Thermal Annealing and Phase Transformation of Serpentine-Like Garnierite
Abstract
1. Introduction
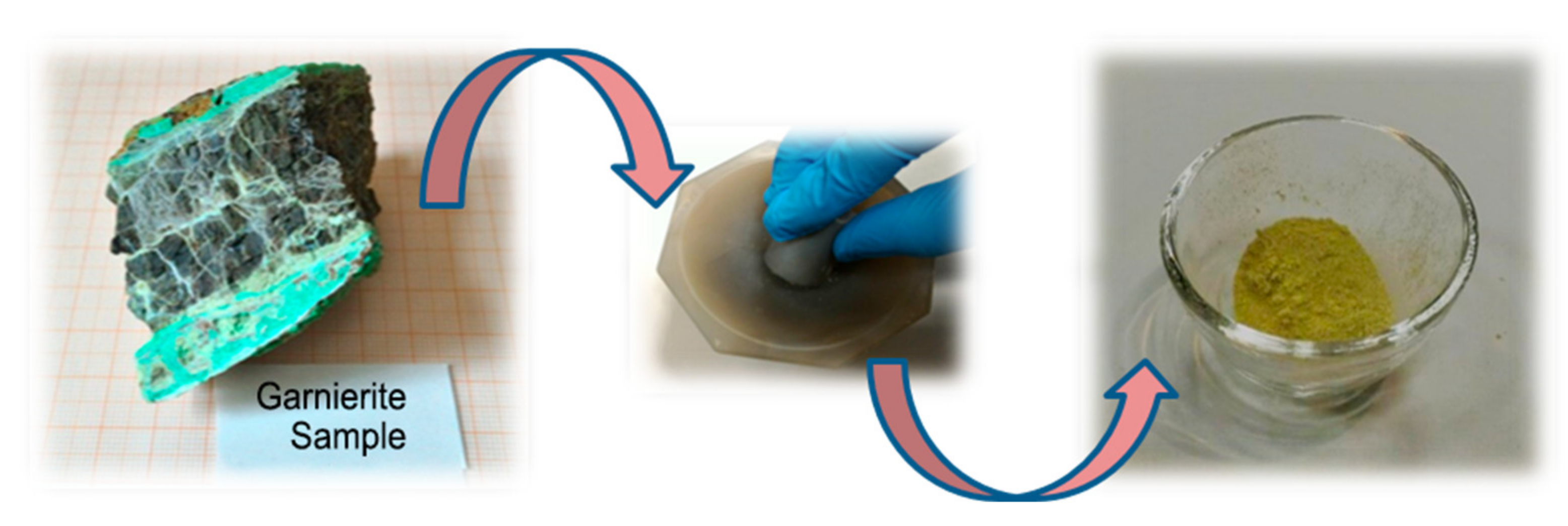
2. Materials and Methods
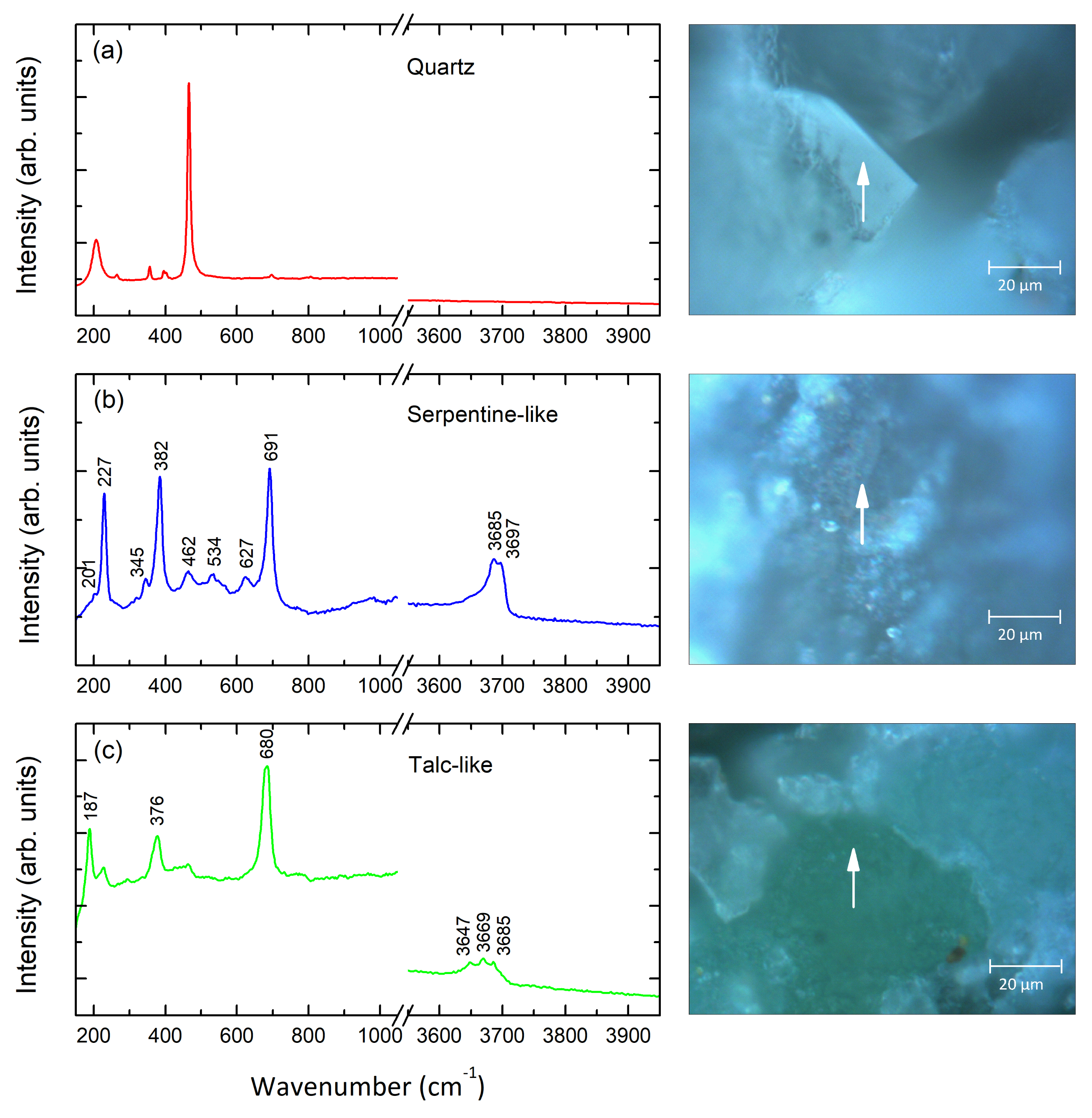
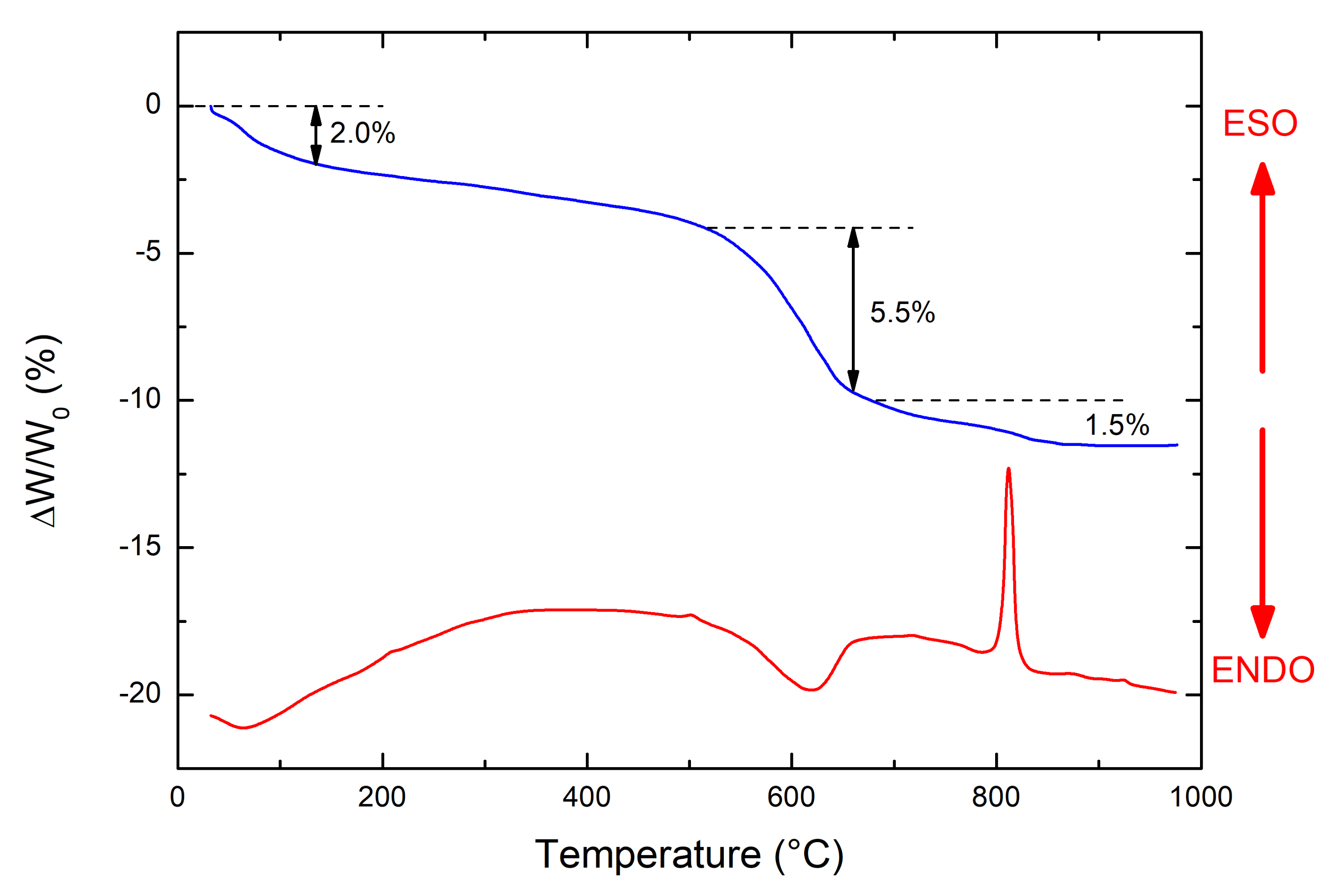
3. Results and Discussion
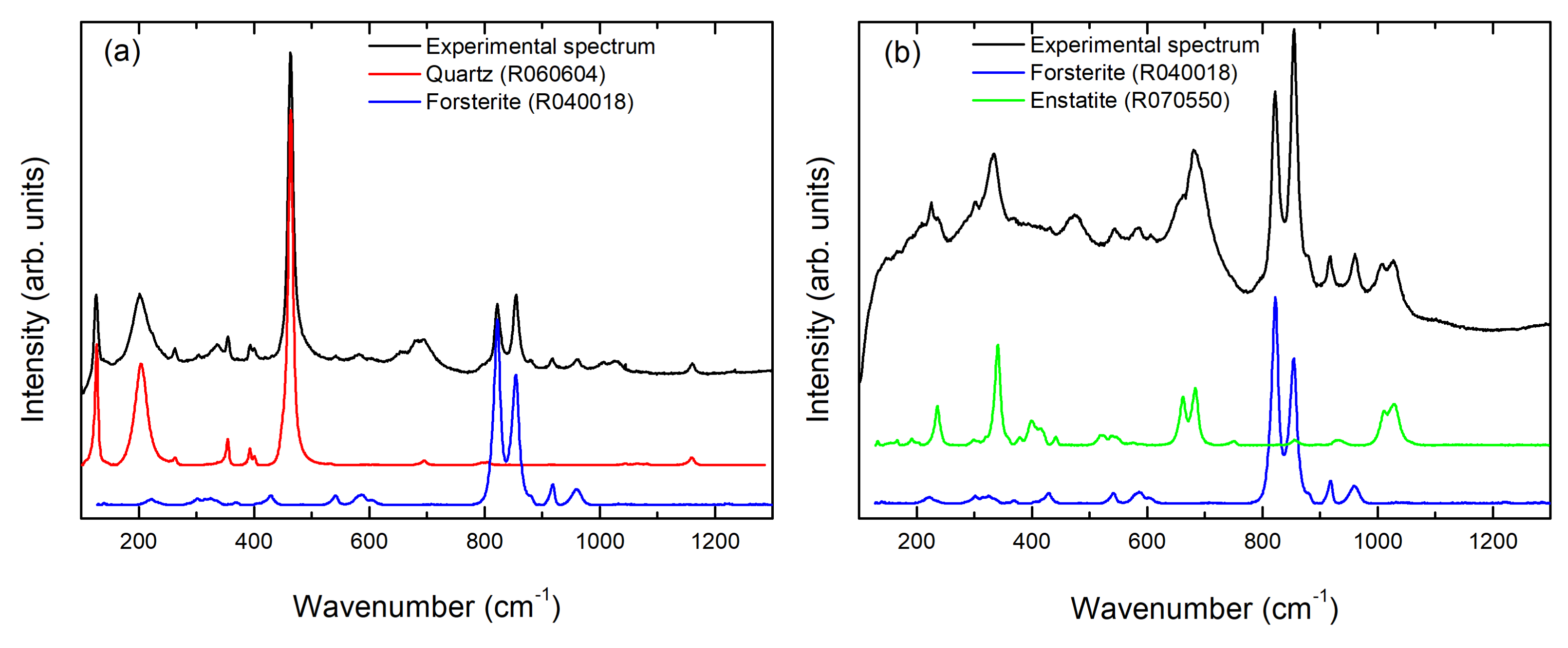
serpentine olivine enstatite
serpentine olivine quartz
talc enstatite
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jun-Hui, X.; Ding, W.; Peng, Y.; Chen, T.; Zou, K.; Wang, Z. Extraction of Nickel from Garnierite Laterite Ore Using Roasting and Magnetic Separation with Calcium Chloride and Iron Concentrate. Minerals 2020, 10, 352. [Google Scholar] [CrossRef]
- Qu, T.; Tian, Y.; Yang, B.; Liu, D.C.; Xu, B.Q.; Dai, Y.N. Study on the thermodynamic and experimental carbothermic reduction of garnierite. In Magnesium Technology; Wiley: Hoboken, NJ, USA, 2012; pp. 505–510. ISBN 9781118291214. [Google Scholar]
- De Bakker, J.; Peacey, J.; Davis, B. Thermal decomposition studies on magnesium hydroxychlorides. Can. Metall. Q. 2012, 51, 419–423. [Google Scholar] [CrossRef]
- Mongelli, G.; Taghipour, B.; Sinisi, R.; Khadivar, S. Mineralization and element redistribution in the Chah-Gheib Ni-laterite ore zone, Bavanat, Zagros Belt, Iran. Ore Geol. Rev. 2019, 111, 102990. [Google Scholar] [CrossRef]
- Villanova-de-Benavent, C.; Jawhari, T.; Roqué-Rosell, J.; Galí, S.; Proenza, J.A. Ni-bearing phyllosilicates (“garnierites”): New insights from thermal analysis, μRaman and IR spectroscopy. Appl. Clay Sci. 2019, 175, 47–66. [Google Scholar] [CrossRef]
- Cathelineau, M.; Quesnel, B.; Gautier, P.; Boulvais, P.; Couteau, C.; Drouillet, M. Nickel dispersion and enrichment at the bottom of the regolith: Formation of pimelite target-like ores in rock block joints (Koniambo Ni deposit, New Caledonia). Miner. Deposita 2015, 51, 271–282. [Google Scholar] [CrossRef]
- Wells, M.A.; Ramanaidou, E.R.; Verrall, M.; Tessarolo, C. Mineralogy and crystal chemistry of "garnierites" in the Goro lateritic nickel deposit, New Caledonia. Eur. J. Miner. 2009, 21, 467–483. [Google Scholar] [CrossRef]
- Brindley, G.W.; Maksimovic, Z. The nature andnomenclature of hydrous nickel-containing silicates. Clay. Miner. 1974, 10, 271–277. [Google Scholar] [CrossRef]
- Hang, P.T.; Brindley, G.W. The nature of garnierites--III thermal transformations. Clays Clay Miner. 1973, 21, 51–57. [Google Scholar] [CrossRef]
- Sazama, U.; Reller, A. The thermochemical reactivity of silicate minerals in hydrogen and methane. J. Therm. Anal. Calorim. 1996, 47, 357–364. [Google Scholar] [CrossRef]
- Duée, C.; Maubec, N.; Laperche, V.; Capar, L.; Bourguignon, A.; Bourrat, X.; Yassine, B.; Mendili, E.; Chateigner, D.; Gascoin, S.; et al. Combined mineralogy and chemistry on drill cores: Challenging for on-line-real-time analyses. In Proceedings of the 14th Biennial SGA Meeting, Québec City, QC, Canada; 2017; Volume 3, pp. 1241–1244. [Google Scholar]
- Secchi, M.; Zanatta, M.; Borovin, E.; Bortolotti, M.; Kumar, A.; Giarola, M.; Sanson, A.; Orberger, B.; Daldosso, N.; Gialanella, S.; et al. Mineralogical investigations using XRD, XRF, and Raman spectroscopy in a combined approach. J. Raman Spectrosc. 2018, 49, 1023–1030. [Google Scholar] [CrossRef]
- El Mendili, Y.; Chateigner, D.; Orberger, B.; Gascoin, S.; Bardeau, J.-F.; Petit, S.; Duée, C.; Le Guen, M.; Pilliere, H. Combined XRF, XRD, SEM-EDS, and Raman Analyses on Serpentinized Harzburgite (Nickel Laterite Mine, New Caledonia): Implications for Exploration and Geometallurgy. ACS Earth Space Chem. 2019, 3, 2237–2249. [Google Scholar] [CrossRef]
- Duée, C.; Orberger, B.; Maubec, N.; Laperche, V.; Capar, L.; Bourguignon, A.; Bourrat, X.; El Mendili, Y.; Chateigner, D.; Gascoin, S.; et al. Impact of heterogeneities and surface roughness on pXRF, pIR, XRD and Raman analyses: Challenges for on-line, real-time combined mineralogical and chemical analyses on drill cores and implication for “high speed” Ni-laterite exploration. J. Geochem. Explor. 2019, 198, 1–17. [Google Scholar] [CrossRef]
- Scott, J.F.; Porto, S.P.S. Longitudinal and Transverse Optical Lattice Vibrations in Quartz. Phys. Rev. 1967, 161, 903–910. [Google Scholar] [CrossRef]
- Shapiro, S.M.; O’Shea, D.C.; Cummins, H.Z. Raman Scattering Study of the Alpha-Beta Phase Transition in Quartz. Phys. Rev. Lett. 1967, 19, 361–364. [Google Scholar] [CrossRef]
- Schmidt, P.; Bellot-Gurlet, L.; Leá, V.; Sciau, P. Moganite detection in silica rocks using Raman and infrared spectros-copy. Eur. J. Mineral. 2014, 25, 797–805. [Google Scholar] [CrossRef]
- Wang, A.; Freeman, J.J.; Jolliff, B.L. Understanding the Raman spectral features of phyllosilicates. J. Raman Spectrosc. 2015, 46, 829–845. [Google Scholar] [CrossRef]
- Rinaudo, C.; Gastaldi, D.; Belluso, E. Characterization of chrysotile, antigorite and lizardite by ft raman spectroscopy. Can. Miner. 2003, 41, 883–890. [Google Scholar] [CrossRef]
- Groppo, C.; Rinaudo, C.; Cairo, S.; Gastaldi, D.; Compagnoni, R. Micro-Raman spectroscopy for a quick and reliable identification of serpentine minerals from ultramafics. Eur. J. Miner. 2006, 18, 319–329. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Frost, R.L.; Rintoul, L. Single crystal Raman microscopic study of the asbestos mineral chrysotile. Phys. Chem. Chem. Phys. 1999, 1, 2559–2564. [Google Scholar] [CrossRef]
- Blaha, J.J.; Rosasco, G.J. Raman microprobe spectra of individual microcrystals and fibers of talc, tremolite, and related silicate minerals. Anal. Chem. 1978, 50, 892–896. [Google Scholar] [CrossRef]
- Rosasco, G.J.; Blaha, J.J. Raman Microprobe Spectra and Vibrational Mode Assignments of Talc. Appl. Spectrosc. 1980, 34, 140–144. [Google Scholar] [CrossRef]
- Wang, A.; Freeman, J.; Kuebler, K.E. Raman Spectroscopic Characterization of Phyllosilicates. In Proceedings of the Lunar and Planetary Science XXXIII, Houston, TX, USA, 11–15 March 2002; p. 1374. [Google Scholar]
- Auzende, A.-L.; Daniel, I.; Reynard, B.; Lemaire, C.; Guyot, F. High-pressure behaviour of serpentine minerals: A Raman spectroscopic study. Phys. Chem. Miner. 2004, 31, 269–277. [Google Scholar] [CrossRef]
- Frost, R.L.; Reddy, B.J.; Dickfos, M.J. Raman spectroscopy of the nickel silicate mineral pecoraite—An analogue of chrysotile (asbestos). J. Raman Spectrosc. 2008, 39, 909–913. [Google Scholar] [CrossRef]
- Cathelineau, M.; Caumon, M.-C.; Massei, F.; Brie, D.; Harlaux, M.; Michel, C. Raman spectra of Ni-Mg kerolite: Effect of Ni-Mg substitution on O-H stretching vibrations. J. Raman Spectrosc. 2015, 46, 933–940. [Google Scholar] [CrossRef]
- Brindley, G.W. Kinetics and Mechanisms of Dehydration and Recrystallization of Serpentine—I. Clays Clay Miner. 1963, 12, 35–47. [Google Scholar] [CrossRef]
- Brindley, G.W.; Thi Hang, P. The nature of garnierites—I structures, chemical compositions and color characteristics. Clays Clay Miner. 1973, 21, 19–26. [Google Scholar] [CrossRef]
- Brindley, G.W.; Hayami, R. Mechanism of formation of forsterite and enstatite from serpentine. Miner. Mag. J. Miner. Soc. 1965, 35, 189–195. [Google Scholar] [CrossRef]
- Quartz R060604—RRUFF Database: Raman, X-ray, Infrared, and Chemistry. Available online: https://rruff.info/quartz/display=default/R060604 (accessed on 21 November 2020).
- Zevgolis, E.N.; Zografidis, C.; Perraki, T.; Devlin, E. Phase transformations of nickeliferous laterites during preheating and reduction with carbon monoxide. J. Therm. Anal. Calorim. 2009, 100, 133–139. [Google Scholar] [CrossRef]
- Fritsch, E.; Juillot, F.; Dublet, G.; Fonteneau, L.; Fandeur, D.; Martin, E.; Caner, L.; Auzende, A.-L.; Grauby, O.; Beaufort, D. An alternative model for the formation of hydrous Mg/Ni layer silicates (’deweylite’/’garnierite’) in faulted peridotites of New Caledonia: I. Texture and mineralogy of a paragenetic succession of silicate infillings. Eur. J. Miner. 2016, 28, 295–311. [Google Scholar] [CrossRef]
- Ovung, T.N.; Ray, J.; Ghosh, B.; Mandal, D.; Dasgupta, P.; Paul, M. Occurrence of népouite in the serpentinite of the Manipur ophiolite belt, Northeastern India: Implication for melt-rock interaction in a supra-subduction zone. J. Geol. Soc. India 2017, 90, 154–158. [Google Scholar] [CrossRef]
- Fu, W.; Zhang, Y.; Pang, C.; Zeng, X.; Huang, X.; Yang, M.; Shao, Y.; Lin, H. Garnierite mineralization from a serpentinite-derived lateritic regolith, Sulawesi Island, Indonesia: Mineralogy, geochemistry and link to hydrologic flow regime. J. Geoc. Explor. 2018, 188, 240–256. [Google Scholar] [CrossRef]
- Forsterite R040018—RRUFF Database: Raman, X-ray, Infrared, and Chemistry. Available online: https://rruff.info/forsterite/display=default/R040018 (accessed on 21 November 2020).
- Enstatite R070550—RRUFF Database: Raman, X-ray, Infrared, and Chemistry. Available online: https://rruff.info/enstatite/display=default/R070550 (accessed on 21 November 2020).
- El Mendili, Y.; Vaitkus, A.; Merkys, A.; Gražulis, S.; Chateigner, D.; Mathevet, F.; Gascoin, S.; Petit, S.; Bardeau, J.-F.; Zanatta, M.; et al. Raman Open Database: First interconnected Raman–X-ray diffraction open-access resource for material identification. J. Appl. Crystallogr. 2019, 52, 618–625. [Google Scholar] [CrossRef]
- Raman Open Database: Information Card for Entry 3500272. Available online: https://solsa.crystallography.net/rod/3500272.html (accessed on 21 November 2020).
- Raman Open Database: Information Card for Entry 3500271. Available online: https://solsa.crystallography.net/rod/3500271.html (accessed on 21 November 2020).
- Raman Open Database: Information Card for Entry 3500029. Available online: https://solsa.crystallography.net/rod/3500029.html (accessed on 21 November 2020).
- Lin, C.-C. High-pressure Raman spectroscopic study of Co- and Ni-olivines. Phys. Chem. Miner. 2001, 28, 249–257. [Google Scholar] [CrossRef]
- Kolesov, B.A.; Geiger, C.A. A Raman spectroscopic study of Fe–Mg olivines. Phys. Chem. Miner. 2004, 31, 142–154. [Google Scholar] [CrossRef]
- Weber, I.; Böttger, U.; Pavlov, S.; Jessberger, E.; Hübers, H.-W. Mineralogical and Raman spectroscopy studies of natural olivines exposed to different planetary environments. Planet. Space Sci. 2014, 104, 163–172. [Google Scholar] [CrossRef]
- Catalano, M.; Bloise, A.; Pingitore, V.; Cazzanelli, E.; Giarola, M.; Mariotto, G.; Barrese, E. Synthesis and characterization of Zn-doped enstatite. Appl. Phys. A 2015, 120, 175–182. [Google Scholar] [CrossRef]
- Zucker, R.; Shim, S.-H. In situ Raman spectroscopy of MgSiO3 enstatite up to 1550 K. Am. Miner. 2009, 94, 1638–1646. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, A.; Cassetta, M.; Giarola, M.; Zanatta, M.; Le Guen, M.; Soraru, G.D.; Mariotto, G. Thermal Annealing and Phase Transformation of Serpentine-Like Garnierite. Minerals 2021, 11, 188. https://doi.org/10.3390/min11020188
Kumar A, Cassetta M, Giarola M, Zanatta M, Le Guen M, Soraru GD, Mariotto G. Thermal Annealing and Phase Transformation of Serpentine-Like Garnierite. Minerals. 2021; 11(2):188. https://doi.org/10.3390/min11020188
Chicago/Turabian StyleKumar, Arun, Michele Cassetta, Marco Giarola, Marco Zanatta, Monique Le Guen, Gian Domenico Soraru, and Gino Mariotto. 2021. "Thermal Annealing and Phase Transformation of Serpentine-Like Garnierite" Minerals 11, no. 2: 188. https://doi.org/10.3390/min11020188
APA StyleKumar, A., Cassetta, M., Giarola, M., Zanatta, M., Le Guen, M., Soraru, G. D., & Mariotto, G. (2021). Thermal Annealing and Phase Transformation of Serpentine-Like Garnierite. Minerals, 11(2), 188. https://doi.org/10.3390/min11020188







