Reduction and Dissolution Behaviour of Manganese Slag in the Ferromanganese Process
Abstract
1. Introduction
ΔG° = −198.79 − 0.0175T (kJ).
ΔG° = −184.15 + 0.0094T (kJ).
ΔG° = −56.60 − 0.0327T (kJ),
ΔG° = −26.82 + 0.0469T (kJ).
ΔG° = 120.65 − 0.1728T (kJ).
ΔG° = 124.91 − 0.1409T (kJ).
ΔG° = 289.54 − 0.1934 T (kJ)
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedure
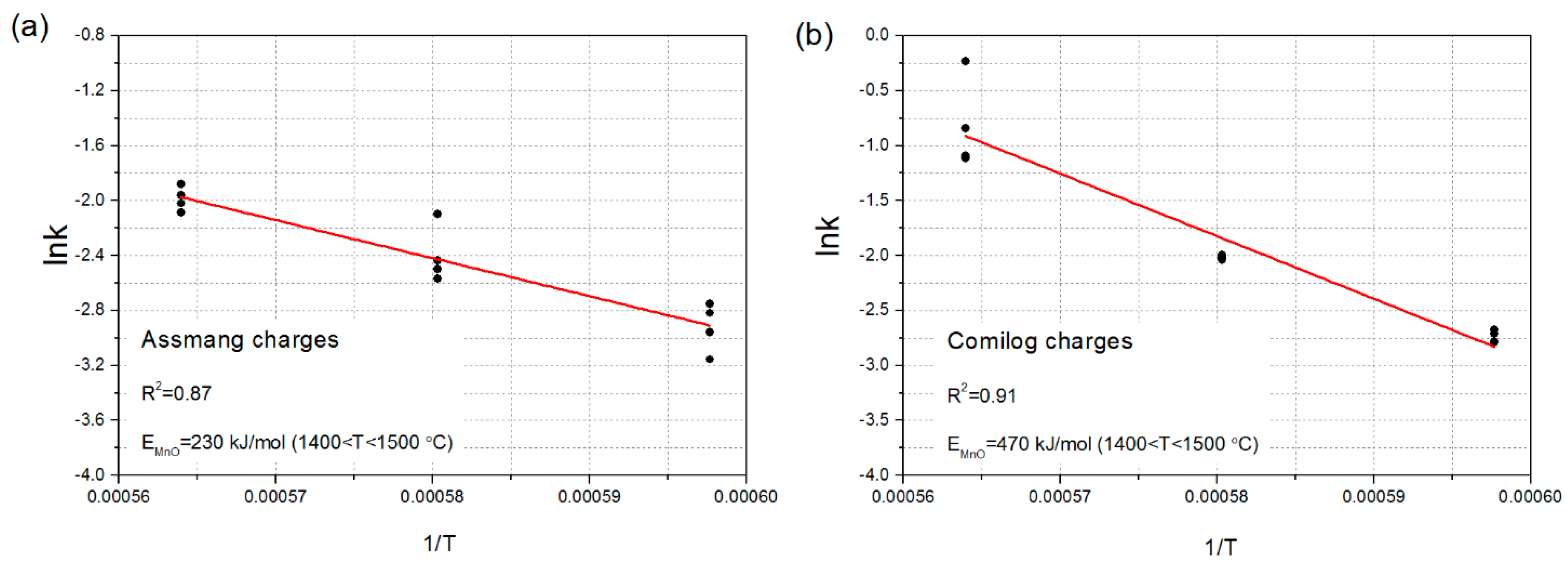
2.3. Kinetic Calculations
3. Results and Discussion
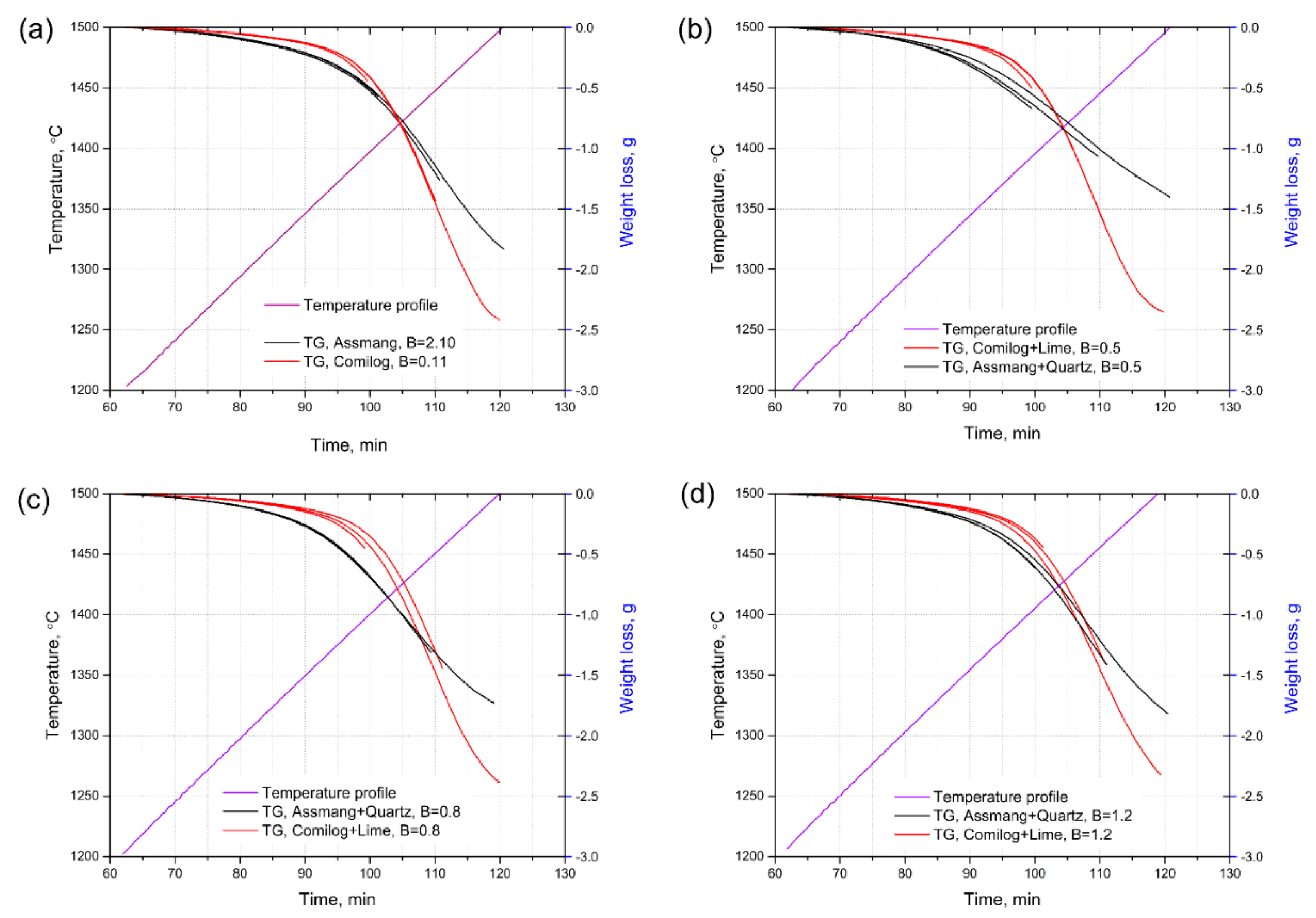
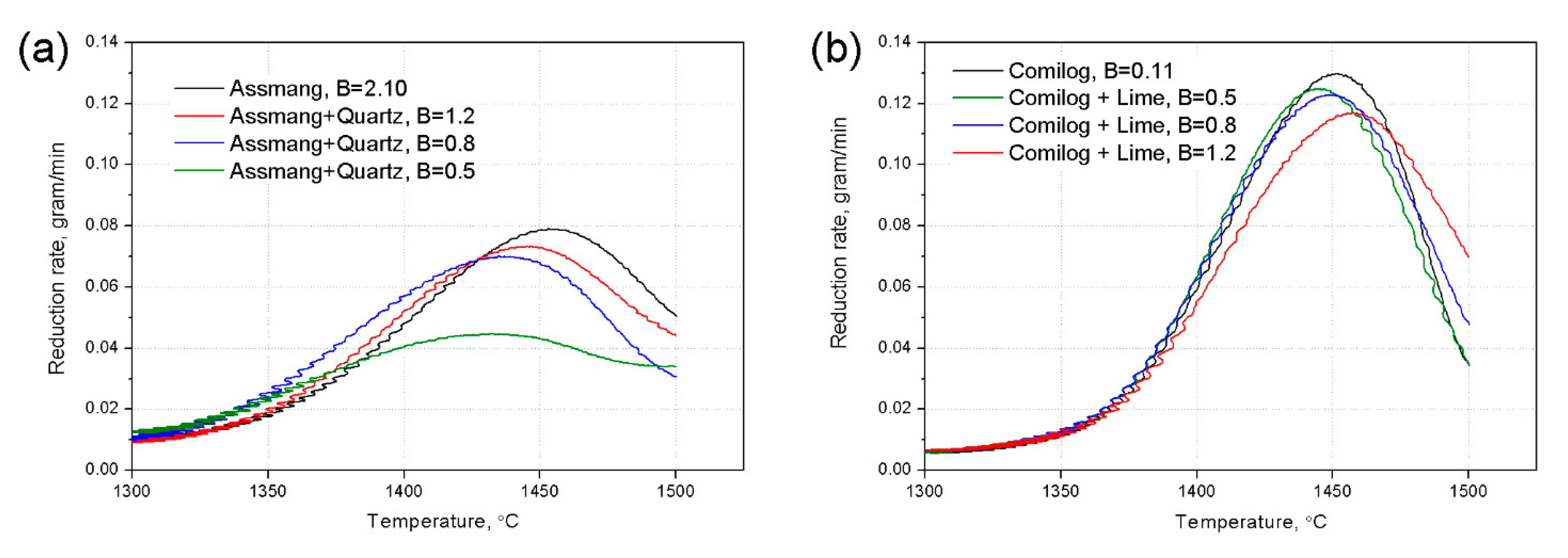
3.1. Reduction Behaviour of Assmang and Comilog Ore
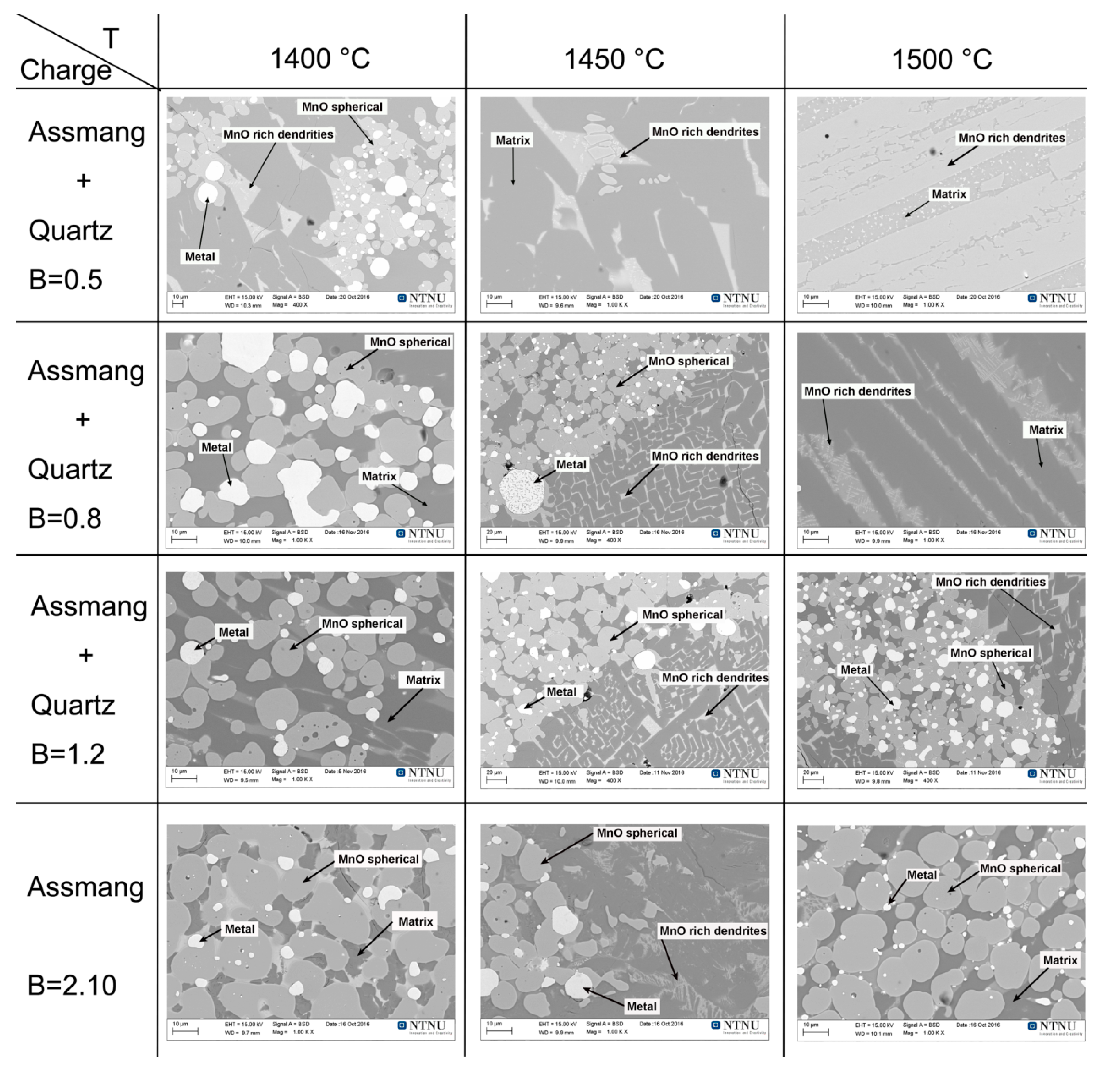
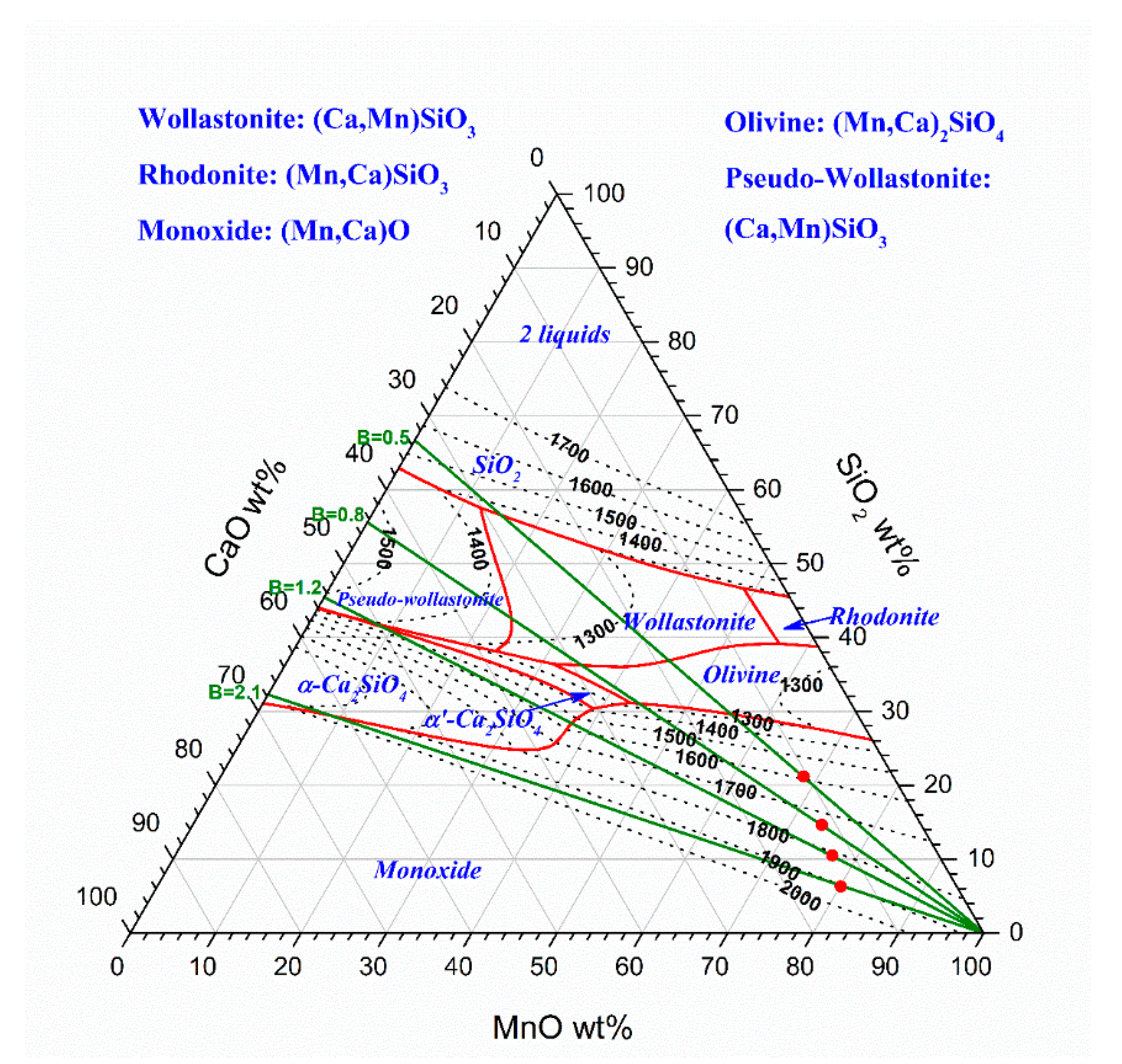
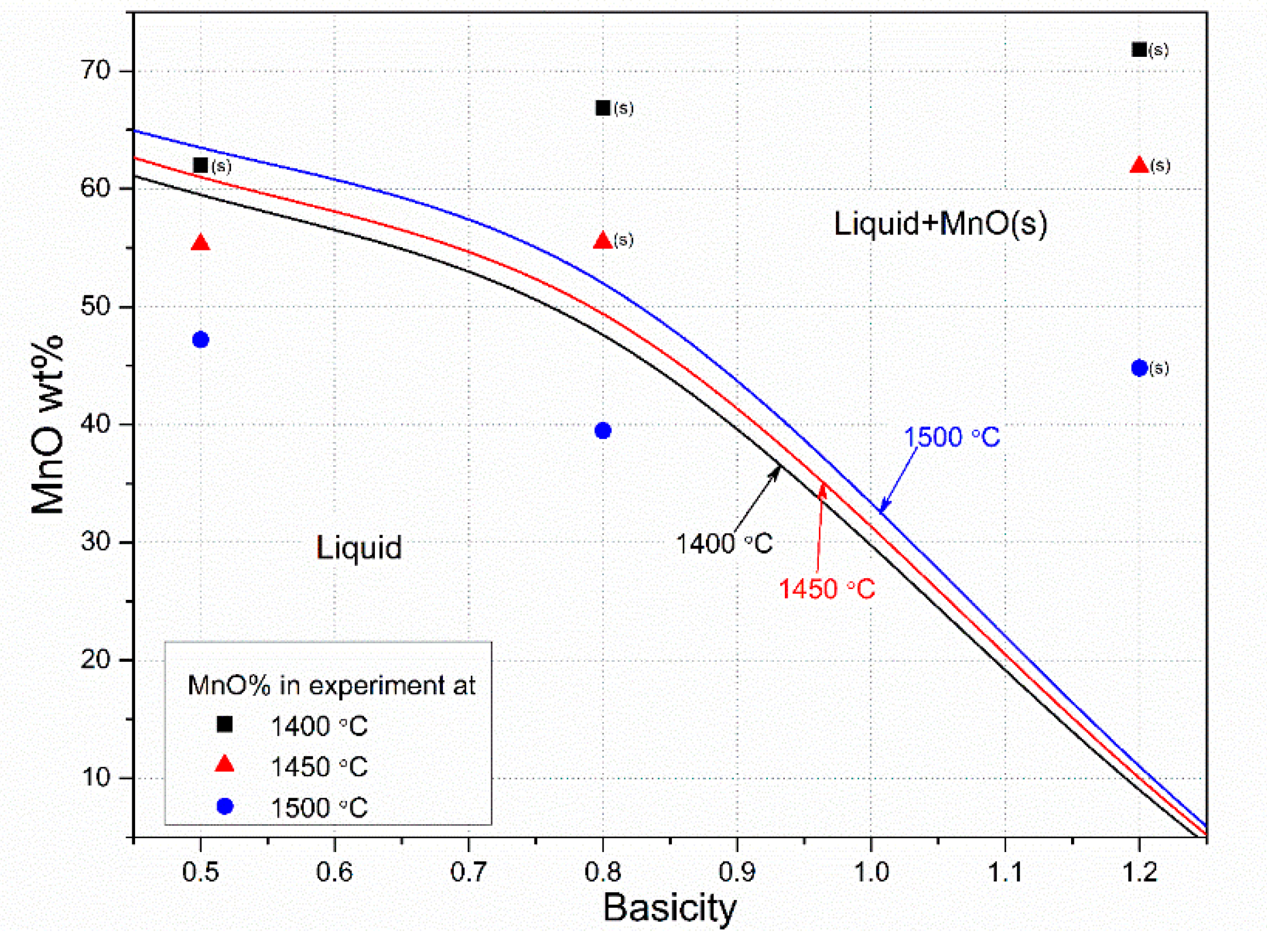
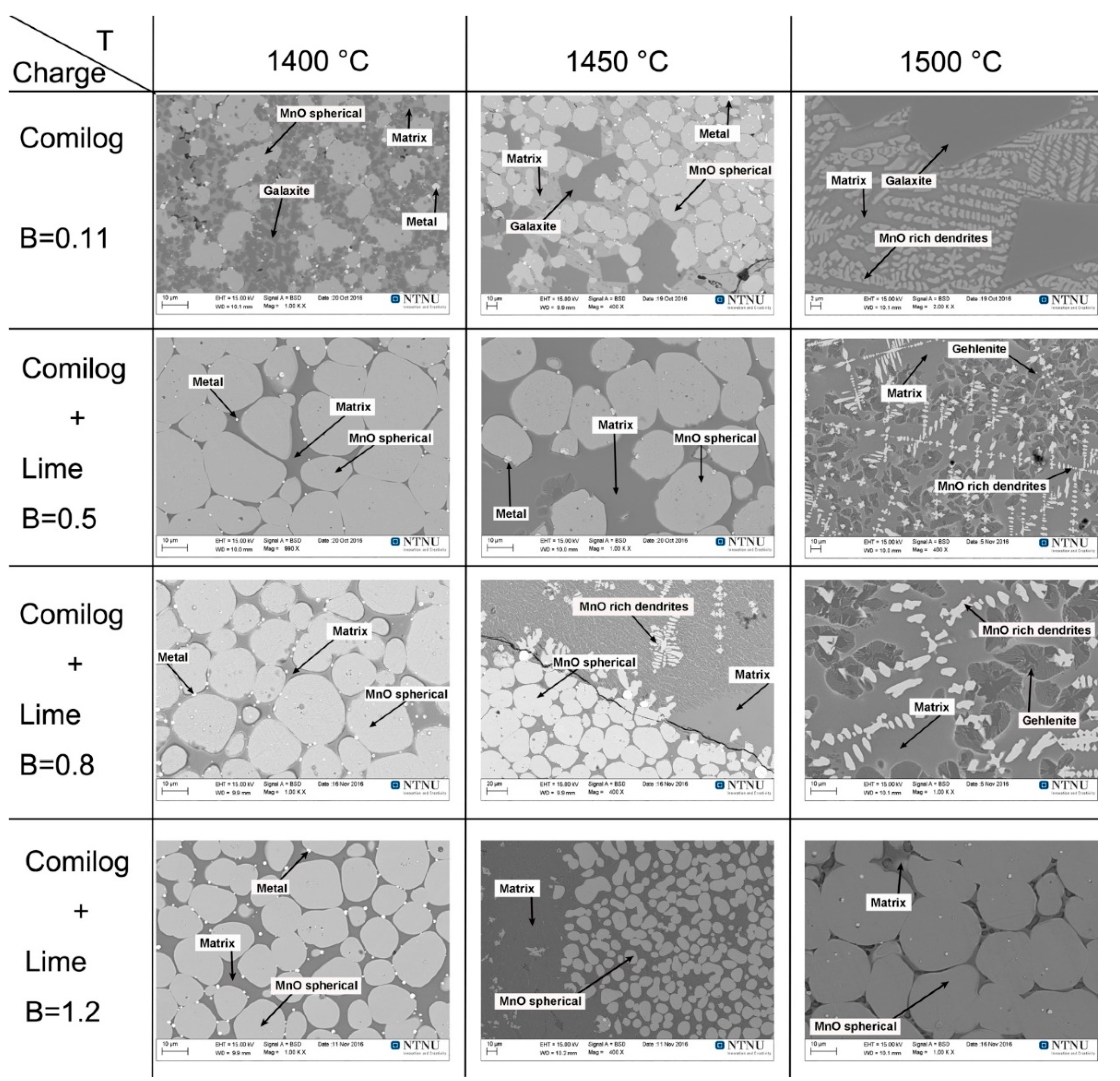
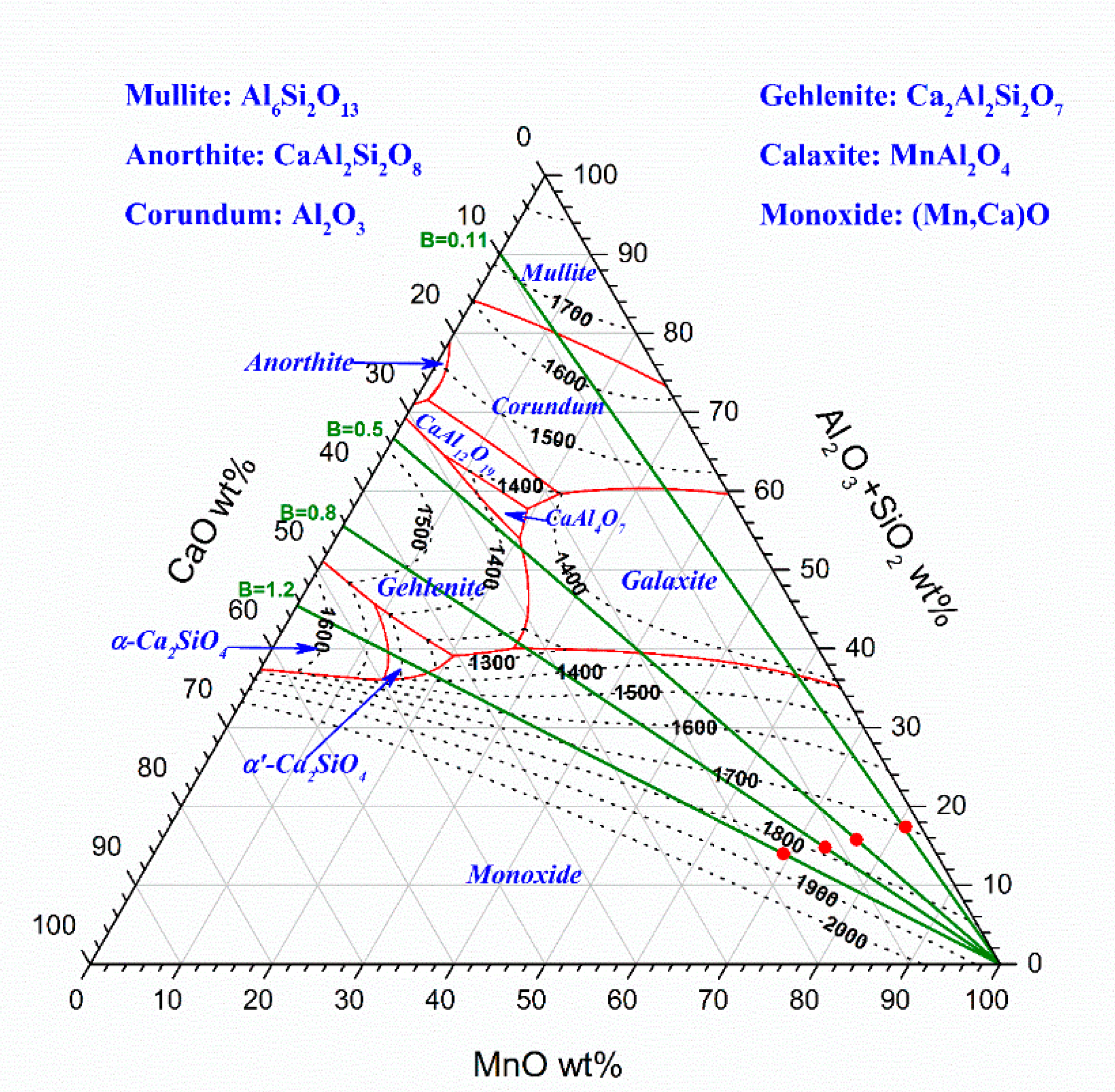
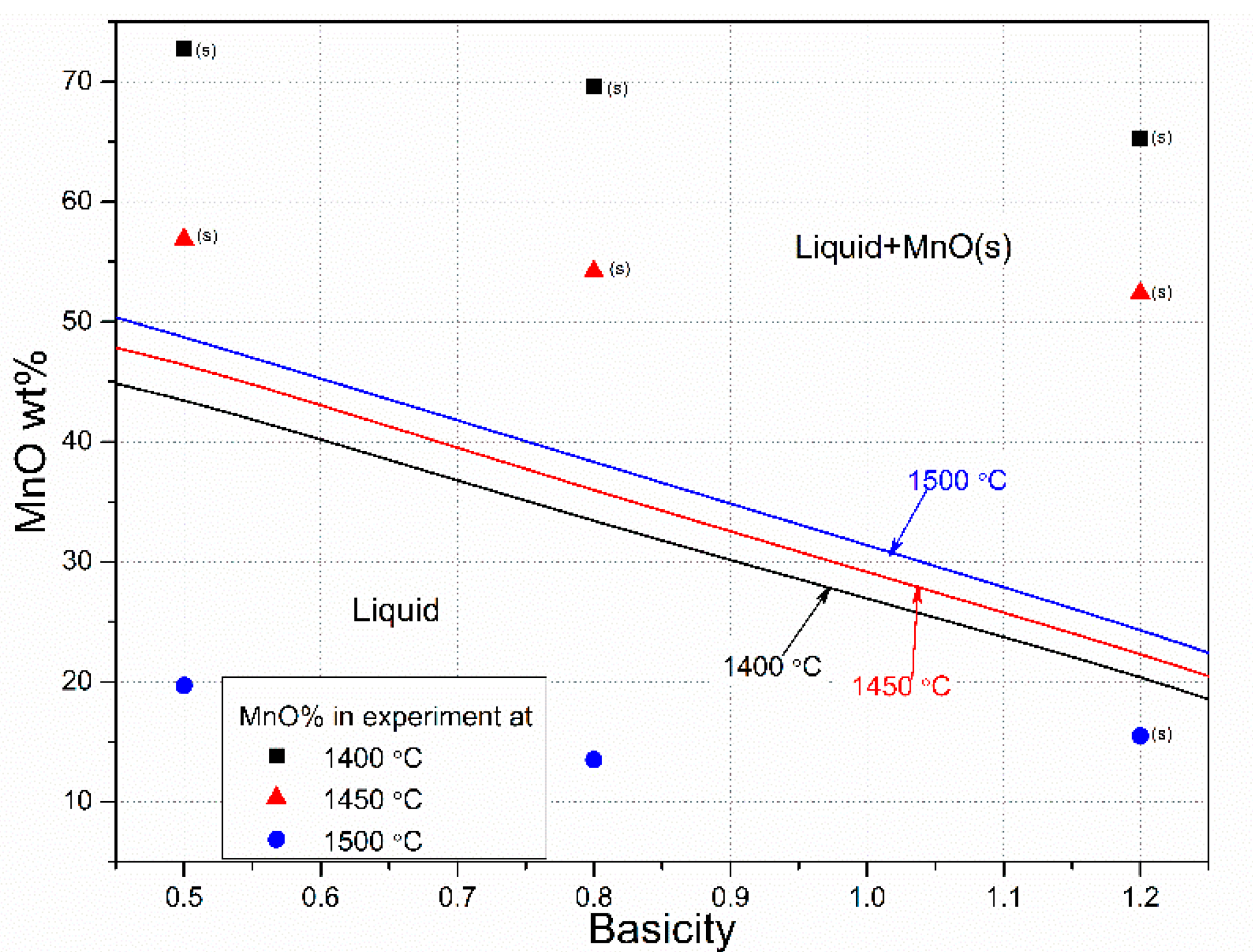
3.2. Slag Melting and Phase Development
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yastreboff, M.; Ostrovski, O.; Ganguly, S. Effect of Gas Composition on the Carbothermic Reduction of Manganese Oxide. ISIJ Int. 2003, 43, 161–165. [Google Scholar] [CrossRef]
- Olsen, S.E.; Tangstad, M.; Lindstad, T. Production of Manganese Ferroalloys; Tapir Academic Press: Trondheim, Norway, 2007. [Google Scholar]
- Hooper, R.T. Production of ferromanganese. J. Met. 1968, 20, 88–92. [Google Scholar] [CrossRef]
- Stansbie, J.H. Iron and Steel; Read Books: Redditch, UK, 2007. [Google Scholar]
- Tangstad, M. The High Carbon Ferromanganese Process–Coke Bed Relations. Ph.D. Thesis, Norwegian Institute of Technology, Trondheim, Norway, 1996. [Google Scholar]
- HSC Chemistry 7. Chemical Reaction and Equilibrium Software with Extensive Thermochemical Database; Outokumpu: Helsinki, Finland, 2009. [Google Scholar]
- Xu, K.; Jiang, G.; Ding, W.; Gu, L.; Guo, S.; Zhao, B. The Kinetics of Reduction of MnO in Molten Slag with Carbon Saturated Liquid Iron. ISIJ Int. 1993, 33, 104–108. [Google Scholar] [CrossRef]
- Olsø, V.; Tangstad, M.; Olsen, S.E. Reduction kinetics of mno-saturated slags. In Proceedings of the 8th International Congress on Ferroalloys (INFACON VIII), Beijing, China, 7–10 June 1998; pp. 259–283. [Google Scholar]
- Skjervheim, T.A. Kinetics and Mechanisms for Transfer of Manganese and Silicon from Melten Oxide to Liquid Manganese Metal. Ph.D. Thesis, Norwegian Institute of Technology, Trondheim, Norway, 1994. [Google Scholar]
- Safarian, J.; Tranell, G.; Kolbeinsen, L.; Tangstad, M.; Gaal, S.; Kaczorowski, J. Reduction Kinetics of MnO from High-Carbon Ferromanganese Slags by Carbonaceous Materials in Ar and CO Atmospheres. Metall. Mater. Trans. B 2008, 39, 702–712. [Google Scholar] [CrossRef]
- Daines, W.L.; Pehlke, R.D. Kinetics of manganese oxide reduction from basic slags by carbon dissolved in liquid iron. Metall. Trans. 1971, 2, 1203–1211. [Google Scholar] [CrossRef]
- Kim, P.P.; Tangstad, M. Kinetic Investigations of SiMn Slags from Different Mn Sources. Metall. Mater. Trans. B 2018, 49, 1185–1196. [Google Scholar] [CrossRef]
- Coetsee, T.; Reinke, C.; Nell, J.; Pistorius, P.C. Reduction Mechanisms in Manganese Ore Reduction. Metall. Mater. Trans. B 2015, 46, 2534–2552. [Google Scholar] [CrossRef]
- Coetsee, T.; Nell, J.; Pistorius, P.C. Phase Characterization of High Basicity Manganese Slags. Metall. Mater. Trans. B 2017, 48, 1463–1485. [Google Scholar] [CrossRef]
- Coetsee, T. The role of manganese ore reduction morphology development in setting reduction mechanisms. Miner. Eng. 2019, 137, 217–231. [Google Scholar] [CrossRef]
- Ostrovski, O.; Olsen, S.E.; Tangstad, M.; Yastreboff, M. Kinetic Modelling of MnO Reduction from Manganese Ore. Can. Metall. Quarte. 2002, 3, 309–318. [Google Scholar] [CrossRef]
- Olsen, H. A Theoretical Study on the Reaction Rates in the SiMn Production Process. Master’s Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2016. [Google Scholar]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Gheribi, A.E.; Hack, K.; Jung, I.-H.; Kang, Y.-B.; Melançon, J.; et al. Reprint of: FactSage thermochemical software and databases, 2010–2016. Calphad J. 2016, 55, 1–19. [Google Scholar] [CrossRef]
- Fenstad, J. Liquidus Relations and Thermochemistry within the System Fe-Mn-C-O. Ph.D. Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2000. [Google Scholar]
- Safarian, J.; Kolbeinsen, L. Microscopic Study of Carbon Surfaces Interacting with High Carbon Ferromanganese Slag. Metall. Mater. Trans. B 2015, 46, 125–134. [Google Scholar] [CrossRef]
- Li, X.; Tangstad, M. The Influence of Sulfur Content on the Carbothermal Reduction of SiMn Slag. Metall. Mater. Trans. B 2019, 50, 136–149. [Google Scholar] [CrossRef]
- Tang, K.; Tangstad, M. Modeling viscosities of ferromanganese slags. In Proceedings of the 11th International Congress on Ferroalloys (INFACON XI), New Delhi, India, 18–21 February 2007; pp. 344–357. [Google Scholar]
- Shimoo, T.; Ando, S.; Kimura, H. Rates and MnO reduction from CaO-Al2O3 slags by solid cabon. J. Japan Inst. Matals. 1984, 48, 285–292. [Google Scholar] [CrossRef]
- Shimoo, T.; Ando, S.; Kimura, H. Rates and MnO reduction from silicate slag with solid carbon. J. Japan Inst. Matals. 1984, 48, 922–929. [Google Scholar] [CrossRef][Green Version]
- Safarian, J. Kinetics and Mechanism of Reduction of MnO-Containing Silicate Slags by Selected Forms of Carbonaceous Materials. Ph.D. Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2000. [Google Scholar]


| Components | Comilog | Assmang | Lime | Quartz | Polish Coke |
|---|---|---|---|---|---|
| MnO | 3.63 | 31.4 | 0.14 | 0.04 | |
| MnO2 | 74.0 | 34.8 | |||
| Fe2O3 | 3.27 | 15.8 | 0.86 | ||
| SiO2 | 4.71 | 3.75 | 1.00 | 93.9 | 5.60 |
| P | 0.11 | 0.032 | 0.00 | 0.05 | |
| Fix C | 87.7 | ||||
| H2O | 4.60 | 0.8 | |||
| CO2 | 0.16 | 3.7 | 47.50 | ||
| Al2O3 | 6.97 | 0.29 | 0.27 | 1.19 | 2.79 |
| MgO | 0.03 | 1.31 | 1.00 | 0.05 | 0.22 |
| CaO | 0.06 | 6.4 | 54.00 | 0.09 | 0.42 |
| BaO | 0.2 | 0.75 | 0.03 | ||
| K2O | 1 | 0.01 | 0.10 | 0.18 | |
| S | 0.009 | 0.21 | 0.011 | 0.44 | |
| Sum, dry basis | 98.8 | 99.3 | 103.9 | 95.4 | 98.3 |
| Ore | Temperature °C | Assmang g | Comilog g | Quartz g | Lime g | Coke g | B * |
|---|---|---|---|---|---|---|---|
| Assmang + Quartz | 1400 | 10.0 | 1.36 | 2.5 | 0.50 | ||
| 1450 | 10.0 | 1.36 | 2.5 | 0.50 | |||
| 1500 | 10.0 | 1.36 | 2.5 | 0.50 | |||
| Assmang + Quartz | 1400 | 10.0 | 0.69 | 2.5 | 0.80 | ||
| 1450 | 10.0 | 0.69 | 2.5 | 0.80 | |||
| 1500 | 10.0 | 0.69 | 2.5 | 0.80 | |||
| Assmang + Quartz | 1400 | 10.0 | 0.32 | 2.5 | 1.20 | ||
| 1450 | 10.0 | 0.32 | 2.5 | 1.20 | |||
| 1500 | 10.0 | 0.32 | 2.5 | 1.20 | |||
| Assmang | 1400 | 10.0 | 2.5 | 2.1 | |||
| 1450 | 10.0 | 2.5 | 2.1 | ||||
| 1500 | 10.0 | 2.5 | 2.1 | ||||
| Comilog | 1400 | 10.0 | 2.5 | 0.11 | |||
| 1450 | 10.0 | 2.5 | 0.11 | ||||
| 1500 | 10.0 | 2.5 | 0.11 | ||||
| Comilog + Lime | 1400 | 10.0 | 0.84 | 2.5 | 0.50 | ||
| 1450 | 10.0 | 0.84 | 2.5 | 0.50 | |||
| 1500 | 10.0 | 0.84 | 2.5 | 0.50 | |||
| Comilog + Lime | 1400 | 10.0 | 1.44 | 2.5 | 0.80 | ||
| 1450 | 10.0 | 1.44 | 2.5 | 0.80 | |||
| 1500 | 10.0 | 1.44 | 2.5 | 0.80 | |||
| Comilog + Lime | 1400 | 10.0 | 2.38 | 2.5 | 1.20 | ||
| 1450 | 10.0 | 2.38 | 2.5 | 1.20 | |||
| 1500 | 10.0 | 2.38 | 2.5 | 1.20 |
| Ore | T °C | MnO wt% | SiO2 wt% | Cao wt% | MgO wt% | Al2O3 wt% | BaO wt% | K2O wt% | Sum wt% |
|---|---|---|---|---|---|---|---|---|---|
| Assmang B = 0.5 | 1200 | 68.3 | 20.4 | 7.4 | 1.6 | 1.3 | 0.9 | 0.1 | 100.0 |
| 1300 | 67.4 | 21.0 | 7.7 | 1.6 | 1.4 | 0.9 | 0.1 | 100.0 | |
| 1400 | 62.0 | 24.5 | 8.9 | 1.9 | 1.6 | 1.0 | 0.1 | 100.0 | |
| 1450 | 55.3 | 28.8 | 10.5 | 2.2 | 1.9 | 1.2 | 0.1 | 100.0 | |
| 1500 | 47.2 | 34.0 | 12.4 | 2.6 | 2.2 | 1.4 | 0.1 | 100.0 | |
| Assmang + Quartz B = 0.8 | 1200 | 73.7 | 14.3 | 8.0 | 1.7 | 1.3 | 0.9 | 0.1 | 100.0 |
| 1300 | 72.8 | 14.8 | 8.3 | 1.7 | 1.4 | 1.0 | 0.1 | 100.0 | |
| 1400 | 66.9 | 18.0 | 10.1 | 2.1 | 1.7 | 1.2 | 0.1 | 100.0 | |
| 1450 | 55.5 | 24.1 | 13.5 | 2.8 | 2.2 | 1.6 | 0.1 | 100.0 | |
| 1500 | 39.5 | 32.9 | 18.4 | 3.9 | 3.0 | 2.1 | 0.2 | 100.0 | |
| Assmang + Quartz B = 1.2 | 1200 | 77.0 | 10.5 | 8.4 | 1.8 | 1.3 | 1.0 | 0.1 | 100.0 |
| 1300 | 76.3 | 10.8 | 8.6 | 1.8 | 1.4 | 1.0 | 0.1 | 100.0 | |
| 1400 | 71.8 | 12.8 | 10.3 | 2.2 | 1.6 | 1.2 | 0.1 | 100.0 | |
| 1450 | 61.9 | 17.4 | 13.9 | 2.9 | 2.2 | 1.6 | 0.1 | 100.0 | |
| 1500 | 44.1 | 25.5 | 20.4 | 4.3 | 3.2 | 2.4 | 0.2 | 100.0 | |
| Assmang + Quartz B = 2.1 | 1200 | 80.1 | 6.9 | 8.7 | 1.8 | 1.3 | 1 | 0.1 | 100 |
| 1300 | 79.5 | 7.1 | 9 | 1.9 | 1.4 | 1 | 0.1 | 100 | |
| 1400 | 75.8 | 8.4 | 10.6 | 2.2 | 1.6 | 1.2 | 0.1 | 100 | |
| 1450 | 66.6 | 11.6 | 14.7 | 3.1 | 2.2 | 1.7 | 0.1 | 100 | |
| 1500 | 44.9 | 19.1 | 24.2 | 5.1 | 3.7 | 2.8 | 0.2 | 100 | |
| Comilog B = 0.11 | 1200 | 80.9 | 7.7 | 0.2 | 0.1 | 9.7 | 0.0 | 1.3 | 100.0 |
| 1300 | 80.6 | 7.9 | 0.2 | 0.1 | 9.9 | 0.0 | 1.3 | 100.0 | |
| 1400 | 78.1 | 8.9 | 0.2 | 0.1 | 11.1 | 0.0 | 1.5 | 100.0 | |
| 1450 | 64.7 | 14.3 | 0.4 | 0.2 | 17.9 | 0.1 | 2.4 | 100.0 | |
| 1500 | 21.2 | 31.9 | 0.9 | 0.4 | 40.0 | 0.1 | 5.5 | 100.0 | |
| Comilog + Lime B = 0.5 | 1200 | 76.3 | 7.4 | 5.6 | 0.2 | 9.2 | 0.0 | 1.3 | 100.0 |
| 1300 | 75.9 | 7.5 | 5.7 | 0.2 | 9.3 | 0.0 | 1.3 | 100.0 | |
| 1400 | 72.8 | 8.5 | 6.5 | 0.2 | 10.6 | 0.0 | 1.4 | 100.0 | |
| 1450 | 56.9 | 13.4 | 10.2 | 0.4 | 16.7 | 0.1 | 2.3 | 100.0 | |
| 1500 | 19.7 | 25.1 | 19.0 | 0.7 | 31.1 | 0.1 | 4.3 | 100.0 | |
| Comilog + Lime B = 0.8 | 1200 | 73.4 | 7.2 | 9.1 | 0.3 | 8.8 | 0.0 | 1.2 | 100.0 |
| 1300 | 73.0 | 7.3 | 9.3 | 0.3 | 9.0 | 0.0 | 1.2 | 100.0 | |
| 1400 | 69.6 | 8.2 | 10.4 | 0.3 | 10.1 | 0.0 | 1.4 | 100.0 | |
| 1450 | 54.2 | 12.3 | 15.7 | 0.5 | 15.2 | 0.1 | 2.1 | 100.0 | |
| 1500 | 13.5 | 23.3 | 29.6 | 0.9 | 28.7 | 0.1 | 3.9 | 100.0 | |
| Comilog + Lime B = 1.2 | 1200 | 69.2 | 6.9 | 14.1 | 0.3 | 8.4 | 0.0 | 1.2 | 100.0 |
| 1300 | 68.7 | 7.0 | 14.3 | 0.4 | 8.5 | 0.0 | 1.2 | 100.0 | |
| 1400 | 65.3 | 7.7 | 15.8 | 0.4 | 9.4 | 0.0 | 1.3 | 100.0 | |
| 1450 | 52.4 | 10.6 | 21.7 | 0.5 | 12.9 | 0.0 | 1.8 | 100.0 | |
| 1500 | 15.5 | 18.8 | 38.6 | 1.0 | 22.9 | 0.1 | 3.2 | 100.0 |
| Ore | T °C | Reduction Rate g/min | aMnO | Metal Mole Composition | aMn | KT | Driving Force | Lnk | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Mn | Fe | C | ||||||||
| Assmang B = 0.5 | 1400 | 0.040 | 0.96 | 0.39 | 0.38 | 0.24 | 0.16 | 22.3 | 0.95 | −3.16 |
| 1450 | 0.043 | 0.36 * | 0.48 | 0.27 | 0.25 | 0.18 | 37.7 | 0.35 | −2.10 | |
| 1500 | 0.034 | 0.23 * | 0.53 | 0.21 | 0.26 | 0.20 | 61.8 | 0.23 | −1.88 | |
| Assmang + Quartz B = 0.8 | 1400 | 0.057 | 0.90 | 0.41 | 0.35 | 0.24 | 0.16 | 22.3 | 0.90 | −2.75 |
| 1450 | 0.068 | 0.89 | 0.52 | 0.22 | 0.25 | 0.20 | 37.7 | 0.89 | −2.57 | |
| 1500 | 0.031 | 0.25 * | 0.57 | 0.17 | 0.26 | 0.22 | 61.8 | 0.25 | −2.09 | |
| Assmang + Quartz B = 1.2 | 1400 | 0.052 | 0.88 | 0.39 | 0.38 | 0.24 | 0.15 | 22.3 | 0.88 | −2.82 |
| 1450 | 0.073 | 0.89 | 0.51 | 0.23 | 0.25 | 0.20 | 37.7 | 0.89 | −2.50 | |
| 1500 | 0.044 | 0.84 | 0.56 | 0.17 | 0.26 | 0.22 | 61.8 | 0.83 | −2.93 | |
| Assmang + Quartz B = 2.1 | 1400 | 0.048 | 0.94 | 0.38 | 0.39 | 0.23 | 0.15 | 22.3 | 0.93 | −2.96 |
| 1450 | 0.079 | 0.91 | 0.51 | 0.24 | 0.25 | 0.2 | 37.7 | 0.9 | −2.44 | |
| 1500 | 0.05 | 0.81 | 0.57 | 0.17 | 0.26 | 0.22 | 61.8 | 0.8 | −2.77 | |
| Comilog B = 0.11 | 1400 | 0.060 | 0.98 | 0.58 | 0.16 | 0.26 | 0.25 | 22.3 | 0.97 | −2.79 |
| 1450 | 0.130 | 0.98 | 0.68 | 0.05 | 0.27 | 0.34 | 37.7 | 0.97 | −2.02 | |
| 1500 | 0.034 | 0.05 * | 0.69 | 0.03 | 0.28 | 0.35 | 61.8 | 0.04 | −0.23 | |
| Comilog + Lime B = 0.5 | 1400 | 0.064 | 0.98 | 0.59 | 0.16 | 0.26 | 0.25 | 22.3 | 0.97 | −2.72 |
| 1450 | 0.123 | 0.96 | 0.68 | 0.05 | 0.27 | 0.34 | 37.7 | 0.95 | −2.04 | |
| 1500 | 0.035 | 0.11 * | 0.69 | 0.03 | 0.28 | 0.35 | 61.8 | 0.11 | −1.11 | |
| Comilog + Lime B = 0.8 | 1400 | 0.063 | 0.92 | 0.59 | 0.16 | 0.26 | 0.25 | 22.3 | 0.91 | −2.68 |
| 1450 | 0.123 | 0.91 | 0.68 | 0.05 | 0.27 | 0.34 | 37.7 | 0.90 | −1.99 | |
| 1500 | 0.048 | 0.12 * | 0.69 | 0.03 | 0.28 | 0.35 | 61.8 | 0.11 | −0.84 | |
| Comilog + Lime B = 1.2 | 1400 | 0.056 | 0.92 | 0.58 | 0.16 | 0.26 | 0.25 | 22.3 | 0.91 | −2.79 |
| 1450 | 0.115 | 0.89 | 0.67 | 0.06 | 0.27 | 0.33 | 37.7 | 0.88 | −2.03 | |
| 1500 | 0.070 | 0.80 | 0.69 | 0.03 | 0.28 | 0.35 | 61.8 | 0.79 | −2.43 | |
| Reference | Initial Metal | Initial MnO Content wt% | Temperature °C | Calculated Activation Energy kJ/mol |
|---|---|---|---|---|
| [23] | None | 8 | 1550–1700 | 397 |
| [24] | None | 10–70 | 1400–1700 | 375 |
| [9] | None | 35 | 1450–1550 | 407 |
| [5] | None | MnO saturated | 1350–1500 | 347–380 |
| [8] | None | 70 | 1450–1550 | 366 |
| [16] | None | 35 | 1250–1400 | 332–345 |
| [11] | Fe | 4.76 | 1550–1600 | 104 ± 27.2 |
| [7] | Fe | 42 | 1520–1580 | 594, 636 |
| [25] | Fe | 35 | 1500–1600 | 227 |
| [12] | Fe | 59 | 1500 | 250 |
| This study | Fe | 62–78 | 1400–1500 | 230, 470 |
| Ore | Assmang + Quartz B = 0.5 | Assmang + Quartz B = 0.8 | Assmang + Quartz B = 1.2 | Assmang B = 2.1 | Comilog B = 0.11 | Comilog + Lime B = 0.5 | Comilog + Lime B = 0.8 | Comilog + Lime B = 1.2 |
|---|---|---|---|---|---|---|---|---|
| Reduction rate, g/min | 0.034 | 0.031 | 0.044 | 0.050 | 0.034 | 0.034 | 0.048 | 0.070 |
| Iso-viscosity, Poise | 0.75 | 0.75 | Not melted | Not melted | 13.00 | 1.35 | 0.50 | Not melted |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Tang, K.; Tangstad, M. Reduction and Dissolution Behaviour of Manganese Slag in the Ferromanganese Process. Minerals 2020, 10, 97. https://doi.org/10.3390/min10020097
Li X, Tang K, Tangstad M. Reduction and Dissolution Behaviour of Manganese Slag in the Ferromanganese Process. Minerals. 2020; 10(2):97. https://doi.org/10.3390/min10020097
Chicago/Turabian StyleLi, Xiang, Kai Tang, and Merete Tangstad. 2020. "Reduction and Dissolution Behaviour of Manganese Slag in the Ferromanganese Process" Minerals 10, no. 2: 97. https://doi.org/10.3390/min10020097
APA StyleLi, X., Tang, K., & Tangstad, M. (2020). Reduction and Dissolution Behaviour of Manganese Slag in the Ferromanganese Process. Minerals, 10(2), 97. https://doi.org/10.3390/min10020097





