Crystal Chemistry and Structural Variations for Zircon Samples from Various Localities
Abstract
1. Introduction
2. Experimental Methods
2.1. Sample Description
2.2. Electron-Probe Microanalysis (EPMA)
2.3. Calculation of α-Radiation Dose
2.4. Single-Crystal X-Ray Diffraction (SCXRD) Data Collection and Structure Refinement for Zircon
2.5. Synchrotron High-Resolution Powder X-ray Diffraction (HRPXRD)
2.6. Rietveld Structure Refinement
3. Results
3.1. Chemical Composition of Zircon
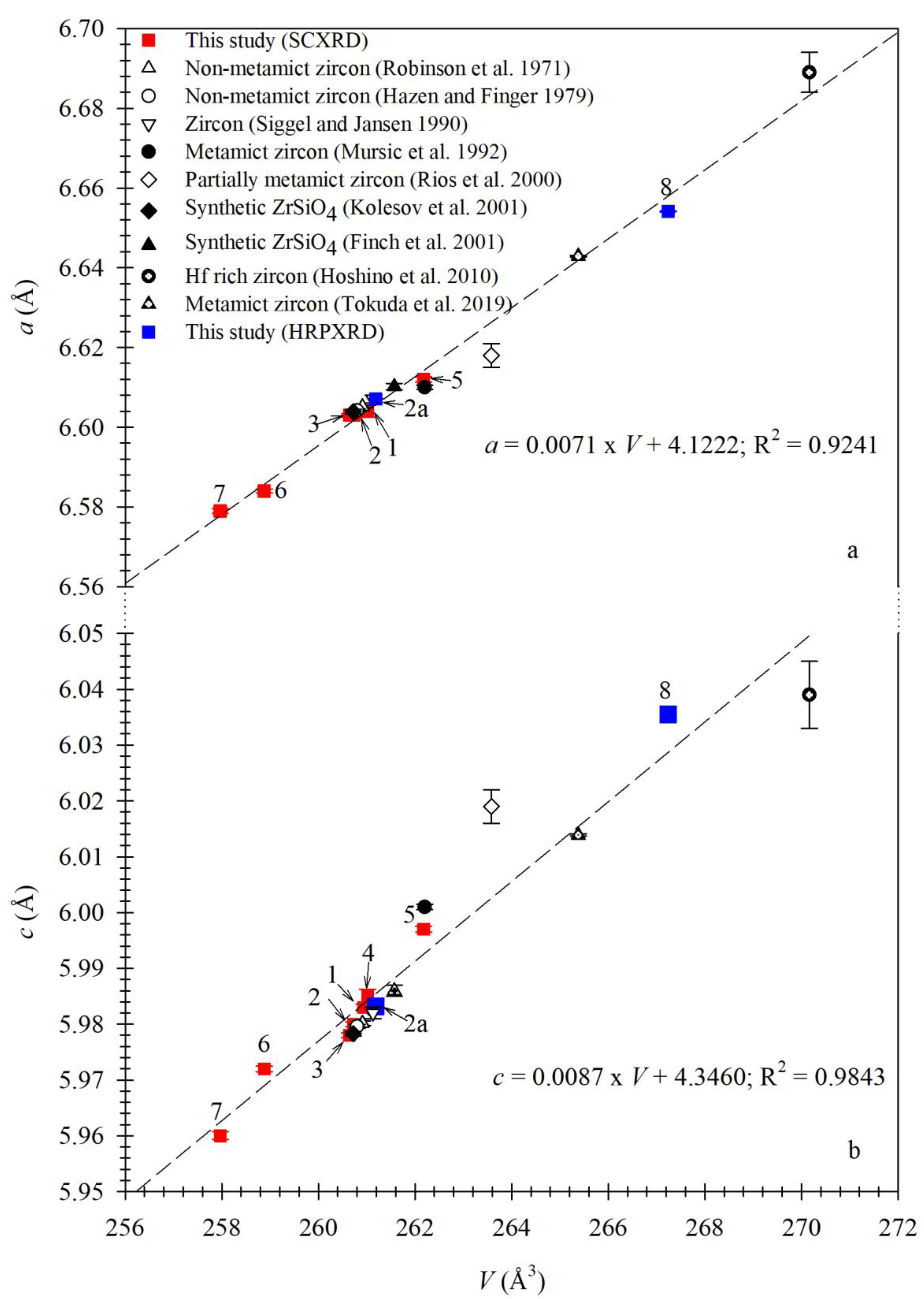
3.2. Variations of Unit-Cell Parameters for Zircon
3.3. Relation between Unit-Cell Parameters and Chemical Composition
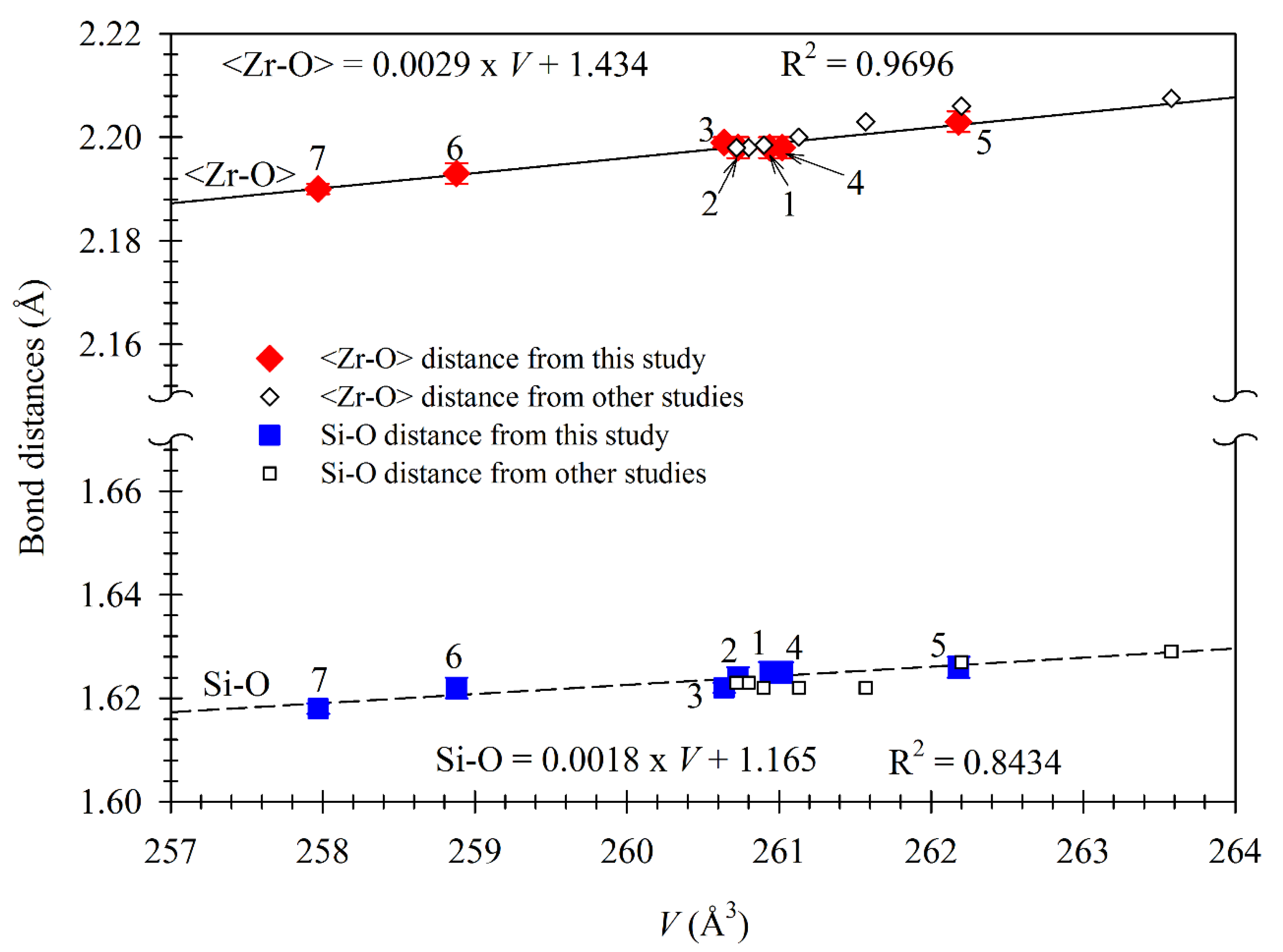
3.4. Relation between Bond Distances and Chemical Composition
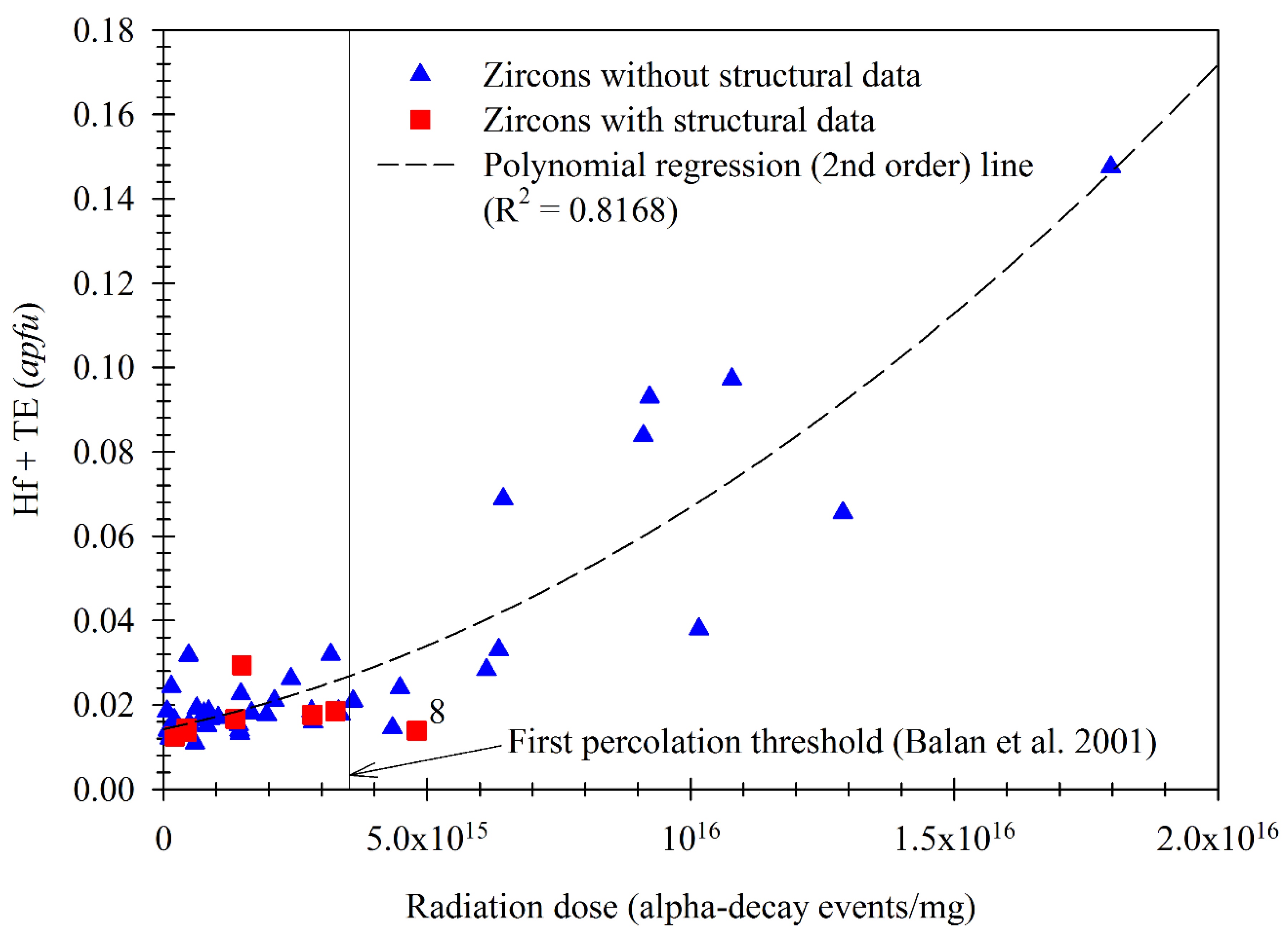
3.5. Effect of α-Radiation Doses in Zircon
3.6. Unit-Cell Volume and Geological Age
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vegard, L. VI Results of crystal analysis. Philos. Mag. 1916, 32, 65–96. [Google Scholar] [CrossRef][Green Version]
- Hassel, O. Die Kristallstruktur einiger Verbindungen von der Zusammensetzung MRO4-I. Zirkon ZrSiO4. Z. Krist. 1926, 63, 247–254. [Google Scholar]
- Krstanovic, L.R. Redetermination of the oxygen parameters in zircon (ZrSiO4). Acta Crystallogr. 1958, 11, 896. [Google Scholar] [CrossRef]
- Wyckoff, R.W.G.; Hendricks, S.B. Die kristallstruktur von zirkon und die kriterien fur spezielle lagen in tetragonalen raurngruppen. Z. Krist. 1928, 66, 73–102. [Google Scholar]
- Finch, R.J.; Hanchar, J.M.; Hoskin, P.W.O.; Burns, P.C. Rare-earth elements in synthetic zircon: Part 2. A single-crystal X-ray study of xenotime substitution. Am. Mineral. 2001, 86, 681–689. [Google Scholar]
- Hazen, R.M.; Finger, L.W. Crystal structure and compressibility of zircon at high pressure. Am. Mineral. 1979, 64, 196–201. [Google Scholar]
- Kolesov, B.A.; Geiger, C.A.; Armbruster, T. The dynamic properties of zircon studied by single-crystal X-ray diffraction and Raman spectroscopy. Eur. J. Mineral. 2001, 13, 939–948. [Google Scholar] [CrossRef]
- Mursic, Z.; Vogt, T.; Boysen, H.; Frey, F. Single-crystal neutron diffraction study of metamict zircon up to 2000 K. J. Appl. Crystallogr. 1992, 25, 519–523. [Google Scholar] [CrossRef]
- Robinson, K.; Gibbs, G.V.; Ribbe, P.H. The structure of zircon: A comparison with garnet. Am. Mineral. 1971, 56, 782–790. [Google Scholar]
- Nyman, H.; Hyde, B.G.; Andersson, S. Zircon, anhydrite, scheelite and some related structures containing bisdisphenoids. Acta Crystallogr. 1984, B40, 441–447. [Google Scholar] [CrossRef]
- Ríos, S.; Salje, E.K.H.; Zhang, M.; Ewing, R.C. Amorphization in zircon: Evidence for direct impact damage. J. Phys. Condens. Matter 2000, 12, 2401–2412. [Google Scholar] [CrossRef]
- Finch, R.J.; Hanchar, J.M. Structure and chemistry of zircon and zircon-group minerals. In Zircon. Rev. Mineral. Geochem. 2003, 53, 1–25. [Google Scholar] [CrossRef]
- Zaman, M.; Schubert, M.; Antao, S. Elevated radionuclide concentrations in heavy mineral-rich beach sands in the Cox’s Bazar region, Bangladesh and related possible radiological effects. Isot. Environ. Health Stud. 2012, 48, 512–525. [Google Scholar] [CrossRef] [PubMed]
- Anfinson, O.A.; Leier, A.L.; Embry, A.F.; Dewing, K. Detrital zircon geochronology and provenance of the Neoproterozoic to Late Devonian Franklinian Basin, Canadian Arctic Islands. Geol. Soc. Am. Bull. 2012, 124, 415–430. [Google Scholar] [CrossRef]
- Holland, H.D.; Gottfried, D. The effect of nuclear radiation on the structure of zircon. Acta Crystallogr. 1955, 8, 291–300. [Google Scholar] [CrossRef]
- Montel, J.M.; Foret, S.; Veschambre, M.; Nicollet, N.; Provost, A. Electron microprobe dating of monazite. Chem. Geol. 1996, 131, 37–53. [Google Scholar] [CrossRef]
- Najman, Y.; Allen, R.; Willett, E.A.F.; Carter, A.; Barfod, D.; Garzanti, E.; Wijbrans, J.; Bickle, M.J.; Vezzoli, G.; Ando, S.; et al. The record of Himalayan erosion preserved in the sedimentary rocks of the Hatia Trough of the Bengal Basin and the Chittagong Hill Tracts, Bangladesh. Basin Res. 2012, 24, 1–21. [Google Scholar] [CrossRef]
- Murakami, T.; Chakoumakos, B.C.; Ewing, R.C.; Lumpkin, G.R.; Weber, W.J. Alpha-decay event damage in zircon. Am. Mineral. 1991, 76, 1510–1532. [Google Scholar]
- Otwinowski, Z.; Minor, W. Processing of X-ray diffraction data collected in oscillation mode. In Methods in Enzymology: Macromolecular Crystallography A276; Carter, C.W., Jr., Sweet, R.M., Eds.; Academic Press: New York, NY, USA, 1997; pp. 307–326. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 1998, 64, 112–122. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Antao, S.M.; Hassan, I.; Wang, J.; Lee, P.L.; Toby, B.H. State-of-the-art high-resolution powder X-ray diffraction (HRPXRD) illustrated with Rietveld structure refinement of quartz, sodalite, tremolite, and meionite. Can. Mineral. 2008, 46, 1501–1509. [Google Scholar] [CrossRef]
- Lee, P.L.; Shu, D.; Ramanathan, M.; Preissner, C.; Wang, J.; Beno, M.A.; Von Dreele, R.B.; Ribaud, L.; Kurtz, C.; Antao, S.M.; et al. A twelve-analyzer detector system for high-resolution powder diffraction. J. Synchrotron Radiat. 2008, 15, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Toby, B.H.; Lee, P.L.; Ribaud, L.; Antao, S.M.; Kurtz, C.; Ramanathan, M.; Von Dreele, R.B.; Beno, M.A. A dedicated powder diffraction beamline at the advanced photon source: Commissioning and early operational results. Rev. Sci. Instrum. 2008, 79, 085105. [Google Scholar] [CrossRef] [PubMed]
- Antao, S.M.; Hassan, I.; Parise, J.B. Cation ordering in magnesioferrite, MgFe2O4, to 982 °C using in situ synchrotron X-ray powder diffraction. Am. Mineral. 2005, 90, 219–228. [Google Scholar] [CrossRef]
- Ehm, L.; Antao, S.M.; Chen, J.H.; Locke, D.R.; Michel, F.M.; Martin, C.D.; Yu, T.; Parise, J.B.; Lee, P.L.; Chupas, P.J.; et al. Studies of local and intermediate range structure in crystalline and amorphous materials at high pressure using high-energy X-rays. Powder Diffr. 2007, 22, 108–112. [Google Scholar] [CrossRef]
- Ehm, L.; Michel, F.M.; Antao, S.M.; Martin, C.D.; Lee, P.L.; Shastri, S.D.; Chupas, P.J.; Parise, J.B. Structural changes in nanocrystalline mackinawaite (FeS) at high pressure. J. Appl. Crystallogr. 2009, 42, 15–21. [Google Scholar] [CrossRef]
- Hassan, I.; Antao, S.M.; Parise, J.B. Haüyne: Phase transition and high-temperature structures obtained from synchrotron radiation and Rietveld refinements. Mineral. Mag. 2004, 68, 499–513. [Google Scholar] [CrossRef]
- Parise, J.B.; Antao, S.M.; Michel, F.M.; Martin, C.D.; Chupas, P.J.; Shastri, S.; Lee, P.L. Quantitative high-pressure pair distribution function analysis. J. Synchrotron Radiat. 2005, 12, 554–559. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Larson, A.C.; Von Dreele, R.B. General Structure Analysis System (GSAS); Los Alamos National Laboratory Report LAUR: Santa Fe, NM, USA, 2000; pp. 86–748. [Google Scholar]
- Toby, B.H. Expgui, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef]
- Finger, L.W.; Cox, D.E.; Jephcoat, A.P. A correction for powder diffraction peak asymmetry due to axial divergence. J. Appl. Crystallogr. 1994, 27, 892–900. [Google Scholar] [CrossRef]
- Hoskin, P.W.O.; Schaltegger, U. The composition of zircon and igneous and metamorphic petrogenesis. In Zircon. Rev. Mineral. Geochem. 2003, 53, 27–62. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Zhenmin, G.; Jingming, P. Factor affecting metamictization in granites. Chin. J. Geochem. 1986, 5, 181–188. [Google Scholar]
- Harley, S.L.; Kelly, N.M. Zircon, tiny but timely. Elements 2007, 3, 13–18. [Google Scholar] [CrossRef]
- Antao, S.M. The mystery of birefringent garnet: Is the symmetry lower than cubic? Powder Diffr. 2013, 28, 281–288. [Google Scholar] [CrossRef]
- Antao, S.M.; Klincker, A.M. Origin of birefringence in andradite from Arizona, Madagascar, and Iran. Phys. Chem. Miner. 2013, 40, 575–586. [Google Scholar] [CrossRef]
- Antao, S.M. Three cubic phases intergrown in a birefringent andradite-grossular garnet and their implications. Phys. Chem. Miner. 2013, 40, 705–716. [Google Scholar] [CrossRef]
- Siggel, A.; Jansen, M. Röntgenographische untersuchungen zur bestimmung der einbauposition von seltenen erden (Pr, Tb) und vanadium in zirkonpigmenten. Z. Anorg. Allg. Chem. 1990, 583, 67–77. [Google Scholar] [CrossRef]
- Hanchar, J.M.; Finch, R.J.; Hoskin, P.W.O.; Watson, E.B.; Cherniak, D.J.; Mariano, A.N. Rare earth elements in synthetic zircon: Part 1. Synthesis, and rare earth element and phosphorus doping. Am. Mineral. 2001, 86, 667–680. [Google Scholar] [CrossRef]
- Tokuda, M.; Yoshiasa, A.; Kojitani, H.; Hashimoto, S.; Uehara, S.; Mashimo, T.; Tobase, T.; Akaogi, M. The importance of cation–cation repulsion in the zircon–reidite phase transition and radiation-damaged zircon. Mineral. Mag. 2019, 83, 561–567. [Google Scholar] [CrossRef]
- Hoshino, M.; Kimata, M.; Nishida, N.; Shimizu, M.; Akasaka, T. Crystal chemistry of zircon from granitic rocks, Japan: Genetic implications of HREE, U and Th enrichment. Neues Jahrb. Mineral. Abh. 2010, 187, 167–188. [Google Scholar] [CrossRef]
- Balan, E.; Neuville, D.R.; Trocellier, P.; Fritsch, E.; Muller, J.P.; Calas, G. Metamictization and chemical durability of detrital zircon. Am. Mineral. 2001, 86, 1025–1033. [Google Scholar] [CrossRef]
- Lumpkin, G.R.; Ewing, R.C. Alpha-decay damage in minerals of the pyrochlore group. Phys. Chem. Miner. 1988, 16, 2–20. [Google Scholar] [CrossRef]






| Wt.% | 1:CB | 2:CB | 3:CB | 4:CB | 5:BIF | 6:HBF | 7:PIF | 8:JN |
|---|---|---|---|---|---|---|---|---|
| ZrO2 | 65.74 | 65.19 | 65.09 | 65.30 | 63.47 | 66.35 | 65.66 | 65.01 |
| HfO2 | 1.11 | 1.37 | 1.59 | 1.36 | 1.33 | 1.11 | 1.19 | 1.19 |
| UO2 | 0.02 | 0.06 | 0.17 | 0.08 | 0.12 | 0.02 | 0.03 | 0.02 |
| ThO2 | 0.01 | 0.06 | 0.01 | 0.08 | 0.06 | 0.02 | 0.03 | 0.16 |
| PbO | bdl | bdl | 0.02 | bdl | 0.04 | 0.01 | bdl | 0.02 |
| CaO | 0.02 | 0.02 | 0.01 | 0.02 | 0.06 | 0.01 | 0.02 | 0.02 |
| Y2O3 | 0.10 | 0.04 | 0.04 | 0.08 | 0.28 | 0.03 | 0.05 | bdl |
| SrO | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| TiO2 | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| FeO | bdl | 0.01 | 0.01 | 0.04 | 0.26 | 0.01 | bdl | 0.01 |
| Cr2O3 | 0.01 | bdl | bdl | bdl | 0.01 | bdl | bdl | 0.01 |
| MnO | 0.01 | 0.01 | 0.01 | bdl | 0.02 | 0.01 | 0.01 | 0.01 |
| MgO | bdl | 0.01 | 0.01 | 0.01 | bdl | 0.01 | 0.01 | bdl |
| NiO | bdl | 0.02 | 0.02 | 0.02 | 0.02 | bdl | 0.01 | bdl |
| SiO2 | 32.67 | 32.11 | 32.28 | 32.75 | 32.16 | 31.84 | 31.34 | 31.28 |
| P2O5 | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| SO3 | 0.03 | 0.04 | 0.03 | 0.03 | 0.03 | 0.01 | 0.02 | 0.01 |
| Al2O3 | bdl | bdl | bdl | bdl | 0.02 | bdl | bdl | bdl |
| Total | 99.71 | 98.93 | 99.29 | 99.78 | 97.89 | 99.43 | 98.38 | 97.75 |
| Atom per formula unit (apfu) based on 4 O atoms | ||||||||
| Zr | 0.984 | 0.987 | 0.983 | 0.978 | 0.969 | 1.002 | 1.004 | 1.000 |
| Hf | 0.010 | 0.012 | 0.014 | 0.012 | 0.012 | 0.010 | 0.011 | 0.011 |
| U | - | - | 0.001 | 0.001 | 0.001 | - | - | - |
| Th | - | - | - | 0.001 | 0.000 | - | - | 0.001 |
| Ca | 0.001 | 0.001 | - | 0.001 | 0.002 | - | 0.001 | 0.001 |
| Y | 0.002 | 0.001 | 0.001 | 0.001 | 0.005 | - | 0.001 | - |
| Fe | - | - | - | 0.001 | 0.007 | - | - | - |
| Mn | - | - | - | - | 0.001 | - | - | - |
| Mg | - | - | - | - | - | 0.001 | 0.001 | - |
| Ni | - | - | - | 0.001 | 0.001 | - | - | - |
| ∑Zr site | 0.997 | 1.003 | 1.000 | 0.995 | 0.998 | 1.014 | 1.018 | 1.014 |
| Si | 1.003 | 0.997 | 0.999 | 1.006 | 1.007 | 0.986 | 0.983 | 0.987 |
| S | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | - | - | - |
| ∑Si site | 1.004 | 0.998 | 1.000 | 1.006 | 1.008 | 0.986 | 0.983 | 0.987 |
| Total * | 2.001 | 2.001 | 2.001 | 2.001 | 2.006 | 2.001 | 2.001 | 2.001 |
| Sample ID | U (ppm) | Th (ppm) | Radiation Dose | Hf + TE (apfu) | Sample ID | U (ppm) | Th (ppm) | Radiation Dose | Hf + TE (apfu) |
|---|---|---|---|---|---|---|---|---|---|
| 1:CB | 144 | 103 | 4.34 × 1014 | 0.014 | 27 | 707 | 186 | 1.67 × 1015 | 0.018 |
| 2:CB | 494 | 528 | 1.36 × 1015 | 0.017 | 28 | 571 | 381 | 1.46 × 1015 | 0.023 |
| 3:CB | 1538 | 98 | 3.26 × 1015 | 0.019 | 29 | 903 | 458 | 2.10 × 1015 | 0.021 |
| 4:CB | 669 | 732 | 2.82 × 1015 | 0.018 | 30 | 400 | 432 | 1.04 × 1015 | 0.017 |
| 5:BIF | 1058 | 542 | 1.48 × 1015 | 0.029 | 31 | 316 | 350 | 8.26 × 1014 | 0.015 |
| 6:HBF | 135 | 149 | 2.12 × 1014 | 0.013 | 32 | 236 | 466 | 7.13 × 1014 | 0.016 |
| 7:PIF | 300 | 254 | 4.42 × 1014 | 0.014 | 33 | 1214 | 286 | 4.34 × 1015 | 0.015 |
| 8:JN | 208 | 1370 | 4.80 × 1015 | 0.014 | 34 | 2900 | 387 | 1.02 × 1016 | 0.038 |
| 9 | 498 | 268 | 1.45 × 1015 | 0.013 | 35 | 1690 | 815 | 6.36 × 1015 | 0.033 |
| 10 | 326 | 19 | 8.57 × 1014 | 0.019 | 36 | 809 | 560 | 3.17 × 1015 | 0.032 |
| 11 | 340 | 0 | 8.83 × 1014 | 0.017 | 37 | 2779 | 1775 | 1.08 × 1016 | 0.097 |
| 12 | 164 | 295 | 5.97 × 1014 | 0.011 | 38 | 2280 | 6861 | 1.29 × 1016 | 0.066 |
| 13 | 848 | 1097 | 2.84 × 1015 | 0.016 | 39 | 4549 | 3334 | 1.80 × 1016 | 0.148 |
| 14 | 702 | 239 | 1.96 × 1015 | 0.018 | 40 | 1672 | 1008 | 6.44 × 1015 | 0.069 |
| 15 | 2221 | 631 | 6.13 × 1015 | 0.028 | 41 | 624 | 379 | 8.89 × 1014 | 0.017 |
| 16 | 1209 | 311 | 3.32 × 1015 | 0.019 | 42 | 90 | 128 | 1.49 × 1014 | 0.024 |
| 17 | 517 | 170 | 1.44 × 1015 | 0.014 | 43 | 275 | 445 | 4.72 × 1014 | 0.032 |
| 18 | 510 | 178 | 1.42 × 1015 | 0.015 | 44 | 118 | 130 | 1.85 × 1014 | 0.016 |
| 19 | 1312 | 334 | 3.60 × 1015 | 0.021 | 45 | 117 | 0 | 1.46 × 1014 | 0.014 |
| 20 | 127 | 271 | 4.86 × 1014 | 0.016 | 46 | 461 | 176 | 6.27 × 1014 | 0.019 |
| 21 | 3082 | 1909 | 9.10 × 1015 | 0.084 | 47 | 33 | 139 | 8.07 × 1013 | 0.014 |
| 22 | 3448 | 475 | 9.22 × 1015 | 0.093 | 48 | 566 | 243 | 7.67 × 1014 | 0.018 |
| 23 | 869 | 1898 | 3.35 × 1015 | 0.018 | 49 | 0 | 230 | 6.53 × 1013 | 0.019 |
| 24 | 1935 | 350 | 4.49 × 1015 | 0.024 | 50 | 104 | 278 | 2.07 × 1014 | 0.017 |
| 25 | 1129 | 582 | 2.81 × 1015 | 0.019 | 51 | 175 | 46 | 2.28 × 1014 | 0.015 |
| 26 | 1070 | 63 | 2.42 × 1015 | 0.026 | 52 | 72 | 91 | 1.15 × 1014 | 0.012 |
| 1:CB | 2:CB | 3:CB | 4:CB | 5:BIF | 6:HBF | 7:PIF | ||
|---|---|---|---|---|---|---|---|---|
| Crystal size (mm) | 0.08 × 0.08 × 0.06 | 0.10 × 0.04 × 0.03 | 0.08 × 0.08 × 0.08 | 0.10 × 0.08 × 0.06 | 0.08 × 0.06 × 0.08 | 0.10 × 0.08 × 0.06 | 0.10 × 0.10 × 0.08 | |
| Color | Gray | Colorless | Pink | Red | Gray | Gray | Gray | |
| Crystal shape | Spherical | Prismatic | Prismatic | Spherical | Spherical | Spherical | Spherical | |
| Unit-cell parameters (Å) | a c | 6.6040(9) 5.9830(6) | 6.6030(7) 5.9800(4) | 6.6030(5) 5.9780(4) | 6.604(2) 5.985(1) | 6.6120(7) 5.9970(5) | 6.5840(5) 5.9720(5) | 6.5790(6) 5.9600(7) |
| Volume, V (Å3) | 260.94(6) | 260.73(4) | 260.64(4) | 261.0(1) | 262.18(4) | 258.88(4) | 257.97(4) | |
| Densitycalc (g/cm3) | 4.666 | 4.670 | 4.672 | 4.665 | 4.644 | 4.703 | 4.720 | |
| Absorption coefficient (mm-1) | 4.461 | 4.464 | 4.466 | 4.459 | 4.440 | 4.496 | 4.512 | |
| 2θ range | 2°–54.34° | 2°–54.70° | 2°–54.70° | 2°–54.70° | 2°–55.16° | 2°–54.87° | 2°–54.96° | |
| Index ranges | −8 ≤ h ≤ 8 −8 ≤ k ≤ 8 −7 ≤ l ≤ 7 | −8 ≤ h ≤ 8 −8 ≤ k ≤ 8 −7 ≤ l ≤ 7 | −8 ≤ h ≤ 8 −8 ≤ k ≤ 8 −7 ≤ l ≤ 7 | −8 ≤ h ≤ 8 −8 ≤ k ≤ 8 −7 ≤ l ≤ 7 | −8 ≤ h ≤ 8 −8 ≤ k ≤ 8 −7 ≤ l ≤ 7 | −8 ≤ h ≤ 8 −8 ≤ k ≤ 8 −6 ≤ l ≤ 7 | −8 ≤ h ≤ 8 −5 ≤ k ≤ 6 −7 ≤ l ≤ 7 | |
| Total reflections | 442 | 487 | 528 | 487 | 556 | 800 | 255 | |
| Unique reflections | 88 | 91 | 91 | 91 | 92 | 88 | 85 | |
| Rint | 0.0270 | 0.0230 | 0.0224 | 0.0267 | 0.0224 | 0.0252 | 0.0183 | |
| R1 | 0.0125 | 0.0170 | 0.0110 | 0.0133 | 0.0114 | 0.0114 | 0.0121 | |
| wR2 | 0.0494 | 0.0558 | 0.0483 | 0.0516 | 0.0556 | 0.0542 | 0.0436 | |
| Extinction coefficient | 0.026(4) | 0.11(1) | 0.076(7) | 0.005(2) | 0.003(2) | 0.019(6) | 0.039(8) | |
| Largest difference peak/hole (e/Å3) | 0.320 −0.330 | 0.825 −0.015 | 0.309 −0.382 | 0.430 −0.295 | 0.250 −0.310 | 0.281 −0.266 | 0.261 −0.268 | |
| Mosaicity (°) | 0.616(6) | 0.540(5) | 0.534(4) | 0.85(1) | 0.733(5) | 0.79(1) | 0.843(7) |
| 1:CB | 2:CB | * 2a:CB | 3:CB | 4:CB | 5:BIF | 6:HBF | 7:PIF | ||
|---|---|---|---|---|---|---|---|---|---|
| Coordinates and Uij for O (x = 0; U12 = U13 = 0) | |||||||||
| y | 0.0656(2) | 0.0657(2) | 0.06609(9) | 0.0657(2) | 0.0659(2) | 0.0658(2) | 0.0654(2) | 0.0659(2) | |
| z | 0.1951(3) | 0.1953(3) | 0.1954(1) | 0.1957(2) | 0.1948(3) | 0.1953(3) | 0.1953(3) | 0.1950(2) | |
| Ueq | 0.0094(5) | 0.0108(6) | 0.0027(2) | 0.0097(5) | 0.0114(5) | 0.0115(5) | 0.0124(5) | 0.0119(4) | |
| U11 | 0.0109(9) | 0.0131(9) | 0.0128(7) | 0.0146(10) | 0.0130(9) | 0.0142(7) | 0.0128(6) | ||
| U22 | 0.0092(8) | 0.0089(8) | 0.0095(6) | 0.0079(8) | 0.0105(8) | 0.0116(6) | 0.0123(6) | ||
| U33 | 0.0080(9) | 0.0104(10) | 0.0067(8) | 0.0117(9) | 0.0110(9) | 0.0116(9) | 0.0105(7) | ||
| U23 | 0.0017(5) | −0.0003(6) | 0.0005(3) | −0.0005(5) | −0.0006(4) | −0.0002(5) | 0.0002(3) | ||
| Uij for Si (x =0, y = 3/4, z = 5/8; U23 = U12 = U13 = 0) | |||||||||
| Ueq | 0.0068(5) | 0.0064(7) | 0.0014(1) | 0.0061(6) | 0.0081(6) | 0.0086(6) | 0.0088(6) | 0.0083(5) | |
| U11 | 0.0070(7) | 0.0072(8) | 0.0070(7) | 0.0083(7) | 0.0090(8) | 0.0093(8) | 0.0090(6) | ||
| U22 | 0.0070(7) | 0.0072(8) | 0.0070(7) | 0.0083(7) | 0.0090(8) | 0.0093(8) | 0.0090(6) | ||
| U33 | 0.0065(12) | 0.0046(14) | 0.0043(12) | 0.0076(12) | 0.0077(13) | 0.0078(13) | 0.0070(11) | ||
| Uij for Zr (x =0, y = 3/4, z = 1/8; U23 = U12 = U13 = 0) | |||||||||
| Ueq | 0.0067(4) | 0.0062(5) | 0.00023(4) | 0.0061(4) | 0.0072(4) | 0.0081(4) | 0.0085(4) | 0.0081(4) | |
| U11 | 0.0068(4) | 0.0066(5) | 0.0065(4) | 0.0072(4) | 0.0080(4) | 0.0083(5) | 0.0083(4) | ||
| U22 | 0.0068(4) | 0.0066(5) | 0.0065(4) | 0.0072(4) | 0.0080(4) | 0.0083(5) | 0.0083(4) | ||
| U33 | 0.0065(6) | 0.0056(7) | 0.0054(6) | 0.0072(5) | 0.0083(6) | 0.0089(6) | 0.0076(5) | ||
| Bond/Angle | # bonds | 1:CB | 2:CB | * 2a:CB | 3:CB | 4:CB | 5:BIF | 6:HBF | 7:PIF |
|---|---|---|---|---|---|---|---|---|---|
| Zr-OI | 4x | 2.126(2) | 2.126(2) | 2.1305(6) | 2.127(1) | 2.128(2) | 2.131(2) | 2.119(1) | 2.120(1) |
| Zr-OII | 4x | 2.269(2) | 2.270(2) | 2.2696(6) | 2.271(1) | 2.267(2) | 2.274(2) | 2.263(2) | 2.259(1) |
| <Zr-O> [8] | 2.198(2) | 2.198(2) | 2.2001(6) | 2.199(2) | 2.198(2) | 2.203(2) | 2.193(2) | 2.190(1) | |
| <O-Zr-O> [18] | 78.77(5) | 78.76(5) | 78.761(1) | 78.75(4) | 78.77(5) | 78.76(5) | 78.76(5) | 78.77(3) | |
| Si-O | 4x | 1.625(2) | 1.624(2) | 1.6220(6) | 1.622(1) | 1.625(2) | 1.626(2) | 1.622(2) | 1.618(1) |
| <O-Si-O> [6] | 109.69(8) | 109.69(9) | 106.69(1) | 109.69(6) | 109.70(8) | 109.70(9) | 109.69(7) | 109.70(6) | |
| Zi-Si | 2x | 2.9920(3) | 2.9900(2) | 2.9915(1) | 2.9890(2) | 2.9930(10) | 2.9990(3) | 2.9860(3) | 2.9800(4) |
| Zr-Zr | 2x | 3.6250(4) | 3.6242(3) | 3.6263(1) | 3.6240(3) | 3.6250(10) | 3.6300(3) | 3.6147(2) | 3.6112(3) |
| Miscellaneous | 8:JN | 2a:CB |
|---|---|---|
| a (Å) | 6.6541(1) | 6.60700(1) |
| c (Å) | 6.03551(6) | 5.98303(1) |
| V (Å3) | 267.237(7) | 261.174(1) |
| 1 Ndata | 26,246 | 44,994 |
| 2 Nobs | 159 | 263 |
| 3R (F2) | 0.0395 | 0.0311 |
| Reduced χ2 | 0.8608 | 2.859 |
| λ (Å) | 0.41417(2) | 0.459001(2) |
| 2θ range | 2°–30° | 2°–50° |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaman, M.M.; Antao, S.M. Crystal Chemistry and Structural Variations for Zircon Samples from Various Localities. Minerals 2020, 10, 947. https://doi.org/10.3390/min10110947
Zaman MM, Antao SM. Crystal Chemistry and Structural Variations for Zircon Samples from Various Localities. Minerals. 2020; 10(11):947. https://doi.org/10.3390/min10110947
Chicago/Turabian StyleZaman, M. Mashrur, and Sytle M. Antao. 2020. "Crystal Chemistry and Structural Variations for Zircon Samples from Various Localities" Minerals 10, no. 11: 947. https://doi.org/10.3390/min10110947
APA StyleZaman, M. M., & Antao, S. M. (2020). Crystal Chemistry and Structural Variations for Zircon Samples from Various Localities. Minerals, 10(11), 947. https://doi.org/10.3390/min10110947





