Oxygen Fugacity and Volatile Content of Syntectonic Magmatism in the Neoarchean Abitibi Greenstone Belt, Superior Province, Canada
Abstract
1. Introduction
2. Geological Setting
2.1. Abitibi Subprovince and Chibougamau Area
2.2. Syntectonic Magmatism in the Chibougamau Area
3. Methodology
3.1. Sampling
3.2. Whole Rock Analyses
3.3. Mineral Chemistry
3.4. Uranium-Th-Pb Isotopic Ratios
3.5. Data Processing
4. Results
4.1. Whole Rock Geochemistry
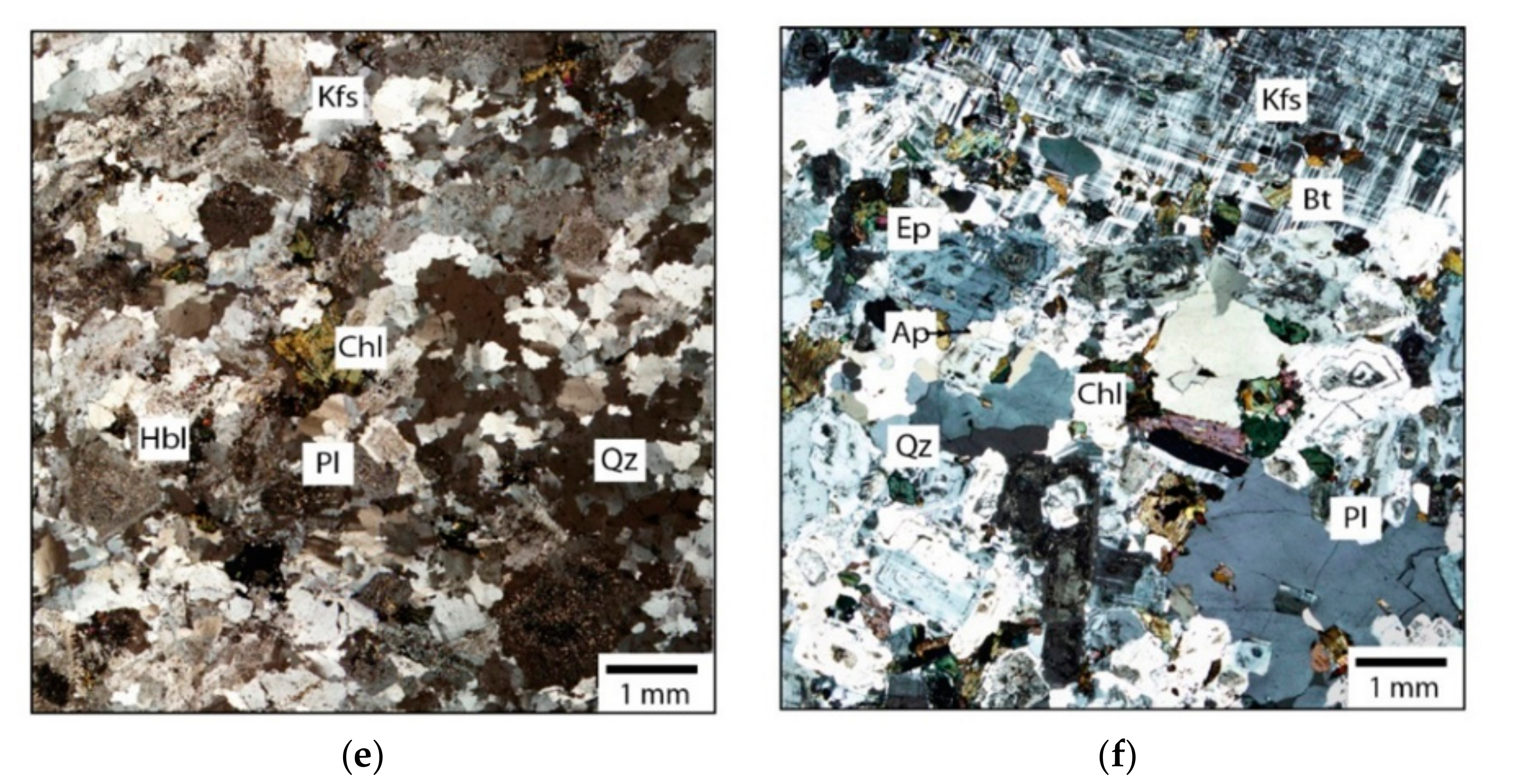
4.2. Petrography
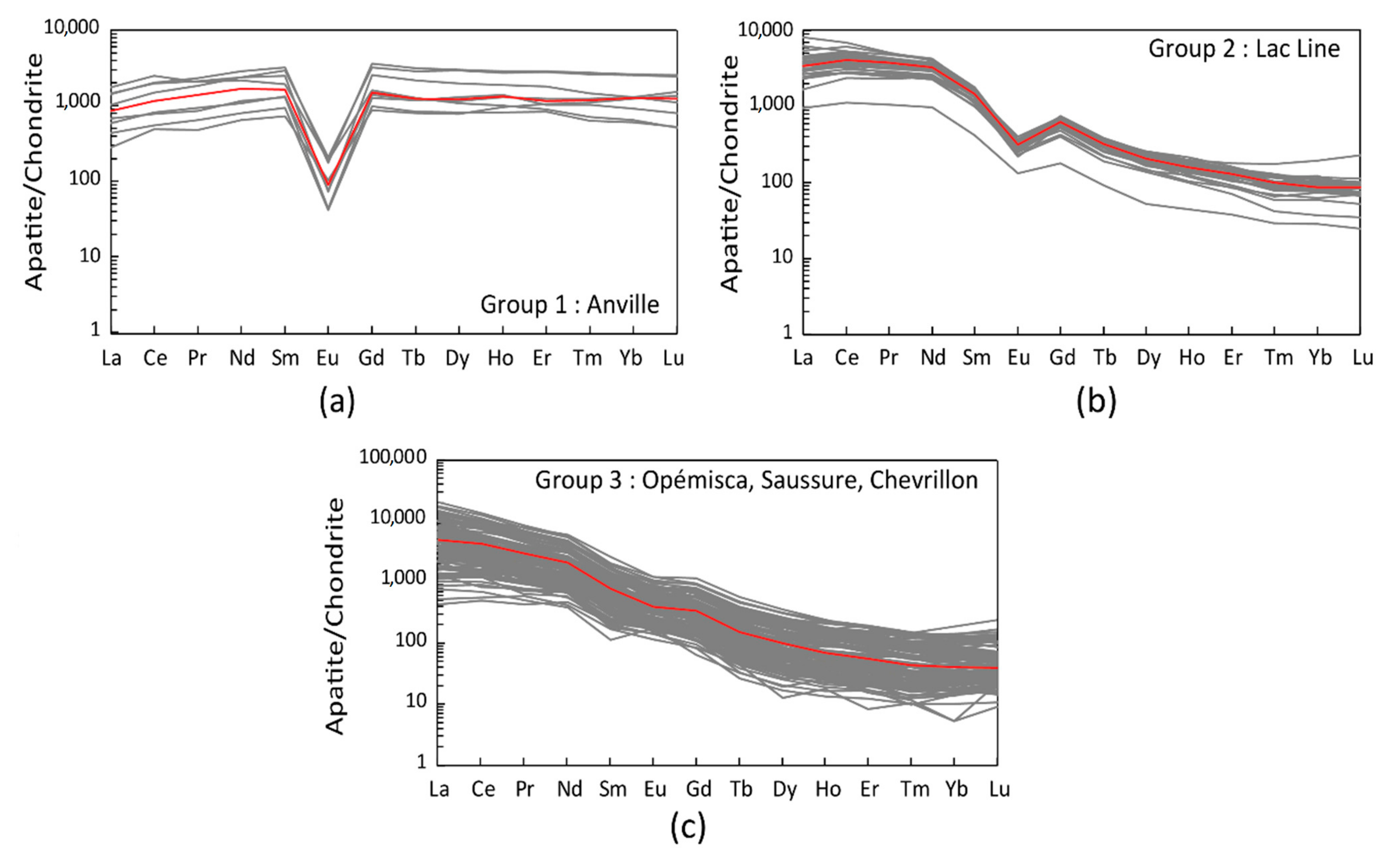
4.3. Volatile Content of the Melt
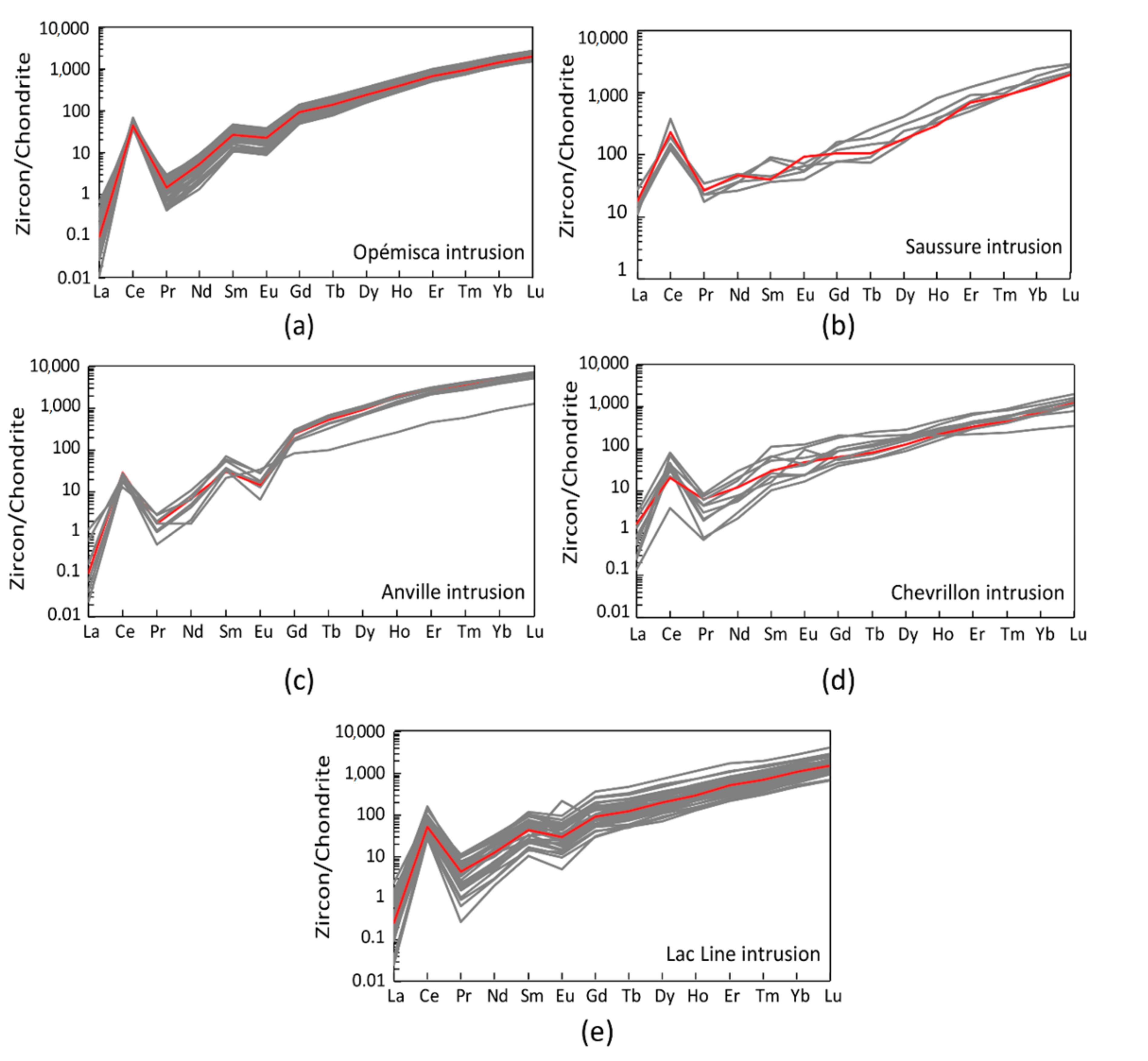
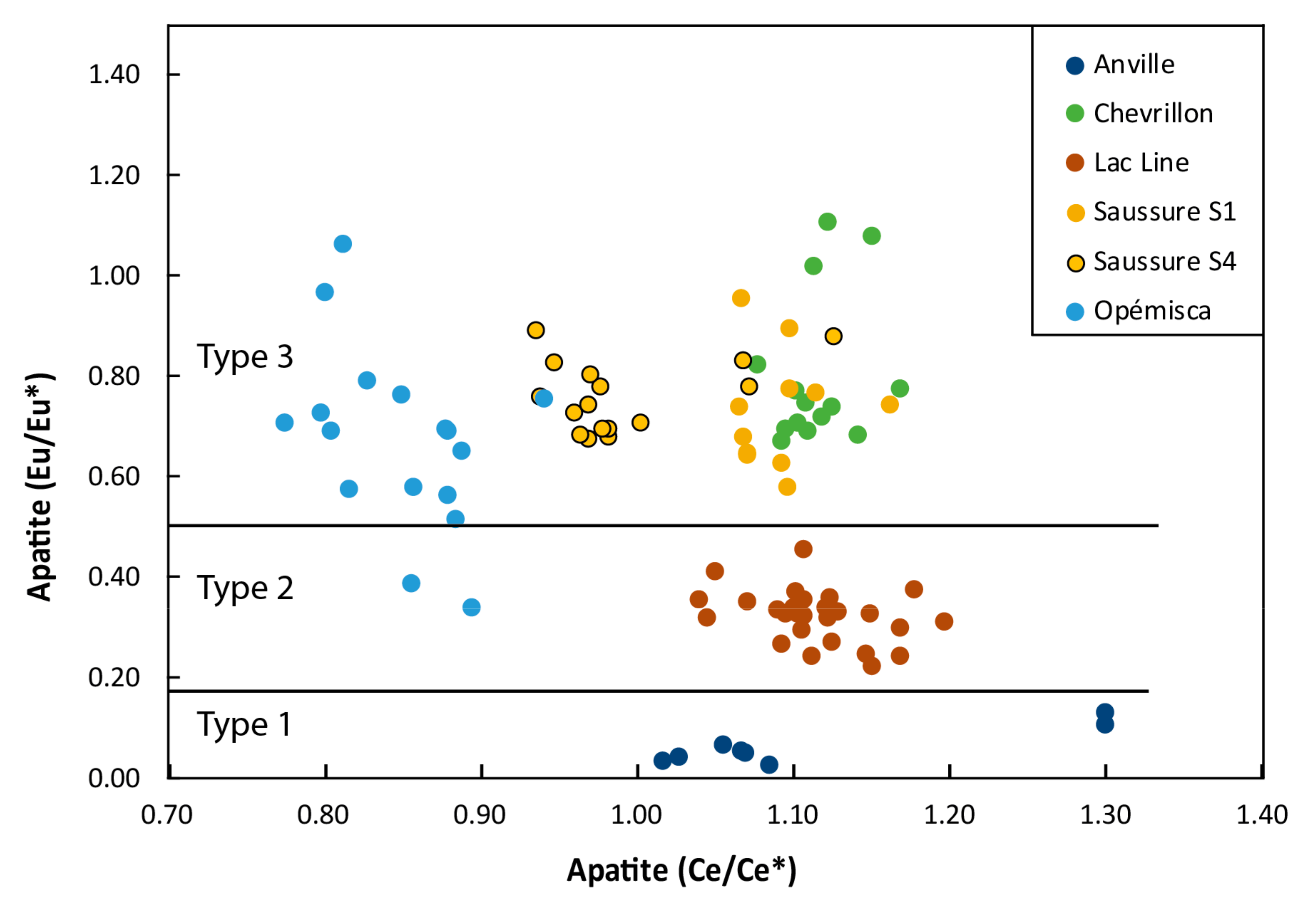
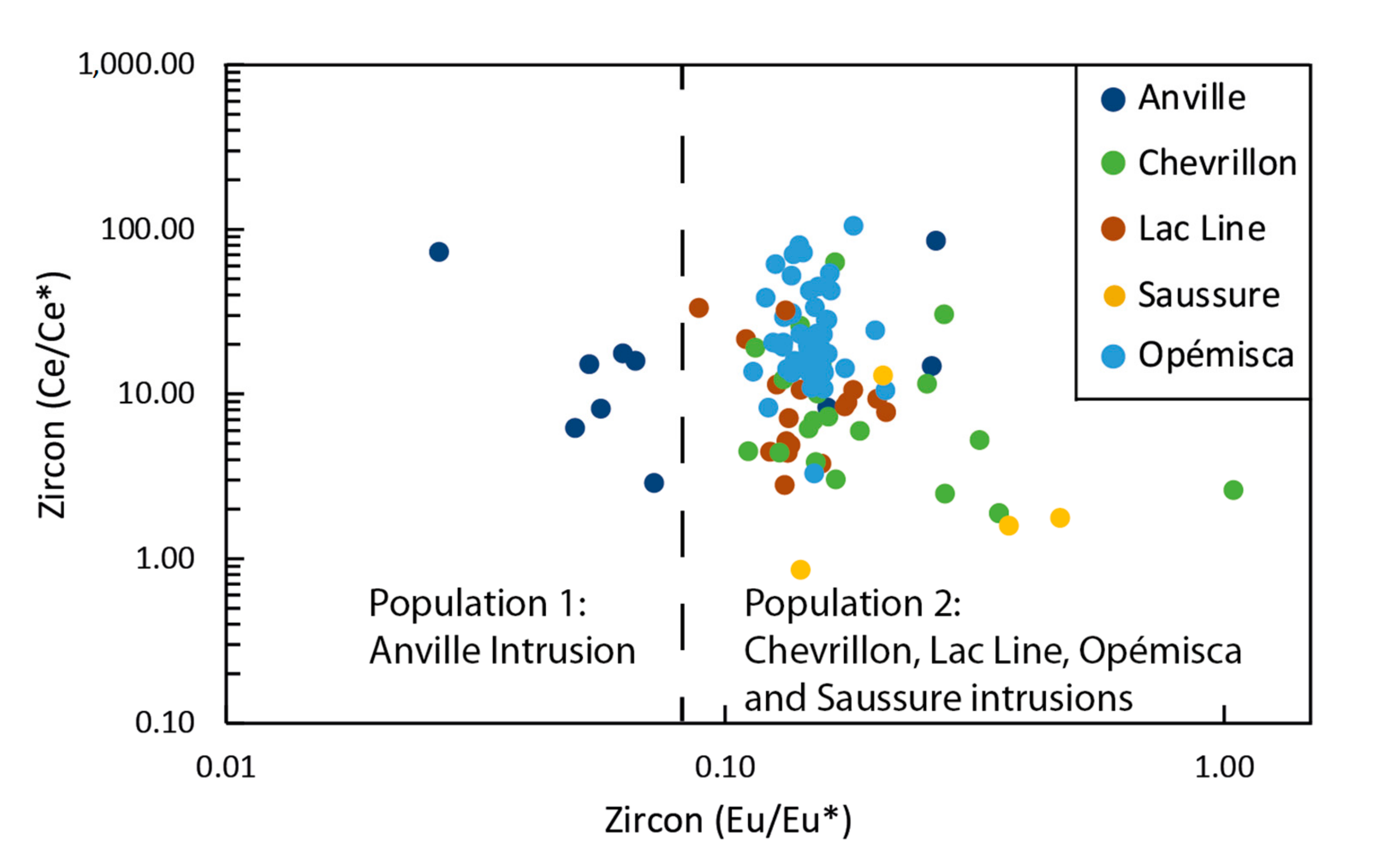
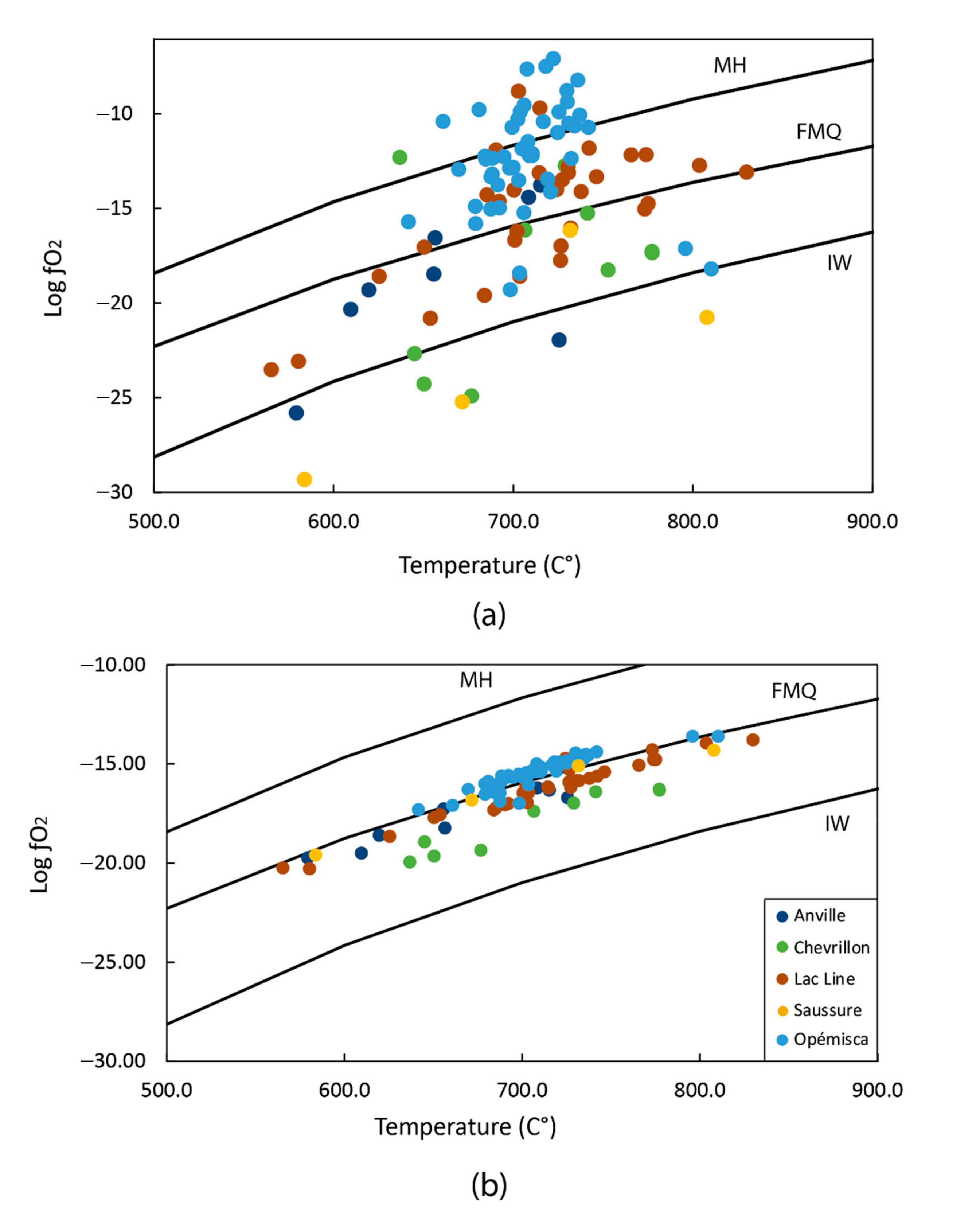
4.4. Oxidation State
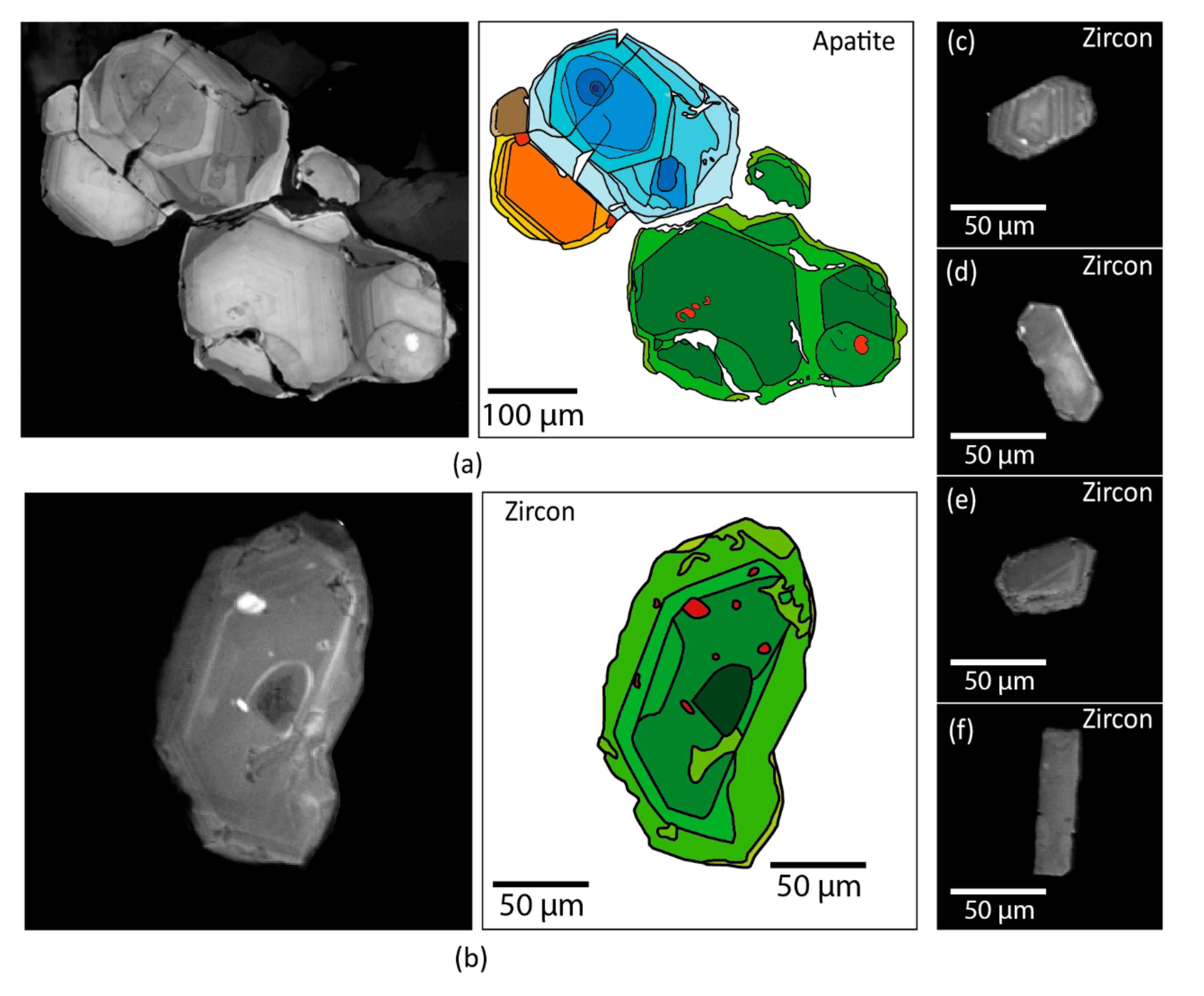
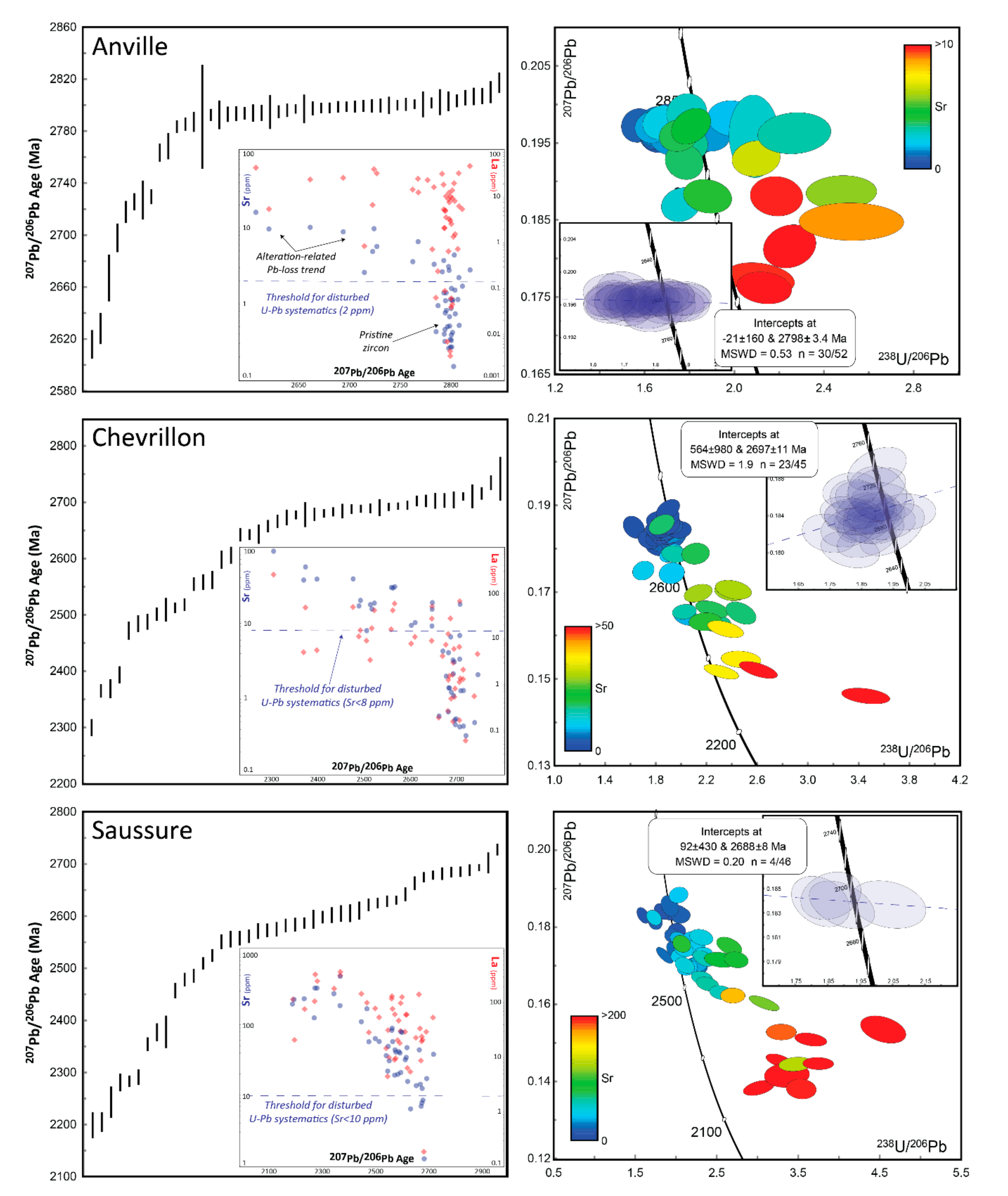
4.5. Geochronology Results
5. Discussion
5.1. Magma Type
5.2. Zircon U-Pb Geochronology
5.3. Impact of Alteration on Mineral Chemistry and ƒO2 Estimates
5.4. Oxidation State and Magma Temperature
5.5. Consequences for IRGS Exploration
5.6. Melts Sources and Oxygen Fugacity
6. Conclusions
- Apatite and zircon are useful to estimate the oxygen fugacity depending on the mineral quality, but further investigations are needed to efficiently measure the ƒO2 parameter of a melt using apatite chemistry;
- There is an increase of the ƒO2 parameter between the synvolcanic and syntectonic periods of the Abitibi Subprovince;
- The studied part of the Anville intrusion was emplaced during the same period as the Chrissie and Des Vents formations, which makes it the oldest subvolcanic complex of the Abitibi Subprovince. Moreover, geochronological analyses of the undated Saussure and Chevrillon intrusions, as well as their stratigraphic relationships with syntectonic sedimentary basins, confirms that they are both syntectonic intrusions;
- Magmas from the Saussure intrusion have the optimal ƒO2 for Au transportation. Moreover, the magma from the Saussure and Lac Line intrusions may have contributed S-rich mineralizing fluids to magmatic-hydrothermal systems. As such, further investigations are needed to evaluate the Au-content of the melt and the size of the magmatic system, and to evaluate the economic potential of the studied intrusions.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lang, J.R.; Baker, T.; Hart, C.; Mortensen, J. An exploration model for intrusion-related gold systems. Soc. Econ. Geol. Newsl. 2000, 40, 6–15. [Google Scholar]
- Hart, C.J.R. Reduced intrusion-related gold systems. In Mineral deposits of Canada: A Synthesis of Major Deposit Types, District Metallogeny, the Evolution of Geological Provinces, and Exploration Methods; Goodfellow, W.D., Ed.; Special Publication No. 5; Geological Association of Canada, Mineral Deposits Division: Saint John’s, NL, Canada, 2007; pp. 95–112. [Google Scholar]
- De Souza, S.; Dubé, B.; McNicoll, V.J.; Dupuis, C.; Mercier-Langevin, P.; A Creaser, R.; Kjarsgaard, I.M. Geology, hydrothermal alteration, and genesis of the world-class Canadian Malartic stockwork-disseminated Archean gold deposit, Abitibi, Quebec. Target. Geosci. Initiat. 2015, 4, 113–126. [Google Scholar] [CrossRef]
- Hart, C.; Goldfarb, R. Distinguishing intrusion-related from orogenic gold systems. In Proceedings of the New Zealand Minerals Conference, Auckland, New Zealand, 13–16 November 2005. [Google Scholar]
- Bigot, L.; Jébrak, M. Gold Mineralization at the Syenite-Hosted Beattie Gold Deposit, Duparquet, Neoarchean Abitibi Belt, Canada. Econ. Geol. 2015, 110, 315–335. [Google Scholar] [CrossRef]
- Mathieu, L. Quantifying hydrothermal alteration with normative minerals and other chemical tools at the Beattie Syenite, Abitibi greenstone belt, Canada. Geochem. Explor. Environ. Anal. 2016, 16, 233–244. [Google Scholar] [CrossRef]
- Botcharnikov, R.E.; Holtz, F.; Mungall, J.E.; Beermann, O.; Linnen, R.L.; Garbe-Schönberg, D. Behavior of gold in a magma at sulfide-sulfate transition: Revisited. Am. Miner. 2013, 98, 1459–1464. [Google Scholar] [CrossRef]
- Botcharnikov, R.E.; Linnen, R.L.; Wilke, M.; Holtz, F.; Jugo, P.J.; Berndt, J. High gold concentrations in sulphide-bearing magma under oxidizing conditions. Nat. Geosci. 2010, 4, 112–115. [Google Scholar] [CrossRef]
- Botcharnikov, R.; Linnen, R.; Holtz, F. Solubility of Au in Cl- and S-bearing hydrous silicate melts. Geochim. Cosmochim. Acta 2010, 74, 2396–2411. [Google Scholar] [CrossRef]
- Mathieu, L.; Racicot, D. Petrogenetic Study of the Multiphase Chibougamau Pluton: Archaean Magmas Associated with Cu–Au Magmato-Hydrothermal Systems. Minerals 2019, 9, 174. [Google Scholar] [CrossRef]
- Côté-Mantha, O. Architecture et Origine du Système de Minéralisation Polymétallique du Secteur lac Line, Région de Chibougamau, Québec; Université du Québec à Chicoutimi: Chicoutimi, QC, Canada, 2009. [Google Scholar] [CrossRef]
- Lépine, S. Le gîte à Au-Cu-Mo de MOP-II (Chibougamau, Québec): Un Porphyre Archéen Déformé. Mémoire. Montréal (Québec, Canada); Université du Québec à Montréal, Maîtrise en Sciences de la Terre: Montréal, QC, Canada, 2009. [Google Scholar]
- Mathieu, L.; Crépon, A.; Kontak, D.J. Tonalite-Dominated Magmatism in the Abitibi Subprovince, Canada, and Significance for Cu-Au Magmatic-Hydrothermal Systems. Minerals 2020, 10, 242. [Google Scholar] [CrossRef]
- Mathieu, L.; Snyder, D.B.; Bedeaux, P.; Cheraghi, S.; Lafrance, B.; Thurston, P.; Sherlock, R. Deep Into the Chibougamau Area, Abitibi Greenstone Belt: Structure of a Neoarchean Crust Revealed by Seismic Reflection Profiling. Tectonics 2020, 39, e2020TC006223. [Google Scholar] [CrossRef]
- Laurent, O.; Martin, H.; Moyen, J.-F.; Doucelance, R. The diversity and evolution of late-Archean granitoids: Evidence for the onset of “modern-style” plate tectonics between 3.0 and 2.5Ga. Lithos 2014, 205, 208–235. [Google Scholar] [CrossRef]
- Thurston, P.C.; Ayer, J.A.; Goutier, J.; Hamilton, M. Depositional Gaps in Abitibi Greenstone Belt Stratigraphy: A Key to Exploration for Syngenetic Mineralization. Econ. Geol. 2008, 103, 1097–1134. [Google Scholar] [CrossRef]
- Dubé, B.; Gosselin, P. Greenstone-hosted quartz-carbonate vein deposits. In Mineral Deposits of Canada: A Synthesis of Major Deposit-Types, District Metallogeny, the Evolution of Geological Provinces, and Exploration Methods; Goodfellow, W.D., Ed.; Special Publication No. 5; Geological Association of Canada, Mineral Deposits Division: Saint John’s, NL, Canada, 2007; Volume 5, pp. 49–73. [Google Scholar]
- Bleeker, W. Synorogenic gold mineralization in granite-greenstone terranes: the deep connection between extension, major faults, synorogenic clastic basins, magmatism, thrust inversion, and long-term preservation. Target. Geosci. Initiat. 2015, 4, 25–47. [Google Scholar]
- Moyen, J.-F. Archean granitoids: Classification, petrology, geochemistry and origin. Geol. Soc. London Spéc. Publ. 2019, 489. [Google Scholar] [CrossRef]
- Robert, F. Syenite-associated disseminated gold deposits in the Abitibi greenstone belt, Canada. Miner. Depos. 2001, 36, 503–516. [Google Scholar] [CrossRef]
- Stern, R.A.; Hanson, G.N.; Shirey, S.B. Petrogenesis of mantle-derived, LILE-enriched Archean monzodiorites and trachyandesites (sanukitoids) in southwestern Superior Province. Can. J. Earth Sci. 1989, 26, 1688–1712. [Google Scholar] [CrossRef]
- Leclerc, F.; Roy, P.; Houle, P.; Pilote, P.; Bédard, J.H.; Harris, L.B.; McNicoll, V.J.; Van Breemen, O.; David, J.; Goulet, N. Géologie de la Région de Chibougamau; Direction Générale de Géologie Québec: Québec, QC, Canada, 2017; RG 2015-03. [Google Scholar]
- Davis, D.W.; Simard, M.; Hammouche, H.; Bandyayera, D.; Goutier, J.; Pilote, P.; Leclerc, F.; Dion, C. Datations U-Pb Effectuées Dans les Provinces du Supérieur et de Churchill en 2011–2012; RP 2014-05; Ministère des Ressources naturelles: Québec, QC, Canada, 2014; p. 62. [Google Scholar]
- David, J.; Mcnicoll, V.; Simard, M.; Bandyayera, D.; Hammouche, H.; Goutier, J.; Pilote, P.; Rhéaume, P.; Leclerc, F.; Dion, C. Datations U-Pb Effectuées Dans les Provinces du Supérieur et de Churchill en 2009–2010; RP 2011-02; Ministère des Ressources naturelles et de la Faune: Québec, QC, Canada, 2011; p. 37. [Google Scholar]
- Mortensen, J.K. U–Pb geochronology of the eastern Abitibi Subprovince. Part 1: Chibougamau–Matagami–Joutel region. Can. J. Earth Sci. 1993, 30, 11–28. [Google Scholar] [CrossRef]
- David, J.; Simard, M.; Bandyayera, D.; Goutier, J.; Hammouche, H.; Pilote, P.; Leclerc, F.; Dion, C. Datations U-Pb effectuées dans les provinces du Supérieur et de Churchill en 2010–2011. MRNF. RP 2012-01, 33 pages. Available online: http://gq.mines.gouv.qc.ca/documents/EXAMINE/RP201201 (accessed on 1 September 2019).
- Leclerc, F.; Harris, L.B.; Bédard, J.H.; dard, J.H.; Van Breemen, O.; Goulet, N. Structural and Stratigraphic Controls on Magmatic, Volcanogenic and Syn-Tectonic Mineralization in the Chapais-Chibougamau Mining Camp, Northeastern Abitibi, Canada. Econ. Geol. 2012, 107, 963–989. [Google Scholar]
- Chown, E.H.; Daigneault, R.; Mueller, W.; Mortensen, J.K. Tectonic evolution of the Northern Volcanic Zone, Abitibi belt, Quebec. Can. J. Earth Sci. 1992, 29, 2211–2225. [Google Scholar] [CrossRef]
- Legault, M.I. Environnement Métallogénique du Couloir de Fancamp avec Emphase sur les Gisements Aurifères de Chevrier, Région de Chibougamau, Québec. Ph.D. Thesis, Université du Québec à Chicoutimi, Chicoutimi, QC, Canada, 2003. [Google Scholar]
- Marie, K. Metal Earth à Chibougamau: Géochimie, Géométrie et Mode de Mise en Place du Complexe d’Eau Jaune; Mémoire de maîtrise, Université du Québec à Chicoutimi: Chicoutimi, QC, Canada, 2019. [Google Scholar]
- David, J.; Davis, D.W.; Dion, C.; Goutier, J.; Legault, M.; Roy, P. Datations U-Pb effectuees dans la Sous-province de l’Abitibi en 2005–2006; MRNF. RP 2007-01; Technical report for Ministère des Ressources naturelles et de la Faune: Quebec, QC, Canada, 2007. [Google Scholar]
- Caty, J.L. Canton de Richardson (comte d’Abitibi-est)—Rapport Interimaire; Ministère des Richesses naturelles du Québec: Quebec, QC, Canada, 1978; p. 38. [Google Scholar]
- Charbonneau, J.M.; Picard, C.; Dupuis-Hebert, L. Synthese geologique de la region de Chapais-Branssat (ABITIBI); MM 88-01; Gouvernement du Québec: Quebec, QC, Canada, 1991; p. 202. [Google Scholar]
- Pierre, S. Pétrographie, Sédimentologie et Analyse des Facies de la Formation de Daubrée, Chapais, Québec; Mémoire de maîtrise, Université du Québec à Chicoutimi: Chicoutimi, QC, Canada, 1986. [Google Scholar]
- McNicoll, V.; Goutier, J. datations U-Pb de la région du lac au Goéland, Sous-province de l’Abitibi, RP 2008-02. In Ressources Naturelles et faune Québec; Quebec, QC, Canada, 2008. [Google Scholar]
- Leclerc, F.; Bédard, J.H.; Harris, L.B.; McNicoll, V.J.; Goulet, N.; Roy, P.; Houle, P. Tholeiitic to calc-alkaline cyclic volcanism in the Roy Group, Chibougamau area, Abitibi Greenstone Belt—Revised stratigraphy and implications for VHMS explorationGeological Survey of Canada Contribution 20100254. Ministère des Ressources naturelles et de la Faune Contribution 8439-2010-2011-17. Can. J. Earth Sci. 2011, 48, 661–694. [Google Scholar] [CrossRef]
- Frarey, M.J.; E Krogh, T. UPb Zircon Ages of Late Internal Plutons of the Abitibi and eastern Wawa Subprovinces, Ontario and Quebec. Curr. Res. Part A Geol. Surv. Can. 1986, 86, 43–48. [Google Scholar] [CrossRef]
- Gariépy, C.; Allègre, C.J. The lead isotope geochemistry and geochronology of late-kinematic intrusives from the Abitibi greenstone belt, and the implications for late Archaean crustal evolution. Geochim. Cosmochim. Acta 1985, 49, 2371–2383. [Google Scholar] [CrossRef]
- Allard, G.O.; Caty, J.-L.; Gobeil, A. The Archean supracrustal rocks of the Chibougamau area. In Evolution of Archean Supracrustal Sequences; Ayres, L.D., Thurston, P.D., Card, K.D., Weber, W., Eds.; Special Paper 28; Geological Association of Canada: Saint John’s, NL, Canada, 1985; pp. 55–63. [Google Scholar]
- Moisan, A. Petrochimie des gres de la Formation de Bordeleau Chibougamau Quebec. Master’s Thesis, Département des Sciences de la Terre, Université du Québec à Chicoutimi, Chicoutimi, QC, Canada, 1992. [Google Scholar] [CrossRef]
- Huguet, J. Pétrographie et géochimie du Pluton de Chevrillon et relation structurale avec le Groupe d’Opémisca (région de Chibougamau, Québec); Mémoire de maîtrise, Université du Québec à Chicoutimi: Chicoutimi, QC, Canada, 2019. [Google Scholar]
- Gervais, D. Le caractère de la zone de contact entre l’intrusion tonalitique syn-à postcinématique d’Anville et le terrain de gneiss du Massif de Lapparent; projet de fin d’études; Université du Québec à Chicoutimi: Chicoutimi, QC, Canada, 1986; p. 56. [Google Scholar]
- Midra, R.; Chown, E.H.; Tait, L. Géologie de la region du lac Dickson (Bande Caopatina-Desmaraisville); MB 91-30; Ministère de l’Énergie et des Ressources: Québec, QC, Canada, 1992; p. 65. [Google Scholar]
- Thiboutot, H.; Rouillard, M. Rapport géologique sur Saussure; service du potentiel mineral; Ministère de l’Energie et des ressources, Gouvernement du Québec: Québec, QC, Canada, 1981. [Google Scholar]
- Pilote, P. Stratigraphie et signification des minéralisations dans le secteur du mont Bourbeau, canton de McKenzie, Chibougamau; mémoire de maîtrise; Université du Québec à Chicoutimi: Chicoutimi, QC, Canada, 1986; p. 182. [Google Scholar]
- Wolhuter, L.E. Le pluton d’Opémisca, une étude pétrologique et géochimique; Ministère des richesses naturelles du Québec, direction générale des mines: Québec, QC, Canada, 1971.
- Wolhuter, L.E. Géologie des quarts NW, SW et SE du canton de Lévy et du quart SE du canton de Daubrée; MB 84-05; Energie et Ressources, Direction de la Recherche Géologique: Québec, QC, Canada, 1984. [Google Scholar]
- Augland, L.E.; David, J.; Pilote, P.; Leclerc, F.; Goutier, J.; Hammouche, H.; Lafrance, I.; Talla Takam, F.; Deschênes, P.L.; Guemache, M. Datations U-Pb dans les provinces de Churchill et du Supérieur effectuées au GÉOTOP en 2012-2013; RP 2015-01; Ministère de l’Énergie et des Ressources Naturelles: Québec, QC, Canada, 2016; p. 43.
- Harmon, R.S.; Lawley, C.J.; Watts, J.; Harraden, C.L.; Somers, A.M.; Hark, R.R. Laser-Induced Breakdown Spectroscopy—An Emerging Analytical Tool for Mineral Exploration. Minerals 2019, 9, 718. [Google Scholar] [CrossRef]
- Gladney, E.S.; Roelandts, I. Distribution of NBS, USGS and CCRMP Reference Material Data in the Literature (1951–1985). Geostand. Newsl. 1987, 11, 133–142. [Google Scholar] [CrossRef]
- Loader, M.A.; Wilkinson, J.J.; Armstrong, R. The effect of titanite crystallisation on Eu and Ce anomalies in zircon and its implications for the assessment of porphyry Cu deposit fertility. Earth Planet. Sci. Lett. 2017, 472, 107–119. [Google Scholar] [CrossRef]
- SIGEOM Système d’information géominière du Québec. Available online: http://sigeom.mines.gouv.qc.ca (accessed on 1 January 2019).
- Whitney, D.L.; Evans, B.W. Abbreviations for names of rock-forming minerals. Am. Miner. 2009, 95, 185–187. [Google Scholar] [CrossRef]
- Chew, D.M.; Babechuk, M.G.; Cogné, N.; Mark, C.; O’Sullivan, G.J.; Henrichs, I.A.; Doepke, D.; McKenna, C.A. (LA,Q)-ICPMS trace-element analyses of Durango and McClure Mountain apatite and implications for making natural LA-ICPMS mineral standards. Chem. Geol. 2016, 435, 35–48. [Google Scholar] [CrossRef]
- Jochum, K.P.; Weis, U.; Stoll, B.; Kuzmin, D.; Yang, Q.; Raczek, I.; Jacob, D.E.; Stracke, A.; Birbaum, K.; Frick, D.A.; et al. Determination of Reference Values for NIST SRM 610–617 Glasses Following ISO Guidelines. Geostand. Geoanalytical Res. 2011, 35, 397–429. [Google Scholar] [CrossRef]
- Zeh, A.; Stern, R.A.; Gerdes, A. The oldest zircons of Africa—Their U–Pb–Hf–O isotope and trace element systematics, and implications for Hadean to Archean crust–mantle evolution. Precambrian Res. 2014, 241, 203–230. [Google Scholar] [CrossRef]
- Turlin, F.; Turlin, F.; Gervais, F.; André-Mayer, A.-S.; Moukhsil, A.; Zeh, A.; André-Mayer, A.-S.; Iptn, I. Petrogenesis of LREE-rich pegmatitic granite dykes in the central Grenville Province by partial melting of Paleoproterozoic-Archean metasedimentary rocks: Evidence from zircon U-Pb-Hf-O isotope and trace element analyses. Precambrian Res. 2019, 327, 327–360. [Google Scholar] [CrossRef]
- Groulier, P.-A.; Turlin, F.; André-Mayer, A.-S.; Ohnenstetter, D.; Crépon, A.; Boulvais, P.; Poujol, M.; Rollion-Bard, C.; Zeh, A.; Moukhsil, A.; et al. Silicate-Carbonate Liquid Immiscibility: Insights from the Crevier Alkaline Intrusion (Quebec). J. Pet. 2020, 61. [Google Scholar] [CrossRef]
- Geisler, T.; Schaltegger, U.; Tomaschek, F. Re-equilibration of Zircon in Aqueous Fluids and Melts. Elements 2007, 3, 43–50. [Google Scholar] [CrossRef]
- McDonough, W.; Sun, S.S. The composition of the Earth. Chem. Geol. 1995, 67, 1050–1056. [Google Scholar] [CrossRef]
- Ferry, J.M.; Watson, E.B. New thermodynamic models and revised calibrations for the Ti-in-zircon and Zr-in-rutile thermometers. Contrib. Miner. Pet. 2007, 154, 429–437. [Google Scholar] [CrossRef]
- Hayden, L.A.; Watson, E.B. Rutile saturation in hydrous siliceous melts and its bearing on Ti-thermometry of quartz and zircon. Earth Planet. Sci. Lett. 2007, 258, 561–568. [Google Scholar] [CrossRef]
- Shen, P.; Hattori, K.; Pan, H.; Jackson, S.; Seitmuratova, E. Oxidation Condition and Metal Fertility of Granitic Magmas: Zircon Trace-Element Data from Porphyry Cu Deposits in the Central Asian Orogenic Belt. Econ. Geol. 2015, 110, 1861–1878. [Google Scholar] [CrossRef]
- Cao, M.; Li, G.; Qin, K.; Seitmuratova, E.Y.; Liu, Y. Major and Trace Element Characteristics of Apatites in Granitoids from Central Kazakhstan: Implications for Petrogenesis and Mineralization. Resour. Geol. 2011, 62, 63–83. [Google Scholar] [CrossRef]
- Drake, M.J. The oxidation state of europium as an indicator of oxygen fugacity. Geochim. Cosmochim. Acta 1975, 39, 55–64. [Google Scholar] [CrossRef]
- Sha, L.-K.; Chappell, B.W. Apatite chemical composition, determined by electron microprobe and laser-ablation inductively coupled plasma mass spectrometry, as a probe into granite petrogenesis. Geochim. et Cosmochim. Acta 1999, 63, 3861–3881. [Google Scholar] [CrossRef]
- Azadbakht, Z.; Lentz, D.; McFarlane, C.R.M. Apatite Chemical Compositions from Acadian-Related Granitoids of New Brunswick, Canada: Implications for Petrogenesis and Metallogenesis. Minerals 2018, 8, 598. [Google Scholar] [CrossRef]
- Trail, D.; Watson, E.B.; Tailby, N.D. Ce and Eu anomalies in zircon as proxies for the oxidation state of magmas. Geochim. Cosmochim. Acta 2012, 97, 70–87. [Google Scholar] [CrossRef]
- Trail, D.; Watson, E.B.; Tailby, N.D. The oxidation state of Hadean magmas and implications for early Earth’s atmosphere. Nat. Cell Biol. 2011, 480, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Smythe, D.J.; Brenan, J.M. Magmatic oxygen fugacity estimated using zircon-melt partitioning of cerium. Earth Planet. Sci. Lett. 2016, 453, 260–266. [Google Scholar] [CrossRef]
- Smythe, D.J.; Brenan, J.M. Cerium oxidation state in silicate melts: Combined f O 2, temperature and compositional effects. Geochim. Cosmochim. Acta 2015, 170, 173–187. [Google Scholar] [CrossRef]
- Zou, X.; Qin, K.; Han, X.; Li, G.; Evans, N.J.; Li, Z.; Yang, W. Insight into zircon REE oxy-barometers: A lattice strain model perspective. Earth Planet. Sci. Lett. 2019, 506, 87–96. [Google Scholar] [CrossRef]
- Li, W.; Yang, Z.; Cao, K.; Lu, Y.; Sun, M. Redox-controlled generation of the giant porphyry Cu–Au deposit at Pulang, southwest China. Contrib. Miner. Pet. 2019, 174, 12. [Google Scholar] [CrossRef]
- Mair, J.L.; Farmer, G.L.; Groves, D.I.; Hart, C.J.R.; Goldfarb, R.J. Petrogenesis of Postcollisional Magmatism at Scheelite Dome, Yukon, Canada: Evidence for a Lithospheric Mantle Source for Magmas Associated with Intrusion-Related Gold Systems. Econ. Geol. 2011, 106, 451–480. [Google Scholar] [CrossRef]
- Ridolfi, F.; Renzulli, A.; Puerini, M. Stability and chemical equilibrium of amphibole in calc-alkaline magmas: An overview, new thermobarometric formulations and application to subduction-related volcanoes. Contrib. Miner. Pet. 2009, 160, 45–66. [Google Scholar] [CrossRef]
- Virgo, D.; Mysen, B.O.; Kushiro, I. Anionic Constitution of 1-Atmosphere Silicate Melts: Implications for the Structure of Igneous Melts. Science 1980, 208, 1371–1373. [Google Scholar] [CrossRef]
- Le Bas, M.J.; Le Maitre, R.W.; Woolley, A.R. The construction of the Total Alkali-Silica chemical classification of volcanic rocks. Miner. Pet. 1992, 46, 1–22. [Google Scholar] [CrossRef]
- Middlemost, E.A.K. Magmas and Magmatic Rocks. An Introduction to Igneous Petrology. x + 266 pp. London, New York: Longman. ISBN 0 582 30080 0. Geol. Mag. 1986, 123, 87–88. [Google Scholar] [CrossRef]
- Shand, S.J. The Eruptive Rocks, 2nd ed.; John Wiley: New York, NY, USA, 1943; p. 444. [Google Scholar]
- Corfu, F.; Hanchar, J.M.; Hoskin, P.W.; Kinny, P. Atlas of Zircon Textures. Rev. Miner. Geochem. 2003, 53, 469–500. [Google Scholar] [CrossRef]
- Scherer, E.E.; Whitehouse, M.J.; Munker, C. Zircon as a Monitor of Crustal Growth. Elements 2007, 3, 19–24. [Google Scholar] [CrossRef]
- Hawthorne, F.C.; Oberti, R.; Harlow, G.E.; Maresch, W.V.; Martin, R.F.; Schumacher, J.C.; Welch, M.D. Nomenclature of the amphibole supergroup. Am. Miner. 2012, 97, 2031–2048. [Google Scholar] [CrossRef]
- Piccoli, P.M.; Candela, P.A. Apatite in Igneous Systems. Rev. Miner. Geochem. 2002, 48, 255–292. [Google Scholar] [CrossRef]
- Parat, F.; Holtz, F.; Streck, M.J. Sulfur-bearing Magmatic Accessory Minerals. Rev. Miner. Geochem. 2011, 73, 285–314. [Google Scholar] [CrossRef]
- Li, H.; Hermann, J. Chlorine and fluorine partitioning between apatite and sediment melt at 2.5 GPa, 800 °C: A new experimentally derived thermodynamic model. Am. Miner. 2017, 102, 580–594. [Google Scholar] [CrossRef]
- Chelle-Michou, C.; Chiaradia, M. Amphibole and apatite insights into the evolution and mass balance of Cl and S in magmas associated with porphyry copper deposits. Contrib. Miner. Pet. 2017, 172, 105. [Google Scholar] [CrossRef]
- Martin, H.; Moyen, J.-F.; Rapp, R. The sanukitoid series: Magmatism at the Archaean–Proterozoic transition. Trans. Royal Soc. Edinb. 2009, 100, 15–33. [Google Scholar] [CrossRef]
- Mondal, M.; Raza, A. Geochemistry of sanukitoid series granitoids from the Neoarchaean Berach granitoid batholiths, Aravalli craton, northwestern Indian shield. Curr. Sci. 2013, 105, 102–108. [Google Scholar]
- Exley, R. Microprobe studies of REE-rich accessory minerals: Implications for Skye granite petrogenesis and REE mobility in hydrothermal systems. Earth Planet. Sci. Lett. 1980, 48, 97–110. [Google Scholar] [CrossRef]
- Cave, B.; Lilly, R.; Glorie, S.; Gillespie, J. Geology, Apatite Geochronology, and Geochemistry of the Ernest Henry Inter-Lens: Implications for a Re-Examined Deposit Model. Minerals 2018, 8, 405. [Google Scholar] [CrossRef]
- Panina, L.I.; Rokosova, E.Y.; Isakova, A.T.; Tolstov, A.V. Lamprophyres of the Tomtor Massif: A result of mixing between potassic and sodic alkaline mafic magmas. Petrology 2016, 24, 608–625. [Google Scholar] [CrossRef]
- Harlov, D.E.; Andersson, U.B.; Förster, H.-J.; Nyström, J.O.; Dulski, P.; Broman, C. Apatite–monazite relations in the Kiirunavaara magnetite–apatite ore, northern Sweden. Chem. Geol. 2002, 191, 47–72. [Google Scholar] [CrossRef]
- Zhong, S.; Feng, C.; Seltmann, R.; Li, D.; Qu, H. Can magmatic zircon be distinguished from hydrothermal zircon by trace element composition? The effect of mineral inclusions on zircon trace element composition. Lithos 2018, 314–315, 646–657. [Google Scholar] [CrossRef]
- Yang, W.-B.; Niu, H.-C.; Shan, Q.; Sun, W.-D.; Zhang, H.; Li, N.-B.; Jiang, Y.-H.; Yu, X.-Y. Geochemistry of magmatic and hydrothermal zircon from the highly evolved Baerzhe alkaline granite: Implications for Zr–REE–Nb mineralization. Miner. Deposita 2013, 49, 451–470. [Google Scholar] [CrossRef]
- Geisler, T.; Pidgeon, R.T.; Kurtz, R.; Van Bronswijk, W.; Schleicher, H. Experimental hydrothermal alteration of partially metamict zircon. Am. Miner. 2003, 88, 1496–1513. [Google Scholar] [CrossRef]
- Hoskin, P.W. Trace-element composition of hydrothermal zircon and the alteration of Hadean zircon from the Jack Hills, Australia. Geochim. et Cosmochim. Acta 2005, 69, 637–648. [Google Scholar] [CrossRef]
- Hoskin, P.W.O.; Schaltegger, U. The composition of zircon and igneous and metamorphic petrogenesis. Rev. Miner. Geochem. 2003, 53, 25–104. [Google Scholar] [CrossRef]
- Nutman, A.P.; Maciejowski, R.; Wan, Y. Protoliths of enigmatic Archaean gneisses established from zircon inclusion studies: Case study of the Caozhuang quartzite, E. Hebei, China. Geosci. Front. 2014, 5, 445–455. [Google Scholar] [CrossRef]
- Fu, B.; Page, F.Z.; Cavosie, A.J.; Fournelle, J.; Kita, N.T.; Lackey, J.S.; Wilde, S.A.; Valley, J.W. Ti-in-zircon thermometry: Applications and limitations. Contrib. Miner. Pet. 2008, 156, 197–215. [Google Scholar] [CrossRef]
- Watson, E.B.; Wark, D.A.; Thomas, J.B. Crystallization thermometers for zircon and rutile. Contrib. Miner. Pet. 2006, 151, 413–433. [Google Scholar] [CrossRef]
- Mathieu, L.; Madon, B.; Hamilton, M.A. Physico-chemical parameters of Neoarchean syntectonic magmatism: The example of the Muscocho Pluton, Abitibi Subprovince. Ore Geol. Rev. 2020, 125, 103670. [Google Scholar] [CrossRef]
- Blundy, J.; Cashman, K. Ascent-driven crystallisation of dacite magmas at Mount St Helens, 1980–1986. Contrib. Miner. Pet. 2001, 140, 631–650. [Google Scholar] [CrossRef]
- Blundy, J.; Cashman, K. Petrologic Reconstruction of Magmatic System Variables and Processes. Rev. Miner. Geochem. 2008, 69, 179–239. [Google Scholar] [CrossRef]
- Li, J.-X.; Qin, K.-Z.; Li, G.-M.; Evans, N.J.; Zhao, J.-X.; Yue, Y.-H.; Xie, J. Volatile variations in magmas related to porphyry Cu-Au deposits: Insights from amphibole geochemistry, Duolong district, central Tibet. Ore Geol. Rev. 2018, 95, 649–662. [Google Scholar] [CrossRef]
- Martin, H.; Moyen, J.-F.; Guitreau, M.; Blichert-Toft, J.; Le Pennec, J.-L. Why Archaean TTG cannot be generated by MORB melting in subduction zones. Lithos 2014, 1–13. [Google Scholar] [CrossRef]
- Moyen, J.-F. The composite Archaean grey gneisses: Petrological significance, and evidence for a non-unique tectonic setting for Archaean crustal growth. Lithos 2011, 123, 21–36. [Google Scholar] [CrossRef]
- Morris, E.M.; Pasteris, J.D. Mantle Metasomatism and Alkaline Magmatism; Geological Society of America: Boulder, CO, USA, 1987. [Google Scholar] [CrossRef]
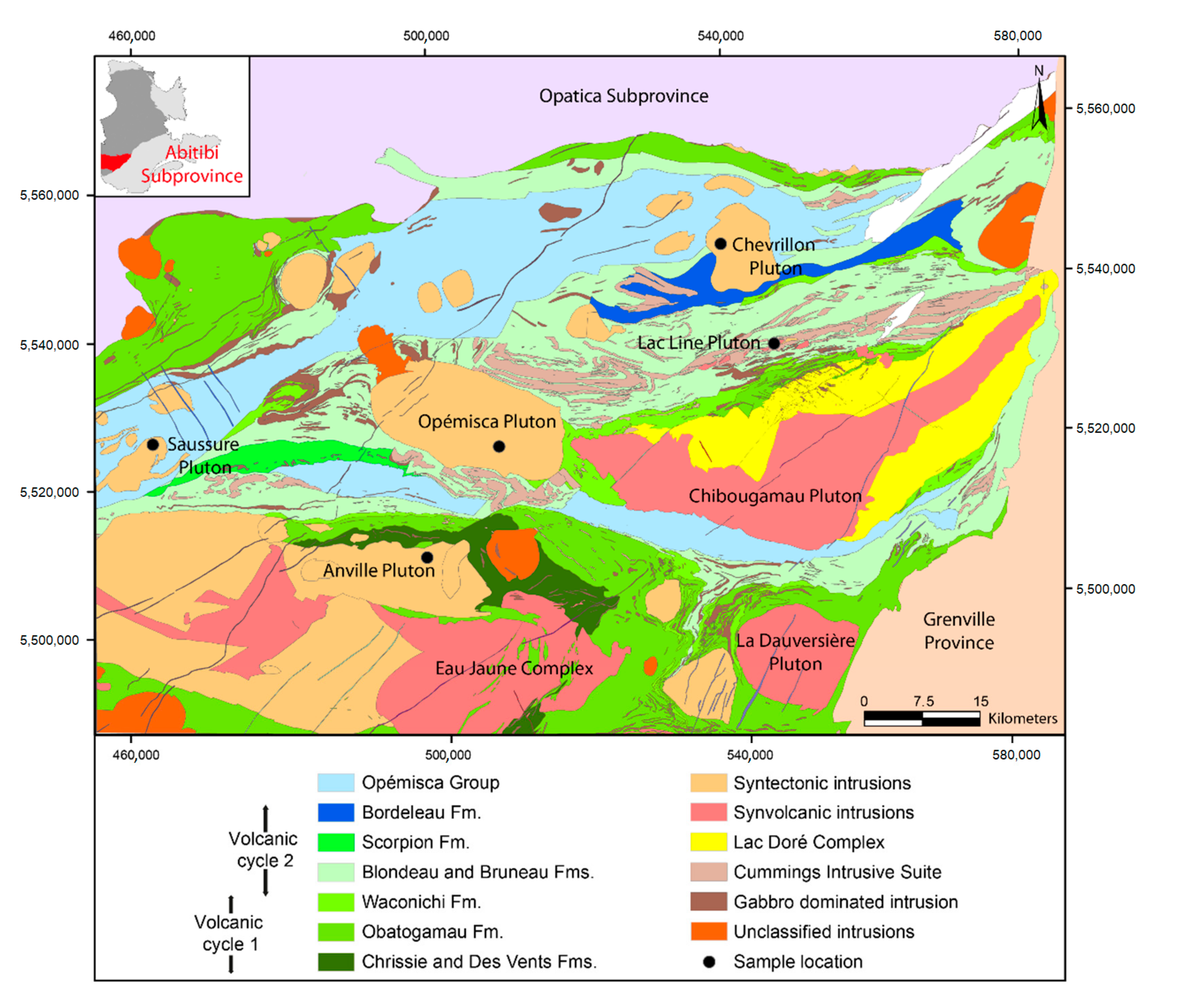



| Intrusions | UTME * | UTMN * | Zone | Sample | Whole-Rock Geochemistry | Apatite and Zircon Compositions | Dating U-Pb Geochronology |
| Saussure | 467,051 | 5,526,000 | 18 | Granite | Figure 2 | Figures 5, 7; Tables 2, 3 | Figure 11 |
| Saussure | 466,402 | 5,526,084 | 18 | Quartz-rich syenite | Figure 2 | Figures 5, 7; Tables 2, 3 | Figure 11 |
| Saussure | 463,895 | 5,526,093 | 18 | Pegmatitic syenite | Figure 2 | Figures 5, 7; Tables 2, 3 | Figure 11 |
| Chevrillon | 540,371 | 5,5473,39 | 18 | Quartz-rich monzodiorite | Figure 2 | Figures 5, 7; Tables 2, 3 | Figure 11 |
| Lac Line | 548,644 | 5,533,262 | 18 | Tonalite | Figure 2 | Figures 5, 7; Tables 2, 3 | - |
| Opémisca | 511,694 | 5,520,891 | 18 | Qz-rich monzonite | Figure 2 | Figures 5, 7; Tables 2, 3 | - |
| Anville | 497,685 | 5,509,637 | 18 | Granodiorite | Figure 2 | Figures 5, 7; Tables 2, 3 | Figure 11 |
| Intrusion | Lithology | Mineralogy | Accessory Minerals |
|---|---|---|---|
| Saussure | Qz-rich syenite | 40% Kfs, 30% Pl, 10–12% Qz, 18% Bt-Hbl | Ttn, Zrn, Ap, Iox |
| Opémisca | Qz-rich monzonite | 50–60% Pl, 15–20% Kfs, 10–15% Qz, 5–10% Hbl | Ep, Ttn, Mag, Ap, Zrn |
| Lac Line | Tonalite | >50% Pl, 30–35% Qz, 10–12% Cb, 5–7% Bt-Chl | Zrn, Ap, |
| Chevrillon | Qz-rich monzodiorite | 50–55% Pl, 10–20% Kfs, 10–20% Qz, 5–8% Bt-Hbl | Ep, Ttn, Mag, Ap, Zrn |
| Anville | Granodiorite | 40–45% Qz, 30–40% Pl, 10–15% Kfs, <5% Bt-Chl | Zrn, Ap, Ep, Ilm, Alu |
| Intrusions | Saussure | Opémisca | Lac Line | Chevrillon | Anville | |
|---|---|---|---|---|---|---|
| Elements | S101 | S401 | U139 | LL01 | CHV01 | U140 |
| SiO2 (wt.%) | 67.40 | 63.3 | 62.50 | 63.10 | 64.20 | 77.00 |
| Al2O3 | 15.55 | 17.3 | 17.10 | 17.60 | 16.00 | 12.30 |
| FeO | 1.38 | 1.26 | 1.47 | 1.64 | 1.50 | 1.45 |
| Fe2O3 | 1.66 | 1.84 | 2.29 | 2.15 | 1.94 | 1.12 |
| CaO | 1.96 | 1.82 | 3.44 | 4.28 | 3.21 | 1.30 |
| MgO | 1.24 | 0.86 | 2.37 | 2.19 | 1.78 | 0.22 |
| Na2O | 5.04 | 5.91 | 6.32 | 5.17 | 5.03 | 4.03 |
| K2O | 4.15 | 5.76 | 2.44 | 1.03 | 2.99 | 2.29 |
| TiO2 | 0.40 | 0.35 | 0.34 | 0.32 | 0.48 | 0.15 |
| MnO | 0.04 | 0.05 | 0.06 | 0.06 | 0.04 | 0.04 |
| P2O5 | 0.29 | 0.21 | 0.19 | 0.14 | 0.23 | 0.02 |
| BaO | 0.20 | 0.150 | 0.10 | 0.06 | 0.18 | 0.05 |
| LOI | 0.31 | 0.21 | 0.56 | 1.92 | 0.60 | 0.44 |
| TOTAL | 99.62 | 99.02 | 99.18 | 99.79 | 98.18 | 100.41 |
| C (ppm) | 0.01 | 0.01 | 0.01 | 0.03 | 0.00 | 0.01 |
| Ce | 152.00 | 170.5 | 54.80 | 75.00 | 101.00 | 72.70 |
| Cr | 40.00 | 20 | 110.00 | 40.00 | 60.00 | 30.00 |
| Cs | 4.14 | 0.46 | 0.35 | 0.50 | 2.46 | 0.38 |
| Dy | 1.72 | 3.37 | 2.02 | 1.23 | 1.81 | 5.61 |
| Er | 0.60 | 1.54 | 1.03 | 0.59 | 0.80 | 3.78 |
| Eu | 1.88 | 2.59 | 1.02 | 1.04 | 1.63 | 0.91 |
| Ga | 22.10 | 20.9 | 20.10 | 20.90 | 24.10 | 16.20 |
| Gd | 4.45 | 6.88 | 2.97 | 2.41 | 4.21 | 5.58 |
| Hf | 5.80 | 5.7 | 4.20 | 2.50 | 4.60 | 5.90 |
| Ho | 0.31 | 0.56 | 0.39 | 0.22 | 0.29 | 1.18 |
| La | 79.80 | 77.8 | 26.90 | 36.00 | 51.00 | 36.80 |
| Lu | 0.05 | 0.12 | 0.15 | 0.08 | 0.07 | 0.58 |
| Nb | 9.90 | 15.2 | 6.90 | 2.80 | 5.40 | 10.30 |
| Nd | 62.20 | 73.7 | 26.50 | 31.90 | 47.60 | 28.80 |
| Pr | 17.00 | 19.95 | 6.63 | 8.36 | 12.50 | 7.83 |
| Rb | 124.00 | 110 | 42.90 | 24.20 | 75.30 | 61.70 |
| Sm | 8.48 | 10.95 | 4.07 | 4.08 | 7.47 | 6.25 |
| Sn | 1.00 | 1 | 1.00 | 1.00 | 2.00 | 3.00 |
| Sr | 1240.00 | 1285 | 1165.00 | 1140.00 | 1410.00 | 79.20 |
| Ta | 0.70 | 0.9 | 0.50 | 0.20 | 0.50 | 1.30 |
| Tb | 0.42 | 0.84 | 0.38 | 0.29 | 0.44 | 0.88 |
| Th | 14.70 | 4.62 | 3.03 | 4.71 | 6.12 | 9.58 |
| Tm | 0.07 | 0.19 | 0.17 | 0.08 | 0.10 | 0.57 |
| U | 1.72 | 0.96 | 0.55 | 0.67 | 1.06 | 1.82 |
| V | 38.00 | 47 | 60.00 | 59.00 | 67.00 | 7.00 |
| Y | 8.70 | 16.2 | 10.60 | 6.40 | 8.40 | 35.90 |
| Yb | 0.48 | 1.06 | 1.14 | 0.54 | 0.55 | 3.95 |
| Zr | 247.00 | 299 | 173.00 | 108.00 | 170.00 | 200.00 |
| Intrusions | Anville | Chevrillon | Lac Line | Opémisca | Saussure | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elements | Min | Mean | Max | Min | Mean | Max | Min | Mean | Max | Min | Mean | Max | Min | Mean | Max |
| Li | ND | 25.8 | 152.4 | ND * | 3.6 | 20.1 | ND | 4.2 | 18.9 | ND | 3.2 | 21.3 | 1.0 | 5.0 | 16.6 |
| Na | 414.2 | 876.8 | 1766.6 | 222.7 | 2432.9 | 29,952.8 | 287.4 | 783.4 | 1816.4 | 194.7 | 816.8 | 2050.3 | 389.2 | 5195.3 | 117,998.2 |
| Mg | 14.2 | 3617.9 | 26,431.3 | 13.0 | 1053.0 | 8621.2 | 10.2 | 939.8 | 11,077.4 | 13.7 | 871.0 | 13,092.0 | 4.2 | 890.1 | 20,498.0 |
| Al | 1006.1 | 17,594.6 | 108,042.4 | 32.4 | 3358.0 | 40,388.3 | 222.0 | 1746.5 | 11,838.2 | 36.8 | 973.2 | 8241.2 | 60.6 | 4860.8 | 133,241.2 |
| Si | 2500.4 | 25,923.9 | 117,541.2 | ND | 8389.0 | 106,053.2 | ND | 3241.3 | 38,686.5 | ND | 2815.7 | 19,774.3 | 781.4 | 19,362.5 | 383,624.1 |
| S | ND | 263.6 | 1038.6 | ND | 464.9 | 916.6 | 298.6 | 1371.4 | 2384.7 | 133.7 | 540.2 | 959.2 | 361.6 | 974.1 | 1645.5 |
| Cl | ND | 232.7 | 592.3 | ND | 180.4 | 1013.2 | 124.0 | 687.4 | 1064.2 | ND | 112.1 | 362.5 | 31.6 | 115.7 | 411.6 |
| K | 3.1 | 129.4 | 369.2 | ND | 151.7 | 990.5 | ND | 38.8 | 254.5 | 0.5 | 255.6 | 2429.0 | ND | 72.4 | 994.1 |
| Ti | ND | 1351.4 | 10,356.4 | ND | 333.7 | 6667.4 | 1.3 | 27.7 | 535.6 | 0.2 | 164.2 | 3149.2 | 0.1 | 22.8 | 554.1 |
| V | 0.2 | 12.6 | 58.6 | 3.1 | 14.8 | 90.2 | 5.0 | 17.9 | 44.6 | 4.9 | 38.2 | 483.0 | 5.3 | 27.7 | 264.3 |
| Mn | 336.0 | 1173.6 | 6223.5 | 158.1 | 308.6 | 711.7 | 303.3 | 553.5 | 777.0 | 213.3 | 327.7 | 741.0 | 124.2 | 317.7 | 2723.0 |
| Fe | 108.8 | 9119.4 | 63,402.0 | 56.2 | 1217.8 | 10,177.3 | 855.7 | 1590.5 | 7864.7 | 43.0 | 4714.6 | 95,786.4 | 49.8 | 624.3 | 14,325.2 |
| Rb | ND | 2.6 | 28.9 | ND | 1.4 | 16.2 | ND | 0.1 | 2.6 | ND | 0.7 | 9.3 | ND | 2.1 | 17.9 |
| Sr | 55.2 | 73.5 | 118.9 | 887.7 | 1116.3 | 1779.2 | 338.2 | 398.4 | 506.9 | 830.9 | 905.5 | 986.0 | 723.9 | 1857.6 | 9637.5 |
| Y | 1382.8 | 2729.3 | 5129.2 | 44.1 | 97.4 | 156.0 | 81.3 | 250.4 | 340.4 | 72.8 | 199.7 | 334.1 | 45.5 | 189.6 | 494.1 |
| Zr | 0.2 | 158.1 | 1224.9 | 0.2 | 1.7 | 7.1 | 0.1 | 0.9 | 5.2 | 0.2 | 2.0 | 23.7 | 0.3 | 24.4 | 1203.2 |
| Sn | ND | 12.8 | 51.0 | ND | 0.4 | 3.0 | 0.0 | 1.0 | 5.7 | 0.0 | 3.6 | 40.7 | 0.0 | 10.0 | 131.9 |
| La | 66.0 | 226.5 | 413.6 | 100.3 | 367.2 | 819.4 | 244.7 | 880.5 | 1943.1 | 517.4 | 1374.7 | 2396.7 | 215.5 | 1773.9 | 5257.8 |
| Ce | 299.7 | 810.0 | 1515.9 | 288.3 | 999.4 | 1981.0 | 737.2 | 2529.5 | 4296.5 | 750.1 | 1997.5 | 3181.5 | 509.4 | 3486.8 | 9158.8 |
| Pr | 44.0 | 130.6 | 214.7 | 39.7 | 118.1 | 236.0 | 106.0 | 342.1 | 490.0 | 58.5 | 178.6 | 261.6 | 56.1 | 341.9 | 866.4 |
| Nd | 293.6 | 772.4 | 1317.8 | 187.6 | 504.4 | 972.9 | 482.7 | 1495.0 | 1994.0 | 271.8 | 678.8 | 1070.4 | 211.9 | 1228.4 | 3090.0 |
| Sm | 106.5 | 275.2 | 477.0 | 27.1 | 73.9 | 137.5 | 67.8 | 218.4 | 281.3 | 28.8 | 90.5 | 160.5 | 20.8 | 151.1 | 446.2 |
| Eu | 2.3 | 6.4 | 11.8 | 8.2 | 16.9 | 28.0 | 8.5 | 19.0 | 24.8 | 8.1 | 17.9 | 32.4 | 9.9 | 32.1 | 80.8 |
| Gd | 172.6 | 387.5 | 722.9 | 18.5 | 49.1 | 91.9 | 40.3 | 130.0 | 161.8 | 27.0 | 66.9 | 124.1 | 16.3 | 94.6 | 270.8 |
| Tb | 29.1 | 60.9 | 114.0 | 1.6 | 4.2 | 8.2 | 3.8 | 12.4 | 15.5 | 2.5 | 6.8 | 12.1 | 1.5 | 8.4 | 24.4 |
| Dy | 192.9 | 399.8 | 736.1 | 6.1 | 18.3 | 31.0 | 15.1 | 55.4 | 70.6 | 10.6 | 35.4 | 61.7 | 6.6 | 37.5 | 107.6 |
| Ho | 45.1 | 88.4 | 156.5 | 1.2 | 3.2 | 5.0 | 2.9 | 9.7 | 13.2 | 2.7 | 6.9 | 11.6 | 1.2 | 6.1 | 16.3 |
| Er | 133.2 | 250.9 | 461.3 | 2.8 | 7.6 | 11.1 | 7.2 | 22.4 | 32.4 | 7.6 | 19.0 | 30.3 | 3.5 | 14.3 | 38.0 |
| Tm | 15.7 | 35.8 | 68.1 | 0.4 | 0.9 | 1.6 | 0.9 | 2.7 | 4.9 | 0.8 | 2.5 | 3.6 | 0.4 | 1.7 | 4.6 |
| Yb | 96.9 | 225.6 | 424.5 | 2.0 | 5.9 | 8.3 | 5.5 | 16.5 | 35.2 | 3.9 | 17.0 | 29.7 | 2.9 | 9.7 | 26.0 |
| Lu | 12.7 | 33.2 | 63.0 | 0.5 | 1.0 | 1.4 | 0.7 | 2.4 | 6.4 | 1.1 | 2.9 | 5.9 | 0.5 | 1.4 | 3.3 |
| Intrusions | Anville | Chevrillon | Lac Line | Opémisca | Saussure | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elements | Min | Mean | Max | Min | Mean | Max | Min | Mean | Max | Min | Mean | Max | Min | Mean | Max |
| P | ND | 533.1 | 1432.5 | ND | 775.0 | 5074.8 | 127.5 | 480.2 | 1049.3 | 279.7 | 704.9 | 2080.4 | ND | 407.4 | 2956.2 |
| Ca | 123.1 | 306.5 | 542.7 | −8.5 | 556.1 | 3128.6 | ND | 43.9 | 856.2 | ND | 60.3 | 298.4 | 209.2 | 777.0 | 1705.9 |
| Ti | ND | 3.8 | 15.5 | 0.4 | 132.3 | 2726.2 | 0.7 | 6.2 | 20.9 | 2.0 | 6.4 | 53.2 | ND | 29.5 | 150.2 |
| V | ND | 0.1 | 4.4 | ND | 1.4 | 19.4 | −0.3 | 0.3 | 2.5 | 0.1 | 0.3 | 1.0 | ND | 3.0 | 11.0 |
| Fe | 57.1 | 464.2 | 1691.1 | 18.2 | 540.6 | 2174.3 | ND | 66.3 | 473.2 | ND | 17.9 | 140.9 | 163.1 | 661.2 | 1536.1 |
| Sr | 0.0 | 1.2 | 7.5 | ND | 4.4 | 37.2 | 0.1 | 1.5 | 23.4 | 0.2 | 0.8 | 14.6 | 1.0 | 23.5 | 78.5 |
| Y | 574.6 | 2379.0 | 3994.1 | 314.5 | 720.7 | 2964.0 | 234.6 | 622.5 | 1936.7 | 441.3 | 772.3 | 1910.1 | 534.5 | 869.3 | 1422.1 |
| Nb | 1.5 | 5.9 | 12.8 | 0.6 | 3.7 | 21.7 | 1.2 | 1.7 | 2.8 | 1.9 | 2.7 | 5.0 | 1.0 | 3.3 | 7.9 |
| Hf | 6554.2 | 8939.7 | 11,773.4 | 6650.0 | 8938.0 | 11,683.0 | 6145.0 | 8442.4 | 10,388.0 | 7282.4 | 8442.1 | 10,974.9 | 5964.2 | 8091.6 | 10,938.2 |
| Ta | 0.1 | 2.3 | 5.6 | 0.0 | 0.3 | 1.8 | 0.0 | 0.3 | 2.7 | 0.2 | 0.4 | 1.6 | ND | 0.4 | 1.5 |
| Th | 140.7 | 221.1 | 315.4 | 50.6 | 204.4 | 540.5 | 34.2 | 154.0 | 641.5 | 31.3 | 69.6 | 185.1 | 237.2 | 498.5 | 960.0 |
| U | 269.4 | 413.8 | 524.0 | 111.7 | 244.8 | 543.7 | 43.9 | 146.8 | 481.3 | 44.5 | 80.9 | 256.8 | 292.8 | 522.8 | 785.0 |
| La | ND | 2.8 | 27.7 | 0.0 | 8.6 | 143.8 | 0.0 | 0.1 | 0.6 | 0.0 | 0.1 | 1.3 | 0.0 | 16.0 | 87.2 |
| Ce | 4.3 | 17.2 | 61.4 | 2.4 | 77.6 | 796.7 | 16.7 | 35.4 | 99.6 | 15.9 | 28.9 | 49.6 | 54.9 | 158.5 | 463.7 |
| Pr | ND | 1.1 | 7.9 | 0.0 | 7.8 | 127.3 | 0.0 | 0.4 | 1.1 | 0.0 | 0.2 | 0.9 | 0.0 | 9.8 | 52.0 |
| Nd | 0.6 | 7.7 | 44.4 | 0.7 | 54.2 | 709.8 | 0.9 | 6.0 | 14.7 | 0.6 | 2.5 | 7.4 | 0.8 | 66.5 | 335.5 |
| Sm | 1.8 | 8.2 | 25.6 | 0.0 | 27.2 | 209.5 | 1.6 | 7.0 | 17.8 | 1.5 | 4.2 | 7.0 | 3.4 | 20.9 | 86.8 |
| Eu | 0.4 | 1.3 | 5.0 | 0.6 | 11.0 | 72.8 | 0.3 | 2.1 | 12.3 | 0.5 | 1.3 | 2.1 | 0.4 | 6.7 | 25.9 |
| Gd | 9.0 | 45.0 | 78.3 | 6.9 | 48.2 | 332.0 | 6.0 | 22.0 | 74.6 | 9.4 | 19.0 | 43.9 | 12.6 | 31.1 | 80.8 |
| Tb | 3.7 | 16.4 | 28.4 | 1.6 | 9.0 | 58.0 | 1.8 | 5.3 | 17.6 | 2.7 | 5.5 | 14.9 | 2.3 | 6.4 | 14.8 |
| Dy | 35.0 | 196.9 | 327.4 | 22.0 | 77.4 | 453.9 | 17.8 | 55.1 | 183.8 | 35.2 | 64.8 | 183.1 | 34.3 | 61.9 | 106.0 |
| Ho | 14.9 | 80.4 | 132.7 | 8.5 | 22.5 | 108.8 | 7.3 | 19.0 | 63.7 | 12.1 | 24.0 | 66.3 | 11.4 | 22.5 | 41.2 |
| Er | 69.1 | 370.4 | 613.4 | 35.6 | 91.9 | 366.8 | 36.2 | 89.1 | 279.6 | 73.0 | 122.4 | 313.9 | 69.7 | 110.2 | 181.3 |
| Tm | 14.4 | 76.0 | 121.7 | 5.6 | 17.8 | 61.5 | 7.9 | 18.7 | 49.7 | 16.0 | 25.8 | 58.0 | 16.4 | 23.9 | 40.6 |
| Yb | 150.6 | 677.9 | 1076.7 | 45.9 | 163.9 | 441.1 | 79.4 | 185.2 | 461.5 | 169.0 | 256.2 | 528.7 | 158.9 | 247.1 | 386.8 |
| Lu | 32.1 | 137.0 | 213.1 | 8.4 | 35.3 | 68.8 | 17.2 | 41.7 | 104.5 | 36.3 | 54.5 | 103.8 | 35.7 | 51.0 | 71.6 |
| La/YbN | 4E-06 | 6E-03 | 6E-02 | 5E-05 | 4E-02 | 6E-01 | 5E-05 | 4E-04 | 1E-03 | 6E-06 | 3E-04 | 4E-03 | 4E-03 | 5E-02 | 2E-01 |
| Eu/Eu* | 0.1 | 0.3 | 0.8 | 0.5 | 1.0 | 3.2 | 0.3 | 0.6 | 4.6 | 0.3 | 0.4 | 0.6 | 0.4 | 0.9 | 1.4 |
| Ce/Ce* | 0.9 | 29.8 | 195.8 | 0.7 | 17.6 | 145.1 | 5.9 | 22.1 | 76.6 | 7.5 | 63.6 | 241.9 | 0.8 | 4.7 | 29.8 |
| T (°C) | 571.2 | 679.9 | 826.3 | 538.1 | 845.0 | 1990.4 | 565.2 | 719.8 | 860.0 | 641.6 | 718.1 | 980.0 | 583.8 | 888.3 | 1147.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madon, B.; Mathieu, L.; Marsh, J.H. Oxygen Fugacity and Volatile Content of Syntectonic Magmatism in the Neoarchean Abitibi Greenstone Belt, Superior Province, Canada. Minerals 2020, 10, 966. https://doi.org/10.3390/min10110966
Madon B, Mathieu L, Marsh JH. Oxygen Fugacity and Volatile Content of Syntectonic Magmatism in the Neoarchean Abitibi Greenstone Belt, Superior Province, Canada. Minerals. 2020; 10(11):966. https://doi.org/10.3390/min10110966
Chicago/Turabian StyleMadon, Baptiste, Lucie Mathieu, and Jeffrey H. Marsh. 2020. "Oxygen Fugacity and Volatile Content of Syntectonic Magmatism in the Neoarchean Abitibi Greenstone Belt, Superior Province, Canada" Minerals 10, no. 11: 966. https://doi.org/10.3390/min10110966
APA StyleMadon, B., Mathieu, L., & Marsh, J. H. (2020). Oxygen Fugacity and Volatile Content of Syntectonic Magmatism in the Neoarchean Abitibi Greenstone Belt, Superior Province, Canada. Minerals, 10(11), 966. https://doi.org/10.3390/min10110966







