Abstract
The aim of this study was to increase feedstock availability for mineral carbonation. Acid dissolution and carbonic acid dissolution approaches were used to achieve higher Mg extractions from peridotites. Acid dissolution studies of raw dunite, heat-activated dunite, heat-transformed dunite, and twin sister dunite have not been reported in the literature. Heat-activated dunite is more reactive as compared to heat-transformed dunite, raw dunite, and twin sister dunite. The fraction of magnesium extracted from heat-activated dunite was 57% as compared to 18% from heat-transformed dunite, 14% from raw dunite, and 11% from twin sister dunite. Similarly, silicon and iron extractions were higher for heat-activated dunite compared to that of heat-transformed dunite, raw dunite, and twin sister dunite. Materials rich in forsterite (twin sister dunite and heat-transformed dunite) showed preferential Mg release and exhibited incongruent dissolution similar to that of forsterite. Heat-activated dunite (amorphous magnesium silicate rich) on the other hand behaved differently and showed congruent dissolution. Olivine did not dissolve under carbonic acid dissolution (with concurrent grinding) and acidic conditions. Under carbonic acid dissolution with concurrent grinding conditions, olivine was partially converted into nanometer sized particles (d10 = 0.08 µm) but still provided 16% Mg extraction during 4 h of dissolution.
1. Introduction
The CO2 concentration in the atmosphere has risen to 411 ppm as compared to 280 ppm in pre-industrial times [1]. Accumulation of CO2 in the atmosphere is considered the main cause of climate change and the global warming phenomenon. Warming of 2 °C would release billions of tons of soil carbon [2]. Geological storage, oceanic storage, and recently mineral carbonation, are among different candidates for CO2 sequestration to prevent its emissions to the atmosphere [3,4]. Mineral carbonation, mainly using naturally abundant Mg-silicates, can offer safe storage of CO2 in the form of mineral carbonates for many centuries [5,6].
Single stage [4,6,7,8,9,10,11,12] and two-stage carbonation [13,14,15,16,17,18,19] are different processes that are being extensively studied for CO2 capture, utilization, and storage. Initial studies on mineral carbonation showed relatively slow reaction kinetics [20]. One of the key considerations for developing efficient mineral carbonation technology is to increase Mg extraction from feedstock. For this purpose, acid dissolution of magnesium silicate minerals was studied previously [15,21,22,23,24]. Park et al. used different acid solutions and grinding media to increase Mg extraction [25,26]. Sulfuric acid, formic acid, acetic acid, hydrochloric acid, and nitric acid were used to extract Mg from serpentine [27,28,29]. Farhang et al. studied acid dissolution of heat-activated lizardite at different pH values, solid-to-liquid ratios, and particle sizes and obtained almost 60% Mg extractions during 1 h of dissolution [22]. Farhang et al. investigated silica precipitation at different pH values and temperatures and determined that re-precipitation of silica retards dissolution of Mg by forming a diffusion barrier [30]. Dunite is partially weathered olivine that is the most abundant ultramafic rock available [31,32], which makes it important for the mineral carbonation process and needs significant consideration. Compared to other magnesium silicate minerals that have been studied for mineral carbonation, dunite is more complex as it is usually a mixture of minerals. One example that has previously been studied contains 61%–62% lizardite, 30%–33% olivine, 3.8%–8.3% brucite, and 0.6% magnetite [4]. Heat activation can be used to convert the lizardite present in dunite into more reactive amorphous Mg-silicate phases. Heat activation is a process in which dehydroxylation of serpentine minerals occurs as hydroxyl groups bound within mineral matrix are destabilized and released from the sample in the form of water vapor. The optimum decomposition temperature for lizardite has been reported as 635 °C [33]. Lizardite dehydroxylation converts the material into MgO. SiO2 motifs, Equation (1), can further crystallize at higher temperatures (or excessive soak times exceeding 4 h) to form forsterite (Mg2SiO4), Equation (2), enstatite (MgSiO3), Equation (3), and silica (SiO2) [34]. The formation of intermediate phases, which tend to be X-ray amorphous, increases the reactivity of the parent mineral [35], while the formation of new crystalline phases, such as forsterite, is detrimental to the reactivity of the material.
Dunite is comprised of approximately 61% lizardite, which can be converted into a more reactive amorphous phase through the heat-activation treatment process. A temperature of 650 °C is sufficient for lizardite to complete dehydroxylation [36]. In our previous publication, we established that heat-activated dunite provided higher magnesite yields compared to that of heat-transformed dunite (forsterite rich) and raw dunite [4]. The aim of this work was to confirm these magnesite yield results through a second approach, i.e., Mg extraction using acid dissolution experiments. Materials that provided a higher carbonation extent also showed higher Mg extractions, indicating a direct relationship between carbonation extent and Mg extractions. Acid dissolution studies of dunite rock, heat-activated dunite, and heat-transformed dunite have not yet been reported in the literature. The purpose of this article was to study acid dissolution of dunite rock at room temperature and to investigate if dunite can be considered as a potential feedstock for mineral carbonation. Furthermore, dissolution of olivine as a relatively pure peridotite mineral in carbonic acid solution with the aid of concurrent grinding was studied in this work, which has not been studied previously in the literature.
2. Material, Methods, and Experimental Set-Up
2.1. Dunite
The dunite used in this study was sourced from a dunite quarry, located close to the township of Bingara (within 5 km), located in The Great Serpentine Belt (GSB) of New South Wales (NSW), Australia. Samples of rock were handpicked (a total mass of approximately 15 kg) from the center and north walls of the quarry and then crushed to 2 to 10 cm in diameter. The dunite was tested by stereomicroscopy and polarized light microscopy (with dispersion staining) to check for the presence of chrysotile, amosite, and crocidolite asbestos and was only used if it was certified to be asbestos free (Pickford & Rhyder Consulting Pty Ltd., New South Wales, Australia). Approximately 2 kg of the dunite was dry crushed in a jaw crusher (200 × 125 mm model, Terex Jaques) to obtain a 2–3 mm size fractions. A portion of this material was transferred to a ball mill (MTI Corporation, CA, USA) to prepare sub-75 micron size fractions. Grinding was performed in a stainless-steel jar for 2 h, where stainless steel balls (diameter, 6 to 20 mm) were used as the grinding media.
2.2. Heat-Activated Dunite
Heat activation of dunite (approximately 150 g) was performed in an electrically heated stainless-steel rotary kiln (Nabertherm, Lilienthal, Germany) at 630 °C under a nitrogen purge flow (80 L/h) for 4 h. A temperature of 650 °C is sufficient for lizardite’s complete dehydroxylation [36] and above this temperature enstatite formation starts [33]. The rotary kiln is shown in Figure 1, and a schematic of the rotary kiln is shown in Figure 2.
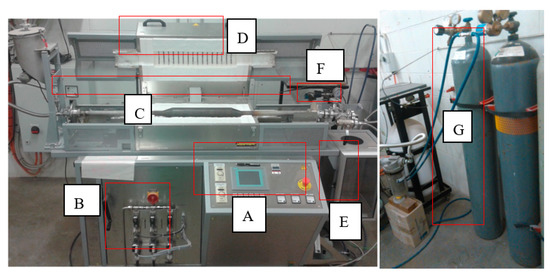
Figure 1.
Photo of the rotary kiln (Nabertherm, Lilienthal, Germany). (A): Control panel, (B): Mass flow controllers, (C): Stainless steel tube, (D): Heating element, (E): Material collection jar, (F): Thermocouple connection, (G): Nitrogen cylinder. Th rotary kiln was used for the heat-activation of dunite and lizardite. It was also used for the dunite heat-transformation.
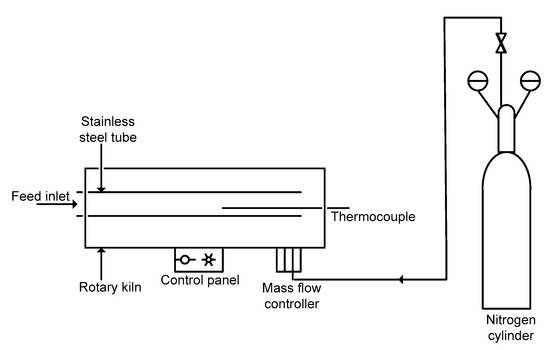
Figure 2.
Simplified process schematic of the rotary kiln and gas control manifold.
2.3. Heat-Transformed Dunite
To study the reactivity of synthesized forsterite and compare it with natural forsterite (i.e., olivine), dunite was heat-transformed to forsterite in a rotary kiln (Nabertherm, Lilienthal, Germany) at 800 °C for 3 h. Lizardite dehydroxylation may result in non-reactive enstatite formation at or above 800 °C [33]. Heat transformation was used to convert the lizardite present in dunite into forsterite to compare its reactivity with other forms of feedstock via dissolution in mild acidic pH. Heat transformation is a process in which serpentine minerals (here the lizardite fraction in dunite rock) are deliberately transformed into forsterite at high temperatures (in excess of 780 °C). During the dunite heat transformation process, lizardite and brucite transform into forsterite and magnesium oxide, respectively, Equations (4) and (5).
We adopted the term “heat-transformed” to clearly distinguish this process from “heat-treatment” that involves heating minerals to a specific temperature and for a specific duration to produce an amorphous phase. The maximum forsterite yield was calculated based on decomposition of lizardite and brucite, which is represented in Equations (6) and (7).
2Mg3Si2O5(OH)4 (s) ⇌ 3Mg2SiO4 (s)+SiO2 (s)+4H2O (g)
Mg(OH)2 (s) ⇌ MgO (s)+H2O (g)
2.4. Twin Sister Dunite
This reference material was collected from a quarry located in the Twin Sisters Range, approximately 20 miles east of Bellingham, Washington, DC, USA. This dunite had more than 90% forsterite with minor chromite and trace amounts of lizardite. It had 29.8 wt/wt% Mg and 18.4 wt/wt% Si.
2.5. Olivine
Two olivine samples were imported from Sibelco (Norway), originating from the Aheim plant. Crushed olivine was ground in a ball mill (MTI Corporation, CA, USA) for 1 h and sub-20 micron size fractions were prepared. Twin sister dunite (>90% forsterite) and olivine were considered standard materials and were used to compare results with the Australian dunite.
2.6. Methods and Experimental Set-Up
2.6.1. Reactivity Test Via Acid Dissolution
An acid dissolution experiment previously developed in our research group [22] was used to determine Mg extraction from different minerals and rocks. The process involved drying solid sample at 100 °C for two days to remove absorbed moisture. Once dried, 1 g of the dried sample was added to 200 mL buffered acid solution (1 M acetic acid, 1 M sodium acetate, constant pH = 4.7) and stirred by a magnetic stirrer (Figure 3). Samples of the supernatant solution were taken using a 1 mL syringe at different time intervals. Samples were filtered immediately using a 0.22 µL syringe filter, diluted by 2% nitric acid, and analyzed by ICP-OES.
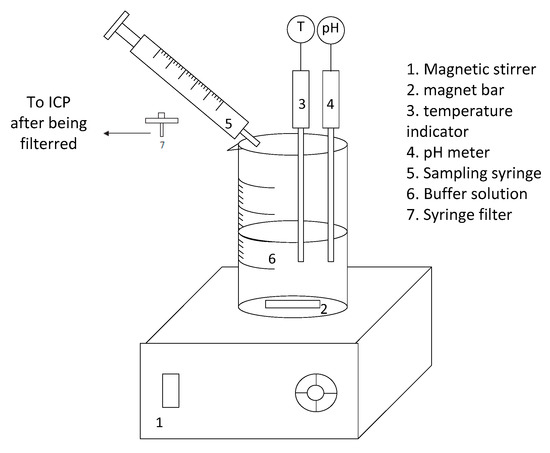
Figure 3.
Schematic of the dissolution set-up [22] used to determine relative reactivity of raw, heat-activated, and heat-transformed dunite.
2.6.2. Carbonic Acid Dissolution
To perform dissolution experiments, the grinding media, distilled water, and mineral (olivine) were added (2% loading) to a liner, the liner was placed inside the reactor (Parr Instrument Company, IL, USA), and reactor was assembled. The back pressure regulator (BPR) was set at 3 bar and cooling water was turned on. The stirrer then commenced at 600 rpm and the reactor was heated to 45 °C. When the temperature stabilized at 45 °C, the CO2 cylinder was opened, the CO2 pressure regulator was set at 3 bar, and the reactor inlet valve opened to feed CO2 into the reactor. Dissolution was performed for 4 h, and the slurry was sampled from the reactor using a sampler (Figure 4). The slurry was then filtered, and the supernatant was diluted with 2% nitric acid to prevent precipitation of magnesium carbonate phases.
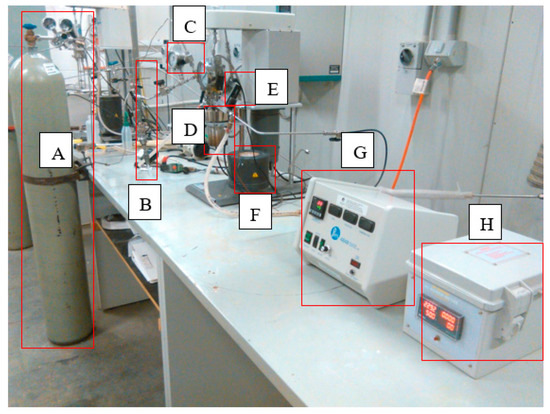
Figure 4.
Photo of the carbonic acid dissolution experimental set-up closed (back pressure regulator (BPR)) system, (A): Carbon dioxide gas cylinder, (B): Sampler to sample slurry, (C): Low pressure gauge, (D): Reactor vessel, (E): Back pressure regulator, (F): Electric heater for heating the slurry during the reaction, (G): Temperature and revolution per minute (RPM) controller, (H): Power meter.
2.6.3. Material Characterization
XRD analyses were performed using Philips X’Pert Pro multi-purpose diffractometer with Cu radiation and the 2 θ used was 10–90°. Collection time and step size were 1 s and 0.02°, respectively. Semi-quantitative XRD was performed by addition of silicon reference material inside the original sample [16]. Inductively coupled plasma-optical emission spectrometry (ICP-OES) (Varian) was used to determine elemental composition of Mg, Si, and Fe in liquid samples. Olivine feed and concurrent ground products were analyzed using scanning electron microscopy (SEM) (Zeiss Sigma VP FESEM). Particle size analyses were performed using Malvern Mastersizer 2000. To identify and quantify the different phases present in dunite, samples were heated from 0–1000 °C in TGA, coupled with a mass spectrometer. For more details about material characterization and techniques used, readers are referred to [14,16].
3. Results and Discussion
3.1. Feedstock Phase Identification and Analysis
X-ray Diffraction (XRD) analysis of the dunite sample indicated that it contained various mineral phases, i.e., lizardite, olivine, brucite, and magnetite. Semi-quantitative XRD and TGA-MS analyses revealed that the dunite sample was composed of 61%–62% lizardite, 30%–33% olivine, 3.8%–8.3% brucite, and a minor quantity of magnetite (0.6%). XRD analysis of heat-activated dunite (Figure 5) shows that heat activation completely transformed lizardite to more reactive Mg-silicate X-ray amorphous phases as observed and reported before [24] and dehydroxylated brucite to MgO. The dominant crystalline phases in the heat-activated dunite were olivine and magnetite, which remained unchanged after heat activation.
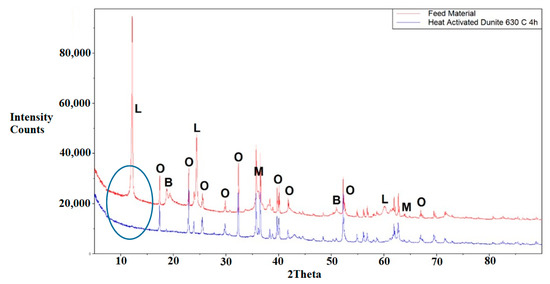
Figure 5.
Raw dunite (top) and heat-treated dunite (bottom). L = Lizardite, B = Brucite, O = Olivine, M = Magnetite.
After 3 h of dunite heat-transformation, a product comprised of 64% (semi-quantitative XRD) forsterite was formed, which was lower than the maximum possible forsterite formation of 85% based on reaction stoichiometry and mass balance shown in Table 1. Much longer (100 h) heat-transformation is required to achieve maximum forsterite formation [16,37].

Table 1.
Mass balance to calculate theoretical forsterite.
XRD patterns of olivine crystals (Sample 1) and crushed olivine (Sample 2) are shown in Figure 6. Olivine crystals were essentially 100% pure as determined by semi-quantitative XRD analysis and the crushed olivine samples were 94% olivine (based on semi-quantitative XRD analysis). The elemental composition of the crushed olivine sample was determined by ICP-OES. The sample was estimated to comprise 30.7% Mg and 19.1% Si, consistent with the XRF analysis that showed Mg and Si compositions of 29.9% and 19.4%, respectively.
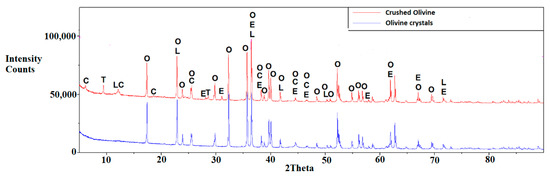
Figure 6.
XRD patterns for crushed olivine (top) and olivine crystals (bottom). O = Olivine, C = Clinochlore, E = Enstatite, L = Lizardite, T = Talc. For crushed olivine, the phases identified are olivine, clinochlore, enstatite, lizardite, and talc. For olivine crystals, the phase identified is olivine.
3.2. Acid Dissolution Experiments
Comparing Mg-leachability of the four feedstocks chosen for this study, i.e., sub-75 μm raw, heat-activated (630 °C, 4 h), heat-transformed (800 °C, 3 h) (64% forsterite), and twin sister dunite (more than 90% forsterite), provided insight into the reactivity of each material. Further dissolution experiments were performed using different size fractions of heat-transformed dunite (800 °C, 4 h) to study the effect of particle size distribution on reactivity and in particular Mg-leachability. The fraction of magnesium, silicon, and iron extracted over a 7 h dissolution period is shown in Figure 7, Figure 8 and Figure 9, respectively. Sub-75 μm fractions of raw, heat-transformed (800 °C, 3 h), heat-activated (630 °C, 4 h), and twin sister dunite had similar particle size distribution (PSD), as shown in Figure 10.
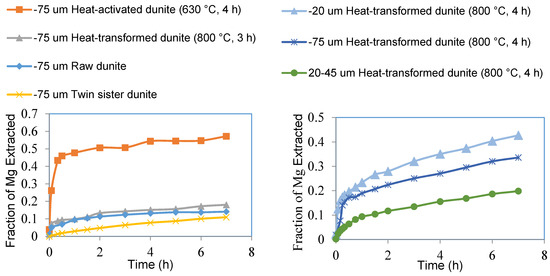
Figure 7.
The extent of Mg extracted from sub-75 μm fractions of raw dunite, heat-activated dunite (630 °C, 4 h), heat-transformed dunite (800 °C, 3 h), and twin sister dunite (left). The extent of Mg extracted from different size fractions of heat-transformed dunite (800 °C, 4 h) (right).
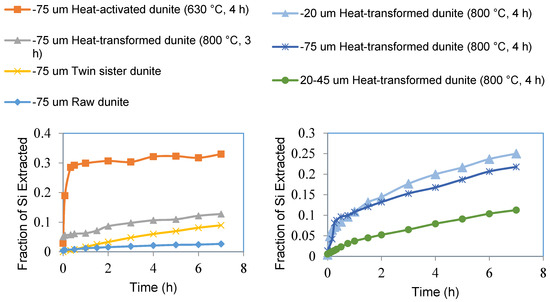
Figure 8.
Fraction of silicon extracted from sub-75 μm fractions of raw dunite, heat-activated dunite (630 °C, 4 h), heat-transformed dunite (800 °C, 3 h), and twin sister dunite (left). Fraction of magnesium extracted from sub-75 μm fraction, sub-20 μm fraction, and 20–45 μm fraction of heat-transformed dunite (800 °C, 4 h) (right).
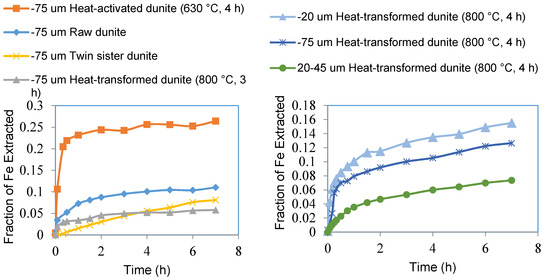
Figure 9.
Fraction of iron extracted from sub-75 μm fractions of raw dunite, heat-activated dunite (630 °C, 4 h), heat-transformed dunite (800 °C, 3 h), and twin sister dunite (left). Fraction of magnesium extracted from sub-75 μm fraction, sub-20 μm fraction, and 20–45 μm fraction of heat-transformed dunite (800 °C, 4 h) (right).
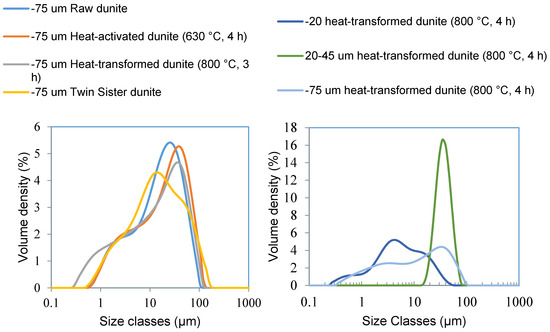
Figure 10.
Particle size distribution (PSD) for sub-75 μm fractions of raw dunite, heat-activated dunite (630 °C, 4 h), heat-transformed dunite (800 °C, 3 h), and twin sister dunite (left), PSD for sub-75 μm fraction, sub-20 μm fraction, and 20–45 μm fraction of heat-transformed dunite (800 °C, 4 h) (right).
The fraction of magnesium extracted from heat-activated dunite was higher than that from other materials, 57% as compared to 18% from heat-transformed dunite, 14% from raw dunite, and 11% from twin sister dunite (Figure 7). Acid-dissolution results indicated higher Mg leachability in materials rich in amorphous magnesium silicates (e.g., heat-activated dunite) compared to that of forsterite-rich materials (e.g., heat-transformed dunite), raw dunite or twin sister dunite (> 90% forsterite). Mg extraction results were consistent with the trends in magnesite yield reported for the carbonation experiments using these same materials [4]. Farhang et.al. [22] has previously obtained 60% Mg extraction during 1 h dissolution using an optimized −75 µm heat-activated lizardite compared to 48% Mg extraction achieved in this work using −75 µm heat-activated dunite. This finding indicates that in addition to serpentine, if dunite is properly heat activated, it can be used as an abundant feedstock for mineral carbonation.
Heat-transformed dunite showed a slightly elevated level of Mg extraction as compared to that of raw and twin sister dunite because it contained an approximately 32% amorphous magnesium silicate (Table 1, mass balance and quantitative XRD) phase. Mg extraction was slightly higher for raw dunite as compared to that of twin sister dunite because of the 8% brucite present in it. The sub-20 micron fraction of heat-transformed dunite (800 °C, 4 h) resulted in Mg extraction levels of 43% as compared to 34% from the sub-75 micron fraction and 20% from the 20–45 micron fraction (Figure 7). The reason for the higher Mg extraction is the higher quantity of fine particles in the sub-20 micron fraction as compared to that in the sub-75 micron and 20–45 micron fractions (Figure 10). This indicates that higher Mg extractions can be achieved by reducing the particle size of the feedstock.
Similar to Mg extraction, the fraction of silicon extracted from heat-activated dunite was higher than that of the other samples, 33% from heat-activated dunite compared to 13% from heat-transformed dunite, 9% from twin sister, and 3% from raw dunite (Figure 8 left). This again highlights that materials rich with amorphous magnesium silicates (e.g., heat-activated dunite) are more reactive (soluble) than forsterite-rich materials such as heat-transformed dunite, twin sister dunite, and raw dunite. Si extraction was significant here compared to <4% extraction observed by Teir et.al. using different acid solutions and particle size fraction [29]. The effect of particle size distribution on Si extraction was confirmed by higher extraction from finer materials. Twenty-five percent Si was extracted from the sub-20 micron fraction of heat-transformed dunite (800 °C, 4 h) as compared to 22% from the sub-75 micron fraction and 11% from the 20–45 micron fraction (Figure 8 bottom). These results clearly demonstrated that Si extraction is a proportion of surface area of the Mg-silicates, which can be modified by the particle size distribution of the feedstock.
The fraction of iron extracted from heat-activated dunite was 26% as compared to 11% from raw dunite, 8% from twin sister dunite, and 6% from heat-transformed dunite (Figure 9 left). Elevated Fe extraction from the twin sister dunite was due to the presence of a small amount of chromite (FeCr2O4) in this mineral. Sub-20 micron fractions of heat-transformed dunite (800 °C, 4 h) provided Fe extraction of 15% as compared to 13% from the sub-75 micron fraction and 7% from 20–45 micron fraction (Figure 9 right).
3.3. Incongruent and Congruent Dissolution
During the dissolution of forsterite under acidic conditions, non-stoichiometric (incongruent) dissolution of Mg occurs, but under alkaline conditions (pH ≥ 10), preferential release of Si is observed [38]. Similar trends for Mg have been reported by Oelkers [39] during the initial two hours of dissolution experiments, but with additional dissolution time they observed a decrease in the Mg/Si ratio, trending towards stoichiometric dissolution. A similar trend for the preferential dissolution of Mg was observed in the current study (see Figure 11a) during the initial period of dissolution for the twin sister dunite (>90% forsterite). This preferential release of Mg continued (but at a reduced rate after the initial 30 min) for 2 h, after which time the dissolution was close to stoichiometric. The incongruent dissolution of Mg from dunite (Figure 11b) is thought to be due to the presence of 30% forsterite and high levels of Mg dissolution is due to the presence of 8% brucite (which lacks silicon in its structure). Heat-transformed dunite (64% forsterite) also showed preferential release of Mg, as did forsterite, but this trend quickly stabilized and trended towards stoichiometric dissolution (Figure 11c–f). This preferential Mg release was higher for sub-20 µm heat-transformed dunite, especially at the initial stages of dissolution and is attributed to the high fraction of smaller particle size material. Materials rich in forsterite (twin sister dunite and heat-transformed dunite) show preferential Mg release and exhibit incongruent dissolution similar to that of forsterite (Figure 11a–f). Heat-activated dunite (amorphous magnesium silicate rich) on the other hand behaves differently and shows congruent dissolution (Figure 11g). This trend of heat-activated dunite has been also observed by others but for heat-activated lizardite [40].
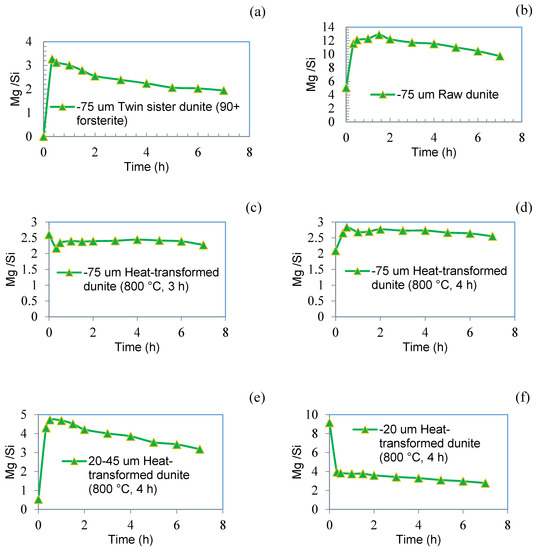
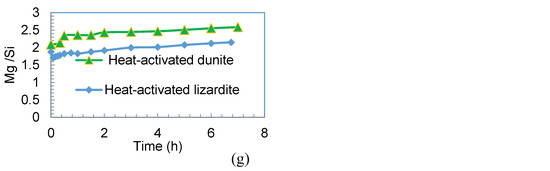
Figure 11.
The Mg/Si ratio for different materials: (a) sub-75 µm twin sister dunite (more than 90% forsterite) taken as reference; (b) sub-75 µm raw dunite; (c) sub-75 µm heat-transformed dunite (800 °C, 3 h); (d) sub-75 µm heat-transformed dunite (800 °C, 4 h); (e) 20–45 µm heat-transformed dunite (800 °C, 4 h); (f) sub-20 µm heat-transformed dunite (800 °C, 4 h); and (g) sub-75 µm heat-activated dunite (630 °C, 4 h).
3.4. Carbonic Acid Dissolution of Raw Olivine
Sub-20 micron olivine was treated by carbonic acid solution (3 bar CO2, 45 °C, 2 wt % solid) with the aid of concurrent grinding (1 mm zirconia, 60 wt %) to enhance Mg extraction from this feedstock. Mg extraction of 16% was achieved in 4 h dissolution (Figure 12 left). The trend of Mg extraction shows that it continuously increased over time. Therefore, a much longer dissolution period is required for higher Mg extractions. PSD analysis (Figure 12, Table 2) showed that olivine was converted into very fine powder (d10 = 0.08 μm (80 nanometres), d50 = 0.32 μm) using concurrent grinding. Very fine concurrent ground product (Figure 13b) was identified in SEM analysis as compared to relatively large feed particles (Figure 13a). Formation of nano particles of olivine was also identified by SEM analysis of concurrent ground product (Figure 13c). Even though olivine was converted into nano particles, the extent of dissolution was still very low, indicating the low reactivity of olivine under these conditions. Formation, presence, and growth of secondary phases on olivine particle surfaces reduce olivine dissolution [41].
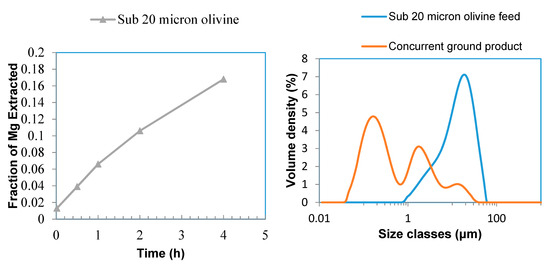
Figure 12.
Mg extraction for sub-20 micron olivine (left), PSD analysis for sub-20 micron olivine feed and concurrent ground product (right).

Table 2.
PSD analysis for sub-20 micron olivine feed and concurrent ground product.
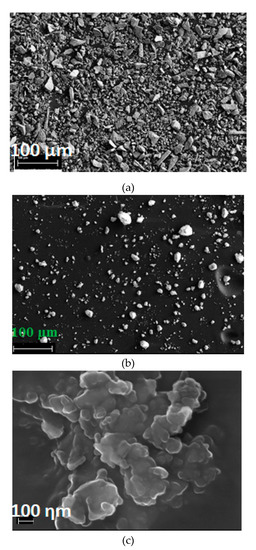
Figure 13.
SEM micrograph for sub-20 micron olivine feed and concurrent ground products: (a) sub-20 micron olivine feed, (b) concurrent ground product, and (c) concurrent ground product nanometer-sized particles.
4. Conclusions
Heat-activated dunite can be used as a feedstock for mineral carbonation. Heat-activated dunite showed higher Mg, Si, and Fe extractions compared to that of heat-transformed dunite, raw dunite, and twin sister dunite. These results are in agreement with magnesite yield results obtained for these materials [4]. This study showed that carbonation extent and Mg extractions have a direct relationship. Elevated Fe extraction from twin sister dunite compared to heat-transformed dunite was due to the presence of a small amount of chromite (FeCr2O4) in this mineral. When different fractions of heat-transformed dunite were dissolved, the sub-20 micron fraction showed higher Mg, Si, and Fe extractions followed by the sub-75 micron and 20–45 micron fractions. Heat-activated dunite showed congruent dissolution while forsterite rich materials showed incongruent dissolution. Forsterite/olivine does not dissolve properly under acidic and carbonic acid dissolution (with concurrent grinding) conditions. Only 16% Mg extraction was achieved from olivine during 4 h of dissolution. Further research is required to investigate different acid dissolution approaches, medias, media mixtures, and reactor configurations to increase Mg extraction from olivine. Different buffer solutions and or acid solutions need to be investigated to increase Mg extraction from peridotites. Outstanding research questions include the following: Why does sub-75 µm twin sister dunite show a higher Si extraction compared to that of sub-75 µm raw dunite? Why does raw dunite show a higher Fe extraction compared to that of twin sister dunite and heat-transformed dunite?
Author Contributions
Conceptualization, M.I.R.; Data curation, M.I.R.; Formal analysis, M.I.R.; Roles/Writing-original draft, M.I.R.; Investigation, E.B. and F.F.; Methodology, E.B. and F.F.; Writing-review and editing, E.B., F.F., M.S., and E.M.K.; Validation, E.B. and F.F.; Visualization, E.B. and F.F.; Funding acquisition, M.S. and E.M.K.; Project administration, M.S. and E.M.K.; Supervision, M.S. and E.M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Mineral Carbonation international.
Acknowledgments
M.I.R. thanks the University of Newcastle, Australia for a postgraduate scholarship. Jennifer Zobec and Yun Lin from EMX unit are acknowledged for support in XRD and SEM, respectively. Kitty Tang is acknowledged for support in particle size analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Global Monitoring Laboratory, Trends in Carbon Dioxide. Available online: https://www.esrl.noaa.gov/gmd/ccgg/trends/ (accessed on 1 November 2020).
- Varney, R.M.; Chadburn, S.E.; Friedlingstein, P.; Burke, E.J.; Koven, C.D.; Hugelius, G.; Cox, P.M. A spatial emergent constraint on the sensitivity of soil carbon turnover to global warming. Nat. Commun. 2020, 11, 5544. [Google Scholar] [CrossRef]
- Rashid, M.I.; Benhelal, E.; Rafiq, S. Reduction of Greenhouse Gas Emissions from Gas, Oil, and Coal Power Plants in Pakistan by Carbon Capture and Storage (CCS): A Review. Chem. Eng. Technol. 2020, 43, 2140–2148. [Google Scholar] [CrossRef]
- Rashid, M.I.; Benhelal, E.; Farhang, F.; Oliver, T.K.; Rayson, M.S.; Brent, G.F.; Stockenhuber, M.; Kennedy, E.M. ACEME: Direct Aqueous Mineral Carbonation of Dunite Rock. Environ. Prog. Sustain. Energy 2019, 38, e13075. [Google Scholar] [CrossRef]
- Lackner, K.S. A Guide to CO2 Sequestration. Science 2003, 300, 1677–1678. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.I.; Benhelal, E.; Farhang, F.; Oliver, T.K.; Rayson, M.S.; Brent, G.F.; Stockenhuber, M.; Kennedy, E.M. Development of Concurrent grinding for application in aqueous mineral carbonation. J. Clean. Prod. 2019, 212, 151–161. [Google Scholar] [CrossRef]
- Farhang, F.; Oliver, T.K.; Rayson, M.; Brent, G.; Stockenhuber, M.; Kennedy, E. Experimental study on the precipitation of magnesite from thermally activated serpentine for CO2 sequestration. Chem. Eng. J. 2016, 303, 439–449. [Google Scholar] [CrossRef]
- Benhelal, E.; Rashid, M.I.; Holt, C.; Rayson, M.S.; Brent, G.; Hook, J.M.; Stockenhuber, M.; Kennedy, E.M. The utilisation of feed and byproducts of mineral carbonation processes as pozzolanic cement replacements. J. Clean. Prod. 2018, 186, 499–513. [Google Scholar] [CrossRef]
- Benhelal, E.; Oliver, T.K.; Farhang, F.; Hook, J.M.; Rayson, M.S.; Brent, G.F.; Stockenhuber, M.; Kennedy, E.M. Structure of Silica Polymers and Reaction Mechanism for Formation of Silica-Rich Precipitated Phases in Direct Aqueous Carbon Mineralization. Ind. Eng. Chem. Res. 2020, 59, 6828–6839. [Google Scholar] [CrossRef]
- Rim, G.; Wang, D.; Rayson, M.; Brent, G.; Park, A.-H.A. Investigation on Abrasion versus Fragmentation of the Si-rich Passivation Layer for Enhanced Carbon Mineralization via CO2 Partial Pressure Swing. Ind. Eng. Chem. Res. 2020. [Google Scholar] [CrossRef]
- Benhelal, E.; Rashid, M.I.; Rayson, M.S.; Prigge, J.-D.; Molloy, S.; Brent, G.F.; Cote, A.; Stockenhuber, M.; Kennedy, E.M. Study on mineral carbonation of heat activated lizardite at pilot and laboratory scale. J. CO2 Util. 2018, 26, 230–238. [Google Scholar] [CrossRef]
- Benhelal, E.; Rashid, M.I.; Rayson, M.S.; Brent, G.F.; Oliver, T.; Stockenhuber, M.; Kennedy, E.M. Direct aqueous carbonation of heat activated serpentine: Discovery of undesirable side reactions reducing process efficiency. Appl. Energy 2019, 242, 1369–1382. [Google Scholar] [CrossRef]
- Werner, M.; Hariharan, S.; Mazzotti, M. Flue gas CO2 mineralization using thermally activated serpentine: From single- to double-step carbonation. Phys. Chem. Chem. Phys. 2014. [Google Scholar] [CrossRef] [PubMed]
- Benhelal, E. Synthesis and Application of Mineral Carbonation By-Products as Portland Cement Substitues. Ph.D. Thesis, University of Newcastle, Newcastle, Australia, 2018. [Google Scholar]
- Benhelal, E.; Rashid, M.I.; Rayson, M.S.; Oliver, T.K.; Brent, G.; Stockenhuber, M.; Kennedy, E.M. “ACEME”: Synthesis and characterization of reactive silica residues from two stage mineral carbonation Process. Environ. Prog. Sustain. Energy 2019, 38, e13066. [Google Scholar] [CrossRef]
- Rashid, M.I. Mineral Carbonation of CO2 Using Alternative Feedstocks. Ph.D. Thesis, The University of Newcastle, Newcastle, Australia, 2019. [Google Scholar]
- Oliver, T.K.; Farhang, F.; Hodgins, T.W.; Rayson, M.S.; Brent, G.F.; Molloy, T.S.; Stockenhuber, M.; Kennedy, E.M. CO2 Capture Modeling Using Heat-Activated Serpentinite Slurries. Energy Fuels 2019, 33, 1753–1766. [Google Scholar] [CrossRef]
- Mouedhen, I.; Kemache, N.; Pasquier, L.-C.; Cecchi, E.; Blais, J.-F.; Mercier, G. Effect of pCO2 on direct flue gas mineral carbonation at pilot scale. J. Environ. Manag. 2017, 198, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.I.; Benhelal, E.; Farhang, F.; Oliver, T.K.; Stockenhuber, M.; Kennedy, E.M. Application of a concurrent grinding technique for two-stage aqueous mineral carbonation. J. CO2 Util. 2020, 42, 101347. [Google Scholar] [CrossRef]
- Hänchen, M.; Prigiobbe, V.; Storti, G.; Mazzotti, M. Mineral carbonation: Kinetic study of olivine dissolution and carbonate precipitation. Proceedings of 8th International Conference On Greenhouse Gas Technology, Trondium, Norway, 19–22 June 2006. [Google Scholar]
- Werner, M.; Hariharan, S.; Zingaretti, D.; Baciocchi, R.; Mazzotti, M. Dissolution of dehydroxylated lizardite at flue gas conditions: I. Experimental study. Chem. Eng. J. 2014, 241, 301–313. [Google Scholar] [CrossRef]
- Farhang, F.; Rayson, M.; Brent, G.; Hodgins, T.; Stockenhuber, M.; Kennedy, E. Insights into the dissolution kinetics of thermally activated serpentine for CO2 sequestration. Chem. Eng. J. 2017, 330, 1174–1186. [Google Scholar] [CrossRef]
- Hariharan, S.; Mazzotti, M. Kinetics of flue gas CO2 mineralization processes using partially dehydroxylated lizardite. Chem. Eng. J. 2017, 324, 397–413. [Google Scholar] [CrossRef]
- Benhelal, E.; Hook, J.M.; Rashid, M.I.; Zhao, G.; Oliver, T.K.; Rayson, M.S.; Brent, G.F.; Stockenhuber, M.; Kennedy, E.M. Insights into chemical stability of Mg-silicates and silica in aqueous systems using 25Mg and 29Si solid-state MAS NMR spectroscopy: Applications for CO2 capture and utilisation. Chem. Eng. J. 2020. [Google Scholar] [CrossRef]
- Park, A.-H.A.; Jadhav, R.; Fan, L.-S. CO2 mineral sequestration: Chemically enhanced aqueous carbonation of serpentine. Can. J. Chem. Eng. 2003, 81, 885–890. [Google Scholar] [CrossRef]
- Park, A.-H.A.; Fan, L.-S. CO2 mineral sequestration: Physically activated dissolution of serpentine and pH swing process. Chem. Eng. Sci 2004, 59, 5241–5247. [Google Scholar] [CrossRef]
- Maroto-Valer, M.M.; Fauth, D.J.; Kuchta, M.E.; Zhang, Y.; Andrésen, J.M. Activation of magnesium rich minerals as carbonation feedstock materials for CO2 sequestration. Fuel Process. Technol. 2005, 86, 1627–1645. [Google Scholar] [CrossRef]
- Teir, S.; Kuusik, R.; Fogelholm, C.-J.; Zevenhoven, R. Production of magnesium carbonates from serpentinite for long-term storage of CO2. Int. J. Miner. Process. 2007, 85, 1–15. [Google Scholar] [CrossRef]
- Teir, S.; Revitzer, H.; Eloneva, S.; Fogelholm, C.J.; Zevenhoven, R. Dissolution of natural serpentinite in mineral and organic acids. Int. J. Miner. Process. 2007, 83, 36–46. [Google Scholar] [CrossRef]
- Farhang, F.; Oliver, T.K.; Rayson, M.S.; Brent, G.F.; Molloy, T.S.; Stockenhuber, M.; Kennedy, E.M. Dissolution of heat activated serpentine for CO2 sequestration: The effect of silica precipitation at different temperature and pH values. J. CO2 Util. 2019, 30, 123–129. [Google Scholar] [CrossRef]
- Li, J.; Hitch, M. Mechanical activation of magnesium silicates for mineral carbonation, a review. Miner. Eng. 2018, 128, 69–83. [Google Scholar] [CrossRef]
- Styles, M.T.; Sanna, A.; Lacinska, A.M.; Naden, J.; Maroto-Valer, M. The variation in composition of ultramafic rocks and the effect on their suitability for carbon dioxide sequestration by mineralization following acid leaching. Greenh. Gases Sci. Technol. 2014, 4, 440–451. [Google Scholar] [CrossRef]
- Dlugogorski, B.Z.; Balucan, R.D. Dehydroxylation of serpentine minerals: Implications for mineral carbonation. Renew. Sustain. Energy Rev. 2014, 31, 353–367. [Google Scholar] [CrossRef]
- Mann, J. Serpentine Activation for CO2 Sequestration. Ph.D. Thesis, The University of Sydney, Sydney, Australia, 2014. [Google Scholar]
- MacKenzie, K.J.D.; Meinhold, R.H. Thermal reactions of chrysotile revisited: A 29Si and 25Mg MAS NMR study. Am. Mineral. 1994, 73, 43–50. [Google Scholar]
- Dahlin, D.C.; O’Connor, W.K.; Nilsen, R.P.; Rush, G.E.; Walters, R.P.; Turner, P.C. A method for permanent CO2 mineral carbonation. In Proceedings of the 17th Annual International Pittsburg Coal Conference, Pittsburgh, PA, USA, 11–15 September 2000. DOE/ARC-2000-012. [Google Scholar]
- Brindley, G.W.; Hayami, R. Kinetics and Mechanisms of Dehydration and Recrystallization of Serpentine—I. Clays Clay Miner. 1963, 12, 35–47. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Schott, J. Kinetics and mechanism of forsterite dissolution at 25 °C and pH from 1 to 12. Geochim. Cosmochim. Acta 2000, 64, 3313–3325. [Google Scholar] [CrossRef]
- Oelkers, E.H. An experimental study of forsterite dissolution rates as a function of temperature and aqueous Mg and Si concentrations. Chem. Geol. 2001, 175, 485–494. [Google Scholar] [CrossRef]
- Hariharan, S.; Werner, M.; Hänchen, M.; Mazzotti, M. Dissolution of dehydroxylated lizardite at flue gas conditions: II. Kinetic modeling. Chem. Eng. J. 2014, 241, 314–326. [Google Scholar] [CrossRef]
- Oelkers, E.H.; Declercq, J.; Saldi, G.D.; Gislason, S.R.; Schott, J. Olivine dissolution rates: A critical review. Chem. Geol. 2018, 500, 1–19. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).