Recovery of Lithium from Lepidolite by Sulfuric Acid and Separation of Al/Li by Nanofiltration
Abstract
1. Introduction
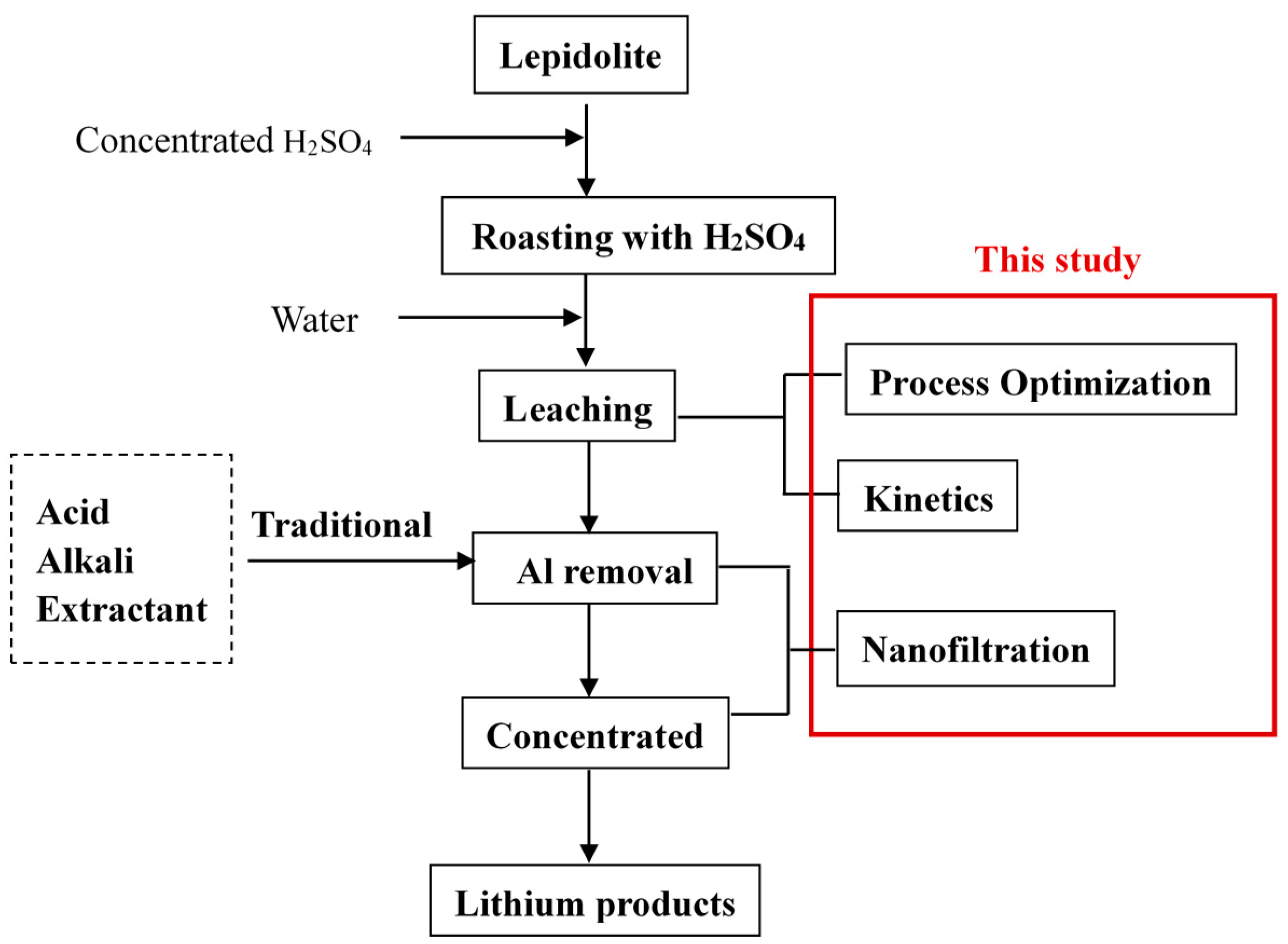
2. Materials and Methods
2.1. Leaching Process of Lepidolite
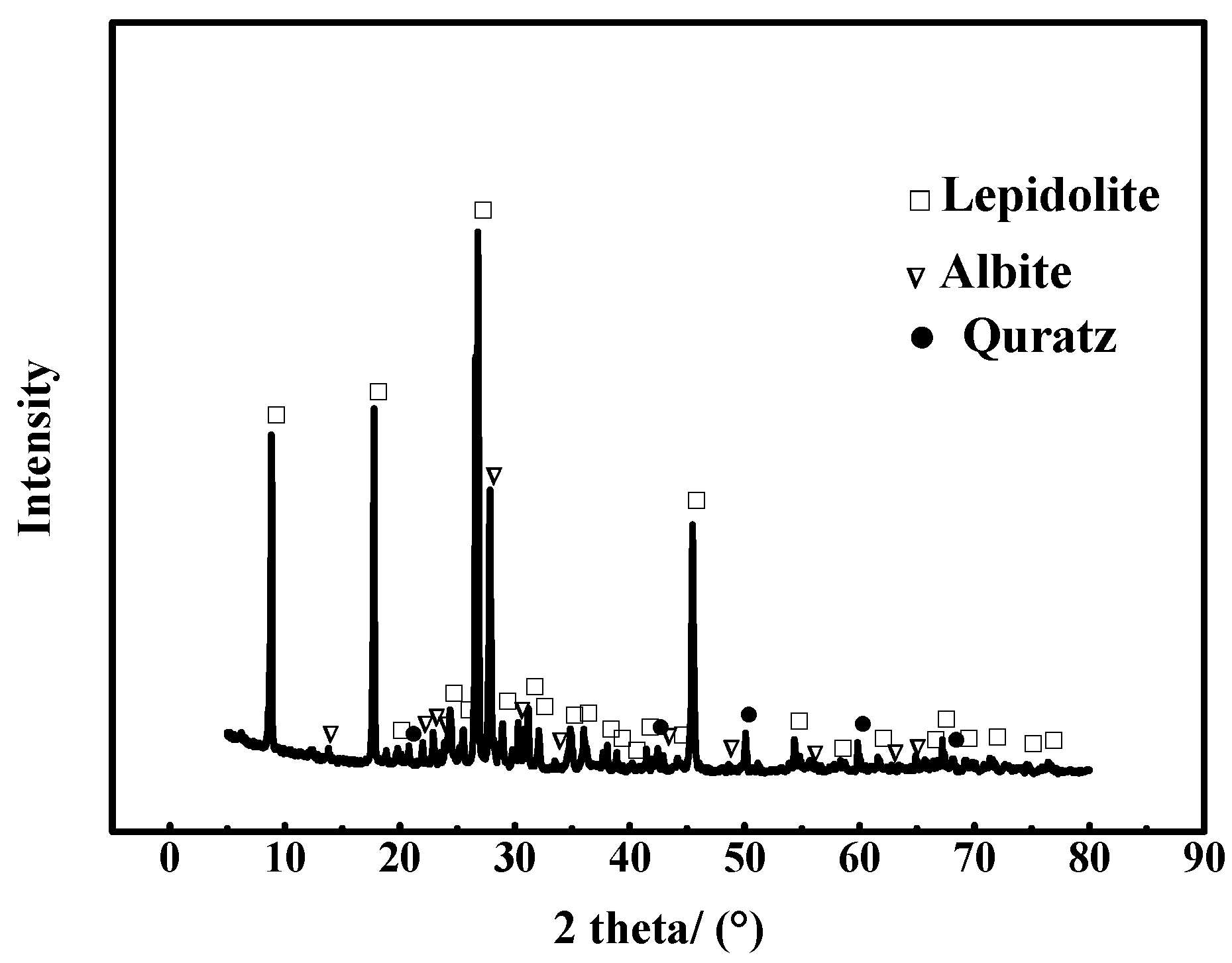
2.1.1. Materials
2.1.2. Experimental Methods and Procedures
2.1.3. Analytical Methods
2.1.4. Calculation
2.2. Nanofiltration Separation
2.2.1. Materials
2.2.2. Experimental Methods and Procedures
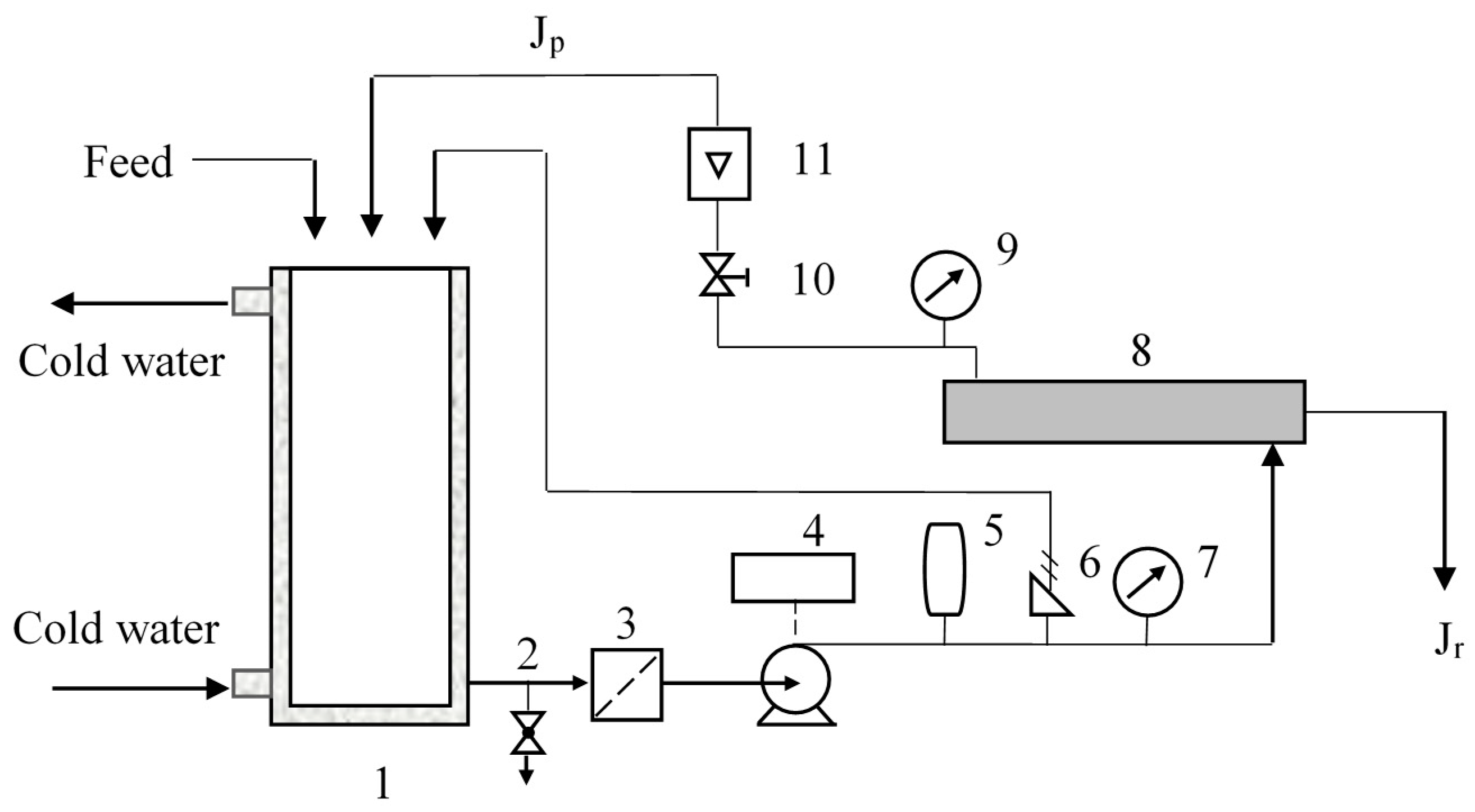
2.2.3. Separation Equipment
2.2.4. Calculation
3. Results and Discussion
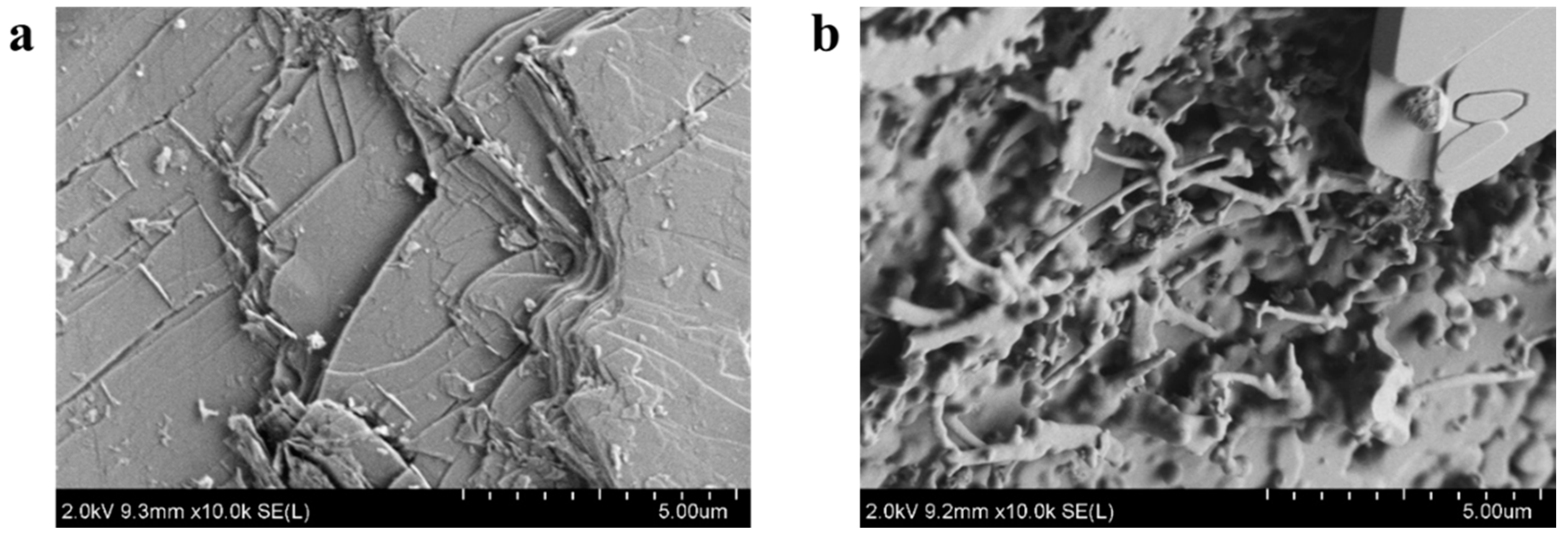
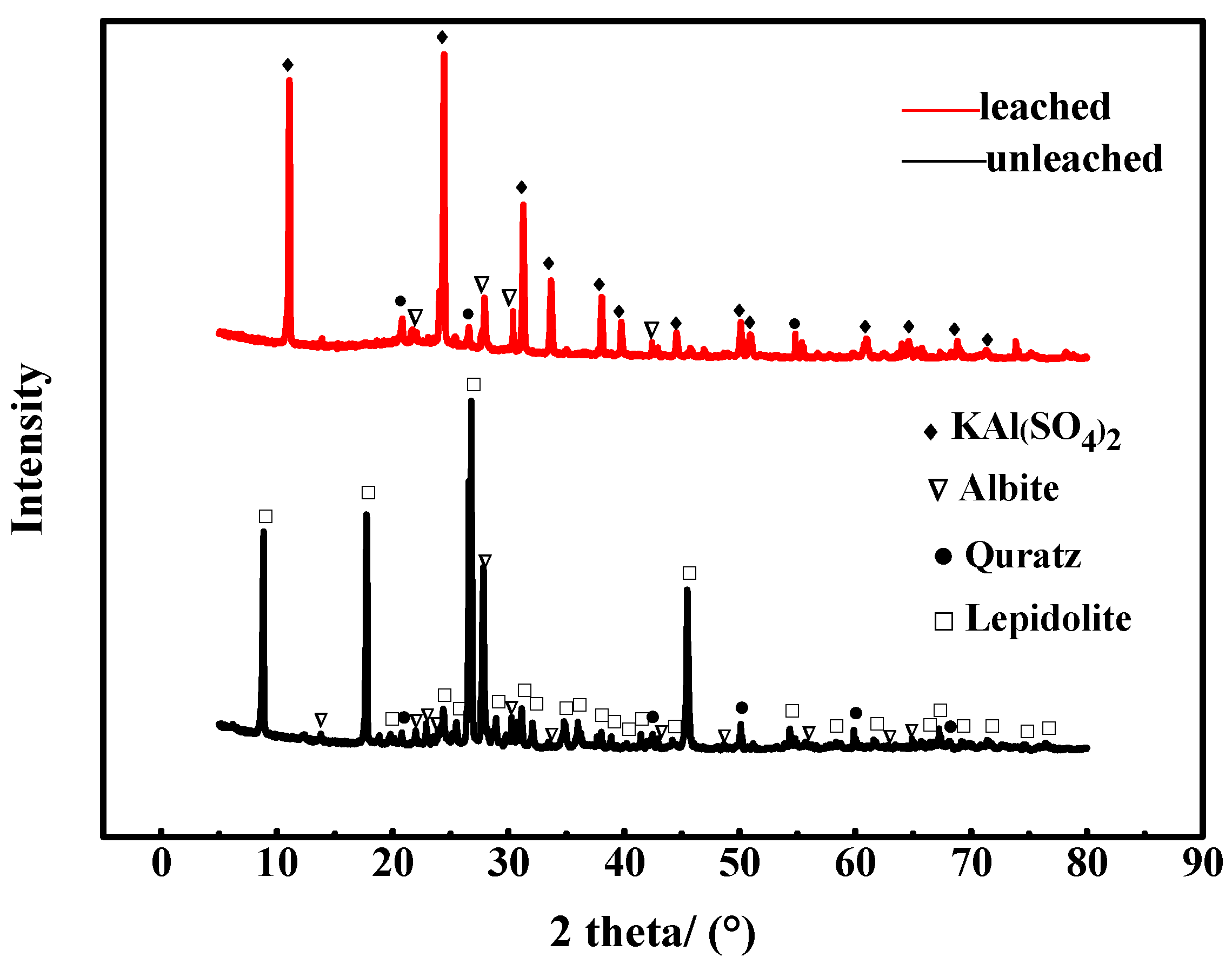
3.1. Morphology and Composition of the Leaching Residue
3.2. Leaching Process Optimization
3.2.1. Effect of Leaching Temperature
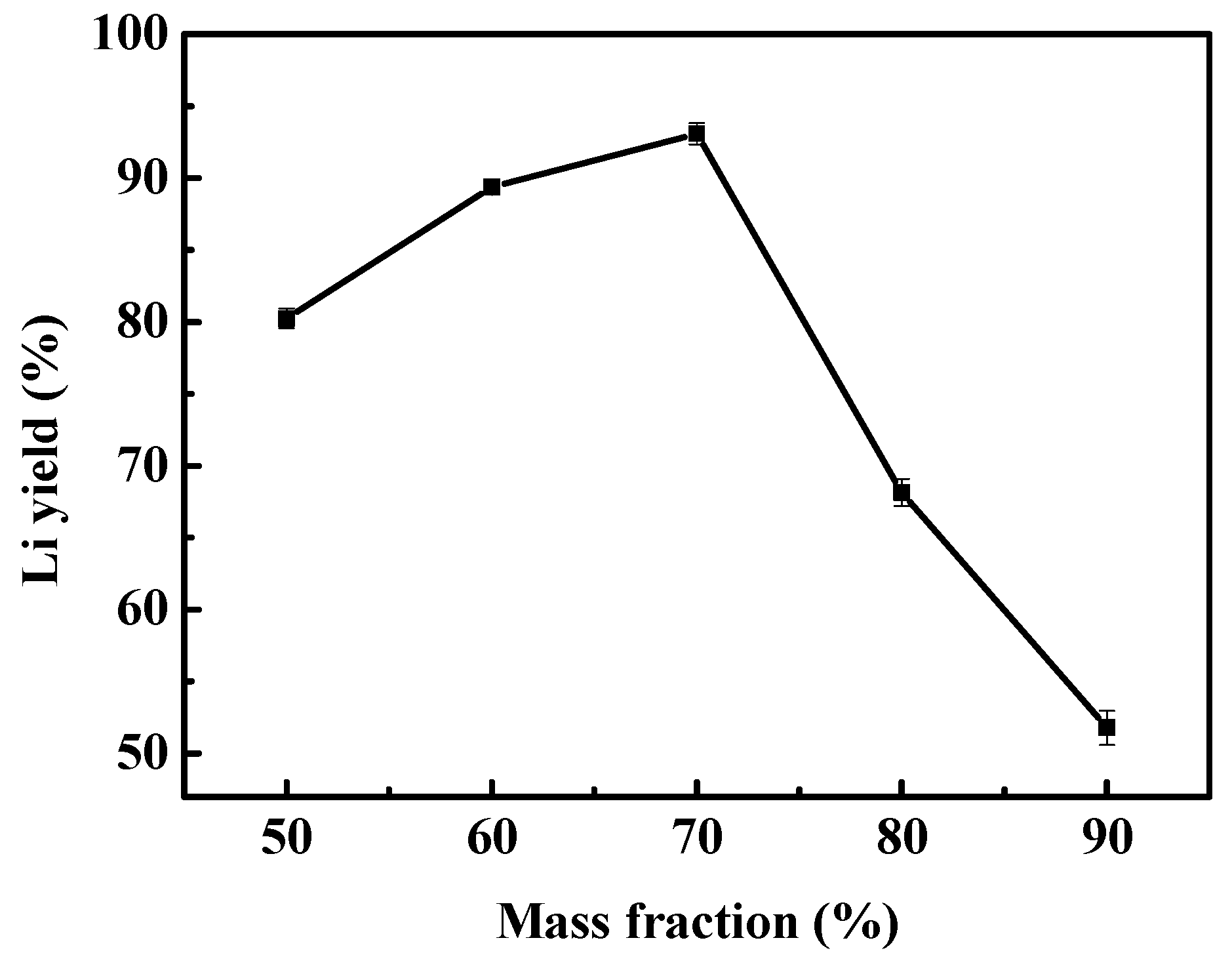
3.2.2. Effect of Sulfuric Acid Mass Fraction
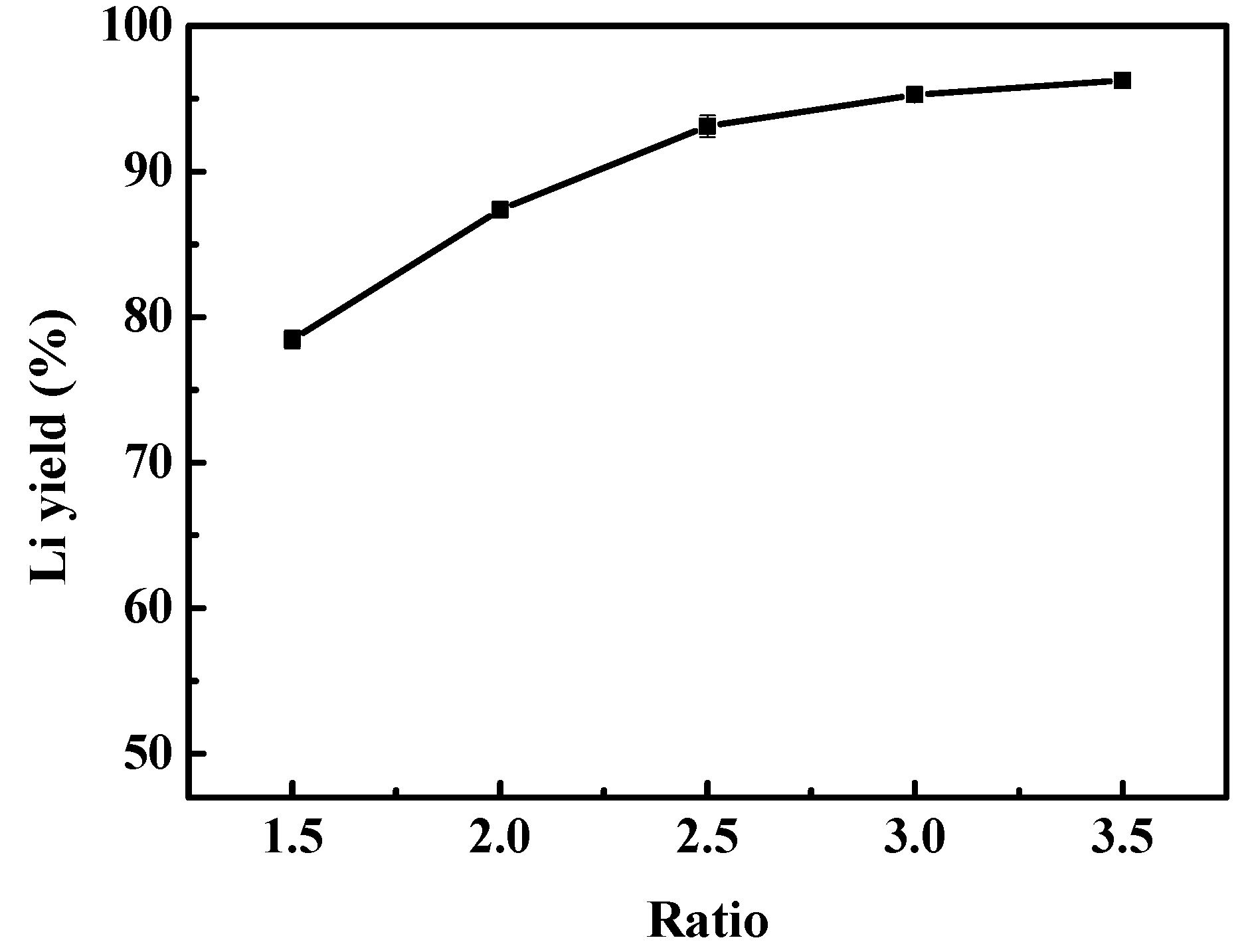
3.2.3. Effect of Liquid-Solid Mass Ratio
3.2.4. Effect of Leaching Time
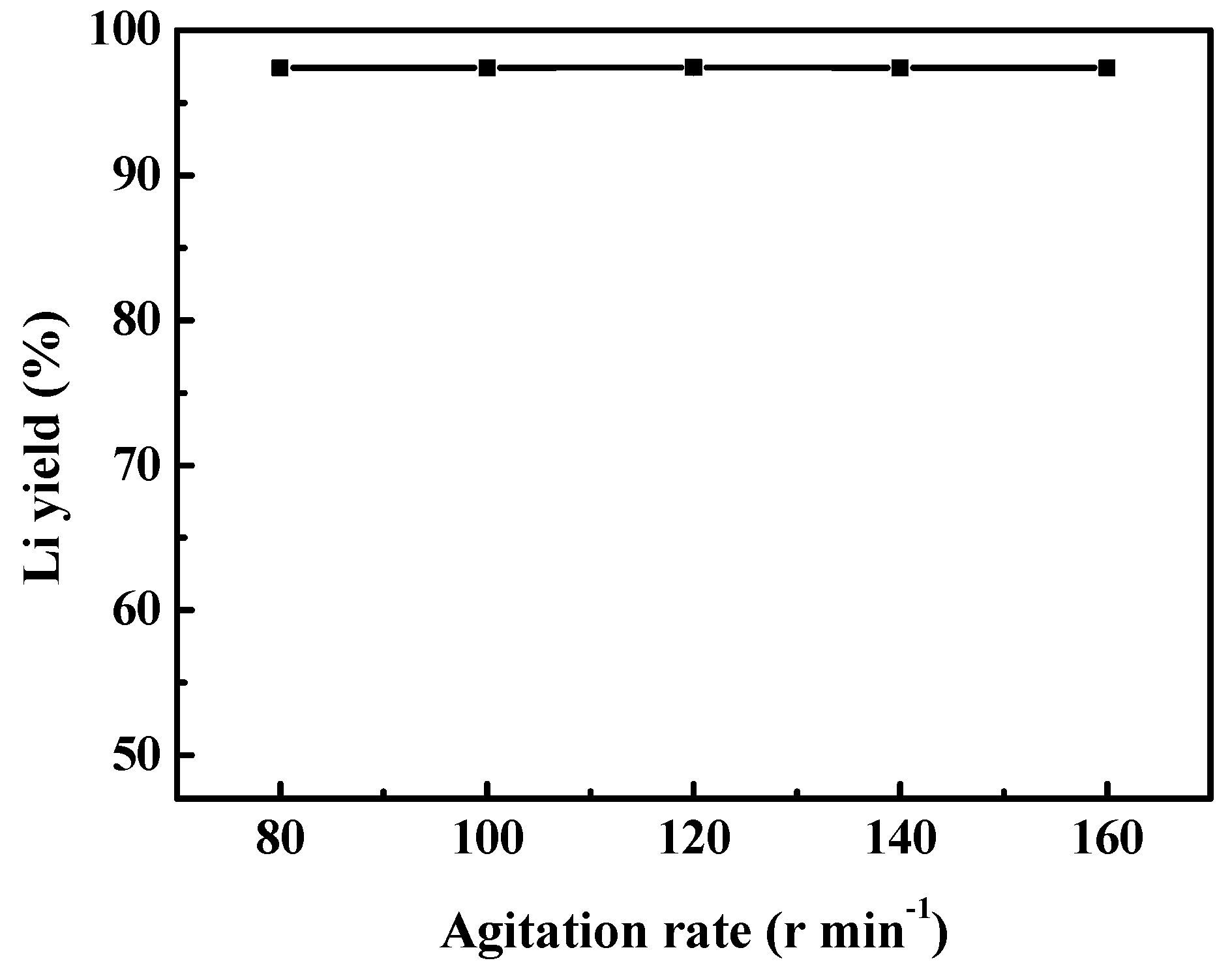
3.2.5. Effect of Agitation Rate
3.2.6. Orthogonal Experiment
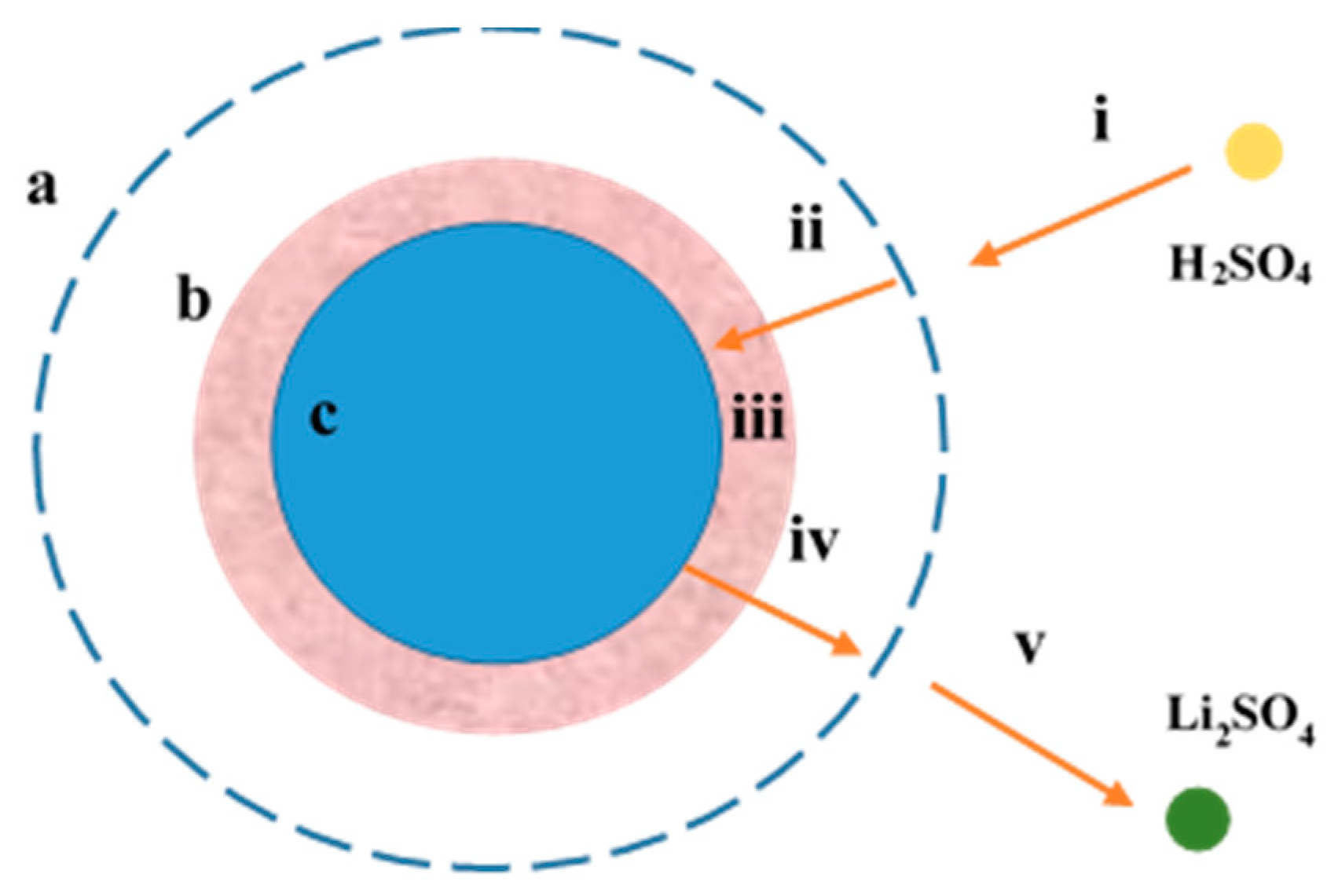
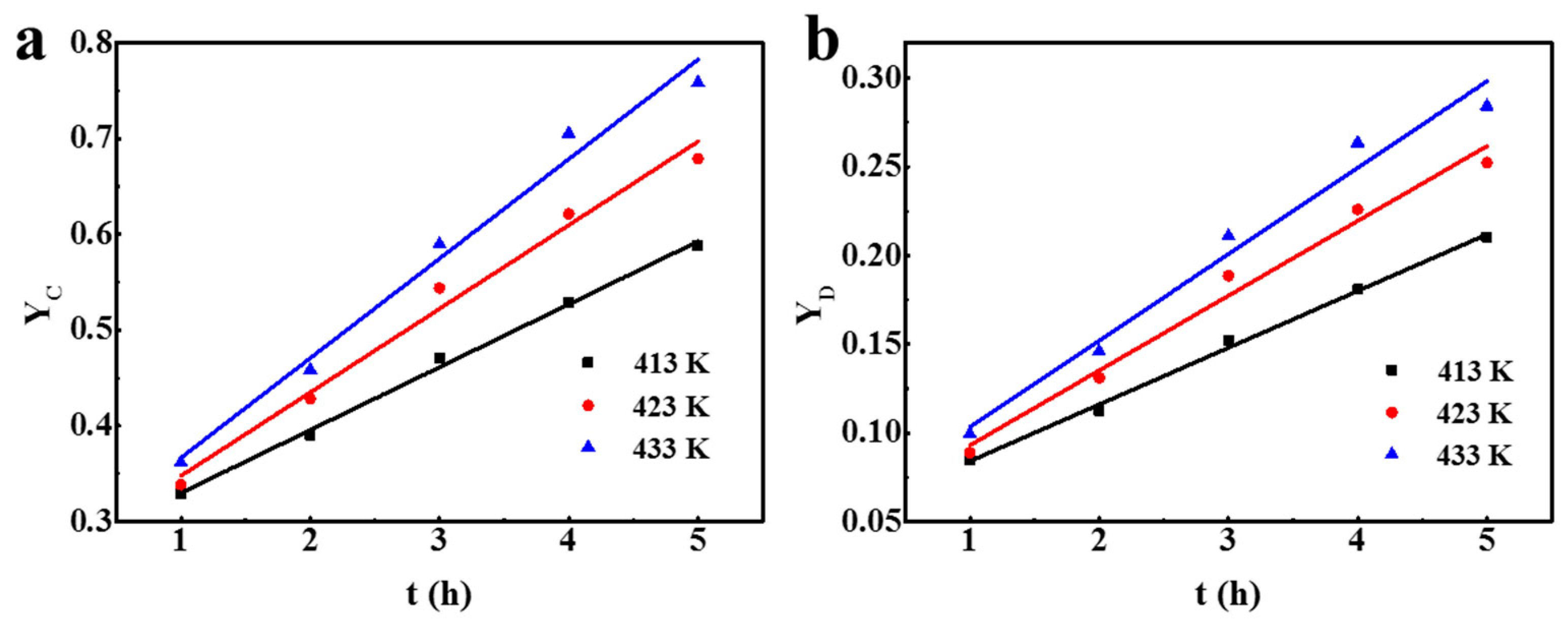
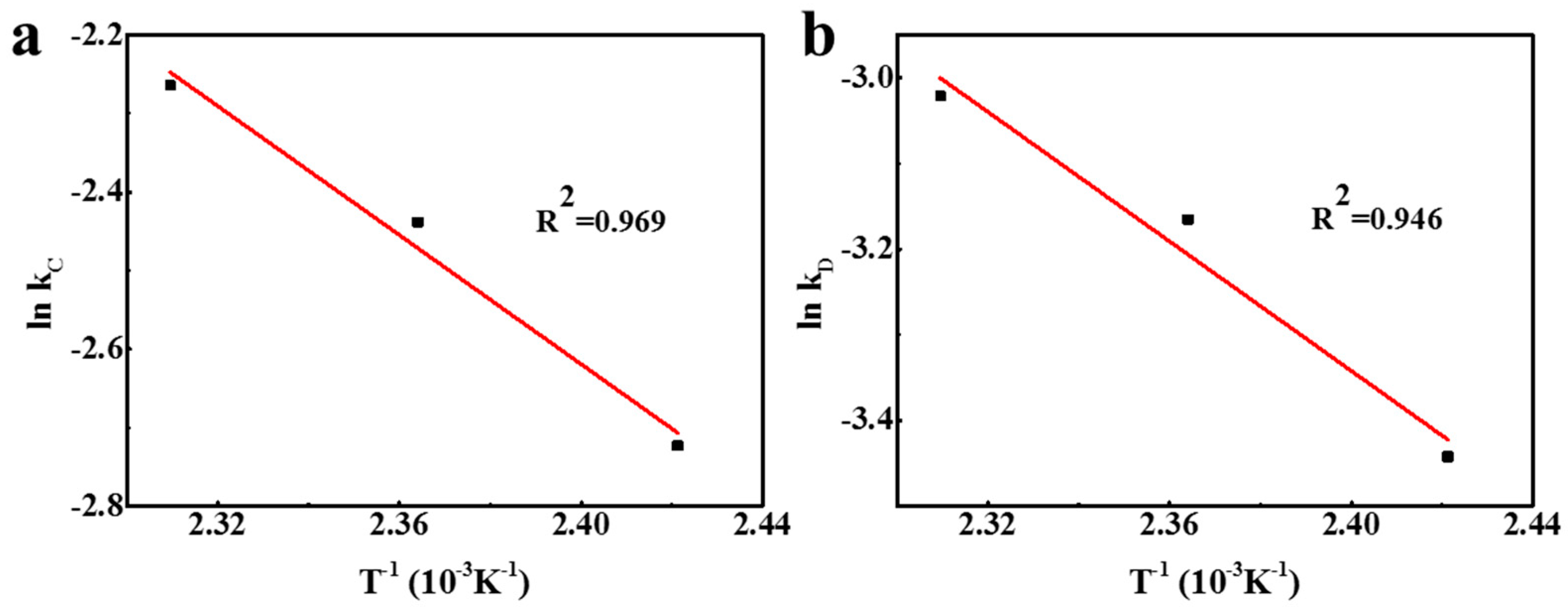
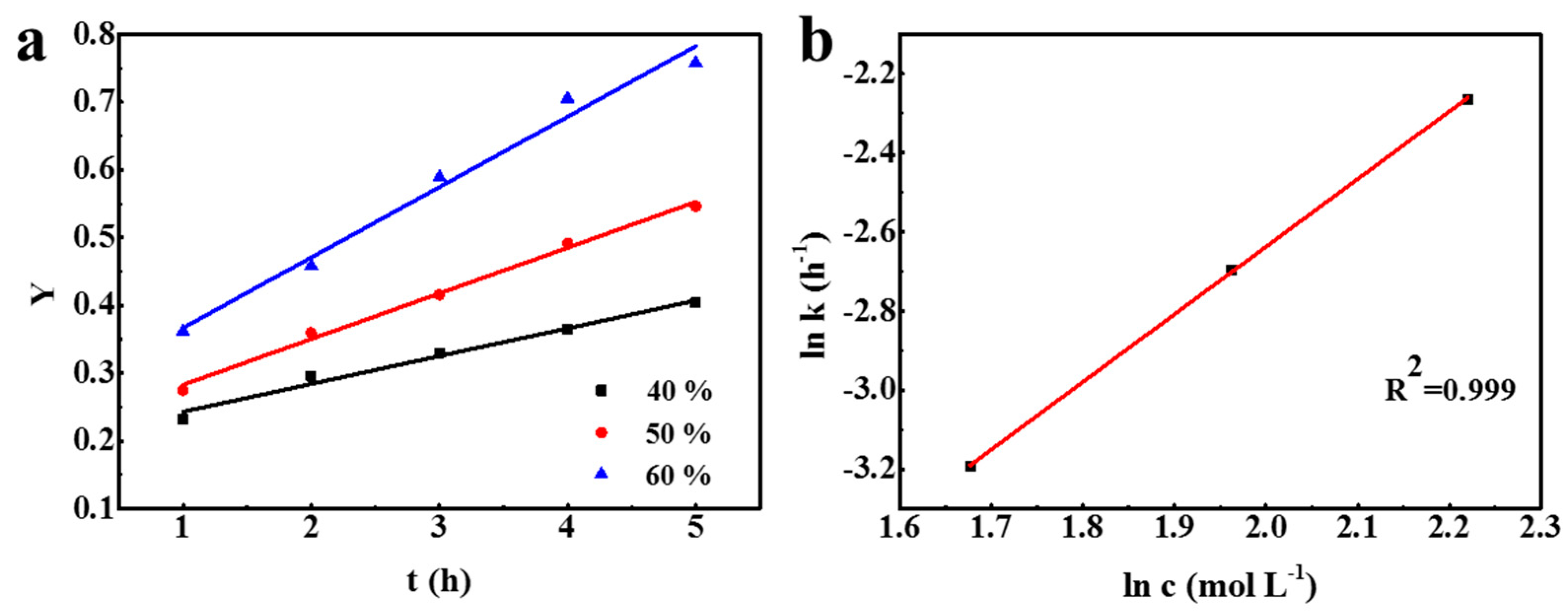
3.3. Kinetic Analysis
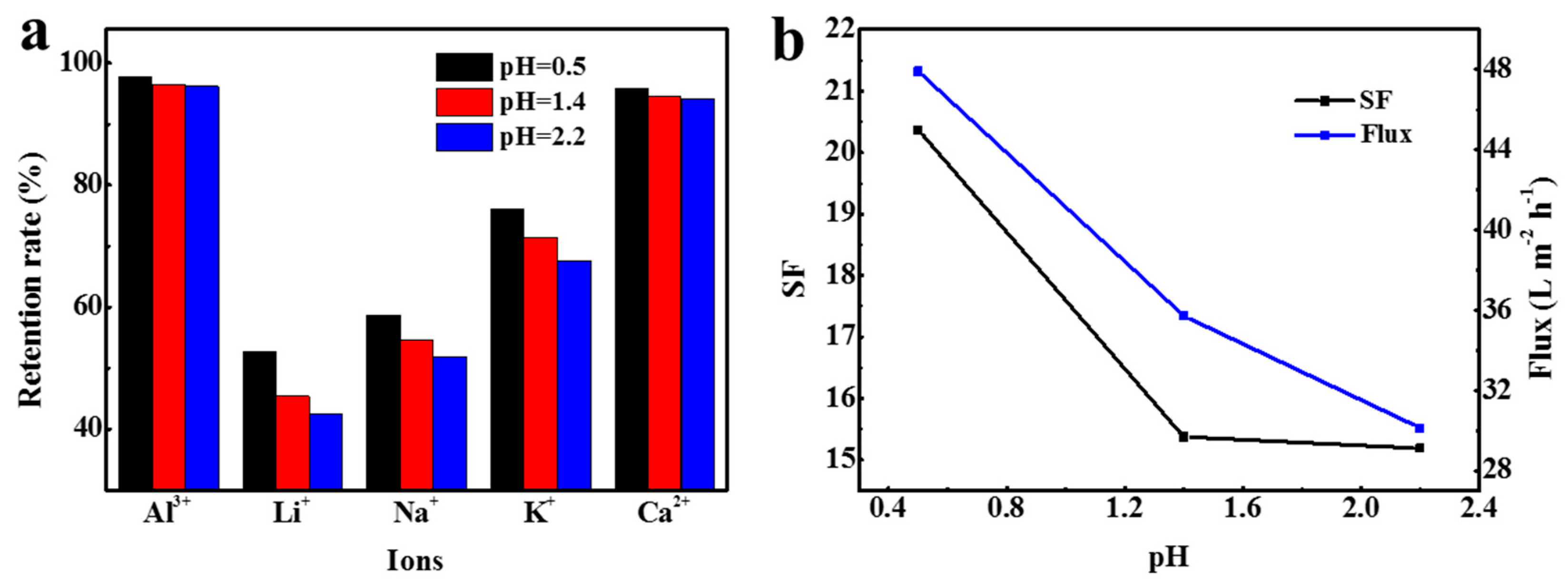
3.4. Nanofiltration Separation Process of Actual Leaching Solution
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Batistoni, P.; Angelone, M.; Carconi, P.; Fischer, U.; Fleischer, K.; Kondo, K.; Klix, A.; Kodeli, I.; Leichtle, D.; Petrizzi, L.; et al. Neutronics experiments on HCPB and HCLL TBM mock-ups in preparation of nuclear measurements in ITER. Fusion Eng. Des. 2010, 85, 1675–1680. [Google Scholar] [CrossRef]
- Xu, W.; Birbilis, N.; Sha, G.; Wang, Y.; Daniels, J.E.; Xiao, Y.; Ferry, M. A high-specific-strength and corrosion-resistant magnesium alloy. Nat. Mater. 2015, 14, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Rioja, R.J.; Liu, J. The Evolution of Al-Li Base Products for Aerospace and Space Applications. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2012, 43, 3325–3337. [Google Scholar] [CrossRef]
- De Laurentis, N.; Cann, P.M.; Lugt, P.M.; Kadiric, A. The Influence of Base Oil Properties on the Friction Behaviour of Lithium Greases in Rolling/Sliding Concentrated Contacts. Tribol. Lett. 2017, 65, 128. [Google Scholar] [CrossRef]
- Bernhardt, D.; Reilly, I.J. Mineral Commodity Summaries 2019; US Geological Survey: Reston, VA, USA, 2019.
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Extraction of lithium from primary and secondary sources by pre-treatment, leaching and separation: A comprehensive review. Hydrometallurgy 2014, 150, 192–208. [Google Scholar] [CrossRef]
- Choubey, P.K.; Kim, M.; Srivastava, R.R.; Lee, J.; Lee, J. Advance review on the exploitation of the prominent energy-storage element: Lithium. Part I: From mineral and brine resources. Miner. Eng. 2016, 89, 119–137. [Google Scholar] [CrossRef]
- Barbosa, L.I.; Gonzalez, J.A.; Ruiz, M.D. Extraction of lithium from beta-spodumene using chlorination roasting with calcium chloride. Thermochim. Acta 2015, 605, 63–67. [Google Scholar] [CrossRef]
- Guo, H.; Kuang, G.; Wang, H.; Yu, H.; Zhao, X. Investigation of Enhanced Leaching of Lithium from α-Spodumene Using Hydrofluoric and Sulfuric Acid. Minerals 2017, 7, 205. [Google Scholar] [CrossRef]
- Luong, V.T.; Kang, D.J.; An, J.W.; Dao, D.A.; Kim, M.J.; Tran, T. Iron sulphate roasting for extraction of lithium from lepidolite. Hydrometallurgy 2014, 141, 8–16. [Google Scholar] [CrossRef]
- Yan, Q.; Li, X.; Wang, Z.; Wu, X.; Guo, H.; Hu, Q.; Peng, W.; Wang, J. Extraction of valuable metals from lepidolite. Hydrometallurgy 2012, 117–118, 116–118. [Google Scholar] [CrossRef]
- Yan, Q.; Li, X.; Yin, Z.; Wang, Z.; Guo, H.; Peng, W.; Hu, Q. A novel process for extracting lithium from lepidolite. Hydrometallurgy 2012, 121–124. [Google Scholar] [CrossRef]
- Yan, Q.-X.; Li, X.-H.; Wang, Z.-X.; Wang, J.-X.; Guo, H.-J.; Hu, Q.-Y.; Peng, W.-J.; Wu, X.-F. Extraction of lithium from lepidolite using chlorination roasting–water leaching process. Trans. Nonferr. Metals Soc. of China 2012, 22, 1753–1759. [Google Scholar] [CrossRef]
- Luong, V.T.; Dong, J.K.; An, J.W.; Kim, M.J.; Tran, T. Factors affecting the extraction of lithium from lepidolite. Hydrometallurgy 2013, 134–135, 54–61. [Google Scholar] [CrossRef]
- Ellestad, R.B.; Milne, L.K. Method of Extracting Lithium Values from Spodumene Ores. U.S. Patent 2516109, 25 July 1950. [Google Scholar]
- Lajoie-Leroux, F.; Dessemond, C.; Soucy, G.; Laroche, N.; Magnan, J.-F. Impact of the impurities on lithium extraction from β-spodumene in the sulfuric acid process. Miner. Eng. 2018, 129, 1–8. [Google Scholar] [CrossRef]
- Tian, Q.; Chen, B.; Chen, Y.; Ma, L.; Shi, X. Roasting and leaching behavior of spodumene in sulphuric acid process. Chin. J. Rare Metals 2011, 35, 118–123. [Google Scholar]
- Vieceli, N.; Nogueira, C.A.; Pereira, M.F.C.; Durão, F.O.; Margarido, F. Recovery of lithium carbonate by acid digestion and hydrometallurgical processing from mechanically activated lepidolite. Hydrometallurgy 2017, 175, 1–10. [Google Scholar] [CrossRef]
- Zhang, X.; Tan, X.; Li, C.; Yi, Y.; Liu, W.; Zhang, L. Energy-efficient and simultaneous extraction of lithium, rubidium and cesium from lepidolite concentrate via sulfuric acid baking and water leaching. Hydrometallurgy 2019, 185, 244–249. [Google Scholar] [CrossRef]
- Jia, J.; Zhang, Y.; Sheng, W.U.; Liao, C.; Yan, C.; Liu, J.; Deng, G.; Rui, X. Distribution and Separation of Aluminum in the Rare Earth Solvent Extraction Separation Processes(I). Chin. Rare Earths 2001, 22, 10–13. [Google Scholar]
- Zhang, Y.; Peng, Q. Method for Extracting Lithium Carbonate and Removing Aluminum from Lepidolite. CN Patent 10222577, 26 October 2011. [Google Scholar]
- Bracken, L.; Bruggen, B.V.D.; Vandecasteele, C. Regeneration of brewery waste water using nanofiltration. Water Res. 2004, 38, 3075–3082. [Google Scholar] [CrossRef]
- Zhi, W.; Liu, G.C.; Fan, Z.F.; Yang, X.T.; Wang, J.X.; Wang, S.C. Experimental study on treatment of electroplating wastewater by nanofiltration. J. Membr. Sci. 2007, 305, 185–195. [Google Scholar]
- Mohammad, A.W.; Teow, Y.H.; Ang, W.L.; Chung, Y.T.; Oatley-Radcliffe, D.L.; Hilal, N. Nanofiltration membranes review: Recent advances and future prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]
- Gao, L.; Wang, H.; Zhang, Y.; Wang, M. Nanofiltration Membrane Characterization and Application: Extracting Lithium in Lepidolite Leaching Solution. Membranes 2020, 10, 178. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Zhang, X.; Tan, X. Extraction of Li, Rb, Cs from lepidolite by sulfuric acid and water leaching. Nonferr. Metals (Extr. Metall.) 2019, 04, 39–42. [Google Scholar]
- Chi, R.; Zhu, G.; Tian, J. Leaching kinetics of rare earth from black weathering mud with hydrochloric acid. Trans. Nonferr. Metals Soc. China 2000, 10, 531–533. [Google Scholar]
- Chen, J.; Yang, S.; Ke, J. The Studies and Progress of Metallurgy; China Metallurgy Industry Press: Beijing, China, 1998. [Google Scholar]
- Tian, J.; Yin, J.; Chi, R.; Rao, G.; Jiang, M.; Ouyang, K. Kinetics on leaching rare earth from the weathered crust elution-deposited rare earth ores with ammonium sulfate solution. Hydrometallurgy 2010, 101, 166–170. [Google Scholar]
- Abdel-Aal, E.A. Kinetics of Sulfuric Acid Leaching of Low-Grade Zinc Silicate Ore. Hydrometallurgy 2000, 55, 247–254. [Google Scholar] [CrossRef]
- Zhu, B. Chemical Reaction Engineering; Chemical Industry Press: Beijing, China, 2012. [Google Scholar]
- Liu, K.; Chen, Q.; Yin, Z.; Hu, H.; Ding, Z. Kinetics of leaching of a Chinese laterite containing maghemite and magnetite in sulfuric acid solutions. Hydrometallurgy 2012, 125–126, 125–136. [Google Scholar] [CrossRef]
- Liu, J.; Yin, Z.; Li, X.; Hu, Q.; Liu, W. Recovery of valuable metals from lepidolite by atmosphere leaching and kinetics on dissolution of lithium. Trans. Nonferr. Metals Soc. China 2019, 29, 641–649. [Google Scholar] [CrossRef]


| Methods | Principle |
|---|---|
| Alkali | Al3+ + 4OH− = AlO2− + 2H2O |
| Acid | 2Al3+ + 3(COOH)2·2H2O = Al2(C2O4)3·2H2O↓ + 6H+ |
| Extractant | Selectively forming a complex with aluminum |
| Li | Al | Cs | Mg | Ca | Na | K | Rb | Si | F | H2O |
|---|---|---|---|---|---|---|---|---|---|---|
| 1.68 | 7.55 | 0.13 | 0.26 | 1.04 | 2.29 | 5.07 | 0.70 | 20.37 | 0.74 | 13.37 |
| Temperature (K) | Mass Fraction of H2SO4 (%) | Liquid-Solid Mass Ratio | Time (h) | Agitation Rate (r min−1) | |
|---|---|---|---|---|---|
| Temperature (K) | 393 | 70 | 2.5:1 | 3 | 120 |
| 413 | |||||
| 433 | |||||
| 453 | |||||
| 473 | |||||
| Mass fraction of H2SO4 (%) | 433 | 50 | 2.5:1 | 3 | 120 |
| 60 | |||||
| 70 | |||||
| 80 | |||||
| 90 | |||||
| Liquid-solid Mass Ratio | 433 | 60 | 1.5:1 | 3 | 120 |
| 2:1 | |||||
| 2.5:1 | |||||
| 3:1 | |||||
| 3.5:1 | |||||
| Time (h) | 433 | 60 | 2.5:1 | 1 | 120 |
| 2 | |||||
| 3 | |||||
| 4 | |||||
| 5 | |||||
| Agitation Rate (r min−1) | 433 | 60 | 2.5:1 | 4 | 80 |
| 100 | |||||
| 120 | |||||
| 140 | |||||
| 160 |
| A Sulfuric Acid Mass Fraction (wt %) | B Liquid-solid Mass Ratio | C Roasting Temperature (K) | |
|---|---|---|---|
| 1 | 50 | 2:1 | 413 |
| 2 | 60 | 2.5:1 | 433 |
| 3 | 70 | 3:1 | 453 |
| No. | A | B | C | Li Yield (%) |
|---|---|---|---|---|
| 1 | 50 | 2:1 | 413 | 70 |
| 2 | 50 | 2.5:1 | 433 | 86 |
| 3 | 50 | 3:1 | 453 | 84 |
| 4 | 60 | 2:1 | 453 | 86 |
| 5 | 60 | 2.5:1 | 413 | 90 |
| 6 | 60 | 3:1 | 433 | 99 |
| 7 | 70 | 2:1 | 433 | 87 |
| 8 | 70 | 2.5:1 | 453 | 85 |
| 9 | 70 | 3:1 | 413 | 83 |
| K1 | 240 | 243 | 243 | Σ = 770.1 |
| K2 | 275 | 261 | 272 | |
| K3 | 255 | 266 | 255 | |
| 1 | 80 | 81 | 81 | |
| 2 | 91.7 | 87 | 90.7 | |
| 3 | 85 | 88.7 | 85 | |
| Rj | 11.7 | 7.7 | 9.7 | |
| Optimal level | A2 | B3 | C2 | |
| Order | A C B | |||
| Li | Al | Mg | Ca | Na | K | Si | |
|---|---|---|---|---|---|---|---|
| Lepidolite | 1.68 | 7.55 | 0.26 | 1.04 | 2.29 | 5.07 | 20.37 |
| Solution | 1.64 | 5.37 | 0.005 | 0.07 | 0.31 | 0.66 | 0.006 |
| Temperature (K) | YC | YD | ||
|---|---|---|---|---|
| kC (h−1) | R2 | kD (h−1) | R2 | |
| 413 | 0.0657 | 0.996 | 0.0320 | 0.996 |
| 423 | 0.0873 | 0.982 | 0.0422 | 0.979 |
| 433 | 0.104 | 0.979 | 0.0487 | 0.970 |
| Mass fraction of H2SO4 (wt%) | k (h−1) | R2 |
|---|---|---|
| 40 | 0.0411 | 0.977 |
| 50 | 0.0675 | 0.993 |
| 60 | 0.1039 | 0.979 |
| 1 | 2 | 3 | |
|---|---|---|---|
| pH | 0.5 | 1.4 | 2.2 |
| Ca(OH)2 solution added (L) | - | 13.1 | 14.7 |
| CaCl2 added (g/L) | 13.3 | 9.4 | 8.9 |
| Total salinity (g/L) | 46.6 | 38.9 | 37.0 |
| SO42− (g/L) | 33.1 | 24.0 | 22.8 |
| Cl− (g/L) | 7.9 | 5.6 | 5.3 |
| Ca2+ (g/L) | 1.1 | 2.5 | 2.7 |
| Li+ (g/L) | 0.3 | 0.3 | 0.3 |
| Al3+ (g/L) | 1.1 | 1.0 | 0.9 |
| K+ (g/L) | 0.1 | 0.1 | 0.1 |
| Na+ (g/L) | 0.06 | 0.06 | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, L.; Wang, H.; Li, J.; Wang, M. Recovery of Lithium from Lepidolite by Sulfuric Acid and Separation of Al/Li by Nanofiltration. Minerals 2020, 10, 981. https://doi.org/10.3390/min10110981
Gao L, Wang H, Li J, Wang M. Recovery of Lithium from Lepidolite by Sulfuric Acid and Separation of Al/Li by Nanofiltration. Minerals. 2020; 10(11):981. https://doi.org/10.3390/min10110981
Chicago/Turabian StyleGao, Lin, Huaiyou Wang, Jinli Li, and Min Wang. 2020. "Recovery of Lithium from Lepidolite by Sulfuric Acid and Separation of Al/Li by Nanofiltration" Minerals 10, no. 11: 981. https://doi.org/10.3390/min10110981
APA StyleGao, L., Wang, H., Li, J., & Wang, M. (2020). Recovery of Lithium from Lepidolite by Sulfuric Acid and Separation of Al/Li by Nanofiltration. Minerals, 10(11), 981. https://doi.org/10.3390/min10110981




