An Experimental Investigation of Interaction between Andesite and Hyperacidic Volcanic Lake Water
Abstract
1. Introduction
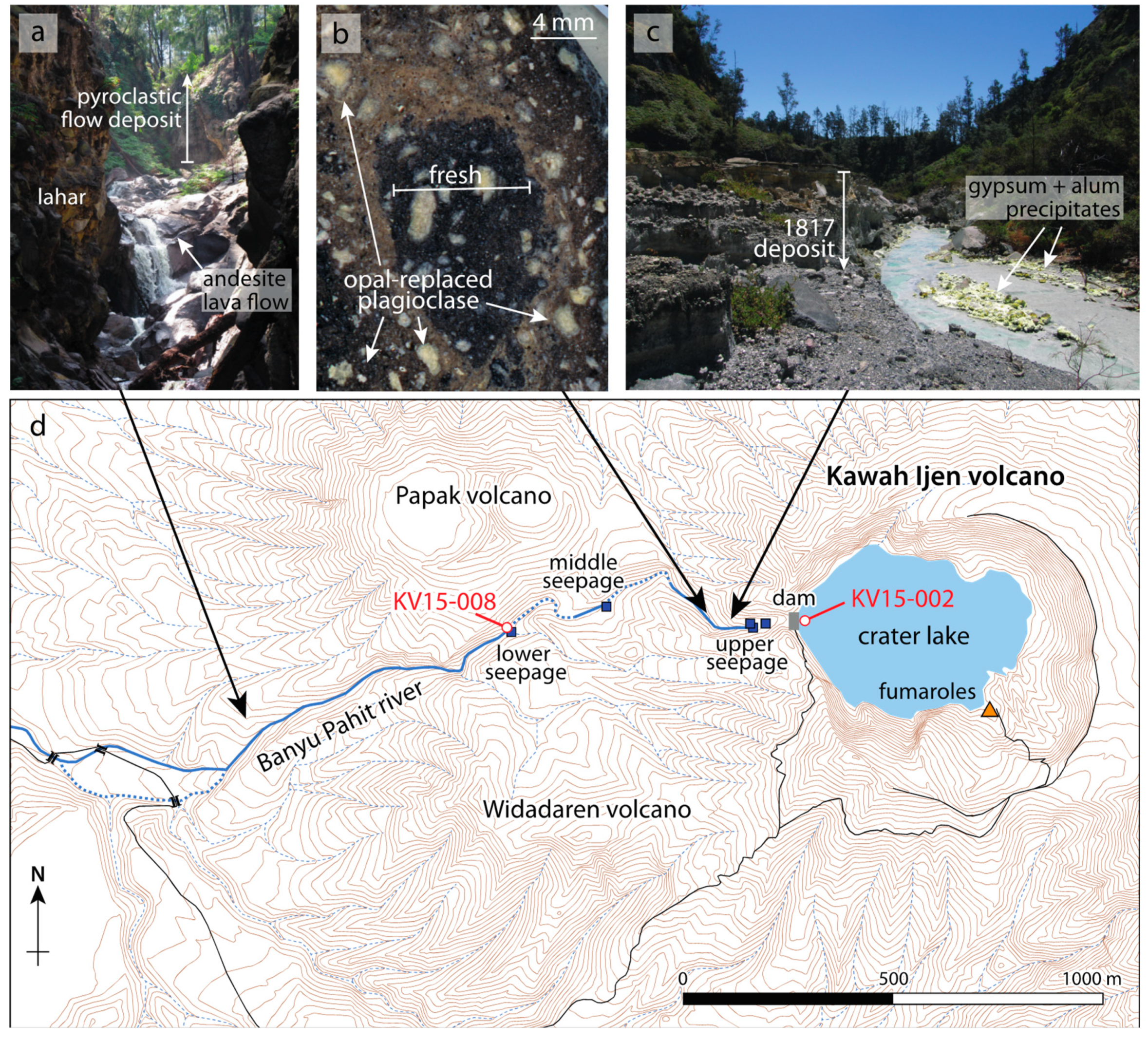
2. The Kawah Ijen–Banyu Pahit System
3. Materials and Methods
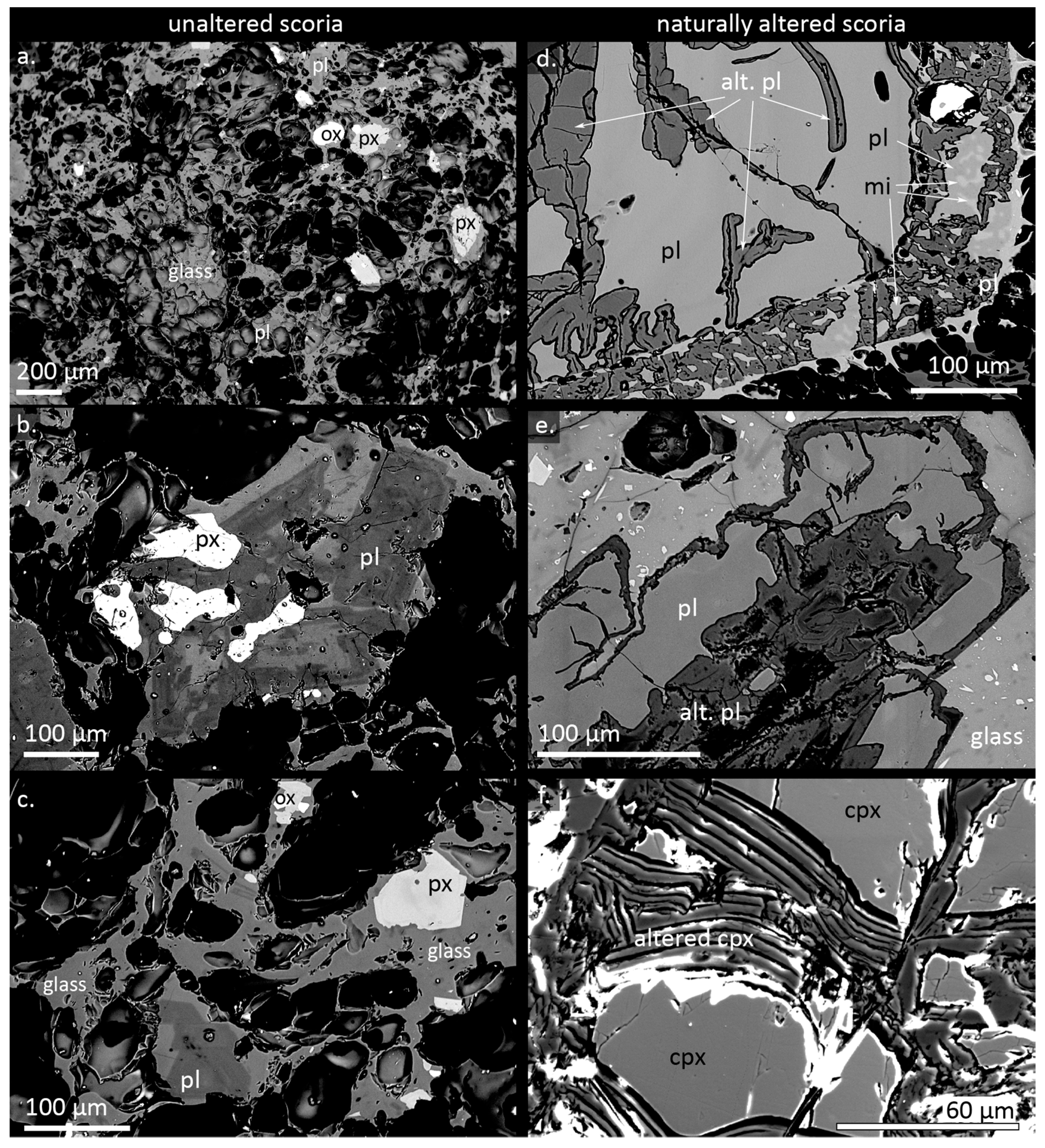
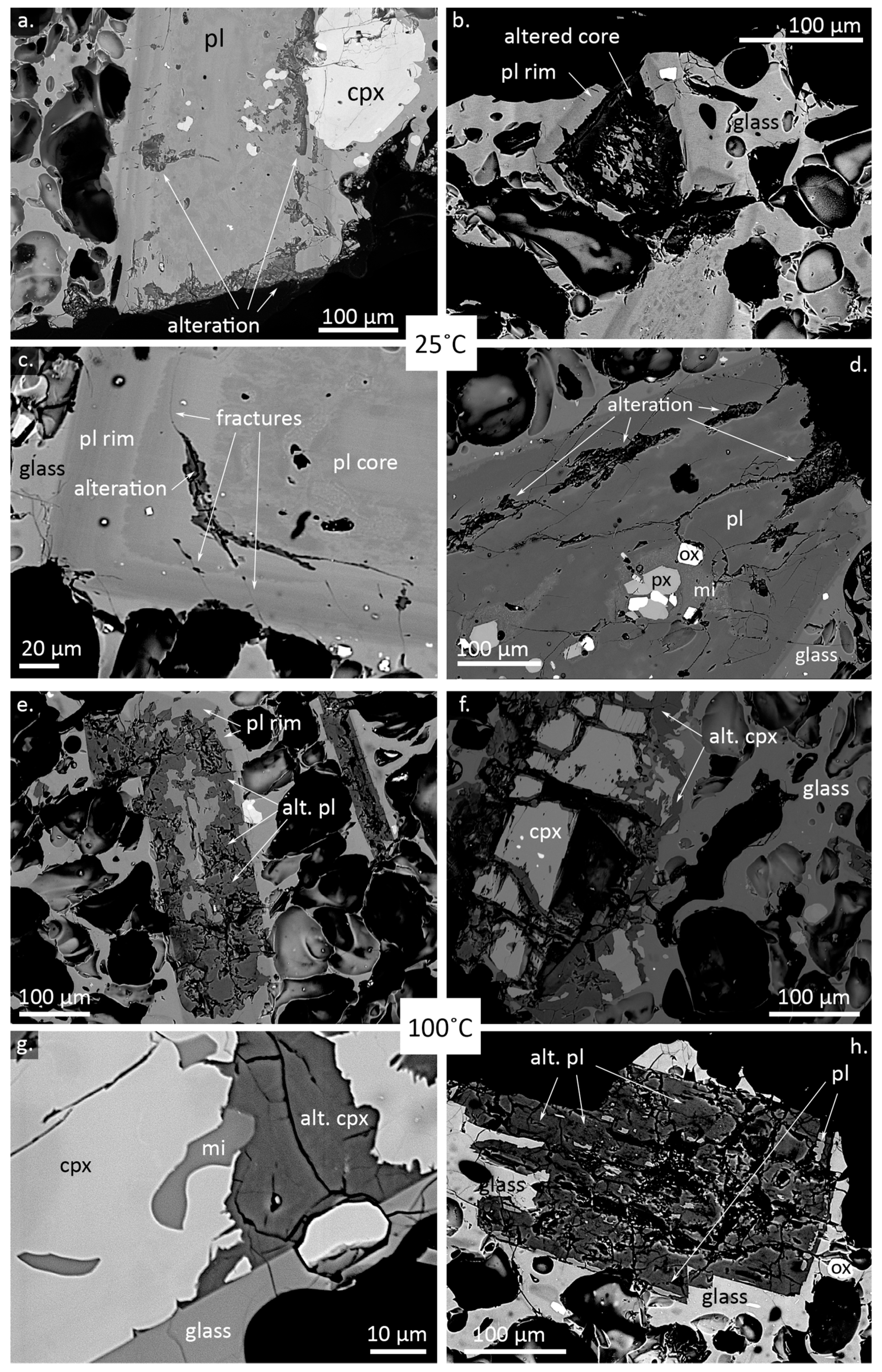
4. Results
5. Discussion
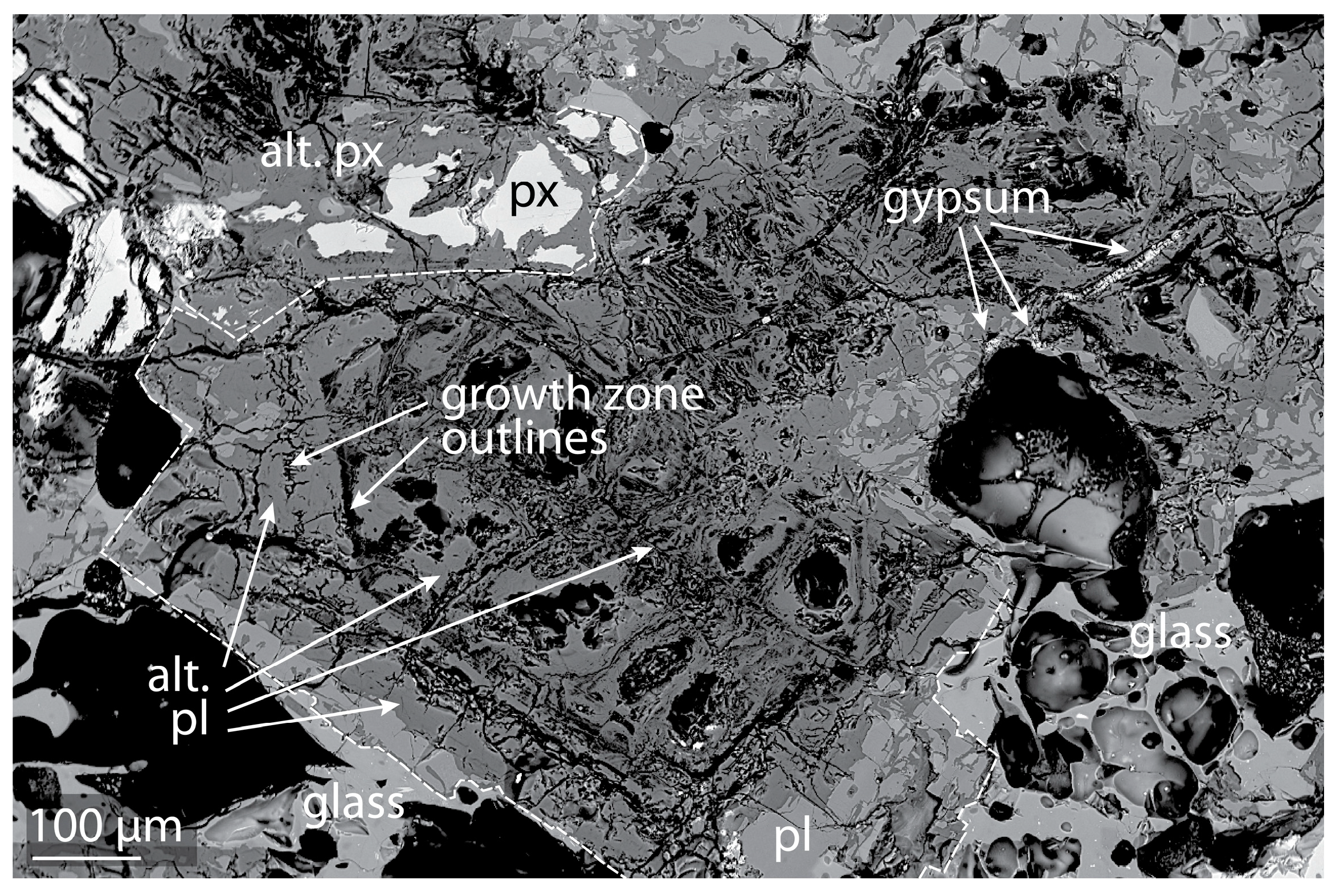
5.1. Alteration Textures
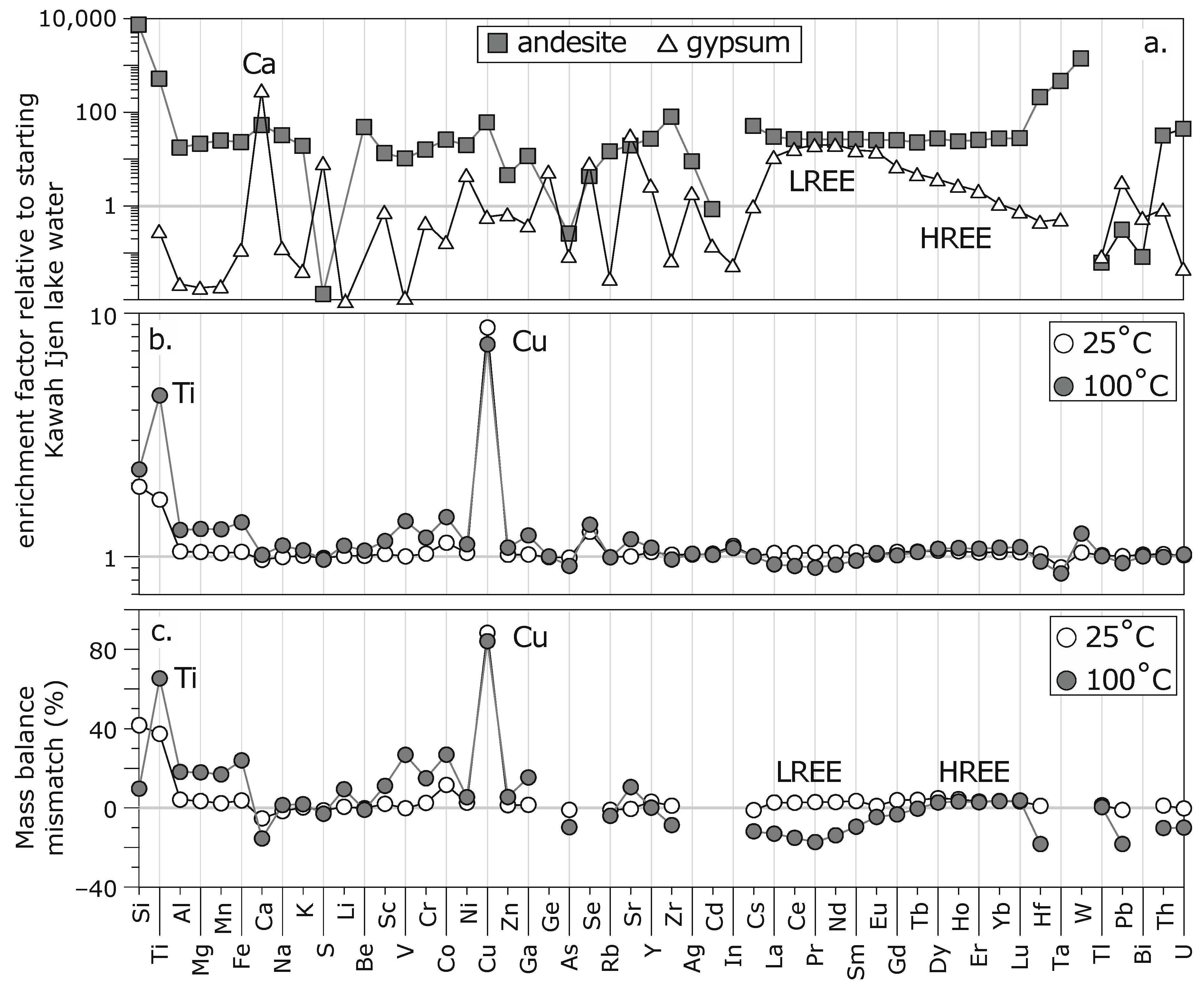
5.2. Compositional Changes
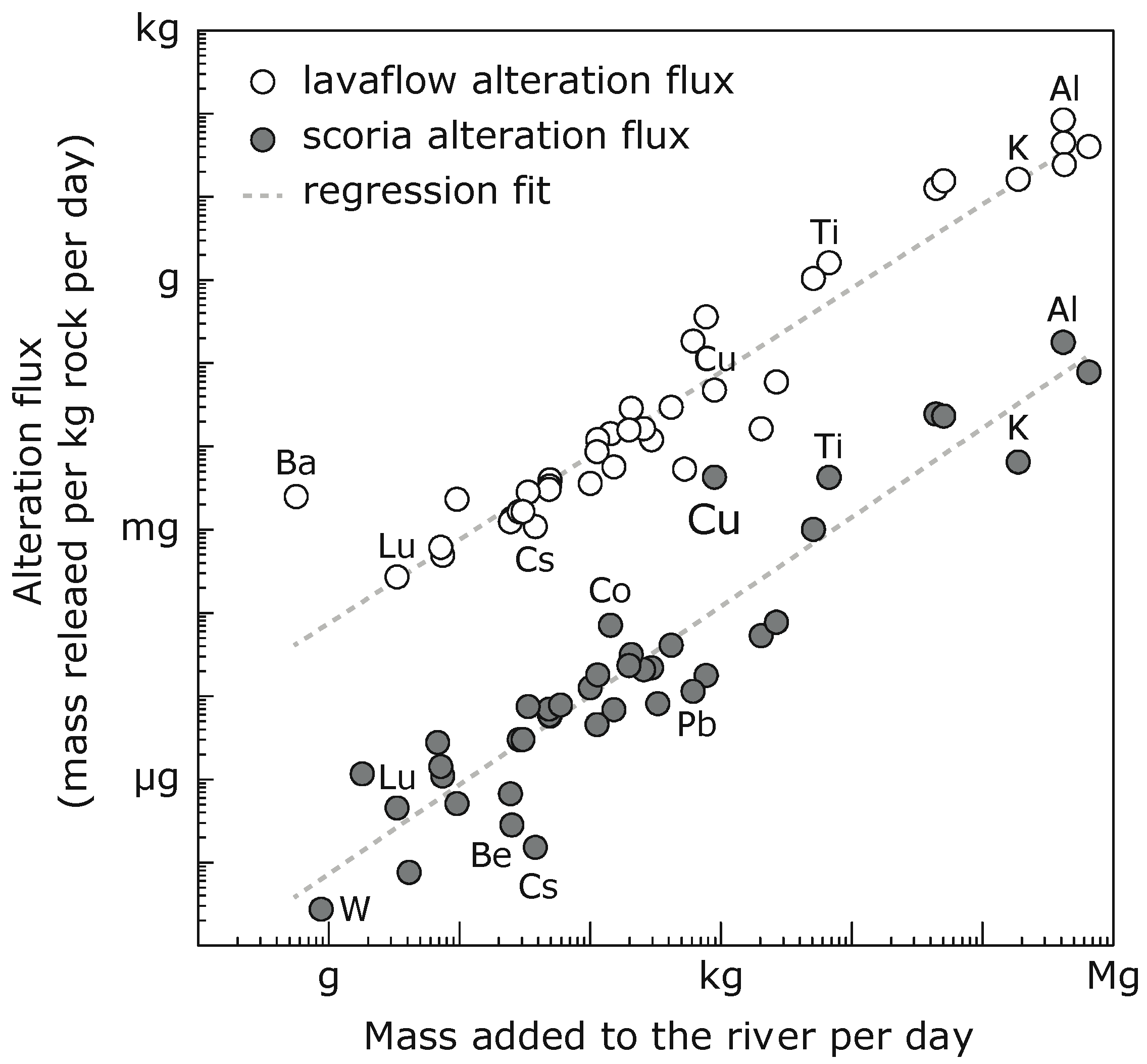
5.3. Alteration Flux
5.4. Alteration Mass Balance
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Henley, R.W.; Berger, B.R. Nature’s refineries—Metals and metalloids in arc volcanoes. Earth Sci. Rev. 2013, 125, 146–170. [Google Scholar] [CrossRef]
- Hedenquist, J.W.; Lowenstern, J.B. The role of magmas in the formation of hydrothermal ore deposits. Nature 1994, 370, 519–527. [Google Scholar] [CrossRef]
- Arribas, A.; Hedenquist, J.W.; ITAYA, T.; Okada, T.; Concepción, R.A.; Garcia, J.S. Contemporaneous formation of adjacent porphyry and epithermal Cu–Au deposits over 300 ka in northern Luzon, Philippines. Geology 1995, 23, 337–340. [Google Scholar] [CrossRef]
- Spilde, M.; Brearley, A.; Papike, J. Alteration of plagioclase and pyroxene phenocrysts in a fissure fumarole, Valley of Ten Thousand Smokes, Alaska. Am. Mineral. 1993, 78, 1066–1081. [Google Scholar]
- Getahun, A.; Reed, M.; Symonds, R. Mount St Augustine volcano fumarole wall rock alteration: Mineralogy, zoning, composition and numerical models of its formation process. J. Volcanol. Geotherm. Res. 1996, 71, 73–107. [Google Scholar] [CrossRef]
- Sillitoe, R.H. Porphyry Copper Systems. Econ. Geol. 2010, 105, 3–41. [Google Scholar] [CrossRef]
- Henley, R.W.; Berger, B.R. Magmatic-vapor expansion and the formation of high-sulfidation gold deposits: Chemical controls on alteration and mineralization. Ore Geol. Rev. 2011, 39, 63–74. [Google Scholar] [CrossRef]
- King, J.; Williams-Jones, A.E.; van Hinsberg, V.; Williams-Jones, G. High-sulfidation epithermal pyrite-hosted Au (Ag–Cu) ore formation by condensed magmatic vapors on Sangihe Island, Indonesia. Econ. Geol. 2014, 109, 1705–1733. [Google Scholar] [CrossRef]
- Stoffregen, R. Genesis of acid-sulfate alteration and Au–Cu–Ag mineralization at Summitville, Colorado. Econ. Geol. 1987, 82, 1575–1591. [Google Scholar] [CrossRef]
- Varekamp, J.C. The chemical composition and evolution of volcanic lakes. In Volcanic Lakes; Rouwet, D., Christenson, B., Tassi, F., Vandemeulebrouck, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 93–123. [Google Scholar]
- Lowenstern, J.B.; van Hinsberg, V.; Berlo, K.; Liesegang, M.; Iacovino, K.; Bindeman, I.N.; Wright, H.M. Opal-A in glassy pumice, acid alteration, and the 1817 phreatomagmatic eruption at kawah ijen (Java), Indonesia. Front. Earth Sci. 2018, 6. [Google Scholar] [CrossRef]
- Scher, S.; Williams-Jones, A.E.; Williams-Jones, G. Fumarolic activity, acid-sulfate alteration, and high sulfidation epithermal precious metal mineralization in the crater of Kawah Ijen volcano, Java, Indonesia. Econ. Geol. 2013, 108, 1099–1118. [Google Scholar] [CrossRef]
- Caudron, C.; Syahbana, D.K.; Lecocq, T.; van Hinsberg, V.; McCausland, W.; Triantafyllou, A.; Camelbeeck, T.; Bernard, A. Surono Kawah Ijen volcanic activity: A review. Bull. Volcanol. 2015, 77, 16. [Google Scholar] [CrossRef]
- van Hinsberg, V.; Berlo, K.; Sumarti, S.; van Bergen, M.; Williams-Jones, A. Extreme alteration by hyperacidic brines at Kawah Ijen volcano, East Java, Indonesia: II Metasomatic imprint and element fluxes. J. Volcanol. Geotherm. Res. 2010, 196, 169–184. [Google Scholar] [CrossRef]
- Handley, H.K.; Macpherson, C.G.; Davidson, J.P.; Berlo, K.; Lowry, D. Constraining fluid and sediment contributions to subduction-related magmatism in Indonesia: Ijen Volcanic Complex. J. Petrol. 2007, 48, 1155–1183. [Google Scholar] [CrossRef]
- Sitorus, K. Volcanic Stratigraphy and Geochemistry of the Idjen Caldera Complex, Indonesia. Master’s Thesis, Victoria University of Wellington, Wellington, New Zealand, 1990; 148p. [Google Scholar]
- Kemmerling, G.L.L. Het Idjen Hoogland. De Geologie en Geomorphologie van den Idjen; G. Kolff & Co.: Batavia, Dutch East Indies, 1921; Volume II, 143p. [Google Scholar]
- Delmelle, P.; Bernard, A. Geochemistry, mineralogy, and chemical modeling of the acid crater lake of Kawah Ijen Volcano, Indonesia. Geochim. Cosmochim. Acta 1994, 58, 2445–2460. [Google Scholar] [CrossRef]
- Delmelle, P.; Bernard, A.; Kusakabe, M.; Fischer, T.P. Geochemistry of the magmatic–hydrothermal system of Kawah Ijen volcano, East Java, Indonesia. J. Volcanol. Geotherm. Res. 2000, 97, 31–53. [Google Scholar] [CrossRef]
- Takano, B.; Suzuki, K.; Sugimori, K.; Ohba, T.; Fazlullin, S.M.; Bernard, A.; Sumarti, S.; Sukhyar, R.; Hirabayashi, M. Bathymetric and geochemical investigation of Kawah Ijen crater lake, east Java, Indonesia. J. Volcanol. Geotherm. Res. 2004, 135, 299–329. [Google Scholar] [CrossRef]
- van Hinsberg, V.; Vigouroux, N.; Palmer, S.; Berlo, K.; Mauri, G.; Williams-Jones, A.; McKenzie, J.M.; Williams-Jones, G.; Fischer, T.P. Element flux to the environment of the passively degassing crater lake-hosting Kawah Ijen volcano, Indonesia, and implications for estimates of the global volcanic flux. Geol. Soc. Lond. Spec. Publ. 2017, 437, 9–34. [Google Scholar] [CrossRef]
- Woudstra, H. Analyse van Merkwaardige Watersoorten op het Idjen-Hoogland; G. Kolff & Co.: Batavia, Dutch East Indies, 1921; Volume II, 17p. [Google Scholar]
- Caudron, C.; Lecocq, T.; Syahbana, D.K.; McCausland, W.; Watlet, A.; Camelbeeck, T.; Bernard, A. Surono Stress and mass changes at a wet volcano: Example during the 2011–2012 volcanic unrest at Kawah Ijen volcano (Indonesia). J. Geophys. Res. Solid Earth 2015, 120, 5117–5134. [Google Scholar] [CrossRef]
- Tonini, R.; Sandri, L.; Rouwet, D.; Caudron, C.; Marzocchi, W. Suparjan A new Bayesian Event Tree tool to track and quantify volcanic unrest and its application to Kawah Ijen volcano. Geochem. Geophys. Geosyst. 2016, 17, 2539–2555. [Google Scholar] [CrossRef]
- Global Volcanism Program Website, Report on Ijen (Indonesia). Available online: https://volcano.si.edu/volcano.cfm?vn=263350# (accessed on 26 November 2019).
- Caudron, C.; Girona, T.; Taisne, B.; Gunawan, H. Change in seismic attenuation as a long-term precursor of gas-driven eruptions. Geology 2019, 47, 632–636. [Google Scholar] [CrossRef]
- Delmelle, P.; Bernard, A. Downstream composition changes of acidic volcanic waters discharged into the Banyupahit stream, Ijen caldera, Indonesia. J. Volcanol. Geotherm. Res. 2000, 97, 55–75. [Google Scholar] [CrossRef]
- Palmer, S.C.J.; van Hinsberg, V.J.; McKenzie, J.M.; Yee, S. Characterization of acid river dilution and associated trace element behavior through hydrogeochemical modeling: A case study of the Banyu Pahit River in East Java, Indonesia. Appl. Geochem. 2011, 26, 1802–1810. [Google Scholar] [CrossRef]
- Sumarti, S. Volcanic Pollutants in Hyperacid River Water Discharged from Ijen Crater Lake, East Java, Indonesia. Master’s Thesis, Utrecht University, Utrecht, The Netherland, 1998. [Google Scholar]
- Löhr, A.; Bogaard, T.; Heikens, A.; Hendriks, M. Natural Pollution Caused by the Extremely Acid Crater Lake Kawah Ijen, East Java, Indonesia. Environ. Sci. Pollut. Res. 2005, 12, 89–95. [Google Scholar] [CrossRef]
- van Rotterdam-Los, A.M.D.; Vriend, S.P.; van Bergen, M.J.; van Gaans, R.F.M. The effect of naturally acidified irrigation water on agricultural volcanic soils. The case of Asembagus, Java, Indonesia. J. Geochem. Explor. 2008, 96, 53–68. [Google Scholar] [CrossRef]
- van Hinsberg, V.; Berlo, K.; van Bergen, M.; Williams-Jones, A. Extreme alteration by hyperacidic brines at Kawah Ijen volcano, East Java, Indonesia I Textural and mineralogical imprint. J. Volcanol. Geotherm. Res. 2010, 198, 253–263. [Google Scholar] [CrossRef]
- Rowe, G.; Brantley, S. Estimation of the dissolution rates of andesitic glass, plagioclase and pyroxene in a flank aquifer of Poás Volcano, Costa Rica. Chem. Geol. 1993, 105, 71–87. [Google Scholar] [CrossRef]
- Africano, F.; Bernard, A. Acid alteration in the fumarolic environment of Usu volcano, Hokkaido, Japan. J. Volcanol. Geotherm. Res/ 2000, 97, 475–495. [Google Scholar] [CrossRef]
- Berlo, K.; van Hinsberg, V.J.; Vigouroux, N.; Gagnon, J.E.; Williams-Jones, A.E. Sulfide breakdown controls metal signature in volcanic gas at Kawah Ijen volcano, Indonesia. Chem. Geol. 2014, 371, 115–127. [Google Scholar] [CrossRef]
- Utami, S.B.; Hinsberg, V.J.; Ghaleb, B.; Pinti, D.L. Growth-zoned gypsum stalactite from the Kawah Ijen volcanic lake, Indonesia, records a >40-year record of volcanic activity. Bull. Volcanol. 2019, 81, 52. [Google Scholar] [CrossRef]
- Casey, W.H.; Westrich, H.R.; Holdren, G.R. Dissolution rates of plagioclase at pH = 2 and 3. Am. Miner. 1991, 76, 211–217. [Google Scholar]
- Parkhurst, D.L.; Appelo, C.A.J. User’s guide to PHREEQC (Version 2): A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. Water Resour. Investig. Rep. 1999, 99, 312. [Google Scholar]
- Bindeman, I.N.; Davis, A.M.; Drake, M.J. Ion microprobe study of plagioclase-basalt partition experiments at natural concentration levels of trace elements. Geochim. Cosmochim. Acta 1998, 62, 1175–1193. [Google Scholar] [CrossRef]
- Bindeman, I.N.; Davis, A.M. Trace element partitioning between plagioclase and melt: Investigation of dopant influence on partition behavior. Geochim. Cosmochim. Acta 2000, 64, 2863–2878. [Google Scholar] [CrossRef]
- Wood, B.J.; Blundy, J.D. Trace Element Partitioning under Crustal and Uppermost Mantle Conditions: The Influences of Ionic Radius, Cation Charge, Pressure, and Temperature. Treatise Geochem. 2003, 2, 395–424. [Google Scholar]
- Bédard, J.H. A catalytic delamination-driven model for coupled genesis of Archaean crust and sub-continental lithospheric mantle. Geochim. Cosmochim. Acta 2006, 70, 1188–1214. [Google Scholar] [CrossRef]
- Putirka, K.D. Thermometers and Barometers for Volcanic Systems. Rev. Mineral. Geochem. 2008, 69, 61–120. [Google Scholar] [CrossRef]
- Dare, S.A.S.; Barnes, S.-J.; Beaudoin, G. Variation in trace element content of magnetite crystallized from a fractionating sulfide liquid, Sudbury, Canada: Implications for provenance discrimination. Geochim. Cosmochim. Acta 2012, 88, 27–50. [Google Scholar] [CrossRef]
- Lawson, C.L.; Hanson, R.J. Solving Least Squares Problems (Classics in applied mathematics); SIAM: Philadelphia, PA, USA, 1995; Volume 15, 337p. [Google Scholar]
- Berlo, K.; van Hinsberg, V.J.; Suparjan; Gunawan, H. Using the composition of fluid seepage from the magmatic-hydrothermal system of Kawah Ijen volcano, Indonesia, as a monitoring tool. J. Volcanol. Geotherm. Res. 2020. in review. [Google Scholar]

| Mass % | Glass | PL | CPX | Opx | Ox | Alt-Pl | Alt-Px |
|---|---|---|---|---|---|---|---|
| SiO2 | 68.56 | 57.23 | 53.38 | 53.44 | 0.10 | 89.76 | 89.53 |
| TiO2 | 0.55 | 0.06 | 0.26 | 0.24 | 12.64 | 0.05 | 0.25 |
| Al2O3 | 14.71 | 26.45 | 0.83 | 0.69 | 2.86 | 0.51 | 0.05 |
| Cr2O3 | 0.007 | n.a. | 0.008 | 0.006 | 0.031 | 0.000 | 0.009 |
| NiO | 0.004 | n.a. | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 |
| MgO | 0.50 | 0.05 | 13.31 | 21.26 | 2.59 | 0.00 | 0.01 |
| MnO | 0.08 | 0.02 | 0.61 | 0.96 | 0.56 | 0.01 | 0.01 |
| FeO | 3.17 | 0.57 | 12.07 | 22.82 | 77.06 | 0.10 | 0.20 |
| CaO | 1.79 | 9.13 | 20.78 | 1.63 | 0.03 | 0.03 | 0.04 |
| Na2O | 3.96 | 5.66 | 0.33 | 0.04 | 0.03 | 0.05 | 0.10 |
| K2O | 5.40 | 0.87 | 0.00 | 0.00 | 0.01 | 0.03 | 0.09 |
| P2O5 | 0.11 | 0.02 | 0.01 | 0.01 | 0.00 | 0.00 | 0.02 |
| SO3 | 0.01 | 0.01 | n.a. | 0.04 | n.a. | 0.72 | n.a. |
| F | 0.14 | 0.03 | 0.01 | 0.00 | 0.01 | 0.07 | 0.03 |
| Cl | 0.11 | n.a. | 0.01 | 0.01 | 0.00 | 0.27 | 0.38 |
| Total | 99.10 | 100.10 | 101.61 | 101.18 | 95.94 | 91.61 | 90.73 |
| Atoms per formula unit (apfu) | |||||||
| Si | 0.65 | 2.58 | 1.98 | 1.98 | 0.00 | 0.99 | 0.99 |
| Ti | 0.00 | 0.00 | 0.01 | 0.01 | 0.35 | 0.00 | 0.00 |
| Al | 0.16 | 1.41 | 0.04 | 0.03 | 0.12 | 0.01 | 0.00 |
| Cr | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Ni | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Mg | 0.01 | 0.00 | 0.74 | 1.18 | 0.14 | 0.00 | 0.00 |
| Mn | 0.00 | 0.00 | 0.02 | 0.03 | 0.02 | 0.00 | 0.00 |
| Fe | 0.02 | 0.02 | 0.37 | 0.71 | 2.36 | 0.00 | 0.00 |
| Ca | 0.02 | 0.44 | 0.83 | 0.06 | 0.00 | 0.00 | 0.00 |
| Na | 0.07 | 0.50 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 |
| K | 0.06 | 0.05 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| P | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| S | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 |
| F | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Cl | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.01 |
| Fe2+ | n.d. | n.d. | 0.36 | 0.71 | 1.19 | n.d. | n.d. |
| Fe3+ | n.d. | n.d. | 0.02 | 0.00 | 1.17 | n.d. | n.d. |
| XMg | 0.22 | 0.13 | 0.67 | 0.62 | 0.11 | 0.00 | 0.10 |
| Concentrations (mg kg−1) | Enrichment Factor | Andesite (mg kg−1) | Gypsum (mg kg−1) | Andesite /KV15-002 | Gypsum /KV15-002 | K mass Balance, ∆ in % | Incongruent Mass Balance, ∆ in % | Alteration Flux (mg kg−1 day−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICP-OES | KV15-002 | WRI-25C | WRI-100C | WRI-25C | WRI-100C | KV15-008 | average | WRI-25C | WRI-100C | WRI-25C | WRI-100C | WRI-25C | WRI-100C | ||
| Si | 37 | 72 | 85 | 1.9 | 2.3 | 273,395 | n.d. | 7366 | − | −97 | −954 | 42 | 10 | 24 | 33 |
| Ti | 8 | 14 | 39 | 1.7 | 4.6 | 4375 | 2 | 519 | 0.3 | 30 | 43 | 37 | 65 | 4 | 21 |
| Al | 5103 | 5356 | 6556 | 1.0 | 1.3 | 89,757 | 110 | 18 | 0.02 | 4 | 18 | 4 | 18 | 178 | 1020 |
| Mg | 746 | 779 | 968 | 1.0 | 1.3 | 15,980 | 13 | 21 | 0.02 | 3 | 18 | 3 | 18 | 23 | 156 |
| Mn | 43 | 45 | 56 | 1.0 | 1.3 | 1084 | 1 | 25 | 0.02 | 2 | 17 | 2 | 17 | 1 | 9 |
| Fe | 2443 | 2554 | 3379 | 1.0 | 1.4 | 56,514 | 285 | 23 | 0.1 | 3 | 22 | 4 | 24 | 78 | 657 |
| Ca | 798 | 774 | 811 | 1.0 | 1.0 | 42,453 | 232,800 | 53 | 292 | −5 | −15 | −5 | −15 | −17 | 9 |
| Na | 809 | 805 | 898 | 1.0 | 1.1 | 26,113 | 102 | 32 | 0.1 | −2 | 1 | −2 | 1 | −3 | 63 |
| K | 1248 | 1257 | 1323 | 1.0 | 1.1 | 24,074 | 51 | 19 | 0.04 | 0 | 0 | 0 | 2 | 6 | 53 |
| S | 22,612 | 22,332 | 21,955 | 1.0 | 1.0 | 300 | 186,200 | 0.01 | 8 | −1 | −3 | −1 | −3 | −196 | −461 |
| ICP-MS | |||||||||||||||
| Li | 12.9 | 12.9 | 14.2 | 1.0 | 1.1 | n.a. | 0.1 | − | 0.01 | − | − | 1 | 9 | 0.1 | 1.0 |
| Be | 0.062 | 0.062 | 0.066 | 1.0 | 1.1 | 3.00 | n.d. | 48 | − | −1 | −9 | 0 | −1 | 0 | 0.003 |
| Sc | 1.26 | 1.30 | 1.46 | 1.0 | 1.2 | 17 | 0.9 | 13 | 0.7 | 2 | 10 | 2 | 11 | 0.02 | 0.14 |
| V | 14.8 | 14.8 | 20.6 | 1.0 | 1.4 | 153 | 0.1 | 10 | 0.01 | 0 | 26 | 0 | 27 | 0.01 | 4.1 |
| Cr | 0.643 | 0.661 | 0.770 | 1.0 | 1.2 | 10 | 0.3 | 16 | 0.4 | 2 | 12 | 2 | 15 | 0.012 | 0.089 |
| Co | 0.718 | 0.818 | 1.043 | 1.1 | 1.5 | 19 | 0.1 | 26 | 0.2 | 11 | 26 | 12 | 27 | 0.070 | 0.228 |
| Ni | 0.282 | 0.291 | 0.316 | 1.0 | 1.1 | 5.6 | 1 | 20 | 5 | 3 | 5 | 3 | 5 | 0.007 | 0.024 |
| Cu | 0.8 | 6.8 | 5.8 | 8.7 | 7.5 | 48 | 0.5 | 61 | 0.6 | 88 | 84 | 88 | 84 | 4.2 | 3.5 |
| Zn | 6.37 | 6.48 | 6.94 | 1.0 | 1.1 | 29 | 4 | 5 | 0.7 | 2 | 7 | 1 | 5 | 0.08 | 0.40 |
| Ga | 1.48 | 1.51 | 1.81 | 1.0 | 1.2 | 17 | 0.6 | 12 | 0.4 | 2 | 15 | 2 | 15 | 0.02 | 0.23 |
| Ge | 0.061 | 0.061 | 0.061 | 1.0 | 1.0 | n.a. | 0.3 | − | 5 | − | − | − | − | 0 | 0 |
| As | 3.49 | 3.46 | 3.19 | 1.0 | 0.9 | 0.9 | 0.3 | 0.3 | 0.1 | −1 | −9 | −1 | −10 | −0.02 | −0.21 |
| Se | 0.06 | 0.07 | 0.08 | 1.3 | 1.4 | <0.5 | 0.5 | 4 | 8 | − | − | − | − | 0.01 | 0.01 |
| Rb | 5.6 | 5.6 | 5.6 | 1.0 | 1.0 | 82 | 0.2 | 15 | 0.03 | −1 | −5 | −1 | −4 | −0.02 | −0.03 |
| Sr | 20.9 | 20.9 | 24.6 | 1.0 | 1.2 | 407 | 664 | 19 | 32 | −1 | 10 | −1 | 11 | 0 | 2.6 |
| Y | 1.03 | 1.08 | 1.12 | 1.0 | 1.1 | 28 | 3 | 27 | 3 | 3 | 0 | 3 | 0 | 0.03 | 0.06 |
| Zr | 2.43 | 2.48 | 2.37 | 1.0 | 1.0 | 197 | 0.2 | 81 | 0.1 | −1 | −29 | 1 | −9 | 0.03 | −0.05 |
| Ag | 0.006 | 0.006 | 0.006 | 1.0 | 1.0 | <0.1 | 0.01 | 9 | 2 | 2 | −2 | − | − | 0 | 0 |
| Cd | 0.059 | 0.060 | 0.060 | 1.0 | 1.0 | <0.1 | 0.01 | 0.9 | 0.1 | 3 | −1 | − | − | 0.001 | 0.001 |
| In | 0.037 | 0.041 | 0.040 | 1.1 | 1.1 | n.a. | 0.002 | − | 0.1 | − | − | − | − | 0.003 | 0.002 |
| Cs | 0.06 | 0.06 | 0.06 | 1.0 | 1.0 | 3.3 | 0.1 | 51 | 1 | −2 | −16 | −1 | −12 | 0 | 0 |
| Ba | <0.2 | <0.2 | <0.4 | − | 620 | 76 | − | − | negative | negative | negative | negative | negative | negative | |
| La | 0.75 | 0.77 | 0.69 | 1.0 | 0.9 | 22.4 | 8 | 30 | 11 | 2 | −18 | 3 | −13 | 0.02 | −0.04 |
| Ce | 1.75 | 1.81 | 1.60 | 1.0 | 0.9 | 46.8 | 29 | 27 | 16 | 2 | −19 | 3 | −15 | 0.04 | −0.11 |
| Pr | 0.219 | 0.227 | 0.197 | 1.0 | 0.9 | 5.7 | 4 | 26 | 20 | 3 | −20 | 3 | −17 | 0.006 | −0.015 |
| Nd | 0.897 | 0.930 | 0.831 | 1.0 | 0.9 | 23.5 | 18 | 26 | 20 | 3 | −17 | 3 | −14 | 0.023 | −0.046 |
| Sm | 0.194 | 0.202 | 0.186 | 1.0 | 1.0 | 5.1 | 3 | 27 | 16 | 3 | −13 | 3 | −10 | 0.006 | −0.005 |
| Eu | 0.048 | 0.049 | 0.050 | 1.0 | 1.0 | 1.23 | 0.7 | 26 | 15 | 1 | −5 | 1 | −5 | 0.001 | 0.001 |
| Gd | 0.213 | 0.223 | 0.215 | 1.0 | 1.0 | 5.4 | 1.5 | 25 | 7 | 3 | −7 | 4 | −3 | 0.007 | 0.002 |
| Tb | 0.032 | 0.034 | 0.033 | 1.0 | 1.0 | 0.73 | 0.2 | 23 | 5 | 4 | −3 | 4 | 0 | 0.001 | 0.001 |
| Dy | 0.184 | 0.195 | 0.199 | 1.1 | 1.1 | 5.1 | 0.7 | 28 | 4 | 4 | −1 | 5 | 3 | 0.007 | 0.010 |
| Ho | 0.038 | 0.040 | 0.042 | 1.1 | 1.1 | 0.92 | 0.1 | 24 | 3 | 4 | 1 | 4 | 3 | 0.001 | 0.002 |
| Er | 0.109 | 0.113 | 0.117 | 1.0 | 1.1 | 2.8 | 0.2 | 26 | 2 | 3 | 0 | 3 | 3 | 0.003 | 0.006 |
| Yb | 0.100 | 0.104 | 0.109 | 1.0 | 1.1 | 2.77 | 0.1 | 28 | 1 | 3 | 0 | 3 | 3 | 0.003 | 0.006 |
| Lu | 0.016 | 0.017 | 0.018 | 1.0 | 1.1 | 0.45 | 0.01 | 28 | 0.8 | 3 | 0 | 3 | 4 | 0.0004 | 0.001 |
| Hf | 0.025 | 0.025 | 0.024 | 1.0 | 1.0 | 5.20 | 0.01 | 211 | 0.5 | −5 | −74 | 1 | −18 | 0 | −0.001 |
| Ta | 0.0011 | 0.0010 | 0.0009 | 0.9 | 0.9 | 0.50 | 0.001 | 466 | 0.5 | −30 | −188 | − | − | −0.0001 | −0.0001 |
| W | 0.0010 | 0.0010 | 0.0012 | 1.0 | 1.2 | 1.40 | n.d. | 1404 | − | −48 | −334 | − | − | 0 | 0.0002 |
| Tl | 0.81 | 0.82 | 0.81 | 1.0 | 1.0 | <0.1 | 0.1 | 0.06 | 0.1 | 1 | −1 | 1 | 0 | 0.01 | 0.002 |
| Pb | 5.12 | 5.14 | 4.82 | 1.0 | 0.9 | 1.60 | 16 | 0.3 | 3 | 0 | −6 | −1 | −18 | 0.01 | −0.21 |
| Bi | 0.61 | 0.62 | 0.61 | 1.0 | 1.0 | <0.1 | 0.3 | 0.08 | 0.6 | 2 | −2 | − | − | 0.01 | 0.001 |
| Th | 0.275 | 0.282 | 0.274 | 1.0 | 1.0 | 9 | 0.2 | 32 | 0.8 | 1 | −10 | 1 | −10 | 0.005 | −0.001 |
| U | 0.058 | 0.059 | 0.060 | 1.0 | 1.0 | 2.6 | 0.003 | 44 | 0.05 | 0 | −11 | 0 | −10 | 0.001 | 0.001 |
| Phase | Glass | Cpx | Opx | Oxide | An40 | An60 |
|---|---|---|---|---|---|---|
| mode (mass %) | 49 | 5 | 8 | 4 | 1.4 | 32 |
| Concentrations (mg kg−1) | ||||||
| Sc | 12 | 171 | 17 | 18 | 6 | 2 |
| V | 90 | 90 | 67 | 2434 | 4 | 2 |
| Cr | 3 | 2 | 21 | 179 | 0.2 | 0.1 |
| Co | 8 | 13 | 141 | 64 | 2 | 2 |
| Ni | 2 | 13 | 16 | 65 | 0.11 | 0.03 |
| Cu | 62 | 61 | 3 | 48 | 37 | 37 |
| Zn | 17 | 62 | 143 | 138 | 5 | 2 |
| Ga | 14 | 4 | 4 | 41 | 21 | 24 |
| Rb | 161 | 2 | 8 | 0.2 | 8 | 6 |
| Sr | 319 | 10 | 15 | 7 | 1218 | 717 |
| Y | 53 | 32 | 3 | 1 | 0.7 | 1 |
| Zr | 387 | 48 | 12 | 76 | 4 | 2 |
| Sn | 8 | 4 | 2 | 7 | 1 | 1 |
| Cs | 6 | 0.02 | 0.3 | 0.01 | 0.6 | 0.5 |
| Ba | 1006 | 6 | 47 | 1 | 716 | 348 |
| La | 41 | 1 | 0.01 | 0.03 | 10 | 7 |
| Ce | 86 | 6 | 0.07 | 0.2 | 18 | 13 |
| Pr | 11 | 1 | 0.02 | 0.04 | 2 | 1 |
| Nd | 44 | 6 | 0.1 | 0.2 | 5 | 5 |
| Sm | 10 | 3 | 0.1 | 0.1 | 0.6 | 0.6 |
| Eu | 2 | 0.8 | 1.7 | 0.04 | 0.1 | 0.1 |
| Gd | 10 | 5 | 0.2 | 0.3 | 0.4 | 0.4 |
| Tb | 1 | 0.8 | 0.05 | 0.05 | 0.03 | 0.04 |
| Dy | 9 | 6 | 0.5 | 0.5 | 0.2 | 0.2 |
| Ho | 2 | 1 | 0.1 | 0.1 | 0.02 | 0.03 |
| Er | 5 | 4 | 0.5 | 0.5 | 0.05 | 0.07 |
| Yb | 5 | 4 | 0.8 | 0.8 | 0.03 | 0.04 |
| Lu | 0.8 | 0.6 | 0.1 | 0.1 | 0.003 | 0.006 |
| Hf | 10 | 2 | 2 | 2 | 0.2 | 0.2 |
| Pb | 2 | 0.05 | 0.1 | 3 | 2 | 1 |
| Th | 16 | 2 | 2 | 0.2 | 0.2 | 2 |
| U | 5 | 0.2 | 0.5 | 0.1 | 0.5 | 0.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Hinsberg, V.; Berlo, K.; Lowenstern, J. An Experimental Investigation of Interaction between Andesite and Hyperacidic Volcanic Lake Water. Minerals 2020, 10, 96. https://doi.org/10.3390/min10020096
van Hinsberg V, Berlo K, Lowenstern J. An Experimental Investigation of Interaction between Andesite and Hyperacidic Volcanic Lake Water. Minerals. 2020; 10(2):96. https://doi.org/10.3390/min10020096
Chicago/Turabian Stylevan Hinsberg, Vincent, Kim Berlo, and Jacob Lowenstern. 2020. "An Experimental Investigation of Interaction between Andesite and Hyperacidic Volcanic Lake Water" Minerals 10, no. 2: 96. https://doi.org/10.3390/min10020096
APA Stylevan Hinsberg, V., Berlo, K., & Lowenstern, J. (2020). An Experimental Investigation of Interaction between Andesite and Hyperacidic Volcanic Lake Water. Minerals, 10(2), 96. https://doi.org/10.3390/min10020096




