1. Introduction
The treatment of extensively distributed solid wastes generated by metallurgical processes is a challenge for the environmental sustainability of mining regions [
1,
2]. Soil characterization is a relevant process for subsequent environmental treatments such as decontamination, stabilization, or remediation [
3,
4]. The physical (pH, density) and the chemical properties of soils are a forcing factor for the growth and pollutant metabolism of plants during phytoremediation, particularly heavy metal content in the sediments at the (superficial) upper levels [
5,
6]. In this context, heavy metal concentration is commonly determined by a chemical analysis involving a high cost and a long period of analysis [
7,
8,
9]; however, a low-cost and fast-application method is necessary for effective sediment characterization prior to remediation.
For a reliable tailing storage facility characterization, a large number of sampling points is required so that results can be representative, a fact that has been limited due to the extensive economic resources needed. Sampling quality is essential for estimating the extent of contamination on-site and, therefore, establishing intervention requirements to protect human health and the ecosystem integrity [
10]. In this regard, sampling design plays a fundamental role in the tailing characterization stage and must be based on the spatial contamination distribution hypothesis formulated from the results of the exploratory phase of the study. This allows for making an appropriate assessment of the site and its management [
11,
12]. It is necessary to design a sampling methodology to establish their presence in the mineral species contained in the tailings [
13,
14]. There are no general rules for soil sampling because each site requires a particular strategy. Therefore, it is important to design a scheme appropriate for each of the tailing storage facilities, considering the optimal location of the sampling points. This scheme must be flexible enough to make adjustments during field activities, owing to, for example, lack of access to preselected sites, unforeseen soil formations, and climatic conditions [
15]. The characterization stage involves sampling and the analysis of physical and chemical properties to determine the nature, extent, and extension of contamination. These data are essential for developing, projecting, analyzing, and selecting cost effective technologies to mitigate contamination [
16].
Magnetic techniques and methodologies are notable for characterizing soils since they can be validated with a small number of fast chemical analyses at a low cost [
17,
18]. Although the magnetic phenomenon was recognized in the early 19th century, its application in the different fields of science and technology has increased in the last few decades [
19,
20,
21]. The magnetic characterization and mapping of the magnetic susceptibility of soils have been widely applied as a proxy to characterize heavy metals and pollutants in soils in urban and industrial areas [
22,
23,
24,
25,
26,
27,
28,
29]. This type of study allows for determining the origin and extension of contaminant agents and their effect on natural soils. Magnetic properties, particularly magnetic susceptibility, are useful tools for identifying and describing ferromagnetic elements (Fe, Ni, Cr). They allow an indirect characterization of the study area because a small number of chemical analyses are needed later.
Magnetic susceptibility is the ability of a material to magnetize itself under the effect of an induced magnetic field, which has a range of values characteristic of the different ferromagnetic elements. It is possible to measure this property in the field by using easy-to-use portable susceptometers at a low cost [
30]. Despite the wide use of magnetic techniques for natural soil contamination, they have been scarcely used for detecting and determining the concentration of metals present in tailing deposits [
31]. Therefore, the application of magnetic techniques is a good alternative for the chemical characterization of tailing storage facilities due to their high resolution and low cost. In addition, these techniques have important advantages such as short measurement time and the repetition of analyses at a low cost.
Several factors take part in the control of the retention and mobility of heavy metals in the soil, mineralogy playing an important role among them [
32]. The bioavailability of most of the elements, particularly heavy metals, is determined by adsorption–desorption, complexation, precipitation, and ion-change processes. The most important surfaces involved in soil metal adsorption are active inorganic colloids such as clayey minerals, metal oxides and hydroxides, metal carbonates and phosphates, and organic colloids [
33]. Regarding texture, clayey soils retain more metals by adsorption or in the exchange complex of the clayey minerals; on the contrary, sandy soils lack this fixing capacity. In particular, each clay mineral is characterized by a specific surface area value and a degree of electrical decompensation, which influence its ability to adsorb or exchange metals [
34].
Chilean mining is paramount for the country’s development and has become part of its identity. Chile has become a world’s copper production leader, with mining being the productive activity contributing most to GDP [
35]. However, it also holds a negative aspect due to the large output of residues and toxic wastes resulting from different operations and processes. Copper sulfide ore processing produces residues called tailings, which contain high heavy metal concentrations [
36]. Many solid tailing deposits extend for kilometers, their characterization currently being made via chemical analyses at a high cost [
9]. There are 742 mine tailing disposal sites in Chile; some of them are abandoned and require an urgent management plan [
37]. To propose remediation plans in order to reduce potential risks associated with tailings, their physicochemical and mineralogical characterization is a priority. These data are not available or are rather scarce, as in the case of geochemical data reported by National Geology and Mining Service (SERNAGEOMIN, for its acronym in Spanish) that determined the geochemical characterization of a number of tailing disposal facilities, based on samples from one to four sampling points [
10].
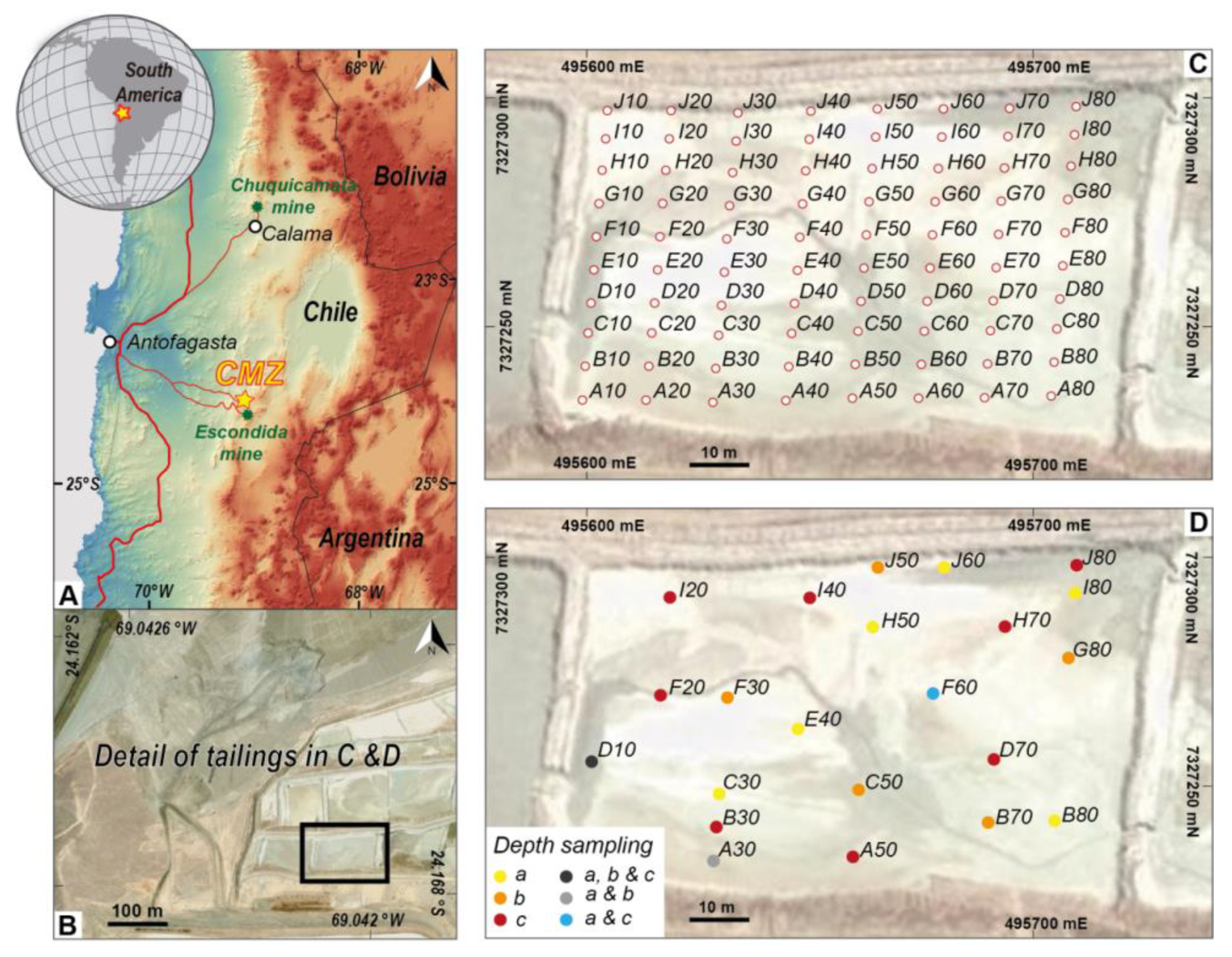
The aim of this work is to discard or validate magnetic susceptibility measurement as a technique for determining the contaminants, concentrations, and/or mobility of the elements in tailing storage facilities. This study, conducted by examining copper mine tailings, intends to validate the techniques measuring magnetic properties as sampling tools, correlating them with a limited number of physicochemical analyses. The objective of this study is to validate the measurement of magnetic susceptibility in the tailing terrace of a copper porphyry-type ore deposit. To attain the objective, heavy metal concentration values in the tailings are correlated with magnetic susceptibility to determine if this technique can be used as a fast and cost effective tool for identifying contaminated areas. Our results are relevant and offer a low cost and effective method to characterize solid wastes generated by copper metallurgical processes prior to their management and treatment in an extensive mining region like northern Chile.
3. Results
Data selected according to the three criteria above (
Section 2.3.3) are shown in
Table 2. The spatial distribution of the sampling points for the three depths are shown in
Figure 1C.
Table 3,
Table 4 and
Table 5 show the results of the concentration analyses of eight heavy metals (As, Cd, Cr, Cu, Fe, Ni, Pb, and Zn) and the magnetic susceptibility (MS) values for the three points measured in the field. The denomination is based on the names of the wells and the sampling depth, as shown in
Table 1.
3.1. Relationship between Magnetic Susceptibility and Depth
Considering the 240 samples, there is a statistical correlation between sampling depth and magnetic susceptibility. For the three depths, the standard deviation and the variance show high values, indicating a high dispersion of the magnetic susceptibility data measured. The mean value of magnetic susceptibility for the three horizons tends to 411–518 µSI, showing a decrease in depth, as illustrated in
Figure 2 and
Figure 3, showing a boxplot and an interval plot, respectively. Given a certain relationship between depth and magnetic susceptibility, a hypothesis test was conducted.
The relationship between MS and depth was analyzed with ANOVA. This test shows the influence of one or more factors, in this case depth, over the mean of a continuous variable, in this case MS.
Table 6 shows the mean, standard deviation, and 95% CI for the mean of each profile. The ANOVA test was conducted with a 95% CI. Results reveal statistically significant differences between at least two groups (df = 2; F = 7.85;
p-value = 0.001). According to the results of the Tukey’s post hoc test [
49], the group under 30 cm present statistically significant differences in the magnetic susceptibility mean when compared with the other two.
3.2. Relationship between Magnetic Susceptibility and pH
MS decreases as tailing depth increases. Kapikca et al. [
50], Hoffmann et al. [
51], Boyko et al. [
52], and Magiera et al. [
53] observed this tendency, associated with the enrichment of anthropogenic particles on the most superficial layers of the soil from nearby industries or plants. The main difference between this study and those mentioned above is the type of soil where samples are collected, since samples are taken from the tailings mass in this study. For this reason, MS decrease is related to chemical processes occurring within the tailings. MS decrease with depth may be explained by the most important reaction in the tailings, i.e., pyrite oxidation (Equations (1)–(3)). As explained by Dold and Fontboté [
54], atmospheric oxygen eruption into the system begins sulfide oxidation, in this case pyrite, the main gangue in the ore deposit.
Pyrite oxidation is the main acidity producer of the system (Equation (1)). The acidity produced by these processes may result in a pH decrease [
55,
56,
57,
58,
59]. Tailings from copper porphyry-type ore deposits show 1–3% pyrite concentrations [
54]. Therefore, ions such as Fe
3+ precipitate at pH > 3.5. The ferric ion, when not involved in sulfide oxidation, precipitates as secondary mineral such as goethite. Dold and Fontboté [
60] conducted chemical, mineralogical, and microbiological analyses on three Chilean copper porphyry ore deposit tailings in different climatic contexts (arid, semiarid, and humid). These tailings were classified as having low sulfidization and carbonate content. The comparison of the behavior of these three tailings shows that the climatic factor controls the direction in which the elements move, free from the chemical reactions in the tailings. For example, in a humid climate, the chemical elements in the tailings move from an oxidizing environment on the upper part to a reducing environment in the direction of the phreatic level. Meanwhile, in regions with an arid climate such as the Atacama Desert, transport is expected to occur in the inverse direction, which is toward a more oxidizing environment due to capillarity, thus forming secondary sulfates on the surface. This effect has also been observed in arid and semiarid environments, favoring oxidation processes and allowing efflorescent mineral formation (hydrated sulfates) on the tailing surface [
61,
62].
In the tailings studied, horizons a and b show a slightly acid pH, while at depth c, the pH is neutral. This suggests:
- (i)
As horizon a is more superficial, pyrite oxidizes, resulting in greater acidity (average pH = 6.1).
- (ii)
There is an enrichment of diamagnetic minerals such as silica toward horizon c; i.e., there is a greater neutralization potential (pH = 7.05), which is supported by Si concentration increase with depth.
- (iii)
The formation of iron hydroxide should occur at the initial stage (pseudominerals) because, if minerals such as goethite were present in horizon c, the signal would increase owing to the high susceptibility of this mineral, which is not correlated with the MS tendency.
Horizontally, MS shows the highest concentration values at the ends and the lowest concentration values in the middle part of the terrace. Dold [
63] indicates that, although tailings particle size is relatively homogeneous, there is a deposition controlled by sedimentological processes resulting in coarse granulometry near the deposition point (sulfides are heavier than silicates). According to this analysis, MS distribution maps show a certain coherence. High MS values may involve the presence of pyrite with positive susceptibility, while low values would be associated with diamagnetic materials such as silica or carbonates.
3.3. Depth Variation of Chemical Elements
As to the variation of chemical elements in the terrace, two factors control element mobility: solubility and pH [
64,
65]. Solubility is a function of the chemical element concentration of the metal. In lower concentration systems, the elements are more mobile than in those with a higher concentration. Thus, mineralogical composition determines what chemical environment predominates and what elements are liberated and can mobilize [
66,
67,
68]. In the terrace, Cu and Cd increase their concentration towards horizon c, similarly to the pH. These behaviors are consistent, as reported by Dold and Fontboté [
54], who determined that in tailings out of operation, the oxidation process occurs in its initial stage. Metals such as Cu, Pb, Cd, Ni, and Ca are quite mobile at a low pH and are adsorbed when pH increases, explaining the lower concentration in horizon a, as compared with horizons b and c. The opposite is observed for Zn behavior, which mainly concentrates in horizon a. Dold and Fontboté [
60] observed the same tendency in Zn and Mg in El Salvador tailings, which concentrate on the most superficial layer, corresponding to an evaporitic horizon with a high pH.
3.4. Relationship between Magnetic Susceptibility and Heavy Metal Concentration
This relationship was analyzed with a MARS (multivariate adaptive regression splines) model suitable for this data structure. MARS was calculated by using the earth [
69] library of the statistical package R [
70], while the other statistical calculations were made with Minitab. Using data from
Table 3,
Table 4 and
Table 5, the relationship between MS and the concentrations of eight heavy metals was analyzed by using MARS, as in other engineering applications [
71,
72,
73,
74,
75] because it fits these data better than other models [
76,
77].
The model is a MARS analysis, i.e., a type of regression introduced by Jerome Friedman [
78]. It is a nonparametric regression technique that may be understood as a linear model extension automatically modeling nonlinear relationships and interactions between variables. In other words, it automatizes prediction model construction by selecting relevant variables, transforming predictive variables, treating missing values, and avoiding overfitting by means of an autotest. MARS is similar to a linear regression without splines.
It is mainly used for predicting a continuous variable , here MS, from a set of explanatory variables , here the concentration of heavy metals. So, the MARS model may be represented by the expression , where is an error vector.
MARS analysis results in both a linear and a second-grade linear model, without higher-grade models.
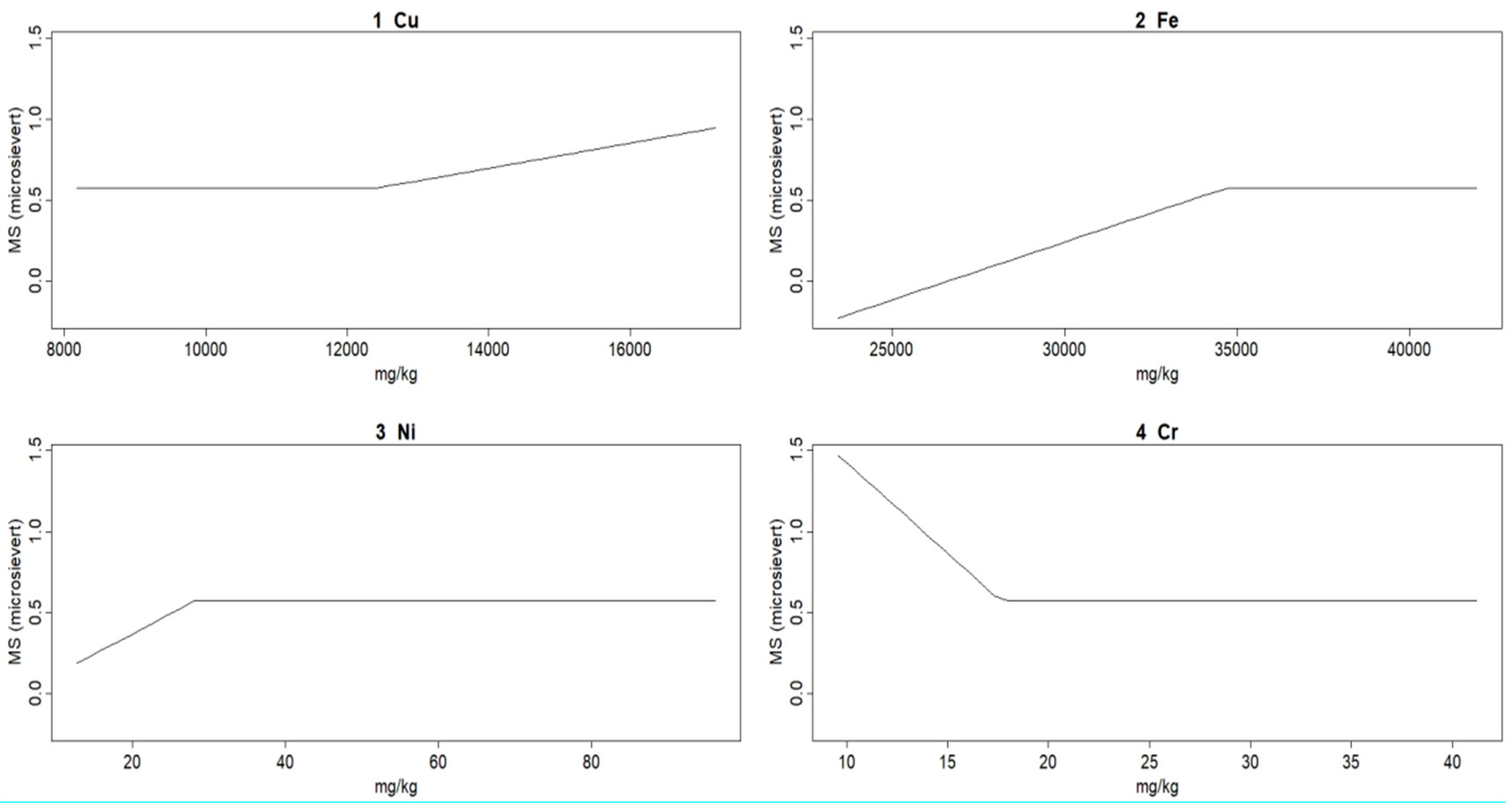
In the linear model, a correlation is established between MS and the concentrations of four metals, Cu, Fe, Ni, and Cr, and no correlation obtained with the other heavy metals Cd, As, Zn, and Pb, meaning that the latter are not relevant for MS.
The correlation in this model is given by Equation (4):
where pmax is 0, if the other value is not positive. For example, pmax (0, 19-Cr) becomes 0; i.e., Cr does not influence the correlation until Cr concentration does not exceed 0.19 mg∙kg
−1.
MARS model parameters GCV = 0.047, RSS = 0.82, GRSq = 0.26, and RSq = 0.58 show the model goodness of fit, RSq = 0.58, is not low for spread data.
The linear model, represented by pmax functions, shows that as Fe and Ni concentration increases, MS increases up to a certain value and then remains constant. For Cu concentration, MS remains constant up to a certain value and then increases. As for Cr, MS decreases to a certain concentration value and then remains constant (
Figure 4).
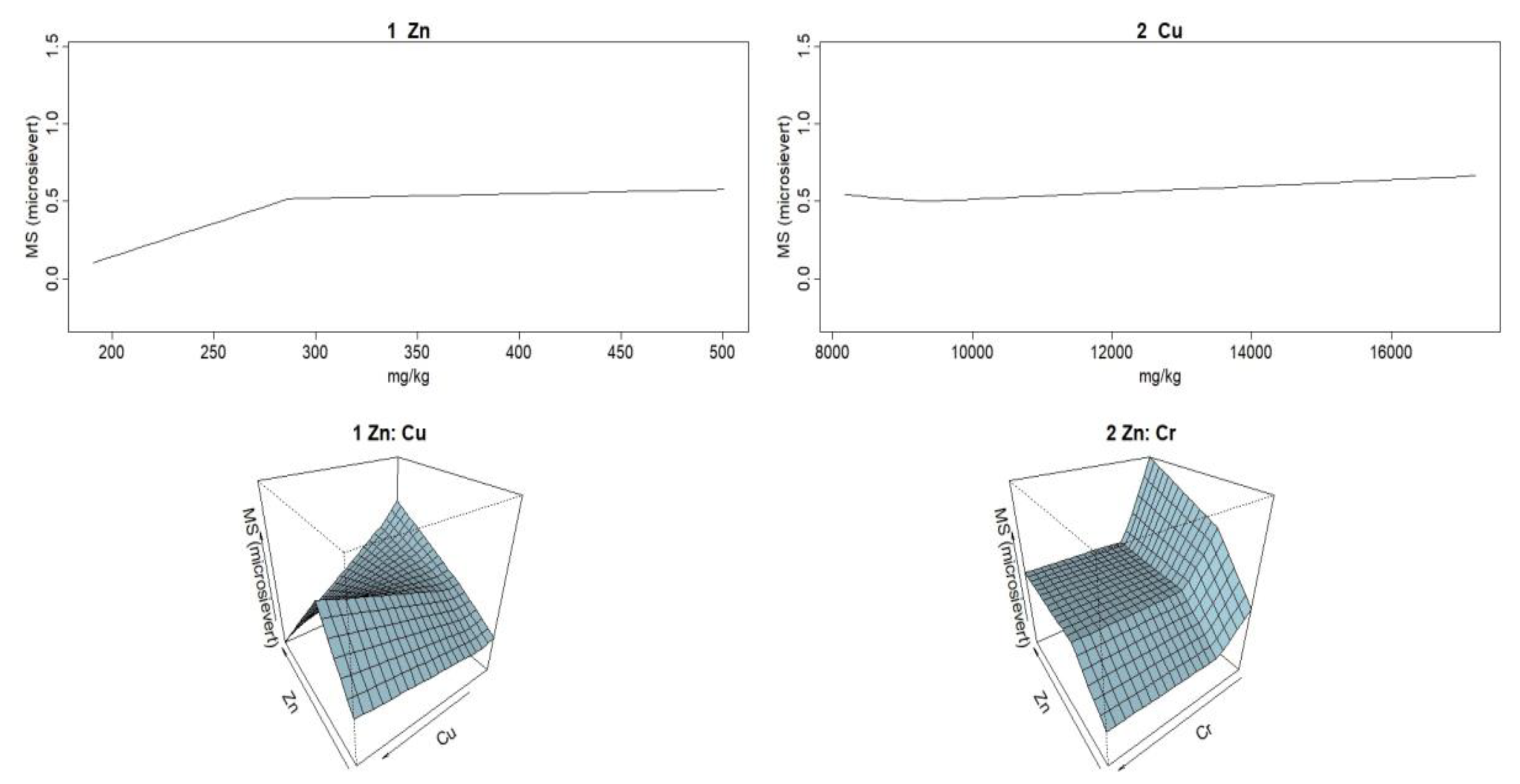
In the second-grade model, the correlation is established between MS and the concentrations of three metals, Cu, Zn, and Cr, the latter only as a second-order term. No correlation is obtained for the other heavy metals. This means that they are not relevant for MS. The fit is better than the one for the first-order model, with RSq slightly over 0.67. The correlation of the second-grade model is given by Equation (5):
MARS model parameters GCV = 0.05631133, RSS = 0.6488601, GRSq = 0.112011, and RSq = 0.670256 show that the model goodness of fit, RSq = 0.67, is better than in the linear model.
Figure 5 shows Zn and Cu linear relationships and Cr second-order relationship.
3.5. MS Advantages over Metal Concentration
The great advantage of susceptibility measurements is their promptness and low cost [
79,
80,
81]. MS was directly measured in the substrate at 0–10, 10–20, and 20–30 cm depths. For each level, MS was measured at least three times at three different points. Then, the average was determined. The sediment was extracted with a plastic shovel to avoid changing magnetic measurements [
82]. The technique results in the lowest cost, as compared with every other type of test and is little invasive for the environment. The chemical analysis was more complex, involving soil homogenization, clod disaggregation, and the removal of larger stones and residues. This was followed by clay content drying and sample sieving. Pretreatment for chemical analysis took about 3 days per sample [
5,
6]. So, preparing the sample for magnetic susceptibility is much simpler than preparing it for a chemical analysis. In addition, time must also be considered. As to MS, it is possible to measure 6 samples in 1 h on an average, considering the pretreatment stage. So, 40 h are required for the 240 sampling points. Thus, by working 8 h/day, 5 days are needed to obtain results. As to concentrations, pretreatment is more demanding and takes longer. For a further description, 6 samples can be collected each hour, the samples requiring at least a 3-day treatment. So, if 48 samples are collected daily, considering 8 h of work/day and 3 additional days for pretreatment, 4 days are required for collecting and treating 48 samples. Hence, for the 240 sampling points 20 days are required. In brief, the MS measurement of 240 points takes 5 days, while collecting and pretreating the 240 samples for measuring concentration takes 20 days. In addition, 3 months are necessary to measure the samples in the laboratory. So, for the time ratio required, concentrations:MS = 110:5 = 22, i.e., 2100% extra time for the characterization process.
Table 7 shows the time necessary for measuring magnetic susceptibility and the chemical analysis of 240 sampling points. The chemical analysis considers the determination of eight heavy metals (As, Cd, Cu, Fe, Hg, Pb, and Zn).
The cost of measuring each MS is about 3 USD, while the cost of the chemical analysis of a sample is about 150 USD. For the cost required relationship, chemical analysis:MS = 1:50 USD, i.e., the cost of chemical analyses would be about 4900% higher than the cost of measuring an MS point.
Although it is not possible to measure all the sampling points only with MS, the number of points may decrease if MS can be correlated with the concentration. This renders significant savings, as in this study, where only 33 out of the 240 points were measured, producing savings of more than 30,000 USD.
4. Conclusions
MS measurements may provide further data about soil pollution to estimate the environmental situation in a study area; i.e., magnetic properties show depth variations, which reflect concentration changes, depth being an environmental soil pollution indicator.
A positive linear correlation of magnetic susceptibility with Cr, Fe, Ni, and Cu was observed in the tailings studied, while in the second-grade model a correlation was found for Cu, Zn, and Cr. These heavy metals, well-known as the most hazardous elements, are easily extracted by plants from the soils in the area studied [
9]. In addition to traditional geochemical mapping, magnetic susceptibility could be successfully used for determining heavy metal soil pollution in the neighborhood of the site under study.
In the second-grade model, R2 is 0.67. For this kind of problem with spread data, this is not a low value and, therefore, indicates a value correlation between heavy metal concentration and magnetic susceptibility. This correlation is important because the cost of measuring MS is much lower than making chemical analyses for heavy metal concentration. Therefore, an indirect method can be used to assess the presence of heavy metals in tailings without conducting chemical analyses, or perhaps just making a few analyses that may serve as a pattern to calibrate magnetic susceptibility, which has been shown to vary with depth.
The concentration and mobility of chemical elements in the tailings is mainly controlled by pH. The interpretation of this and magnetic data enables detecting the chemical processes in the tailings, such as pyrite oxidation. MS is a good indicator because, as it decreases with depth, it is possible to interpret a greater concentration of diamagnetic minerals toward horizon c, which is complemented by the increasing depth of Cu concentrations.
Tailings sediments correspond to paramagnetic rather than diamagnetic arrangements, where ferromagnetic materials are present in small amounts within the paramagnetic matrix. The grain size of the tailings sediments does not allow a macroscopic recognition. So, for interpreting and understanding the magnetic signal, a detailed mineralogy control is necessary.
The correlation functions obtained can be used as semiquantitative tools for detecting toxic substance formations resulting from chemical reactions.
Magnetic methodologies, along with a small number of chemical analyses on representative samples, make it possible to develop sampling grids with a high spatial resolution at a low cost, thus decreasing costs associated with characterization.
Moreover, the potential use of these measurements to assess the metallic values contained in disposal facilities makes up an issue for further studies.