Study of Copper Leaching from Mining Waste in Acidic Media, at Ambient Temperature and Atmospheric Pressure
Abstract
1. Introduction
- The Copper minerals present. Obtaining Copper from mining waste by hydrometallurgical techniques is obviously influenced by the percentage of Copper minerals present. Usually they will be in combination with other types of minerals without value in greater or lesser proportions. At the same time, not all Copper minerals produce the same recovery rates, so it is interesting for its classification and quantification. Impurities will also influence the recovery of Copper, although never to the same extent as in pyrometallurgical processes.
- The mineral associations in the existing rocks in the landfills and the easy release of the Copper minerals. There must be an initial study of the landfill to evaluate this factor directly related to that mentioned above.
- The particle size to be processed [17]. The absence of a concentrate in the mining waste and the combination with other non-valuable minerals means that leaching does not occur at its maximum recovery rate if these Copper minerals are not in contact with the acidic solution. Therefore, a crushing of the landfill materials will influence a higher probability of contact of the Copper ores with the acidic solution and, consequently, will result in a higher recovery rate. The crushing process is therefore essential if the aim is to obtain the highest recovery rate in the shortest time [18]. At the same time, it must be taken that milling very fine crushing would damage the industrial process, due to problems in the recirculation of the leachate and the creation of contaminating sludges. These sludges produced should be evaluated and reused in other industrial processes to avoid their deposition in landfills and the associated environmental pollution. In other words, it is important to evaluate the appropriate particle size to obtain an adequate recovery rate, a lower amount of contaminating waste and a more energetically optimized leaching process [19,20].
2. Materials and Methods
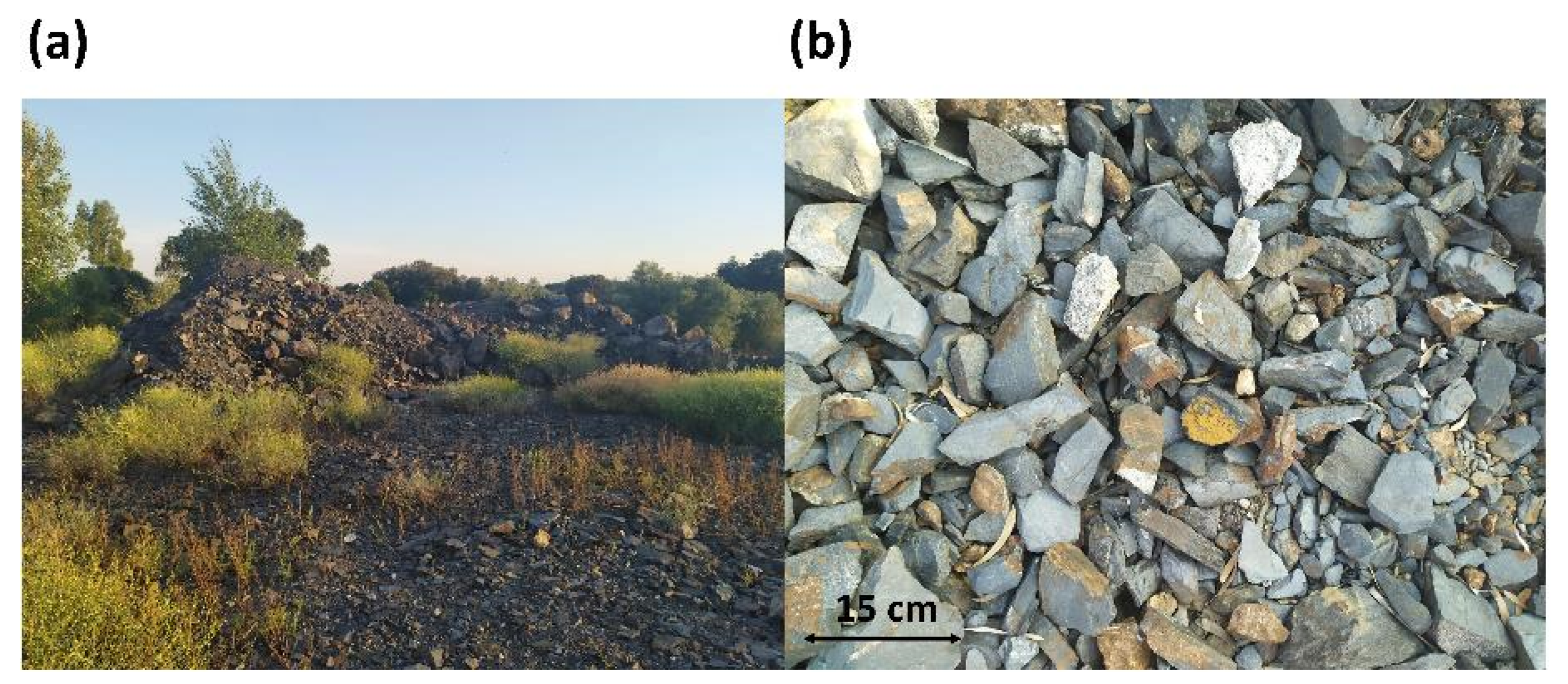
2.1. Materials
2.2. Methodology
2.2.1. Analysis of the Chemical Composition of the Waste
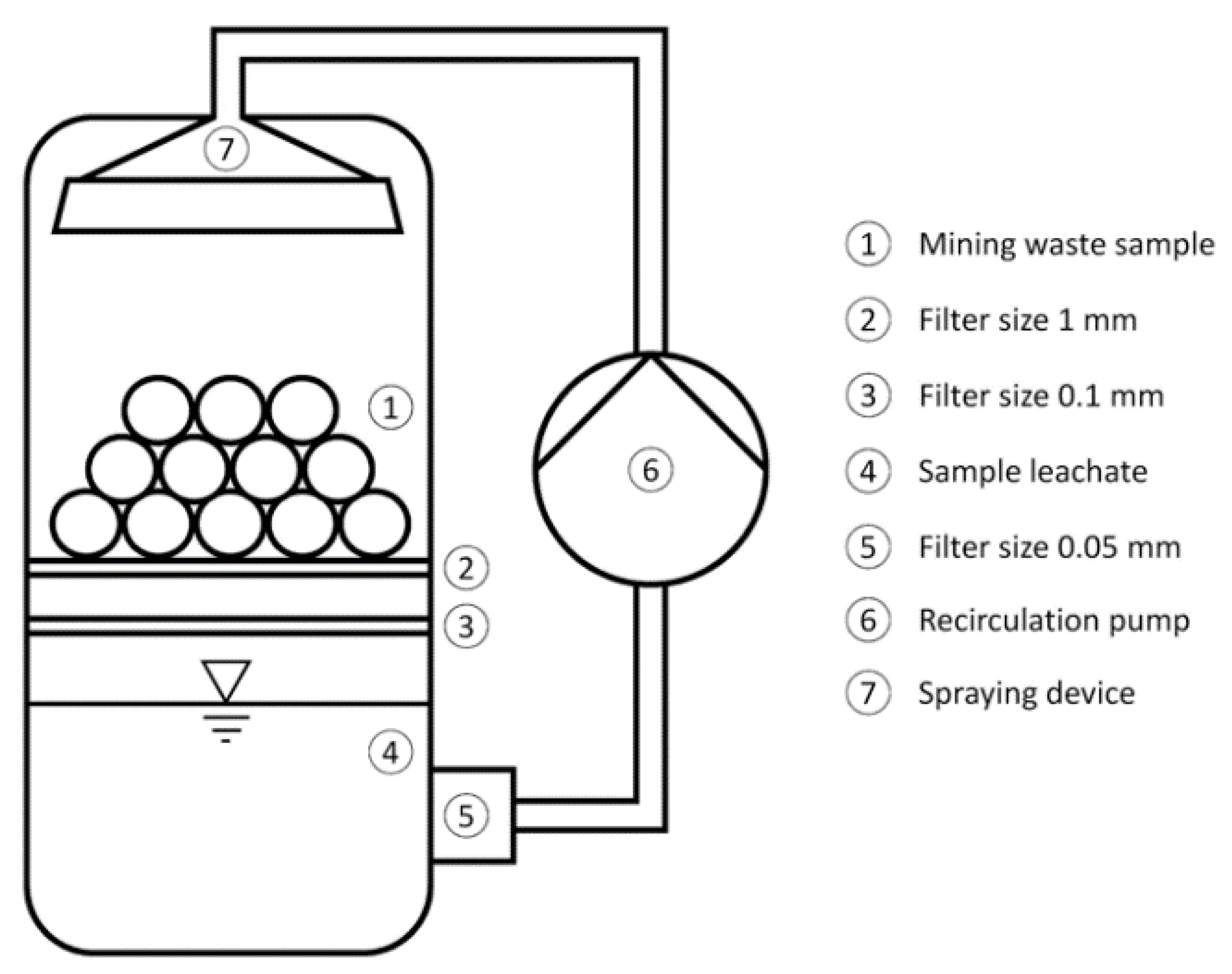
2.2.2. Leaching Process and Concentration Measurement
3. Results and Discussions
3.1. Analysis of the Chemical Composition of the Waste
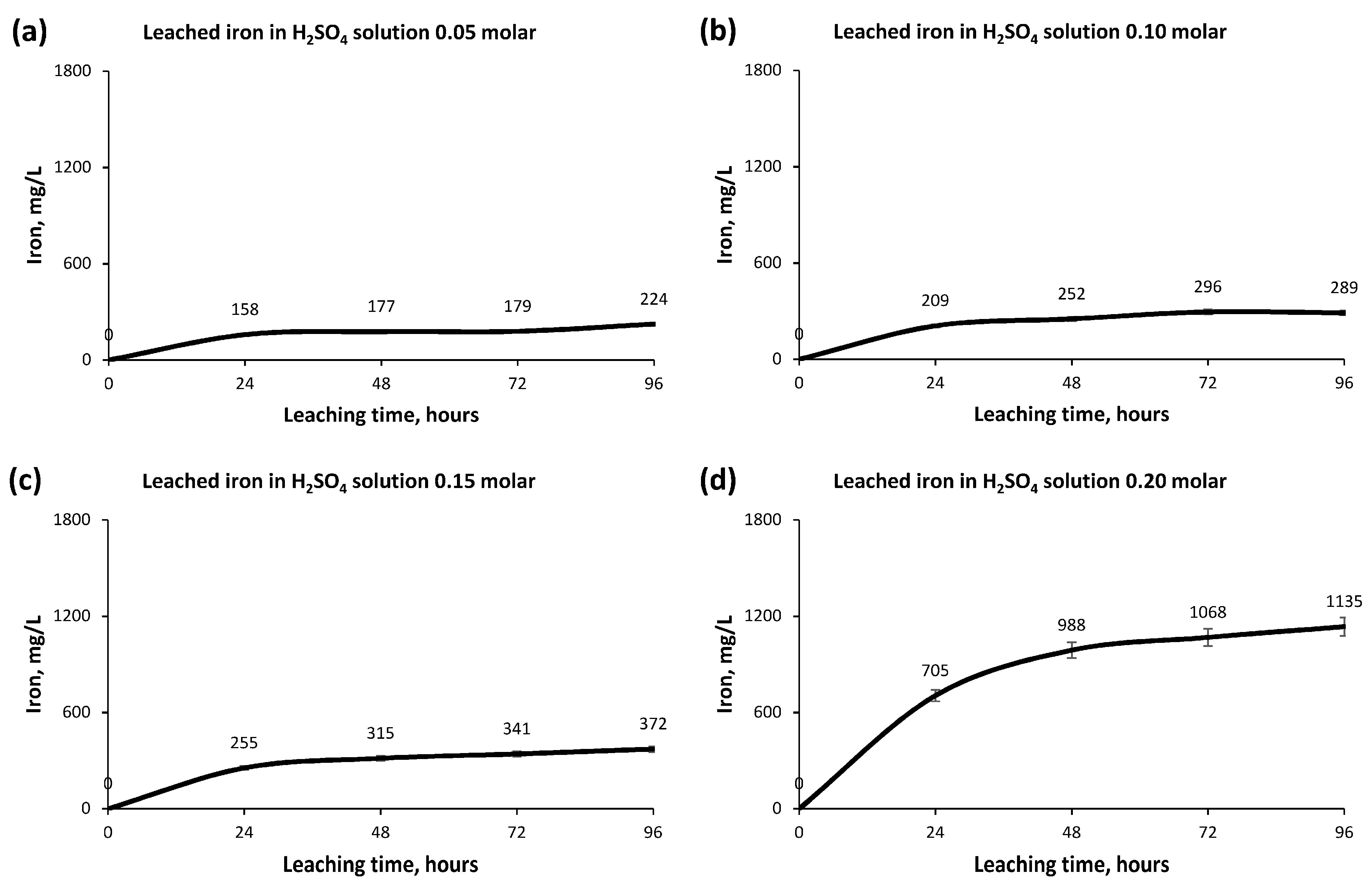
3.2. Leaching Process and Concentration Measurement
4. Conclusions
- The mine waste dump from which the sample was taken for analysis contained mainly granite, Iron Sulphides, Copper Sulphides, Lead Sulphides and, to a lesser proportion, Zinc Sulphides. The Polymetallic Sulphides had been transformed into Oxides by their continuous exposure to the atmospheric conditions and their treatment.
- The chemical composition of the sample under study reflected a low percentage of carbonates and organic matter, with the presence of Copper in a proportion of 4.67%, being a useful sample for the extraction of Copper by hydrometallurgical techniques. There were also percentages of Iron, Lead and Zinc, as well as a low proportion of Arsenic.
- The leaching process was done at ambient temperature so as not to increase the production costs of the process.
- The low molarities of the Sulphuric Acid solutions were selected to avoid subsequent environmental problems with the leachate after the extraction of Copper and elements of interest. In addition, higher molarities of Sulphuric Acid in the solution would have directly caused higher leaching of other elements that are not of interest to extract and would impair the process of extracting Copper from the leachate.
- The particle size between 6 mm and 10 mm of the mine waste sample did not produce sludge at the end of the leaching process for any of the Sulphuric Acid solutions evaluated.
- The leaching times were limited to 96 h, not being necessary longer times as in other hydrometallurgical processes, existing at present in which the leaching time is of weeks and even months.
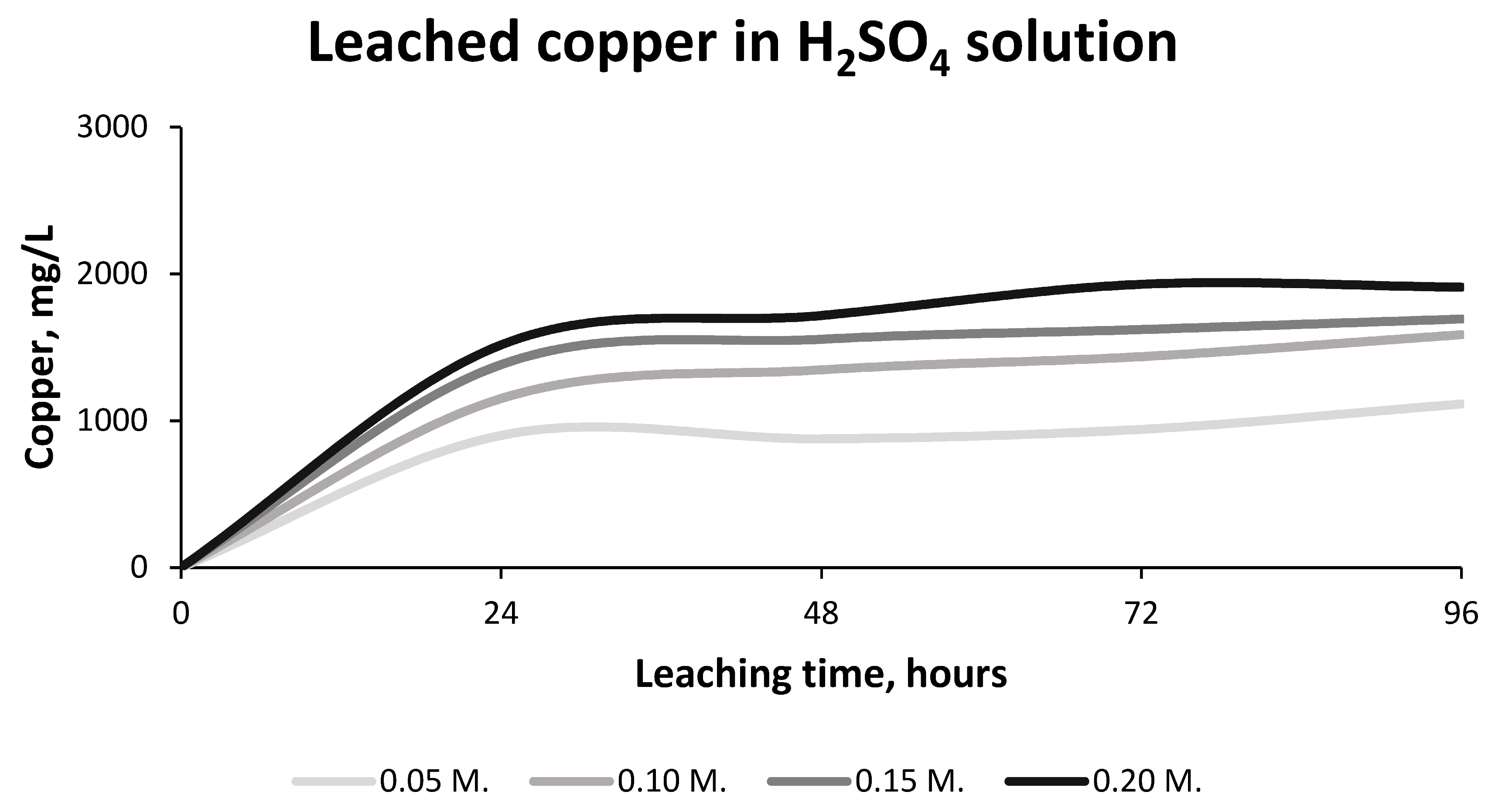
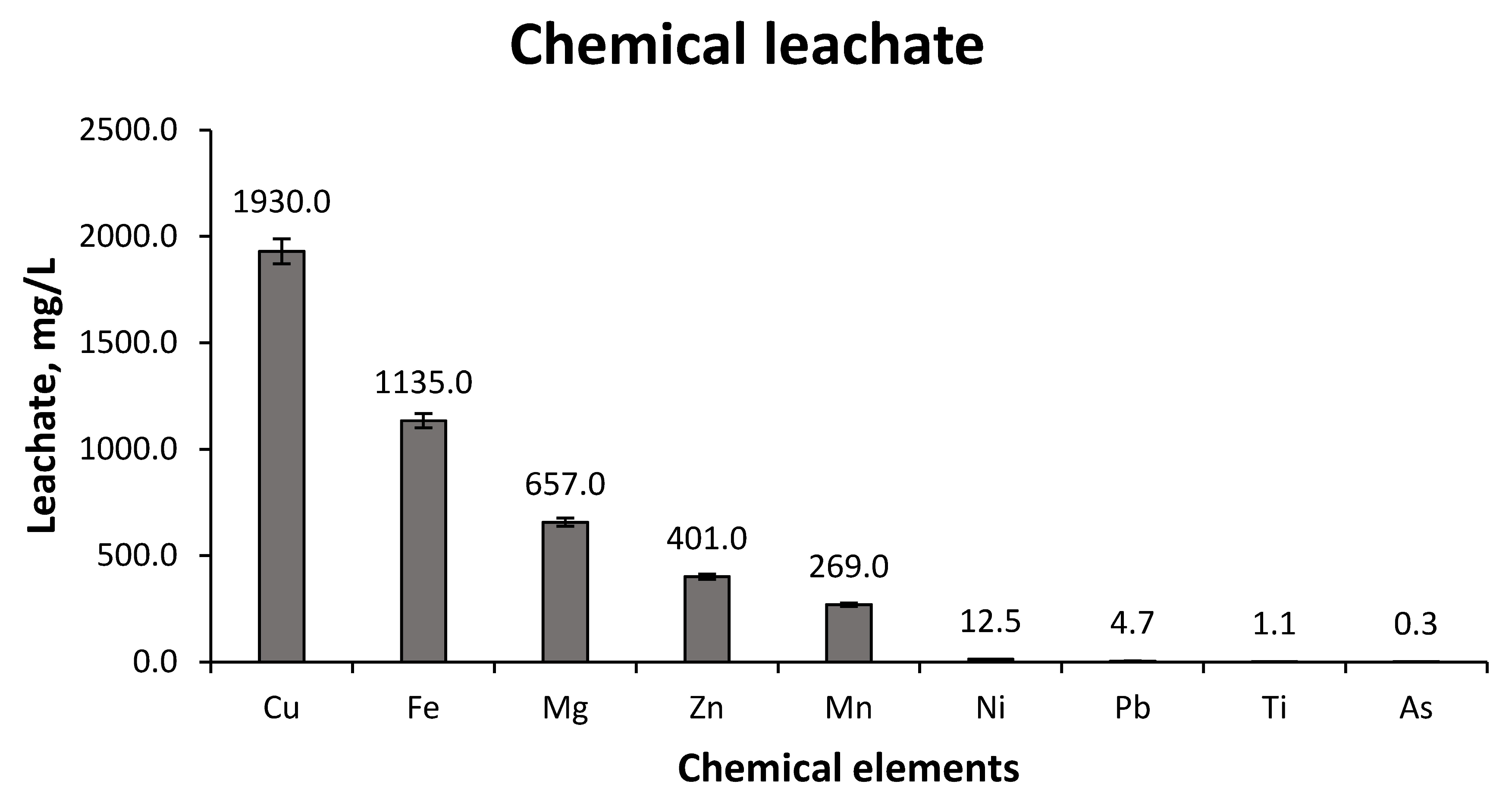
- The 0.20 molar Sulphuric Acid solution yielded results at 72 h of Copper concentrations of 1.930 ± 0.007 g/L and with recovery rates of approximately 80% of the Copper in the sample. This value is a good result for the subsequent hydrometallurgical stages
- The 0.15 and 0.10 molar Sulphuric Acid solutions give similar results to those of the 0.20 molar Sulphuric Acid solution; nonetheless, which is higher.
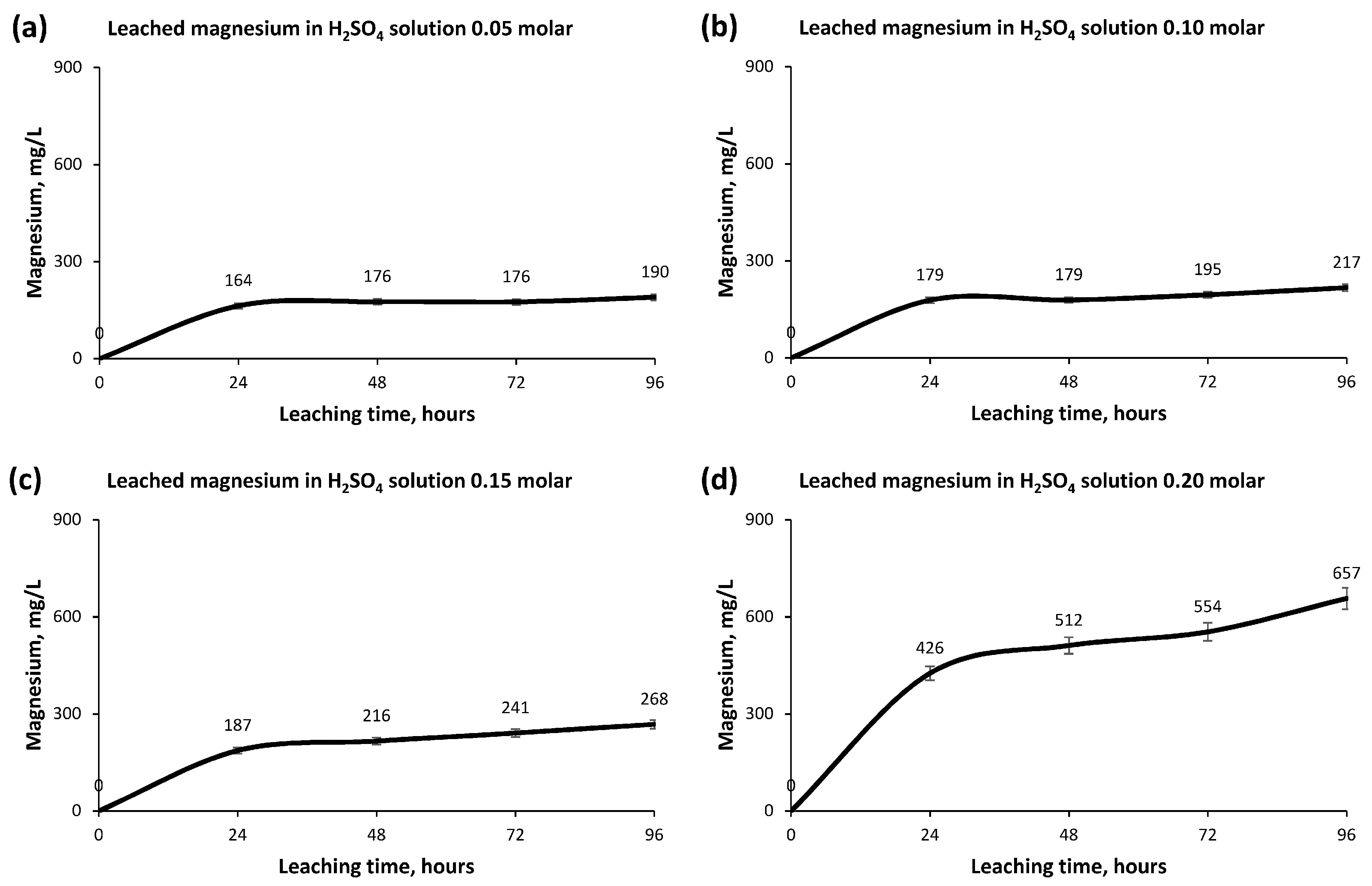
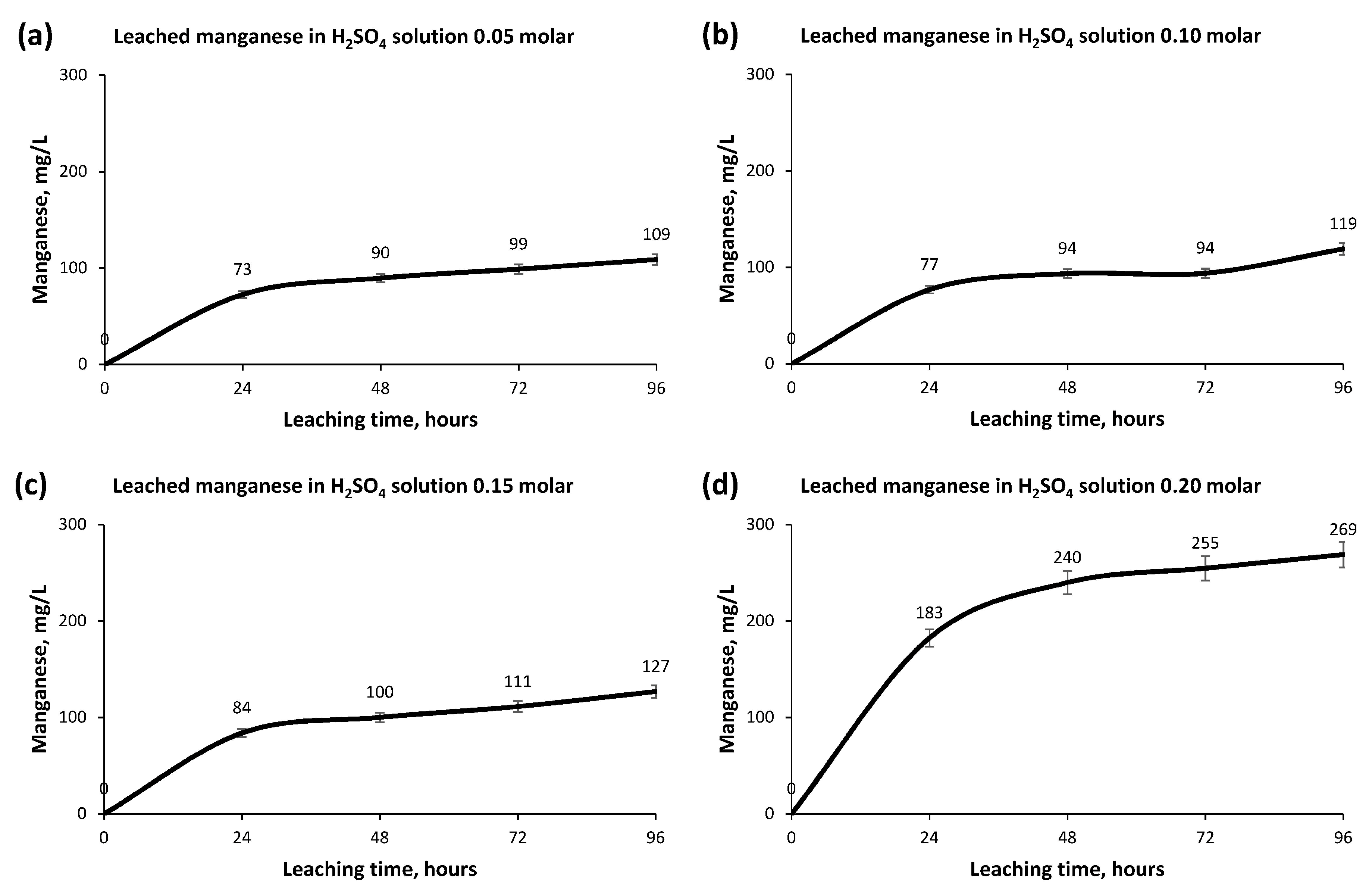
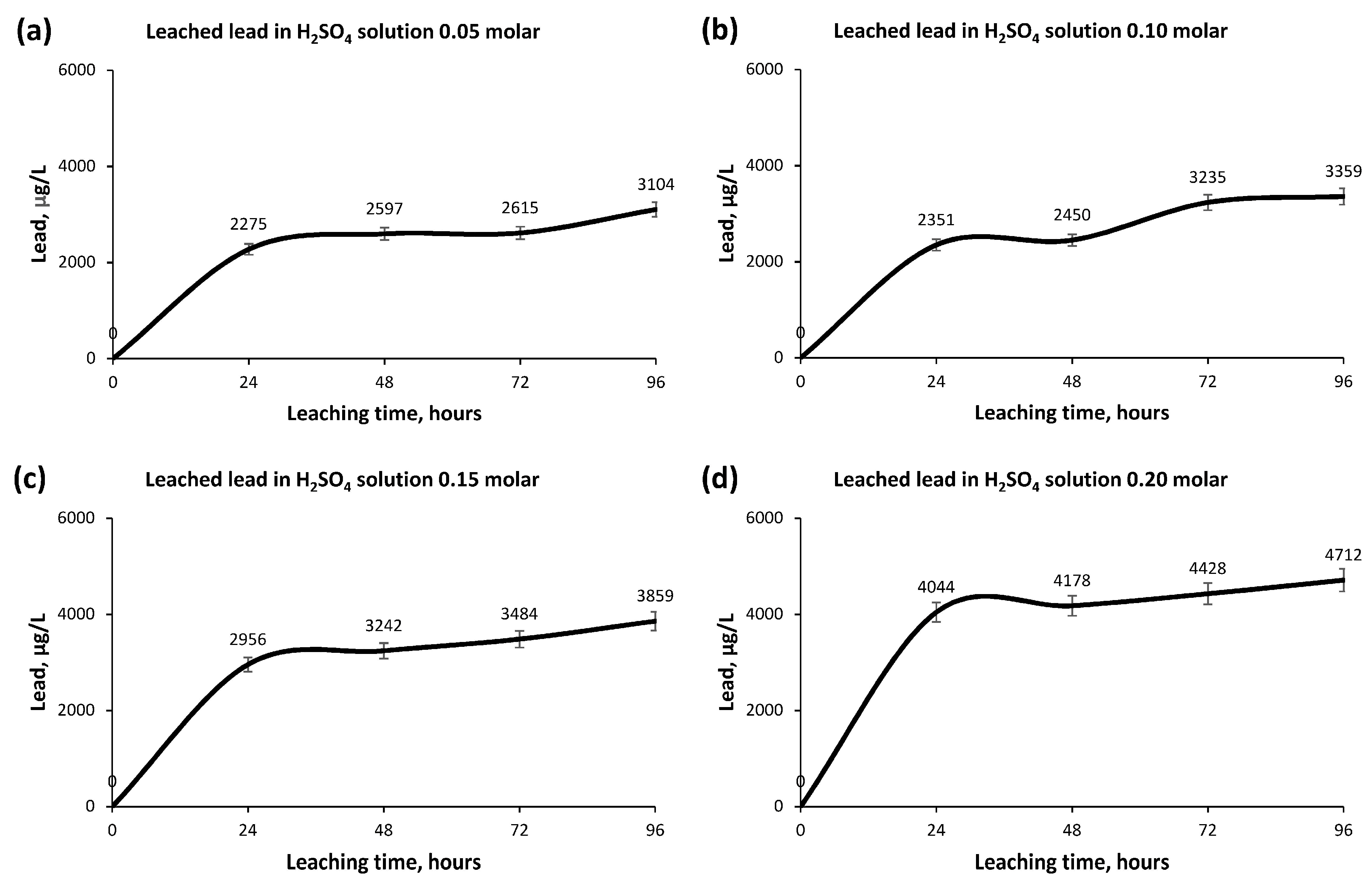
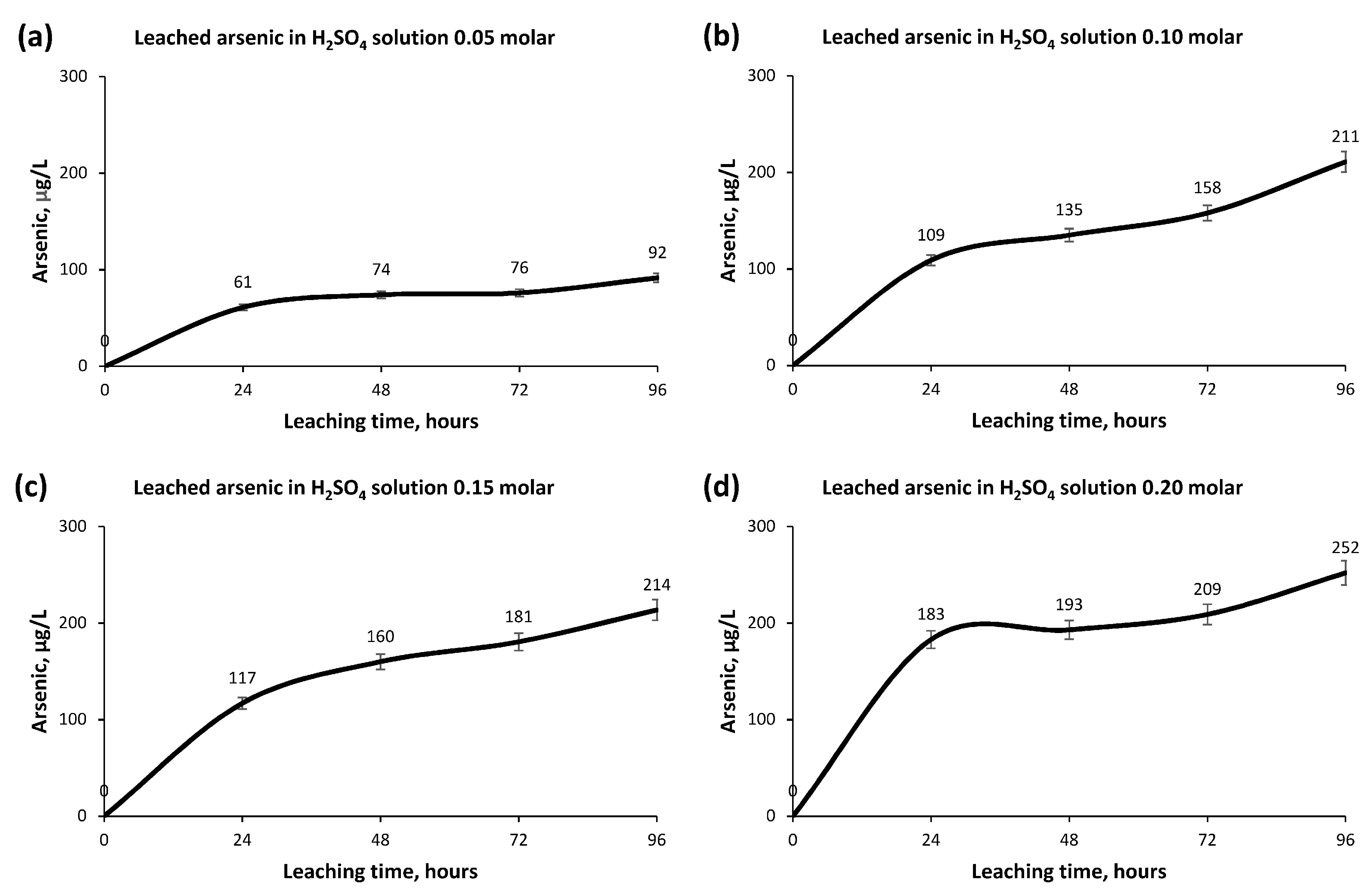
- The greatest leaching of Copper occurs in the first 24 h, producing a small increase in concentration afterwards until its stabilization after 72 h.
- Zinc with the 0.20 molar Sulphuric Acid solution has an almost complete recovery rate, obtaining maximum concentrations of 0.401 ± 0.002 g/L. It is therefore a secondary element that can be economically evaluated the interest of its extraction. A similar process occurs with Nickel, but its concentration is very low, so its extraction is of no interest.
- Lead reflected a total incompatibility with the leaching process; therefore, it achieved the objective of not obtaining its leaching and that it remained in the mining sample after the leaching process.
- The other elements, Magnesium, Manganese and Titanium, did not obtain important rates of recovery, a fact that favors the hydrometallurgical process in its later extraction and allows us to recognize the aptitude of the Copper leaching.
- Arsenic, a very harmful element in Copper pyrometallurgical processes and, to a lesser extent, in hydrometallurgical processes, obtained a very low concentration in the leachate and a very low recovery rate. This fact benefits the hydrometallurgical technique.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, L.; Pan, D.; Li, B.; Wu, Y.; Wang, H.; Gu, Y.; Zuo, T. Patterns and challenges in the copper industry in China. Resour. Conserv. Recycl. 2017, 127, 1–7. [Google Scholar] [CrossRef]
- Wang, M.; Chen, W.; Zhou, Y.; Li, X. Assessment of potential copper scrap in China and policy recommendation. Resour. Policy 2017, 52, 235–244. [Google Scholar] [CrossRef]
- Wang, H.T.; Liu, Y.; Gong, X.Z.; Wang, Z.H.; Gao, F.; Nie, Z.R. Life Cycle Assessment of Metallic Copper Produced by the Pyrometallurgical Technology of China. Mater. Sci. Forum 2015, 814, 559–563. [Google Scholar] [CrossRef]
- Bonnin, M.; Azzaro-Pantel, C.; Domenech, S.; Villeneuve, J. Multicriteria optimization of copper scrap management strategy. Resour. Conserv. Recycl. 2015, 99, 48–62. [Google Scholar] [CrossRef][Green Version]
- Shuva, M.A.H.; Rhamdhani, M.A.; Brooks, G.A.; Masood, S.; Reuter, M.A. Thermodynamics data of valuable elements relevant to e-waste processing through primary and secondary copper production: A review. J. Clean. Prod. 2016, 131, 795–809. [Google Scholar] [CrossRef]
- Copper: World Mine Production, By Country. Available online: https://www.indexmundi.com/en/commodities/minerals/copper/copper_t20.html (accessed on 19 May 2020).
- Kowalczuk, P.; Bouzahzah, H.; Kleiv, R.; Aasly, K. Simultaneous Leaching of Seafloor Massive Sulfides and Polymetallic Nodules. Minerals 2019, 9, 482. [Google Scholar] [CrossRef]
- Watling, H.R. Chalcopyrite hydrometallurgy at atmospheric pressure: 1. Review of acidic sulfate, sulfate-chloride and sulfate-nitrate process options. Hydrometallurgy 2013, 140, 163–180. [Google Scholar] [CrossRef]
- Meshram, P.; Prakash, U.; Bhagat, L.; Abhilash; Zhao, H.; van Hullebusch, E.D. Processing of Waste Copper Converter Slag Using Organic Acids for Extraction of Copper, Nickel, and Cobalt. Minerals 2020, 10, 290. [Google Scholar] [CrossRef]
- Hernández, P.C.; Dupont, J.; Herreros, O.O.; Jimenez, Y.P.; Torres, C.M. Accelerating copper leaching from sulfide ores in acid-nitrate-chloride media using agglomeration and curing as pretreatment. Minerals 2019, 9, 250. [Google Scholar] [CrossRef]
- Hernández, P.; Taboada, M.; Herreros, O.; Graber, T.; Ghorbani, Y. Leaching of Chalcopyrite in Acidified Nitrate Using Seawater-Based Media. Minerals 2018, 8, 238. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Z.; Wu, Y.; Li, L.; Li, B.; Pan, D.; Zuo, T. Environmental benefits of secondary copper from primary copper based on life cycle assessment in China. Resour. Conserv. Recycl. 2019, 146, 35–44. [Google Scholar] [CrossRef]
- Jones, D.J. CESL Copper Process. In Proceedings of the Alta Copper Hydrometallurgy Forum, Brisbane, Australia, 14–15 October 1996. [Google Scholar]
- Watling, H.R.; Elliot, A.D.; Maley, M.; van Bronswijk, W.; Hunter, C. Leaching of a low-grade, copper-nickel sulfide ore. 1. Key parameters impacting on Cu recovery during column bioleaching. Hydrometallurgy 2009, 97, 204–212. [Google Scholar] [CrossRef]
- Wang, S. Copper leaching from chalcopyrite concentrates. JOM 2005, 57, 48–51. [Google Scholar] [CrossRef]
- Dreisinger, D. Copper leaching from primary sulfides: Options for biological and chemical extraction of copper. Hydrometallurgy 2006, 83, 10–20. [Google Scholar] [CrossRef]
- van Staden, P.J. The Mintek/Bactech copper bioleach process. In Proceedings of the ALTA Copper Hydrometallurgy Forum, Brisbane, Australia, 19–21 October 1998. [Google Scholar]
- Gericke, M.; Govender, Y.; Pinches, A. Tank bioleaching of low-grade chalcopyrite concentrates using redox control. Hydrometallurgy 2010, 104, 414–419. [Google Scholar] [CrossRef]
- Hourn, M.; Halbe, D. The Nena Tech Process: Results on Frieda River copper gold concentrates. In Proceedings of the International Conference of Randol Copper Hydromet Roundtable 1999, Phoenix, AZ, USA, 10–13 October 1999; pp. 97–102. [Google Scholar]
- Hourn, M.M.; Turner, D.W.; Holzberger, I.R. Atmospheric Mineral Leaching Process. U.S. Patent 5,993,635, 30 November 1999. [Google Scholar]
- Cerda, C.; Taboada, M.; Jamett, N.; Ghorbani, Y.; Hernández, P. Effect of Pretreatment on Leaching Primary Copper Sulfide in Acid-Chloride Media. Minerals 2017, 8, 1. [Google Scholar] [CrossRef]
- Arroyo, F.; Fernández-Pereira, C.; Bermejo, P. Demonstration Plant Equipment Design and Scale-Up from Pilot Plant of a Leaching and Solvent Extraction Process. Minerals 2015, 5, 298–313. [Google Scholar] [CrossRef]
- Peacey, J.; Guo, X.J.; Robles, E. Copper hydrometallurgy—Current status, preliminary economics, future direction and positioning versus smelting. Trans. Nonferrous Met. Soc. China (Engl. Ed.) 2004, 14, 560–568. [Google Scholar]




| Samples | Nitrogen, % | Carbon, % | Hydrogen, % | Sulfur, % |
|---|---|---|---|---|
| Mining waste | 0.001 ± 0.002 | 3.735 ± 0.051 | 0.393 ± 0.014 | 0.647 ± 0.024 |
| Compound | wt, % | Est. Error |
|---|---|---|
| CaO | 31.40 | 0.23 |
| SiO2 | 19.49 | 0.20 |
| Fe2O3 | 9.01 | 0.14 |
| Al2O3 | 6.10 | 0.12 |
| CuO | 5.85 | 0.12 |
| MgO | 2.73 | 0.08 |
| K2O | 1.52 | 0.06 |
| MnO | 1.37 | 0.06 |
| PbO | 1.06 | 0.05 |
| ZnO | 0.989 | 0.049 |
| S | 0.440 | 0.022 |
| TiO2 | 0.317 | 0.016 |
| BaO | 0.209 | 0.010 |
| P2O5 | 0.0704 | 0.0035 |
| NiO | 0.0354 | 0.0018 |
| As2O3 | 0.0353 | 0.0026 |
| Co3O4 | 0.0179 | 0.0013 |
| SnO2 | 0.0159 | 0.0014 |
| SrO | 0.0149 | 0.0007 |
| Cr2O3 | 0.0140 | 0.0014 |
| ZrO2 | 0.0117 | 0.0009 |
| Y2O3 | 0.0114 | 0.0008 |
| Rb2O | 0.0111 | 0.0006 |
| CdO | 0.0088 | 0.0010 |
| V2O5 | 0.0042 | 0.0015 |
| Ag2O | 0.0024 | 0.0009 |
| Element | wt, % | Est. Error |
|---|---|---|
| Ca | 22.45 | 0.17 |
| Si | 9.11 | 0.09 |
| Fe | 6.30 | 0.10 |
| Al | 3.23 | 0.06 |
| Cu | 4.67 | 0.09 |
| Mg | 1.65 | 0.05 |
| K | 1.26 | 0.05 |
| Mn | 1.06 | 0.05 |
| Pb | 0.98 | 0.05 |
| Zn | 0.794 | 0.040 |
| S | 0.440 | 0.022 |
| Ti | 0.1900 | 0.0095 |
| Ba | 0.1870 | 0.0093 |
| Px | 0.0307 | 0.0015 |
| Ni | 0.0278 | 0.0014 |
| As | 0.0267 | 0.0020 |
| Co | 0.0132 | 0.0009 |
| Sn | 0.0125 | 0.0011 |
| Sr | 0.0126 | 0.0006 |
| Cr | 0.0096 | 0.0010 |
| Zr | 0.0087 | 0.0006 |
| Y | 0.0090 | 0.0006 |
| Rb | 0.0101 | 0.0005 |
| Cd | 0.0077 | 0.0009 |
| V | 0.0023 | 0.0008 |
| Ag | 0.0022 | 0.0009 |
| Total Weight% Oxygen | 28.23 | 0.33 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terrones-Saeta, J.M.; Suárez-Macías, J.; Río, F.J.L.d.; Corpas-Iglesias, F.A. Study of Copper Leaching from Mining Waste in Acidic Media, at Ambient Temperature and Atmospheric Pressure. Minerals 2020, 10, 873. https://doi.org/10.3390/min10100873
Terrones-Saeta JM, Suárez-Macías J, Río FJLd, Corpas-Iglesias FA. Study of Copper Leaching from Mining Waste in Acidic Media, at Ambient Temperature and Atmospheric Pressure. Minerals. 2020; 10(10):873. https://doi.org/10.3390/min10100873
Chicago/Turabian StyleTerrones-Saeta, Juan María, Jorge Suárez-Macías, Francisco Javier Linares del Río, and Francisco Antonio Corpas-Iglesias. 2020. "Study of Copper Leaching from Mining Waste in Acidic Media, at Ambient Temperature and Atmospheric Pressure" Minerals 10, no. 10: 873. https://doi.org/10.3390/min10100873
APA StyleTerrones-Saeta, J. M., Suárez-Macías, J., Río, F. J. L. d., & Corpas-Iglesias, F. A. (2020). Study of Copper Leaching from Mining Waste in Acidic Media, at Ambient Temperature and Atmospheric Pressure. Minerals, 10(10), 873. https://doi.org/10.3390/min10100873








