3.1. Crystallization From Mine Waters
The calculations included 12 independent (Ar, As, C, Cu, Fe, N, Pb, S, Zn, H, O, and ē, where ē is electron) and 274 dependent components: 210 dissolved particles, 18 gases, and 45 solid phases (most probable hypogene and hypergene minerals and ice).
The results of sulfide oxidation modeling in the mine workings within the positive temperature range have been partially published for many districts [
16]. The results of modeling 21 sulfide oxidation variants in the considered temperature range are presented below. The formation of highly concentrated mine waters led to crystallization of the following minerals: fibroferrite, chalcantite, posnjakite, wroewolfeite, antlerite, anglesite, bayldonite, and olivenite. In cryogenesis conditions, additional goslarite and duftite precipitate (
Table 1).
Formation of fibroferrite from the simulated solutions occurs at Eh-pH values from 0.74 to 1.24 V and from 0.1 to 5.7: chalcantite = 0.74–1.20 V, 0.6–3.3; wroewolfeite = 0.74–1.05 V, 3.2–3.3 (in the temperature range from −25 to 30 °C); posnjakite = 1.02–1.03 V, 3.2 (20–35 °C); antlerite = 1.02–1.06 V, 2.4–3.1 (35–45 °C); goslarite = 1.06–1.17 V, 1.3–1.4 (from 25 to −20 °C); anglesite = 1.04–1.18 V, 0.8–2.7; bayldonite = 1.09–1.14 V, 2.0–2.3 (−25 to 20 °C); olivenite = 1.08–1.09 V, 2.0–2.3 (15–45 °C); and duftite = 1.12–1.16 V, 2.0 (−25 to −15 °C). The exception is crystallization from highly alkaline solutions (pH 7 to 10) of fibroferrite, chalcantite, and wroewolfeite during oxidation of chalcocite and bornite in the temperature range from −5 to −25 °C. Probably, the presence of single-valent copper in structure of chalcosine and boronite promotes the formation of such solutions. This contradicts the generally accepted concepts of crystallization of sulfates in the range of positive temperatures from acid solutions.
Crystallization of wroewolfeite, posnjakite, and antlerite is observed only in the presence of secondary sulfide enrichment (cementation) zone minerals in the ore body: chalcocite and bornite, both separately and together. Chalcantite crystallization occurs in all cases in the presence of copper-bearing sulfides. Olivenite, bayldonite, and duftite are observed only in the primary association of sulfides without pyrrhotite, but in the presence of all minerals in the cementation zone. Goslarite forms only in the case of sphalerite oxidation under cryogenic conditions.
The modeled systems have the following masses of minerals (g): antlerite, 52.0–104.0; chalcanthite, 0.0003–261.0; posnjakite, 0.0001–91.0; wroewolfeite, 0.0001–99.0; goslarite, 0.00008–0.0002; anglesite, 3.0–159.0; fibroferrite, 17.0–294.0; bayldonite, 0.00008–18.0; olivenite, 0.00001–5.0; and duftite, 0.001–22.0. As the temperature rises, the masses of antlerite, wroewolfeite, posnjakite, goslarite, anglesite, and olivenite increase; chalcantite, duftite, and bayldonite decrease; and fibroferrite remains constant.
The formation of mine water occurs when all micropore solutions, the variants of which are described above, are merged. The study of hydrochemical samples of mine waters has shown that in the studied area, the pH value is in the range from 4.5 to 8.1 [
17].
3.2. Crystallization From Tailings Dumps Sludge and Drainage
The calculations of technogenic waters (sludge and drainage) modeling included 19 independent (Al, Ar, As, B, C, Ca, Cu, Fe, K, Mg, N, Na, Pb, S, Si, Zn, H, O, and ē) and 371 dependent components: 283 dissolved particles, 18 gases, and 70 solid phases. The obtained results were partly published in works [
16,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27]. Modeling showed that most of the hypergenic minerals (
Table 1) crystallize from highly concentrated solutions in the temperature range from −25 to 45 °C.
To create models of sulfide oxidation in contact with host rocks minerals, the composition listed in
Table 2 and
Table 3 was used. As the waste composition in different parts of the tailings dump varies, systems with host-rock–sulfide ratios of 95:5, 90:10, 80:20, and 20:80 were modeled.
The processes of tailings oxidation and technogenic minerals formation at Khrustalnoe and Dubrovskoe deposits tailings dumps (
Table 2 and
Table 3) were modeled in the temperature range from 0 to 45 °C. The mineral composition presented in this work is similar to previous works on minerals association but differs in the details (for example, alunogen is observed only in the models of the Khrustalnoe tailings dump). Therefore, we combined all the available results.
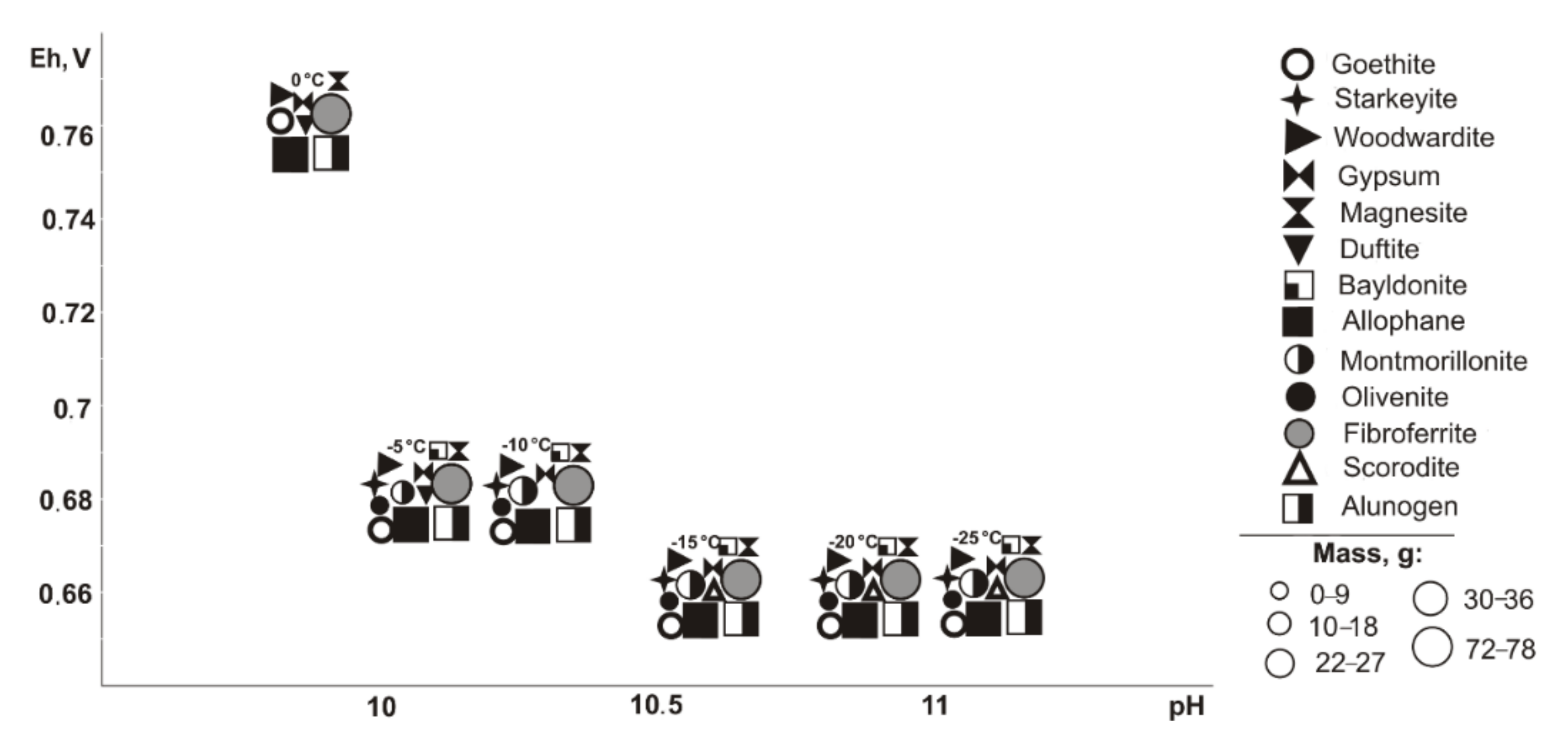
From saturated solutions, Cu, Fe, Pb, Mg, and Al minerals of the following classes precipitate: oxides and hydroxides, sulfates, carbonates, arsenates, and silicates. These are goethite (Eh-pH parameters: 0.42–0.74 V and 7.5–12.6), tenorite and woodwardite (0.42–0.52 V, 11.8–13.3), fibroferrite (0.63–0.86 V, 5.6–13.3), gypsum (0.59–0.86 V, 5.6–11.2), alunogen (0.8–0.86 V, 5.6–7.9), magnesite (0.42–0.66 V, 7.5–13.3), duftite and allophane (0.42–0.86 V, 5.6–13.3), and montmorillonite (0.42–0.65 V, 9.3–13.3). Notably, formation of duftite and allophane in the system does not depend on the total sulfide content in tailings; tenorite crystalizes only at 5–10% sulfide ratios; goethite, woodwardite, and magnesite from 5% to 60%; fibroferrite and alunogen within 40–80%; and anglesite at 80%. The mass of tenorite is 0.20 g, which accounts for 0.1% of the total precipitated minerals mass.
Tailings oxidation of the Vysokogorsk deposit (
Table 2 and
Table 3) was modeled in the temperature range from −25 to 45 °C. At positive temperatures, the following minerals were established (
Table 1): goethite (Eh-pH parameters 0.57–0.75 V, 9.5–10.1, sulfide content in the system from 5% to 60%); gypsum and duftite (0.57–1.08 V, 2.7–11.7, from 5% to 80% sulfide); woodwardite, magnesite, and allofan (0.57–0.75 V, 7.4–11.6, 5–60%); alunogen, anglesite, and bayldonite (1.04–1.08 V, 2.7–2.8, 80%); fibroferrite (0.64–1.08 V, 2.7–10.2, 10–80%); and montmorillonite (0.57–0.62 V, 9.5–11.6, 5%). Montmorillonite disappears at a sulfide content of 10%, but fibroferrite appears at 80%, no goethite, woodwardite, and magnesite are found, but alunogen, anglesite, and bayldonite are noted.
At negative temperatures, the following minerals are found (
Table 1): goethite and magnesite (0.58–0.74 V, 9.3–10.8, 5–40%); woodwardite, starkeyite, duftite, and allophane (0.58–1.15 V, 1.8–10.8); wroewolfeite and chalcantite (1.1–1.15 V, 1.8–2.2, 80%); gypsum, olivenite, and bayldonite (0.71–1.15 V, 1.8–10.8, 10–80%); fibroferrite and alunogen (0.68–1.15 V, 1.8–10.8, 40–80%); and montmorillonite (0.58–0.71 V, 10.6–12.5%). At a 10% sulfide content, the montmorillonite disappears, but fibroferrite and scorodite occur; at 80%, no goethite, woodwardite, olivenite, scorodite, magnesite, or allophane exist, but alunogen is precipitated. Woodwardite and duftite only form at 0 °C, and starkeyite, olivenite, bayldonite form in the range from −25 to −5 °C.
Therefore, oxidation of the total tailings sulfide composition in deposits in the entire temperature range led the system’s Eh-pH parameters to change from 0.42 to 1.15 V and 1.8 to 13.3. High alkaline pH values are typical for systems with the content of host rocks from 60% to 95% in negative temperature range, but such simulated systems also contain sulfates (fibroferrite, chalcantite, etc.) with insignificant masses (from 0.0001 to 0.5 g). Total dissolved solids (TDS) in the positive temperature range after minerals crystallization varies from 11.7 to 72.7 g/L. The TDS increases significantly under cryogenesis conditions with ice crystallization and liquid phase reduction.
3.3. Successive Sulfide Minerals Exclusion
Next, we considered the models with successive exclusion of each sulfide mineral (
Figure 2,
Figure 3,
Figure 4,
Figure 5,
Figure 6,
Figure 7,
Figure 8,
Figure 9,
Figure 10,
Figure 11,
Figure 12 and
Figure 13).
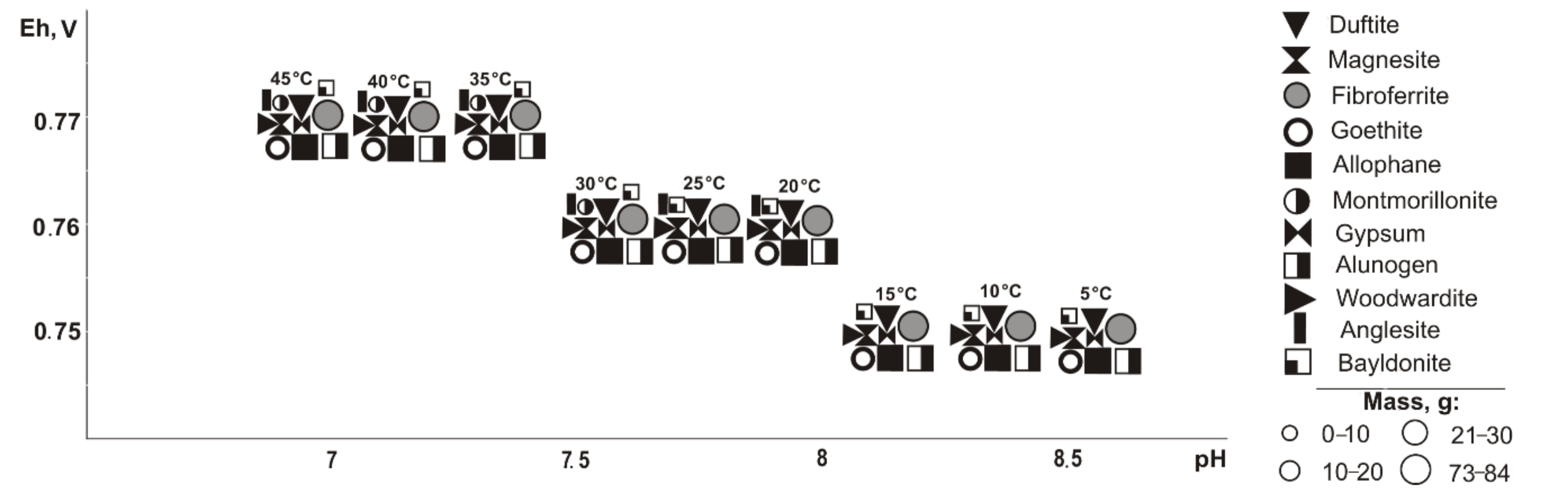
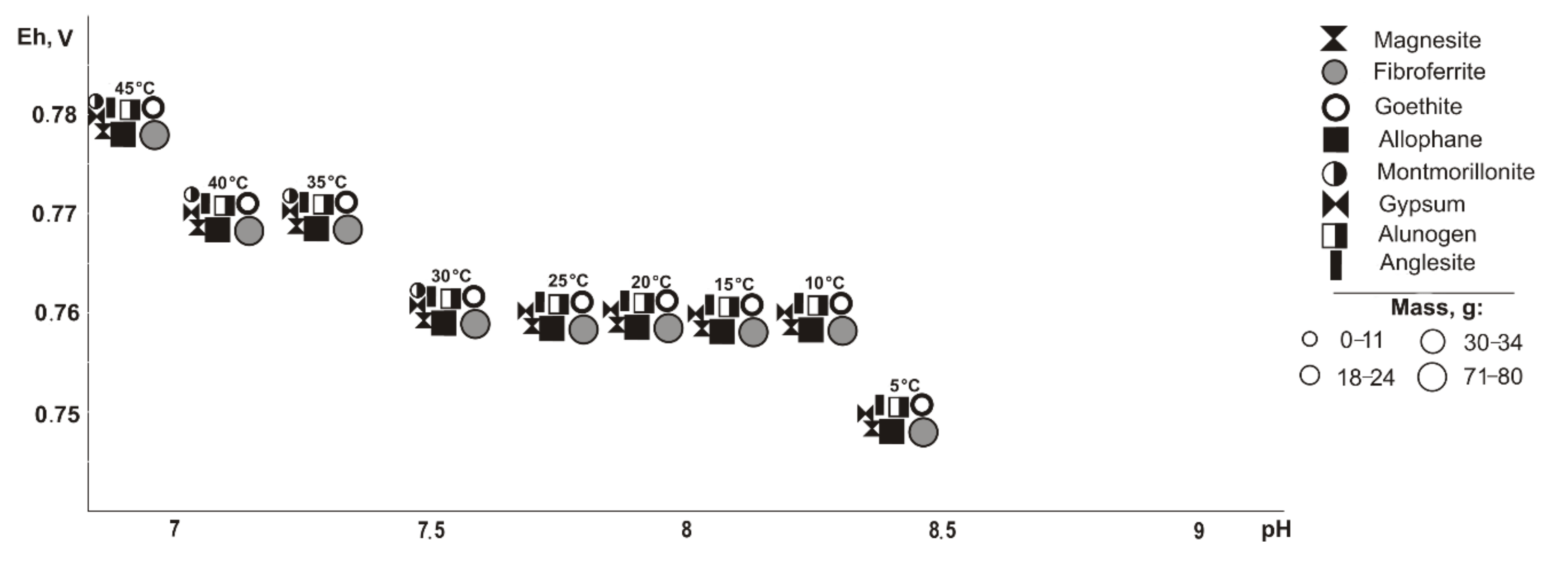
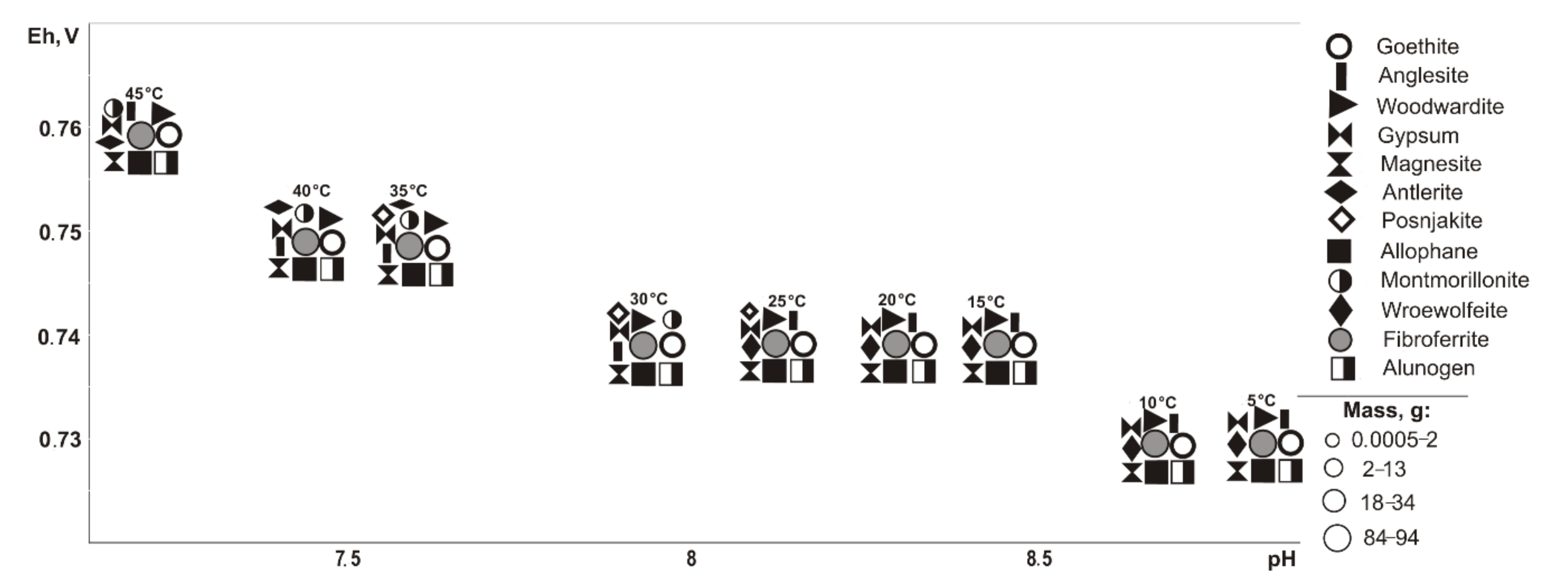
If pyrite (
Figure 2) is excluded from the primary composition, in the positive temperature range, Fe, Cu, Pb, Al, and Mg minerals of oxides and hydroxides, sulfates, arsenates, carbonates, and silicates precipitate from the saturated solution (Eh-pH parameters): goethite and magnesite (0.58–0.75 V, 7.4–11.7); fibroferrite (0.67–1.09 V, 2.5–10.2); woodwardite (0.56–0.85 V, 7.4–11.7); gypsum, duftite, and allophane (0.58–1.09 V, 2.5–11.7); bayldonite and anglesite (1.05–1.09 V, 2.5–2.6); and montmorillonite (0.59–0.62 V, 9.4–10.3). Changes in the qualitative composition of hypergenic minerals depend on the concentration of the sulfide component in the system: some minerals disappear, including montmorillonite at 20%, goethite and magnesite at 60%, woodwardite and allophane at 80%; and others appear, including gypsum at 10%, fibroferrite at 20%, alunogen at 60%, and anglesite with bayldonite at 80%. Anglesite exists in the range from 20 to 45 °C.
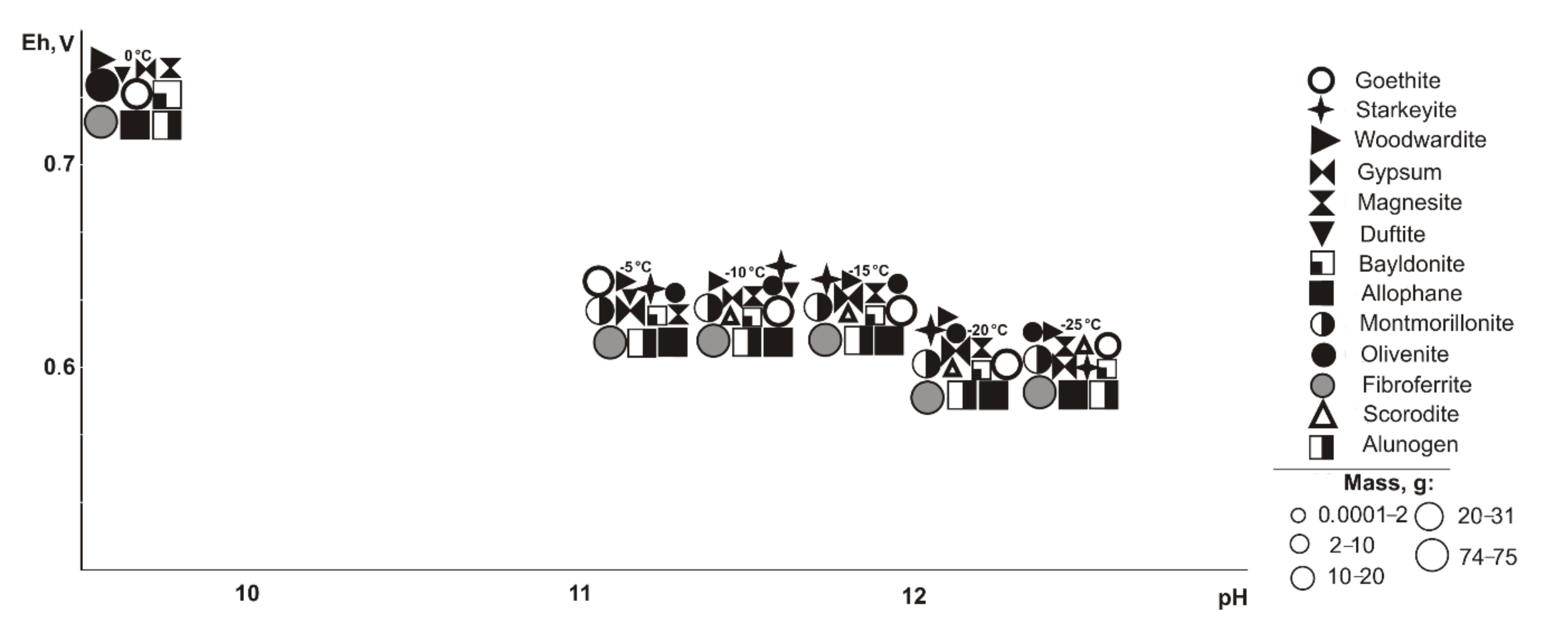
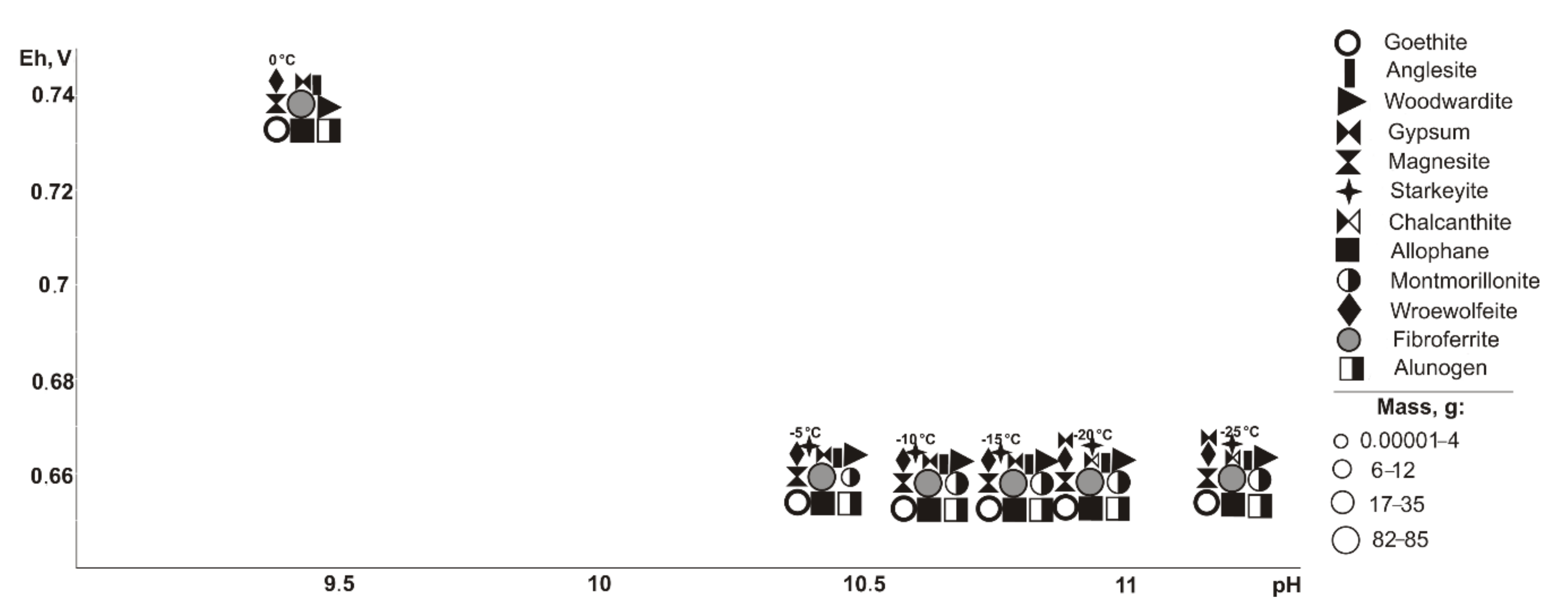
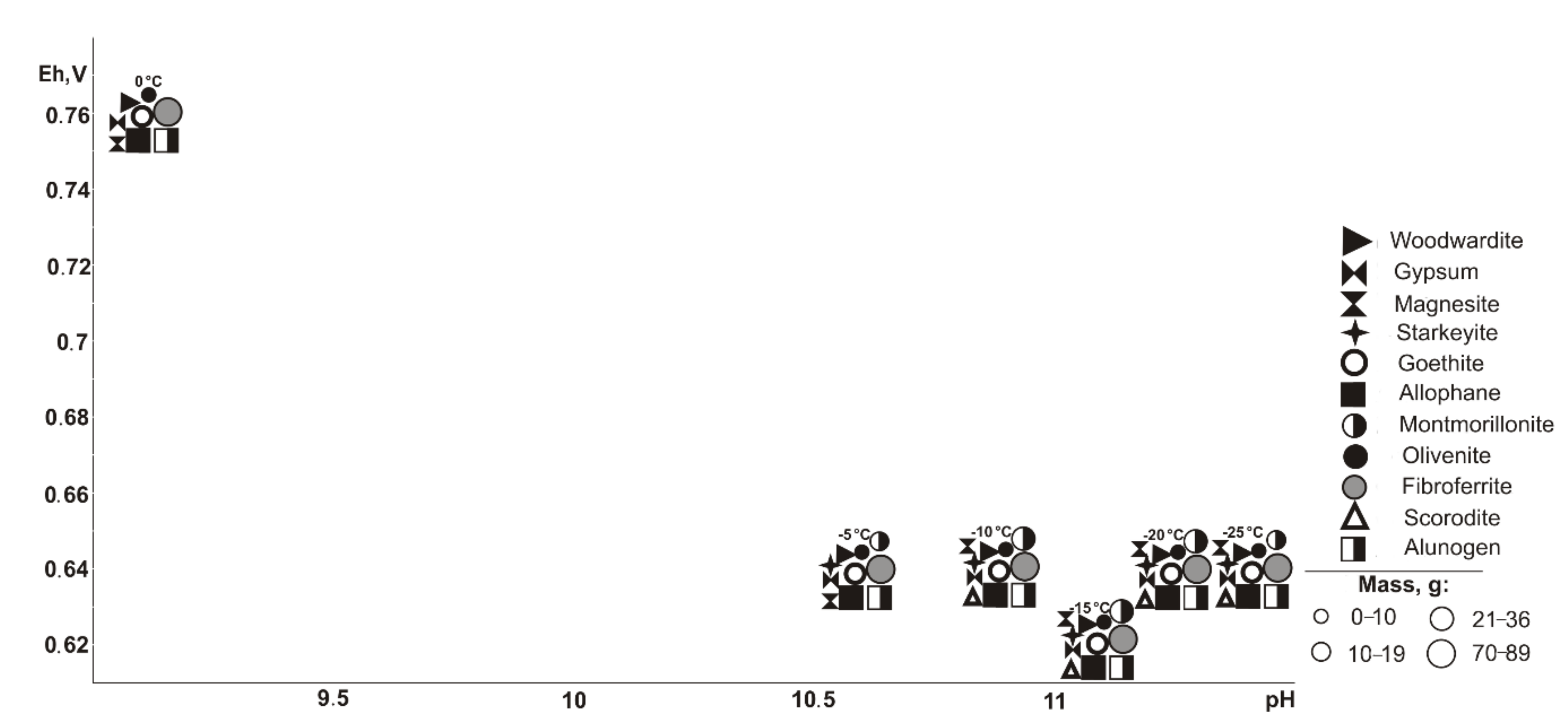
In the range of negative temperatures (
Figure 3), the following minerals crystallize (Eh-pH): goethite, starkeyite, bayldonite, and olivenite (0.5–1.1 V, 2.4–14.3); fibroferrite (0.6–1.1 V, 2.4–12.5), woodwardite, magnesite, and allophane (0.5–0.7 V, 10.1–14.3); gypsum (0.6–1.1 V, 2.4–14.0); alunogen (1.1 V, 2.4–2.5); scorodite (0.6–0.7 V, 10.1–12.5); duffitite (0.5–0.7, 10.3–14.3); and montmorillonite (0.5–0.6 V, 12.3–14.3). The following minerals disappear from the association at the following sulfide contents: woodwardite at 10%, montmorillonite at 20%, allophane at 40%, and goethite, magnesite, baildonite, and soononite at 80%; however, the following appear: olivenite at 10%, and fibroferrite and soonite at 20%. Woodwardite occurs within the temperature range from −25 to −15 °C, and duftite from −10 to −5 °C and alunogen at −25 to 0 °C.
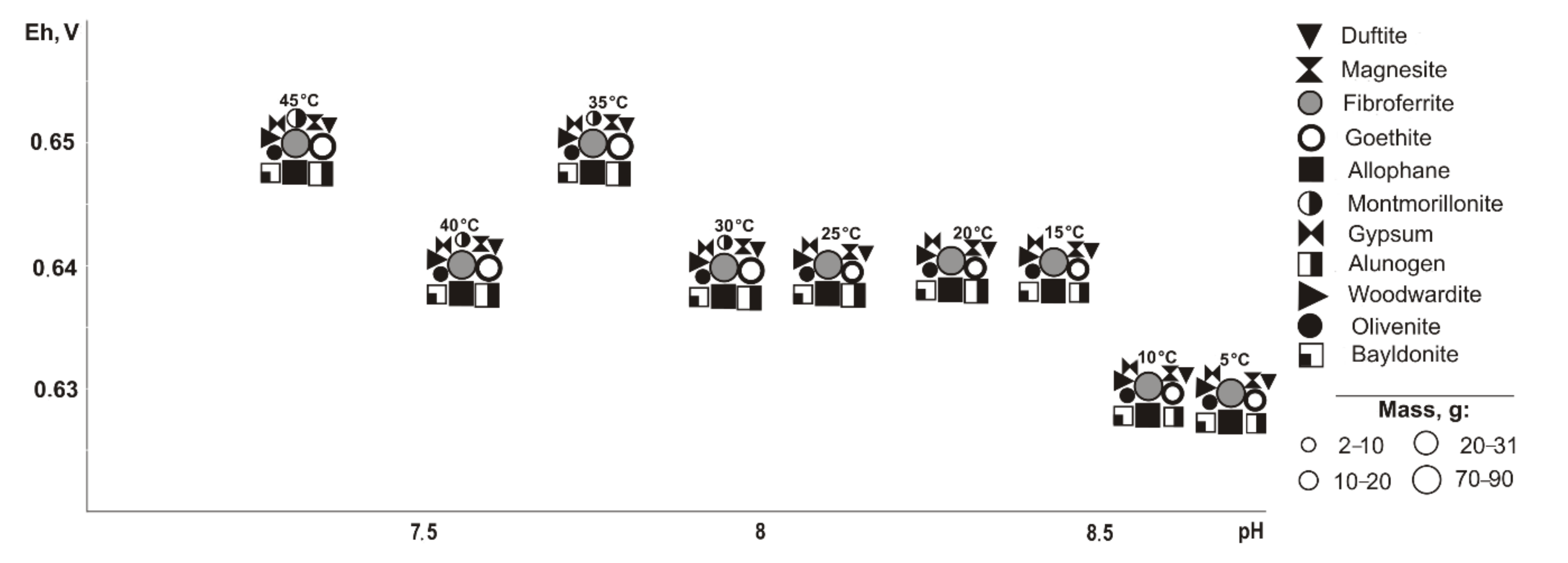
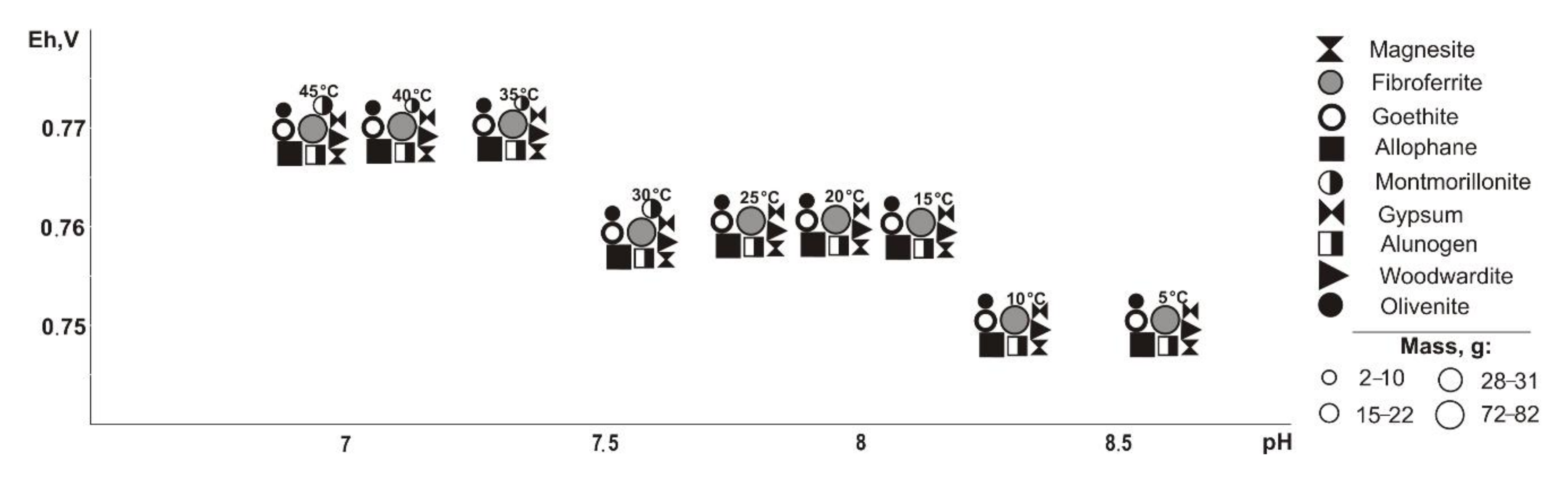
When pyrrhotite is removed from the systems (
Figure 4), the same minerals form as when pyrite is excluded, but the parameters of their crystallization are slightly different (Eh-pH): goethite and magnesite (0.58–0.75 V, 7.4–11); fibroferrite (0.67–1.0 V, 3.9–10.2); gypsum (0.61–1.0 V, 3.9–10.5); woodwardite, duftite, and allophane (0.58–0.81 V, 6.6–11.8); alunogen (0.8–1.0 V, 3.9–7.9); olivenite and baildonite (0.97–1.0 V, 3.9–4.1); and montmorillonite (0.58–0.62 V, 9.7–9.9). The qualitative composition of hypergenic minerals differs from the previous variant (without pyrite) as olivenite, instead of anglesite, precipitates at a sulfide content of 80%. Montmorillonite crystallizes in the range from 30 to 45 °C.
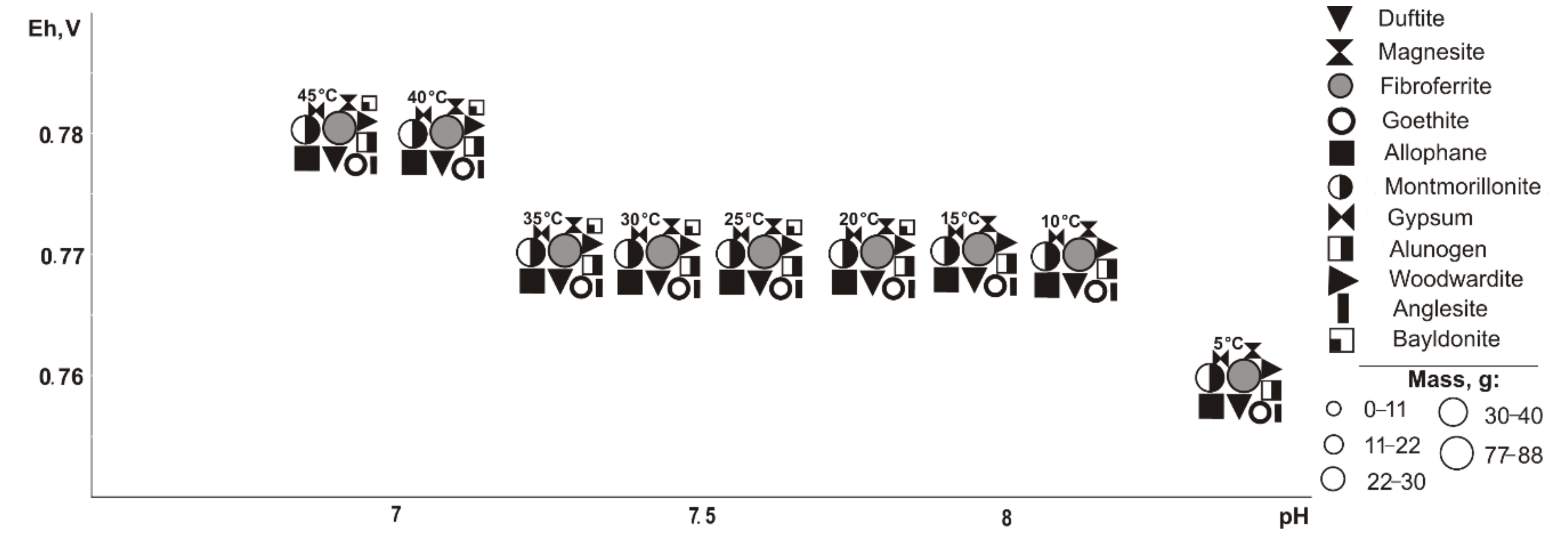
Under cryogenic conditions (
Figure 5), the following minerals (Eh-pH) are determined in the association: goethite, magnesite, and allophane (0.5–0.7 V, 10.1–14.3); gypsum (0.5–1.0 V, 4.1–14.3), woodwardite (0.5–0.7 V, 10.5–14.3); starkeyite and baildonite (0.5–1.0 V, 4.1–14.3); fibroferrite (0.6–1.0 V, 4.1–12.5); alunogen (0.8–1.0 V, 1.1–8.9); duffitite (0.5 V, 13.5); olivenite (0.5–1.0 V, 4.1–14.0); scorodite (0.6–1.0 V, 4.1–12.2); and montmorillonite (0.5–0.7 V, 12.3–14.3). Olivenite appears at a 10% sulfide content, fibroferrite and scorodite at 20%, alunogen at 80%, duftite disappears at 10%, woodwardite and montmorillonite at 20%, and goethite, magnesite, speed, and allophane at 80%. Duftite is seen in the range from −10 to −5 °C.
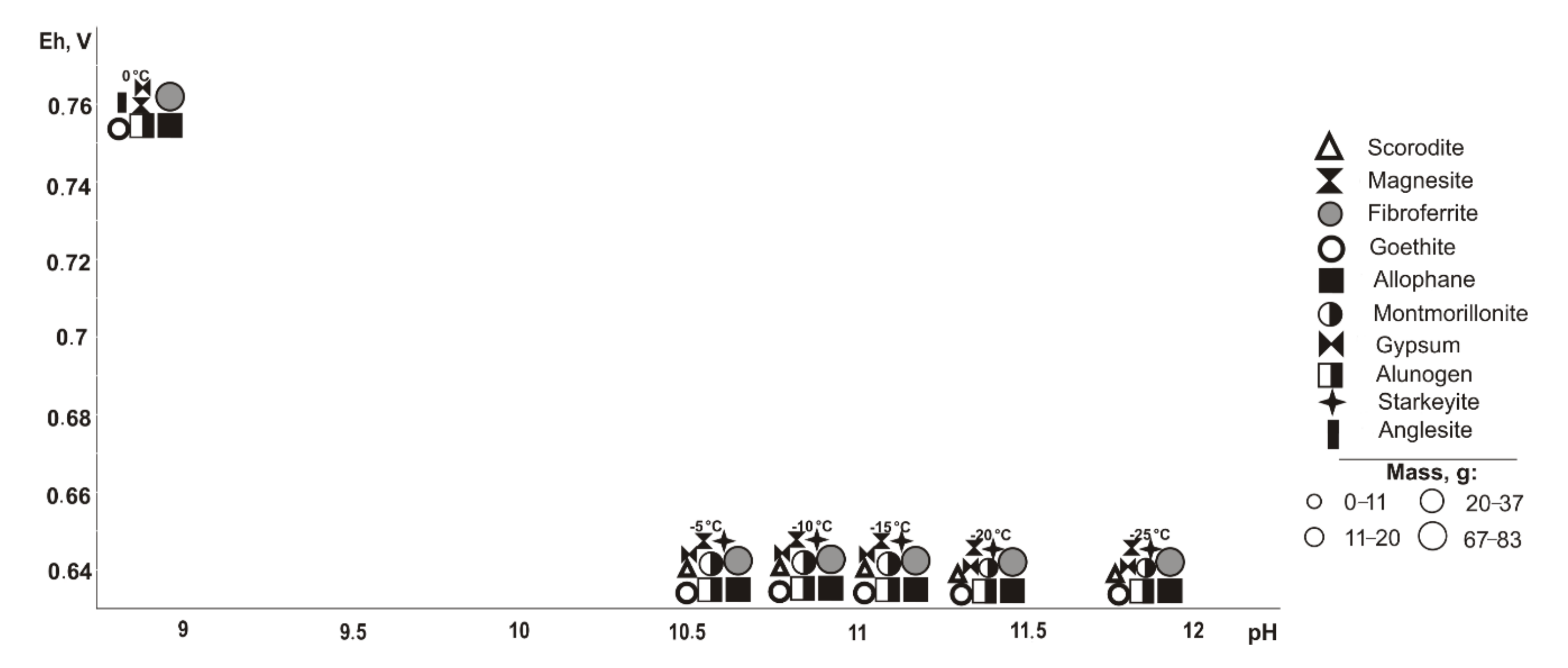
In the absence of chalcopyrite (
Figure 6), all copper-containing minerals disappear from the models. In the range of positive temperatures in the models, the following are formed (Eh-pH): goethite and magnesite (0.59–0.95 V, 7.4–11.7), gypsum (0.59–1.11 V, 2.1–11.7), fibroferrite (0.67–1.11 V, 2.1–11.7), alunogen and anglesite (0.8–1.11 V, 2.1–7.9), allophane (0.59–0.86 V, 5.7–11.7), and montmorillonite (0.57–0.61 V, 9.5–10.8). The sulfide component influences the qualitative composition of hypergenic minerals as follows: montmorillonite (at 10% sulfide content), goethite and magnesite (60%), and allophane (80%), however, the following appear: fibroferrite (10%), alunogen, and anglesite (60%). Montmorillonite was detected only at a 5% sulfide content and temperatures between 30 and 45 °C.
At negative temperatures (
Figure 7), the paragenesis of minerals includes (Eh-pH): goethite, magnesite, and allophane (0.5–0.7 V, 10.2–14.1); gypsum and starkeyite (0.5–1.1 V, 2.1–14.1); fibroferrite (0.6–1.1 V, 2.1–13.3); alunogen (0.9–1.1 V, 2.1–6.8); scorodite (0.6–0.7 V, 10.2–12.5); and montmorillonite (0.5–0.6 V, 12.2–14.1). Fibroferrite appears in association at a 10% sulfide content in the system and alunogen at 80%, scorodite appears at 20–40%, and goethite, magnesite, and allophane disappear at 80%.
Models without arsenopyrite (
Figure 8) do not contain arsenate-class minerals. Minerals (Eh-pH) that crystallize at temperatures from 0 to 45 °C include: goethite and magnesite (0.58–0.74 V, 7.4–11.8), gypsum (0.62–1.01 V, 3.4–10.4), woodwardite and allophane (0.58–0.85 V, 5.8–11.8), fibroferrite (0.67–1.01 V, 3.4–10.2), alunogen and anglesite (0.75–1.01 V, 3.4–8.0), antlerite (0.98–1.01 V, 3.9–4.0), posnjakite (0.98 V, 3.9), wroewolfeite (0.98–1.01 V, 3.4–4.0), and montmorillonite (0.57–0.61 V, 9.5–10.7). The following minerals disappear from the system at various sulfide contents (%): montmorillonite (20%), goethite and magnesite (60%), and woodwardite and allophane (80%); however, the following appear: gypsum (10%), fibroferrite (20%), alunogen and anglesite (60%), as well as antlerite, posnjakite, and wroewolfeite (80%). Posnjakite forms in the temperature range from 25 to 45 °C, antlerite at 35–45 °C, and wroewolfeite at 0–25 °C.
At temperatures below 0 °C (
Figure 9), the models show (Eh-pH): goethite, woodwardite, magnesite, and allophane (0.5–0.7 V, 10.3–14.7); gypsum (0.6–0.7 V, 10.3–12.4); fibroferrite (0.6–1.1 V, 2.0–12.7); starkeyite (0.5–1.1 V, 2.0–14.7); alunogen, wroewolfeite, and anglesite (1.0–1.1 V, 2.0–4.0), chalcantite (1.1 V, 2.0–2.1); and montmorillonite (0.5 V, 13.2–14.7). Montmorillonite is absent in the models with a 20% sulfide content, and goethite, woodwardite, magnesite and allophane are absent in the models with a 80% sulfide content, but in the latter variant, alunogen, wroewolfeite, and chalcantite crystallize only in the range from −25 to −20 °C, as does anglesite.
Removal of galena (
Figure 10) resulted in the absence of lead minerals in the models. In the interval of positive temperatures (Eh-pH), the following minerals form: goethite and magnesite (0.59–0.75 V, 7.4–11.6), gypsum (0.59–1.07 V, 2.8–11.6), fibroferrite (0.67–1.07 V, 2.8–10.2), woodwardite and allofan (0.59–0.85 V, 5.7–11.8), alunogen (0.8–1.07 V, 2.8–7.9), olivenite (1.03–1.07 V, 2.7–2.8), and montmorillonite (0.57–0.62 V, 9.5–10.8). The following minerals (sulfide content, in %) disappear: montmorillonite at 10%; goethite and magnesite at 60%; and gypsum, woodwardite, and allophane at 80%; and the following appear: fibroferrite at 10%, alunogen at 60%, and olivenite at 80%. Montmorillonite crystallizes in the range from 30 to 45 °C.
In the range of negative temperatures (
Figure 11), the associations are as follows (Eh-pH): goethite, magnesite and allophane (0.5–0.7 V, 10.0–14.2), gypsum (0.5–1.1 V, 2.9–13.0), starkeyite and olivenite (0.5–1.1 V, 2.9–14.2), fibroferrite (0.6–1.1 V, 2.9–13.7), woodwardite (0.6–0.7 V, 10.0–13.1), alunogen (0.9–1.1 V, 2.9–6.1), scorodite (0.6–0.9 V, 5.5–12.5), and montmorillonite (0.5–0.6 V, 12.7–14.2). Montmorillonite disappears in the systems at a 20% sulfide content, and goethite, woodwardite, magnesite, and allophane at 80%; however, the following appear: fibroferrite at 10%, scorodite at 20–80% in the temperature range from −25 to −10 °C, and alunogen at 80%.
When sphalerite is excluded (
Figure 12) from the composition of oxidizing sulfides in the positive temperature range, the paragenesis of minerals (Eh-pH) forms as follows: goethite and magnesite (0.57–0.75 V, 7.4–11.5), gypsum (0.57–1.09 V, 2.5–11.4), fibroferrite (0.67–1.09 V, 2.5–10.1), woodwardite and allophane (0.57–0.85 V, 5.7–11.5), alunogen (0.79–1.09 V, 2.5–7.9), anglesite and baildonite (1.04–1.09 V, 2.5–2.8), duftite (0.67–1.09 V, 2.5–10.1), and montmorillonite (0.57–0.62 V, 9.5–11.5). Depending on the sulfide content in the system, the following disappear: montmorillonite at 10%, goethite and magnesite at 60%, woodwardite and allophane at 80%; and fibroferrite and duftite appear at 10%, alunogen at 60%, and anglesite with baildonite at 80%.
In the range of low temperatures (
Figure 13) in the simulated systems, the following are found (Eh-pH): goethite, magnesite, and allophane (0.5–0.7 V, 9.3–14.4); starkeyite (0.5–1.1 V, 2.5–14.4); woodwardite (0.5–0.7 V, 10.1–14.4); gypsum (0.5–1.1 V, 2.5–13.0); fibroferrite (0.7–1.1 B, 2.5–10.1), duftite (0.5–0.7 V, 9.3–13.2); baildonite (0.5–1.1 V, 2.5–14.4); olivenite (0.5–0.9 V, 6.1–13.8); scorodite (0.6 V, 11.2–12.4); chalcantite (0.9 V, 6.1–7.1); alunogen (0.9–1.1 V, 2.5–7.1); anglesite (1.1 V, 2.5); and montmorillonite (0.5–0.6 V, 12.7–14.4). High pH values were found in models with high content of host rocks under cryogenic conditions; such systems contain sulfates (woodwardite, gypsum, etc.) with insignificant masses (from 0.00001 to 0.8 g)
In the system, the following disappear with different sulfide contents: montmorillonite at 20%, goethite; and woodwardite, magnesite, duftite, bayldonite, and allophane at 80%; and the following appear: fibroferrite and olivenite at 10%, calcite only at 40%, as well as chalcantite, alunogen, and anglesite at 80%. Crystallization of a number of minerals is noted in a certain temperature range: starkeyite, olivenite, bayldonite, and montmorillonite at −25 to −5 °C; chalcantite at −25 to −10 °C; anglesite only at 0 °C.
3.4. Tailings Oxidation With Cementation Zone Sulfides
We also examined the processes of sulfide oxidation in contact with the host rock (at the ratios of 5:95, 10:90, 20:80, 40:60, and 80:20) at the tailings dump of the Vysokogorsk deposit with the participation of cementation zone sulfides in the temperature range from 0 to 45 °C. Calculations included 19 independent (Al, Ar, As, B, C, Ca, Cu, Fe, K, Mg, N, Na, Pb, S, Si, Zn, H, O, and ē) and 368 dependent components: 283 dissolved particles, 18 gases, and 67 solid phases.
The following minerals crystallize from solutions in the following Eh-pH conditions: goethite, woodwardite, magnesite, duftite, and allophane (0.58–0.75 V, 7.4–11.8); fibroferrite and gypsum (0.67–1.0 V, 3.5–10.1), alunogen, olivenite, and baildonite (1.04–1.09 V, 2.5–2.8); antlerite (1.04–1.05 V, 2.8); posnjakite (1.05–1.06 V, 2.7); wroewolfeite (1.06–1.09 V, 2.5–2.6); and montmorillonite (0.56–0.62 V, 9.5–11.2). As it was seen previously, the masses of sulfates in high alkaline pH cases are insignificant. Montmorillonite is absent in systems with a sulfide content of 20% or more, and goethite, woodwardite, magnesite, duftite, and allophane at 80%. The following minerals appear at various sulfide contents: gypsum at 10%, fibroferrite at 20%, and alunogen, antlerite, posnyakite, rovolfit, olivionite, and baildonite at 80%.
Thus, the hypergenic minerals paragenesis formed from mine waters in mine workings (adits and quarries) is represented by the following minerals: fibroferrite, chalcantite, posnyakite, wroewolfeite, antlerite, goslarite, anglesite, baildonite, duftite, and olivenite. During oxidation of tailings in the temperature range from −25 to 45 °C, the association is characterized by goethite, gypsum, woodwardite, alunogen, fibroferrite, magnesite, allophane, and montmorillonite.
In the range of positive temperatures, in the absence of pyrite in the composition of sulfides, alunogen does not precipitate, and crystallization of anglesite is possible only in the presence of the enrichment of sulfide containing Pb (galena), duftite, and baildonite: Cu, Pb, and As. The presence of tenorite in paragenesis is observed only in the Khrustalnoe deposit. Antlerite, posnjakite, and wroewolfeite are present in the association when the tailings of the enrichment include minerals of the cementation zone or no arsenopyrite is present. Olivenite is deposited in the absence of galena or pyrrhotite in the system, as well as in the presence of minerals from the secondary sulfide enrichment zone.
In the range of negative temperatures, the association additionally includes starkeyite (−25 to −5 °C). The models do not contain sulfide; if all sulfides are present in the enrichment wastes, there is no As (arsenopyrite), and olivenite, baildonite, and duftite do not precipitate without Cu and As (chalco- and arsenopyrite), nor do baildonite and duftite without Pb (galena). Anglesite crystallizes only in models without arsenopyrite and sphalerite, as well as wroewolfeite and chalcantite in the presence of all sulfides or in the absence of arsenopyrite.
The factors determining hypergenic and technogenic minerals paragenesis formation during the sulfide ores oxidation in the mining industrial technogenic system are the presence of sulfide in the simulated system, the relationship between sulfides and the host rock, and temperature.
Comparison with previously reported findings [
2] showed that all hypergenic minerals formed during modeling were found and diagnosed in the mining and industrial technogenic system in the Kavalerovsky district.
The formation of mine water occurs when all micropore solutions, the variants of which are described above, are merged. The study of hydrochemical samples of mine waters has shown that in the studied area, the pH values are in the range from 4.5 to 8.1 [
17].