Pickeringite from the Stone Town Nature Reserve in Ciężkowice (the Outer Carpathians, Poland)
Abstract
1. Introduction
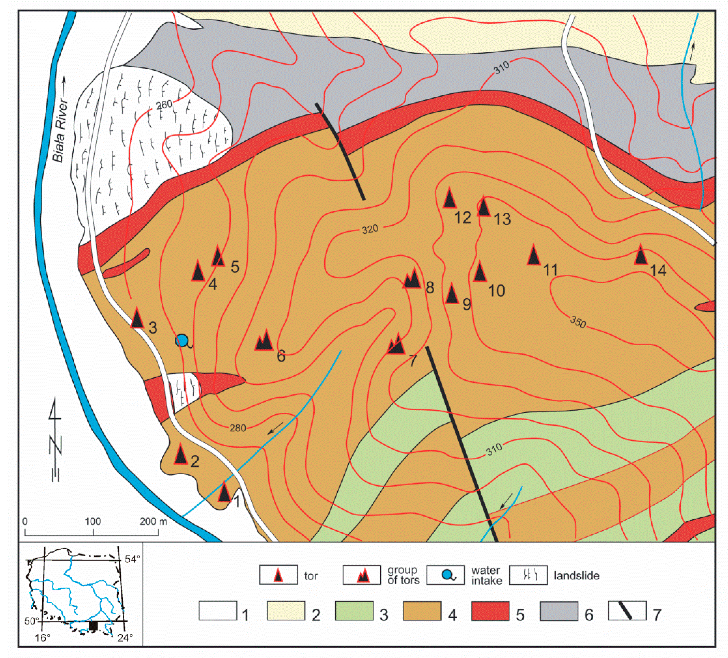
2. Geological Setting
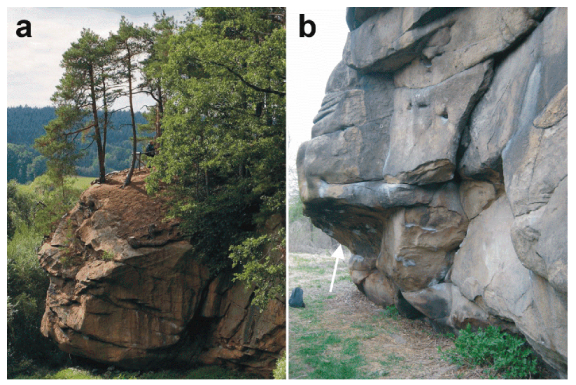
3. The Ciężkowice Sandstones and their Efflorescences
4. Materials and Methods
5. Results
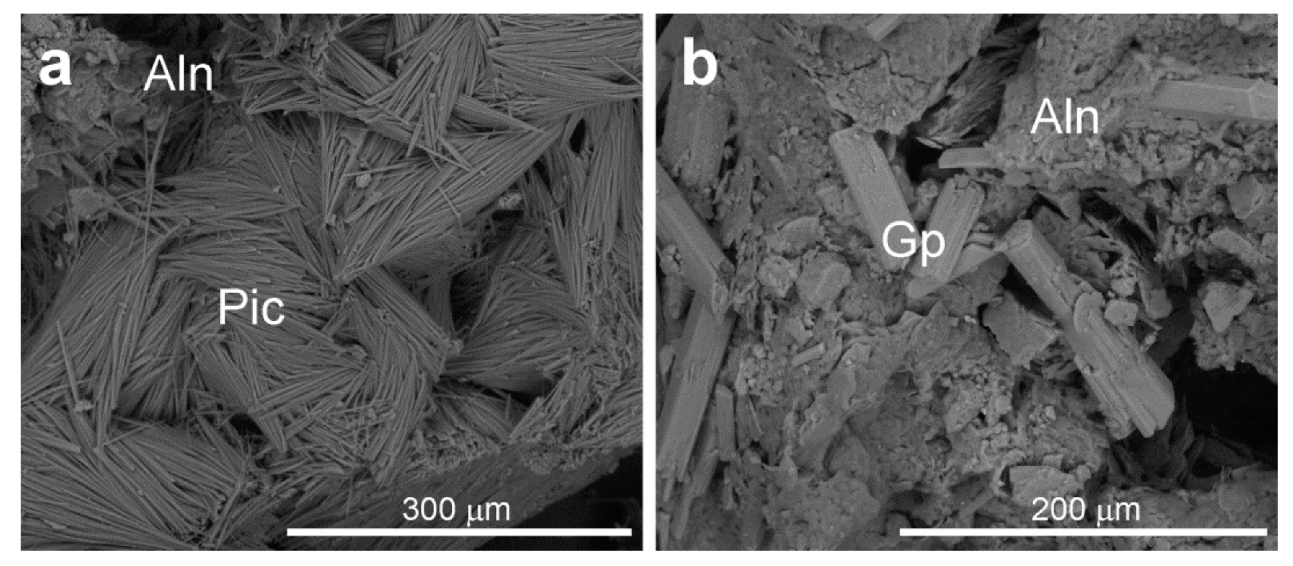
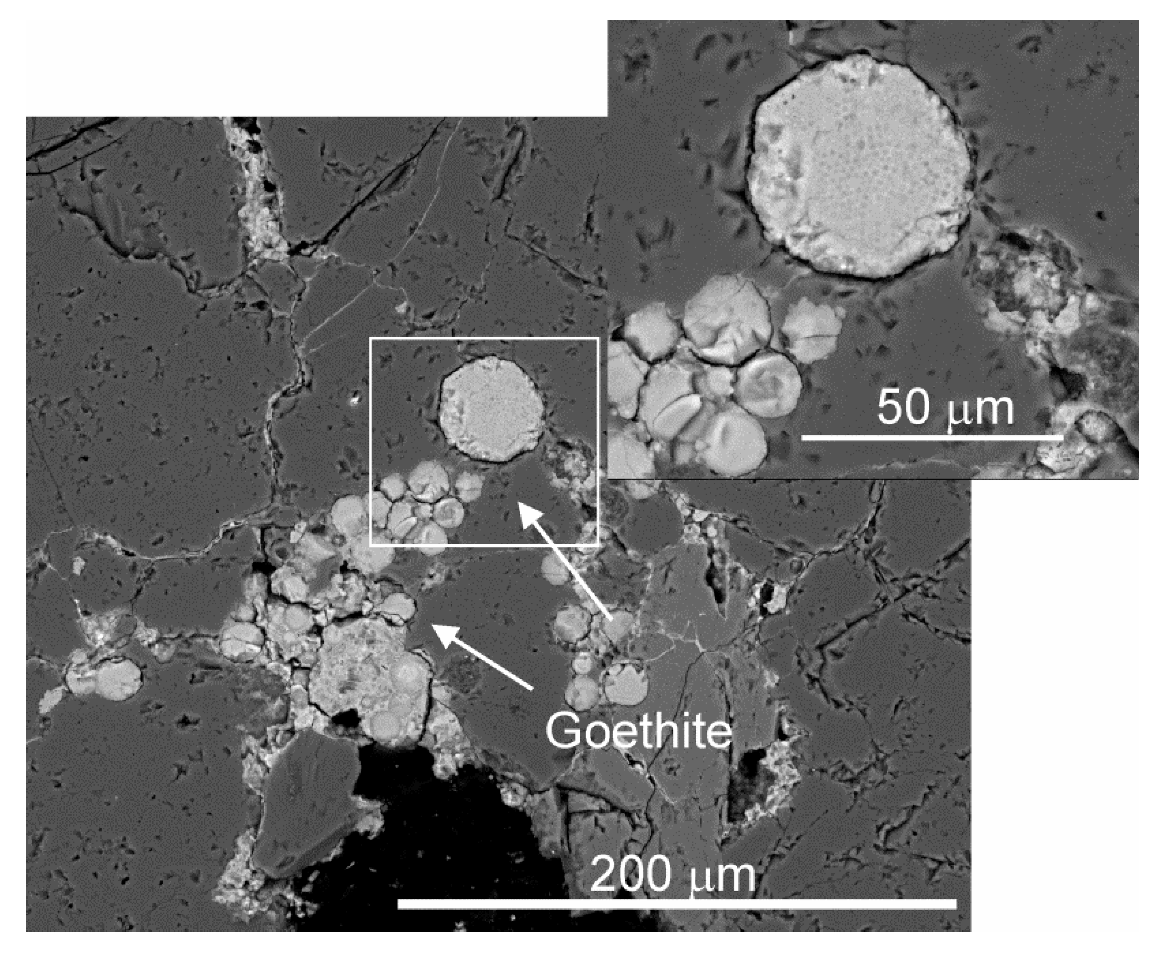
5.1. Scanning Electron Microscopy (SEM-EDS)
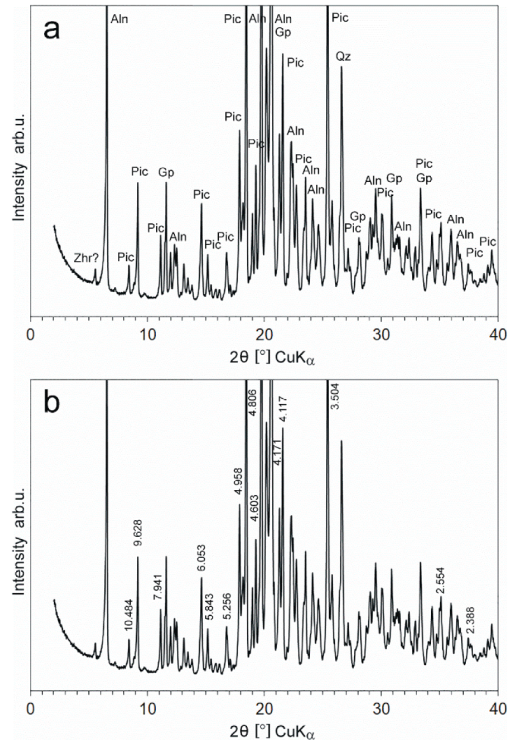
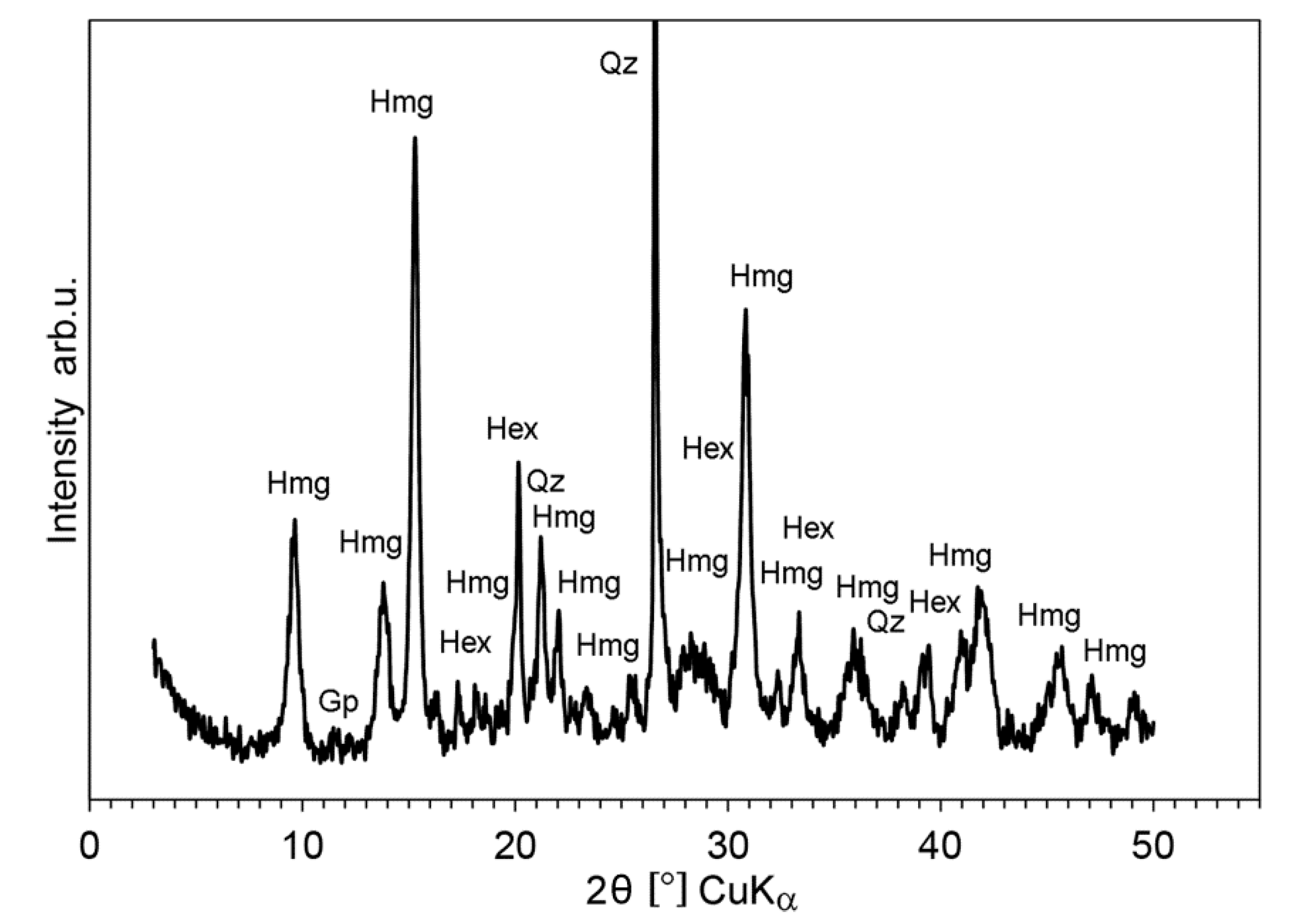
5.2. X-Ray Diffractometry (XRPD)
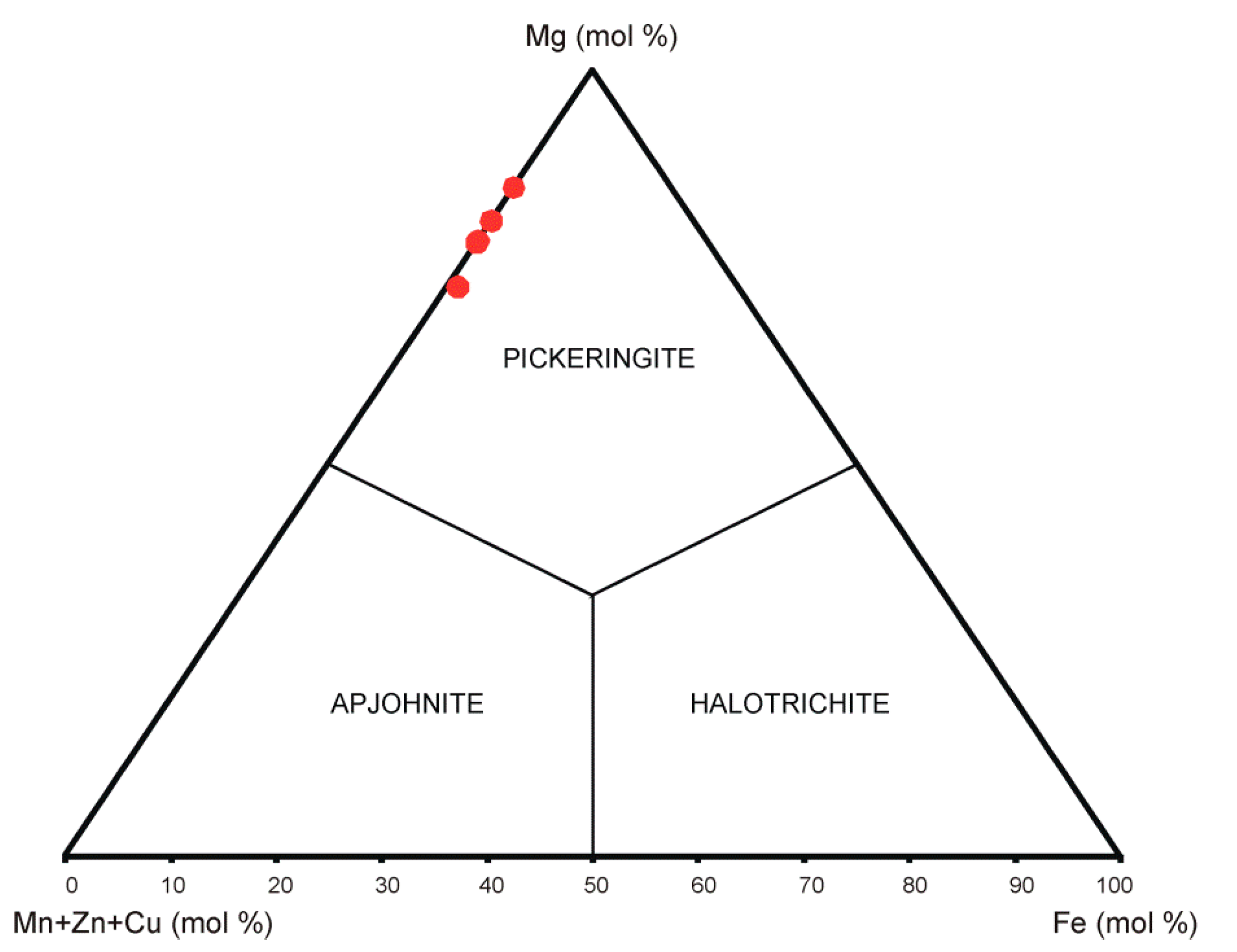
5.3. Chemical Analyses (EPMA)
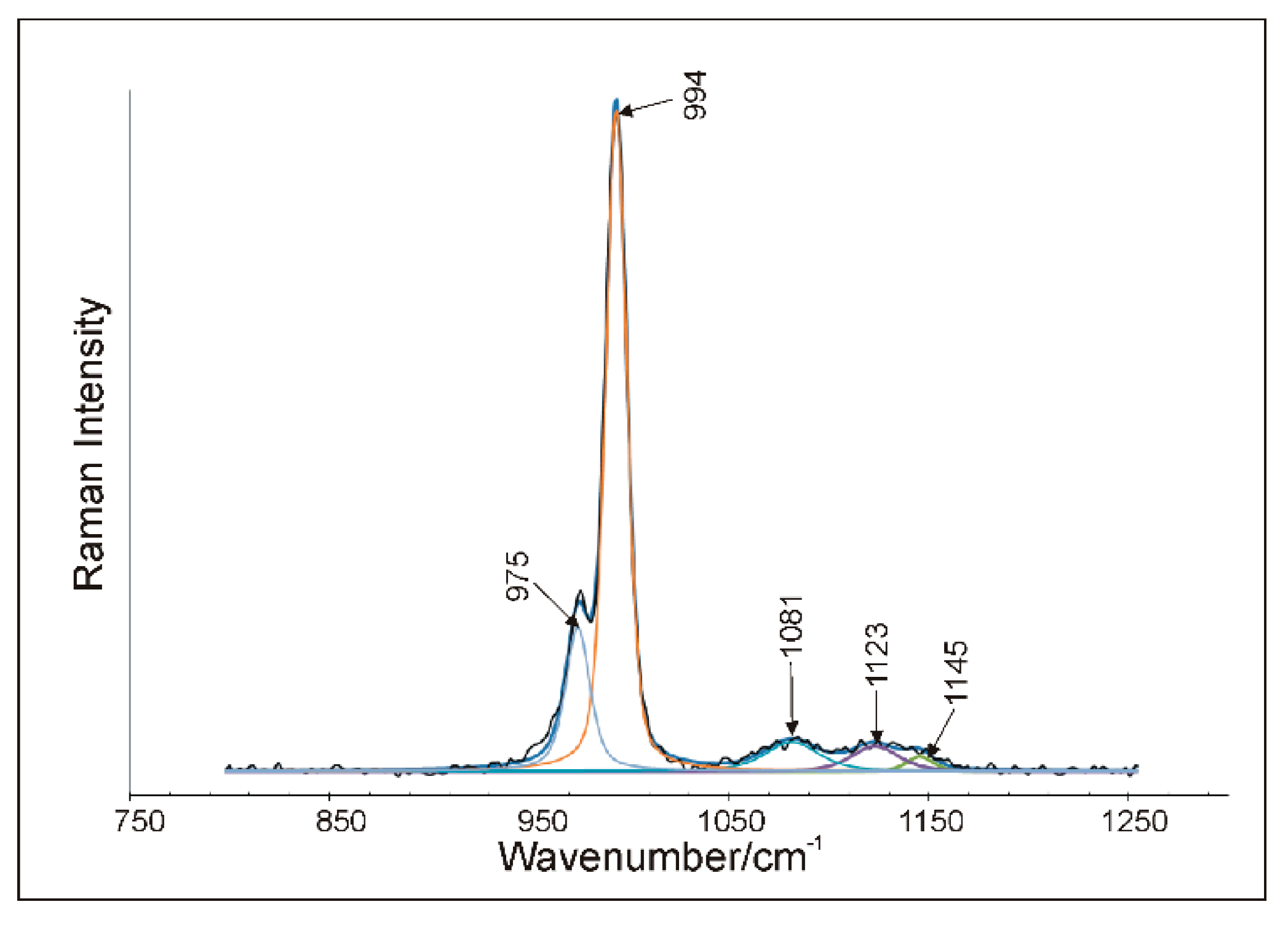
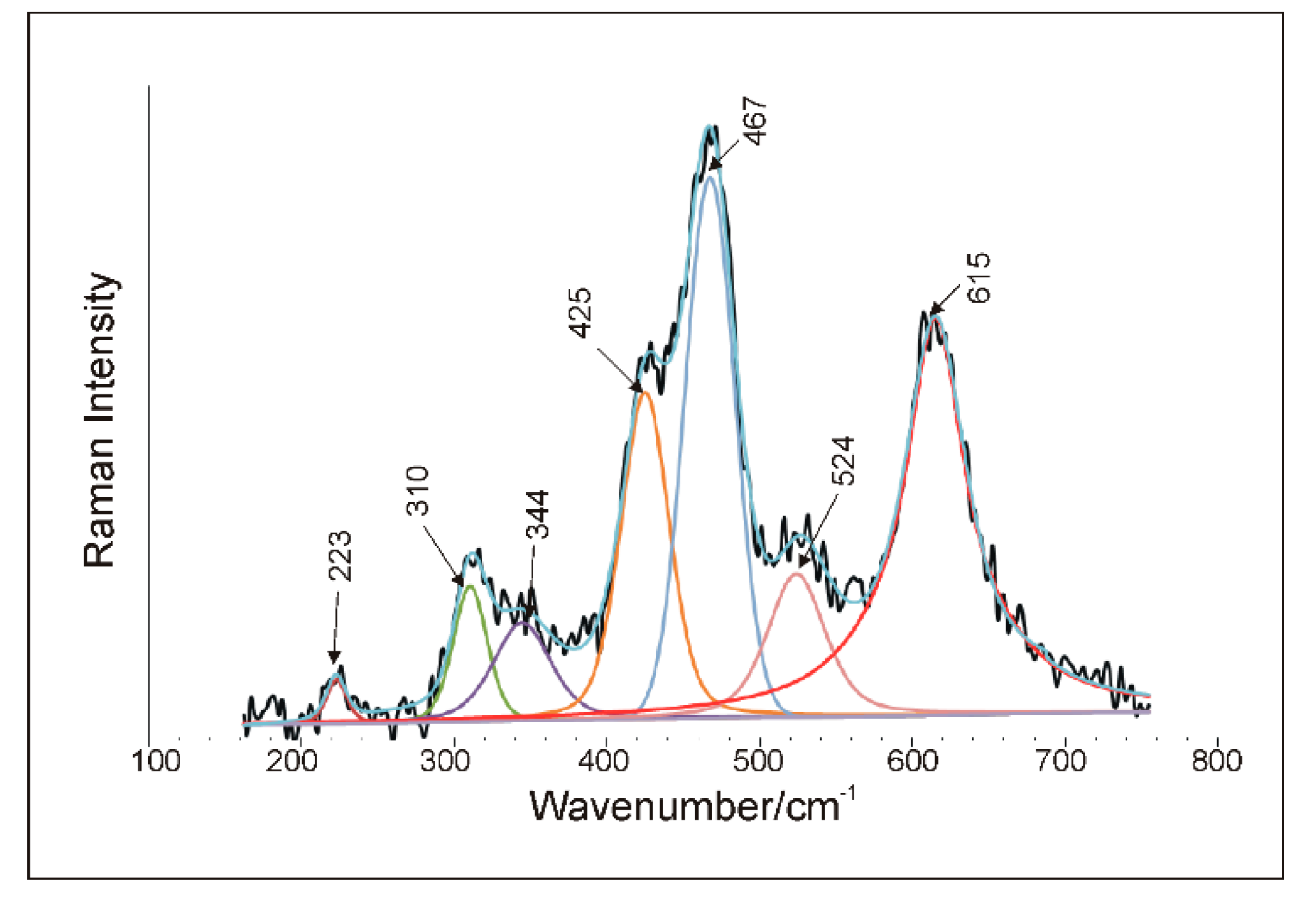
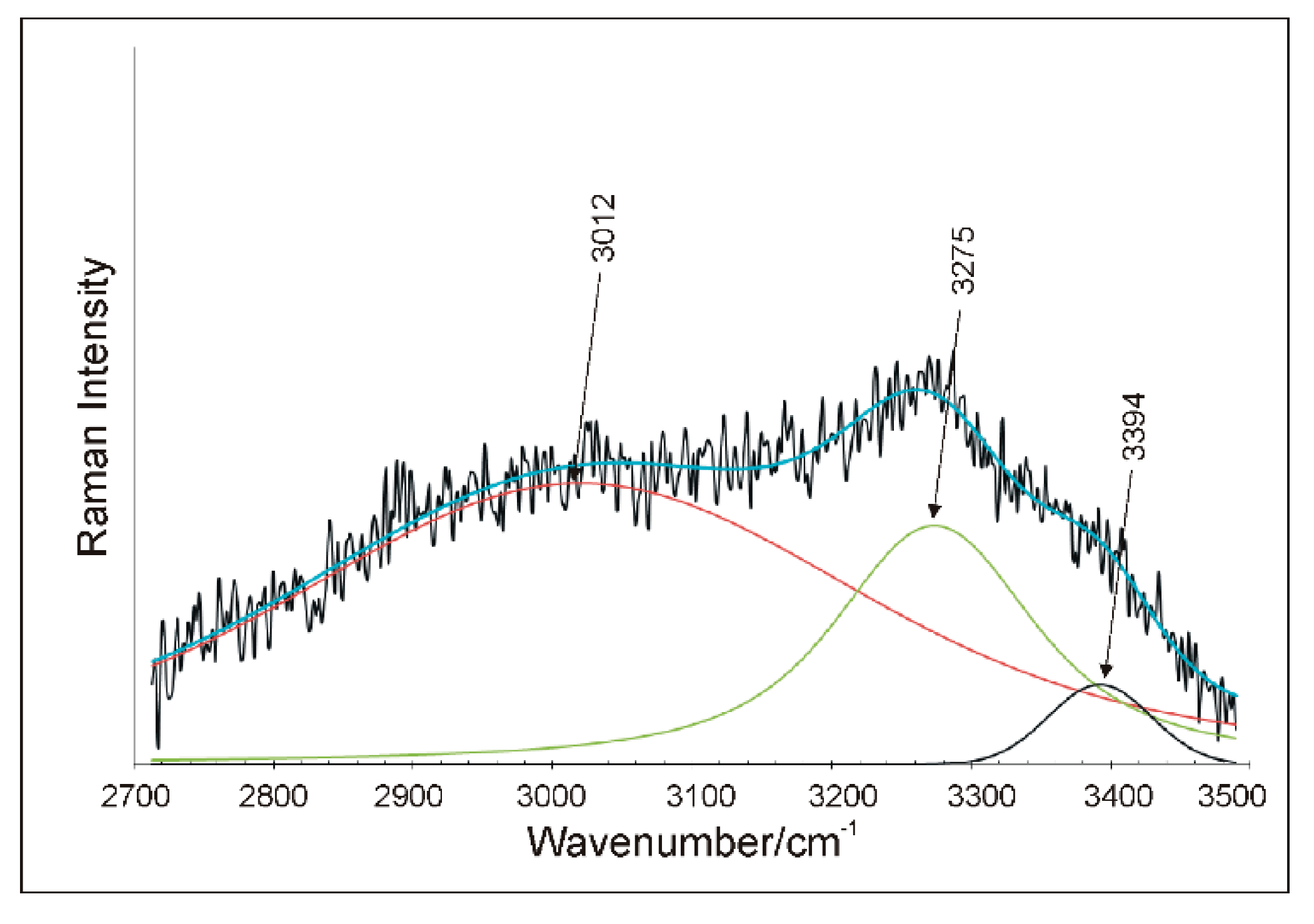
5.4. Raman Microspectroscopy (RS)
6. Discussion and Concluding Remarks
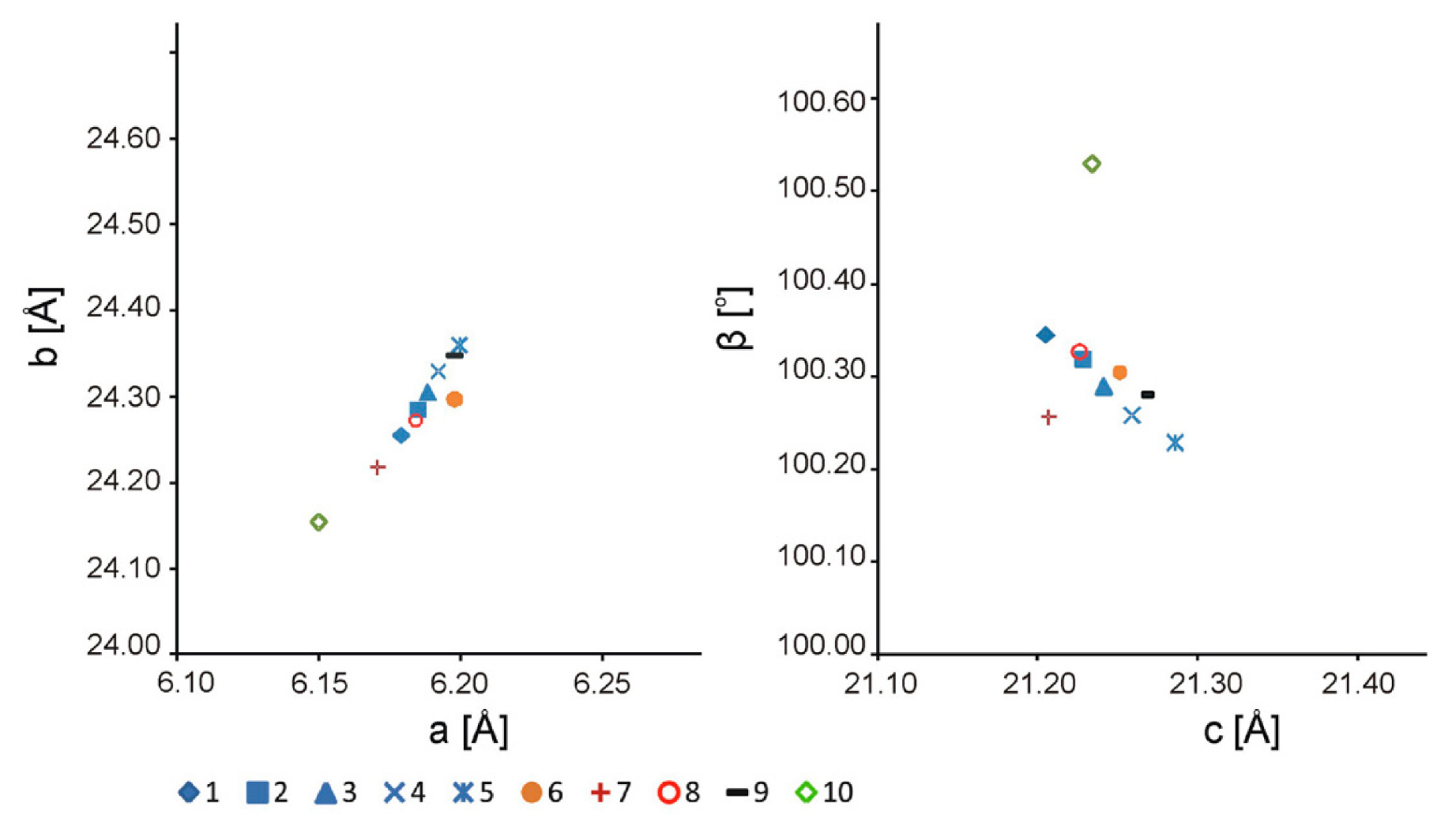
6.1. Chemical Characteristics and Unit Cell Parameters of Pickeringite
6.2. Spectroscopic Characteristics
6.3. Possible Origin
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Quartieri, S.; Triscari, M.; Viani, A. Crystal structure of the hydrated sulphate pickeringite (MgAl2(SO4)4 22H2O): X-ray powder diffraction study. Eur. J. Miner. 2000, 12, 1131–1138. [Google Scholar] [CrossRef]
- Frost, R.L.; Kloprogge, J.T.; Williams, P.A.; Leverett, P. Raman microscopy of some natural pseudo-alums: Halotrichite, apjohnite and wupatkiite, at 298 and 77 K. J. Raman Spectrosc. 2000, 31, 1083–1087. [Google Scholar] [CrossRef]
- Palmer, S.; Frost, R.L. Synthesis and spectroscopic characterization of apjohnite and pickingerite. Polyhedron 2006, 25, 3379–3386. [Google Scholar] [CrossRef][Green Version]
- Menchetti, S.; Sabelli, C. The halotrichite group: The crystal structure of apjohnite. Mineral. Mag. 1976, 40, 599–608. [Google Scholar] [CrossRef]
- Ballirano, P. Crystal chemistry of the halotrichite group XAl2(SO4)4·22H2O: The X = Fe-Mg-Mn-Zn compositional tetrahedron. Eur. J. Miner. 2006, 18, 463–469. [Google Scholar] [CrossRef]
- Hammarstrom, J.M.; Smith, K.S. Geochemical and mineralogical characterization of solids and their effects on waters in metal-mining environments. In Progress on Geoenvironmental Models for Selected Mineral Deposit Types; Seal, R.R., II, Foley, N.K., Eds.; US Geological Survey Open-File Report 02-195; US Geological Survey: Reston, VA, USA, 2002; pp. 9–54. [Google Scholar]
- Locke, A.J.; Martens, W.N.; Frost, R.L. Natural halotrichites-an EDX and Raman spectroscopic study. J. Raman Spectrosc. 2007, 38, 1429–1435. [Google Scholar] [CrossRef]
- Kruszewski, Ł. Supergene sulphate minerals from the burning coal mining dumps in the Upper Silesian Coal Basin, South Poland. Int. J. Coal Geol. 2013, 105, 91–109. [Google Scholar] [CrossRef]
- Parnell, R.A., Jr. Weathering processes and pickeringite formation in a sulfidic schist: A consideration in acid precipitation neutralization studies. Environ. Geol. 1983, 4, 209–215. [Google Scholar] [CrossRef]
- Hammarstrom, J.M.; Seal, R.R., II; Meier, A.L.; Kornfeld, J.M. Secondary sulfate minerals associated with acid drainage in the eastern, US: Recycling of metals and acidity in surficial environments. Chemic. Geol. 2005, 215, 407–431. [Google Scholar] [CrossRef]
- Farkas, I.M.; Weiszburg, T.G.; Pekker, P.; Kuzmann, E. A half-century of environmental mineral formation on a pyrite-bearing waste dump in the Mátra mountains, Hungary. Can. Mineral. 2009, 47, 509–524. [Google Scholar] [CrossRef]
- Mladenova, V.; Dimitrova, D.; Schmitt, R.F. Efflorescent minerals formed during intensive weathering of phyllites, Chiprovtsi ore district, Northwestern Bulgaria. In Proceedings of the Annual Conference of Bulgarian Geological Society (Geosciences-2007), Sofia, Bulgaria, 13–14 December 2007; pp. 63–64. [Google Scholar]
- Gomes, P.; Valente1, T.; Grande, J.A.; Cordeiro, M. Occurrence of sulphate efflorescences in São Domingos mine. Comun. Geológicas 2017, 104, 83–89. [Google Scholar]
- Martin, R.; Rodgers, K.A.; Browne, P.R.L. The nature and significance of sulphate-rich, aluminous efflorescences from the Te Kopia geothermal field, Taupo Volcanic Zone, New Zealand. Mineral. Mag. 1999, 63, 413–419. [Google Scholar] [CrossRef][Green Version]
- Parafiniuk, J. Fibroferrite, slavikite and pickeringite from the oxidation zone of pyrite-bearing schists in Wieściszowice (Lower Silesia). Mineral. Pol. 1991, 22, 3–15. [Google Scholar]
- Parafiniuk, J. Sulphate minerals and their origin in the weathering zone of the pyrite-bearing schist at Wieściszowice (Rudawy Janowickie Mts., Western Sudetes, Poland). Acta Geol. Pol. 1996, 46, 353–414. [Google Scholar]
- Naglik, B.; Natkaniec-Nowak, L. Pickeringite from the Pieprzowe Mts. (the Holy Cross Mts., Central Poland). Geol. Geoph. Environ. 2015, 41, 114–115. [Google Scholar] [CrossRef][Green Version]
- Jambor, J.L.; Nordstrom, D.K.; Alpers, C.N. Metal-sulfate salts from sulfide mineral oxidation. In Sulfate Minerals Crystalography, Geochemistry and Environmental Significance; Alpers, C.N., Jambor, J.L., Nordstrom, D.K., Eds.; Reviews in Mineralogy & Geochemistry; Mineralogical Society of America, Geochemical Society: Washington, DC, USA, 2000; Volume 40, pp. 303–350. [Google Scholar] [CrossRef]
- Blass, G.; Strehler, H. Mineralbildungen in einer durch Selbstentzündung brennenden Bergehalde des Aachener Steinkohlenreviers. Miner. Welt 1993, 4, 35–42. (In German) [Google Scholar]
- Bouška, V.; Dvořák, Z. Minerals of the North Bohemian Lignite Basins; Nakl.: Dick, Praha, 1997; pp. 1–159. (In Czech) [Google Scholar]
- Jírasek, J. Thermal Changes of the Rocks in the Dump Pile of the Kateřina Colliery in Radvanice (Eastern Bohemia); VSB-Technical University of Ostrava, Institute of Geological Engineering: Ostrava, Czechia, 2001; Volume 541, p. 69. [Google Scholar]
- Szakáll, S.; Kristály, F. Ammonium sulphates from burning coal dumps at Komló and Pécs-Vasas, Mecsek Mts., South Hungary. Mineral. Spec. Papers 2008, 32, 155. [Google Scholar]
- Brant, R.A.; Foster, W.R. Magnesian Halotrichite from Vinton County, Ohio. Ohio J. Sci. 1959, 59, 187–192. [Google Scholar]
- Vorma, A. Sulfide-bearing mica gneiss containing pickeringite. Bull. Comm. Geol. Finlinde 1966, 38, 51–53. [Google Scholar]
- Suchý, V.; Sýkorová, I.; Zachariáš, J.; Filip, J.; Machovič, V.; Lapčák, L. Hypogene Features in Sandstones: An Example from Carboniferous Basins of Central and Western Bohemia, Czech Republic. In Hypogene Karst Regions and Caves of the World; Klimchouk, A.W., Palmer, A., De Waele, J., Auler, A.S., Audra, P., Eds.; Springer International Publishing AG: New York, NY, USA, 2017; pp. 313–328. [Google Scholar] [CrossRef]
- Lazaridis, G.; Melfos, V.; Papadopoulou, L. The first cave occurrence of orpiment (As2S3) from the sulfuric acid caves of Aghia Paraskevi (Kassandra Peninsula, N. Greece). Int. J. Speleol. 2011, 40, 133–139. [Google Scholar] [CrossRef][Green Version]
- Sulovsky, P.; Gregerova, M.; Pospisil, P. Mineralogical Study of Stone Decay in Charles Bridge, Prague. In Proceedings of the 8th International Congress on the Deterioration and Conservation of Stone, Berlin, Germany, 30 September–4 October 1996; Riederer, J., Ed.; Möller Druck und Verlag: Berlin, Germany, 1996; pp. 29–36. [Google Scholar]
- Küng, A.; Zehnder, K. Pickeringite: A deleterious salt on buildings. Stud. Conserv. 2017, 62, 304–308. [Google Scholar] [CrossRef]
- Wieser, T. Siarczanowe produkty wietrzenia na złożu żelaza Gór Świętokrzyskich. Ann. Soc. Geol. Pol. 1949, 19, 445–477. (In Polish) [Google Scholar]
- Kubisz, J. Studies of supergene sulphate minerals occurring in Poland. Prace Geol. 1964, 26, 1–76. (In Polish) [Google Scholar]
- Biłoniżka, P.M. O pochodzeniu mioceńskich soli potasowo-magnezowych Przedkarpacia. Prz. Geol. 1996, 44, 1029–1031. (In Polish) [Google Scholar]
- Gajdówna, E. Gips i Towarzyszące mu Minerały w Dobrzyniu Nad Wisłą; Muzeum Ziemi: Warszawa, Poland, 1952; p. 6. (In Polish) [Google Scholar]
- Mazur, L. Rentgenograficzno-chemiczne badania siarczanowych produktów wietrzenia pirytu występującego w Dobrzyniu nad Wisłą. Stud. Soc. Sci. Tor. 1962, IV, 2. (In Polish) [Google Scholar]
- Parafiniuk, J. Sulphate weathering minerals from the mica schist quarry in Krobica (West Sudeten, SW Poland). Prz. Geol. 1994, 7, 536–538. (In Polish) [Google Scholar]
- Ciesielczuk, J.; Kruszewski, Ł.; Fabiańska, M.J.; Misz-Kennan, M.; Kowalski, A.; Mysza, B. Efflorescences and gas composition emitted from the burning coal-waste dump in Słupiec, Lower Silesian Coal Basin, Poland. In Proceedings of the International Symposium CEMC 2014, Skalský Dvůr, Czech Republic, 23–26 April 2014; pp. 26–27. [Google Scholar]
- Naglik, B.; Heflik, W.; Natkaniec-Nowak, L. Charakterystyka mineralogiczno-petrograficzna utworów klastycznych Gór Pieprzowych (Wyżyna Sandomierska) i produktów ich wietrzenia. Prz. Geol. 2016, 64, 338–343. (In Polish) [Google Scholar]
- Naglik, B.; Natkaniec-Nowak, L.; Heflik, W. Mineral assemblages as a record of the evolutionary history of the Pepper Mts. Shale Formation (the Holy Cross Mts.). Geol. Geoph. Envir. 2016, 42, 161–173. [Google Scholar] [CrossRef][Green Version]
- Alexandrowicz, Z.; Marszałek, M. Efflorescences on weathered sandstone tors in the Stone Town Nature Reserve in Ciężkowice the Outer Carpathians, Poland-their geochemical and geomorphological controls. Envir. Sci. Poll. Res. 2019, 26, 37254–37274. [Google Scholar] [CrossRef]
- Alexandrowicz, Z. Sandstone rocky forms in Polish Carpathians attractive for education and tourism. Prz. Geol. 2008, 56, 680–687. [Google Scholar]
- Alexandrowicz, Z. Sandstone tors of the Western Flysch Carpathians. Pr. Geol. Kom. Nauk Geol. PAN 1978, 113, 87. (In Polish) [Google Scholar]
- Alexandrowicz, Z. Inanimate nature in the Czarnorzecki Landscape Park. Ochr. Przyr. 1987, 45, 263–293. (In Polish) [Google Scholar]
- Alexandrowicz, Z. Rezerwaty i pomniki przyrody nieożywionej województwa krośnieńskiego. In System Ochrony Przyrody i Krajobrazu Województwa Krośnieńskiego; Michalik, S., Ed.; Studia Naturae B: Kraków, Poland, 1987; Volume 32, pp. 23–72. (In Polish) [Google Scholar]
- Alexandrowicz, Z. Sandstone rocks in the vicinity of Ciężkowice on the Biała River. Ochr. Przyr. 1970, 35, 281–335. (In Polish) [Google Scholar]
- Cieszkowski, M.; Koszarski, A.; Leszczyński, S.; Michalik, M.; Radomski, A.; Szulc, J. Detailed Geological Map of Poland 1:50 000 Sheet Ciężkowice; Państwowy Instytut Geologiczny: Warszawa, Poland, 1991. (In Polish) [Google Scholar]
- Leszczyński, S. Ciężkowice Sandstones of the Silesian Unit in the Polish Carpathians: A study of coarse-clastic sedimentation in the deep-water. Ann. Soc. Geol. Pol. 1981, 51, 435–502. (In Polish) [Google Scholar]
- Koszarski, L. Observation on the Sedimentation of the Ciężkowice sandstone near Ciężkowice (Carpathian Flysch). Bull. Acad. Pol. Sci. 1956, 3, 393–398. [Google Scholar]
- Leszczyński, S. Characteristics and origin of flxoturbidites from the Carpathian flysch (Cretaceous–Palaeogene). South Poland. Ann. Soc. Geol. Pol. 1989, 59, 351–390. [Google Scholar]
- Jurkiewicz, H. Foraminifers in the sub-Menilitic Paleogene of the Polish Middle Carpathians. Inst. Geol. Biul. 1967, 210, 5–128. (In Polish) [Google Scholar]
- Pettijohn, F.J.; Potter, P.E.; Siever, R. Sand and Sandstone; Springer: New York, NY, USA, 1972. [Google Scholar]
- Leszczyński, S.; Dziadzio, P.S.; Nemec, W. Some current sedimentological controversies in the Polish Carpathian flysch. In Proceedings of the Guidebook for Field Trips Accompanying 31st IAS Meeting of Sedimentology Held in Kraków, Kraków, Poland, 22–25 June 2015; Haczewski, G., Ed.; Polish Geological Society: Kraków, Poland, 2015; pp. 247–287. [Google Scholar]
- Bromowicz, J.; Górniak, K.; Przystaś, G.; Rembiś, M. Wyniki badań petrograficznych typowych litofacji zbiornikowych fliszu karpackiego. In Charakterystyka Parametrów Petrofizycznych Fliszowych Serii Ropogazonośnych Karpat Polskich; Kuśmierek, W., Ed.; Polish Journal of Mineral Resources: Kraków, Poland, 2001; Volume 4, pp. 31–75. (In Polish) [Google Scholar]
- Alexandrowicz, Z.; Marszałek, M.; Rzepa, G. Distribution of secondary minerals in crusts developed on sandstone exposures. Earth Surf. Process. Landf. 2014, 39, 320–335. [Google Scholar] [CrossRef]
- Alexandrowicz, Z.; Marszałek, M.; Rzepa, G. The role of weathering crust in the Evolution of surfaces on the Carpathian sandstone tors. Chrońmy Przyr. Ojczystą 2012, 68, 163–174, (In Polish with English Abstract). [Google Scholar]
- Alexandrowicz, Z.; Pawlikowski, M. Mineral crust of the surface weathering zone of sandstone tors in the Polish Carpathians. Mineral. Pol. 1982, 13, 41–59. [Google Scholar]
- Wilczyńska-Michalik, W. Influence of Atmospheric Pollution on the Weathering of Stones Monuments in Cracow and Rock Outcrops in Cracow, Cracow-Częstochowa Upland and the Carpathians; Prace Monograficzne 377 Akademia Pedagogiczna: Kraków, Poland, 2004. [Google Scholar]
- Ruotsala, A.P.; Babcock, L.L. Zaherite, a new hydrated aluminum sulfate. Am. Mineral. 1977, 62, 1125–1128. [Google Scholar]
- Ross, S.D. Sulphates and other oxy-anions of Group VI. In V.C. In The Infrared Spectra of Minerals; Farmer, V.C., Ed.; The Mineralogical Society: London, UK, 1974; pp. 423–444. [Google Scholar]
- Griffith, W.P. Advances in Raman and infrared spectroscopy of minerals. In Spectroscopy of Inorganic-Based Materials; Clark, R.J.H., Hester, R.E., Eds.; John Wiley & Sons: Chichester, UK, 1987; pp. 119–186. [Google Scholar]
- Hawthorn, F.C.; Krivovichev, S.V.; Burns, P.C. The crystal chemistry of sulfate minerals. In Sulfate Minerals-Crystallography, Geochemistry, and Environmental Significance; Reviews in Mineralogy and Geochemistry 40; Alpers, C.N., Jambor, J.L., Nordstrom, D.K., Eds.; Mineralogical Society of America: Washington, DC, USA, 2000; pp. 1–112. [Google Scholar]
- Palmer, S.; Frost, R.L. Molecular structure of Mg–Al, Mn–Al and Zn–Al halotrichites-type double sulfates—An infrared spectroscopic study. Spectrochim. Acta Part A 2011, 78, 1633–1639. [Google Scholar] [CrossRef][Green Version]
- Alpers, C.N.; Blowes, D.W.; Nordstrom, D.K.; Jambor, J.L. Secondary minerals and acid mine-water chemistry. In Short Course Handbook on Environmental Geochemistry of Sulphide Mine Wastes; Jambor, J., Blowes, D., Eds.; Mineralogical Association of Canada: Waterloo, ON, Canada, 1994; pp. 247–270. [Google Scholar]
- Frost, R.L. Raman spectroscopic study of the magnesium carbonate mineral hydromagnesite (Mg5(CO3)4(OH)2.·4H2O). J. Raman Spectrosc. 2011, 42, 1690–1694. [Google Scholar] [CrossRef]
- Hopkinson, L.; Kristova, P.; Rutt, K.; Cressey, G. Phase transitions in the system MgO–CO2–H2O during CO2 degassing of Mg-bearing solutions. Geochim. Cosmochim. Acta 2012, 76, 1–13. [Google Scholar] [CrossRef]
- Gautier, Q.; Bénézeth, P.; Mavromatis, V.; Schott, J. Hydromagnesite solubility product and growth kinetics in aqueous solution from 25 to 75 °C. Geochim. Cosmochim. Acta 2014, 138, 1–20. [Google Scholar] [CrossRef]
- Rajchel, J.; Marszałek, M.; Rajchel, L. Deposits of sulphurous spring waters from the Carpathians and the Carpathian Foredeep (southern Poland). Prz. Geol. 2000, 48, 1174–1180. (In Polish) [Google Scholar]
- Rajchel, L.; Zuber, A.; Duliński, L.; Rajchel, J. Izotope and chemical composition and water ages of sulphide springs in the Polish Carpathians. In Współczesne Problem Hydrogeologii; Sadurski, A., Krawiec, A., Eds.; UMK Toruń: Toruń, Poland, 2005; Volume 12, pp. 583–588. (In Polish) [Google Scholar]
- Přikryl, R.; Melounová, L.; Vařilová, Z.; Weishauptová, Z. Spatial relationships of salt distribution and related physical changes of underlying rocks on naturally weathered sandstone exposures (Bohemian Switzerland National Park, Czech Republic). Environ. Geol. 2007, 52, 409–420. [Google Scholar] [CrossRef]
- Přikryl, R.; Svobodová, J.; Žák, K.; Hradil, D. Anthropogenic origin of salt crusts on sandstone sculptures of Prague’s Charles Bridge (Czech Republic): Evidence of mineralogy and stable isotope geochemistry. Eur. J. Miner. 2004, 16, 609–618. [Google Scholar] [CrossRef]
- Schweigstillová, J.; Přikryl, R.; Novotná, M. Isotopic composition of salt efflorescence from the sandstone castellated rocks of the Bohemian Cretaceous Basin (Czech Republic). Environ. Geol. 2009, 58, 217–225. [Google Scholar] [CrossRef]
- Navrátil, T.; Vařilová, Z.; Rohovec, J. Mobilization of aluminum by the acid percolates within unsaturated zone of sandstones. Environ. Monit. Assess. 2013, 185, 7115–7131. [Google Scholar] [CrossRef]
| Element | Signal | WDXS Crystal | Calibrant | Detection Limit [ppm] 1 |
|---|---|---|---|---|
| Na | Kα | TAP | albite | 1780 |
| Si | Kα | TAP | albite | 1260 |
| P | Kα | PET | fluorapatite | 3150 |
| Mn | Kα | LIF | rhodonite | 4510 |
| Fe | Kα | LIF | fayalite | 2000 |
| Sr | Lα | PET | celestine | 3850 |
| Cu | Kα | LIF | cuprite | 2780 |
| K | Kα | PET | sanidine | 470 |
| Ba | Lα | PET | barite | 1350 |
| Ca | Kα | PET | diopside | 530 |
| Zn | Kα | LIF | willemite | 3000 |
| Al | Kα | TAP | albite | 1600 |
| Pb | Mα | PET | crocoite | 2900 |
| S | Kα | PET | anhydrite | 2260 |
| Mg | Kα | TAP | diopside | 1260 |
| Analysis Number | 20 | 31 | 32 | 35 | Average | sd |
|---|---|---|---|---|---|---|
| wt. % | ||||||
| SO3 | 36.49 | 36.33 | 36.35 | 37.14 | 36.58 | 0.38 |
| P2O5 | 0.03 | 0.11 | 0.03 | 0.00 | 0.04 | 0.05 |
| Al2O3 | 11.64 | 11.87 | 12.22 | 11.65 | 11.85 | 0.27 |
| CuO | 0.00 | 0.06 | 0.11 | 0.13 | 0.08 | 0.06 |
| ZnO | 0.18 | 0.39 | 0.20 | 0.10 | 0.22 | 0.11 |
| SrO | 0.00 | 0.00 | 0.06 | 0.23 | 0.07 | 0.11 |
| MnO | 2.31 | 1.50 | 1.75 | 1.12 | 1.67 | 0.50 |
| FeO | 0.07 | 0.00 | 0.05 | 0.00 | 0.03 | 0.03 |
| CaO | 0.06 | 0.07 | 0.01 | 0.08 | 0.05 | 0.03 |
| BaO | 0.08 | 0.00 | 0.07 | 0.00 | 0.04 | 0.04 |
| MgO | 3.26 | 3.50 | 3.51 | 3.73 | 3.50 | 0.19 |
| Na2O | 0.00 | 0.63 | 0.14 | 0.21 | 0.25 | 0.27 |
| K2O | 0.02 | 0.05 | 0.06 | 0.07 | 0.05 | 0.02 |
| H2O * | 45.31 | 45.56 | 45.66 | 45.91 | 45.61 | 0.25 |
| Total | 99.44 | 100.01 | 100.23 | 100.36 | 100.01 | 0.41 |
| Apfu ** | ||||||
| S6+ | 3.99 | 3.97 | 3.95 | 4.02 | 3.98 | 0.03 |
| P5+ | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 |
| ∑ | 3.99 | 3.98 | 3.95 | 4.02 | 3.98 | 0.02 |
| Al3+ | 2.00 | 2.04 | 2.08 | 1.98 | 2.02 | 0.04 |
| ∑Y | 2.00 | 2.04 | 2.08 | 1.98 | 2.02 | 0.04 |
| Cu2+ | 0.00 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Zn2+ | 0.02 | 0.04 | 0.02 | 0.01 | 0.02 | 0.01 |
| Mn2+ | 0.29 | 0.19 | 0.22 | 0.14 | 0.21 | 0.05 |
| Fe2+ | 0.01 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 |
| Mg2+ | 0.71 | 0.76 | 0.76 | 0.80 | 0.75 | 0.03 |
| ∑X | 1.03 | 1.00 | 1.02 | 0.96 | 1.00 | 0.03 |
| H2O | 22 | 22 | 22 | 22 | 22 |
| Raman Bands (cm−1) | ||||||
|---|---|---|---|---|---|---|
| Pickeringite | Apjohnite 1 | Apjohnite 2 | Pickeringite 2 | Apjohnite 3 | Pickeringite 4 | Assignments 5 |
| This Study | Frost et al. [4] | Palmer & Frost [3] | Locke et al. [7] | |||
| 3394 | 3490 | 3554 | 3506 | 3548 | 3449 | OH stretch ν3 (Bg) H2O |
| 3486 | 3437 | |||||
| 3275 | 3394 | 3404 | 3289 | 3426 | 3279 | OH stretch ν3 (Ag) H2O |
| 3012 | 3490 | 3282 | 3270 | 3082 | ||
| 3281 | 3175 | OH stretch ν1 H2O | ||||
| 3281 | 3053 | |||||
| 2925 | 2900 | |||||
| 1658 | ν2 (Bg) H2O | |||||
| 1620 | ν2 (Ag) H2O | |||||
| 1145 | 1113 | 1145 | ν3 (Ag) SO4 | |||
| 1123 | 1148 | 1141 | 1096 | 1147 | 1114 | |
| 1081 | 1088 | 1086 | 1071 | ν3 (Bg) SO4 | ||
| 1070 | 1080 | 1058 | ||||
| 1051 | 1031 | |||||
| 994 | 997 | 995 | 984 | 985 | 996 | ν1 (Ag) SO4 |
| 991 | 990 | 971 | ||||
| 975 | 975 | 975 | 975 | |||
| 963 | ||||||
| 615 | 617 | 618 | 611 | 622 | 621 | ν4 (Bg) SO4 |
| 459 | ν4 (Au) SO4 or νϒ (Bg) H2O | |||||
| 524 | 530 | 445 | 530 | ν2 (Ag) SO4 | ||
| 467 | 466 | 474 | 366 | 467 | 468 | |
| 425 | 427 | 460 | ||||
| 423 | 443 | 424 | ||||
| 344 | 336 | 364 | 344 | νg (Ag) H2O | ||
| 310 | 313 | 315 | ||||
| 264 | 251 | 247 | ||||
| 223 | 204 | 233 | 214 | 221 | lattice modes | |
| 173 | 167 | |||||
| 154 | 151 | |||||
| 141 | ||||||
| 112 | ||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marszałek, M.; Gaweł, A.; Włodek, A. Pickeringite from the Stone Town Nature Reserve in Ciężkowice (the Outer Carpathians, Poland). Minerals 2020, 10, 187. https://doi.org/10.3390/min10020187
Marszałek M, Gaweł A, Włodek A. Pickeringite from the Stone Town Nature Reserve in Ciężkowice (the Outer Carpathians, Poland). Minerals. 2020; 10(2):187. https://doi.org/10.3390/min10020187
Chicago/Turabian StyleMarszałek, Mariola, Adam Gaweł, and Adam Włodek. 2020. "Pickeringite from the Stone Town Nature Reserve in Ciężkowice (the Outer Carpathians, Poland)" Minerals 10, no. 2: 187. https://doi.org/10.3390/min10020187
APA StyleMarszałek, M., Gaweł, A., & Włodek, A. (2020). Pickeringite from the Stone Town Nature Reserve in Ciężkowice (the Outer Carpathians, Poland). Minerals, 10(2), 187. https://doi.org/10.3390/min10020187





