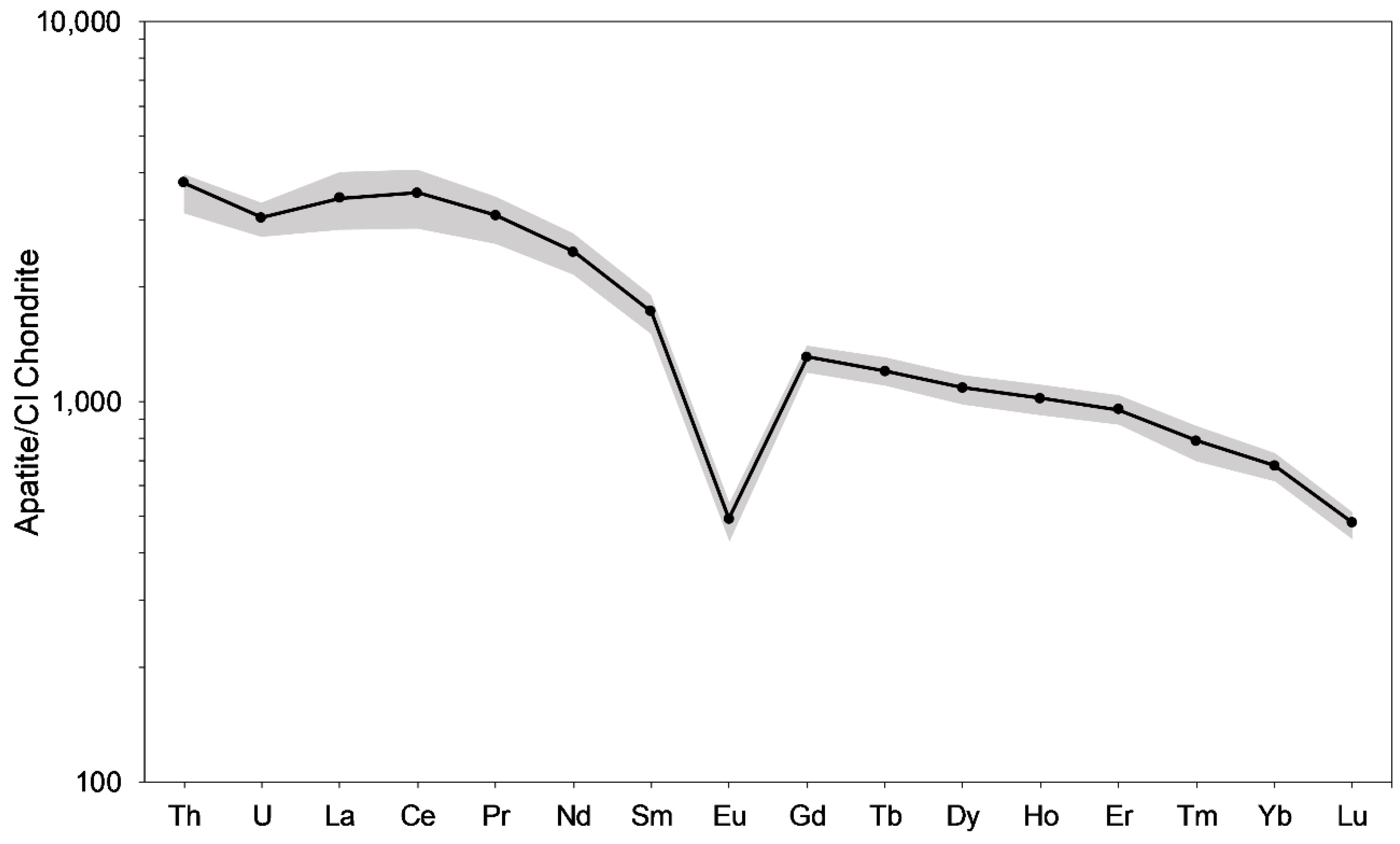
3.4. Mass Spectrometry Analyses
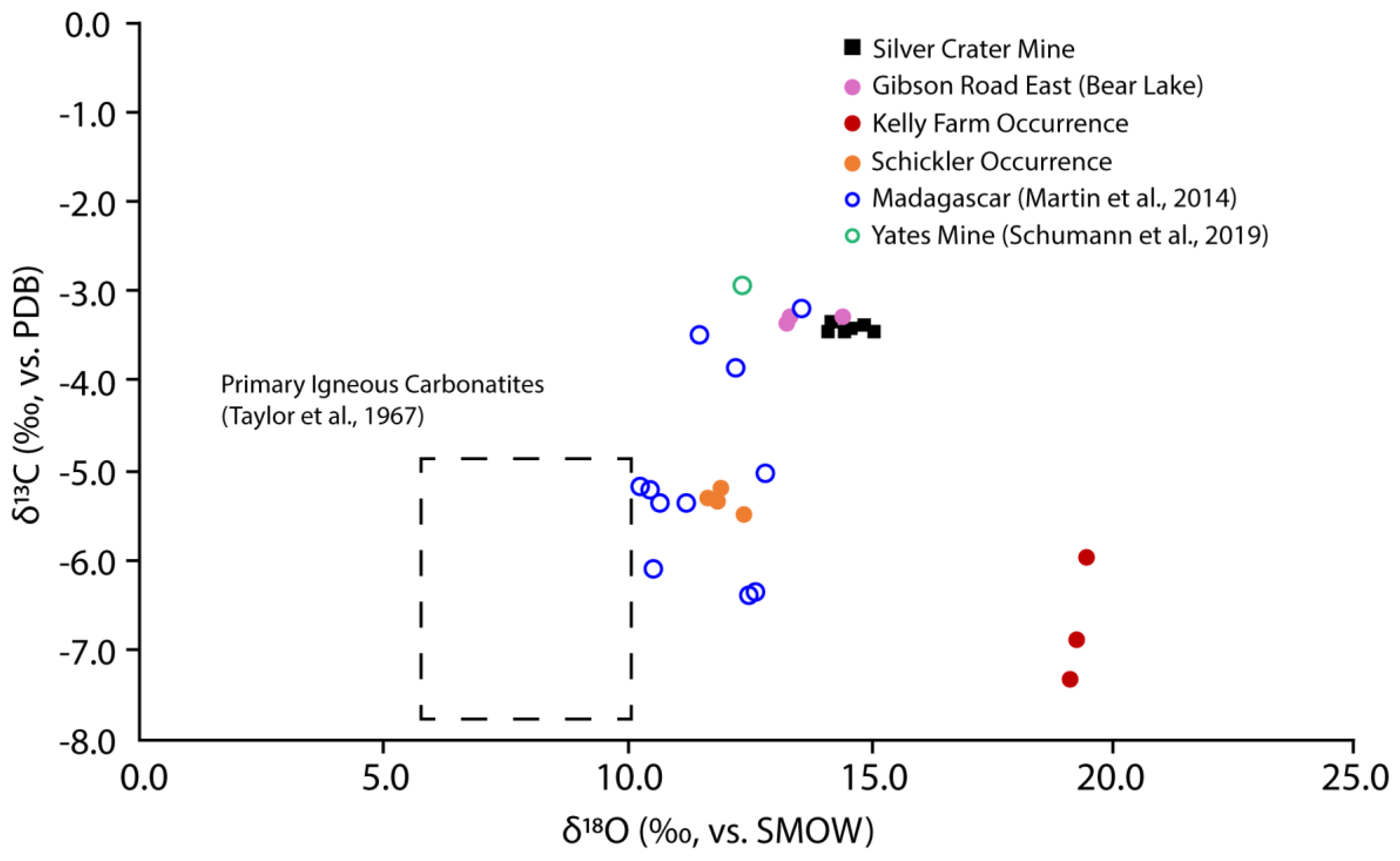
3.5. Stable Isotope Analyses
4. Discussion
4.1. History of the Silver Crater Carbonatite
4.2. Crystallinity of Samples
4.3. Significance of Data
4.4. Future Work
5. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weber, W.J.; Ewing, R.C. The kinetics of alpha-decay-induced amorphization in zircon and apatite containing weapons-grade plutonium or other actinides. J. Nucl. Mater. 1997, 250, 147–155. [Google Scholar] [CrossRef]
- Ewing, R.C.; Wang, L. Phosphates as nuclear waste forms. Rev. Mineral. Geochem. 2002, 48, 673–699. [Google Scholar] [CrossRef]
- Chaumont, J.; Soulet, S.; Krupa, J.C.; Carpena, J. Competition between disorder creation and annealing in fluoroapatite nuclear waste forms. J. Nucl. Mater. 2002, 301, 122–128. [Google Scholar] [CrossRef]
- Oelkers, E.H.; Montel, J.M. Phosphates and nuclear waste storage. Elements 2008, 4, 113–116. [Google Scholar] [CrossRef]
- Bros, R.; Carpena, J.; Sere, V.; Beltritti, A. Occurrence of Pu and fissiogenic REE in hydrothermal apatites from the fossil nuclear reactor 16 at Oklo (Gabon). Radiochim. Acta 1996, 74, 277–282. [Google Scholar] [CrossRef]
- Ewing, R.C.; Haaker, R.F. The metamict state: Implications for radiation damage in crystalline waste forms. Nucl. Chem. Waste Manag. 1980, 1, 51–57. [Google Scholar] [CrossRef]
- Ewing, R.C.; Chakoumakos, B.C.; Lumpkin, G.R.; Murakami, T. The metamict state. MRS Bull. 1987, 12, 58–66. [Google Scholar] [CrossRef]
- Ewing, R.C.; Meldrum, A.; Wang, L.; Wang, S. Radiation-induced amorphization. Rev. Mineral. Geochem. 2000, 39, 319–361. [Google Scholar] [CrossRef]
- Lumpkin, G.R.; Gao, Y.; Gieré, R.; Williams, C.T.; Mariano, A.N.; Geisler, T. The role of Th-U minerals in assessing the performance of nuclear waste forms. Mineral. Mag. 2014, 78, 1071–1095. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Shen, Y.; Zhou, Y.; Nan, S.; Chen, C.H.; Ewing, R.C. In situ TEM observation of alpha-particle induced annealing of radiation damage in Durango apatite. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Johnson, T.A.; Vervoort, J.D.; Ramsey, M.J.; Aleinikoff, J.N.; Southworth, S. Constraints on the timing and duration of orogenic events by combined Lu–Hf and Sm–Nd geochronology: An example from the Grenville orogeny. Earth Planet. Sci. Lett. 2018, 501, 152–164. [Google Scholar] [CrossRef]
- Chiarenzelli, J.; Lupulescu, M.; Robinson, G.; Bailey, D.; Singer, J. Age and origin of silicocarbonate pegmatites of the Adirondack region. Minerals 2019, 9, 508. [Google Scholar] [CrossRef] [Green Version]
- Sabina, A.P. Rocks and Minerals for the Collector: Bancroft—Parry Sound and Southern Ontario; Geological Survey of Canada: Ottawa, ON, Canada, 1986; Volume 39. [Google Scholar]
- Spence, H.S. Phosphate in Canada; Report; Canada Department of Mines, Mines Branch: Ottawa, ON, Canada, 1920; Volume 396. [Google Scholar]
- McDougall, R. Mineral highlights from the Bancroft area, Ontario, Canada. Rocks Miner. 2019, 94, 408–419. [Google Scholar] [CrossRef]
- Joyce, D.K. Calcite vein-dikes of the Grenville geological province, Ontario, Canada. Rocks Miner. 2000, 81, 34–42. [Google Scholar] [CrossRef]
- Satterly, J.; Hewitt, D.F. Some Radioactive Mineral Occurrences in the Bancroft Area. Report; Ontario Department of Mines, Mines Branch: Ottawa, ON, Canada, 1955; p. 2. [Google Scholar]
- Russell, R.; Guggenheim, S. Crystal structures of near end-member phlogopite at high temperatures and heat-treated Fe-rich phlogopite: The influence of the O, OH, F site. Can. Mineral. 1999, 37, 711–720. [Google Scholar]
- Gulbrandsen, R. Carbonate-bearing apatite from Faraday Township, Ontario, Canada. Am. Mineral. 1966, 51, 819–824. [Google Scholar]
- Martin, R.F.; Randrianandraisana, A.; Boulvais, P. Ampandrandava and similar phlogopite deposits in southern Madagascar: Derivation from a silicocarbonatitic melt of crustal origin. J. Afr. Earth Sci. 2014, 94, 111–118. [Google Scholar] [CrossRef]
- Schumann, D.; Martin, R.F.; Fuchs, S.; Fourestier, J. Silicocarbonatitic melt inclusions in fluorapatite from the Yates prospect, Otter Lake, Québec: Evidence of marble anatexis in the central metasedimentary belt of the Grenville Province. Can. Mineral. 2019, 57, 583–604. [Google Scholar] [CrossRef]
- Martin, R.F.; Schumann, D. A Silicocarbonatitic Melt and Spinel-Bearing Dunite of Crustal Origin at the Parker Phlogopite Mine, Notre-Dame-du-Laus, Quebec, Canada. Minerals 2019, 9, 613. [Google Scholar] [CrossRef] [Green Version]
- Hogarth, D.D. A Mineralogical Study of Pyrochlore and Betafite. Ph.D. Thesis, McGill University, Montreal, QC, Canada, 1959. [Google Scholar]
- Josey, S.D. Bedrock Geology of Ontario. 2011. Available online: https://www.mndm.gov.on.ca/en/mines-and-minerals/applications/ogsearth (accessed on 17 February 2020).
- Hogarth, D.D. Classification and nomenclature of the pyrochlore group. Am. Mineral. 1977, 62, 403–410. [Google Scholar]
- Williams, T.; Bailey, F. The Photon Detector and the Variable-Pressure Scanning Electron Microscope an Alternative Method for Cathodoluminescence Imaging of Geologic Materials. Microsc. Microanal. 2006, 12, 1530–1531. [Google Scholar] [CrossRef] [Green Version]
- Jochum, K.P.; Weis, U.; Stoll, B.; Kuzmin, D.; Yang, Q.; Raczek, I.; Jacob, D.E.; Stracke, A.; Birbaum, K.; Frick, D.A.; et al. Determination of Reference Values for NIST SRM 610–617 Glasses Following ISO Guidelines. Geostand. Geoanal. Res. 2011, 35, 397–429. [Google Scholar] [CrossRef]
- Hellstrom, J.; Paton, C.; Woodhead, J.; Hergt, J. Iolite: Software for spatially resolved LA-(quad and MC) ICPMS analysis. Mineral. Assoc. Can. Short Course Ser. 2008, 40, 343–348. [Google Scholar]
- McDonough, W.F.; Sun, S.-S. The composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Lumpkin, G.R.; Ewing, R.C. Geochemical alteration of pyrochlore group minerals: Betafite subgroup. Am. Mineral. 1996, 81, 1237–1248. [Google Scholar] [CrossRef]
- Taylor, H.P., Jr.; Frechen, J.; Degens, E.T. Oxygen and carbon isotope studies of carbonatites from the Laacher See District, West Germany and the Alnö District, Sweden. Geochim. Cosmochim. Acta 1967, 31, 407–430. [Google Scholar] [CrossRef]
- Bermanec, V.; Tomašić, N.; Kniewald, G.; Back, M.E.; Zagler, G. Nioboaeschynite-(Y), A new member of the aeschynite group from Bear Lake Diggings, Haliburton County, Ontario, Canada. Can. Mineral. 2008, 46, 395–402. [Google Scholar] [CrossRef] [Green Version]
- Hayase, I. The development of pleochroic haloes and the alpha radioactivity of the nucleus minerals. Mineral. J. 1955, 1, 213–223. [Google Scholar] [CrossRef] [Green Version]
- Owen, M.R. Radiation-damage halos in quartz. Geology 1988, 16, 529–532. [Google Scholar] [CrossRef]
- Wisniewski, W.; Rüssel, C. An experimental viewpoint on the information depth of EBSD. Scanning 2016, 38, 164–171. [Google Scholar] [CrossRef]
- Wisniewski, W.; Saager, S.; Böbenroth, A.; Rüssel, C. Experimental evidence concerning the significant information depth of electron backscatter diffraction (EBSD). Ultramicroscopy 2017, 173, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.M.; Cameron, M.; Weber, W.J.; Crowley, K.D.; Ewing, R.C. In situ TEM observation of radiation induced amorphization of crystals with apatite structure. In Hydroxyapatite and Related Materials; Brown, P.W., Constanz, B., Eds.; CRC Press: Boca Raton, FL, USA, 1994; pp. 243–249. [Google Scholar]
- Meldrum, A.; Boatner, L.A.; Zinkle, S.J.; Wang, S.X.; Wang, L.M.; Ewing, R.C. Effects of dose rate and temperature on the crystalline-to-metamict transformation in the ABO orthosilicates. Can. Mineral. 1999, 37, 207–221. [Google Scholar]
- Bae, I.T.; Zhang, Y.; Weber, W.J.; Higuchi, M.; Gianuzzi, L.A. Electron-beam induced recrystallization in amorphous apatite. Appl. Phys. Lett. 2007, 90, 021912. [Google Scholar] [CrossRef]
- Lupulescu, M.V.; Chiarenzelli, J.R.; Pullen, A.T.; Price, J.D. Using pegmatite geochronology to constrain temporal events in the Adirondack Mountains. Geosphere 2011, 7, 23–39. [Google Scholar] [CrossRef] [Green Version]
- Horie, K.; Hidaka, H.; Gauthier-Lafaye, F. Isotopic evidence for trapped fissiogenic REE and nucleogenic Pu in apatite and Pb evolution at the Oklo natural reactor. Geochim. Cosmochim. Acta 2004, 68, 115–125. [Google Scholar] [CrossRef]
- Gieré, R.; Guggenheim, R.; Düggelin, M.; Mathys, D.; Williams, C.T.; Lumpkin, G.R.; Smith, K.L.; Blackford, M.G.; Hart, K.P.; McGlinn, P. Retention of actinides during alteration of aperiodic zirconolite. In Proceedings of the 13th Conference on Electron Microscopy, Paris, France, 17–22 July 1994; pp. 1269–1270. [Google Scholar]
- Rakovan, J.; Pasteris, J.D. A Technological Gem: Materials, Medical, and Environmental Mineralogy of Apatite. In Harlov, D. and Rakovan, J. (Guest Editors) Apatite—A mineral for All Seasons. Elements 2015, 11, 195–200. [Google Scholar] [CrossRef]
- Luo, Y.; Rakovan, J.; Hughes, J.; Pan, Y. Site preference of U and Th in Cl, F, Sr apatites. Am. Mineral. 2009, 94, 345–351. [Google Scholar] [CrossRef]
- Luo, Y.; Rakovan, J.; Elzinga, E.; Pan, Y.; Lupulescu, M.V.; Hughes, J. Crystal chemistry of Th in fluorapatite. Am. Mineral. 2011, 96, 23–33. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emproto, C.; Alvarez, A.; Anderkin, C.; Rakovan, J. The Crystallinity of Apatite in Contact with Metamict Pyrochlore from the Silver Crater Mine, ON, Canada. Minerals 2020, 10, 244. https://doi.org/10.3390/min10030244
Emproto C, Alvarez A, Anderkin C, Rakovan J. The Crystallinity of Apatite in Contact with Metamict Pyrochlore from the Silver Crater Mine, ON, Canada. Minerals. 2020; 10(3):244. https://doi.org/10.3390/min10030244
Chicago/Turabian StyleEmproto, Christopher, Austin Alvarez, Christian Anderkin, and John Rakovan. 2020. "The Crystallinity of Apatite in Contact with Metamict Pyrochlore from the Silver Crater Mine, ON, Canada" Minerals 10, no. 3: 244. https://doi.org/10.3390/min10030244
APA StyleEmproto, C., Alvarez, A., Anderkin, C., & Rakovan, J. (2020). The Crystallinity of Apatite in Contact with Metamict Pyrochlore from the Silver Crater Mine, ON, Canada. Minerals, 10(3), 244. https://doi.org/10.3390/min10030244



