Automated Quantitative Mineralogy Applied to Metamorphic Rocks
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Material
2.2. Whole Rock Geochemistry
2.3. Mineralogic Method
2.3.1. Zeiss Mineralogic Mining
2.3.2. Image Navigation
2.3.3. SEM Recipe
2.3.4. Holder Recipe
2.3.5. Calibration Recipe
2.3.6. Image Processing Recipe
2.3.7. Morphology Recipe
2.3.8. Mineral Recipe
- Mapping analysis, where the user defines the step size (pixel size) for the EDS map, and an analysis is performed for every single point (pixel) of the map. Typical pixel sizes are 5–30 µm, but can be larger for coarse grained, homogeneous samples, or as small as 200 nm [20]. This method presents the full detail of the sample but is also the most time-consuming method.
- Spot centroid analysis, where the sample is segmented by BSE value of the grains. The method is typically used on grains in an epoxy matrix. For each grain determined, the geometrical centre of the grain is calculated, and a single EDS analysis on this point is performed. This method is especially suitable for homogeneous grains, as a small variation in the chemistry, like inclusions or zonations, will not be picked up.
- Feature scan analysis, where—like for the spot centroid analysis—the grain boundaries are determined by segmenting the BSE image. In this analysis mode, the beam is rastering within the boundaries of the grain, giving an average chemical composition. In case of zonations, a more correct average grain chemistry will be found; for heavily included samples, a false mineral classification might be generated. The method can be nearly as fast as a spot centroid analysis, depending on the pre-set amount of counts in the spectrum.
- Fast scan is an intermediate form of Feature scan and spot centroid analysis. It scatters a series of point analyses across a grain and thus arrives at an average composition of the grain. The user gets to determine the density of the spots for the analyses in an area, but each grain is analysed at least once, independent of its size.
- Line scan analysis, where all EDS analyses are made along a line with a pre-defined step-size across the centre of a particle or frame. All variations in composition are accounted for, and a first impression on the grain size and texture can be obtained. The method is very fast compared to a full Mapping analysis.
- Grey level mapping, where the sample is imaged (usually with BSE), without applying EDS measurements. For samples with a simple chemistry, grey values can be used to separate individual minerals. The method is very fast, but less precise than EDS-based investigations.
2.3.9. Mining Recipe
3. Examples of Applications
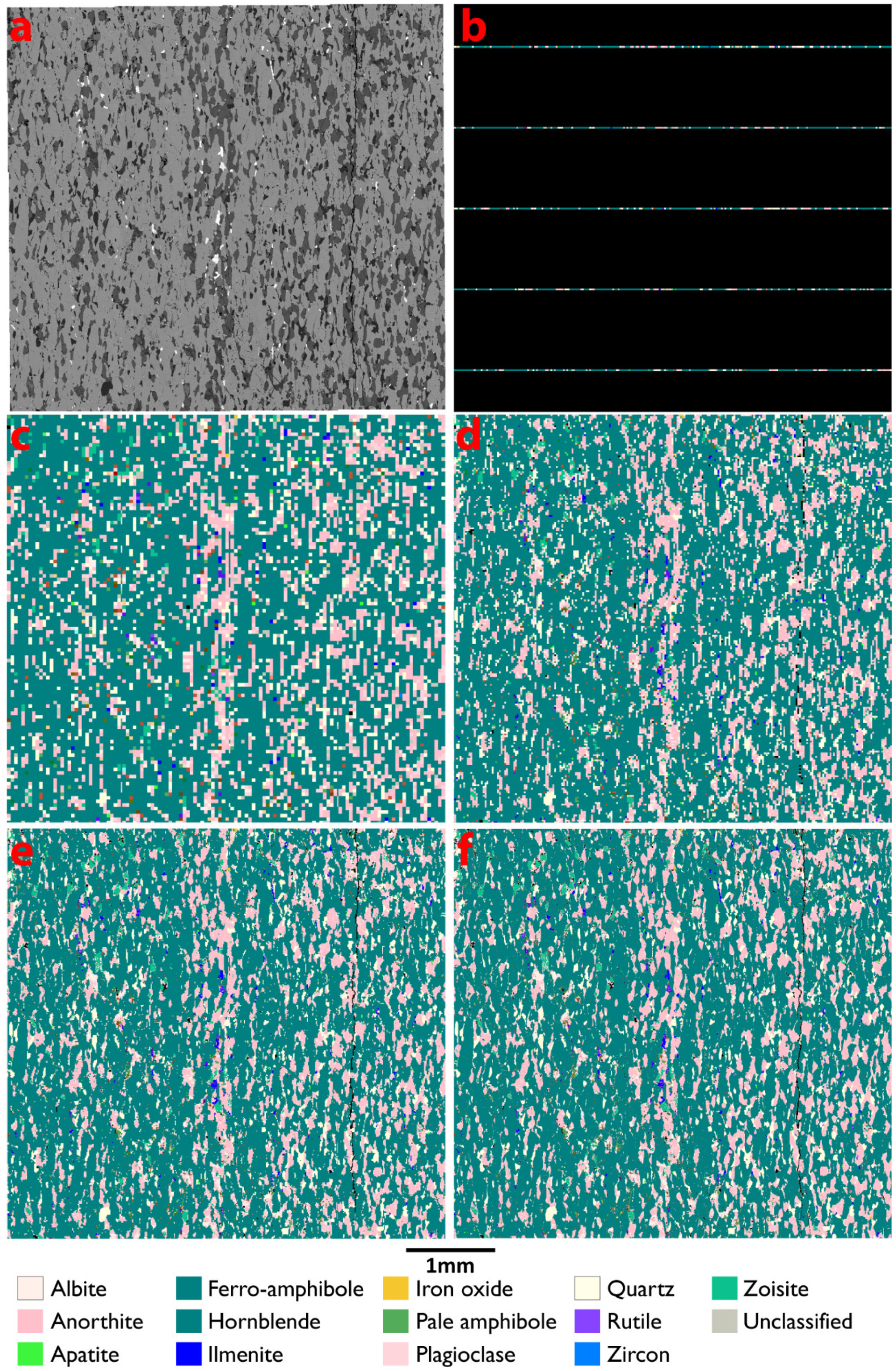
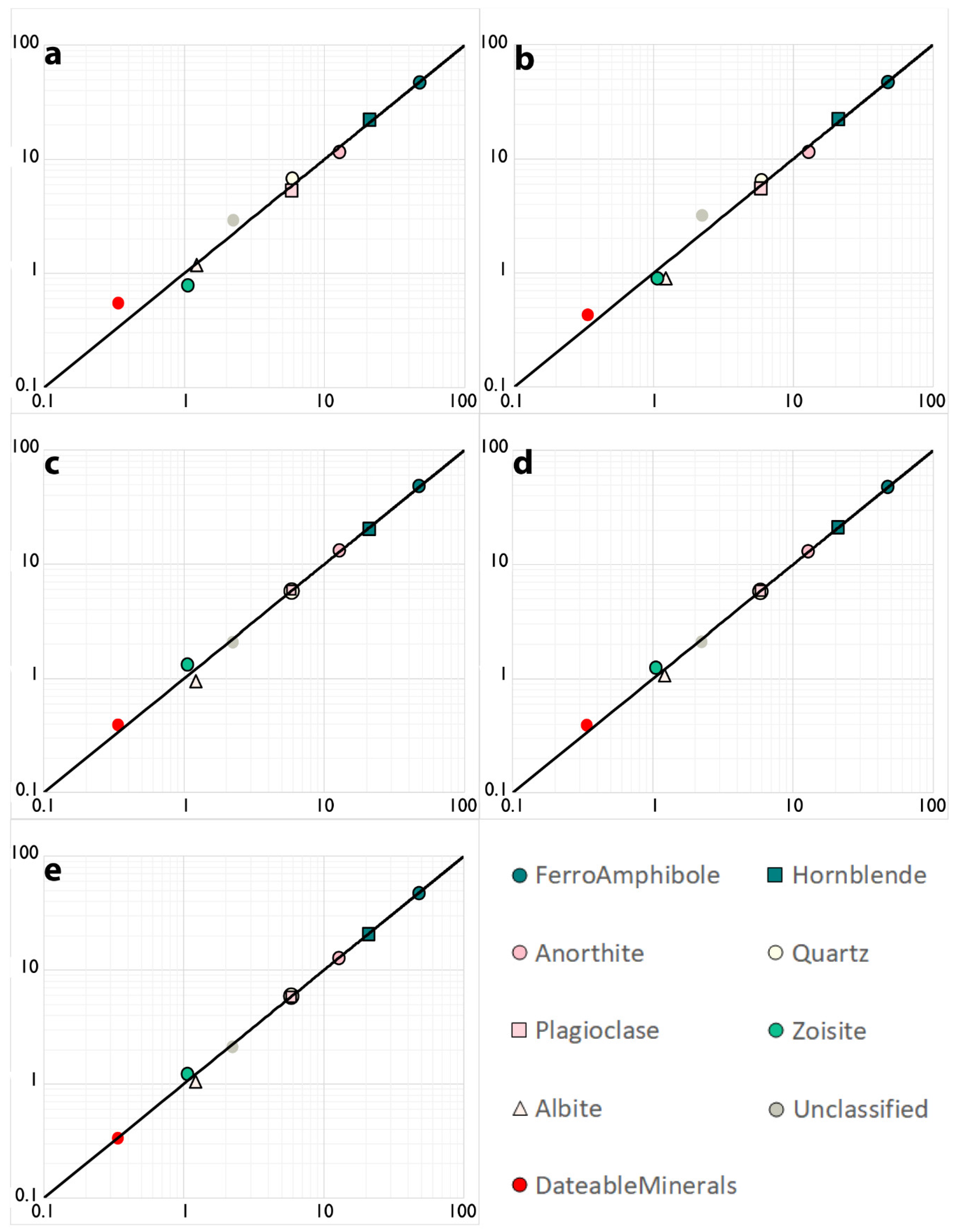
3.1. Mapping Speed and Composition
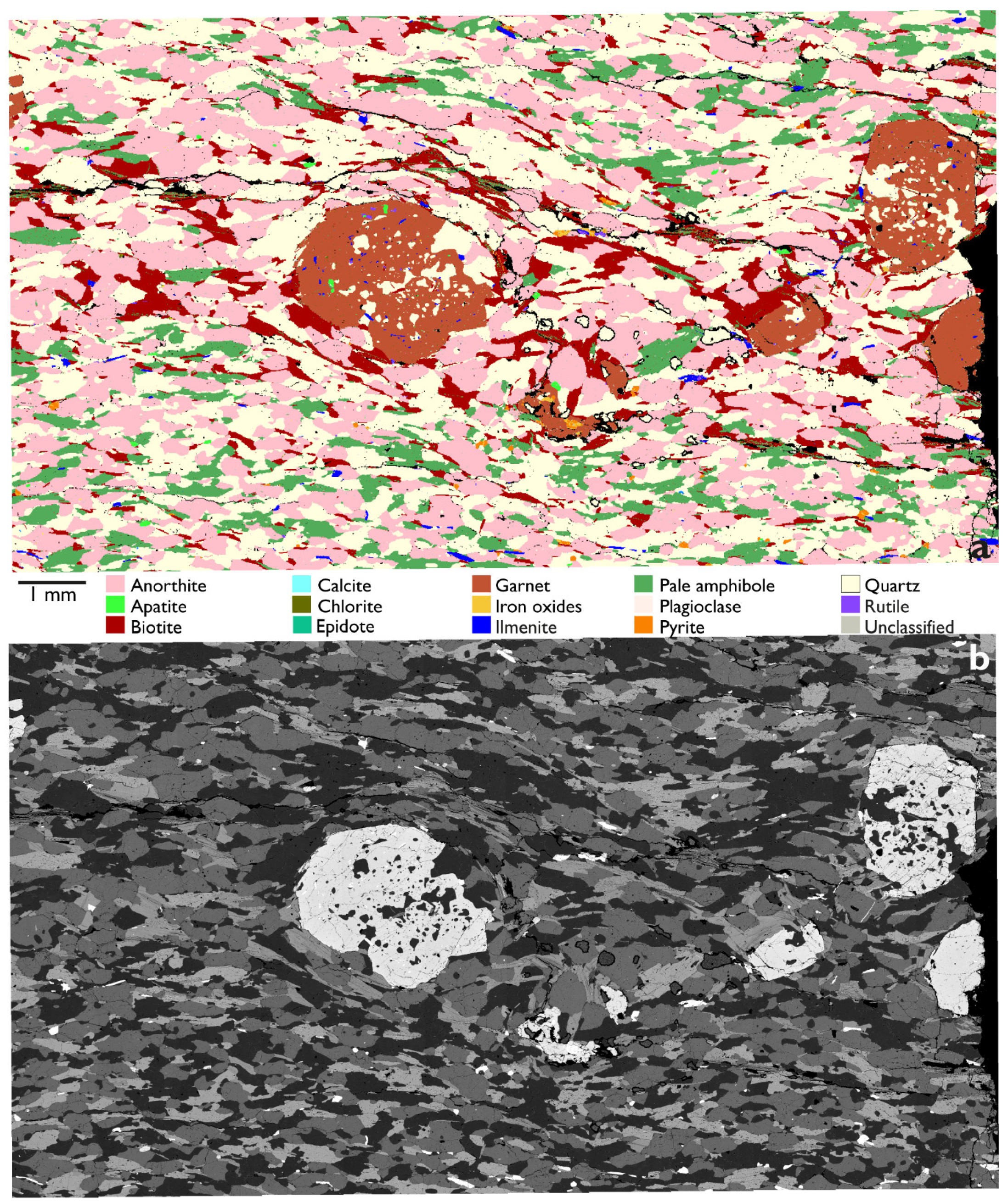
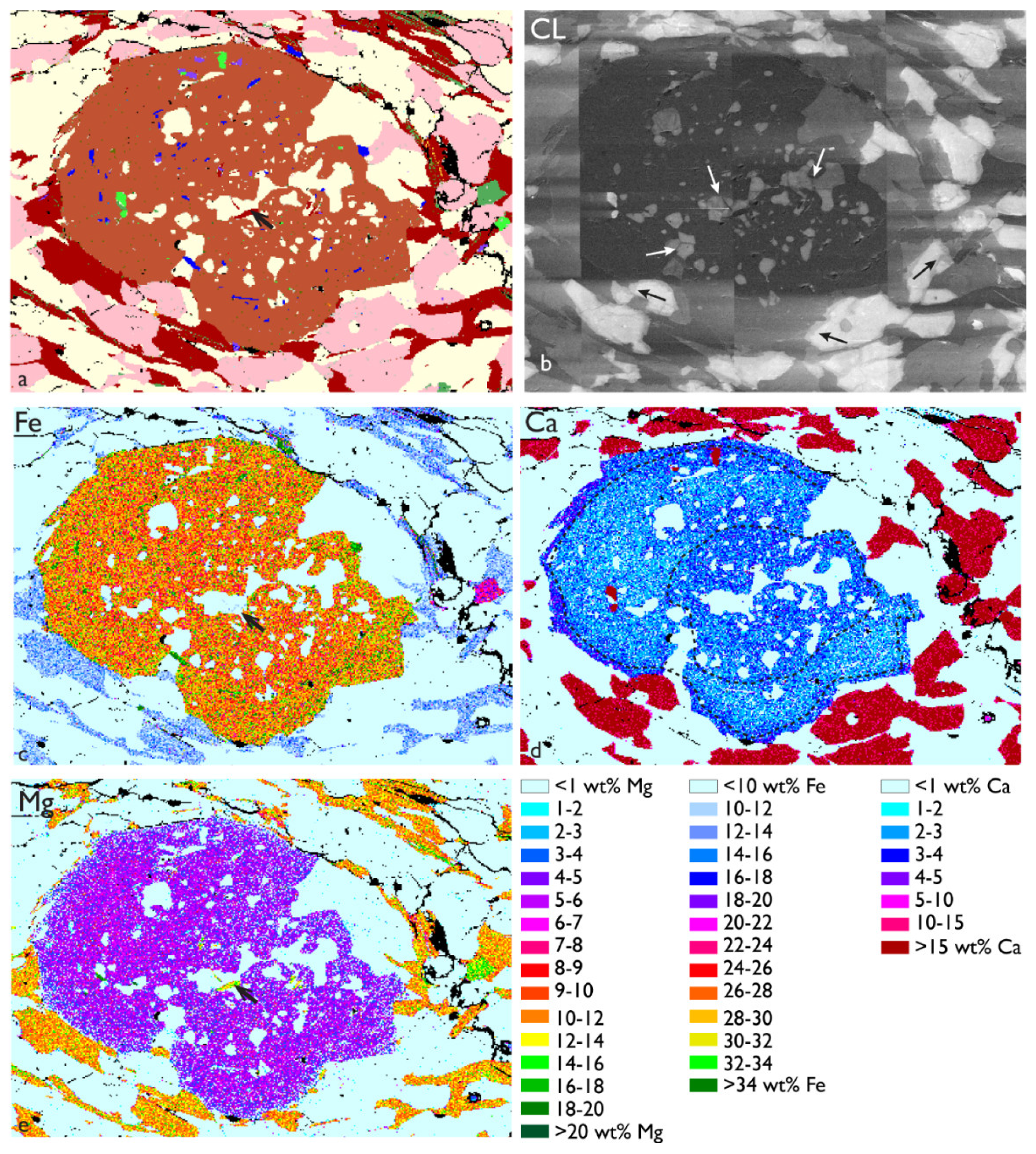
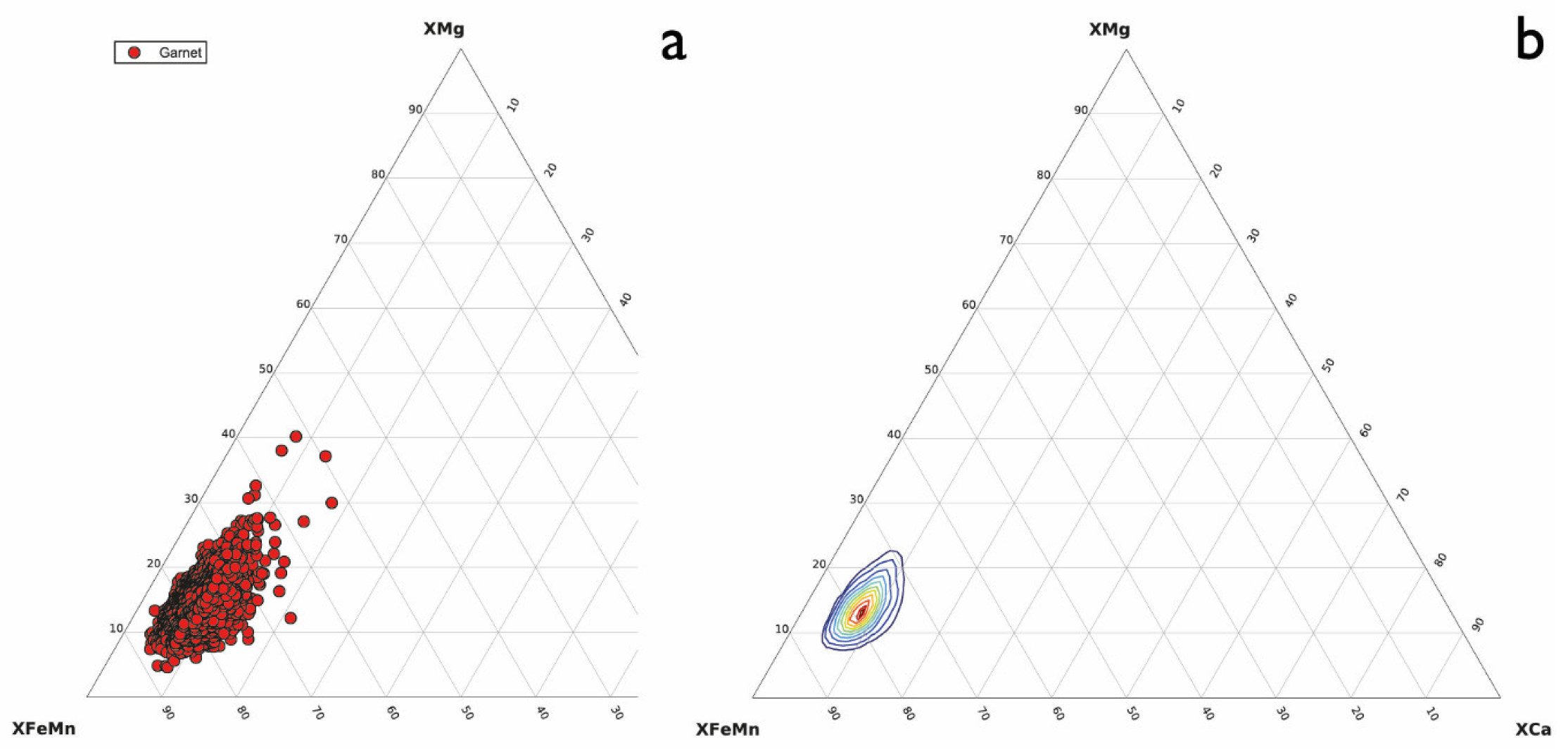
3.2. Garnet with Quartz Inclusions
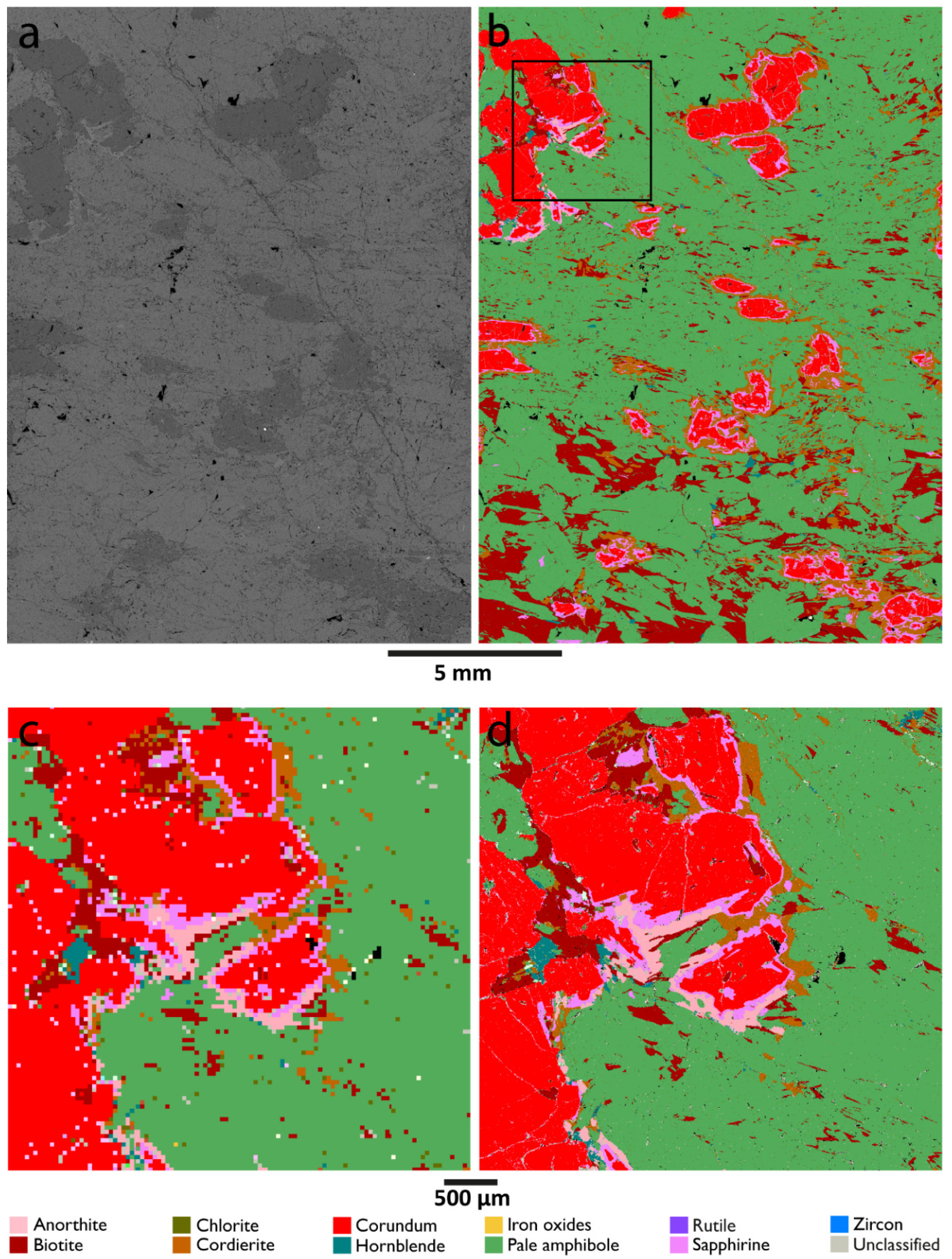
3.3. Reaction Rims Around Rubies
3.4. Grain Size Distribution of Chromite in Leucogabro
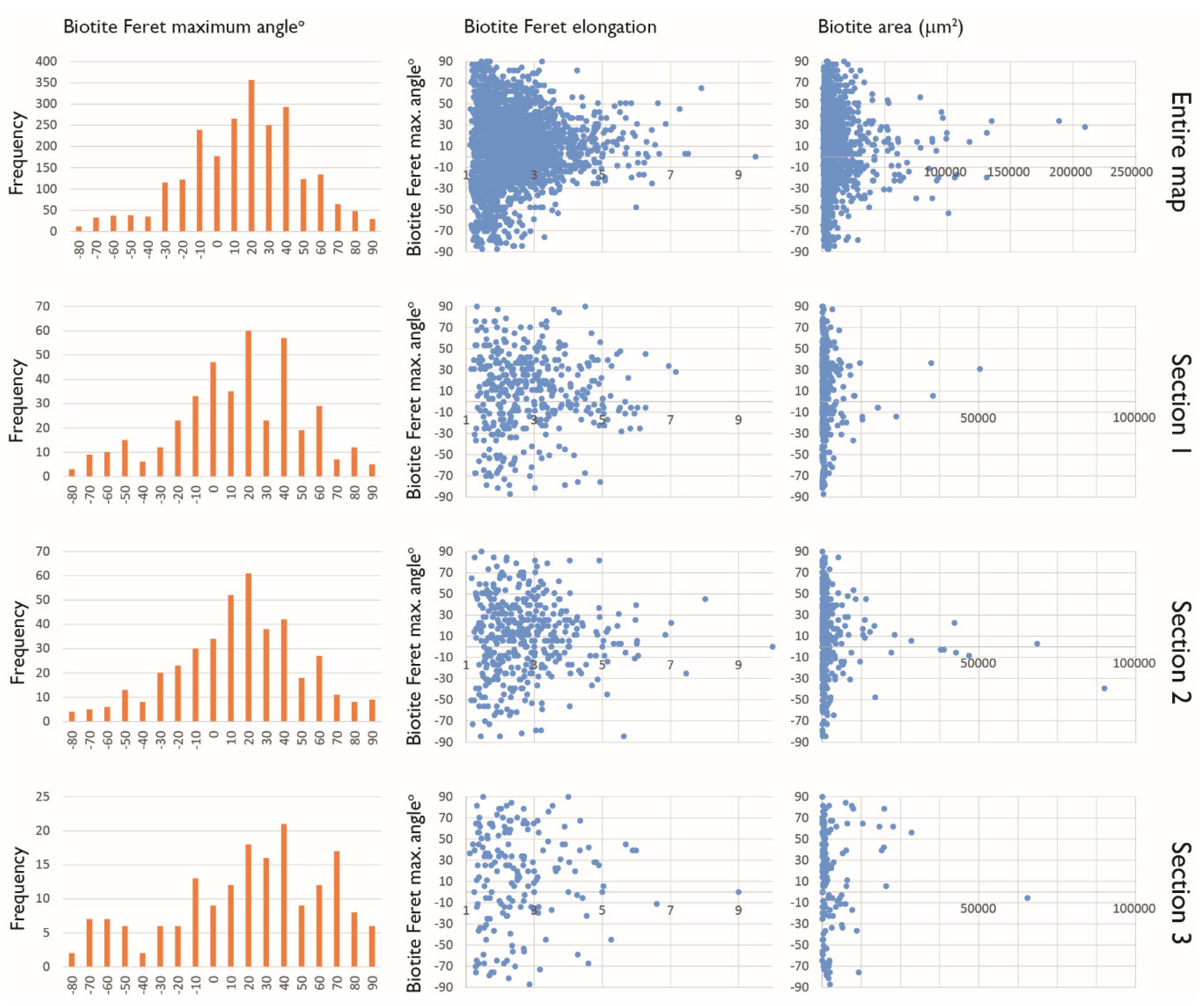
3.5. Maximum Feret Angle Determination
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huggins, F.E.; Kosmack, D.A.; Huffman, G.P.; Lee, R.J. Coal mineralogy by SEM analysis. Scanning Electron Microsc. 1980, 1, 531–540. [Google Scholar]
- Friedrichs, K.H. Electron microscopic analyses of dust from the lungs and the lymph nodes of talc-mine employees. Am. Ind. Hyg. Assoc. J. 1987, 48, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Heasman, I.; Watt, J. Particulate pollution case studies which illustrate uses of individual particle analysis by scanning electron microscopy. Environ. Geochem. Health 1989, 11, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Steffen, S.; Otto, M.; Niewoehner, L.; Barth, M.; Brozek-Mucha, Z.; Biegstraaten, J.; Horvath, R. Chemometric classification of gunshot residues based on energy dispersive X-ray microanalysis and inductively coupled plasma analysis with mass-spectrometric detection. Spectrochim. Acta 2007, 62, 1028–1036. [Google Scholar] [CrossRef]
- Hrtska, T.; Gottlieb, P.; Skála, R.; Breiter, K.; Motl, D. Automated mineralogy and petrology—Applications of Tescan Integrated Mineral Analyzer (TIMA). J. Geosci. 2018, 63, 47–63. [Google Scholar]
- Pirrie, D.; Rollinson, G.K. Unlocking the applications of automated mineral analysis. Geol. Today 2011, 27, 226–235. [Google Scholar] [CrossRef]
- Gäbler, H.-E.; Melcher, F.; Graupner, T.; Bahr, A.; Sitnikova, M.A.; Henjes-Kunst, F.; Brätz, H.; Gerdes, A. Speeding up the analytical workflow for Coltan Fingerprinting by an Integrated Mineral Liberation Analysis/LA-ICP-MS approach. Geostand. Geoanal. Res. 2011, 35, 431–448. [Google Scholar] [CrossRef]
- Graham, S.D.; Brough, C.; Cropp, A. An Introduction to ZEISS Mineralogic Mining and the correlation of light microscopy with automated mineralogy: A case study using BMS and PGM analysis of samples from a PGE-bearing chromitite prospect. Precious Met. 2015, 1–2. Available online: https://www.researchgate.net/profile/Christopher_Brough2/publication/277669986_An_Introduction_to_ZEISS_Mineralogic_Mining_and_the_correlation_of_light_microscopy_with_automated_mineralogy_a_case_study_using_BMS_and_PGM_analysis_of_samples_from_a_PGE-bearing_chromitite_prospect/links/5570388208aeccd77741818c.pdf (accessed on 15 June 2019).
- Keulen, N.; Frei, D.; Riisager, P.; Knudsen, C. Analysis of heavy minerals in sediments by Computer-Controlled Scanning Electron Microscopy (CCSEM): Principles and applications. In Quantitative Mineralogy and Microanalysis of Sediments and Sedimentary Rocks; Sylvester, P., Ed.; Mineralogical Association of Canada Short Course: Toronto, ON, Canada, 2012; Volume 42, pp. 167–184. [Google Scholar]
- Elghali, A.; Benzaazoua, M.; Bouzahzah, H.; Bussiere, B.; Villarraga-Gomez, H. Determination of the available acid-generating potential of waste rock, part 1: Mineralogical approach. Appl. Geochem. 2018, 99, 31–41. [Google Scholar] [CrossRef]
- Sandmann, D. Method Development in Automated Mineralogy. Ph.D. Thesis, Technischen Universität Bergakademie Freiberg, Freiberg, Germany, 2015. Available online: https://pdfs.semanticscholar.org/6c7d/fc4f887906a3c987f47277981fbec453538f.pdf (accessed on 1 June 2019).
- Scheller, S.; Tagle, R.; Gloy, G.; Barraza, M.; Menzies, A. Advancements in Minerals Identification and Characterization in Geo-Metallurgy: Comparing E-Beam and Micro-X-ray-Fluorescence Technologies. Microsc. Microanal. 2017, 23, 2168–2169. [Google Scholar] [CrossRef][Green Version]
- Holwell, D.A.; Adeyemi, Z.; Ward, L.A.; Graham, S.D.; Smith, D.J.; McDonald, I.; Smith, J.W. Low temperature alteration and upgrading of magmatic Ni-Cu-PGE sulfides as a source for hydrothermal Ni and PGE ores: A quantitative approach using automated mineralogy. Ore Geol. Rev. 2017, 91, 718–740. [Google Scholar] [CrossRef]
- Bernstein, S.; Frei, D.; McLimans, R.K.; Knudsen, C.; Vasudev, V.N. Application of CCSEM to heavy mineral deposits: Source of high-Ti ilmenite sand deposits of South Kerala beaches, SW India. J. Geochem. Explor. 2008, 96, 25–42. [Google Scholar] [CrossRef]
- Gu, Y. Automated Scanning Electron Microscope Based Mineral Liberation Analysis—An introduction to JKMRC/FEI Mineral Liberation Analyser. J. Miner. Mater. Charact. Eng. 2003, 2, 33–41. [Google Scholar] [CrossRef]
- Olivarius, M.; Rasmussen, E.S.; Siersma, V.; Knudsen, C.; Kokfelt, T.F.; Keulen, N. Provenance signal variations caused by facies and tectonics: Zircon age and heavy mineral evidence from Miocene sand in the north-eastern North Sea Basin. Mar. Pet. Geol. 2014, 49, 1–14. [Google Scholar] [CrossRef]
- Sylvester, P. Use of the Mineral Liberation Analyzer (MLA) for mineralogical Studies of sediments and sedimentary rocks. In Quantitative Mineralogy and Microanalysis of Sediments and Sedimentary Rocks; Sylvester, P., Ed.; Mineralogical Association of Canada Short Course: Toronto, ON, Canada, 2012; Volume 42, pp. 1–16. [Google Scholar]
- Ma, K.; Jiang, H.; Li, J.; Zhao, L. Experimental study on the micro alkali sensitivity damage mechanism in low-permeability reservoirs using QEMSCAN. J. Nat. Gas Sci. Eng. 2016, 36, 1004–1017. [Google Scholar] [CrossRef]
- Ayling, B.; Rose, P.; Petty, S.; Zemach, E.; Drakos, P. Qemscan° (Quantitative evaluation of minerals by scanning electron microscopy): Capability and application to fracture characterization in Geothermal systems. In Proceedings of the 37th Workshop Geothermal Reservoir Engineering 2012, Stanford, CA, USA, 30 January–1 February 2012. SGP-TR-194. [Google Scholar]
- Mackay, D.A.R.; Simandl, G.J.; Ma, W.; Redfearn, M.; Gravel, J. Indicator mineral-based exploration for carbonatites and related specialty metal deposits—A Qemscan orientation survey, British Columbia, Canada. J. Geochem. Explor. 2016, 165, 159–173. [Google Scholar] [CrossRef]
- Graham, S.; Keulen, N. Nano-scale automated quantitative mineralogy: A 200 nm quantitative mineralogy assessment of fault gouge using Mineralogic. Minerals 2019, 9, 665. [Google Scholar] [CrossRef]
- Nikonow, W.; Rammlmair, D. Automated mineralogy based on micro-energy dispersive X-ray fluorescence microscopy (micro-EDXRF) applied to plutonic rock thin sections in comparison to a mineral liberation analyzer. Geosci. Instrum. Methods Data Syst. 2017, 6, 429–437. [Google Scholar] [CrossRef]
- Nikonow, W.; Rammlmaier, D.; Meima, J.A.; Schodlok, M.C. Advanced mineral characterization and petrographic analysis by µ-EDXRF, LIBS, HIS and hyperspectral data merging. Miner. Petrol. 2019, 113, 417–431. [Google Scholar] [CrossRef]
- Senesi, G.S. Laser-Induced Breakdown Spectroscopy (LIBS) applied to terrestrial and extraterrestrial analogue geomaterials with emphasis to minerals and rocks. Earth Sci. Rev. 2014, 139, 231–267. [Google Scholar] [CrossRef]
- Gottlieb, P.; Wilkie, G.; Sutherland, D.; Ho-Tun, E.; Suthers, S.; Perera, K.; Jenkins, B.; Spencer, S.; Butcher, A.; Rayner, J. Using quantitative electron microscopy for process mineralogy applications. JOM 2000, 52, 24–25. [Google Scholar] [CrossRef]
- Andersen, J.C.Ø.; Rollinson, G.K.; Snook, B.; Herrington, R.; Fairhurst, R.J. Use of QEMSCAN® for the characterization of Ni-rich and Ni-poor goethite in laterite ores. Miner. Eng. 2009, 22, 1119–1129. [Google Scholar] [CrossRef]
- Schulz, B. Polymetamorphism in garnet micaschists of the Saualpe Eclogite Unit (Eastern Alps, Austria), resolved by automated SEM methods and EMP–Th–U–Pb monazite dating. J. Metamorph. Geol. 2016. [Google Scholar] [CrossRef]
- Kern, M.; Mõckel, R.; Krause, J.; Teichmann, J.; Gutzmer, J. Calculating the deportment of a fine-grained and compositionally complex Sn skarn with a modified approach for automated mineralogy. Miner. Eng. 2018, 116, 213–225. [Google Scholar] [CrossRef]
- Williams, M.L.; Jercinovic, M.J. Tectonic interpretation of metamorphic tectonites: Integrating compositional mapping, microstructural analysis and in situ monazite dating. J. Metamorph. Geol. 2012, 30, 739–752. [Google Scholar] [CrossRef]
- Windley, B.F.; Garde, A.A. Arc-generated blocks with crustal sections in the North Atlantic craton of West Greenland: New mechanisms of crustal growth in the Archaean with modern analogues. Earth Sci. Rev. 2009, 93, 1–30. [Google Scholar] [CrossRef]
- Friend, C.R.L.; Nutman, A.P. New pieces to the Archaean terrane jigsaw puzzle in the Nuuk region, southern West Greenland: Steps in transforming a simple insight into a complex regional tectonothermal model. J. Geol. Soc. Lond. 2005, 162, 147–162. [Google Scholar] [CrossRef]
- Kalsbeek, F. Metamorphism of Archaean rocks of West Greenland. In The Early History of the Earth; Windley, B.F., Ed.; Wiley: London, UK, 1976; pp. 225–235. [Google Scholar]
- Henriksen, N.; Higgins, A.K.; Kalsbeek, F.; Pulvertaft, T.C.R. Greenland from Archaean to Quaternary Descriptive Text to the 1995 Geological Map of Greenland, 1:2,500,000, 2nd ed.; Geological Survey of Denmark and Greenland Bulletin: Copenhagen, Denmark, 2009; Volume 18, pp. 1–93. [Google Scholar]
- Myers, J.S. Stratigraphy and structure of the Fiskenæsset Complex, southern West Greenland. Grønlands Geol. Undersøgelse 1985, 150, 1–72. [Google Scholar]
- Keulen, N.; Næraa, T.; Kokfelt, T.F.; Schumacher, J.C.; Scherstén, A. Zircon record of the igneous and metamorphic history of the Fiskenæsset anorthosite complex in southern West Greenland. Geol. Surv. Den. Greenl. Bull. 2010, 20, 67–70. [Google Scholar]
- Polat, A.; Frei, R.; Scherstén, A.; Appel, P.W.U. New age (ca. 2970 Ma), mantle source composition and geodynamic constraints on the Archean Fiskenæsset anorthosite complex, SW Greenland. Chem. Geol. 2010, 277, 1–20. [Google Scholar] [CrossRef]
- Keulen, N.; Schumacher, J.C.; Næraa, T.; Kokfelt, T.F.; Schersten, A.; Szilas, K.; van Hinsberg, V.J.; Schlatter, D.M.; Windley, B.F. Meso- and Neoarchaean geological history of the Bjørnesund and Ravns Storø Supracrustal Belts, southern West Greenland: Settings for gold enrichment and corundum formation. Precambrian Res. 2014, 254, 36–58. [Google Scholar] [CrossRef]
- Keulen, N.; Thomsen, T.B.; Schumacher, J.C.; Poulsen, M.D.; Kalvig, P.; Vennemann, T.; Salimi, R. Formation, origin and geographic typing of corundum (ruby and pink sapphire) from the Fiskenæsset complex, Greenland. Lithos 2019. submitted. [Google Scholar]
- Szilas, K.; Hoffmann, J.E.; Scherstén, A.; Rosing, M.T.; Windley, B.F.; Kokfelt, T.F.; Keulen, N.; van Hinsberg, V.J.; Næraa, T.; Frei, R.; et al. Complexcalc-alkaline volcanism recorded in Mesoarchaean supracrustal belts north of Frederikshåb Isblink, southern West Greenland: Implications for subductionzone processes in the early Earth. Precambrian Res. 2012, 208, 90–123. [Google Scholar] [CrossRef]
- Keulen, N.; Kokfelt, T.F.; The Homogenisation Team. A Seamless, Digital, Internet-Based Geological Map of South-West and Southern West Greenland, 1:100,000, 61°30′–64°; Geological Survey of Denmark and Greenland: Copenhagen, Denmark, 2011. [Google Scholar]
- Schumacher, J.C.; van Hinsberg, V.J.; Keulen, N. Metamorphism in Supracrustaland Ultramafic Rocks in Southern; Geological Survey of Denmark and Greenland: Copenhagen, Denmark, 2011; Volume 6, pp. 1–29. [Google Scholar]
- Keulen, N.; Heijboer, T. The provenance of garnet: Semi-automatic plotting and classification of garnet compositions. Geophys. Res. Abstr. 2011, 13, EGU 2011-4716. [Google Scholar]
- Gublin, Y.L. Garnet–Biotite Geothermometer and Estimation of Crystallization Temperature of Zoned Garnets from Metapelites: I. Reconstruction of Thermal History of Porphyroblast Growth. Geol. Ore Depos. 2012, 54, 602–615. [Google Scholar] [CrossRef]
- Stünitz, H.; Fitzgerald, J.D. Deformation of granitoids at low metamorphic grade. II: Granular flow in albite-rich mylonites. Tectonophysics 1983, 221, 299–324. [Google Scholar] [CrossRef]
- Tullis, J.; Yund, R.A. Dynamic recrystallization of feldspar: A mechanism for ductile shear zone formation. Geology 1985, 13, 238–241. [Google Scholar] [CrossRef]
- Herd, R.K.; Windley, B.F.; Ghisler, M. The mode of occurrence and petrogenisesis of the sapphirine-bearing and associated rocks of West Greenland. Rapp. Grønlands Geol. Undersøgelse 1969, 24, 1–44. [Google Scholar]
- Appel, P.W.U.; Dahl, O.; Kalvig, P.; Polat, A. Discovery of new PGE mineralisation in the Precambrian Fiskenaesset anorthosite complex, West Greenland. Geol. Surv. Den. Greenl. Rep. 2011, 3, 1–48. [Google Scholar]
- Ghisler, M. Pre-metamorphic folded chromite deposits of stratiform type in the early Precambrian of West Greenland. Miner. Depos. 1970, 5, 223–236. [Google Scholar] [CrossRef]
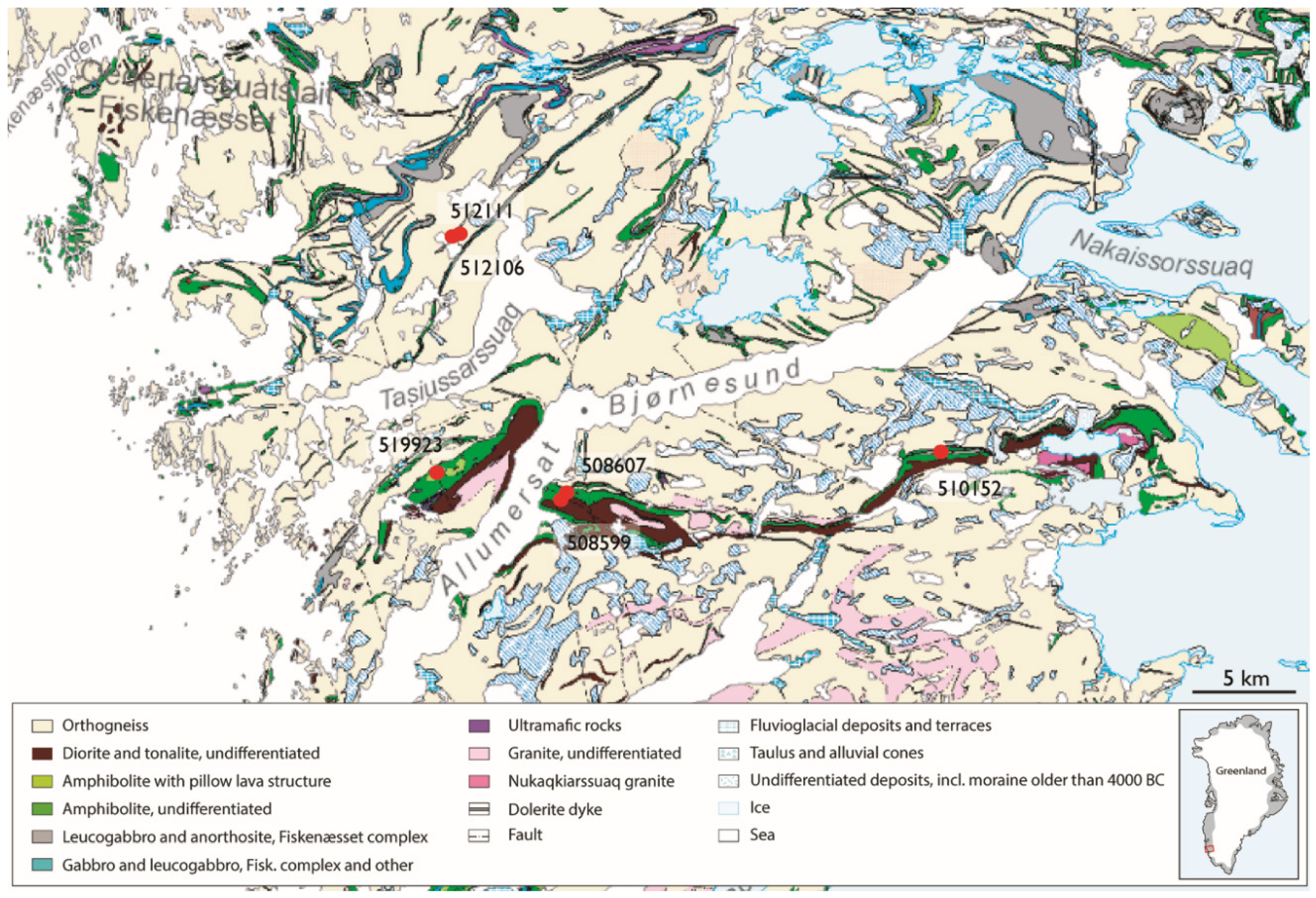
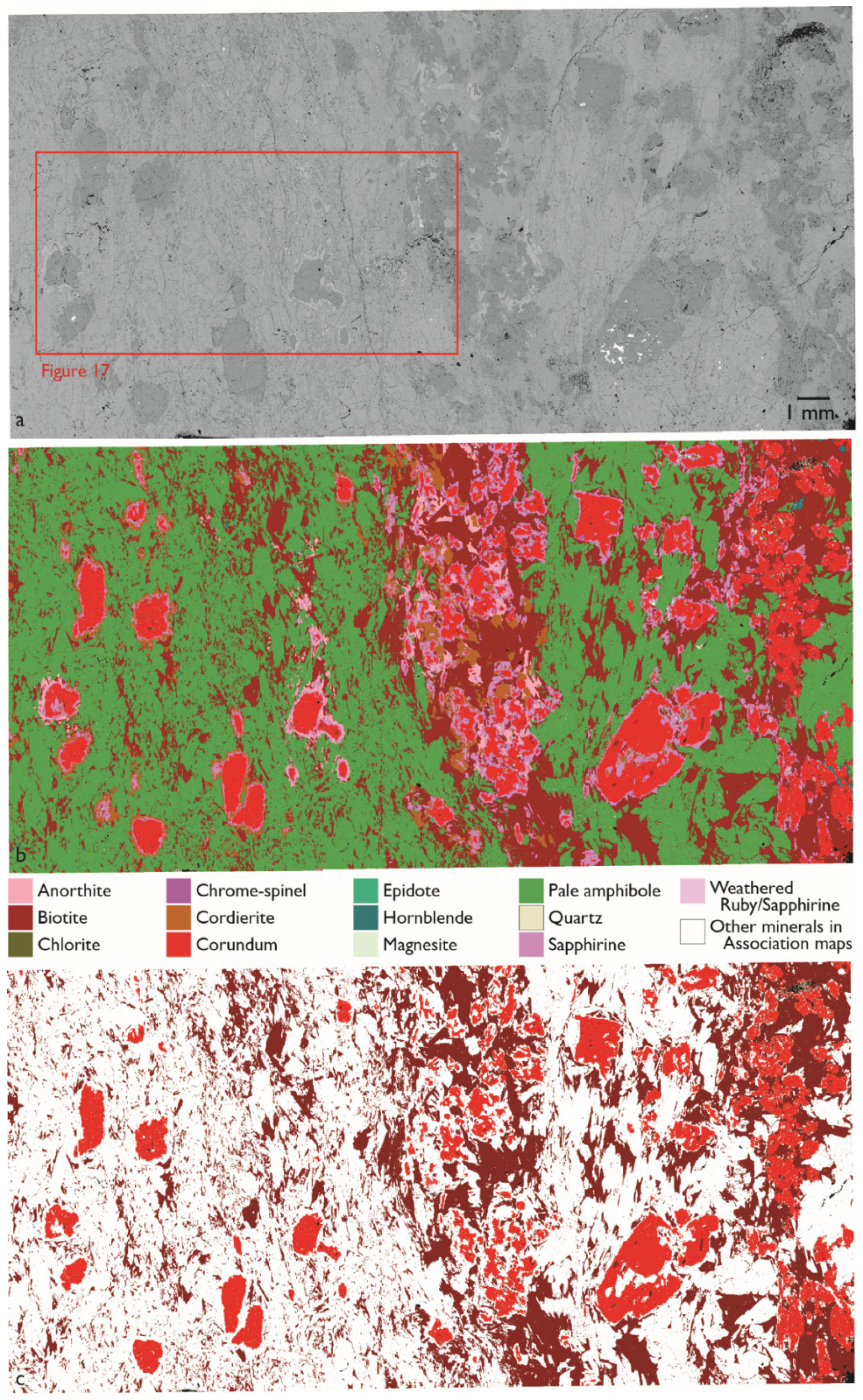
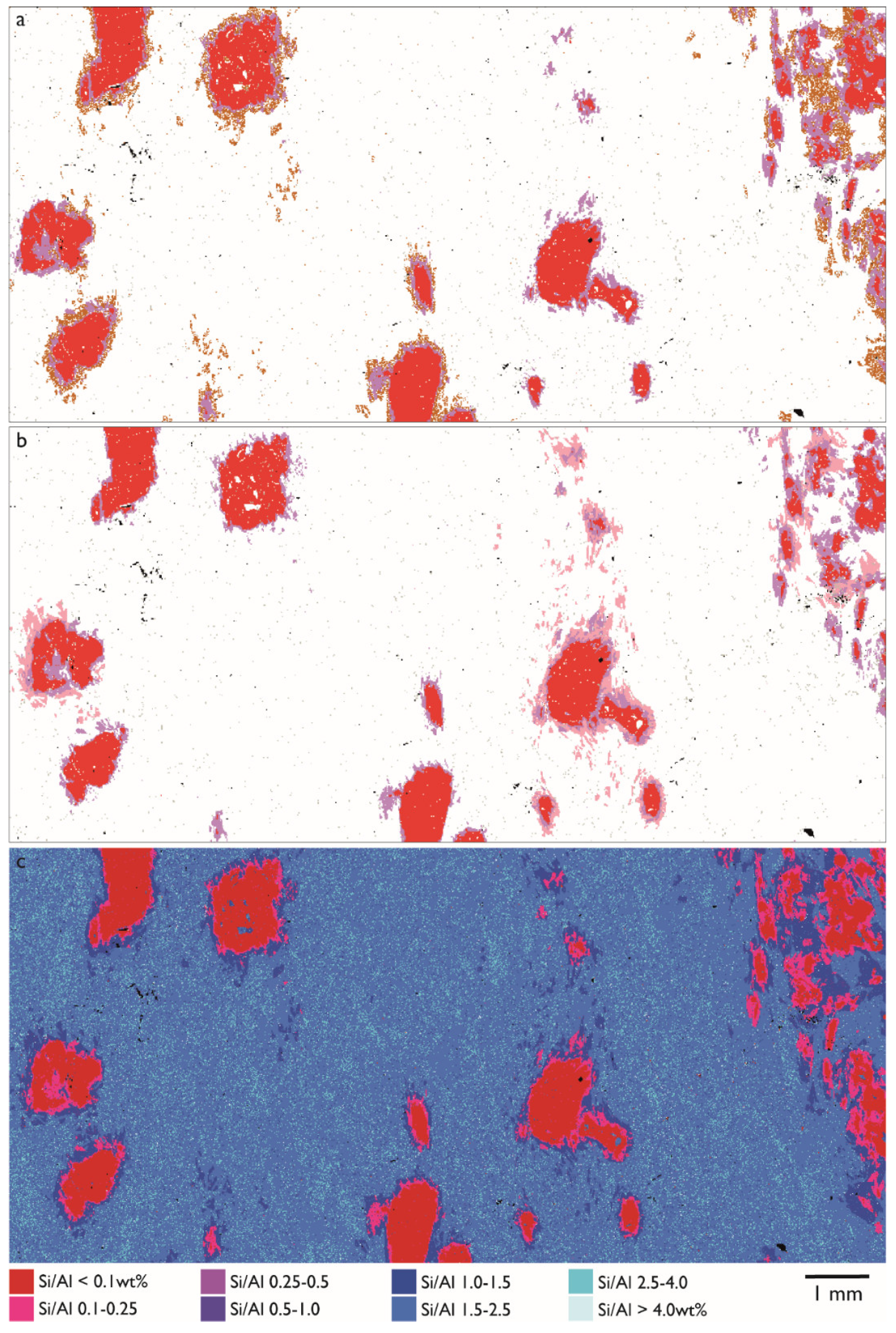
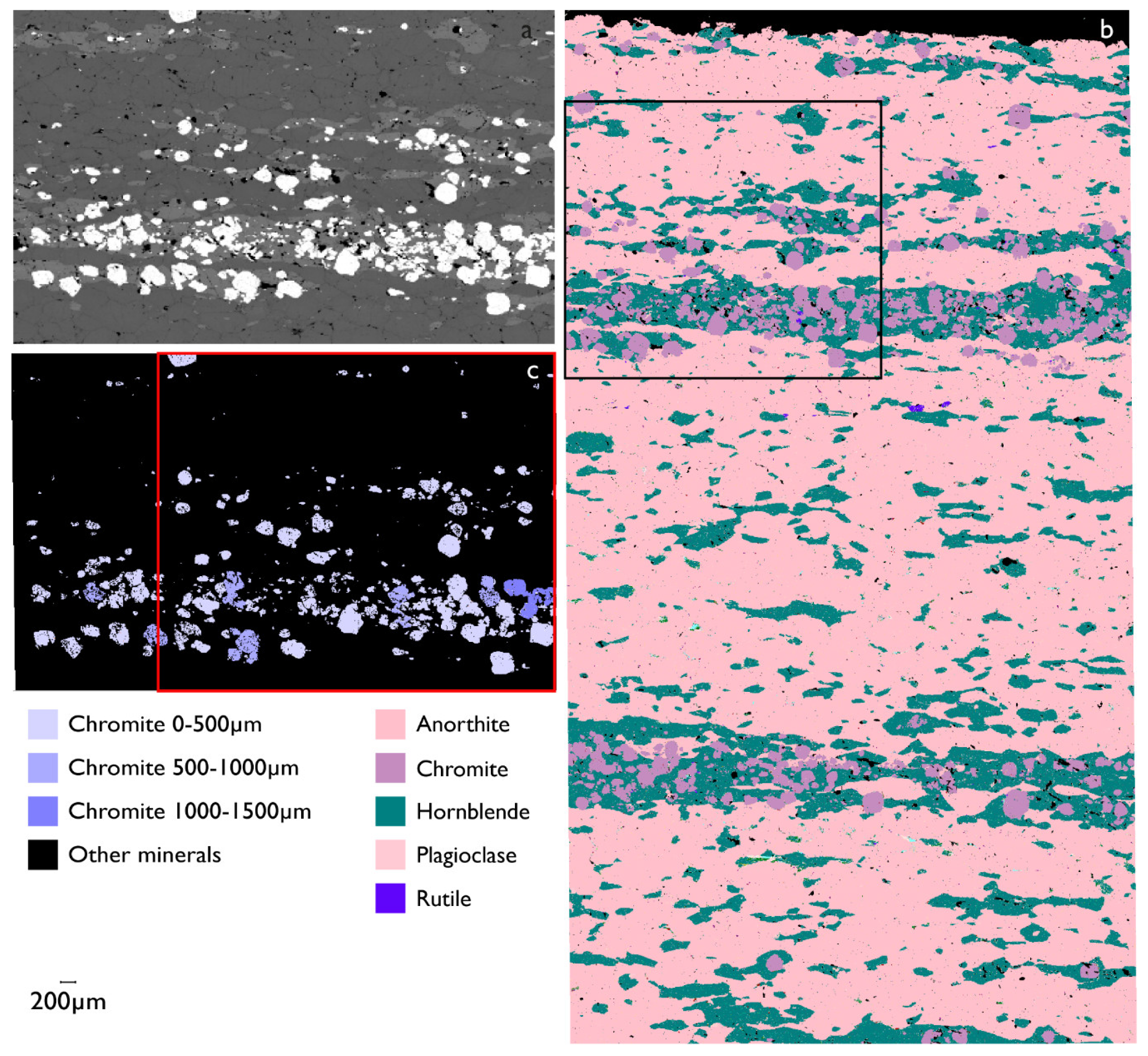
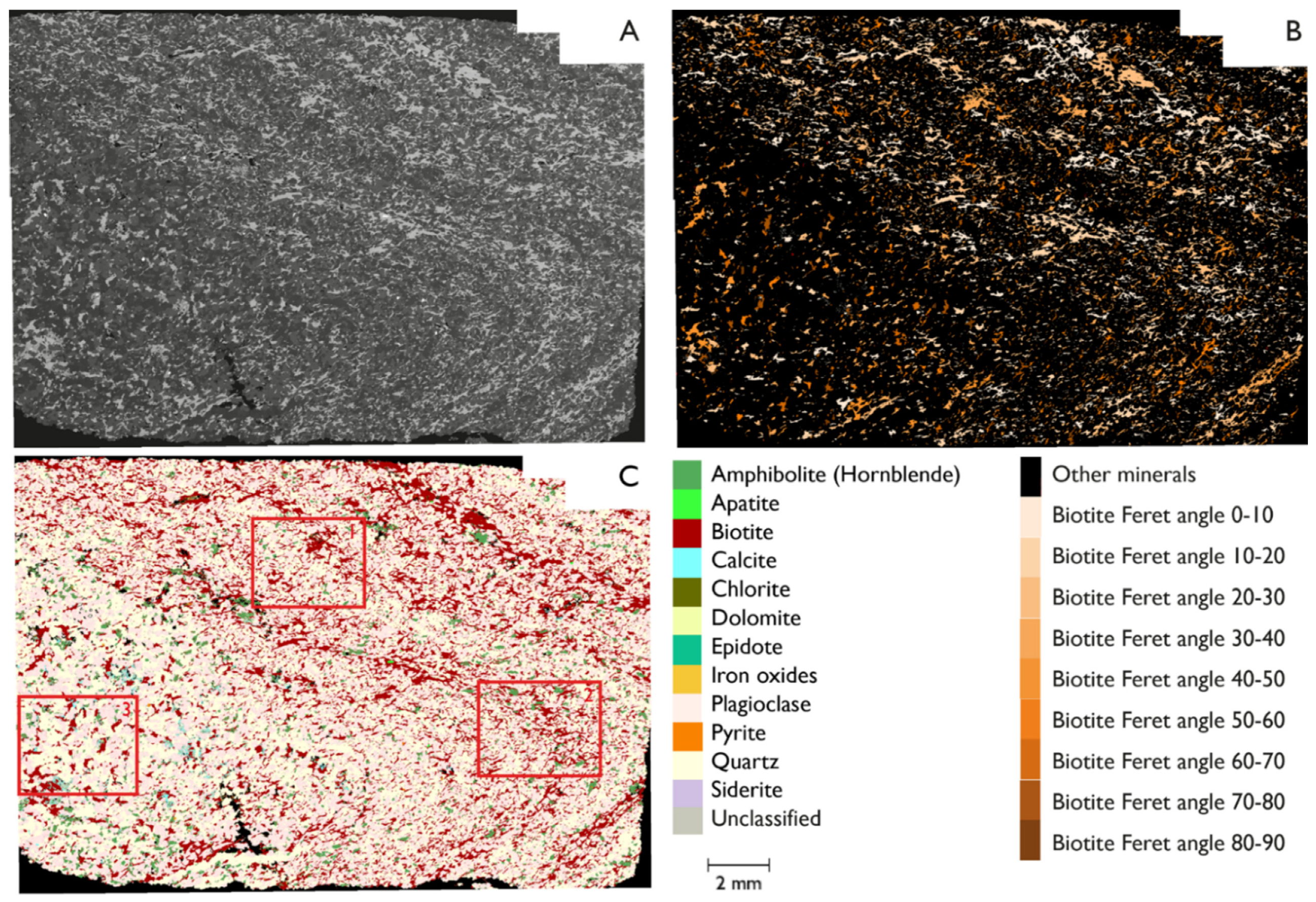
| Sample No. | Acceleration Voltage (kV) | Aperture Size (µm) | Mapping Step Size (µm) | Magnification | Through-Put Rate (kcps) | Dwell Time (s) |
|---|---|---|---|---|---|---|
| 508599 | 20 | 120 | 15 | 160 | 275 | 0.006 |
| 508607 | 15 | 120 | 5 & 20 | 235 | 400 | 0.008 |
| 510152 | 20 | 120 | 20 | 235 | 275 | 0.006 |
| 511923 | 20 | 120 | 5–40 | 235 | 275 | 0.004 |
| 521106 | 20 | 120 | 5–40 | 235 | 275 | 0.004 |
| 521111 | 15 | 120 | 20 | 235 | 400 | 0.007 |
| 511923 | Al% | Ca% | Cl% | Cr% | Cu% | F% | Fe% | K% | Mg% | Mn% | Na% | O% | P% | S% | Si% | Ti% | Zr% | LOI% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIN: element | 8.05 | 10.2 | 0.01 | 0.02 | 0.03 | 0.01 | 10.6 | 0 | 5.22 | 0.01 | 0.86 | 38.6 | 0.03 | 0 | 26.1 | 0.36 | 0.01 | |
| MIN: oxide | 13.9 | 13 | 12.4 | 0 | 7.91 | 0.02 | 1.06 | 0.06 | 0.01 | 51 | 0.55 | |||||||
| MIN: ppm | 295 | 316 | 116 | |||||||||||||||
| Actlab: oxide | 14.9 | 11 | x | x | 13.2 | 0.12 | 8 | 0.2 | 2.01 | 0.07 | x | 49 | 1.02 | 0.72 | ||||
| Actlab: ppm | 250 | 70 | 62 | |||||||||||||||
| 508599 | ||||||||||||||||||
| MIN: element | 7.74 | 2.25 | 0.01 | 0.01 | 0.04 | 0.03 | 3.96 | 1.59 | 2.17 | 0.07 | 2.94 | 41.43 | 0.01 | 0.06 | 37.51 | 0.05 | 0.00 | |
| MIN: oxide | 12.94 | 2.79 | 0.01 | 0.04 | 4.51 | 1.70 | 3.19 | 0.08 | 3.50 | 0.02 | 0.13 | 71.02 | 0.07 | 0.00 | ||||
| 508607 | ||||||||||||||||||
| MIN: element | 9.55 | 5.03 | 0.02 | 0.02 | 0.03 | 0.03 | 6.45 | 0.75 | 2.99 | 0.02 | 0.30 | 39.95 | 0.01 | 0.09 | 34.25 | 0.35 | 0.00 | |
| MIN: oxide | 15.85 | 6.18 | 0.02 | 0.04 | 7.29 | 0.80 | 4.36 | 0.02 | 0.35 | 0.03 | 0.21 | 64.35 | 0.52 | 0.00 | ||||
| 510152 | ||||||||||||||||||
| MIN: element | 16.88 | 11.31 | 0.01 | 2.00 | 0.03 | 0.02 | 4.42 | 0.01 | 3.13 | 1.08 | 38.28 | 0.00 | 22.66 | 0.02 | 0.00 | |||
| MIN: oxide | 27.37 | 13.59 | 2.51 | 0.03 | 4.88 | 0.01 | 4.46 | 1.25 | 0.01 | 41.62 | 0.02 | 0.00 | ||||||
| 521106 | ||||||||||||||||||
| MIN: element | 15.28 | 0.11 | 0.02 | 0.12 | 0.04 | 0.07 | 4.03 | 0.96 | 14.92 | 0.01 | 0.12 | 38.95 | 0.00 | 0.02 | 25.08 | 0.05 | 0.00 | |
| MIN: oxide | 25.26 | 0.14 | 0.16 | 0.04 | 4.54 | 1.01 | 21.64 | 0.02 | 0.14 | 0.00 | 0.04 | 46.95 | 0.07 | 0.00 | ||||
| 521111 | ||||||||||||||||||
| MIN: element | 15.10 | 0.23 | 0.04 | 0.11 | 0.03 | 0.06 | 4.67 | 1.52 | 14.38 | 0.01 | 0.14 | 40.58 | 0.00 | 0.01 | 22.87 | 0.02 | 0.00 | |
| MIN: oxide | 25.96 | 0.29 | 0.15 | 0.04 | 5.46 | 1.66 | 21.69 | 0.02 | 0.17 | 0.00 | 0.03 | 44.51 | 0.03 | 0.00 |
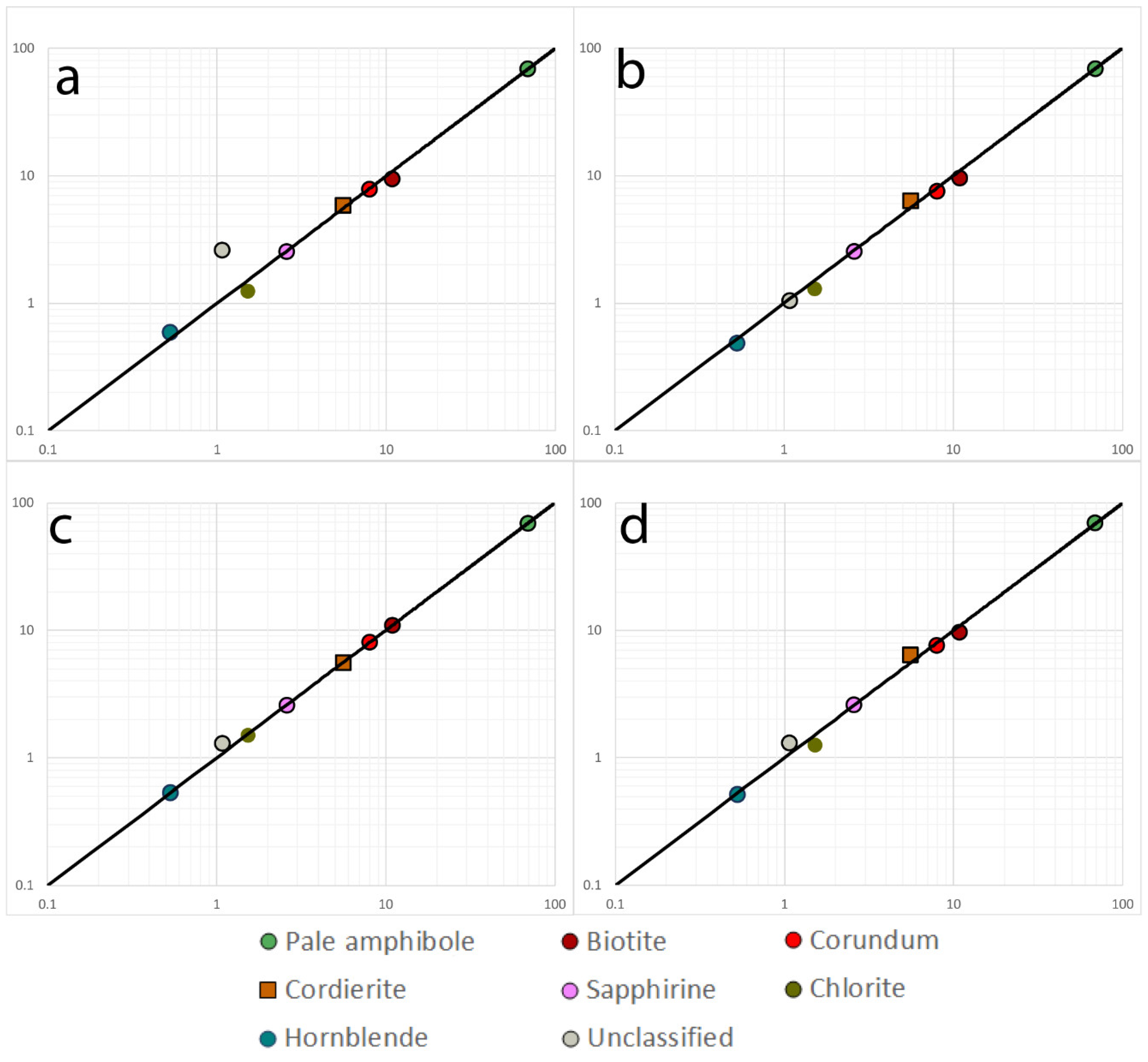
| Sample 519923 | 10 µm Line Scan | 20 µm Line Scan | 40 µm Map | 20 µm Map | 10 µm Map | 5 µm Map | STD |
|---|---|---|---|---|---|---|---|
| Time (minutes) | 4 | 3 | 8 | 21 | 73 | 282 | |
| FerroAmphibole | 46.9 | 46.8 | 48.0 | 47.4 | 47.6 | 47.6 | 0.009 |
| Hornblende | 22.1 | 22.1 | 20.2 | 21.0 | 20.9 | 20.9 | 0.03 |
| Anorthite | 11.6 | 11.6 | 13.2 | 13.0 | 12.8 | 12.8 | 0.05 |
| Quartz | 6.5 | 6.7 | 5.7 | 5.8 | 5.9 | 5.9 | 0.06 |
| Plagioclase | 5.5 | 5.3 | 5.8 | 5.9 | 5.9 | 5.8 | 0.04 |
| Zircon | 0 | 0 | 0.007 | 0.005 | 0.004 | 0.005 | 0.17 |
| Apatite | 0.27 | 0.31 | 0.15 | 0.15 | 0.13 | 0.14 | 0.32 |
| Rutile | 0 | 0 | 0.04 | 0.02 | 0.03 | 0.03 | 0.19 |
| Sphene | 0.16 | 0.23 | 0.20 | 0.16 | 0.17 | 0.16 | 0.14 |
| Unclassified | 2.9 | 3.2 | 2.1 | 2.1 | 2.1 | 2.2 | 0.17 |
| Sample 521106 | |||||||
| Time (minutes) | 40 | 83 | 298 | 765 | 3589 | ||
| Pale orthoamphibole | 69.0 | 69.8 | 68.7 | 69.6 | 68.8 | 0.006 | |
| Biotite | 9.4 | 9.7 | 10.9 | 9.7 | 10.9 | 0.07 | |
| Corundum | 7.8 | 7.6 | 8.0 | 7.6 | 8.0 | 0.02 | |
| Cordierite | 5.8 | 6.4 | 5.6 | 6.4 | 5.6 | 0.06 | |
| Sapphirine | 2.5 | 2.6 | 2.6 | 2.6 | 2.6 | 0.01 | |
| Chlorite | 1.2 | 1.3 | 1.5 | 1.3 | 1.5 | 0.09 | |
| Hornblende | 0.59 | 0.49 | 0.53 | 0.52 | 0.53 | 0.06 | |
| Rutile | 0.007 | 0.02 | 0.02 | 0.02 | 0.02 | 0.29 | |
| Zircon | 0 | 0.005 | 0.003 | 0.004 | 0.003 | 0.24 | |
| IronOxide | 0.007 | 0.001 | 0.001 | 0.0005 | 0.0001 | 1.50 | |
| Unclassified | 2.6 | 1.1 | 1.3 | 1.3 | 1.1 | 0.40 |
| Mineral | Area in TS (%) | Association Data (%) | ||
|---|---|---|---|---|
| Plagioclase | Quartz | Biotite | ||
| Area 1 | ||||
| Biotite | 19.8 | 45.3 | 35.4 | - |
| Plagioclase | 40.2 | - | 40.9 | 37.8 |
| Quartz | 35.6 | 46.6 | - | 34.2 |
| Area 2 | ||||
| Biotite | 23.1 | 48.7 | 31.7 | - |
| Plagioclase | 40.3 | - | 34.5 | 45.3 |
| Quartz | 31.1 | 43.5 | - | 37.7 |
| Area 3 | ||||
| Biotite | 11.3 | 34.0 | 40.8 | - |
| Plagioclase | 36.1 | - | 51.6 | 21.3 |
| Quartz | 45.5 | 47.7 | - | 23.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keulen, N.; Malkki, S.N.; Graham, S. Automated Quantitative Mineralogy Applied to Metamorphic Rocks. Minerals 2020, 10, 47. https://doi.org/10.3390/min10010047
Keulen N, Malkki SN, Graham S. Automated Quantitative Mineralogy Applied to Metamorphic Rocks. Minerals. 2020; 10(1):47. https://doi.org/10.3390/min10010047
Chicago/Turabian StyleKeulen, Nynke, Sebastian Næsby Malkki, and Shaun Graham. 2020. "Automated Quantitative Mineralogy Applied to Metamorphic Rocks" Minerals 10, no. 1: 47. https://doi.org/10.3390/min10010047
APA StyleKeulen, N., Malkki, S. N., & Graham, S. (2020). Automated Quantitative Mineralogy Applied to Metamorphic Rocks. Minerals, 10(1), 47. https://doi.org/10.3390/min10010047




