Abstract
The rare earth elements (REEs) have unique and diverse properties that make them function as an “industrial vitamin” and thus, many countries consider them as strategically important resources. China, responsible for more than 60% of the world’s REE production, is one of the REE-rich countries in the world. Most REE (especially light rare earth elements (LREE)) deposits are closely related to carbonatite in China. Such a type of deposit may also contain appreciable amounts of industrially critical metals, such as Nb, Th and Sc. According to the genesis, the carbonatite-related REE deposits can be divided into three types: primary magmatic type, hydrothermal type and carbonatite weathering-crust type. This paper provides an overview of the carbonatite-related endogenetic REE deposits, i.e., primary magmatic type and hydrothermal type. The carbonatite-related endogenetic REE deposits are mainly distributed in continental margin depression or rift belts, e.g., Bayan Obo REE-Nb-Fe deposit, and orogenic belts on the margin of craton such as the Miaoya Nb-REE deposit. The genesis of carbonatite-related endogenetic REE deposits is still debated. It is generally believed that the carbonatite magma is originated from the low-degree partial melting of the mantle. During the evolution process, the carbonatite rocks or dykes rich in REE were formed through the immiscibility of carbonate-silicate magma and fractional crystallization of carbonate minerals from carbonatite magma. The ore-forming elements are mainly sourced from primitive mantle, with possible contribution of crustal materials that carry a large amount of REE. In the magmatic-hydrothermal system, REEs migrate in the form of complexes, and precipitate corresponding to changes of temperature, pressure, pH and composition of the fluids. A simple magmatic evolution process cannot ensure massive enrichment of REE to economic values. Fractional crystallization of carbonate minerals and immiscibility of melts and hydrothermal fluids in the hydrothermal evolution stage play an important role in upgrading the REE mineralization. Future work of experimental petrology will be fundamental to understand the partitioning behaviors of REE in magmatic-hydrothermal system through simulation of the metallogenic geological environment. Applying “comparative metallogeny” methods to investigate both REE fertile and barren carbonatites will enhance the understanding of factors controlling the fertility.
1. Introduction
Rare earth elements (REEs) are a group of 17 chemically similar metallic elements (scandium, yttrium and lanthanide series in the periodic table IIIB). Scandium and yttrium are included in the REE group considering their comparable nature to the lanthanide elements and their common occurrence in a deposit [1,2]. The REE group is usually divided into two subgroups: light rare earth elements (LREEs, La-Eu) and heavy rare earth elements (HREEs, Gd-Lu and Y). Scandium is not included in the two subgroups because of its much smaller ion radius [3,4]. Rare earth elements are not at all rare in the Earth’s crust (especially LREEs), but are rather dispersed. The crustal abundance of REE is 0.017%, and the abundance of Ce, La and Nd is higher than that of W, Sn, Mo, Pb and Co. In comparison, heavy rare earth elements such as Tb and Tm are two to five times less abundant than Mo in the continental crust [5]. Under the upper mantle conditions, the trend of REE distribution into melts decreases with the decrease in ion radius from La to Lu (i.e., HREEs are generally more compatible with mantle peridotites). REEs are vital to modern technologies and society and are among the most critical of all raw materials. The demand for REE is constantly growing, driven particularly by their application in a range of modern and green technologies, including electric and conventional vehicles, communication technologies, and production and storage of renewable energy [4,6]. Supply of REE resources is largely restricted to China, along with a minor contribution from Brazil, Australia, the United States, Canada, India [7,8]. Although there is a substantial development on recycling of REE from end-products [9], the majority of demand still depends on the production from natural source of REE. The major REE deposits that are economically exploitable are restricted to a few localities in the world (Table 1), while the economic potential of a REE deposit is strongly influenced by the mineralization processes and its mineralogy [8]. The most commercially important REE deposits are associated with magmatic processes and are found in, or related to, alkaline igneous rocks and carbonatites. The supergene REE deposits also represent a large REE resource such as placer-type and ion adsorption-type (IAR) deposits. The IAR deposits distributing over a wide area of southern China are usually rich in middle and heavy REE, which meet almost all the needs of HREE such as Gd, Tb, Dy in the development of emerging industries around the world and also having extremely high economic value [10]. The reserves, production scale, and export volume of rare earth resources in China rank first in the world, which has become the center of many economic and political controversies. Rare earth resources are widely distributed in China and relatively concentrated in the individual deposits. According to statistics, rare earth resources have been found in more than 2/3 of the provinces (regions) in China, among which the Bayan Obo in Inner Mongolia, Mianning in Sichuan province, the Gannan in Jiangxi province and northern Guangdong province are the major areas [11].

Table 1.
Major economically exploitable rare earth element (REE) deposits in the world.
Worldwide, carbonatites are major sources of both REE and niobium, and are characterized by significant enrichment in the LREEs (La-Gd) over the HREEs (Tb-Lu). The Bayan Obo REE-niobium-iron deposit in Inner Mongolia, China, the world’s largest REE deposit, is an important example [12,13]. Whereas, the alkaline oversaturated rocks associated with REE deposits represent one of the most economically important resources of heavy REE and yttrium. Alkaline oversaturated rocks form from magma so enriched in alkalis that they crystallize sodium- and potassium-bearing minerals (e.g., feldspathoids, alkali amphiboles). The source magma is usually mantle-derived and highly differentiated. The world-class Tanbreez REE deposit, which is hosted in the Ilímaussaq complex of southern Greenland, is a typical example [14,15].
Carbonatite usually refers to igneous rocks derived from the mantle with carbonate mineral volume > 50% and SiO2 < 20wt.% [16,17]. Based on wt.% ratios of the major elements (MgO, CaO, FeO, MnO, Fe2O3, etc.), carbonatites are subdivided into magnesiocarbonatites, ferrocarbonatites and calciocarbonatites (Table 2) by the International Union of Geological Sciences (IUGS) [12]. Two unique types of carbonatite are identified to occur locally: silicocarbonatite (carbonatite with more than 20% SiO2) existing in the Afrikanda of the Kola Peninsula (Russia), and natrocarbonatite in the Ol Doinyo Lengai volcano (Tanzania) mainly consisting of Na-K-Ca carbonates, such as nyerereite [(Na, K)2Ca(CO3)2] and gregoryite [(Na, K, Cax)2−x(CO3)] [12,18,19]. Recent works on the different types of carbonatite in the Bayan Obo REE-Nb-Fe deposit reveal that the evolutionary sequence of carbonatitic magma is from ferroan through magnesian to calcic in composition, accompanied with an increase in LREE enrichment [20].

Table 2.
Classification of carbonatites (adapted from Simandl and Paradis [12] and Gou et al. [17]).
The types of REE deposits are complex and diverse, and the ones related to carbonatite–alkaline complexes are the most typical and abundant, including the Bayan Obo REE-Nb-Fe deposit in Inner Mongolia, Maoniuping REE deposit in Sichuan, Mountain Pass REE deposit in the USA and Araxá Nb-P-REE deposit in Brazil [21]. Carbonatite-related REE deposits comprising the main source of LREE and Nb resources in the world refer to REE deposits that are closely related to a set of carbonatite or alkaline rocks (usually coexisting) in genesis and space. Although carbonatite-related REE deposits are particularly crucial, there are still many debates and controversies about their geological background and genetic characteristics, and the detailed metallogenic process is not clear. Therefore, it is necessary to summarize the tectonic background, petrology and geochemical characteristics of carbonatite-related REE deposits, so as to better understand the genesis of the deposits and provide guidance for mineral exploration.
This article first summarizes the geological characteristics of carbonatite-related REE deposits, then summarizes and briefly introduces the typical carbonatite-related REE deposits in China. In combination with previous studies, we present a full discussion of the genetic characteristics and the mechanism of massive REE enrichment of carbonatite-related REE deposits. Ultimately, the existing problems are discussed and the future research directions are proposed to address the problems.
2. Types and Basic Geological Characteristics of Carbonatite-Related REE Deposits
2.1. Types of Carbonatite-Related REE Deposits
Carbonatite usually coexists with alkaline silicate igneous rocks to form carbonatite–alkaline (ring) complexes, but some appear in the form of isolated batholiths, dykes, intrusions, lava flows and pyroclastic overburdens [22]. Three types of carbonatite-related REE deposits are recognized according to the varied characteristics of mineralization, namely primary magmatic type, hydrothermal type and carbonatite weathering-crust type [23]. The former two types, collectively called carbonatite-related endogenetic REE deposits, are mainly formed in relation to carbonatite magma and its derived hydrothermal fluids, forming REE mineral accumulation and mineralization. The mineralization of primary magmatic type REE deposits mainly occur in the magmatic stage. REE minerals such as bastnäsite, monazite, allanite, xenotime, parasite occur in carbonatite or magmatic phosphorite, and the entire rock body is mineralized. REE minerals of hydrothermal type deposits are formed by hydrothermal fluids that are evolved from magmas, which are usually associated with calcite, barite, fluorite, quartz and other minerals to form major or net veins. The mineralized veins frequently interpenetrate in the contact zone and surrounding rock of carbonatite complexes. The hydrothermal REE minerals can also occur as crack fillings superimposed on the minerals that are formed during the magmatic stage. This type of deposit is usually large in scale, and the REE minerals are relatively simple, mainly bastnäsite [24]. Carbonatite weathering-crust type is secondary carbonatite REE deposits, which comprise laterite crust after long-term weathering and fluid leaching under particular environmental conditions that are hot, humid and suitable for weathering crust preservation. REEs in the weathered crust section are remobilized, enriched, and adsorbed on the surface of clay minerals such as kaolinite in the form of ions. This type of REE deposit is usually HREE-enriched due to the higher mobility of LREE during weathering [23,25].
2.2. Spatial and Temporal Distribution
Worldwide there are more than 500 proven carbonatites, only a small proportion of which is fertile with REEs (Figure 1) [26,27]. The ages of carbonatites range from the Archean to Cenozoic. The mineralization of carbonatite-related REE deposits occurs over a long period of time but is mostly concentrated in the Mesozoic. Carbonatite-related REE deposits in China are formed during the Proterozoic to Mesozoic. Among those, two major mineralization events occurred in the Mesoproterozoic and Mesozoic (Figure 2), which are represented by the Bayan Obo REE deposit in Inner Mongolia (~1.32 Ga, [20]) and the Miaoya REE deposit in Hubei province (232 Ma, [28]), respectively.
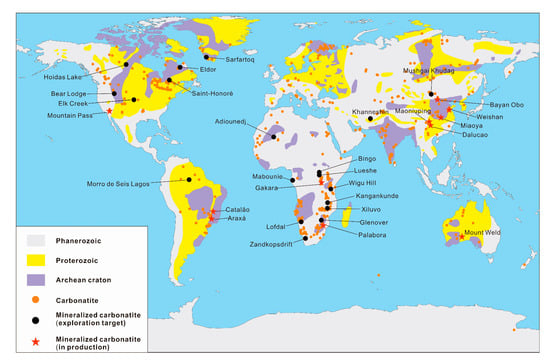
Figure 1.
Distribution of carbonatites and carbonatite-related REE deposits, including deposits in production and exploration targets discussed in this paper (modified from Woolley and Kjarsgaard [26], Liu and Hou [27] and Verplanck et al. [29]).
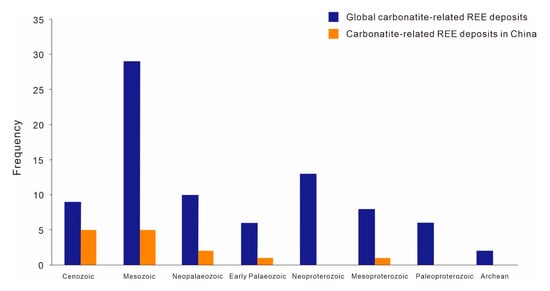
Figure 2.
Age distribution histograms of major carbonatite-related REE deposits in the world and China (based on data from Woolley and Kjarsgaard [26], with minor updates).
Globally, carbonatite-related REE deposits mostly occur in continental marginal depression belts and rift belts along craton margins controlled by large-scale deep lithosphere faults (e.g., the Mountain Pass REE deposit in the USA). Subordinately, REE mineralization also occurs at stable geological structural units (platform or paraplatform) such as the Araxá Nb-P-REE deposit in Brazil and the Sarfartoq REE deposit in Greenland (Figure 1) [17,29,30]. The distribution of carbonatite-related REE deposits in China is rather restricted to continental margin rifts or orogenic belts at the margin of cratons [21]. For example, the Bayan Obo and Weishan REE deposits are located on the northern and southeastern margins of the North China Craton, respectively; the Maoniuping REE deposit is located in the rift belt of the western margin of the Yangtze Craton; the Miaoya and Shaxiongdong REE deposits in Hubei Province are located in the Qinling orogenic belt [31,32,33,34,35].
2.3. Ore and Orebody Characteristics
The vast majority of carbonatite-related REE deposits in China belong to endogenetic deposits including both the primary magmatic type and hydrothermal type REE deposits. Large and super large-scale REE deposits often have both magmatic and hydrothermal features, reflecting an interplay of both processes in contribution to the ore genesis [23]. The Bayan Obo REE-Nb-Fe deposit is a great example [36]. Previous studies suggest that two major mineralization events occurred at 1400 Ma and 440 Ma, respectively, at the giant Bayan Obo REE-Nb-Fe deposit, which correspond to an early emplacement of large-scale carbonatitic magma (1.4-1.3 Ga) and a late intense hydrothermal activity (440 Ma). The early carbonatitic magma is fertile in REEs, and the REEs are captured in primary REE-minerals such as bastnäsite and monazite. The late hydrothermal fluids released the REEs from its original host minerals, and upgraded the reserves, forming REE minerals such as secondary bastnäsite, parasite and cerianite, which are usually coarse-grained and appear in veins [37,38,39,40].
Carbonatite-related primary magmatic type REE deposits are mainly hosted in a set of ring-shaped complexes composed of carbonatites and alkaline rocks. The entire carbonatite body is commonly mineralized, and mineralization tends to extend into the alkaline complexes appearing as lenticular or irregular lenses [23]. The occurrence of ore bodies is usually controlled by regional deep faults existing in the extensional lithosphere. In this context, the regional deep faults act as conduits for the migration of deeply seated and fertile magma [17]. For example, the Mountain Pass REE deposit, United States, is controlled by a large deep fault that crosses the west coast of North America. The Miaoya REE deposit in China occurs in an extensional tectonic setting derived from fault zones or orogenic belts [41,42,43]. The ores usually are fine-grained and massive, disseminated, or striped in texture. The REE minerals are generally formed from crystallization in the late stage of magma evolution, mainly including bastnäsite, monazite, xenotime, parisite, allanite, and frequently in association with Nb-containing minerals such as pyrochlore, aeschynite, niobite. Other existing minerals include magnetite, hematite, apatite, barite, calcite, dolomite, mica and zircon [23,25].
The orebodies of hydrothermal type REE deposits are usually in the form of veins or stockwork that occur near the contact zone between orebodies and host rocks or in the host rocks. The mineralization is also controlled by regional deep faults. For example, the Maoniuping REE deposit in Sichuan Province and Weishan REE deposit in Shandong Province are typical hydrothermal type REE mineralization. They are located in the Panxi Rift Valley and Tanlu Fault Zone, respectively [44,45]. Compared with REE deposits of primary magmatic type, the REE minerals in hydrothermal type deposits are mineralogically simpler, typically containing bastnäsite and parasite. The REE minerals appear either as filling in veins or disseminated or as overgrowth on the early magmatic minerals in carbonatite [23]. The gangue minerals mainly comprise of fluorite, quartz, barite, calcite, apatite and aegirine-augite. The hydrothermal fluids exsolved from the late stage magma tend to overprint early magmatic mineralization, a process that is crucial for the massive enrichment of REEs in carbonatite-related REE deposits.
2.4. Geochemical Characteristics
The chemical composition of carbonatite varies greatly. Typical carbonatites are SiO2 unsaturated, rich in CO2 and CaO, and some are rich in Mg, Fe and alkalis [17,46]. Trace elements in carbonatite mainly include LREE, Nb, Ta, Th, Zr, Hf, Sr, Ba, F, and P. Carbonatite is one of the rocks with the highest REE content and the highest LREE differentiation on the Earth. It has become an essential prospecting indicator for large-scale, high-grade and LREE-enriched REE deposits. As the most characteristic and significant elements in carbonatite, LREEs are enriched to a higher level than other trace elements as main ore-forming elements in some carbonatite-related REE deposits, e.g., Bayan Obo REE-Nb-Fe deposit [17]. Niobium and Ta often appear as associated elements in the deposits and are enriched in the late petrogenic stage. The precipitation of Nb and Ta often forms pyrochlore, aeschynite, etc. Alkaline-earth metals such as Sr, Ba also show enrichment and are incorporated in carbonates. Substantial accumulation of Fe can occur to a level that is economically exploitable (e.g., Bayan Obo REE-Nb-Fe deposit) [17,40,47]. The contents of F and Cl in carbonatite are higher compared to those of other magmatic rocks, which is essential for the efficient migration and precipitation of REEs in the fluids. Occasionally, F is highly enriched in carbonatite-forming carbonatite-type fluorite deposits [48,49,50]. Phosphorus is also one of the most characteristic trace element in carbonatite, and often occurs in the form of apatite.
2.5. Alteration Characteristics
In the process of ascending and emplacement of carbonatitic magma, the temperature and pressure of fluids decrease, along with release of volatiles (F, Cl, P and S) and elements such as REE, Na, K, and Fe. The fluids reach the upper crust and metasomatize surrounding rocks, forming a lithological belt of fenite with asymmetric zonal distribution in the orebody, which is called fenitization (Figure 3). Fenitization is an alkali metasomatism, which is caused by alkali-rich fluids exsolved from igneous carbonatitic or alkaline magma. It is a typical and unique alteration phenomenon of carbonatite and alkaline igneous rocks [47,51,52,53]. McKie (1966) defined fenite as the rock produced by the metasomatism of igneous carbonatite with surrounding rocks, mainly composed of alkaline feldspar and alkaline mafic minerals [54]. During the metasomatism, a large amount of Si and substantial Al are released into the solution, while K, Na, Ca, and Fe are retained in the fenite forming new minerals. The degree of metasomatism varies according to the distance to the causative rocks, resulting in halo-like patterns of fenitization. The main minerals of fenite include Na- and K-amphiboles, Na-pyroxene, aegirine-augite, K-feldspar, albite, perthite, nepheline and pale brown mica. The accessory minerals include apatite and REE minerals such as titanite, pyrochlore, monazite and bastnäsite [12].

Figure 3.
Fenitization in the wallrock quartz sandstone from the Bayan Obo REE-Nb-Fe deposit.
According to the Na2O/K2O ratio of whole rock, fenitization can be divided into three main types: sodic, potassic, and sodic-potassic. Potassic fenite is dominated by K-rich feldspars (orthoclase or microcline). In some cases, there are also low-Al phlogopite or biotite, which is usually developed near the upper part of intrusive calcite/dolomite carbonatite. Sodic fenite mainly consists of Na-rich amphibole, sodic pyroxene, alkali feldspar or albite. Sodic-potassic fenitization is an intermediate between the two endmembers [12,55]. In addition, sodic fenite usually occurs in the contact zones between carbonatite bodies and surrounding rocks in the early stage or deep emplacement, while potassic fenite is often found in the contact zones in the late stage or shallow emplacement [56]. Previous studies have suggested that the initial carbonatite contains a considerable amount of alkali, and the hydrothermal fluid rich in Na and K can be derived in the process of evolution and metasomatic reaction with surrounding rocks [46]. This initial carbonatite is named sodic carbonatite, such as Oldoinyo Lengai sodic carbonatite in northern Tanzania [19,57,58]. However, carbonatites exposed in nature are mostly ferroan, magnesian, and calcic carbonatites, rarely alkali in composition, although they spatially closely coexist with alkali-rich fenite. In addition to Na and K, other elements such as Fe, Zr, V, Zn, Rb and Ba are incorporated into the fluids derived from carbonatite magma [59,60]. During the separation of fluid phase, REEs, especially LREEs, prefer to partition into the liquid and vapor phases. The fenitizing fluids derived from carbonatite magma are characterized by strong enrichment of LREEs. REE-bearing minerals such as bastnäsite and monazite crystallize and accumulate from the fenitizing fluids during its auto-metasomatism to carbonatite, which favors for the formation of large REE deposits [20].
3. Typical Carbonatite-Related REE Deposits
3.1. Bayan Obo REE-Nb-Fe Deposit, China
The Bayan Obo deposit is the largest REE deposit in the world and an important Fe and Nb deposit in China (Figure 4a). It is located in the Wulanchabu grassland, Baotou City, Inner Mongolia Autonomous Region [61,62]. The ore-forming process is extremely complex, which is mainly due to the extended and intense hydrothermal alterations and transformation during the late mineralization period [63,64]. In the deposit, REE orebodies are mainly hosted in a set of dolomites in the south of Kuangou anticline (Figure 4b). The dolomite is divided into fine-grained and coarse-grained lithofacies. Mineralization is absent in the dolomite (also known as limestone) in the northern flank of the Kuangou anticline. The contrast of mineralized and barren dolomite may reflect key factors that control the formation of the large Bayan Obo deposit. In addition, various carbonatite dykes are developed in the mining area, which are divided into three categories: dolomite type (ferroan), calcite-dolomite type (magnesian), and calcite type (calcic) based on their mineralogical composition [65]. The extensive development of carbonatite dykes has long been believed to account for the massive enrichment of REE. In addition to the mineralogical variation, the REE content is also different for the three types of carbonatite dykes. The calcic carbonatite dykes contain the highest REE content relative to the other two types (REE2O3 reaches up to 20%) [20,65]. This may suggest that the elemental differentiation process of different types of carbonatite dykes is crucial in controlling the massive enrichment of REE. However, the evolution mechanism of the Bayan Obo carbonatite magma is still unclear, and the magmatic affinity of the ore-bearing carbonatites is also controversial [66,67].
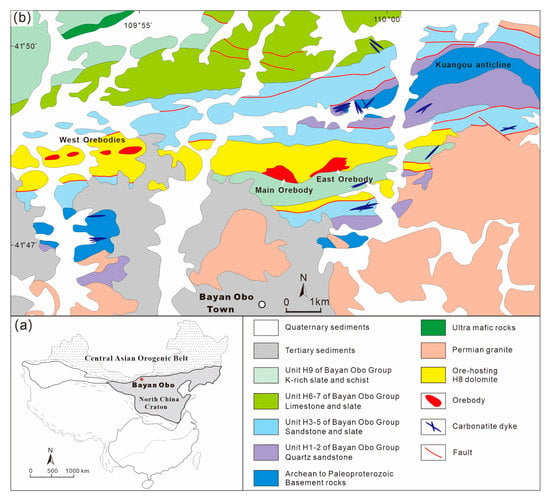
Figure 4.
The location of the Bayan Obo deposit in China is marked in the interior plot (a). The geological setting of the Bayan Obo REE-Nb-Fe deposit (adapted from Yang et al. [65]) has been plotted in (b).
3.2. Miaoya Nb-REE Deposit, China
The Miaoya Nb-REE deposit is located in the southern margin of the Qinling Orogen, Central China. It was first discovered by the Northwest Geological Bureau in 1963 during the regional radiological survey. The deposit contains more than 40 REE-Nb orebodies with an estimated reserve of 1.21 Mt REE2O3, which is the largest REE deposit in the Qinling orogenic belt [68]. The Miaoya syenite–carbonatite complex hosts the entire mineralization (Figure 5). The Miaoya complex is mainly syenitic in composition and has banded structures featured by being fine-grained in the center, xenomorphic granular in the middle and porphyritic at the edge [68]. The mineralogical composition of syenite outcrops at different locations is relatively homogenous, mainly composed of K-feldspar, microcline, biotite, albite, plagioclase, quartz, and sericite, together with a small amount of zircon, monazite and kaolinite. The carbonatite occurs as stocks intruding into the alkaline syenite, and comprise both calciocarbonatite and ferrrocarbonate in composition. The main constitutional minerals are calcite, ankerite and apatite; the accessory minerals include biotite, alkaline feldspar, magnetite, ilmenite, perovskite, zircon, and Nb-rich rutile. A variety of REE-bearing minerals are formed including bastnäsite, parasite, monazite, allanite and REE-rich apatite [69]. The occurrence of syenite and carbonatite is spatially closely associated. It is speculated that they have a common source and are the products of partial melting of the mantle [41].
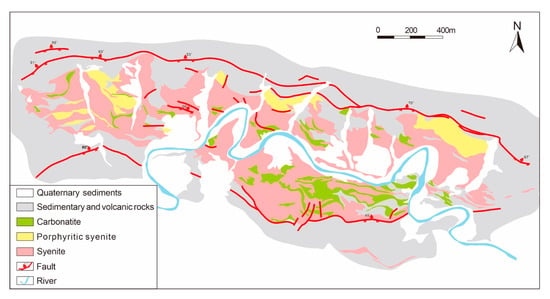
Figure 5.
Simplified geological map of the Miaoya syenite–carbonatite complex (adapted from Su et al. [41]).
3.3. Maoniuping REE Deposit, China
The Maoniuping REE deposit is the second largest REE deposit in China. It is located in the east of the Tibetan Plateau and the west of the Yangtze Plate, and controlled by the Panxi rift. The Maoniuping REE deposit is also the largest and most typical REE deposit in the Mianning–Dechang REE metallogenic belt [70]. The orebodies are mainly in the form of veins, veinlets, and net veins interspersed in syenite (Figure 6). Fluoritization, carbonation and REE minerals usually occur in the contact zone between veins and surrounding rocks. The carbonatite intrudes along the center of the expanded part of syenite and is distributed in the contact zones around the orebodies. The REE minerals are mainly bastnäsite, together with minor parisite and cerianite. The gangue minerals mainly include calcite, quartz, barite, fluorite, albite, arfvedsonite, and a small amount of metal oxides and metal sulfides. The deposit is a typical hydrothermal carbonatite-related REE deposit. During the migration of hydrothermal fluids, REEs were transported by complexing with the F−, Cl−, (SO4)2−and (CO3)2− ligands in the fluids. The hydrothermal fluids gradually cool down, together with crystallization and precipitation of fluorite, barite and calcite. The gradual cooling of fluids and the crystallization of fluorite, barite and calcite lead to the breakdown of REE complexes and precipitation of the main REE ore mineral bastnäsite [71].
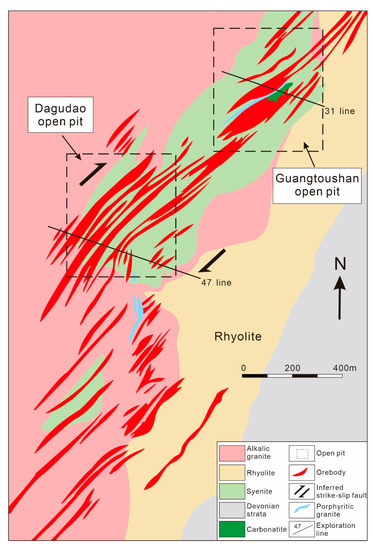
Figure 6.
Simplified geological map of the Maoniuping REE deposit, showing the location of the Dagudao and Guangtoushan section (adapted from Liu et al. [71]).
3.4. Weishan REE Deposit, China
The Weishan REE deposit is one of the three major LREE bases in China. It is a typical carbonatite-related hydrothermal REE deposit, located in the Weishan County, southern Shandong Province. Tectonically it is located at the southeast margin of North China Craton and western part of the Tanlu fault zone. The carbonatite orebodies contain veins and veinlets, as well as disseminated mineralization that occur within the faults of rock mass and coexist with ijolite syenite (Figure 7) [44]. The large vein-type orebodies have the highest economic value, and the net veins and disseminated orebodies are distributed at the edge of the large veins [34]. The REE minerals mainly include bastnäsite, parisite, Ce-apatite, and a small amount of allanite, monazite, and titanite. The gangue minerals include fluorite, quartz, barite, calcite, muscovite, limonite, aegirine augite, orthoclase, albite. The deposit also contains substantial sulfides, including pyrite, galena, pyrrhotite, chalcopyrite, molybdenite. The formation of the Weishan deposit requires two enrichment processes: generation of mineralized carbonatite and subsequent enrichment from magmatic–hydrothermal processes. The carbonate magma is sourced from enriched lithospheric mantle. Liquid immiscibility between the carbonatite melt and CO2-rich silicate melt resulted in the separation of REE-rich carbonatite melt, from which the REE-rich magmatic–hydrothermal fluids were derived. In the late stage of hydrothermal evolution, a large number of REE minerals were precipitated. The hydrothermal fluids evolved to lower temperatures and became sulfidic, which allows for the silver mineralization to occur [34,44,72].
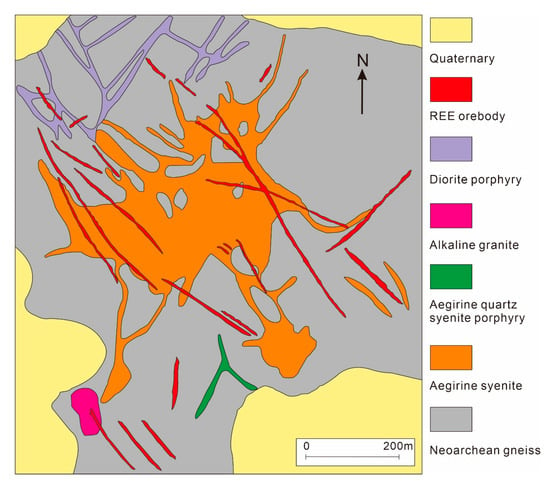
Figure 7.
Simplified geological sketch map of the Weishan rare earth element (REE) deposit in Shandong Province (adapted from Jia et al. [44]).
3.5. Mountain Pass REE Deposit, USA
The Mountain Pass REE deposit, located at the southern border of California to Nevada, is the second largest REE deposit in the world following the Bayan Obo [25]. Around 16 million tons of REE oxide reserves have been proven [73,74]. The deposit is hosted in the Sulphide Queen carbonatite (Figure 8). The mineralized rocks include the shoshonite, syenite, granite, and the Sulphide Queen carbonatite. However, only the carbonatite orebodies are economically viable [75]. The orebodies intrude into the Precambrian metamorphic basement in plate or lenticular form, mainly composed of calcite, dolomite, barite, bastnäsite in mineralogical composition, and bastnäsite as the main ore mineral accounts for about 10–15%. The existing data show that cerium (Ce) is the main rare earth element in primary bastnäsite and other REE minerals in the Sulphide Queen carbonatite [73,76]. The Sulphide Queen carbonatite is distinct from other carbonatites in the world. Carbonatites are usually spatially and genetically associated with a set of sodic alkaline rocks in a ring-shaped and concentric circle, and rich in LREE, Nb, Ta, Zr, and P. However, the formation of the Sulphide Queen carbonatite is associated with a suite of ultrapotassic intrusive rocks in plate-like form, being rich in LREE and Ba and depleted in Nb and P. Previous studies suggest that the abnormal nature of Sulphide Queen carbonatite is due to the fact that the carbonatite magma and associated ultrapotassic magma have a common source, or alternatively, the carbonatite magma evolved from the ultrapotassic magma [73,75,77].
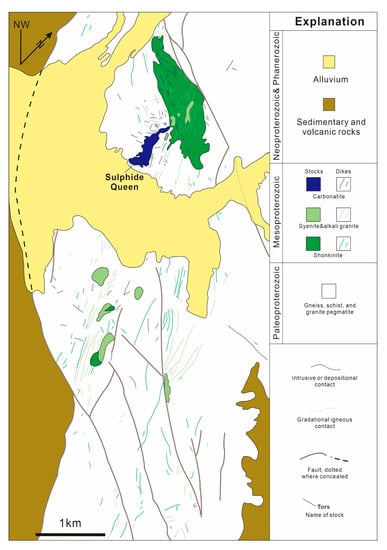
Figure 8.
Generalized geological map of the Mountain Pass area (adapted from Poletti et al. [77]).
3.6. Araxá Nb-P-REE Deposit, Brazil
Brazil is the second largest REE-enriched country in the world, with about 22 million tons of REE reserves [78]. Among them, the Araxá carbonatite Nb-P-REE deposit is a main supplier of REE resources [78]. The deposit is located near the Araxá City, Minas Gerais State in the southeast of Brazil. It is the largest Nb deposit in the world, and produces a significant amount of P, Ti and REE. The orebody appears as a concentric ring complex, intruding into the Mesoproterozoic quartzite and schist strata (Figure 9). The core of the complex is composed of fine-grained dolomite carbonatite, which gradually transits to mica schists. The main constitutional minerals are dolomite, calcite and ankerite, and the accessory minerals are mica, apatite, alkaline amphibole, magnetite, monazite, pyrochlore, perovskite and zircon [79]. The main REE minerals are monazite (over 70%) and plumbogummite group minerals. In contrast, bastnäsite and cerianite only take up about 1% of the REE minerals. Pyrochlore is the main Nb-bearing mineral [80]. The periphery of the complex underwent intense fenitization, which is manifested in abundant alkaline feldspar, arfvedsonite and aegirine in the surrounding quartzite. Supergene enrichment in which Nb, P and REE form high-grade ores is well developed at the mining area [79].
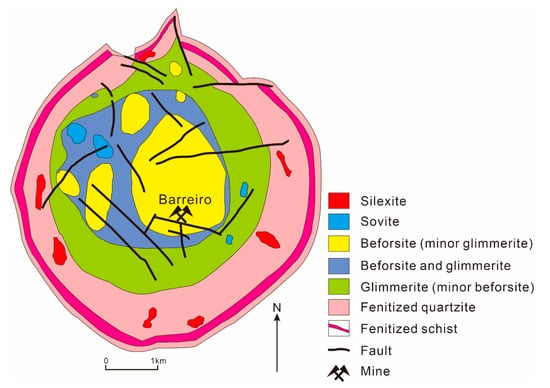
Figure 9.
Simplified geological map of the alkaline–carbonatitic complex, Araxá (adapted from Li et al. [79]).
3.7. Gakara REE Deposit, Burundi
The Gakara REE deposit, located in the Republic of Burundi in east-central Africa (Figure 10), was discovered in 1936. It is one of the highest-grade REE deposits in the world, with an estimated in-situ grade of 47–67% of the total REE oxides [81,82]. The orebodies mainly occur in the wall rocks of quartzite and schist and in the form of net veins. The main constitutional minerals are bastnäsite, monazite, quartz, biotite, barite, K-feldspar and pyrite. The accessory minerals are cerianite, galena, anhydrite, rutile, molybdenite and chalcopyrite. According to previous work, the formation of the deposit includes three stages: Stage I: formation of primary coarse-grained bastnäsite, accompanied by gangue minerals such as quartz, biotite, and barite; Stage II: replacement of the primary bastnäsite by microcrystalline monazite that hosts most of the REE; microcrystalline quartz matrix was developed; silicification and brecciation occurred locally; Stage III: formation of La-rich monazite and supergene minerals such as cerianite, rhabdophane, goethite and kaolinite [81,82,83].
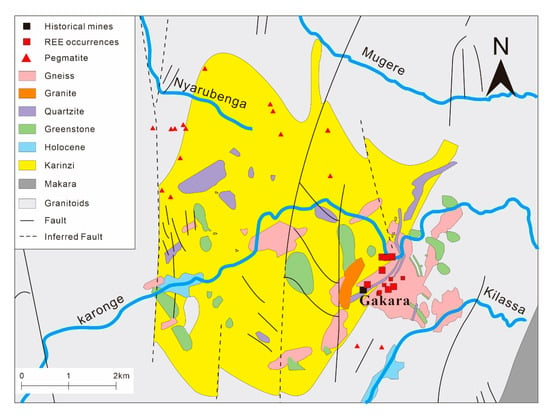
Figure 10.
Simplified geological map of the Gakara REE deposit (adapted from Buyse et al. [81]).
3.8. Sarfartoq REE Deposit, Greenland
The Sarfartoq carbonatite, located in the southwest of Greenland, was discovered by the Geological Survey of Greenland in regional aerial radioactivity surveys from 1975 to 1976. Since the discovery, the Sarfartoq carbonatite has become the target of mineral exploration for resources such as diamond, Nb, U, P, REE [84]. It is located in the transitional zone between the Archean gneiss and the Paleoproterozoic Nagssugtoqidian active zone. The complex is composed of a carbonatite central core, fenitization zones and ring-like gneiss edge zones, with local intrusion of carbonate breccia veins (Figure 11). The carbonatite core area is mainly composed of rauhaugite; a small amount of sovite occurs sporadically. Extensive fenitization is developed around the core, within which a shear zone of 50–200m in width appears. Other characteristic features of the Sarfartoq REE deposit include the development of limonitization of the gneisses that is abundant in Th, U and REE. Niobium and REE mineralization, which is precipitated in the form of pyrochlore, are found in the outer fenitization zones. Hematitization and limonitization alteration are generally restricted to gneiss at the outer zones due to cataclastic deformation and hydrothermal metasomatism. Late stage of carbonatite veins, breccias and agglomerates of various shapes are developed in the alteration zone. The REE minerals in the Sarfartoq carbonatite are mainly bastnäsite, monazite and pyrochlore [14,84,85,86].
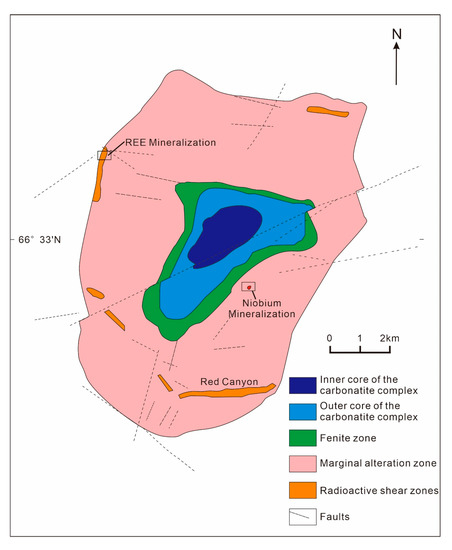
Figure 11.
Geological sketch map of the Sarfartoq carbonatite complex (adapted from Bedini et al. [86]).
4. Genesis of Carbonatite-Related REE Deposits
4.1. Origin of Carbonatites
The evidence for the genesis of carbonatite mainly comes from experimental petrology, which shows that the formation of carbonatite magma is related to the mantle (lithospheric mantle or asthenosphere mantle) [87]. However, the specific evolution process of the magma is still controversial. At present, there are three main models explaining the formation of carbonatitic magma (Figure 12): (1) immiscible separation of primary carbonate–silicate magma under crustal or mantle pressure [88,89,90,91,92]; (2) fractional crystallization of parental carbonate–silicate magma [93]; (3) low-degree (generally < 1%) partial melting of the mantle rich in H2O and CO2 [88,89,94]. In addition, a recent study based on the boron (B) isotopes of global carbonatites suggests that although most carbonatites may have originated in the upper mantle, young carbonatites (<300 Ma) may contain at least part of the subducted crustal materials [95], and it is likely that the source of melts forming carbonatites is diverse. However, it is generally agreed that alkali elements (Na and K) play a significant role in the formation of calcite/dolomite carbonatite, and ferrocarbonatite intrusions, regardless of their formation mode [12].
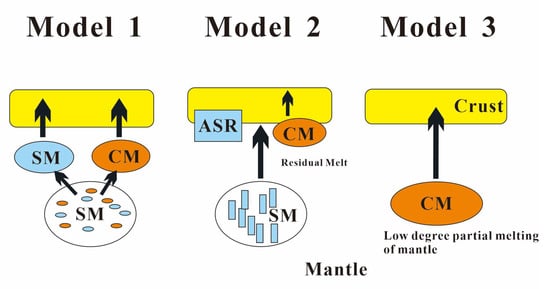
Figure 12.
Lithogenesis of carbonatites. Model 1—immiscible separation of primary carbonate–silicate magma; Model 2—fractional crystallization of parental carbonate–silicate magma; Model 3—low-degree (generally < 1%) partial melting of the mantle rich in H2O and CO2; CM—carbonate melts; SM—silicate melts; ASR—alkaline silicate rocks (adapted from Ye et al. [87]).
4.2. Source of Ore-Forming Materials
High REE enrichment is a prerequisite for carbonatites to become a source of ore-forming materials. The genesis of carbonatites is generally recognized to be derived from the enriched mantle and related to mantle plume activities [95]. However, the source of REE in carbonatites is still controversial, including two main theories on the basis of previous research results.
The first theory suggests that the initial carbonated magma was formed by low-degree partial melting of the mantle which is rich in H2O and CO2, preceding the immiscible separation of primary silicate magmas that contain a large amount of REE (especially LREE) and carbonate melt. Incompatible elements such as REE preferentially partition into the carbonatite melt phase, resulting in an enrichment of REE in the melt relative to the primitive mantle [96]. For example, previous work on the Bayan Obo deposit generally suggests that the huge amount of REE in the deposit has a rich mantle source unaffected by crustal contamination, and the potential contribution of any external materials is trivial [40,97]. However, most carbonatite melts from mantle xenoliths are not enriched in incompatible elements [92,98], which is against this assumption. Enrichment of incompatible elements was otherwise discovered to exist in silicate minerals [99,100]. The carbonatite melts from mantle xenoliths have low REE content and relatively flat REE distribution patterns (i.e., low differentiation degree of LREE and HREE). At the same time, the carbonatite melt produced by partial melting of carbonated primitive mantle also has significantly lower REE content than that of carbonatite in the surface [98,101,102]. In addition, the partitioning of REE in immiscible melts is not in agreement with that of the interpretation of carbonatite formation process. The immiscibility process of carbonated silicate melts was simulated at high temperature and pressure, and it was found that REEs (especially HREE) were more inclined to enter the silicate melt [100]. Even in the case that a small quantity of volatiles (such as F, Cl, etc.) is added into carbonatite melt, the REEs will be slightly promoted into the carbonatite melt, but the overall enrichment trend of REEs in silicate melt remains unchanged [100]. Nevertheless, carbonatites are obviously more enriched in REE than the associated alkaline silicate rocks in a natural environment. Therefore, carbonatites formed by low-degree partial melting of mantle and immiscibility of carbonated silicate magma tend to be mildly enriched in REE, which is insufficient for REE mineralization to occur. Other sources of REEs are therefore required.
The second theory advocates that crustal sediments enriched in REE were subducted to the mantle, resulting in partial melting of the mantle. This process is of fundamental importance to enrich REE for the carbonatite magma, which is particularly the case for the carbonatite-related REE deposits near orogenic belts, where the subducted sediments are inevitably involved [103,104]. Kato et al. (2011) reported high concentrations of REE and Y in the deep-sea sediments at numerous sites such as the eastern South and central North Pacific [105]. Mimura et al. (2019) reported pelagic clay has high concentrations (>400 ppm) of REEs and Y in the western North Pacific Ocean, which has a significant impact on chemical composition of subducting sediments [106]. Yasukawa et al. (2014) also reported that there are remarkably high concentrations of REE and Y of potential economic significance in the deep-sea sediments of the Pacific and Indian oceans. The sediments are mainly composed of siliceous ooze with subordinate zeolitic clay, although seawater is proved to be the ultimate source of REE in the sediments [107]. This study provides a theoretical premise of reinforcing crustal sediments to be the REE source materials of carbonatite-related REE deposits. Moreover, it is found that the formation of typical carbonatite-related REE deposits in China is related to plate subduction and the associated circulation of crustal materials [30]. For example, the Bayan Obo deposit, located near the Central Asian orogenic belt, was formed during the closure of the Paleo-Asian Ocean. During the subduction of the Paleo-Asian plate, Si-rich fluid from the subducting slab was released and reacted with the calcic carbonatite pluton, from which REEs, Nb, Th, and Sr were collected. Then the fluid caused metasomatism with the overlying H8 dolomite to form a super large-scale deposit [108,109]. The Miaoya REE deposit in Hubei Province, located in the southern margin of Qinling orogenic belt, experienced complex orogenies such as continental collision, oceanic subduction, terrane accretion, and ocean basin opening and closing [110]. In the process of producing carbonatite magma, the contamination of crustal materials seems unavoidable and can contribute an additional amount of REE for the mineralization [41,111].
4.3. Nature of Ore-Forming Fluids
In carbonatite-related REE deposits (especially hydrothermal type), the ore-forming fluids play an important role in metasomatic wall-rock alteration, REE enrichment and mineralization. The ore-forming fluid derived at the late stage of carbonatite magma evolution is mainly composed of alkaline carbonate brine, which is rich in H2O, high-density CO2, Na, K, Ca, SO42−, as well as REE, Nb and other ore-forming elements [45,112,113,114,115,116,117]. The fluids, which are freshly derived from carbonatite melts, are supercritical and high in temperature, pressure and solubility [45]. This fluid is also rich in alkalis, carbon and volatiles (CO2, CH4, C2H6, N2, etc.), and has low viscosity. The nature of the fluids is ideal for establishing transport efficiency of the ore-forming elements in the fluids. The ore-forming fluids migrate along fractures at a high rate of speed and rise to the shallow surface along major structures [112,115]. In the early stage, the hydrothermal fluid is characterized by magmatic signatures, and gradually becomes mixed and diluted by meteoric water. In consequence, the fluids evolve to an aqueous, low temperature, low pressure, low salinity phase, in which the ore-forming elements are precipitated [116].
The study of fluid inclusions in carbonatites reflects the above-mentioned changes of fluid properties in different stages. Ting et al. (1994) studied fluid inclusions in the Sukulu carbonatite complex in Uganda, and found that the fluid inclusions evolve from CO2-bearing, mid-high salinity to aqueous-rich and eventually to a CH4-bearing composition from early to late stages, while CO2-rich and CH4-rich inclusions generally do not coexist [118]. Hong et al. (2014) also found a complete magmatic–hydrothermal evolution sequence from melt inclusions to melt-fluid inclusions and then to fluid inclusions for the Maoniuping REE deposit [119].
4.4. Migration and Precipitation Mechanism
REEs, as high field strength elements (HFSE), are traditionally regarded to be scarce in the form of simple ions in hydrothermal system and rarely migrate or mineralize [120]. However, it is found that REEs in certain hydrothermal fluids can be particularly enriched and migrate in the form of complexes [48,50,121]. Based on the Hard-Soft-Acid-Base (HSAB) principle, the REE ions belong to hard acid ions and are more likely to form stable complexes with hard base ions, e.g., F−, SO42−, Cl−, CO32−, PO43−, OH−. Previous studies suggest that REE mainly migrate in the form of chloride, sulfate and complexes in hydrothermal systems [48,122,123,124,125]. The proportions of various complexes vary with the temperature and pH of the hydrothermal fluids. In a highly acidic environment, the chlorine REE complexes (mainly REECl2+) play a dominant role. With the increase in pH to weakly acidic or neutral conditions, the chlorine REE complexes are gradually replaced by sulfate complexes (mainly REE(SO)42−). In low-chlorine and alkaline environments, REEs mainly migrate in the form of hydroxy complexes (mainly REE(OH)03) [125,126,127]. In addition, temperature is critical to the transport efficiency of REEs. With the increase in fluid temperature, the complexation of REEs with chlorine and sulfate is enhanced to different extents that allows for higher capacity of REEs transportation in the fluids.
In hydrothermal systems, hard base ions complexed with REEs are not all conducive to the migration of ore-forming elements. Previous studies have found that when REEs form complexes with fluorine, carbonate and phosphate, it is more likely leading to rapid precipitation of REEs rather than migration [48,50,127]. Especially in the fluorine rich system, the REE-fluorine complex has low solubility and high-temperature instability, and the precipitation and mineralization of REEs are more likely to occur in this condition [50]. In the field investigation, we found that REEs are mainly enriched in the fluorine-enriched carbonate, which is consistent with the view that fluorine is more critical in precipitating REE than transporting REE.
The precipitation of REE in hydrothermal fluids is collectively controlled by temperature, pressure, pH, oxygen fugacity and chemical compositions [128]. The hydrothermal fluids that are initially exsolved are high in temperature and pressure, and inversely low in the pH value. The stability and solubility of REE complexes are high in the early stage of hydrothermal fluids, in which the migration of REEs is enhanced. In the process of migration, the fluid temperature and pressure decrease, leading to the separation of CO2 and other acid gas phases. This process is accompanied with metasomatic reactions. Consequently, the pH of the hydrothermal system increases while the REE complexes (especially the fluorine complexes) become unstable and precipitated. During the metasomatism phosphorus can be introduced into the hydrothermal fluids, resulting in the precipitation of REEs in the form of REE-phosphates, e.g., xenotime and monazite [30,48,50,126]. In the late stage of fluid evolution, the temperature further decreases, and the frequent brecciations near the surface introduce meteoric water in the hydrothermal system. The fluid composition changes dramatically. Carbon dioxide is available and involved in the formation of bastnäsite that precipitates REEs [112,129]. For example, the Maoniuping REE deposit in Sichuan Province is a typical carbonatite-related hydrothermal deposit. A rich body of research has been carried out to understand the migration and precipitation mechanism of REEs in the deposit. The migration of REEs in the hydrothermal system was controlled by complexing agents such as F−, Cl−, SO42−, CO32−. At the initial stage of hydrothermal activity, REEs are mainly transported as chlorine and sulfate complexes. As the fluids advance, CO2 became immiscible and the fluid pH increases. Corresponding to the change of pH the gangue minerals such as fluorite, celestite and barite began to precipitate, decreasing the buffering capacity of fluids and reducing the activity of fluorine complexing with REE. In consequence, a small amount of REEs was precipitated in REE minerals such as bastnäsite. During the late stage of hydrothermal activity, the fluid temperature continues to decrease, and the addition of external fluids such as meteoric water and atmospheric components such as CO2 induces the major precipitation of gangue minerals, along with significant deposition of bastnäsite. This paragenesis is readily reflected in field observation where bastnäsite is often coexisting with fluorite, calcite and barite. To conclude, the late stage of hydrothermal evolution corresponds to the major precipitation of REE minerals, and therefore most likely represents the main mineralization stage [70,71].
4.5. REE Enrichment Mechanism
4.5.1. Fractional Crystallization of Magma
In the primary magmatic carbonatite-related REE deposits, it is common that REEs, volatiles and alkali elements are enriched in the residual magma despite the fact that primary REE minerals can also incorporate a small amount of REEs during crystallization from the carbonatite magma. This explains the close association of REE mineralization with the late stage of magmatic evolution. In particular, fractional crystallization of carbonates is important for enriching REE in the residual melts for mineralization. The carbonate minerals (such as calcite and dolomite) that are crystallized in the early stage of magmatic evolution usually have low REE content because REEs are incompatible with carbonate minerals and are therefore enriched in the residual melts. In addition to carbonate minerals, it can happen that ore minerals such as magnetite and hematite crystallize prior to the REE minerals during the fractional crystallization process. The Miaoya REE deposit in Hubei Province is a good example. Two REE mineralization events occurred during the formation of the deposit at about 420 Ma (in situ Th-Pb age of zircon from carbonatites) and 240 Ma (in situ Th-Pb age of monazite from carbonatites), respectively [28]. Zhang et al. (2019) applied monazite U-Th-Pb dating to constrain the age of the Miaoya syenite–carbonatite complexes, which yields two groups of U-Pb ages at 414 ± 11Ma (n = 5; MSWD = 0.91) and 231 ± 2Ma (n = 21; MSWD = 3.1), respectively, in addition to an age of 206 ± 4 Ma for the REE mineral bastnäsite [68]. The age of 414 Ma was obtained from homogeneous monazite grains, which is consistent with the zircon U-Pb ages of the syenite from the Miaoya syenite–carbonatite complexes (445.2 ± 2.6 Ma, MSWD = 0.66) and the carbonatite (434.3 ± 3.2 Ma, MSWD = 1.08) previously dated by Zhu et al. (2016) [33]. The two episodes of REE mineralization, in combination with field observation, are illustrated as follows. The first stage of REE mineralization is represented by the crystallization of REE minerals and phosphates (mainly monazite and fluorapatite) from early carbonatite magma, which is high in P and REE contents. The residual magma became depleted in REEs. The second stage of mineralization is characterized by fractional crystallization of calcite that endowed the residual magma with REEs (especially LREE), from which sufficient bastnäsite and a small amount of monazite were crystallized [43]. Thus, the fractional crystallization of carbonatite magma plays an important role in upgrading the REE mineralization to giant and super giant scale.
4.5.2. Immiscibility of Melts and Hydrothermal Fluids
In the carbonatite-related hydrothermal REE deposits, REEs are introduced into the carbonate fluids through immiscibility of melts and hydrothermal fluids. The REE-rich carbonate fluids migrate along fractures and precipitate under the given ambient condition, forming veins- and net veins-orebodies that are composed of REE minerals and accessory minerals such as fluorite, barite and calcite [23]. The Maoniuping REE deposit is a typical hydrothermal deposit, in which the REEs are mostly introduced and enriched in the hydrothermal evolution stage. Field observation and study of fluid inclusions in fluorite from the orebodies reveals that the evolutionary sequence of the magmatic–hydrothermal system in the Maoniuping deposit encloses three stages, namely an early magmatic stage, an intermediate high-temperature hydrothermal stage, and a late low-temperature hydrothermal stage. The carbonatite magma was initially formed by low-degree partial melting of the mantle; the immiscibility of silicate melt and carbonate melt triggered the formation of the initial ore-forming fluids. The initial ore-forming fluids, which are high in temperature, pressure and density and rich in CO2, H2O, H2S, sulfates, were able to carry a large amount of REE and migrate them in gas phase. Ultimately, corresponding to the phase separation of CO2 and aqueous fluids the temperature and pressure of the ore-forming fluids dropped, which resulted in REE precipitation and mineralization [70,71,117,119,130]. The enrichment, migration and precipitation of REEs mainly occur in the CO2-rich fluids and at medium-high temperature, although a prerequisite for securing sufficient REEs in the early stage of fluid evolution is the liquid immiscibility between carbonatite melts and hydrothermal fluids.
5. Conclusions and Remarks
Carbonatite-related REE deposit, which provides the majority of LREE and Nb resources in the world, is a significant global deposit type. There are still many controversies about the ore genesis and enrichment mechanisms of REEs in this type of deposits. At present, we believe that the formation of carbonatites is closely related to the mantle, and there are three main mechanisms of forming REE-enriched carbonatites: (1) immiscibility of primary carbonate–silicate magma under crustal or mantle pressure; (2) fractional crystallization of parental carbonate–silicate magma; (3) low-degree (generally < 1%) partial melting of the mantle which is rich in H2O and CO2. However, the former two mechanisms cannot explain the massive enrichment of REEs (especially LREE) as observed in the field. Therefore, we believe that the REE enrichment occurs after the formation of carbonatite magma, and most likely during the gradual evolution of the carbonatite magma by means of fractional crystallization of carbonate minerals and immiscibility of melt and hydrothermal fluids.
Considering the existing controversies, we propose the following directions for future work:
- (1)
- At present, there is still a lack of reliable evidence to constrain the genesis of carbonatite. Experimental work needs to be designed to verify the influence of immiscibility on rock formation and REE enrichment. Meanwhile, isotope analysis, especially the non-traditional stable isotopes (such as Fe, Mg, Ba) can be carried out to examine the source of magma and its relationship with the mantle.
- (2)
- In view of the unclear REE partitioning behaviors in a magmatic–hydrothermal system, experimental petrology work on this aspect will enhance the understanding of the distribution of REEs in different systems, such as between carbonatite melts rich in F, S, P and silicate melts, between carbonates (its symbiotic precipitates) and carbonatite melts, between carbonatite melts and fluid or gas phase. This development will provide a better understanding of REE enrichment mechanisms.
- (3)
- Research on carbonatite-related REE deposits should expand to both REE fertile and barren carbonatites. Although carbonatite is absent in some REE deposits, the formation of those deposits is still closely related to carbonatite magma (such as the Nolans Bore P-REE-Th deposit, Australia) [131,132,133]. By using the method of “comparative metallogeny”, a comparative study on the metallogenic characteristics of carbonatites under different or the same tectonic background should be strengthened. In combination with better knowledge of the REE partitioning behavior and the sequence of mineral formation in high temperature and pressure experiments, a critical understanding of the factors controlling the fertility of carbonatites will eventually be achieved.
Author Contributions
Conceptualization, Z.-Y.W.; resources, Z.-Y.W and H.-R.F; writing—original draft preparation, Z.-Y.W.; writing—review and editing, H.-R.F., L.Z., K.-F.Y. and H.-D.S.; funding acquisition, H.-R.F and K.-F.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by research grants from National Natural Science Foundation of China (Grant Nos. 41930430, 91962103), National Key R&D Program of China (grant 2017YFC0602302), the Key Research Program of the Innovation Academy for Earth Science, CAS (IGGCAS-201901) and the Zhongke Developing Science and Technology Co., Ltd. (2017H1973, ZK2018H003).
Acknowledgments
Thomas Ulrich is thanked for his constructive feedback that helped to improve an earlier draft of the manuscript. We are grateful to three anonymous reviewers for thoughtful suggestions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Loges, A.; Migdisov, A.A.; Wagner, T.; Williams-Jones, A.E.; Markl, G. An experimental study of the aqueous solubility and speciation of Y(III) fluoride at temperatures up to 250 °C. Geochim. Cosmochim. Acta 2013, 123, 403–415. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Yang, Y.; Walton, A.; Buchert, M. Recycling of rare earths: A critical review. J. Clean. Prod. 2013, 51, 1–22. [Google Scholar] [CrossRef]
- Jha, M.K.; Kumari, A.; Panda, R.; Rajesh Kumar, J.; Yoo, K.; Lee, J.Y. Review on hydrometallurgical recovery of rare earth metals. Hydrometallurgy 2016, 161, 77. [Google Scholar] [CrossRef]
- Jordens, A.; Cheng, Y.P.; Waters, K.E. A review of the beneficiation of rare earth element bearing minerals. Miner. Eng. 2013, 41, 97–114. [Google Scholar] [CrossRef]
- Chakhmouradian, A.R.; Wall, F. Rare Earth Elements: Minerals, Mines, Magnets (and More). Elements 2012, 8, 333–340. [Google Scholar] [CrossRef]
- Kumari, A.; Panda, R.; Jha, M.K.; Kumar, J.R.; Lee, J.Y. Process development to recover rare earth metals from monazite mineral: A review. Miner. Eng. 2015, 79, 102–115. [Google Scholar] [CrossRef]
- Zhang, S.J.; Zhang, L.W.; Zhang, Y.W.; Shang, L.; Li, J.B. Summarize on rare earth mineral resources and their distribution at home and abroad. Inorg. Chem. Ind. 2020, 52, 9–16, (In Chinese with English abstract). [Google Scholar]
- Li, Z.; Hu, J.Z. World rare earth resources survey and development utilization trend. Mod. Min. 2017, 33, 97–105, (In Chinese with English abstract). [Google Scholar]
- Li, F.Q.; Dai, T.; Wang, G.S. A review on recycling and reuse of rare earth metals. Conserv. Util. Min. Resour. 2019, 39, 84–89, (In Chinese with English abstract). [Google Scholar]
- Wang, D.H.; Zhao, Z.; Yu, Y.; Wang, C.H.; Dai, J.J.; Sun, Y.; Zhao, T.; Li, J.K.; Huang, F.; Chen, Z.Y.; et al. A review of the achievements in the survey and study of ion-absorption type REE deposits in China. Acta Geosci. Sin. 2017, 38, 317–325, (In Chinese with English abstract). [Google Scholar]
- Fu, T.Y.; Li, B.H.; Dong, X.Y.; Xu, L. Analysis on the distribution, classification and characteristics of rare earth deposits in China. J. Henan Sci. Technol. 2015, 14, 124–126, (In Chinese with English abstract). [Google Scholar]
- Simandl, G.J.; Paradis, S. Carbonatites: Related ore deposits, resources, footprint, and exploration methods. Appl. Earth Sci. 2018, 127, 123–152. [Google Scholar] [CrossRef]
- Sun, J.; Zhu, X.K.; Chen, Y.L.; Fang, N.; Li, S.Z. Is the Bayan Obo ore deposit a micrite mound? A comparison with the Sailinhudong micrite mound. Int. Geol. Rev. 2014, 56, 1720–1731. [Google Scholar] [CrossRef]
- Liu, Q.P.; Zhao, Y.Y.; Liu, C.H. REE resources potential in Greenland and the availability evaluation in favor of China. Geol. Bull. China 2019, 38, 1386–1395, (In Chinese with English abstract). [Google Scholar]
- Schonwandt, H.K.; Barnes, G.B.; Ulrich, T. Chapter 5—A Description of the World-Class Rare Earth Element Deposit, Tanbreez, South Greenland. In Rare Earths Industry: Technological, Economic, and Environmental Implications; Elsevier: Amsterdam, The Netherlands, 2016; pp. 73–85. [Google Scholar]
- Streckeisen, A. Classification and nomenclature of volcanic rocks, lamprophyres, carbonatites and melilitic rocks IUGS Subcommission on the Systematics of Igneous Rocks. Geol. Rundsch. 1980, 69, 194–207. [Google Scholar] [CrossRef]
- Gou, R.T.; Zeng, P.S.; Liu, S.W.; Ma, J.; Wang, J.J.; Dai, Y.J.; Wang, Z.Q. Distribution characteristics of carbonatites of the world and its metallogenic significance. Acta Geol. Sin. 2019, 93, 2348–2361, (In Chinese with English abstract). [Google Scholar]
- Chakhmouradian, A.R.; Cooper, M.A.; Medici, L.; Abdu, Y.A.; Shelukhina, Y.S. Anzaite-(Ce), a new rare-earth mineral and structure type from the Afrikanda silicocarbonatite, Kola Peninsula, Russia. Mineral. Mag. 2018, 79, 1231–1244. [Google Scholar] [CrossRef]
- Mattsson, H.B.; Balashova, A.; Almqvist, B.S.G.; Bosshard-Stadlin, S.A.; Weidendorfer, D. Magnetic mineralogy and rock magnetic properties of silicate and carbonatite rocks from Oldoinyo Lengai volcano (Tanzania). J. Afr. Earth Sci. 2018, 142, 193–206. [Google Scholar] [CrossRef]
- Yang, K.F.; Fan, H.R.; Pirajno, F.; Li, X.C. The Bayan Obo (China) giant REE accumulation conundrum elucidated by intense magmatic differentiation of carbonatite. Geology 2019, 47, 1198–1202. [Google Scholar] [CrossRef]
- Yang, Z.M.; Woolley, A. Carbonatites in China: A review. J. Asian Earth Sci. 2006, 27, 559–575. [Google Scholar] [CrossRef]
- Linnen, R.L.; Samson, I.M.; Williams-Jones, A.E.; Chakhmouradian, A.R. Geochemistry of the Rare-Earth Element, Nb, Ta, Hf, and Zr Deposits. In Treatise on Geochemistry; Elsevier: Amsterdam, The Netherlands, 2014; pp. 543–568. [Google Scholar]
- Song, W.L.; Xu, C.; Wang, L.J.; Wu, M.; Zeng, L.; Wang, L.Z.; Feng, M. Review of the metallogenesis of the endogenetic rare earth elements deposits related to carbonatite-alkaline complex. Acta Sci. Nat. Univ. Pekin. 2013, 49, 725–740, (In Chinese with English abstract). [Google Scholar]
- Weng, Z.H.; Jowitt, S.M.; Mudd, G.M.; Haque, N. A Detailed Assessment of Global Rare Earth Element Resources: Opportunities and Challenges. Econ. Geol. 2015, 110, 1925–1952. [Google Scholar] [CrossRef]
- Kanazawa, Y.; Kamitani, M. Rare earth minerals and resources in the world. J. Alloy. Compd. 2006, 408–412, 1339–1343. [Google Scholar] [CrossRef]
- Woolley, A.R.; Kjarsgaard, B.A. Carbonatite occurrences of the world: Map and database. Geol. Surv. Can. Open File 2008, 5796, 1–21. [Google Scholar]
- Liu, Y.; Hou, Z.Q. A synthesis of mineralization styles with an integrated genetic model of carbonatite-syenite-hosted REE deposits in the Cenozoic Mianning-Dechang REE metallogenic belt, the eastern Tibetan Plateau, southwestern China. J. Asian Earth Sci. 2017, 137, 35–79. [Google Scholar] [CrossRef]
- Ying, Y.C.; Chen, W.; Lu, J.; Jiang, S.Y.; Yang, Y.H. In situ U–Th–Pb ages of the Miaoya carbonatite complex in the South Qinling orogenic belt, central China. Lithos 2017, 290–291, 159–171. [Google Scholar] [CrossRef]
- Verplanck, P.L.; Mariano, A.N.; Mariano, A., Jr. Rare Earth Element Ore Geology of Carbonatites. In Rare Earth and Critical Elements in Ore Deposits; Verplanck, P.L., Hitzman, M.W., Eds.; Soc Economic Geologists, Inc.: Littleton, CO, USA, 2016; pp. 5–32. [Google Scholar]
- Xie, Y.L.; Hou, Z.Q.; Goldfarb, R.J.; Guo, X.; Wang, L. Rare Earth Element Deposits in China. In Rare Earth and Critical Elements in Ore Deposits; Verplanck, P.L., Hitzman, M.W., Eds.; Soc Economic Geologists, Inc.: Littleton, CO, USA, 2016; pp. 115–136. [Google Scholar]
- Lai, X.D.; Yang, X.Y. U-Pb Ages and Hf Isotope of Zircons from a Carbonatite Dyke in the Bayan Obo Fe-REE Deposit in Inner Mongolia: Its Geological Significance. Acta Geol. Sin. Engl. Ed. 2019, 93, 1783–1796. [Google Scholar] [CrossRef]
- Xie, Y.L.; Li, Y.X.; Cooke, D.; Kamenetsky, V.; Chang, Z.S.; Danyushevsky, L.; Dominy, S.; Ryan, C.; Laird, J. Geochemical characteristics of carbonatite fluids at the Maoniuping REE deposit, Western Sichuan, China. In Let’s Talk Ore Deposits. In Proceedings of the Eleventh Biennial SGA Meeting, Antofagasta, Chile, 26–29 September 2011; Volume 1, pp. 196–198. [Google Scholar]
- Zhu, J.; Wang, L.X.; Peng, S.G.; Peng, L.H.; Wu, C.X.; Qiu, X.F. U-Pb zircon age, geochemical and isotopic characteristics of the Miaoya syenite and carbonatite complex, central China. Geol. J. 2017, 52, 938–954. [Google Scholar] [CrossRef]
- Wang, C.; Liu, J.C.; Zhang, H.D.; Zhang, X.Z.; Zhang, D.M.; Xi, Z.; Wang, Z.X.; Wang, Z.J. Geochronology and mineralogy of the Weishan carbonatite in Shandong province, eastern China. Geosci. Front. 2019, 10, 769–785. [Google Scholar] [CrossRef]
- Xu, C.; Campbell, I.H.; Allen, C.M.; Chen, Y.J.; Huang, Z.L.; Qi, L.; Zhang, G.S.; Yan, Z.F. U-Pb zircon age, geochemical and isotopic characteristics of carbonatite and syenite complexes from the Shaxiongdong, China. Lithos 2008, 105, 118–128. [Google Scholar] [CrossRef]
- Liu, S.; Fan, H.R.; Groves, D.I.; Yang, K.F.; Yang, Z.F.; Wang, Q.W. Multiphase carbonatite-related magmatic and metasomatic processes in the genesis of the ore-hosting dolomite in the giant Bayan Obo REE-Nb-Fe deposit. Lithos 2020, 354–355, 105359. [Google Scholar] [CrossRef]
- Yang, X.Y.; Lai, X.Y.; Pirajno, F.; Liu, Y.L.; Ling, M.X.; Sun, W.D. Genesis of the Bayan Obo Fe-REE-Nb formation in Inner Mongolia, North China Craton: A perspective review. Precambrian Res. 2017, 288, 39–71. [Google Scholar] [CrossRef]
- Song, W.L.; Xu, C.; Smith, M.P.; Chakhmouradian, A.R.; Brenna, M.; Kynický, J.; Chen, W.; Yang, Y.H.; Deng, M.; Tang, H.Y. Genesis of the world’s largest rare earth element deposit, Bayan Obo, China: Protracted mineralization evolution over ~1 b.y. Geology 2018, 46, 323–326. [Google Scholar] [CrossRef]
- Hu, L.; Li, Y.K.; Wu, Z.J.; Bai, Y.; Wang, A.J. Two metasomatic events recorded in apatite from the ore-hosting dolomite marble and implications for genesis of the giant Bayan Obo REE deposit, Inner Mongolia, Northern China. J. Asian Earth Sci. 2019, 172, 56–65. [Google Scholar] [CrossRef]
- Fan, H.R.; Yang, K.F.; Hu, F.F.; Liu, S.; Wang, K.Y. The giant Bayan Obo REE-Nb-Fe deposit, China: Controversy and ore genesis. Geosci. Front. 2016, 7, 335–344. [Google Scholar] [CrossRef]
- Su, J.H.; Zhao, X.F.; Li, X.C.; Hu, W.; Chen, M.; Xiong, Y.L. Geological and geochemical characteristics of the Miaoya syenite-carbonatite complex, Central China: Implications for the origin of REE-Nb-enriched carbonatite. Ore Geol. Rev. 2019, 113, 103101. [Google Scholar] [CrossRef]
- Ying, Y.C.; Chen, W.; Simonetti, A.; Jiang, S.Y.; Zhao, K.D. Significance of hydrothermal reworking for REE mineralization associated with carbonatite: Constraints from in situ trace element and C-Sr isotope study of calcite and apatite from the Miaoya carbonatite complex (China). Geochim. Cosmochim. Acta 2020, 280, 340–359. [Google Scholar] [CrossRef]
- Xu, C.; Kynicky, J.; Chakhmouradian, A.R.; Campbell, I.H.; Allen, C.M. Trace-element modeling of the magmatic evolution of rare-earth-rich carbonatite from the Miaoya deposit, Central China. Lithos 2010, 118, 145–155. [Google Scholar] [CrossRef]
- Jia, Y.H.; Liu, Y. REE Enrichment during Magmatic-Hydrothermal Processes in Carbonatite-Related REE Deposits: A Case Study of the Weishan REE Deposit, China. Minerals 2019, 10, 25. [Google Scholar] [CrossRef]
- Hou, Z.Q.; Tian, S.H.; Xie, Y.L.; Yang, Z.; Yuan, Z.S.; Yin, S.P.; Yi, L.S.; Fei, H.C.; Zou, T.R.; Bai, G.; et al. The Himalayan Mianning-Dechang REE belt associated with carbonatite-alkaline complexes, eastern Indo-Asian collision zone, SW China. Ore Geol. Rev. 2009, 36, 65–89. [Google Scholar] [CrossRef]
- Woolley, A.R. A discussion of carbonatite evolution and nomenclature, and the generation of sodic and potassic fenites. Mineral. Mag. 1982, 46, 13–17. [Google Scholar] [CrossRef]
- Liu, S.; Fan, H.R.; Yang, K.F.; Hu, F.F.; Rusk, B.; Liu, X.; Li, X.C.; Yang, Z.F.; Wang, Q.W.; Wang, K.Y. Fenitization in the giant Bayan Obo REE-Nb-Fe deposit: Implication for REE mineralization. Ore Geol. Rev. 2018, 94, 290–309. [Google Scholar] [CrossRef]
- Migdisov, A.A.; Williams-Jones, A.E. Hydrothermal transport and deposition of the rare earth elements by fluorine-bearing aqueous liquids. Miner. Depos. 2014, 49, 987–997. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.Y.; Yang, Z.S.; Sun, X.; Zhu, Z.M.; Zhang, Q.C. Mineralogical and geochemical studies of brecciated ores in the Dalucao REE deposit, Sichuan Province, southwestern China. Ore Geol. Rev. 2015, 70, 613–636. [Google Scholar] [CrossRef]
- Williams-Jones, A.E.; Migdisov, A.A.; Samson, I.M. Hydrothermal Mobilisation of the Rare Earth Elements—A Tale of “Ceria” and “Yttria”. Elements 2012, 8, 355–360. [Google Scholar] [CrossRef]
- Cooper, A.F.; Palin, J.M.; Collins, A.K. Fenitization of metabasic rocks by ferrocarbonatites at Haast River, New Zealand. Lithos 2016, 244, 109–121. [Google Scholar] [CrossRef]
- Currie, K.L.; Ferguson, J. A Study of Fenitization Around the Alkaline Carbonatite Complex at Callander Bay, Ontario, Canada. Can. J. Earth Sci. 1971, 8, 498–517. [Google Scholar] [CrossRef]
- Currie, K.L.; Ferguson, J. A Study of Fenitization in Mafic Rocks, with Special Reference to the Callander Bay Complex. Can. J. Earth Sci. 1972, 9, 1254–1261. [Google Scholar] [CrossRef]
- McKie, D. Fenitization. In Tuttle of Gittins J. (eds.) & Carbonatites; Interscience: New York, NY, USA, 1966; pp. 261–294. [Google Scholar]
- Le Bas, M.J. Fenites Associated with Carbonatites. Can. Mineral. 2008, 46, 915–932. [Google Scholar] [CrossRef]
- Yang, X.M.; Yang, X.Y.; Fan, H.R.; Guo, F.; Zhang, Z.F.; Zheng, Y.F. Petrological characteristics of fenites and their geological significance. Geol. Rev. 2000, 46, 481–490, (In Chinese with English abstract). [Google Scholar]
- Potter, N.J.; Kamenetsky, V.S.; Simonetti, A.; Goemann, K. Different types of liquid immiscibility in carbonatite magma: A case study of the Oldoinyo Lengai 1993 lava and melt inclusions. Chem. Geol. 2017, 455, 376–384. [Google Scholar] [CrossRef]
- Mitchell, R.H. Peralkaline nephelinite-natrocarbonatite immiscibility and carbonatite assimilation at Oldoinyo Lengai, Tanzania. Contrib. Mineral. Petrol. 2009, 158, 589–598. [Google Scholar] [CrossRef]
- Kresten, P.; Morogan, V. Fenitization at the Fen complex, southern Norway. Lithos 1986, 19, 27–42. [Google Scholar] [CrossRef]
- Kresten, P. The chemistry of fenitization: Examples from Fen, Norway. Chem. Geol. 1988, 68, 329–349. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, X.Q.; Nie, L.S.; Jennifer, M.M.; Liu, H.L.; Zhang, B.M.; Han, Z.X. Geochemical background and dispersion pattern of the world’s largest REE deposit of Bayan Obo, China. J. Geochem. Explor. 2020, 215, 106545. [Google Scholar] [CrossRef]
- Zhang, S.H.; Zhao, Y.; Liu, Y. A precise zircon Th-Pb age of carbonatite sills from the world’s largest Bayan Obo deposit: Implications for timing and genesis of REE-Nb mineralization. Precambrian Res. 2017, 291, 202–219. [Google Scholar] [CrossRef]
- Smith, M.P.; Campbell, L.S.; Kynicky, J. A review of the genesis of the world class Bayan Obo Fe-REE-Nb deposits, Inner Mongolia, China: Multistage processes and outstanding questions. Ore Geol. Rev. 2015, 64, 459–476. [Google Scholar] [CrossRef]
- Chen, W.; Liu, H.Y.; Lu, J.; Jiang, S.Y.; Simonetti, A.; Xu, C.; Zhang, W. The formation of the ore-bearing dolomite marble from the giant Bayan Obo REE-Nb-Fe deposit, Inner Mongolia: Insights from micron-scale geochemical data. Miner. Depos. 2019, 55, 131–146. [Google Scholar] [CrossRef]
- Yang, K.F.; Fan, H.R.; Santosh, M.; Hu, F.F.; Wang, K.Y. Mesoproterozoic carbonatitic magmatism in the Bayan Obo deposit, Inner Mongolia, North China: Constraints for the mechanism of super accumulation of rare earth elements. Ore Geol. Rev. 2011, 40, 122–131. [Google Scholar] [CrossRef]
- Deng, M.; Xu, C.; Song, W.L.; Tang, H.Y.; Liu, Y.; Zhang, Q.; Zhou, Y.; Feng, M.; Wei, C.W. REE mineralization in the Bayan Obo deposit, China: Evidence from mineral paragenesis. Ore Geol. Rev. 2017, 91, 100–109. [Google Scholar] [CrossRef]
- Sun, J.; Zhu, X.K.; Chen, Y.L.; Fang, N. Iron isotopic constraints on the genesis of Bayan Obo ore deposit, Inner Mongolia, China. Precambrian Res. 2013, 235, 88–106. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, W.T.; Gao, J.F.; Chen, H.K.; Li, J.H. Two episodes of REE mineralization in the Qinling Orogenic Belt, Central China: In-situ U-Th-Pb dating of bastnäsite and monazite. Miner. Depos. 2019, 54, 1265–1280. [Google Scholar] [CrossRef]
- Li, S. Geochemical features and petrogenesis of Miaoya carbonatites, Hubei. Geochimica 1980, 1, 345–355, (In Chinese with English abstract). [Google Scholar]
- Zheng, X.; Liu, Y. Mechanisms of element precipitation in carbonatite-related rare-earth element deposits: Evidence from fluid inclusions in the Maoniuping deposit, Sichuan Province, southwestern China. Ore Geol. Rev. 2019, 107, 218–238. [Google Scholar] [CrossRef]
- Liu, Y.; Chakhmouradian, A.R.; Hou, Z.Q.; Song, W.L.; Kynický, J. Development of REE mineralization in the giant Maoniuping deposit (Sichuan, China): Insights from mineralogy, fluid inclusions, and trace-element geochemistry. Miner. Depos. 2018, 54, 701–718. [Google Scholar] [CrossRef]
- Li, J.K.; Yuan, Z.X.; Bai, G.; Chen, Y.C.; Wang, D.H.; Ying, L.J.; Zhang, J. Ore-forming fluid evolvement and its controlling to REE(Ag) mineralizing in the Weishan deposit, Shandong. Jmineral Pet. 2009, 29, 60–68, (In Chinese with English abstract). [Google Scholar]
- Castor, S.B. The Mountain Pass Rare-Earth Carbonatite and Associated Ultrapotassic Rocks, California. Can. Mineral. 2008, 46, 779–806. [Google Scholar] [CrossRef]
- Mariano, A.N.; Mariano, A. Rare Earth Mining and Exploration in North America. Elements 2012, 8, 369–376. [Google Scholar] [CrossRef]
- Denton, K.M.; Ponce, D.A.; Peacock, J.R.; Miller, D.M. Geophysical characterization of a Proterozoic REE terrane at Mountain Pass, eastern Mojave Desert, California, USA. Geosphere 2019, 16, 456–471. [Google Scholar] [CrossRef]
- Jones, A.P.; Wyllie, P.J. Low-temperature glass quenched from a synthetic, rare earth carbonatite; implications for the origin of the Mountain Pass Deposit, California. Econ. Geol. 1983, 78, 1721–1723. [Google Scholar] [CrossRef]
- Poletti, J.E.; Cottle, J.M.; Hagen-Peter, G.A.; Lackey, J.S. Petrochronological Constraints on the Origin of the Mountain Pass Ultrapotassic and Carbonatite Intrusive Suite, California. J. Petrol. 2016, 57, 1555–1598. [Google Scholar] [CrossRef]
- Nascimento, M.; Lemos, F.; Guimarães, R.; Sousa, C.; Soares, P. Modeling of REE and Fe Extraction from a Concentrate from Araxá (Brazil). Minerals 2019, 9, 451. [Google Scholar] [CrossRef]
- Li, Y.K.; Chen, R.Y.; Ke, C.H.; Chen, J.; Hao, M.Z.; Li, R.P. The strategic and critical minerals associated with alkaline and alkaline-carbonatite complexes Brazil. Acta Geol. Sin. 2019, 93, 1422–1443, (In Chinese with English abstract). [Google Scholar]
- Neumann, R.; Medeiros, E.B. Comprehensive mineralogical and technological characterisation of the Araxá (SE Brazil) complex REE (Nb-P) ore, and the fate of its processing. Int. J. Miner. Process. 2015, 144, 1–10. [Google Scholar] [CrossRef]
- Buyse, F.; Dewaele, S.; Decrée, S.; Mees, F. Mineralogical and geochemical study of the rare earth element mineralization at Gakara (Burundi). Ore Geol. Rev. 2020, 124, 103659. [Google Scholar] [CrossRef]
- Ntiharirizwa, S.; Boulvais, P.; Poujol, M.; Branquet, Y.; Morelli, C.; Ntungwanayo, J.; Midende, G. Geology and U-Th-Pb Dating of the Gakara REE Deposit, Burundi. Minerals 2018, 8, 394. [Google Scholar] [CrossRef]
- Lehmann, B.; Nakai, S.i.; Höhndorf, A.; Brinckmann, J.; Dulski, P.; Hein, U.F.; Masuda, A. REE mineralization at Gakara, Burundi: Evidence for anomalous upper mantle in the western Rift Valley. Geochim. Cosmochim. Acta 1994, 58, 985–992. [Google Scholar] [CrossRef]
- Secher, K.; Larsen, L.M. Geology and mineralogy of the Sarfartôq carbonatite complex, southern West Greenland. Lithos 1980, 13, 199–212. [Google Scholar] [CrossRef]
- Goodenough, K.M.; Schilling, J.; Jonsson, E.; Kalvig, P.; Charles, N.; Tuduri, J.; Deady, E.A.; Sadeghi, M.; Schiellerup, H.; Müller, A.; et al. Europe’s rare earth element resource potential: An overview of REE metallogenetic provinces and their geodynamic setting. Ore Geol. Rev. 2016, 72, 838–856. [Google Scholar] [CrossRef]
- Bedini, E.; Rasmussen, T.M. Use of airborne hyperspectral and gamma-ray spectroscopy data for mineral exploration at the Sarfartoq carbonatite complex, southern West Greenland. Geosci. J. 2018, 22, 641–651. [Google Scholar] [CrossRef]
- Ye, H.M.; Zhang, X. Advances on the carbonatite research in recent years. Resour. Surv. Environ. 2015, 36, 21–27, (In Chinese with English abstract). [Google Scholar]
- Wallace, M.E.; Green, D.H. An experimental determination of primary carbonatite magma composition. Nature 1988, 335, 343–346. [Google Scholar] [CrossRef]
- Wyllie, P.J.; Lee, W.J. Model System Controls on Conditions for Formation of Magnesiocarbonatite and Calciocarbonatite Magma from the Mantle. J. Petrol. 1998, 39, 1885–1893. [Google Scholar] [CrossRef]
- Hamilton, D.L.; Freestone, I.C.; Dawson, J.B.; Donaldson, C.H. Origin of carbonatites by liquid immiscibility. Nature 1979, 279, 52–54. [Google Scholar] [CrossRef]
- Harmer, R. The Case for Primary, Mantle-derived Carbonatite Magma. J. Petrol. 1998, 39, 1895–1903. [Google Scholar] [CrossRef]
- Lee, W.J.; Wyllie, P.J. Processes of Crustal Carbonatite Formation by Liquid Immiscibility and Differentiation, Elucidated by Model Systems. J. Petrol. 1998, 39, 2005–2013. [Google Scholar] [CrossRef]
- Veksler, I.; Lentz, D. Parental magma of plutonic carbonatites, carbonate-silicate immiscibility and decarbonation reactions: Evidence from melt and fluid inclusions. Melt Incl. Plutonic Rocks 2006, 36, 123–150. [Google Scholar]
- Manthilake, M.A.G.M.; Sawada, Y.; Sakai, S. Genesis and evolution of Eppawala carbonatites, Sri Lanka. J. Asian Earth Sci. 2008, 32, 66–75. [Google Scholar] [CrossRef]
- Hulett, S.R.W.; Simonetti, A.; Rasbury, E.T.; Hemming, N.G. Recycling of subducted crustal components into carbonatite melts revealed by boron isotopes. Nat. Geosci. 2016, 9, 904–908. [Google Scholar] [CrossRef]
- Nelson, D.R.; Chivas, A.R.; Chappell, B.W.; McCulloch, M.T. Geochemical and isotopic systematics in carbonatites and implications for the evolution of ocean-island sources. Geochim. Cosmochim. Acta 1988, 52, 1–17. [Google Scholar] [CrossRef]
- Zhu, X.K.; Sun, J.; Pan, C.X. Sm-Nd isotopic constraints on rare-earth mineralization in the Bayan Obo ore deposit, Inner Mongolia, China. Ore Geol. Rev. 2015, 64, 543–553. [Google Scholar] [CrossRef]
- Ionov, D. Trace Element Composition of Mantle-derived Carbonates and Coexisting Phasesin Peridotite Xenoliths from Alkali Basalts. J. Petrol. 1998, 39, 1931–1941. [Google Scholar] [CrossRef]
- Veksler, I.V.; Petibon, C.; Jenner, G.A.; Dorfman, A.M.; Dingwell, D.B. Trace Element Partitioning in Immiscible Silicate-Carbonate Liquid Systems: An Initial Experimental Study Using a Centrifuge Autoclave. J. Petrol. 1998, 39, 2095–2104. [Google Scholar] [CrossRef]
- Veksler, I.V.; Dorfman, A.M.; Dulski, P.; Kamenetsky, V.S.; Danyushevsky, L.V.; Jeffries, T.; Dingwell, D.B. Partitioning of elements between silicate melt and immiscible fluoride, chloride, carbonate, phosphate and sulfate melts, with implications to the origin of natrocarbonatite. Geochim. Cosmochim. Acta 2012, 79, 20–40. [Google Scholar] [CrossRef]
- Foley, S.F.; Yaxley, G.M.; Rosenthal, A.; Buhre, S.; Kiseeva, E.S.; Rapp, R.P.; Jacob, D.E. The composition of near-solidus melts of peridotite in the presence of CO2 and H2O between 40 and 60 kbar. Lithos 2009, 112, 274–283. [Google Scholar] [CrossRef]
- Ionov, D.; Harmer, R.E. Trace element distribution in calcite-dolomite carbonatites from Spitskop: Inferences for differentiation of carbonatite magma and the origin of carbonates in mantle xenoliths. Earth Planet. Sci. Lett. 2002, 198, 495–510. [Google Scholar] [CrossRef]
- Banerjee, A.; Chakrabarti, R. A geochemical and Nd, Sr and stable Ca isotopic study of carbonatites and associated silicate rocks from the ~65 Ma old Ambadongar carbonatite complex and the Phenai Mata igneous complex, Gujarat, India: Implications for crustal contamination, carbonate recycling, hydrothermal alteration and source-mantle mineralogy. Lithos 2019, 326–327, 572–585. [Google Scholar]
- Hou, Z.Q.; Tian, S.H.; Yuan, Z.X.; Xie, Y.L.; Yin, S.P.; Yi, L.S.; Fei, H.C.; Yang, Z.M. The Himalayan collision zone carbonatites in western Sichuan, SW China: Petrogenesis, mantle source and tectonic implication. Earth Planet. Sci. Lett. 2006, 244, 234–250. [Google Scholar] [CrossRef]
- Kato, Y.; Fujinaga, K.; Nakamura, K.; Takaya, Y.; Kitamura, K.; Ohta, J.; Toda, R.; Nakashima, T.; Iwamori, H. Deep-sea mud in the Pacific Ocean as a potential resource for rare-earth elements. Nat. Geosci. 2011, 4, 535–539. [Google Scholar] [CrossRef]
- Mimura, K.; Nakamura, K.; Yasukawa, K.; Machida, S.; Ohta, J.; Fujinaga, K.; Kato, Y. Significant impacts of pelagic clay on average chemical composition of subducting sediments: New insights from discovery of extremely rare-earth elements and yttrium-rich mud at Ocean Drilling Program Site 1149 in the western North Pacific Ocean. J. Asian Earth Sci. 2019, 186, 104059. [Google Scholar] [CrossRef]
- Yasukawa, K.; Liu, H.; Fujinaga, K.; Machida, S.; Haraguchi, S.; Ishii, T.; Nakamura, K.; Kato, Y. Geochemistry and mineralogy of REY-rich mud in the eastern Indian Ocean. J. Asian Earth Sci. 2014, 93, 25–36. [Google Scholar] [CrossRef]
- Ling, M.X.; Liu, Y.L.; Williams, I.S.; Teng, F.Z.; Yang, X.Y.; Ding, X.; Wei, G.J.; Xie, L.H.; Deng, W.F.; Sun, W.D. Formation of the world’s largest REE deposit through protracted fluxing of carbonatite by subduction-derived fluids. Sci. Rep. 2013, 3, 1776. [Google Scholar] [CrossRef]
- Ni, P.; Zhou, J.; Chi, Z.; Pan, J.-Y.; Li, S.-N.; Ding, J.-Y.; Han, L. Carbonatite dyke and related REE mineralization in the Bayan Obo REE ore field, North China: Evidence from geochemistry, C O isotopes and Rb Sr dating. J. Geochem. Explor. 2020, 215, 106560. [Google Scholar] [CrossRef]
- Wu, Y.B.; Zheng, Y.F. Tectonic evolution of a composite collision orogen: An overview on the Qinling-Tongbai-Hong’an-Dabie-Sulu orogenic belt in central China. Gondwana Res. 2013, 23, 1402–1428. [Google Scholar] [CrossRef]
- Xu, C.; Kynicky, J.; Chakhmouradian, A.R.; Li, X.H.; Song, W.L. A case example of the importance of multi-analytical approach in deciphering carbonatite petrogenesis in South Qinling orogen: Miaoya rare-metal deposit, central China. Lithos 2015, 227, 107–121. [Google Scholar] [CrossRef]
- Fan, H.R.; Xie, Y.H.; Wang, K.Y.; Yang, X.M. Carbonatitic Fluids and REE Mineralization. Earth Science Frontiers. 2001, 8, 289–295, (In Chinese with English abstract). [Google Scholar]
- Zhou, J.; Ni, P.; Ding, J.Y.; Zhu, X.T. Fluid inclusion study related to cabonatitic magmatic process. Geol. J. China Univ. 2003, 9, 293–301, (In Chinese with English abstract). [Google Scholar]
- Xie, Y.L.; Xu, J.H.; Chen, W.; He, J.P.; Hou, Z.Q.; Xu, W.Y. Characteristics of carbonatite fluid in the Maoniuping REE deposit, Mianning, China. In Mineral Deposit Research: Meeting the Global Challenge; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Xie, Y.L.; Yin, S.P.; Xu, J.H.; Chen, W.; Yi, L.S. A Study on the fluids in carbonatite of the Mianning-Dechang REE metallogenic belt. Bull. Mineral. Petrol. Geochem. 2006, 25, 66–74, (In Chinese with English abstract). [Google Scholar]
- Shu, X.C.; Liu, Y. Fluid inclusion constraints on the hydrothermal evolution of the Dalucao Carbonatite-related REE deposit, Sichuan Province, China. Ore Geol. Rev. 2019, 107, 41–57. [Google Scholar] [CrossRef]
- Xie, Y.L.; Hou, Z.Q.; Yin, S.P.; Dominy, S.C.; Xu, J.H.; Tian, S.H.; Xu, W.Y. Continuous carbonatitic melt–fluid evolution of a REE mineralization system: Evidence from inclusions in the Maoniuping REE Deposit, Western Sichuan, China. Ore Geol. Rev. 2009, 36, 90–105. [Google Scholar] [CrossRef]
- Ting, W.P.; Burke, E.A.J.; Rankin, A.H.; Woolley, A.R. Characterisation and petrogenetic significance of CO2, H2O and CH4 fluid inclusions in apatite from the Sukulu carbonatite, Uganda. Eur. J. Mineral. 1994, 6, 787–803. [Google Scholar] [CrossRef]
- Kong, L.R.; Zhang, G.R. Ore Types and Characteristics of Melt (Fluid) Inclusions on the Maoniuping REE Deposit Mianning, Sichuan, China. Appl. Mech. Mater. 2014, 675–677, 1308–1311. [Google Scholar] [CrossRef]
- She, H.D.; Fan, H.R.; Hu, F.F.; Yang, K.F.; Yang, Z.F.; Wang, Q.W. Migration and precipitation of rare earth elements in the hydrothermal fluids. Acta Petrol. Sin. 2018, 34, 3567–3581, (In Chinese with English abstract). [Google Scholar]
- Migdisov, A.; Williams-Jones, A.E.; Brugger, J.; Caporuscio, F.A. Hydrothermal transport, deposition, and fractionation of the REE: Experimental data and thermodynamic calculations. Chem. Geol. 2016, 439, 13–42. [Google Scholar] [CrossRef]
- Migdisov, A.A.; Williams-Jones, A.E. An experimental study of the solubility and speciation of neodymium (III) fluoride in F-bearing aqueous solutions. Geochim. Cosmochim. Acta 2007, 71, 3056–3069. [Google Scholar] [CrossRef]
- Migdisov, A.A.; Williams-Jones, A.E. A spectrophotometric study of Nd(III), Sm(III) and Er(III) complexation in sulfate-bearing solutions at elevated temperatures. Geochim. Cosmochim. Acta 2008, 72, 5291–5303. [Google Scholar] [CrossRef]
- Migdisov, A.A.; Williams-Jones, A.E.; Wagner, T. An experimental study of the solubility and speciation of the Rare Earth Elements (III) in fluoride- and chloride-bearing aqueous solutions at temperatures up to 300°C. Geochim. Cosmochim. Acta 2009, 73, 7087–7109. [Google Scholar] [CrossRef]
- Debruyne, D.; Hulsbosch, N.; Muchez, P. Unraveling rare earth element signatures in hydrothermal carbonate minerals using a source–sink system. Ore Geol. Rev. 2016, 72, 232–252. [Google Scholar] [CrossRef]
- Louvel, M.; Bordage, A.; Testemale, D.; Zhou, L.; Mavrogenes, J. Hydrothermal controls on the genesis of REE deposits: Insights from an in situ XAS study of Yb solubility and speciation in high temperature fluids (T < 400 °C). Chem. Geol. 2015, 417, 228–237. [Google Scholar]
- Liu, Y.; Chen, C.; Shu, X.C.; Guo, D.X.; Li, Z.J.; Zhao, H.X.; Jia, Y.H. The formation model of the carbonatite-syenite complex REE deposits in the east of Tibetan Plateau: A case study of Dalucao REE deposit. Acta Petrol. Sin. 2017, 33, 1978–2000, (In Chinese with English abstract). [Google Scholar]
- Verplanck, P.L. The Role of Fluids in the Formation of Rare Earth Element Deposits. Procedia Earth Planet. Sci. 2017, 17, 758–761. [Google Scholar] [CrossRef]
- Chikanda, F.; Otake, T.; Ohtomo, Y.; Ito, A.; Yokoyama, T.D.; Sato, T. Magmatic-Hydrothermal Processes Associated with Rare Earth Element Enrichment in the Kangankunde Carbonatite Complex, Malawi. Minerals 2019, 9, 442. [Google Scholar] [CrossRef]
- Qin, C.J.; Qiu, Y.Z.; Wen, H.J.; Xu, C. Genesis of Maoniuping REE deposit, Sichuan: Evidence from inclusions. Acta Petrol. Sin. 2008, 24, 2155–2162, (In Chinese with English abstract). [Google Scholar]
- Anenburg, M.; Burnham, A.D.; Mavrogenes, J.A. REE Redistribution Textures in Altered Fluorapatite: Symplectites, Veins, and Phosphate-Silicate-Carbonate Assemblages from the Nolans Bore P-REE-Th Deposit, Northern Territory, Australia. Can. Mineral. 2018, 56, 331–354. [Google Scholar] [CrossRef]
- Anenburg, M.; Mavrogenes, J.A.; Bennett, V.C. The Fluorapatite P-REE-Th Vein Deposit at Nolans Bore: Genesis by Carbonatite Metasomatism. J. Petrol. 2020, 61, egaa003. [Google Scholar] [CrossRef]
- Anenburg, M.; Mavrogenes, J.A. Carbonatitic versus hydrothermal origin for fluorapatite REE-Th deposits: Experimental study of REE transport and crustal “antiskarn” metasomatism. Am. J. Sci. 2018, 318, 335–366. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).