The Tres Arroyos Granitic Aplite-Pegmatite Field (Central Iberian Zone, Spain): Petrogenetic Constraints from Evolution of Nb-Ta-Sn Oxides, Whole-Rock Geochemistry and U-Pb Geochronology
Abstract
1. Introduction
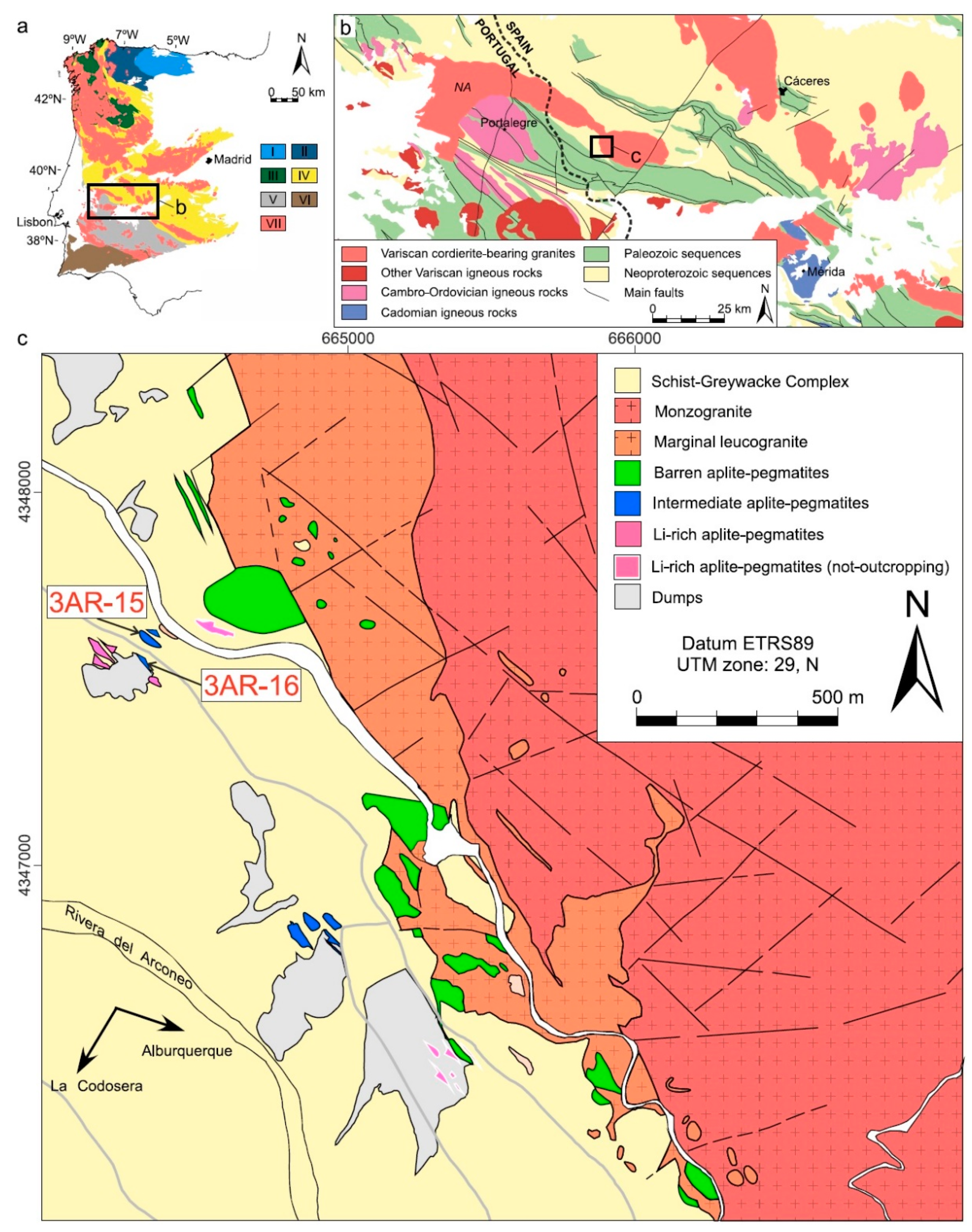
2. Geological Setting and Lithotypes of Tres Arroyos
3. Materials and Methods
4. Results
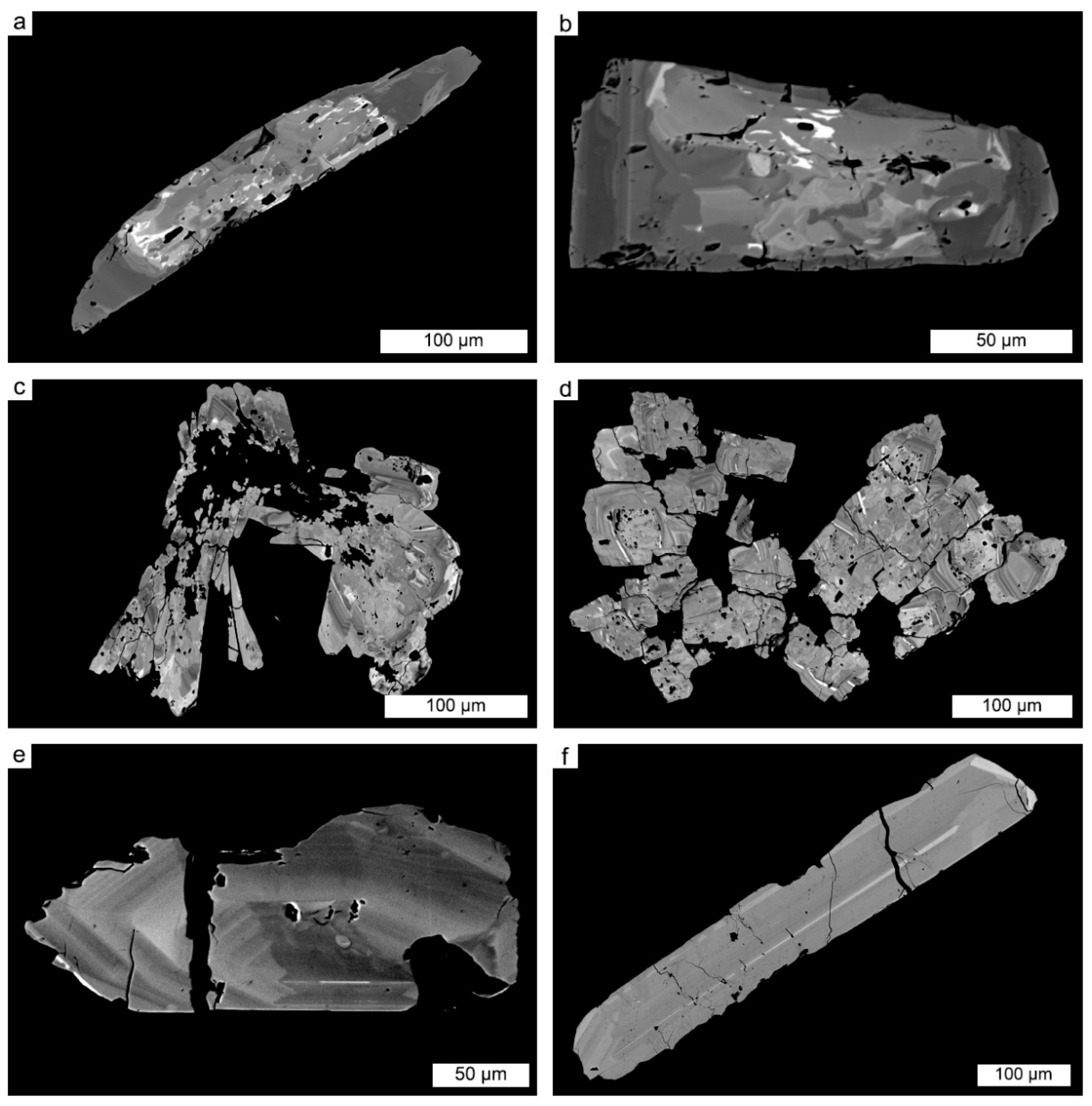
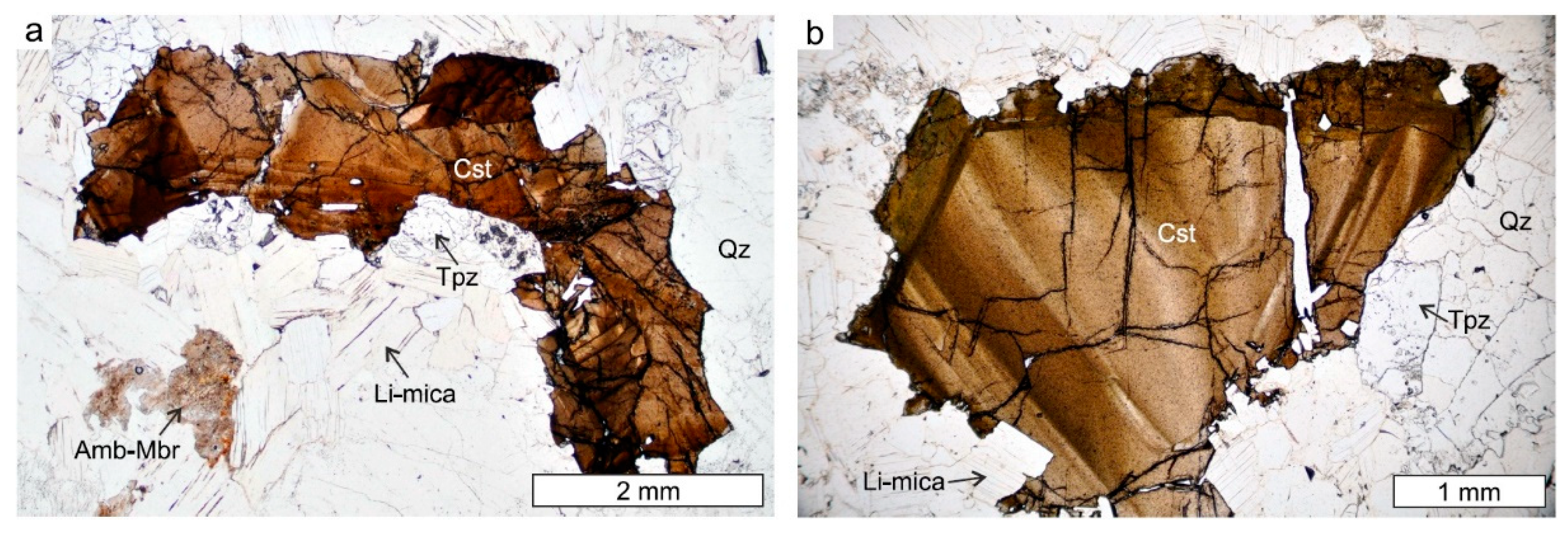
4.1. Petrography of Nb-Ta-Sn Oxides
4.2. Mineral Chemistry
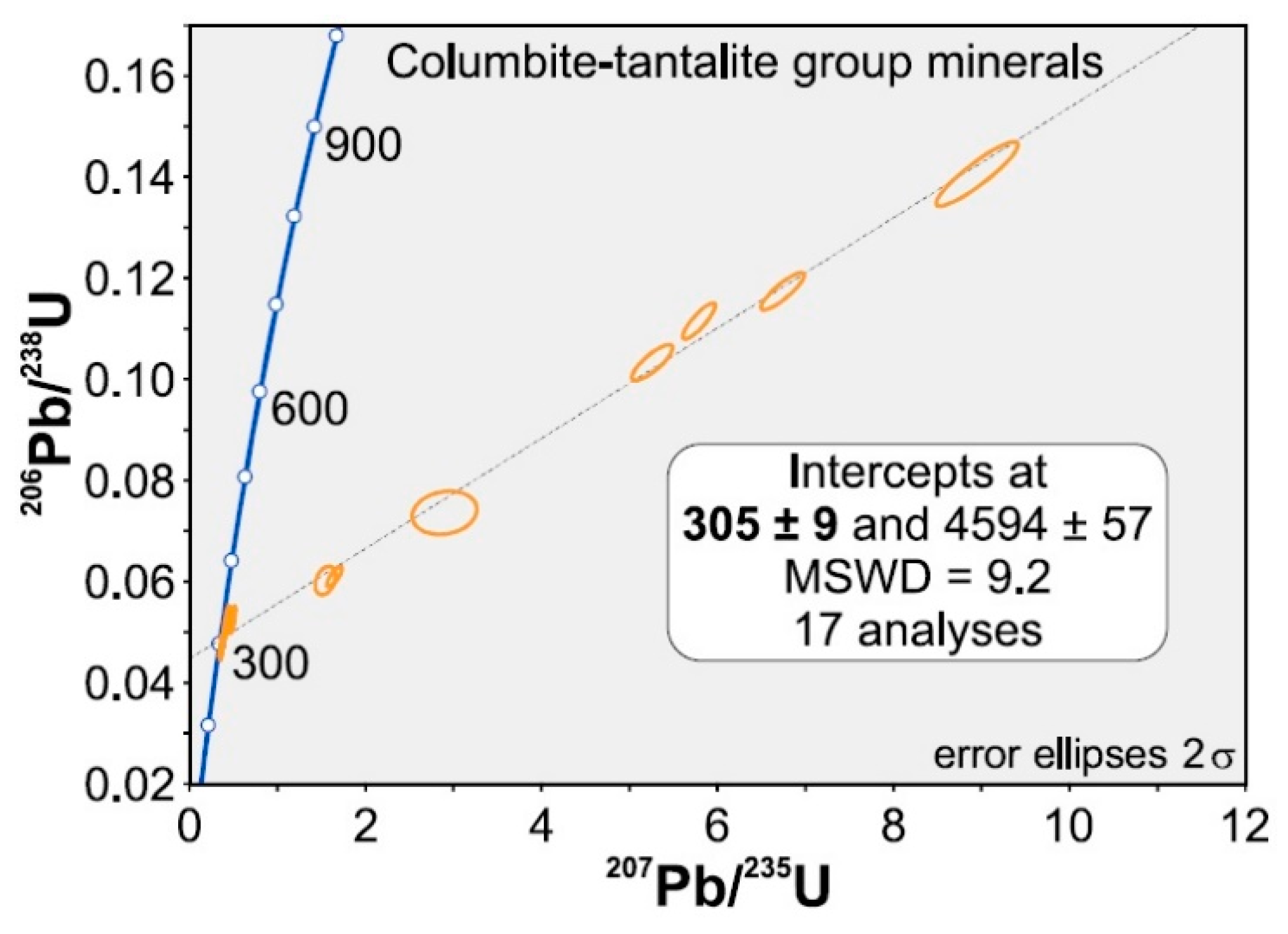
4.3. Geochronology
4.4. Whole-Rock Geochemistry
5. Discussion
5.1. Contraints on Mineral Chemistry of Nb-Ta-Sn Oxides
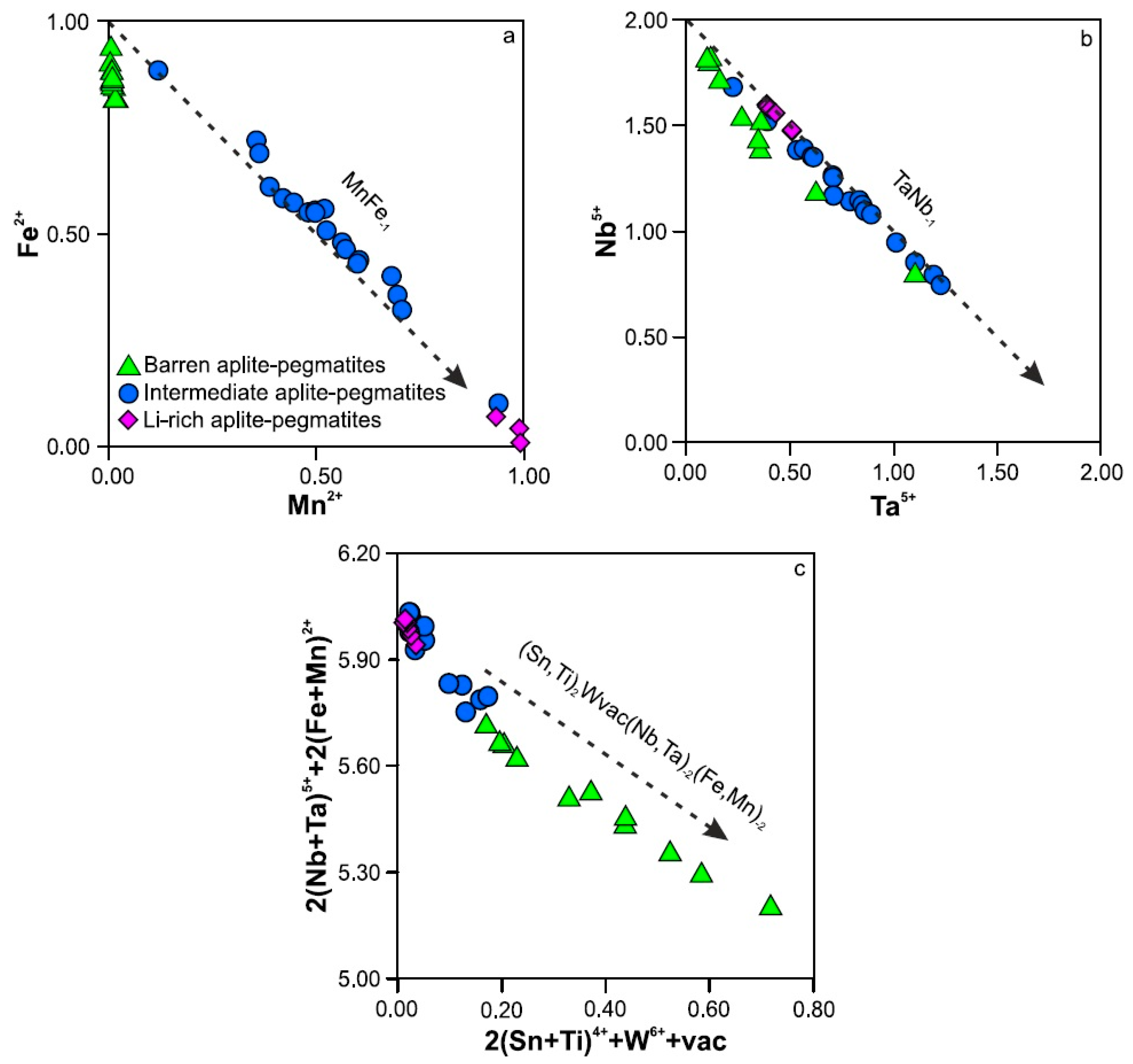
5.1.1. Substitution Mechanisms in Nb-Ta-Sn Oxides
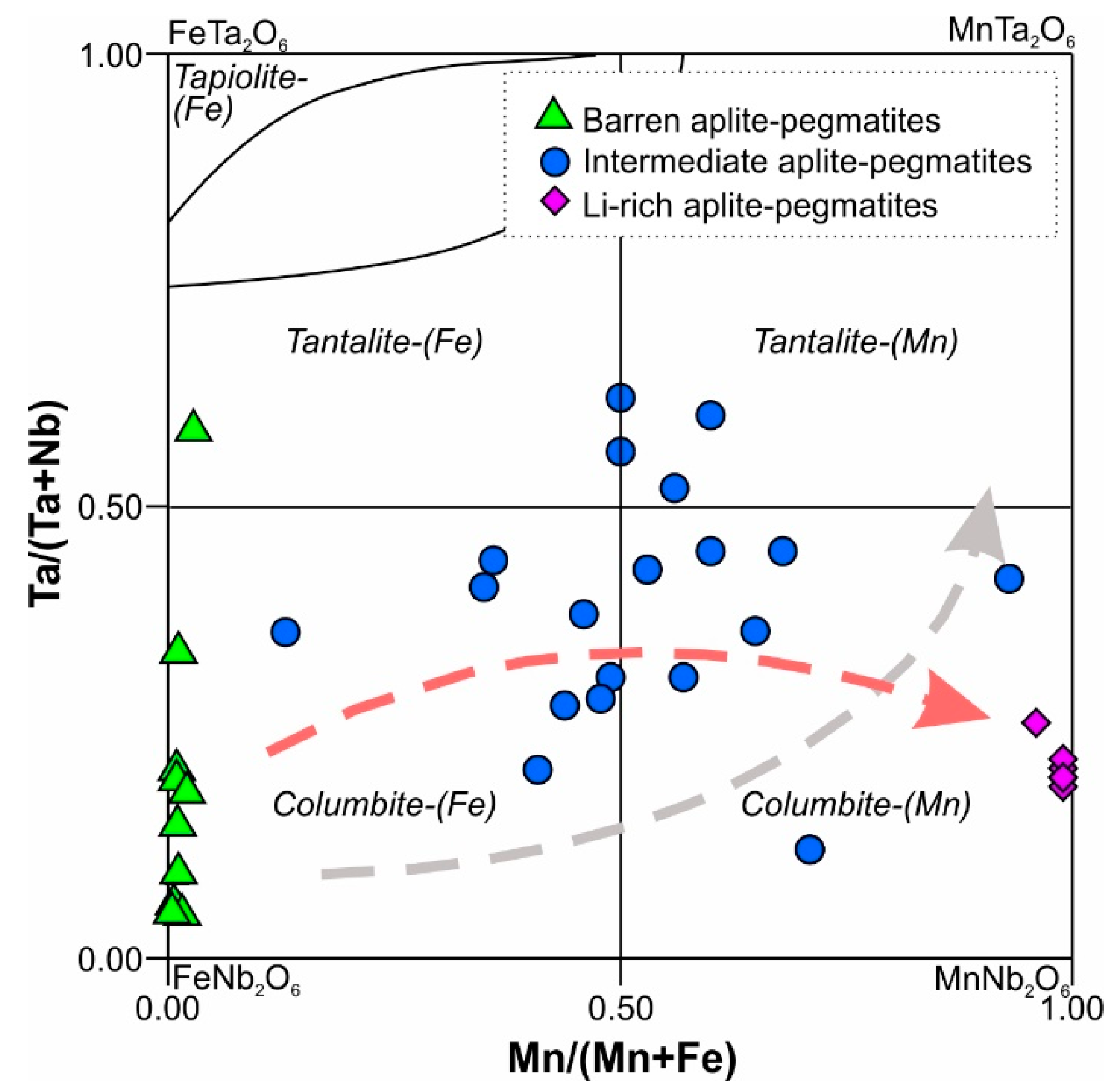
5.1.2. Fractionation of Nb-Ta and Fe-Mn in Columbite-Tantalite Group Minerals
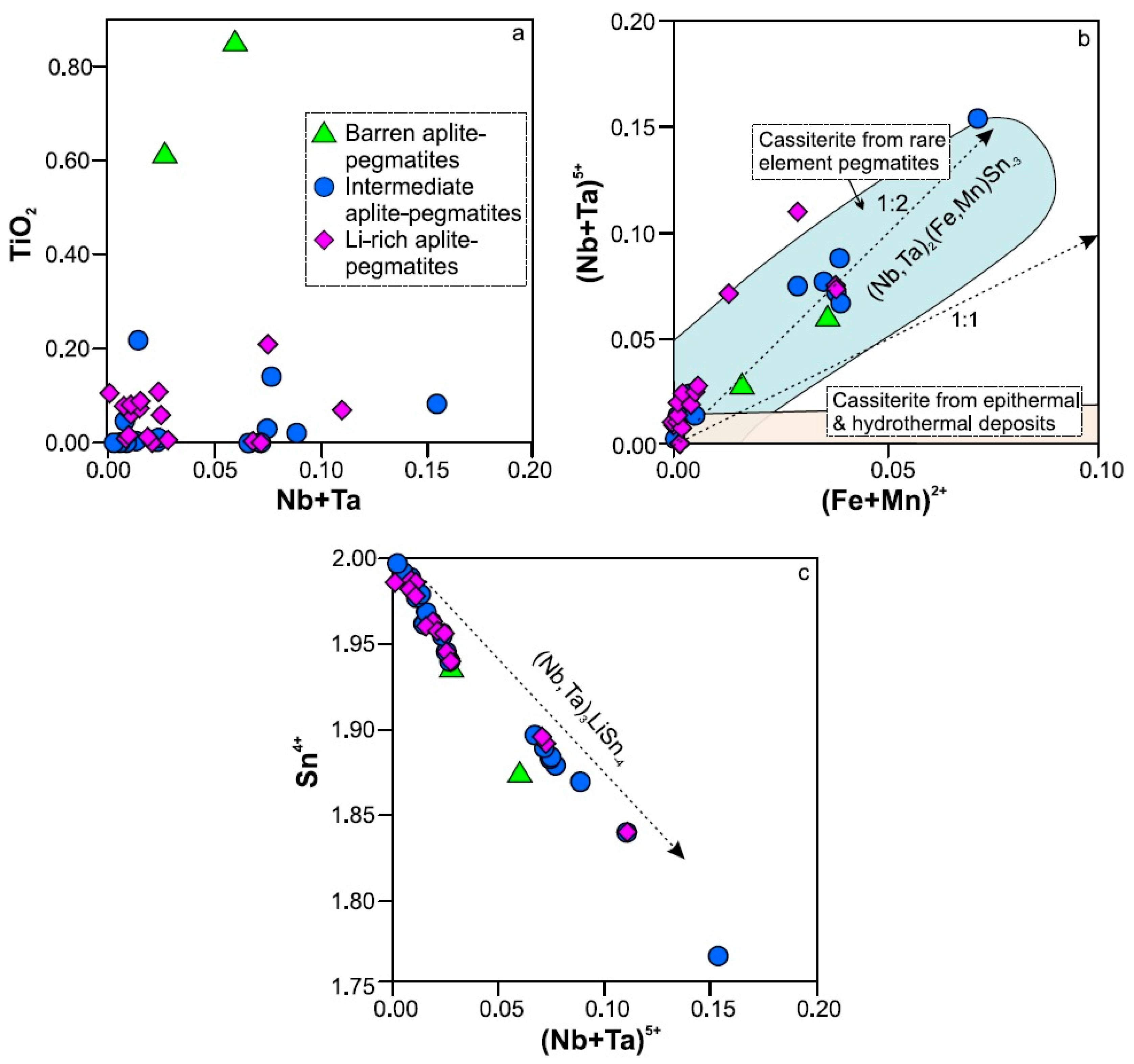
5.1.3. Formation of Primary Cassiterite
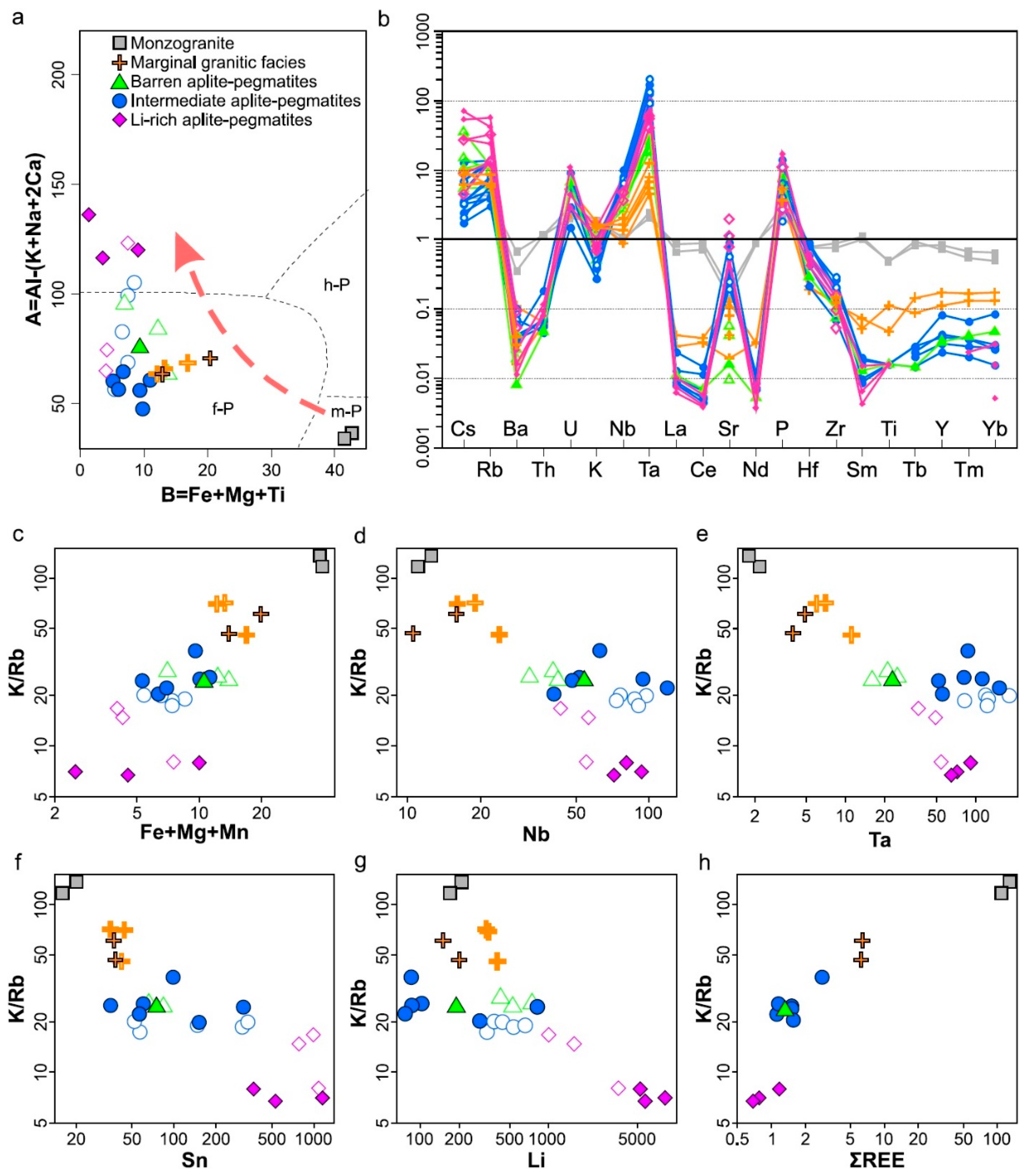
5.2. Petrogenesis of the Aplite-Pegmatites from Tres Arroyos
6. Conclusions
- (1)
- The main substitution mechanism for the CT group minerals seems to be (Ti4+,Sn4+)2W6+1vacancy1(Nb5+,Ta5+), whereas that for cassiterite would be (Nb5+,Ta5+)2(Fe2+,Mn2+)1Sn4+-3.
- (2)
- The Mn/(Mn+Fe) ratio in CT oxides can be used as a petrogenetic indicator in the Tres Arroyos system, suggesting a single sequence of magmatic evolution from the less evolved to the most evolved bodies. The high F and P availability in the parental pegmatitic melts may have caused the lack of an expected progressive Ta/Nb increase with the fractionation.
- (3)
- The fractionation of Nb-Ta and Fe-Mn in the pegmatitic melts reflected in CT oxides seems to be controlled by tourmaline, phosphates and mica crystallization.
- (4)
- Some of the columbite-(Fe) and columbite-(Mn) crystals would have been crystallized by punctual chemical variations in the boundary layer, whereas the crystallization of other CT oxides, mainly those richer in Ta, would be controlled by Ta saturation in the pegmatitic melt. On the other hand, the formation of primary cassiterite may have been related to a high undercooling of the system.
- (5)
- Whole-rock geochemical data of the studied aplite-pegmatites and granitic units mirror the trends defined by mineral chemistry and strengthen the observed geochemical evolution from the NA monzogranite up to the Li-rich bodies.
- (6)
- The newly obtained U-Pb age of 305 ± 9 Ma on CT minerals from the Tres Arroyos aplite-pegmatites reinforces the genetic relationship between the studied aplite-pegmatites and the NA monzogranite, which would represent the parental granitic magma.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kendall-Langley, L.A.; Kemp, A.I.S.; Grigson, J.L.; Hammerli, J. U-Pb and reconnaissance Lu-Hf isotope analysis of cassiterite and columbite group minerals from Archean Li-Cs-Ta type pegmatites of Western Australia. Lithos 2020, 352. [Google Scholar] [CrossRef]
- Stepanov, A.; Mavrogenes, J.; Meffre, S.; Davidson, P. The key role of mica during igneous concentration of tantalum. Contrib. Mineral. Petrol. 2014, 167, 1009. [Google Scholar] [CrossRef]
- Van Lichtervelde, M.; Holtz, F.; Hanchar, J.M. Solubility of manganotantalite, zircon and hafnon in highly fluxed peralkaline to peraluminous pegmatitic melts. Contrib. Mineral. Petrol. 2010, 160, 17–32. [Google Scholar] [CrossRef]
- Küster, D.; Romer, R.L.; Tolessa, D.; Zerihun, D.; Bheemalingeswara, K.; Melcher, F.; Oberthür, T. The Kenticha rare-element pegmatite, Ethiopia: Internal differentiation, U-Pb age and Ta mineralization. Mineral. Depos. 2009, 44, 723–750. [Google Scholar] [CrossRef]
- Mackay, D.A.R.; Simandl, G.J. Geology, market and supply chain of niobium and tantalum-a review. Mineral. Depos. 2014, 49, 1025–1047. [Google Scholar] [CrossRef]
- Che, X.-D.; Wu, F.-Y.; Wang, R.-C.; Gerdes, A.; Ji, W.-Q.; Zhao, Z.-H.; Yang, J.-H.; Zhu, Z.-Y. In situ U-Pb isotopic dating of columbite-tantalite by LA-ICP-MS. Ore Geol. Rev. 2015, 65, 979–989. [Google Scholar] [CrossRef]
- Ballouard, C.; Elburg, M.A.; Tappe, S.; Reinke, C.; Ueckermann, H.; Doggart, S. Magmatic-hydrothermal evolution of rare metal pegmatites from the Mesoproterozoic Orange River pegmatite belt (Namaqualand, South Africa). Ore Geol. Rev. 2020, 116, 103252. [Google Scholar] [CrossRef]
- Breiter, K.; Ackerman, L.; Ďurišová, J.; Svojtka, M.; Novák, M. Trace element composition of quartz from different types of pegmatites: A case study from the Moldanubian Zone of the Bohemian Massif (Czech Republic). Mineral. Mag. 2014, 78, 703–722. [Google Scholar] [CrossRef]
- Fuchsloch, W.C.; Nex, P.A.M.; Kinnaird, J.A. The geochemical evolution of Nb–Ta–Sn oxides from pegmatites of the Cape Cross–Uis pegmatite belt, Namibia. Mineral. Mag. 2019, 83, 161–179. [Google Scholar] [CrossRef]
- Galliski, M.A.; Marquez-Zavalia, M.F.; Černý, P.; Martínez, V.A.; Chapman, R. The Ta-Nb-Sn-Ti oxide-mineral paragenesis from La Viquita, a spodumene-bearing rare-element granitic pegmatite, San Luis, Argentina. Can. Mineral. 2008, 46, 379–393. [Google Scholar] [CrossRef]
- Garate-Olave, I.; Müller, A.; Roda-Robles, E.; Gil-Crespo, P.P.; Pesquera, A. Extreme fractionation in a granite–pegmatite system documented by quartz chemistry: The case study of Tres Arroyos (Central Iberian Zone, Spain). Lithos 2017, 286, 162–164. [Google Scholar] [CrossRef]
- Llorens, T.; Moro, M.C. Oxide minerals in the granitic cupola of the Jálama Batholith, Salamanca, Spain. Part II: Sn, W and Ti minerals in intra-granitic quartz veins. J. Geosci. 2012, 155–171. [Google Scholar] [CrossRef]
- López-Moro, F.J.; Garcia Polonio, F.; Llorens González, T.; Sanz Contreras, J.L.; Fernández Fernández, A.; Moro Benito, M.C. Ta and Sn concentration by muscovite fractionation and degassing in a lens-like granite body: The case study of the Penouta rare-metal albite granite (NW Spain). Ore Geol. Rev. 2017, 82, 10–30. [Google Scholar] [CrossRef]
- Novák, M.; Černý, P.; Uher, P. Extreme variation and apparent reversal of Nb-Ta fractionation in columbite-group minerals from the Scheibengraben beryl-columbite granitic pegmatite, Maršíkov, Czech Republic. Eur. J. Mineral. 2003, 15, 565–574. [Google Scholar] [CrossRef]
- Černý, P.; Goad, B.E.; Hawthorne, F.C.; Chapman, R. Fractionation trends of the Nb- and Ta-bearing oxide minerals in the Greer Lake pegmatitic granite and its pegmatite aureole, southeastern Manitoba. Am. Mineral. 1986, 71, 501–517. [Google Scholar]
- Mulja, T.; Williams-Jones, A.E.; Martin, R.F.; Wood, S.A. Compositional variation and structural state of columbite-tantalite in rare-element granitic pegmatites of the Preissac-Lacorne Batholith, Quebec, Canada. Am. Mineral. 1996, 81, 146–157. [Google Scholar] [CrossRef]
- Linnen, R.L.; Cuney, M. Granite-related rare-element deposits and experimental constraints on Ta-Nb-W-Sn-Zr-Hf mineralization. In Rare-Element Geochemistry and Mineral Deposits; Linnen, R.L., Sammson, I.M., Eds.; Geological Society: London, UK, 2005; pp. 45–68. [Google Scholar]
- Černý, P.; Ercit, T.S. The classification of granitic pegmatites revisited. Can. Mineral. 2005, 43, 2005–2026. [Google Scholar] [CrossRef]
- London, D. Pegmatites; The Canadian Mineralogist, Special Publication nº 10: Ottawa, ON, Canada, 2008; p. 347. [Google Scholar]
- Roda-Robles, E.; Villaseca, C.; Pesquera, A.; Gil-Crespo, P.P.; Vieira, R.; Lima, A.; Garate-Olave, I. Petrogenetic relationships between Variscan granitoids and Li-(F-P)-rich aplite-pegmatites in the Central Iberian Zone: Geological and geochemical constraints and implications for other regions from the European Variscides. Ore Geol. Rev. 2018, 95, 408–430. [Google Scholar] [CrossRef]
- Breiter, K.; Durisova, J.; Hrstka, T.; Korbelova, Z.; Galiova, M.V.; Muller, A.; Simons, B.; Shail, R.K.; Williamson, B.J.; Davies, J.A. The transition from granite to banded aplite-pegmatite sheet complexes: An example from Megiliggar Rocks, Tregonning topaz granite, Cornwall. Lithos 2018, 302, 370–388. [Google Scholar] [CrossRef]
- Galliski, M.Á.; Márquez-Zavalía, M.F.; Pagano, D.S. Metallogenesis of the Totoral LCT rare-element pegmatite district, San Luis, Argentina: A review. J. S. Am. Earth. Sci. 2019, 90, 423–439. [Google Scholar] [CrossRef]
- Müller, A.; Romer, R.L.; Pedersen, R.-B. The Sveconorwegian Pegmatite Province-Thousands of Pegmatites Without Parental Granites. Can. Mineral. 2017, 55, 283–315. [Google Scholar] [CrossRef]
- Konzett, J.; Schneider, T.; Nedyalkova, L.; Hauzenberger, C.; Melcher, F.; Gerdes, A.; Whitehouse, M. Anatectic Granitic Pegmatites from the Eastern Alps: A Case of Variable Rare-Metal Enrichment During High-Grade Regional Metamorphism–I: Mineral Assemblages, Geochemical Characteristics, and Emplacement Ages. Can. Mineral. 2018, 56, 555–602. [Google Scholar] [CrossRef]
- Feng, Y.; Liang, T.; Yang, X.; Zhang, Z.; Wang, Y. Chemical Evolution of Nb-Ta Oxides and Cassiterite in Phosphorus-Rich Albite-Spodumene Pegmatites in the Kangxiwa–Dahongliutan Pegmatite Field, Western Kunlun Orogen, China. Minerals 2019, 9, 166. [Google Scholar] [CrossRef]
- Roda-Robles, E.; Pesquera Pérez, A.; Velasco Roldan, F.; Fontan, F. The granitic pegmatites of the Fregeneda area (Salamanca, Spain): Characteristics and petrogenesis. Mineral. Mag. 1999, 63, 535–558. [Google Scholar] [CrossRef]
- Vieira, R.; Roda-Robles, E.; Pesquera, A.; Lima, A. Chemical variation and significance of micas from the Fregeneda-Almendra pegmatitic field (Central-Iberian Zone, Spain and Portugal). Am. Mineral. 2011, 96, 637–645. [Google Scholar] [CrossRef]
- Tang, Y.; Zhao, J.-Y.; Zhang, H.; Cai, D.-W.; Lv, Z.-H.; Liu, Y.-L.; Zhang, X. Precise columbite-(Fe) and zircon U-Pb dating of the Nanping No. 31 pegmatite vein in northeastern Cathaysia Block, SE China. Ore Geol. Rev. 2017, 83, 300–311. [Google Scholar] [CrossRef]
- Vieira, R. Aplitopegmatitos com Elementos Raros da Região Entre Almendra (V. N. de Foz-Côa) e Barca d’Alba (Figueira de Castelo Rodrigo). Campo Aplitopegmatítico da Fregeneda-Almendra. Ph.D. Thesis, Universidade do Porto, Porto, Portugal, 2010. [Google Scholar]
- Antunes, I.M.H.R.; Neiva, A.M.R.; Farinha Ramos, J.M.; Silva, P.B.; Silva, M.M.V.G.; Corfu, F. Petrogenetic links between lepidolite-subtype aplite-pegmatite, aplite veins and associated granites at Segura (central Portugal). Chem. Erde Geochem. 2013, 73, 323–341. [Google Scholar] [CrossRef]
- Roda-Robles, E.; Pesquera, A.; Gil-Crespo, P.P.; Vieira, R.; Lima, A.; Garate-Olave, I.; Martins, T.; Torres-Ruiz, J. Geology and mineralogy of Li mineralization in the Central Iberian Zone (Spain and Portugal). Mineral. Mag. 2016, 80, 103–126. [Google Scholar] [CrossRef]
- Legros, H.; Mercadier, J.; Villeneuve, J.; Romer, R.L.; Deloule, E.; Van Lichtervelde, M.; Dewaele, S.; Lach, P.; Che, X.-D.; Wang, R.-C.; et al. U-Pb isotopic dating of columbite-tantalite minerals: Development of reference materials and in situ applications by ion microprobe. Chem. Geol. 2019, 512, 69–84. [Google Scholar] [CrossRef]
- Martínez Catalán, J.R.; Fernández-Suárez, J.; Jenner, G.A.; Belousova, E.; Díez Montes, A. Provenance constraints from detrital zircon U-Pb ages in the NW Iberian Massif: Implications for Palaeozoic plate configuration and Variscan evolution. J. Geol. Soc. 2004, 161, 463–476. [Google Scholar] [CrossRef]
- Martínez Catalán, J.R.; Rubio Pascual, F.J.; Díez Montes, A.; Díez Fernández, R.; Gómez Barreiro, J.; Dias Da Silva, I.; González Clavijo, E.; Ayarza, P.; Alcock, J.E. The late Variscan HT/LP metamorphic event in NW and Central Iberia: Relationships to crustal thickening, extension, orocline development and crustal evolution. In Variscan Orogeny: Extent, Timescale and the Formation of the European Crust; Schulmann, K., Martínez Catalán, J.R., Lardeaux, J.M., Janoušek, V., Oggiano, G., Eds.; Geological Society: London, UK, 2014; Volume 405, pp. 225–247. [Google Scholar]
- Talavera, C.; Martínez Poyatos, D.; Francisco, G. SHRIMP U–Pb geochronological constraints on the timing of the intra-Alcudian (Cadomian) angular unconformity in the Central Iberian Zone (Iberian Massif, Spain). Int. J. Earth Sci. 2015, 104, 1739–1757. [Google Scholar] [CrossRef]
- Bea, F.; Montero, P.; González-Lodeiro, F.; Talavera, C. Zircon inheritance reveals exceptionally fast crustal magma generation processes in central iberia during the cambro-ordovician. J. Petrol. 2007, 48, 2327. [Google Scholar] [CrossRef]
- Martínez-Poyatos, D.; Carbonell, R.; Palomeras, I.; Simancas, J.F.; Ayarza, P.; Martí, D.; Azor, A.; Jabaloy, A.; González-Cuadra, P.; Tejero, R.; et al. Imaging the crustal structure of the Central Iberian Zone (Variscan Belt): The ALCUDIA deep seismic reflection transect. Tectonics 2012, 31, TC3017. [Google Scholar] [CrossRef]
- Villaseca, C.; Merino Martínez, E.; Orejana, D.; Andersen, T.; Belousova, E. Zircon Hf signatures from granitic orthogneisses of the Spanish Central System: Significance and sources of the Cambro-Ordovician magmatism in the Iberian Variscan Belt. Gondwana Res. 2016, 34, 60–83. [Google Scholar] [CrossRef]
- Castro, A.; Patiño Douce, A.E.; Corretgé, L.G.; De La Rosa, J.D.; El-Biad, M.; El-Hmidi, H. Origin of peraluminous granites and granodiorites, Iberian massif, Spain: An experimental test of granite petrogenesis. Contrib. Mineral. Petrol. 1999, 135, 255–276. [Google Scholar] [CrossRef]
- Errandonea-Martin, J.; Sarrionandia, F.; Janoušek, V.; Carracedo-Sánchez, M.; Gil-Ibarguchi, J.I. Origin of cordierite-bearing monzogranites from the southern Central Iberian Zone-Inferences from the zoned Sierra Bermeja Pluton (Extremadura, Spain). Lithos 2019, 342, 440–462. [Google Scholar] [CrossRef]
- González-Menéndez, L. Petrología del batolitio granítico de Nisa-Alburquerque. Rev. Soc. Geol. España 1998, 15, 233–246. [Google Scholar]
- Solá, A.R.; Ribeiro, M.L.; Moreira, M.E.; Moreira, M. Complexo eruptivo de Nisa cartografia geoquímica e mecanismo de implantação. In Actas do V Congresso Nacional de Geologia; Instituto Geológico e Mineiro: Lisboa, Portugal, November 1998; pp. B39–B42. [Google Scholar]
- González-Menéndez, L.; Azor, A.; Rubio-Ordóñez, Á.; Sánchez-Almazo, I. The metamorphic aureole of the Nisa-Alburquerque batholith (SW Iberia): Implications for deep structure and emplacement mode. Int. J. Earth Sci. 2011, 100, 1533–1550. [Google Scholar] [CrossRef]
- Gutiérrez-Alonso, G.; Fernández-Suárez, J.; Jeffries, T.E.; Johnston, S.T.; Pastor-Galán, D.; Murphy, J.B.; Franco, M.P.; Gonzalo, J.C. Diachronous post-orogenic magmatism within a developing orocline in Iberia, European Variscides. Tectonics 2011, 30, TC5008. [Google Scholar] [CrossRef]
- Solá, A.R.; Williams, I.S.; Neiva, A.M.R.; Ribeiro, M.L. U-Th-Pb SHRIMP ages and oxygen isotope composition of zircon from two contrasting late Variscan granitoids, Nisa-Albuquerque batholith, SW Iberian Massif: Petrologic and regional implications. Lithos 2009, 111, 156–167. [Google Scholar] [CrossRef]
- Gallego Garrido, M. Las mineralizaciones de Li asociadas a magmatismo ácido en Extremadura y su encuadre en la Zona Centro-Ibérica. Ph.D. Thesis, Complutense University of Madrid, Madrid, Spain, 1992. [Google Scholar]
- Garate-Olave, I.; Roda-Robles, E.; Gil-Crespo, P.P.; Pesquera, A. Mica and feldspar as indicators of the evolution of a highly evolved granite-pegmatite system in the Tres Arroyos area (Central Iberian Zone, Spain). J. Iberian Geol. 2018, 44, 375–403. [Google Scholar] [CrossRef]
- London, D.; Wolf, M.B.; Morgan, G.B.; Gallego-Garrido, M. Experimental Silicate–Phosphate Equilibria in Peraluminous Granitic Magmas, with a Case Study of the Alburquerque Batholith at Tres Arroyos, Badajoz, Spain. J. Petrol. 1999, 40, 215–240. [Google Scholar] [CrossRef]
- Garate-Olave, I.; Roda-Robles, E.; Gil-Crespo, P.P.; Pesquera, A. The phosphate mineral associations from the Tres Arroyos aplite-pegmatites (Badajoz, Spain): Petrography, mineral chemistry and petrogenetic implications. Can. Mineral. 2020, in press. [Google Scholar]
- Farias, P.; Gallastegui, G.; González-Lodeiro, F.; Marquínez, J.; Martín-Parra, L.M.; Martínez Catalán, J.R.; de Pablo Maciá, J.G.; Rodríguez-Fernández, L.R. Aportaciones al conocimiento de la litoestratigrafia y estructura de Galicia central. Mem. Museo e Lab. Miner. Geol. Fac. Ciencias, Univ. Oporto 1987, 1, 411–431. [Google Scholar]
- Julivert, M.; Marcos, A.; Truyols, J. L’évolution paléogéographique du NW de l’Espagne pendant l’Ordovicien–Silurien. Bull. Soc. Geol. Min. Bret. 1972, 4, 1–7. [Google Scholar]
- Lotze, F. Zur Gliederung der Varisziden in der Iberischen Meseta. Geoteckt Forsch 1945, 6, 78–92. [Google Scholar]
- Rodríguez Fernández, L.R.; Oliveira, J.T. Mapa Geológico de España y Portugal a escala 1:1.000.000; Instituto Geológico y Minero de España, Laboratório Nacional de Energía e Geología de Portugal: Madrid, Spain, 2015. [Google Scholar]
- Pouchou, J.L.; Pichoir, F. “PAP” φ(ρZ) procedure for improved quantitative microanalysis. In Microbean Analysis; Armstrong, J.T., Ed.; San Francisco Press: San Francisco, CA, USA, 1985; pp. 104–106. [Google Scholar]
- Jackson, S.E.; Pearson, N.J.; Griffin, W.L.; Belousova, E.A. The application of laser ablation-inductively coupled plasma-mass spectrometry to in situ U-Pb zircon geochronology. Chem. Geol. 2004, 211, 47–69. [Google Scholar] [CrossRef]
- Paton, C.; Hellstrom, J.; Paul, B.; Woodhead, J.; Hergt, J. Iolite: Freeware for the visualisation and processing of mass spectrometric data. J. Anal. At. Spectrom. 2011, 26. [Google Scholar] [CrossRef]
- Chew, D.M.; Petrus, J.A.; Kamber, B.S. U-Pb LA-ICPMS dating using accessory mineral standards with variable common Pb. Chem. Geol. 2014, 363, 185–199. [Google Scholar] [CrossRef]
- García de Madinabeitia, S.; Garate-Olave, I.; Roda-Robles, E.; Gil-Crespo, P.P.; Gil-Ibarguchi, J.I. Datación U-Pb de columbita-tantalita mediante LA-Q-ICP-MS. Ejemplo: Granito/aplopegmatitas de Tres Arroyos (Zona Centro-Ibérica). Macla 2019, 24, 23. [Google Scholar]
- Whitney, D.L.; Evans, B.W. Abbreviations for names of rock-forming minerals. Am. Mineral. 2010, 95, 185–187. [Google Scholar] [CrossRef]
- Černý, P.; Ercit, T.S. Mineralogy of niobium and tantalum: Crystal chemistry relationship, paragenetic aspects and their economic implications. In Lanthanides, Tantalum and Niobium; Moller, P., Černý, P., Saupé, F., Eds.; Spring: Berlin, Germany, 1989; pp. 27–29. [Google Scholar]
- Tindle, A.G.; Breaks, F.W. Oxide minerals of the Separation Rapids rare-element granitic pegmatite group, northwestern Ontario. Can. Mineral. 1998, 36, 609–635. [Google Scholar]
- Shand, S.J. The Eruptive Rocks, 2nd ed.; John Wiley: New York, NY, USA, 1943. [Google Scholar]
- Debon, F.; Lefort, P. A chemical-mineralogical classification of common plutonic rocks and associations. Trans. R. Soc. Edinb. Earth Sci. 1983, 73, 135–149. [Google Scholar] [CrossRef]
- Villaseca, C.; Barbero, L.; Herreros, V. A re-examination of the typology of peraluminous granite types in intracontinental orogenic belts. Trans. R. Soc. Edinb. Earth Sci. 1998, 89, 113–119. [Google Scholar] [CrossRef]
- Rudnick, R.L.; Gao, S. 4.1-Composition of the Continental Crust. In Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Oxford, UK, 2014; Volume 4, pp. 1–51. [Google Scholar]
- Pistorino, M.; Nestola, F.; Boffa Ballaran, T.; Domeneghetti, M.C. The effect of composition and cation ordering on the compressibility of columbites up to 7 GPa. Phys. Chem. Minerals 2006, 33, 593–600. [Google Scholar] [CrossRef]
- Izoret, L.; Marnier, G.; Dusausoy, Y. Crystallochemical classification of cassiterite from tin and tungsten deposits in Galicia, Spain. Can. Mineral. 1985, 23, 221–231. [Google Scholar]
- Raimbault, L. Composition of complex lepidolite-type granitic pegmatites and of constituent columbite-tantalite, Chèdeville, Massif Central, France. Can. Mineral. 1998, 36, 563–583. [Google Scholar]
- Linnen, R.; Keppler, H. Columbite solubility in granitic melts: Consequences for the enrichment and fractionation of Nb and Ta in the Earth’s crust. Contrib. Mineral. Petrol. 1997, 128, 213–227. [Google Scholar] [CrossRef]
- Bartels, A.; Vetere, F.; Holtz, F.; Behrens, H.; Linnen, R.L. Viscosity of flux-rich pegmatitic melts. Contrib. Mineral. Petrol. 2011, 162, 51–60. [Google Scholar] [CrossRef]
- Breiter, K.; Škoda, R.; Uher, P. Nb-Ta-Ti-W-Sn-oxide minerals as indicators of a peraluminous P- and F-rich granitic system evolution: Podlesi, Czech Republic. Mineral. Petrol. 2007, 91, 225–248. [Google Scholar] [CrossRef]
- Keppler, H. Influence of fluorine on the enrichment of high field strength trace elements in granitic rocks. Contrib. Mineral. Petrol. 1993, 114, 479–488. [Google Scholar] [CrossRef]
- Linnen, R.L. Depth of emplacement, fluid provenance and metallogeny in granitic terranes: A comparison of western Thailand with other tin belts. Mineral. Depos. 1998, 33, 461–476. [Google Scholar] [CrossRef]
- Wolf, M.B.; London, D.; Morgan, G.B.V. Effects of boron on the solubility of cassiterite and tantalite in granitic liquids. Soc. Am. Prog. Abstr. 1994, 26, A450. [Google Scholar]
- London, D.; Morgan Vi, G.B.; Wolf, M.B. Amblygonite-montebrasite solid solutions as monitors of fluorine in evolved granitic and pegmatitic melts. Am. Mineral. 2001, 86, 225–233. [Google Scholar] [CrossRef]
- Lukkari, S. Magmatic Evolution of Topaz-Bearing Granite Stocks within the Wiborg Rapakivi Granite Batholith. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 2007. [Google Scholar]
- Černý, P.; Novák, M.; Chapman, R. Effects of sillimanite-grade metamorphism and shearing on Nb-Ta oxide minerals in granitic pegmatites, Marsikov, northern Moravia, Czechoslovakia. Can. Mineral. 1992, 30, 699–718. [Google Scholar]
- Lahti, S.I. Zoning in columbite-tantalite crystals from the granitic pegmatites of the Eräjärvi area, southern Finland. Geochim. Cosmochim. Acta 1987, 51, 509–517. [Google Scholar] [CrossRef]
- Tindle, A.G.; Breaks, F.W. Columbite-tantalite mineral chemistry from rare-element granitic pegmatites: Separation Lake area, N.W. Ontario, Canada. Mineral. Petrol. 2000, 70, 165–198. [Google Scholar] [CrossRef]
- Aseri, A.A.; Linnen, R.L.; Che, X.D.; Thibault, Y.; Holtz, F. Effects of fluorine on the solubilities of Nb, Ta, Zr and Hf minerals in highly fluxed water-saturated haplogranitic melts. Ore Geol. Rev. 2015, 64, 736–746. [Google Scholar] [CrossRef]
- Fiege, A.; Kirchner, C.; Holtz, F.; Linnen, R.L.; Dziony, W. Influence of fluorine on the solubility of manganotantalite (MnTa2O6) and manganocolumbite (MnNb2O6) in granitic melts-An experimental study. Lithos 2011, 122, 165–174. [Google Scholar] [CrossRef]
- Raimbault, L.; Burnol, L. The Richemont rhyolite dyke, Massif Central, France: A subvolcanic equivalent of rare-metal granites. Can. Mineral. 1998, 36, 265–282. [Google Scholar]
- Llorens González, T.; García Polonio, F.; López Moro, F.J.; Fernández Fernández, A.; Sanz Contreras, J.L.; Moro Benito, M.C. Tin-tantalum-niobium mineralization in the Penouta deposit (NW Spain): Textural features and mineral chemistry to unravel the genesis and evolution of cassiterite and columbite group minerals in a peraluminous system. Ore Geol. Rev. 2017, 81, 79–95. [Google Scholar] [CrossRef]
- Wise, M.A.; Francis, C.A.; Černý, P. Compositional and structural variations in columbite-group minerals from granitic pegmatites of the Brunswick and Oxford fields, Maine: Differential trends in F-poor and F-rich environments. Can. Mineral. 2012, 50, 1515–1530. [Google Scholar] [CrossRef]
- Beurlen, H.; Da Silva, M.R.R.; Thomas, R.; Soares, D.R.; Olivier, P. Nb-Ta-(Ti-Sn) oxide mineral chemistry as tracer of rare-element granitic pegmatite fractionation in the Borborema Province, Northeastern Brazil. Mineral. Depos. 2008, 43, 207–228. [Google Scholar] [CrossRef]
- Pieczka, A. Primary Nb-Ta minerals in the Szklary pegmatite, Poland: New insights into controls of crystal chemistry and crystallization sequences. Am. Mineral. 2010, 95, 1478–1492. [Google Scholar] [CrossRef]
- Van Lichtervelde, M.; Salvi, S.; Beziat, D.; Linnen, R.L. Textural features and chemical evolution in tantalum oxides: Magmatic versus hydrothermal origins for Ta mineralization in the Tanco Lower Pegmatite, Manitoba, Canada. Econ. Geol. 2007, 102, 257–276. [Google Scholar] [CrossRef]
- Jolliff, B.L.; Papike, J.J.; Laul, J.C. Mineral recorders of pegmatite internal evolution; REE contents of tourmaline from the Bob Ingersoll Pegmatite, South Dakota. Geochim. Cosmochim. Acta 1987, 51, 2225–2232. [Google Scholar] [CrossRef]
- Selway, J.B.; Novák, M.; Černý, P.; Hawthorne, F.C. The Tanco pegmatite at Bernic Lake, Manitoba. XIII. Exocontact tourmaline. Can. Mineral. 2000, 38, 869–876. [Google Scholar] [CrossRef]
- Monier, G.; Charoy, B.; Cuney, M.; Ohnenstetter, D.; Robert, J.L. Évolution spatiale et temporelle de la composition des micas du granite albitique á topaze-lépidolite de Beauvoir. Géol. France 1987, 2, 179–188. [Google Scholar]
- Bhalla, P.; Holtz, F.; Linnen, R.L.; Behrens, H. Solubility of cassiterite in evolved granitic melts: Effect of T, fO2, and additional volatiles. Lithos 2005, 80, 387–400. [Google Scholar] [CrossRef]
- Xie, L.; Wang, R.-C.; Groat, L.A.; Zhu, J.-C.; Huang, F.-F.; Cempírek, J. A combined EMPA and LA-ICP-MS study of Li-bearing mica and Sn-Ti oxide minerals from the Qiguling topaz rhyolite (Qitianling District, China): The role of fluorine in origin of tin mineralization. Ore Geol. Rev. 2015, 65, 779–792. [Google Scholar] [CrossRef]
- Heinrich, C.A. Geochemical evolution and hydrothermal mineral deposition in Sn(–W-base metal) and other granite-related ore systems: Some conclusions from Australian examples. In Magmas, Fluids, and Ore Deposits; Thompson, J.F.H., Ed.; Mineralogical Association of Canada Short Course: Québec, QC, Canada, 1995. [Google Scholar]
- Candela, P.A. A review of shallow, ore-related granites: Textures, volatiles, and ore metals. J. Petrol. 1997, 38, 1619–1633. [Google Scholar] [CrossRef]
- Linnen, R.; Williams-Jones, A.E.; Martin, R.F. Evidence of magmatic cassiterite mineralization at the Nong Sua aplite-pegmatite complex, Thailand. Can. Mineral. 1992, 30, 739–761. [Google Scholar]
- London, D. Ore-forming processes within granitic pegmatites. Ore Geol. Rev. 2018, 101, 349–383. [Google Scholar] [CrossRef]
- Simmons, W.B.; Webber, K.L. Pegmatite genesis: State of the art. Eur. J. Mineral. 2008, 20, 421–438. [Google Scholar] [CrossRef]
- Roda-Robles, E.; Vieira, R.; Pesquera, A.; Lima, A. Chemical variations and significance of phosphates from the Fregeneda-Almendra pegmatite field, Central Iberian Zone (Spain and Portugal). Mineral. Petrol. 2010, 100, 23–34. [Google Scholar] [CrossRef]
- Deng, X.D.; Li, J.W.; Zhao, X.F.; Hu, Z.C.; Hu, H.; Selby, D.; de Souza, Z.S. U-Pb isotope and trace element analysis of columbite-(Mn) and zircon by laser ablation ICP-MS: Implications for geochronology of pegmatite and associated ore deposits. Chem. Geol. 2013, 344, 1–11. [Google Scholar] [CrossRef]
- Romer, R.L.; Wright, J.A. U-Pb dating of columbites: A geochronologic tool to date magmatism and ore deposits. Geochim. Cosmochim. Acta 1992, 56, 2137–2142. [Google Scholar] [CrossRef]
- Melleton, J.; Gloaguen, E. Timing of rare-elements (Li-Be-Ta-Sn-Nb) magmatism in the European Variscan belt The Variscan Belt: Correlations and plate dynamics. Geol. France 2015, 1, 100. [Google Scholar]
- Villaseca, C. On the origin of granite types in the Central Iberian Zone: Contribution from integrated U-Pb and Hf isotope studies of zircon. In Proceedings of the VIII Congresso Ibérico de Geoquímica, Castelo Branco, Portugal, 24 September 2011; pp. 29–34. [Google Scholar]
- Dingwell, D.B.; Hess, K.U.; Knoche, R. Granite and granitic pegmatite melts: Volumes and viscosities. Trans. Roy. Soc. Edinb. Earth Sci. 1996, 87, 65–72. [Google Scholar] [CrossRef]
- Holtz, F.; Johannes, W.; Tamic, N.; Behrens, H. Maximum and minimum water contents of granitic melts generated in the crust: A reevaluation and implications. Lithos 2001, 56, 1–14. [Google Scholar] [CrossRef]
- Tartèse, R.; Boulvais, P. Differentiation of peraluminous leucogranites “en route” to the surface. Lithos 2010, 114, 353–368. [Google Scholar] [CrossRef]
- Roda-Robles, E.; Pesquera, A.; Gil-Crespo, P.P.; Torres-Ruiz, J. From granite to highly evolved pegmatite: A case study of the Pinilla de Fermoselle granite–pegmatite system (Zamora, Spain). Lithos 2012, 153, 192–207. [Google Scholar] [CrossRef]

| Lithology | Main Minerals | Accessory Minerals | Oxides Crystal Size | Oxides Texture | Main Petrographic Textures |
|---|---|---|---|---|---|
| Barren aplite-pegmatites | Quartz, plagioclase, K-feldspar, muscovite, tourmaline | ‘Zinnwaldite’ mineral series, topaz, Fe-Mn-(± Mg ± Al) phosphates, fluorapatite, Sn-Nb-Ta oxides | CT: Very fine < 2 mm Cassiterite: Very fine < 2 mm | Sub-anhedral, elongated-prismatic to acicular CT Slight concentric zoning, irregular patches and corrosion gulfs in CT | Layered texture, alternating pegmatitic and aplitic layers; locally greenish colour in hand sample due to Fe-Mn phosphates alteration; feldspars display comb-texture |
| Intermediate aplite-pegmatites | Quartz, plagioclase, K-feldspar, muscovite | Topaz, Li-Al phosphates, fluorapatite, Sn-Nb-Ta oxides | CT: Very fine < 2 mm Cassiterite: Very fine < 2 mm | Sub-anhedral Well-develop primary faces in CT Concentric zoning, irregular patches and corrosion gulfs in CT | Layering: alternating albite-rich and quartz-rich layers, grain size also may change; aplitic albite: wedged crystals; coarse feldspars display comb-texture |
| Li-rich aplite-pegmatites | Quartz, plagioclase, K-feldspar, Li-Al-mica | Topaz, Li-Al phosphates, Sn-Nb-Ta oxides, fluorapatite | CT: Very fine < 3 mm Cassiterite: Very fine < 5 mm | Subhedral elongated-prismatic to acicular CT Anhedral-subhedral cassiterite with chromatic zoning Concentric zoning, slight irregular patches and less corrosion gulfs in CT | Rhythmic layering, some with a complex pattern, with alternating albite-rich and Li-mica rich layers; feldspars display comb-texture |
| Lithology | Barren Aplite-Pegmatites | Intermediate Aplite-Pegmatites | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mineral | Tnf | Clf | Clf | Clf | Clf | Tnm | Tnm | Tnf | Clm | Clm |
| Number | 1 | 5 | 6 | 8 | 9 | 12 | 13 | 15 | 17 | 18 |
| wt% | ||||||||||
| TiO2 | 2.19 | 1.37 | 1.35 | 4.77 | 1.26 | - | 0.00 | 0.02 | 0.15 | 0.96 |
| MnO | 0.23 | 0.09 | 0.16 | 0.14 | 0.19 | 9.77 | 8.35 | 7.88 | 10.30 | 13.92 |
| FeO | 13.17 | 18.54 | 18.13 | 16.43 | 18.12 | 6.49 | 8.46 | 8.14 | 6.95 | 5.78 |
| Nb2O5 | 23.01 | 69.16 | 69.12 | 50.81 | 68.91 | 23.72 | 25.10 | 21.60 | 33.88 | 61.61 |
| SnO2 | 0.86 | 0.00 | 0.04 | 0.92 | 0.02 | 0.11 | 0.09 | 0.19 | 0.16 | 0.19 |
| Ta2O5 | 52.97 | 6.64 | 7.53 | 20.66 | 6.42 | 59.40 | 53.99 | 59.18 | 46.75 | 13.74 |
| WO3 | 1.34 | 3.54 | 2.96 | 3.74 | 4.33 | 0.89 | 1.21 | 0.92 | 0.67 | 2.55 |
| MgO | 0.00 | 0.03 | 0.00 | 0.00 | 0.02 | - | - | - | - | 0.00 |
| Total | 93.77 | 99.37 | 99.28 | 97.48 | 99.26 | 100.38 | 97.20 | 97.93 | 98.85 | 98.79 |
| apfu | ||||||||||
| Ti | 0.127 | 0.060 | 0.059 | 0.224 | 0.055 | 0.000 | 0.000 | 0.001 | 0.008 | 0.044 |
| Mn | 0.015 | 0.004 | 0.008 | 0.008 | 0.009 | 0.608 | 0.529 | 0.507 | 0.612 | 0.713 |
| Fe | 0.845 | 0.902 | 0.884 | 0.857 | 0.883 | 0.399 | 0.529 | 0.517 | 0.408 | 0.292 |
| Nb | 0.797 | 1.819 | 1.822 | 1.433 | 1.817 | 0.788 | 0.848 | 0.741 | 1.075 | 1.684 |
| Sn | 0.026 | 0.000 | 0.001 | 0.023 | 0.000 | 0.003 | 0.003 | 0.006 | 0.004 | 0.005 |
| Ta | 1.104 | 0.105 | 0.119 | 0.350 | 0.102 | 1.187 | 1.098 | 1.222 | 0.892 | 0.226 |
| W | 0.027 | 0.053 | 0.045 | 0.060 | 0.065 | 0.017 | 0.023 | 0.018 | 0.012 | 0.040 |
| Mg | 0.000 | 0.003 | 0.000 | 0.000 | 0.002 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| XTa | 0.581 | 0.055 | 0.061 | 0.197 | 0.053 | 0.601 | 0.564 | 0.622 | 0.454 | 0.118 |
| XMn | 0.017 | 0.005 | 0.009 | 0.009 | 0.011 | 0.604 | 0.500 | 0.495 | 0.600 | 0.709 |
| Ʃ Cations | 2.941 | 2.943 | 2.938 | 2.955 | 2.932 | 3.001 | 3.030 | 3.012 | 3.012 | 3.004 |
| Ʃ Charges | 11.998 | 11.997 | 11.997 | 11.996 | 11.996 | 11.999 | 11.999 | 11.999 | 11.999 | 11.994 |
| Lithology | Intermediate Aplite-Pegmatites | Li-Rich Aplite-Pegmatites | ||||||||
| Mineral | Clm | Clm | Clf | Clf | Clf | Clm | Clm | Clm | Clm | Clm |
| Number | 19 | 20 | 23 | 27 | 30 | 31 | 32 | 33 | 34 | 37 |
| wt% | ||||||||||
| TiO2 | 0.36 | 0.22 | - | 0.34 | 0.79 | 0.03 | 0.02 | - | 0.02 | 0.01 |
| MnO | 10.40 | 16.31 | 7.98 | 6.38 | 7.45 | 17.36 | 18.67 | 19.12 | 18.98 | 18.99 |
| FeO | 7.86 | 1.22 | 9.68 | 11.37 | 11.08 | 0.73 | 0.13 | 0.16 | 0.21 | 0.21 |
| Nb2O5 | 45.22 | 36.79 | 38.37 | 35.06 | 53.52 | 50.85 | 55.86 | 56.61 | 56.38 | 54.51 |
| SnO2 | 0.10 | 0.37 | 0.66 | 0.19 | 1.19 | 0.49 | 0.30 | 0.25 | 0.13 | 0.12 |
| Ta2O5 | 34.21 | 44.05 | 38.59 | 44.98 | 22.96 | 29.22 | 23.50 | 22.59 | 22.88 | 25.00 |
| WO3 | 0.65 | 0.36 | 5.45 | 0.57 | 2.06 | 0.60 | 0.58 | 0.44 | 0.42 | 0.41 |
| MgO | 0.00 | - | - | - | 0.00 | - | - | - | - | - |
| Total | 98.81 | 99.31 | 100.72 | 98.89 | 99.09 | 99.28 | 99.05 | 99.17 | 99.00 | 99.26 |
| apfu | ||||||||||
| Ti | 0.018 | 0.011 | 0.000 | 0.018 | 0.037 | 0.001 | 0.001 | 0.000 | 0.001 | 0.000 |
| Mn | 0.581 | 0.950 | 0.455 | 0.376 | 0.397 | 0.943 | 0.991 | 1.010 | 1.005 | 1.013 |
| Fe | 0.434 | 0.070 | 0.544 | 0.661 | 0.582 | 0.039 | 0.007 | 0.009 | 0.011 | 0.011 |
| Nb | 1.350 | 1.143 | 1.166 | 1.103 | 1.521 | 1.474 | 1.582 | 1.596 | 1.593 | 1.551 |
| Sn | 0.003 | 0.010 | 0.018 | 0.005 | 0.030 | 0.013 | 0.007 | 0.006 | 0.003 | 0.003 |
| Ta | 0.614 | 0.824 | 0.705 | 0.851 | 0.392 | 0.510 | 0.400 | 0.383 | 0.389 | 0.428 |
| W | 0.011 | 0.006 | 0.095 | 0.010 | 0.034 | 0.010 | 0.009 | 0.007 | 0.007 | 0.007 |
| Mg | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| XTa | 0.313 | 0.419 | 0.377 | 0.436 | 0.205 | 0.257 | 0.202 | 0.194 | 0.196 | 0.216 |
| XMn | 0.573 | 0.931 | 0.455 | 0.362 | 0.405 | 0.960 | 0.993 | 0.992 | 0.989 | 0.989 |
| Ʃ Cations | 3.010 | 3.015 | 2.982 | 3.025 | 2.993 | 2.990 | 2.998 | 3.011 | 3.009 | 3.014 |
| Ʃ Charges | 11.998 | 12.000 | 11.994 | 11.999 | 11.994 | 11.999 | 11.999 | 12.000 | 12.000 | 12.000 |
| Lithology | Barren Aplite- Pegmatites | Intermediate Aplite-Pegmatites | Li-Rich Aplite-Pegmatites | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | 1 | 2 | 4 | 5 | 7 | 16 | 17 | 18 | 19 | 24 | 29 |
| wt% | |||||||||||
| TiO2 | 0.85 | 0.61 | 0.22 | 0.14 | 0.08 | 0.01 | 0.09 | 0.21 | 0.00 | 0.01 | 0.06 |
| MnO | 0.01 | 0.00 | 0.00 | 0.10 | 0.17 | 0.01 | 0.60 | 0.02 | 0.01 | 0.02 | 0.09 |
| FeO | 0.86 | 0.39 | 0.03 | 0.73 | 1.55 | 0.01 | 0.09 | 0.00 | 0.00 | 0.02 | 0.06 |
| Nb2O5 | 0.72 | 0.27 | 0.27 | 0.72 | 3.45 | 0.22 | 3.00 | 0.11 | 0.04 | 0.31 | 1.13 |
| SnO2 | 95.21 | 97.53 | 97.95 | 93.62 | 90.01 | 98.90 | 92.18 | 98.86 | 99.50 | 99.20 | 96.52 |
| Ta2O5 | 3.30 | 1.61 | 0.55 | 4.43 | 5.80 | 0.20 | 3.06 | 0.61 | 0.60 | 0.06 | 0.17 |
| WO3 | 0.14 | 0.01 | 0.00 | 0.11 | 0.02 | 0.09 | 0.06 | 0.00 | 0.05 | 0.34 | 0.98 |
| MgO | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| BaO | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Total | 101.07 | 100.42 | 99.33 | 99.84 | 101.07 | 99.44 | 99.16 | 99.82 | 100.21 | 99.95 | 99.00 |
| apfu | |||||||||||
| Ti | 0.031 | 0.023 | 0.008 | 0.005 | 0.003 | 0.000 | 0.003 | 0.008 | 0.000 | 0.000 | 0.002 |
| Mn | 0.000 | 0.000 | 0.000 | 0.004 | 0.007 | 0.000 | 0.025 | 0.001 | 0.000 | 0.001 | 0.004 |
| Fe | 0.035 | 0.016 | 0.001 | 0.031 | 0.064 | 0.000 | 0.004 | 0.000 | 0.000 | 0.001 | 0.003 |
| Nb | 0.016 | 0.006 | 0.006 | 0.016 | 0.077 | 0.005 | 0.068 | 0.002 | 0.001 | 0.007 | 0.026 |
| Sn | 1.873 | 1.934 | 1.962 | 1.879 | 1.768 | 1.988 | 1.840 | 1.978 | 1.987 | 1.982 | 1.940 |
| Ta | 0.044 | 0.022 | 0.008 | 0.061 | 0.078 | 0.003 | 0.042 | 0.008 | 0.008 | 0.001 | 0.002 |
| W | 0.002 | 0.000 | 0.000 | 0.001 | 0.000 | 0.001 | 0.001 | 0.000 | 0.001 | 0.004 | 0.013 |
| Mg | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Ba | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| XTa | 0.734 | 0.784 | 0.549 | 0.787 | 0.503 | 0.356 | 0.380 | 0.777 | 0.903 | 0.095 | 0.083 |
| XMn | 0.008 | 0.000 | 0.000 | 0.122 | 0.099 | 0.453 | 0.868 | 0.795 | 0.791 | 0.603 | 0.577 |
| Ʃ Cations | 2.002 | 2.001 | 1.985 | 1.998 | 1.997 | 1.998 | 1.983 | 1.998 | 1.997 | 1.997 | 1.990 |
| Ʃ Charges | 8.000 | 8.000 | 7.952 | 8.000 | 8.000 | 8.000 | 7.986 | 8.000 | 7.998 | 8.000 | 7.999 |
| Spot Name | Data for Wetherill Plot | Data for Tera-Wasserburg Plot | Elemental Concentration (µg g−1) a | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 207Pb/235U | 2s (abs) | 206Pb/238U | 2s (abs) | Rho | 238U/206Pb | 2s (abs) | 207Pb/206Pb | 2s (abs) | Rho | U | Th | Pb | |
| CT-01 | 0.361 | 0.012 | 0.046 | 0.001 | 0.74 | 21.9 | 0.527 | 0.056 | 0.002 | 0.27 | 254 | 0.72 | 1.83 |
| CT-02 | 0.414 | 0.017 | 0.051 | 0.002 | 0.72 | 19.8 | 0.664 | 0.058 | 0.002 | 0.26 | 308 | 1.55 | 9.6 |
| CT-03 | 0.381 | 0.012 | 0.048 | 0.001 | 0.71 | 20.8 | 0.478 | 0.057 | 0.002 | 0.36 | 254 | 1.06 | 4.09 |
| CT-04 | 0.414 | 0.015 | 0.052 | 0.002 | 0.84 | 19.1 | 0.548 | 0.056 | 0.001 | 0.42 | 488 | 3.14 | 12.6 |
| CT-05 | 0.367 | 0.015 | 0.048 | 0.001 | 0.61 | 21.1 | 0.620 | 0.055 | 0.002 | 0.24 | 493 | 2.15 | 7.51 |
| CT-06 | 0.385 | 0.013 | 0.049 | 0.001 | 0.79 | 20.6 | 0.550 | 0.056 | 0.002 | 0.23 | 359 | 1.04 | 4.20 |
| CT-07 | 0.360 | 0.012 | 0.047 | 0.001 | 0.77 | 21.4 | 0.548 | 0.055 | 0.001 | 0.24 | 626 | 2.31 | 6.50 |
| CT-08 | 0.389 | 0.015 | 0.049 | 0.001 | 0.70 | 20.5 | 0.588 | 0.057 | 0.002 | 0.25 | 348 | 1.39 | 3.50 |
| CT-09 | 0.494 | 0.026 | 0.053 | 0.002 | 0.72 | 19.0 | 0.798 | 0.068 | 0.003 | 0.06 | 123 | 1.28 | 9.6 |
| CT-10 | 0.448 | 0.019 | 0.053 | 0.002 | 0.20 | 18.8 | 0.528 | 0.061 | 0.003 | 0.23 | 264 | 0.45 | 30.0 |
| CT-11 | 1.65 | 0.069 | 0.061 | 0.002 | 0.78 | 16.4 | 0.430 | 0.194 | 0.005 | −0.36 | 138 | 0.20 | 139 |
| CT-12 | 5.79 | 0.150 | 0.112 | 0.003 | 0.86 | 8.97 | 0.233 | 0.376 | 0.005 | 0.17 | 783 | 2.04 | 3200 |
| CT-13 | 8.95 | 0.380 | 0.141 | 0.005 | 0.91 | 7.11 | 0.263 | 0.453 | 0.006 | −0.26 | 2655 | 8.89 | 16,700 |
| CT-14 | 6.74 | 0.200 | 0.117 | 0.003 | 0.87 | 8.52 | 0.218 | 0.414 | 0.005 | −0.02 | 2207 | 14.0 | 10,350 |
| CT-15 | 1.55 | 0.094 | 0.060 | 0.002 | 0.33 | 16.6 | 0.635 | 0.186 | 0.011 | 0.33 | 103 | 0.31 | 93.6 |
| CT-16 | 2.90 | 0.300 | 0.074 | 0.004 | 0.16 | 13.6 | 0.646 | 0.288 | 0.031 | 0.10 | 124 | 0.20 | 199 |
| CT-17 | 5.26 | 0.190 | 0.103 | 0.003 | 0.82 | 9.68 | 0.272 | 0.370 | 0.006 | −0.14 | 433 | 2.71 | 1737 |
| Sample | 3AR-1 | 3AR-2 | 3AR-3 | 3AR-4 | 3AR-5 | 3AR-6 | 3AR-7 | 3AR-8 ** | 3AR-9 | 3AR-10 | 3AR-11 | 3AR-12 ** | 3AR-13 ** | 3AR-14 ** |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lithotype | 1 | 1 | 2 | 2 | 3 | 4 | 4 | 4 | 4 | 4 | 4 | 5 | 5 | 5 |
| SiO2 | 72.85 | 72.37 | 72.63 | 74.00 | 72.13 | 70.81 | 68.86 | 73.74 | 72.40 | 72.01 | 70.64 | 68.88 | 67.17 | 71.13 |
| Al2O3 | 14.17 | 14.75 | 14.86 | 14.86 | 16.23 | 16.55 | 16.50 | 16.13 | 17.04 | 16.97 | 16.29 | 17.54 | 18.50 | 16.64 |
| Fe2O3t | 2.17 | 2.30 | 0.96 | 1.38 | 0.66 | 0.63 | 0.30 | 0.72 | 0.45 | 0.83 | 0.41 | 0.70 | 0.06 | 0.23 |
| MnO | 0.03 | 0.03 | 0.06 | 0.02 | 0.07 | 0.03 | 0.02 | 0.02 | 0.02 | 0.02 | 0.03 | 0.07 | 0.09 | 0.08 |
| MgO | 0.47 | 0.36 | 0.04 | 0.09 | 0.04 | 0.05 | 0.05 | 0.03 | 0.04 | 0.02 | 0.03 | 0.01 | 0.02 | 0.02 |
| CaO | 0.84 | 0.89 | 0.22 | 0.39 | 0.21 | 0.93 | 0.64 | 0.25 | 0.28 | 0.23 | 0.39 | 0.17 | 0.31 | 0.35 |
| Na2O | 3.30 | 3.55 | 3.53 | 3.67 | 6.36 | 6.35 | 5.24 | 7.54 | 7.29 | 7.47 | 5.85 | 5.50 | 4.04 | 4.27 |
| K2O | 4.93 | 5.13 | 4.94 | 4.16 | 1.38 | 1.41 | 3.34 | 0.75 | 1.13 | 1.06 | 2.79 | 1.90 | 4.00 | 2.80 |
| TiO2 | 0.31 | 0.30 | 0.03 | 0.07 | 0.01 | 0.01 | 0.01 | 0.00 | 0.01 | 0.00 | 0.01 | 0.01 | 0.01 | 0.01 |
| P2O5 | 0.48 | 0.33 | 1.04 | 0.60 | 1.17 | 0.99 | 2.08 | 0.46 | 0.49 | 0.46 | 1.79 | 2.50 | 0.80 | 0.57 |
| F | 0.08 | 0.07 | 0.13 | 0.10 | 0.48 | 0.24 | 0.20 | 0.04 | 0.08 | 0.08 | 0.24 | 1.25 | 1.80 | 1.44 |
| LOI | 1.29 | 0.63 | 1.40 | 0.84 | 1.15 | 1.70 | 2.02 | 0.90 | 0.97 | 1.24 | 0.92 | 2.11 | 3.88 | 2.60 |
| Total | 100.92 | 100.71 | 99.84 | 100.18 | 99.89 | 99.70 | 99.26 | 100.58 | 100.20 | 100.39 | 99.39 | 100.64 | 100.68 | 100.14 |
| Ag | 0.8 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 |
| As | 17 | 19 | 86 | 102 | 90 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 |
| Ba | 216 | 412 | 10 | 68 | 5 | 27 | 43 | 20.5 | 22.5 | 25.6 | 22 | 10 | 16 | 7 |
| Be | 5 | 6 | 2 | 3 | 4 | 7 | 5 | 6 | 8 | 6 | 7 | 10 | 7 | 11 |
| Bi | 0.8 | 0.4 | 1.4 | 1.5 | 0.6 | 1.0 | 0.8 | 0.15 | <0.1 | <0.1 | 0.2 | <0.1 | <0.1 | 0.1 |
| Co | 34 | 2 | 64 | <1 | 24 | <1 | 26 | <1 | <1 | <1 | <1 | <1 | 33 | 32 |
| Cr | <20 | 50 | <20 | 120 | <20 | 90 | <20 | <20 | 40 | <20 | 90 | <20 | <20 | <20 |
| Cs | 22 | 19.6 | 48 | 29.2 | 26.1 | 15.4 | 23.8 | 8.43 | 16.8 | 11.8 | 61.7 | 136 | 260 | 341 |
| Cu | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 |
| Ga | 24 | 24 | 35 | 32 | 36 | 52.8 | 45 | 41.5 | 46.5 | 42.2 | 45 | 50 | 65 | 50 |
| Ge | 2.0 | 2.3 | 3.7 | 3.4 | 8.5 | 5.26 | 5 | 4.33 | 4.5 | 4.8 | 7.4 | 7.4 | 10.0 | 9.0 |
| Hf | 3.9 | 4.1 | 1.1 | 1.0 | 1.5 | 4.2 | 2.2 | 4.73 | 4.4 | 3.04 | 1.1 | 2.7 | 3.2 | 2.2 |
| In | 0.2 | 0.2 | 0.4 | 0.6 | 0.3 | 0.34 | 1.1 | 0.15 | 0.25 | 0.22 | 0.6 | 1.2 | 3.6 | 1.7 |
| Li | 170 | 210 | 200 | 150 | 190 | 84 | 810 | 85 | 75 | 102 | 290 | 5260 | 8230 | 5730 |
| Mo | <2 | <2 | <2 | <2 | <2 | 3 | <2 | <2 | <2 | <2 | 5 | <2 | <2 | <2 |
| Nb | 11.0 | 12.5 | 10.5 | 15.9 | 53.9 | 62.3 | 48.0 | 93.9 | 119 | 51.2 | 40.2 | 80.7 | 93.1 | 71.5 |
| Ni | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 |
| Pb | 20 | 22 | 8 | 11 | 5 | 11 | 14 | 21 | 24 | 20 | 12 | 6 | 8 | <5 |
| Rb | 352 | 314 | 881 | 568 | 459 | 318 | 1130 | 248 | 421 | 342 | 1130 | 1990 | 4710 | 3450 |
| Sb | 0.4 | <0.2 | 0.5 | <0.2 | 0.7 | 0.4 | 0.5 | 0.4 | 0.4 | 0.44 | <0.2 | 1.1 | 0.4 | 0.6 |
| Sc | 5 | 4 | 4 | 6 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| Sn | 16 | 20 | 38 | 37 | 75 | 98 | 311 | 35 | 56 | 60 | 150 | 370 | 1150 | 530 |
| Sr | 45 | 61 | 6 | 40 | 5 | 82.4 | 113 | 66.3 | 89 | 83.2 | 285 | 169 | 171 | 156 |
| Ta | 2.19 | 1.81 | 3.94 | 4.86 | 22.9 | 86.4 | 51.6 | 111 | 151 | 80.6 | 54.9 | 91 | 71.6 | 64.8 |
| Th | 12.2 | 12.0 | 0.62 | 0.67 | 0.49 | 1.89 | 0.47 | 0.77 | 0.73 | 0.71 | 0.58 | 0.83 | 1.21 | 0.95 |
| Tl | 1.69 | 1.86 | 4.11 | 3.31 | 2.24 | 1.4 | 6.49 | 0.92 | 1.80 | 1.24 | 10.1 | 8.06 | 20.5 | 15.3 |
| U | 5.33 | 7.85 | 13.8 | 6.22 | 17.6 | 24.3 | 7.59 | 3.98 | 7.83 | 14.8 | 12.9 | 29.0 | 11.7 | 7.72 |
| V | 22 | 18 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 |
| W | 265 | 3 | 449 | 11.9 | 238 | 12.5 | 123 | 6.08 | 7.8 | 4.54 | 6.2 | 34.6 | 249 | 301 |
| Y | 17.2 | 14.8 | 3.6 | 2.3 | 0.7 | 1.68 | 0.8 | 0.9 | 0.5 | 0.65 | <0.5 | <0.5 | <0.5 | <0.5 |
| Zn | 70 | 80 | 80 | 60 | 70 | 30 | <30 | <30 | <30 | <30 | <30 | 140 | 80 | 150 |
| Zr | 144 | 169 | 22 | 26 | 19 | 50 | 19 | 39 | 32 | 28 | 13 | 28 | 36 | 24 |
| La | 20.2 | 26.1 | 0.89 | 1.26 | 0.34 | 0.73 | 0.24 | 0.313 | 0.275 | 0.25 | 0.4 | 0.31 | 0.23 | 0.19 |
| Ce | 44.1 | 55.2 | 2.01 | 2.36 | 0.43 | 0.918 | 0.34 | 0.403 | 0.315 | 0.278 | 0.7 | 0.36 | 0.26 | 0.24 |
| Pr | 5.53 | 6.31 | 0.24 | 0.26 | 0.05 | 0.084 | 0.05 | 0.063 | 0.045 | 0.04 | 0.08 | 0.04 | 0.03 | 0.02 |
| Nd | 22.8 | 24.1 | 0.87 | 0.85 | 0.14 | 0.274 | 0.3 | 0.295 | 0.195 | 0.184 | 0.23 | 0.17 | 0.1 | 0.1 |
| Sm | 4.65 | 5.08 | 0.34 | 0.24 | 0.06 | 0.084 | 0.09 | 0.093 | 0.045 | 0.072 | 0.04 | 0.07 | 0.02 | 0.03 |
| Eu | 0.49 | 0.715 | 0.014 | 0.036 | <0.005 | 0.187 | 0.05 | 0.027 | 0.02 | 0.019 | 0.005 | 0.011 | 0.015 | 0.01 |
| Gd | 3.8 | 3.9 | 0.44 | 0.29 | 0.05 | 0.09 | 0.12 | 0.098 | 0.065 | 0.092 | 0.04 | 0.06 | 0.04 | 0.03 |
| Tb | 0.56 | 0.64 | 0.1 | 0.06 | 0.01 | 0.02 | 0.02 | 0.017 | 0.01 | 0.014 | <0.01 | <0.01 | <0.01 | <0.01 |
| Dy | 2.98 | 3.24 | 0.55 | 0.41 | 0.08 | 0.108 | 0.13 | 0.078 | 0.065 | 0.078 | 0.04 | 0.05 | 0.03 | 0.04 |
| Ho | 0.5 | 0.48 | 0.09 | 0.07 | 0.02 | 0.024 | 0.02 | 0.02 | 0.01 | 0.014 | <0.01 | <0.01 | <0.01 | <0.01 |
| Er | 1.36 | 1.15 | 0.25 | 0.18 | 0.05 | 0.082 | 0.06 | 0.043 | 0.025 | 0.044 | 0.03 | 0.03 | 0.02 | 0.02 |
| Tm | 0.197 | 0.16 | 0.049 | 0.038 | 0.012 | 0.020 | 0.011 | 0.011 | 0.006 | 0.008 | <0.005 | 0.007 | <0.005 | <0.005 |
| Yb | 1.24 | 0.96 | 0.33 | 0.25 | 0.09 | 0.164 | 0.06 | 0.05 | 0.03 | 0.052 | <0.01 | 0.06 | 0.03 | 0.01 |
| Lu | 0.172 | 0.14 | 0.039 | 0.034 | 0.013 | 0.026 | 0.007 | 0.007 | 0.004 | 0.007 | <0.002 | 0.008 | 0.004 | <0.002 |
| A/CNK | 1.15 | 1.13 | 1.29 | 1.32 | 1.32 | 1.21 | 1.23 | 1.18 | 1.24 | 1.23 | 1.22 | 1.54 | 1.60 | 1.56 |
| K/Rb | 116 | 136 | 46.6 | 60.8 | 25.0 | 36.8 | 24.5 | 25.1 | 22.3 | 25.7 | 20.5 | 7.93 | 7.05 | 6.74 |
| Nb/Ta | 5.02 | 6.91 | 2.67 | 3.27 | 2.35 | 0.722 | 0.930 | 0.844 | 0.787 | 0.635 | 0.732 | 0.887 | 1.30 | 1.10 |
| Zr/Hf | 36.9 | 41.2 | 20.0 | 26.0 | 12.7 | 11.9 | 8.64 | 8.10 | 7.27 | 9.15 | 11.8 | 10.4 | 11.3 | 10.9 |
| (La/Lu)N | 12.2 | 19.4 | 2.37 | 3.85 | 2.72 | 2.87 | 3.56 | 4.81 | 8.16 | 3.82 | ND | 4.02 | 5.97 | ND |
| Eu/Eu * | 0.355 | 0.490 | 0.110 | 0.416 | ND | 6.56 | 1.47 | 0.851 | 1.13 | 0.697 | 0.381 | 0.517 | 1.62 | 1.02 |
| ΣREE | 108 | 128 | 6.21 | 6.34 | 1.35 | 2.81 | 1.50 | 1.51 | 1.11 | 1.15 | 1.57 | 1.18 | 0.779 | 0.690 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garate-Olave, I.; Roda-Robles, E.; Gil-Crespo, P.P.; Pesquera, A.; Errandonea-Martin, J. The Tres Arroyos Granitic Aplite-Pegmatite Field (Central Iberian Zone, Spain): Petrogenetic Constraints from Evolution of Nb-Ta-Sn Oxides, Whole-Rock Geochemistry and U-Pb Geochronology. Minerals 2020, 10, 1008. https://doi.org/10.3390/min10111008
Garate-Olave I, Roda-Robles E, Gil-Crespo PP, Pesquera A, Errandonea-Martin J. The Tres Arroyos Granitic Aplite-Pegmatite Field (Central Iberian Zone, Spain): Petrogenetic Constraints from Evolution of Nb-Ta-Sn Oxides, Whole-Rock Geochemistry and U-Pb Geochronology. Minerals. 2020; 10(11):1008. https://doi.org/10.3390/min10111008
Chicago/Turabian StyleGarate-Olave, Idoia, Encarnación Roda-Robles, Pedro Pablo Gil-Crespo, Alfonso Pesquera, and Jon Errandonea-Martin. 2020. "The Tres Arroyos Granitic Aplite-Pegmatite Field (Central Iberian Zone, Spain): Petrogenetic Constraints from Evolution of Nb-Ta-Sn Oxides, Whole-Rock Geochemistry and U-Pb Geochronology" Minerals 10, no. 11: 1008. https://doi.org/10.3390/min10111008
APA StyleGarate-Olave, I., Roda-Robles, E., Gil-Crespo, P. P., Pesquera, A., & Errandonea-Martin, J. (2020). The Tres Arroyos Granitic Aplite-Pegmatite Field (Central Iberian Zone, Spain): Petrogenetic Constraints from Evolution of Nb-Ta-Sn Oxides, Whole-Rock Geochemistry and U-Pb Geochronology. Minerals, 10(11), 1008. https://doi.org/10.3390/min10111008





