Influence of Salinity on the Removal of Ni and Zn by Phosphate-Intercalated Nano Montmorillonite (PINM)
Abstract
1. Introduction
2. Experimental
2.1. Chemicals
2.2. The Adsorbent Preparation and Characterization
2.3. Adsorption Isotherm Experiments
2.4. Isotherm Equations and Fitting Method
3. Results and Discussion
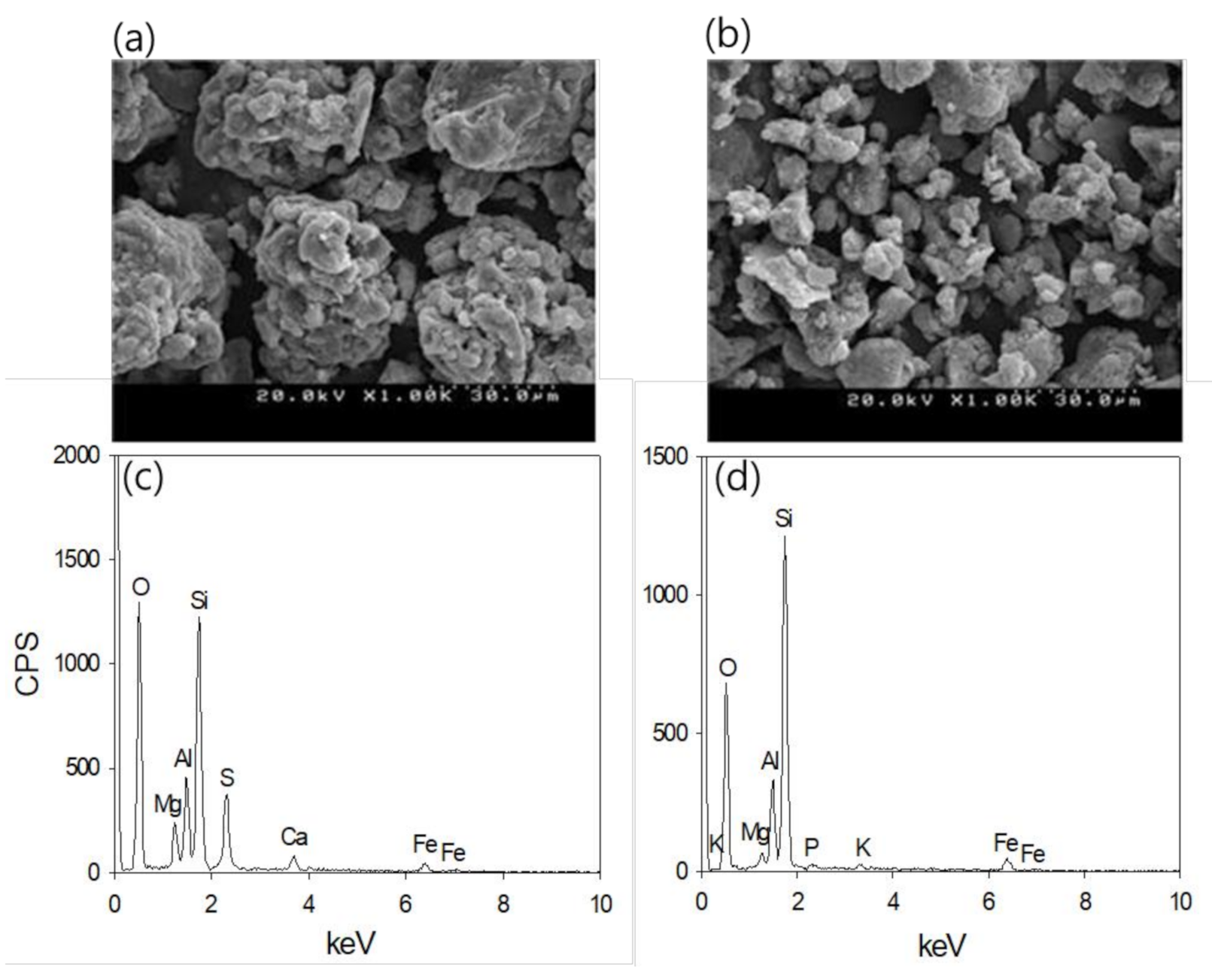
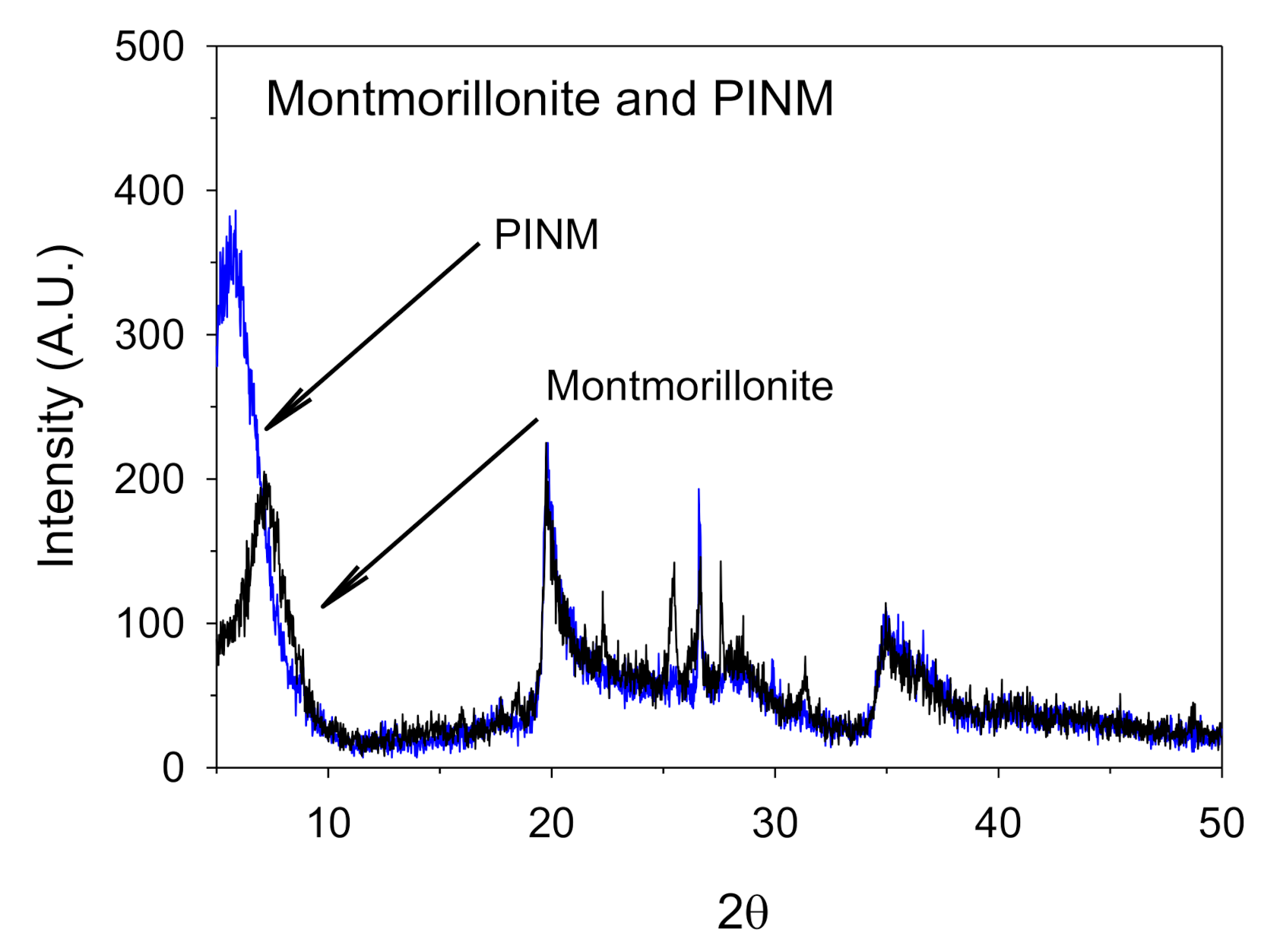
3.1. Adsorbent Characteristics
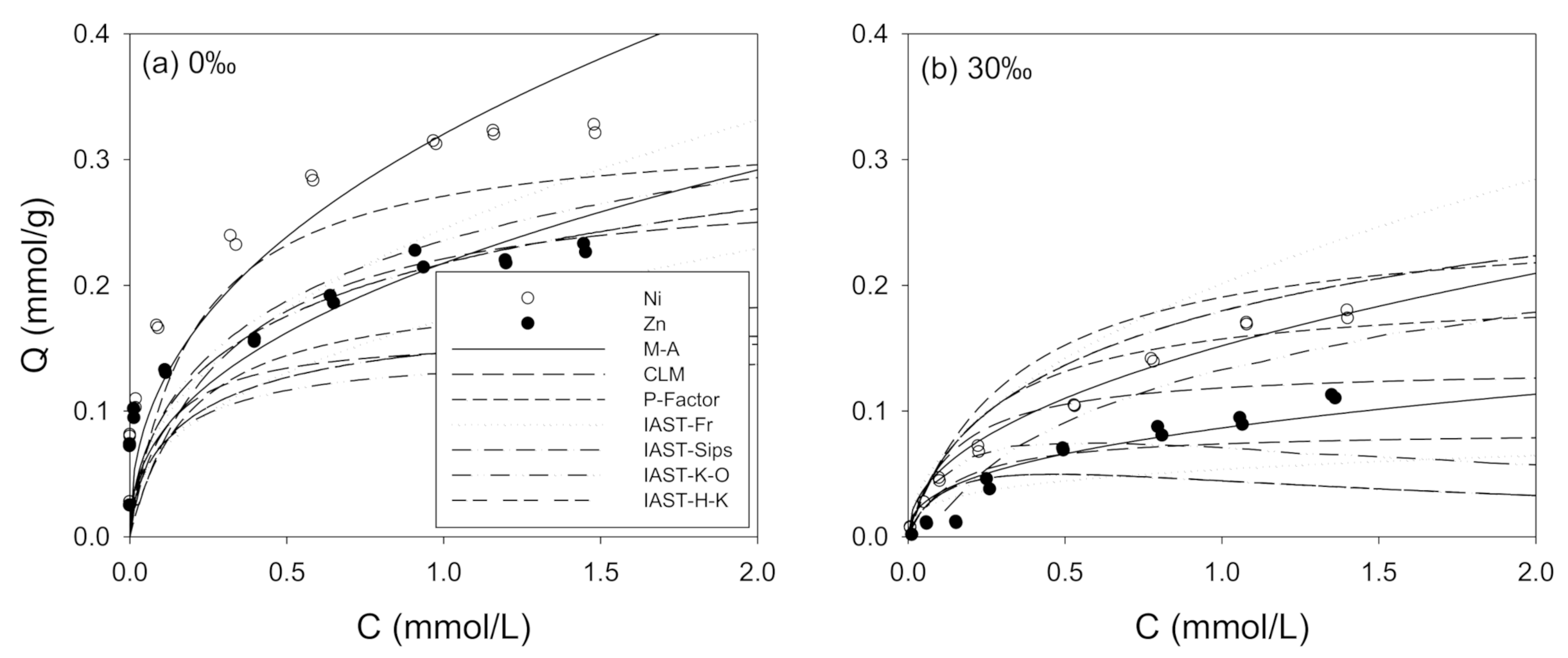
3.2. Ni and Zn Adsorption
3.3. Ni/Zn Adsorption
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| A | interfacial area between the solution and solid adsorbent |
| aij | competition coefficient between the solutes; subscripts i and j denote the solutes |
| bHK | constant in the H−K model [(L/mmol)] |
| bL | site energy factor (L/mmol) |
| bL,i | Langmuir model parameter obtained from single adsorption (L/mmol) |
| bS | Sips isotherm constant (L/mmol) |
| β | constant related to the mean free energy of adsorption per mole of the adsorbate (mol2/J2) |
| Ci | aqueous solution concentration of solute i at multi-solute competitive adsorption equilibrium (mmol/L) |
| Cs | solid-phase equilibrium concentration (mmol/g) |
| Cs,i | adsorbed amount of solute i at multi-solute competitive adsorption equilibrium (mmol/g) |
| Cw | aqueous-phase equilibrium concentration (mmol/g) |
| E | mean free energy [(2β)−0.5] (kJ/mol) |
| ε | adsorption potential [=RT ln(1 + 1/Cw)] (J/mol) |
| K | saturation constant (mmol/L) |
| KF | Freundlich adsorption coefficient (sorption affinity) [(mmol/kg)/(mmol/L)] |
| N | total number of solutes |
| NF | linearity coefficient (−) |
| NG | cooperative binding constant (–) |
| NHK | constant in the H−K model (−) |
| NS | (−) is Sips isotherm exponential constant |
| R | ideal gas constant (J/mol·K) |
| T | absolute temperature (K) |
| QmD | theoretical saturation capacity (mg/kg) |
| QmG | maximum adsorption capacity of the adsorbent (mmol/g) |
| QmHK | constant in the H−K model [] |
| QmL | maximum adsorption capacity (mmol/kg) |
| QmL,I | maximum adsorption capacity for component i in a single system (mmol/g) |
| maximum adsorption capacity for component i in a single system (mmol/g) | |
| QmS | maximum adsorption capacity (mmol/g) |
| π | spreading pressure. |
References
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Malamis, S.; Katsou, E. A review on zinc and nickel adsorption on natural and modified zeolite, bentonite and vermiculite: Examination of process parameters, kinetics and isotherms. J. Hazard. Mater. 2013, 252–253, 428–461. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Hu, W.; Tian, K.; Huang, B.; Zhao, Y.; Wang, X.; Zhou, Y.; Shi, B.; Kwon, B.-O.; Choi, K.; et al. Accumulation and ecological risk of heavy metals in soils along the coastal areas of the Bohai Sea and the Yellow Sea: A comparative study of China and South Korea. Environ. Int. 2020, 137, 105519. [Google Scholar] [CrossRef] [PubMed]
- Kabir, E.; Ray, S.; Kim, K.-H.; Yoon, H.-O.; Jeon, E.-C.; Kim, Y.S.; Cho, Y.-S.; Yun, S.-T.; Brown, R.J.C. Current status of trace metal pollution in soils affected by industrial activities. Sci. World J. 2012, 916705. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Habib-Ur-Rehman, M.; Khanam, T.; Sheer, A.; Kebin, Z.; Jianjun, Y. Health risk assessment of different heavy metals dissolved in drinking water. Int. J. Environ. Res. Public Health 2019, 16, 1737. [Google Scholar] [CrossRef] [PubMed]
- Wongsasuluk, P.; Chotpantarat, S.; Siriwong, W.; Robson, M. Heavy metal contamination and human health risk assessment in drinking water from shallow groundwater wells in an agricultural area in Ubon Ratchathani province, Thailand. Environ. Geochem. Health 2014, 36, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Fajčíková, K.; Cvečková, V.; Stewart, A.; Rapant, S. Health risk estimates for groundwater and soil contamination in the Slovak Republic: A convenient tool for identification and mapping of risk areas. Environ. Geochem. Health 2014, 36, 973–986. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Chan, G.Y.S.; Lo, W.H.; Babel, S. Physico-chemical treatment techniques for wastewater laden with heavy metals. Chem. Eng. J. 2006, 118, 83–98. [Google Scholar] [CrossRef]
- Wu, J.; Lu, J.; Li, L.; Min, X.; Luo, Y. Pollution, ecological-health risks, and sources of heavy metals in soil of the northeastern Qinghai-Tibet Plateau. Chemosphere 2018, 201, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Vetrimurugan, E.; Brindha, K.; Elango, L.; Ndwandwe, O.M. Human exposure risk to heavy metals through groundwater used for drinking in an intensively irrigated river delta. Appl. Water Sci. 2017, 7, 3267–3280. [Google Scholar] [CrossRef]
- Mulligan, C.N.; Yong, R.N.; Gibbs, B.F. Remediation technologies for metal-contaminated soils and groundwater: An evaluation. Eng. Geol. 2001, 60, 193–207. [Google Scholar] [CrossRef]
- Oliva, J.; De Pablo, J.; Cortina, J.-L.; Cama, J.; Ayora, C. The use of Apatite IITM to remove divalent metal ions zinc(II), lead(II), manganese(II) and iron(II) from water in passive treatment systems: Column experiments. J. Hazard. Mater. 2010, 184, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Abollino, O.; Giacomino, A.; Malandrino, M.; Mentasti, E. Interaction of metal ions with montmorillonite and vermiculite. Appl. Clay Sci. 2008, 38, 227–236. [Google Scholar] [CrossRef]
- Cabrera, C.; Gabaldon, C.; Marzal, P. Sorption characteristics of heavy metal ions by a natural zeolite. J. Chem. Technol. Biotechnol. 2005, 80, 477–481. [Google Scholar] [CrossRef]
- Katsou, E.; Malamis, S.; Haralambous, K.J.; Loizidou, M. Use of ultrafiltration membranes and aluminosilicate minerals for nickel removal from industrial wastewater. J. Membr. Sci. 2010, 360, 234–249. [Google Scholar] [CrossRef]
- Katsou, E.; Malamis, S.; Haralambous, K.J. Examination of zinc uptake in a combined system using sludge, minerals and ultrafiltration membranes. J. Hazard. Mater. 2010, 182, 27–38. [Google Scholar] [CrossRef]
- Lin, S.H.; Juang, R.S. Heavy metal removal from water by sorption using surfactant-modified montmorrilonite. J. Hazard. Mater. 2002, 92, 315–326. [Google Scholar] [CrossRef]
- Abollino, O.; Aceto, M.; Malandrino, M.; Sarzanini, C.; Mentasti, E. Adsorption of heavy metals on Na-montmorillonite: Effect of pH and organic substances. Water Res. 2003, 37, 1619–1627. [Google Scholar] [CrossRef]
- Almasri, D.A.; Rhadfia, T.; Atieh, M.A.; McKay, G.; Ahzi, S. High performance hydroxyiron modified montmorillonite nanoclay adsorbent for arsenite removal. Chem. Eng. J. 2018, 335, 1–12. [Google Scholar] [CrossRef]
- Yang, J.; Yu, K.; Liu, C. Chromium immobilization in soil using quaternary ammonium cations modified montmorillonite: Characterization and mechanism. J. Hazard. Mater. 2017, 321, 73–80. [Google Scholar] [CrossRef]
- Ma, B.; Oh, S.; Shin, W.S.; Choi, S.-J. Removal of Co2+, Sr2+ and Cs+ from aqueous solution by phosphate-modified montmorillonite (PMM). Desalination 2011, 276, 336–346. [Google Scholar] [CrossRef]
- Wen, X.; Lu, J.; Wu, J.; Lin, Y.; Luo, Y. Influence of coastal groundwater salinization on the distribution and risks of heavy metals. Sci. Total Environ. 2019, 652, 267–277. [Google Scholar] [CrossRef]
- Kester, D.R.; Duedall, I.W.; Connors., D.N.; Pytkowicz, R.M. Preparation of artificial seawater. Limno. Oceanogr. 1967, 12, 176–179. [Google Scholar] [CrossRef]
- U.S. EPA. Method 9081: Cation-Exchange Capacity (Sodium Acetate), Test Methods for the Evaluation of Solid Waste: Laboratory Manual Physical Chemical Methods; SW 846; U.S. EPA, Office of Solid Waste: Washington, DC, USA, 2003.
- Wolff-Boenisch, D.; Traina, S.J. A comparative study of the effect of desferrioxamine B, oxalic acid, and Na-alginate on the desorption of U(VI) from goethite at pH 6 and 25 °C. Geochim. Cosmochim. Acta 2006, 70, 4356–4366. [Google Scholar] [CrossRef]
- Dubinin, M.M. The potential theory of adsorption of gases and vapors for adsorbents with energetically non-uniform surface. Chem. Rev. 1960, 60, 235–266. [Google Scholar] [CrossRef]
- Kundu, S.; Gupta, A.K. Arsenic adsorption onto iron oxide-coated cement (IOCC): Regression analysis of equilibrium data with several isotherm models and their optimization. Chem. Eng. J. 2006, 122, 93–106. [Google Scholar] [CrossRef]
- Ko, D.C.K.; Cheung, C.W.; Choy, K.K.H.; Porter, J.F.; McKay, G. Sorption equilibria of metal ions on bone char. Chemosphere 2004, 54, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Kargi, F.; Ozmıhci, S. Biosorption performance of powdered activated sludge for removal of different dyestuffs. Enzym. Microb. Technol. 2004, 35, 267–271. [Google Scholar] [CrossRef]
- Parker, G.R., Jr. Optimum isotherm equation and thermodynamic interpretation for aqueous 1,1,2-trichloroethene adsorption isotherms on three adsorbents. Adsorption 1995, 1, 113–132. [Google Scholar] [CrossRef]
- Khan, A.R.; Ataullah, R.; Al-Haddad, A. Equilibrium adsorption studies of some aromatic pollutants from dilute aqueous solutions on activated carbon at different temperatures. J Colloid Interface Sci. 1997, 194, 154–165. [Google Scholar] [CrossRef]
- Srivastava, V.C.; Mall, I.D.; Mishra, I.M. Equilibrium modelling of single and binary adsorption of cadmium and nickel onto bagasse fly ash. Chem. Eng. J. 2006, 117, 79–91. [Google Scholar] [CrossRef]
- Choy, K.K.H.; Porter, J.F.; McKay, G. Langmuir isotherm models applied to the multicomponent sorption of acid dyes from effluent onto activated carbon. J. Chem. Eng. Data 2000, 45, 575–584. [Google Scholar] [CrossRef]
- Murali, V.; Aylmore, A.G. Competitive adsorption during solute transport in soils: 1. Mathematical models. Soil Sci. 1983, 135, 143–150. [Google Scholar] [CrossRef]
- Radke, C.J.; Prausnitz, J.M. Thermodynamics of multi-solute adsorption from dilute liquid solutions. AIChE J. 1972, 18, 761–768. [Google Scholar] [CrossRef]
- Yen, C.-Y.; Singer, P.C. Competitive adsorption of phenols on activated carbon. J. Environ. Eng. 1984, 110, 976–983. [Google Scholar] [CrossRef]
- Shin, W.S. Competitive sorption of anionic and cationic dyes onto cetylpyridinium-modified montmorillonite. J. Environ. Sci. Heal. A 2008, 43, 1459–1470. [Google Scholar] [CrossRef]
- Sparks, D.L. Environmental Soil Chemistry, 2nd ed.; Academic Press: Cambridge, MA, USA, 2003. [Google Scholar]
- Suteu, D.; Bilba, D.; Dan, F. Synthesis and characterization of polyamide powders for sorption of reactive dyes from aqueous solutions. J. Appl. Polym. Sci. 2007, 105, 1833–1843. [Google Scholar] [CrossRef]
- Hilal, N.; Busca, G.; Rozada, F.; Hankins, N. Use of activated carbon to polish effluent from metalworking treatment plant: Comparison of different streams. Desalination 2005, 185, 297–306. [Google Scholar] [CrossRef]
- Ghosh, G.C.; Chakraborty, T.K.; Zaman, S.; Nahar, M.N.; Kabir, A.H.M.E. Removal of Methyl Orange Dye from Aqueous Solution by a Low-Cost Activated Carbon Prepared from Mahagoni (Swietenia mahagoni) Bark. Pollution 2020, 6, 171–184. [Google Scholar] [CrossRef]
- McKay, G.; Blair, H.S.; Gardner, J.R. Adsorption of dyes on chitin. I. Equilibrium studies. J. Appl. Polym. Sci. 1982, 27, 3040–3057. [Google Scholar] [CrossRef]
- Adlnasab, L.; Nader, D.; Akram, M. A new magnetic bio-sorbent for arsenate removal from the contaminated water: Characterization, isotherms, and kinetics. Environ. Health Eng. Manag. J. 2020, 7, 49–58. [Google Scholar] [CrossRef]
- Apiratikula, R.; Pavasant, P. Sorption of Cu2+, Cd2+, and Pb2+ using modified zeolite from coal fly ash. Chem. Eng. J. 2008, 144, 245–258. [Google Scholar] [CrossRef]
- Rivas, B.L.; Quilodrán, B.; Quiroz, E. Removal properties of crosslinked poly(2-acrylamidoglycolic acid) for trace heavy metal ions: Effect of pH, temperature, contact time, and salinity on the adsorption behavior. J. Appl. Polym. Sci. 2003, 88, 2614–2621. [Google Scholar] [CrossRef]
- Phillips, I.R.; Lamb, D.T.; Hawker, D.W.; Burton, E.D. Effects of pH and salinity on copper, lead, and zinc sorption rates in sediments from Moreton bay, Australia. Bull. Environ. Contam. Toxicol. 2004, 73, 1041–1048. [Google Scholar] [CrossRef]
- Choi, J.; Septian, A.; Shin, W.S. The influence of salinity on the removal of Ni and Zn by sorption onto iron oxide- and manganese oxide-coated sand. Sustainability 2020, 12, 5815. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Gupta, S.S. Uptake of Ni(II) ions from aqueous solution by kaolinite and montmorillonite: Influence of acid activation of the clays. Sep. Sci. Technol. 2008, 43, 3221–3250. [Google Scholar] [CrossRef]
- Gupta, S.S.; Bhattacharyya, K.G. Immobilization of Pb(II), Cd(II) and Ni(II) ions on kaolinite and montmorillonite surfaces from aqueous medium. J. Environ. Manag. 2008, 87, 46–58. [Google Scholar] [CrossRef]
- Baeyens, B.; Bradbury, M.H. A mechanistic description of Ni and Zn sorption on Na-montmorillonite Part I: Titration and sorption measurements. J. Contam. Hydrol. 1997, 27, 199–222. [Google Scholar] [CrossRef]
- Musso, T.B.; Parolo, M.E.; Pettinari, G. pH, ionic strength, and ion competition effect on Cu(II) and Ni(II) sorption by a Na-bentonite used as liner material. Pol. J. Environ. Stud. 2019, 28, 2299–2309. [Google Scholar] [CrossRef]
- Van Riemsdijk, H.; Bolt, G.H.; Koopel, L.K.; Blaakmeer, J. Electrolyte adsorption on heterogeneous surfaces: Adsorption models. J. Colloid Interface Sci. 1985, 109, 219–228. [Google Scholar] [CrossRef]
- Adhikari, R.; Singh, M.V. Adsorption characteristics of lead and cadmium in some soils of India. Geoderma 2003, 114, 81–92. [Google Scholar] [CrossRef]

| Ingredient | Concentration (g/L) |
|---|---|
| NaCl | 24.72 |
| KCl | 0.67 |
| CaCl2⋅2H2O | 1.36 |
| MgCl2 | 2.18 |
| MgSO4 | 3.07 |
| NaHCO3 | 0.18 |
| Model | Equation | Reference |
|---|---|---|
| Single Adsorption | ||
| Freundlich | (1) | |
| Langmuir | (2) | |
| Dubinin-Radushkevich (D−R) | (3) | [26,27] |
| (4) | ||
| Sips | (5) | [28] |
| Kargi–Ozmıhci (K–O) | (6) | [29] |
| Holl-Kirch (H−K) | (7) | [30,31] |
| Binary Adsorption | ||
| Competitive Langmuir model (CLM) | (8) | [32] |
| P-factor | (9) (10) | [33] |
| Murali−Aylmore (M−A) | (11) | [34] |
| Ideal adsorbed solution theory (IAST) or | (12) | [35,36,37] |
| Freundlich | Metal | Salinity (‰) | NF (-) | R2 | SSE | ||
|---|---|---|---|---|---|---|---|
| Ni | 0 | 0.337 ± 0.005 | 0.425 ± 0.016 | 0.994 | 0.001 | ||
| 30 | 0.239 ± 0.008 | 0.464 ± 0.038 | 0.957 | 0.006 | |||
| Zn | 0 | 0.282 ± 0.006 | 0.423 ± 0.021 | 0.987 | 0.002 | ||
| 30 | 0.115 ± 0.003 | 0.392 ± 0.033 | 0.950 | 0.002 | |||
| Langmuir | Metal | Salinity (‰) | QmL (mmol/g) | bL (L/mmol) | R2 | SSE | Sf |
| Ni | 0 | 0.383 ± 0.017 | 4.937 ± 0.767 | 0.986 | 0.003 | 0.910 | |
| 30 | 0.319 ± 0.015 | 2.988 ± 0.378 | 0.982 | 0.002 | 0.944 | ||
| Zn | 0 | 0.314 ± 0.014 | 5.171 ± 0.751 | 0.983 | 0.003 | 0.906 | |
| 30 | 0.147 ± 0.005 | 4.055 ± 0.490 | 0.977 | 0.001 | 0.925 | ||
| D−R | Metal | Salinity (‰) | QmD (mmol/g) | β (mol2/J2, ×10−8), | R2 | SSE | E (kJ/mol) |
| Ni | 0 | 0.330 ± 0.011 | 2.394 ± 0.264 | 0.975 | 0.006 | 4.57 | |
| 30 | 0.263 ± 0.008 | 3.799 ± 0.319 | 0.972 | 0.004 | 3.63 | ||
| Zn | 0 | 0.274 ± 0.009 | 2.443 ± 0.242 | 0.973 | 0.004 | 4.52 | |
| 30 | 0.128 ± 0.004 | 3.170 ± 0.251 | 0.970 | 0.001 | 3.97 | ||
| Sips | Metal | Salinity (‰) | QmS (mmol/g) | bS (L/mmol) | NS | R2 | SSE |
| Ni | 0 | 0.670 ± 0.087 | 0.945 ± 0.387 | 0.604 ± 0.036 | 0.998 | 0.000 | |
| 30 | 0.323 ± 0.041 | 2.897 ± 0.911 | 0.982 ± 0.147 | 0.979 | 0.002 | ||
| Zn | 0 | 0.492 ± 0.096 | 1.407 ± 0.854 | 0.640 ± 0.069 | 0.993 | 0.001 | |
| 30 | 0.153 ± 0.017 | 3.627 ± 1.094 | 0.928 ± 0.150 | 0.972 | 0.001 | ||
| K−O | Metal | Salinity (‰) | QmKO (mmol/g) | bKO (L/mmol) | NKO | R2 | SSE |
| Ni | 0 | 0.670 ± 0.087 | 1.034 ± 0.254 | 0.604 ± 0.036 | 0.998 | 0.000 | |
| 30 | 0.323 ± 0.041 | 0.352 ± 0.160 | 0.982 ± 0.147 | 0.978 | 0.002 | ||
| Zn | 0 | 0.492 ± 0.096 | 0.804 ± 0.330 | 0.640 ± 0.069 | 0.993 | 0.001 | |
| 30 | 0.153 ± 0.017 | 0.303 ± 0.139 | 0.928 ± 0.150 | 0.972 | 0.001 | ||
| H−K | Metal | Salinity (‰) | QmHK (mmol/g) | bHK (L/mmol) | NHK | R2 | SSE |
| Ni | 0 | 0.670 ± 0.087 | 0.967 ± 0.237 | 0.604 ± 0.036 | 0.998 | 0.000 | |
| 30 | 0.323 ± 0.041 | 2.844 ± 1.292 | 0.982 ± 0.147 | 0.978 | 0.002 | ||
| Zn | 0 | 0.492 ± 0.096 | 1.244 ± 0.511 | 0.640 ± 0.069 | 0.993 | 0.010 | |
| 30 | 0.153 ± 0.017 | 3.305 ± 1.518 | 0.928 ± 0.150 | 0.972 | 0.001 |
| M−A model | Salinity (‰) | a12 | a21 | R2 | SSE | RMSE |
|---|---|---|---|---|---|---|
| 0 | 0.053 ± 0.042 | 0.297 ± 0.069 | 0.866/0.749 | 0.028/0.021 | 0.041/0.035 | |
| 30 | 0.576 ± 0.028 | 0.333 ± 0.090 | 0.984/0.820 | 0.001/0.004 | 0.008/0.017 | |
| CLM | Salinity (‰) | - | - | R2 | SSE | RMSE |
| 0 | 0.869/0.861 | 0.128/0.067 | 0.090/0.065 | |||
| 30 | 0.934/0.908 | 0.013/0.006 | 0.030/0.021 | |||
| P−Factor model | Salinity (‰) | Pi | - | R2 | SSE | RMSE |
| 0 | 1.175/1.570 | 0.948/0.894 | 0.050/0.051 | 0.058/0.058 | ||
| 30 | 1.253/0.747 | 0.929/0.299 | 0.014/0.048 | 0.033/0.061 | ||
| IAST−Fr | Salinity (‰) | - | - | R2 | SSE | RMSE |
| 0 | 0.918/0.924 | 0.080/0.037 | 0.073/0.049 | |||
| 30 | 0.881/0.809 | 0.091/0.013 | 0.084/0.032 | |||
| IAST−Sips | Salinity (‰) | - | - | R2 | SSE | RMSE |
| 0 | 0.882/0.867 | 0.115/0.064 | 0.088/0.065 | |||
| 30 | 0.964/0.699 | 0.007/0.021 | 0.023/0.040 | |||
| IAST−K−O | Salinity (‰) | - | - | R2 | SSE | RMSE |
| 0 | 0.905/0.823 | 0.093/0.085 | 0.079/0.075 | |||
| 30 | 0.965/0.832 | 0.007/0.012 | 0.023/0.030 | |||
| IAST−H−K | Salinity (‰) | - | - | R2 | SSE | RMSE |
| 0 | 0.882/0.867 | 0.115/0.064 | 0.088/0.065 | |||
| 30 | 0.964/0.700 | 0.007/0.021 | 0.023/0.040 |
| Salinity (‰) | Solute | (mmol/g) | (L/mmol) | R2 | SSE |
|---|---|---|---|---|---|
| 0 | Ni in Ni/Zn | 0.326 ± 0.015 | 14.38 ± 3.946 | 0.918 | 0.017 |
| Zn in Ni/Zn | 0.200 ± 0.011 | 64.31 ± 28.61 | 0.794 | 0.017 | |
| 30 | Ni in Ni/Zn | 0.254 ± 0.016 | 1.616 ± 0.232 | 0.986 | 0.001 |
| Zn in Ni/Zn | 0.196 ± 0.026 | 0.922 ± 0.227 | 0.973 | 0.001 |
| Salinity (‰) | QmL,Ni/QmL,Zn | / | / | / |
| 0 | 1.219 | 1.630 | 0.851 | 0.637 |
| 30 | 2.175 | 1.297 | 0.798 | 1.338 |
| Salinity (‰) | bL,Ni/bL,Zn | / | / | / |
| 0 | 0.955 | 0.224 | 2.913 | 12.44 |
| 30 | 0.737 | 1.752 | 0.541 | 0.228 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.; Septian, A.; Shin, W.S. Influence of Salinity on the Removal of Ni and Zn by Phosphate-Intercalated Nano Montmorillonite (PINM). Minerals 2020, 10, 980. https://doi.org/10.3390/min10110980
Choi J, Septian A, Shin WS. Influence of Salinity on the Removal of Ni and Zn by Phosphate-Intercalated Nano Montmorillonite (PINM). Minerals. 2020; 10(11):980. https://doi.org/10.3390/min10110980
Chicago/Turabian StyleChoi, Jiyeon, Ardie Septian, and Won Sik Shin. 2020. "Influence of Salinity on the Removal of Ni and Zn by Phosphate-Intercalated Nano Montmorillonite (PINM)" Minerals 10, no. 11: 980. https://doi.org/10.3390/min10110980
APA StyleChoi, J., Septian, A., & Shin, W. S. (2020). Influence of Salinity on the Removal of Ni and Zn by Phosphate-Intercalated Nano Montmorillonite (PINM). Minerals, 10(11), 980. https://doi.org/10.3390/min10110980






