1. Introduction
The world’s largest alkaline massifs—Khibiny and Lovozero—are located in Kola Peninsula (Murmansk Region, northwest of Russia). Over the past few decades, the development of apatite and rare-metal deposits of these massifs has accumulated a huge amount of mining waste. Among them, the largest ones are flotation tailings dumps of apatite-nepheline dressing plants of Joint-stock company “Apatit”. The technological process of the production of apatite concentrate in apatite–nepheline dressing plants is accompanied by the accumulation of huge masses of fine sandy waste, about half consisting of nepheline. In the apatite–nepheline dressing plant ANDP-2 tailing dumps alone (
Figure 1), which area is about 10 km
2, by various estimations 550–630 million tons of nepheline containing tailings (NT) are accumulated. The total amount of such waste in the area of Kirovsk and Apatity (Murmansk Region, Russia) ranges between 800–900 million tons [
1,
2].
The nepheline containing tailings of the apatite-nepheline dressing plant ANDP-2 are a fine gray sand with a significant content of dusty fractions. The granulometric composition of these tailings sampled in 2005 is presented in
Table 1. The mineral composition of the nepheline containing tailings of apatite-nepheline dressing plant ANDP-2, wt. %: nepheline 50–55, feldspars 2.5–4, secondary minerals with respect to nepheline 0.5–2, aegirine 23–27, titanomagnetite 4–5, apatite 2–3.5, titanite 4–5 [
3].
Table 2 presents the chemical composition of the nepheline containing tailings.
There are several technological schemes for obtaining nepheline concentrate (NC) from the nepheline containing tailings. The chemical composition of the nepheline concentrate is given in
Table 2. The mineral composition of the nepheline concentrate, wt. %: nepheline 70–85, feldspars 8–16, secondary minerals with respect to nepheline 1.5–5, aegirine 23–27, titanomagnetite 0.4–0.6, apatite 0.2–0.8, titanite 0.5–1.0 [
3]. The nepheline concentrate is processed in limited quantities into alumina, soda, potash, and Portland cement. The major part of nepheline is stored in the tailings. The proportion of nepheline produced in the form of the nepheline concentrate does not exceed 10% of its content in the mined apatite-nepheline ore [
4].
The nepheline containing tailings, on the one hand, pose a real threat to the environment (mainly as a source of air pollution due to dusting). On the other hand, the nepheline containing tailings are a man-made deposit that contains reserves of valuable components (Na2O, K2O, Al2O3, etc). The need for nepheline as a source of alumina, soda and potash is very limited. Therefore, it is relevant to search for alternative ways of disposing of the nepheline containing mining waste. The use of nepheline in the production of new binders can be very much in demand.
Nepheline is a rock-forming mineral, a framework aluminosilicate of potassium and sodium with idealized composition Na
3KAl
4Si
4O
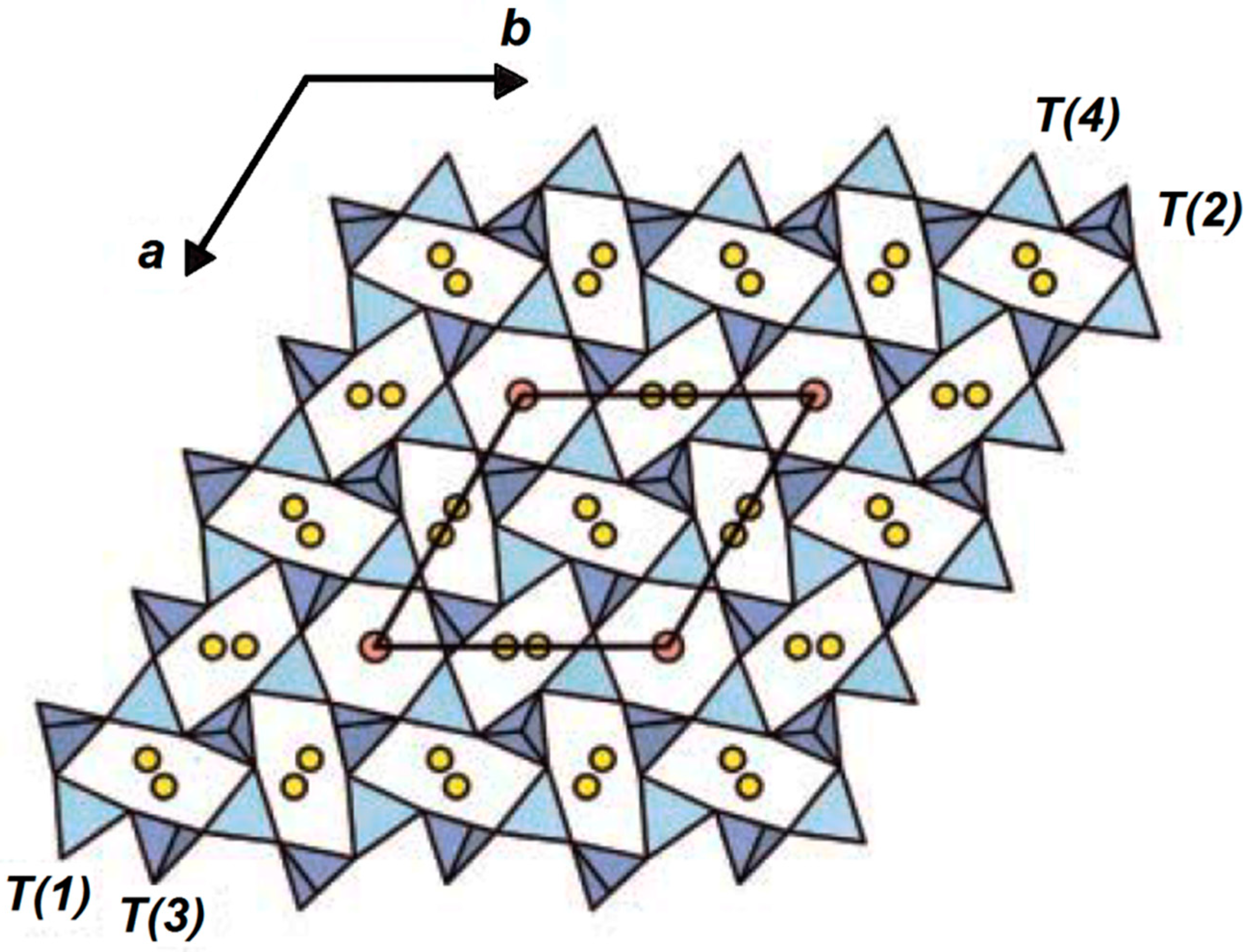
16. The crystal structure of nepheline (
Figure 2) belongs to the type of trimidite where half of the silicon atoms are replaced by aluminum. As a result of such a replacement, the alumina–silicon–oxygen group acquires negative charge, which is compensated by sodium and potassium cations. The mineral framework is formed by distorted 6-membered rings of SiO
4 and AlO
4 tetrahedra connected to each other by their vertices, and alkali metal ions are in the cavities of the structure. There are two types of 6-membered rings of tetrahedra: one quarter of the rings is almost regular, and three quarters of the rings are highly distorted. The general pattern of Al–Si ordering in nepheline is shown in
Figure 2 by T(1), T(2), T(3), and T(4) tetrahedra.
In the middle of the last century, Glukhovsky and co-workers showed that highly basic minerals, such as nepheline, can be used to produce cements, if they were previously transferred from a stable crystalline state to a more active vitreous form [
6]. Finely ground glasses of an alkaline aluminosilicate with a composition close to natural nepheline exhibited hydraulic activity not only during heat and moisture treatment, but also at ambient curing conditions. According to the authors of [
6], nepheline cement is a fine ground glass of nepheline composition, obtained by melting of natural nepheline. The nepheline cement is a hydraulic binder. It gained strength and water resistance due to the formation of alkaline hydroaluminosilicates similar in composition and structure to natural minerals such as zeolites. The hydraulic properties of the nepheline cement were enhanced by activating it with dilute alkali solutions. The degree of hydration of the nepheline cement increased by autoclaving. A characteristic feature of the nepheline cement was that, when heated, its hydration products first transformed into anhydrous amorphous phases without a significant change in the volume of the solid phase and loss of strength. With a further increase in temperature, these phases recrystallized into nepheline. Because of this, the nepheline cement was recommended for preparation of heat-resistant concrete [
6].
In addition to vitrification, another effective tool to increase the reactivity of solids is mechanical activation (MA) [
7,
8,
9,
10]. The term mechanical activation is applied here to the structural–chemical changes on the surface and in the bulk of solid caused by milling. In this review, binding properties of geopolymers and blended cements prepared using the mechanically activated nepheline containing tailings and nepheline concentrate are presented.
2. Geopolymers Prepared Using the Nepheline Concentrate and the Nepheline Containing Tailings Dumps
Geopolymers (inorganic polymers) are a subclass of alkali activated binders. Geopolymers are prepared by mixing of natural or technogenic low-calcium aluminosilicates with alkaline agents (sodium or potassium hydroxide solutions, liquid glass). They harden at ambient temperature and are used as environmentally friendly building materials alternative to Portland cement [
11,
12,
13,
14,
15,
16]. The advantages of geopolymer binders for replacing traditional Portland cement in particular are supported by the fact that in various industries there are many by-products that are suitable for use as raw materials for geopolymers [
17,
18,
19]. In recent years, there has been an increasing number of studies on the use of mining waste in the preparation of geopolymer materials both as a component of an alkali-activated binder and as an aggregate [
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32,
33,
34]. Geopolymer materials display special physical-mechanical and technical properties: high durability and strength, particularly bending strength; resistance to chemical aggressive environments, and high temperatures. They can be used as a matrix for immobilization of toxic waste, as sorbents for waste water treatment and for other applications [
35,
36,
37,
38,
39]. All this has stimulated in recent years the rapid growth of research and development on the geopolymers. It should be noted that, for improving the mechanical properties of the geopolymers, the efficiency of mechanical activation is well established [
34,
40,
41,
42,
43,
44,
45,
46].
Nepheline contains all the components necessary for geopolymer synthesis: aluminum, silicon, and alkali metals. However, crystalline nepheline itself exhibited a very weak hydraulic activity. The compressive strength of samples based on the nepheline concentrate milled to specific surface area ca. 1000 m
2∙kg
−1 and cured under relative humidity of 95 ± 5% at temperature of 20–22 °C for 180 days was 0.9 MPa only [
47]. This necessitated the increase of reactivity of nepheline-containing raw materials. As indicated above, an increase in the hydraulic activity of nepheline can be achieved by its transformation to a metastable vitreous form or by its mechanical activation.
Another valuable technogenic mineral raw material which is of interest for the production of binders is granulated magnesia-ferrous slag of copper-nickel smelter plants in the Murmansk region, Russia. It is well known that alkali activated binders based on blast furnace slag have been extensively investigated since the 1960s when Glukhovsky and coworkers developed their synthesis and applied it in the construction industry [
6,
48,
49]. The granulated Cu–Ni magnesia-ferrous slag is different in chemical composition from blast furnace slag. Magnesium, silicon, and iron oxides prevail in its composition and the content of CaO is only about 2–3 wt. %. Because of this, the hydraulic activity of the Cu–Ni slag is lower relative to that of blast furnace slag. However, Gurevich and Zosin [
50] as long ago as in 1965 developed the slag–alkali binder based on the granulated Cu–Ni slag. Later, the lime–slag binder and the Portland cement–slag binder were developed based on the Cu–Ni slag [
51]. Hydration activity of the Cu–Ni slag can be significantly increased by MA in a CO
2 atmosphere. Compressive strengths of the alkali activated binders based on the slag mechanically activated in air after curing at room temperature for 1, 7, 28, 150, and 360 days were 51, 75, 81, 83, and 90 MPa, respectively. When using mechanical activation in CO
2, the corresponding values were 54, 77, 94, 106, and 119 MPa, respectively [
52].
In this section, the binding properties of the following nepheline containing compositions prepared using mechanical activation of the solid components in air and in CO2 are reviewed:
- 1)
NC–Cu–Ni slag–water;
- 2)
NC–Cu–Ni slag–liquid glass;
- 3)
NT–alkali agent (liquid glass or NaOH solution);
- 4)
NT–Cu–Ni slag–liquid glass.
2.1. The Nepheline Concentrate–Cu–Ni Slag–Water Binder
The binding properties of the nepheline concentrate–Cu–Ni slag–water composition were studied in [
47]. In this mixture, the nepheline concentrate substituted 20, 30, 50, and 80% of the slag. The Cu–Ni granulated (water-cooled) slag was obtained from Pechenganickel smelter plant (Murmansk Region, Russia). The slag mainly consisted of magnesia-ferriferous glass (95–98 wt. %) with minor amount of the crystalline phases of olivine (1–5 wt. %) and ore minerals (1–3 wt. %). Chemical composition of the slag is given in
Table 2.
Mechanical activation of the nepheline concentrate–Cu–Ni slag mixture was carried out in an AGO-2 laboratory centrifugal-planetary mill [
8] at a centrifugal factor of 40 g for 270 s in air and in CO
2 atmosphere (P = 10
5 Pa). Steel balls 8 mm in diameter were used as milling bodies. The ratio between the masses of the balls and a slag sample was 6. The specific surface area was measured by Blaine method.
The mechanically activated nepheline concentrate—Cu–Ni slag blend was mixed with water to prepare cubic specimens. The specimens were cured under relative humidity of 95 ± 5% at temperature of 20–22 °C until tested in compression [
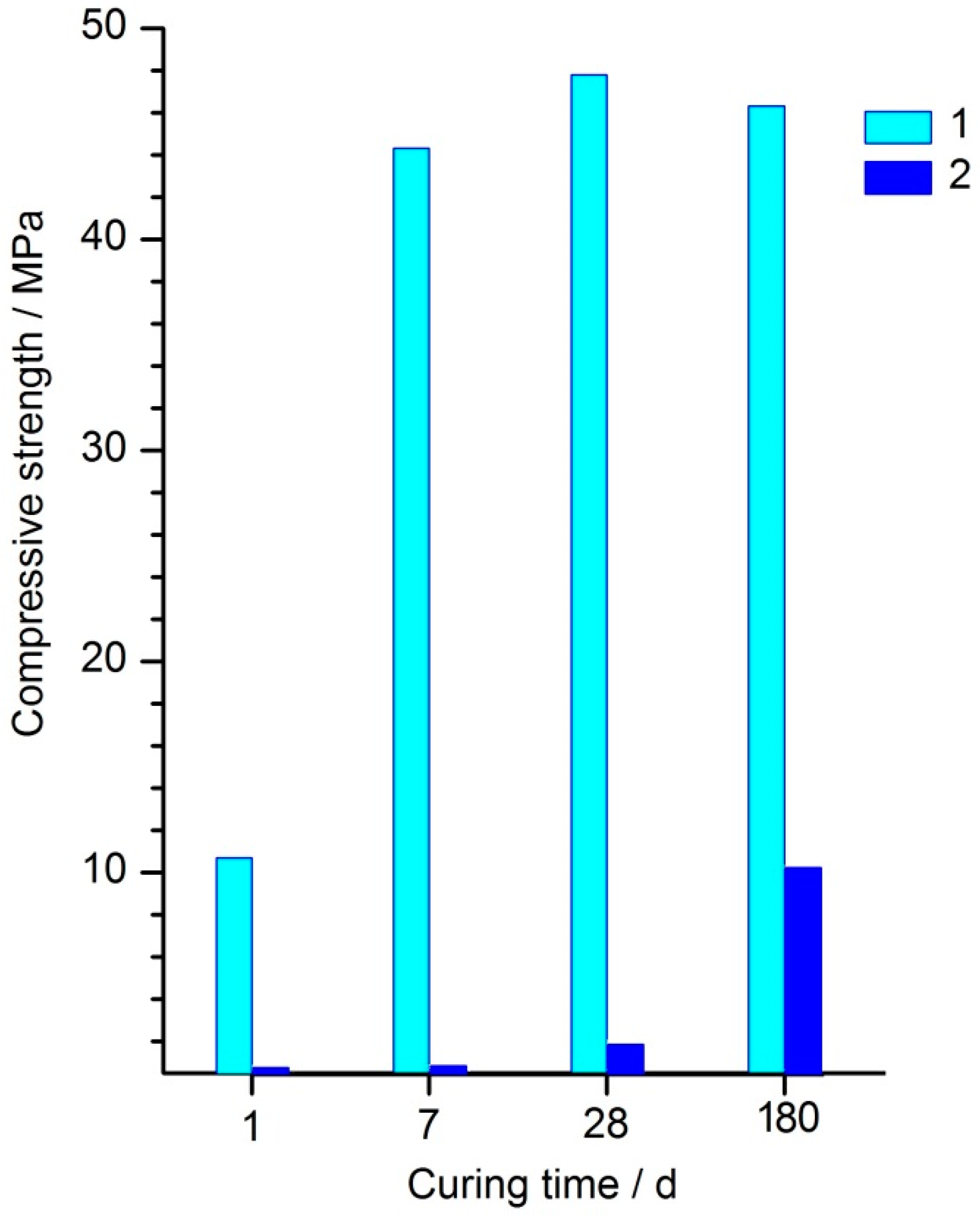
47]. Compressive strength of the nepheline concentrate—Cu–Ni slag—water binder is presented in
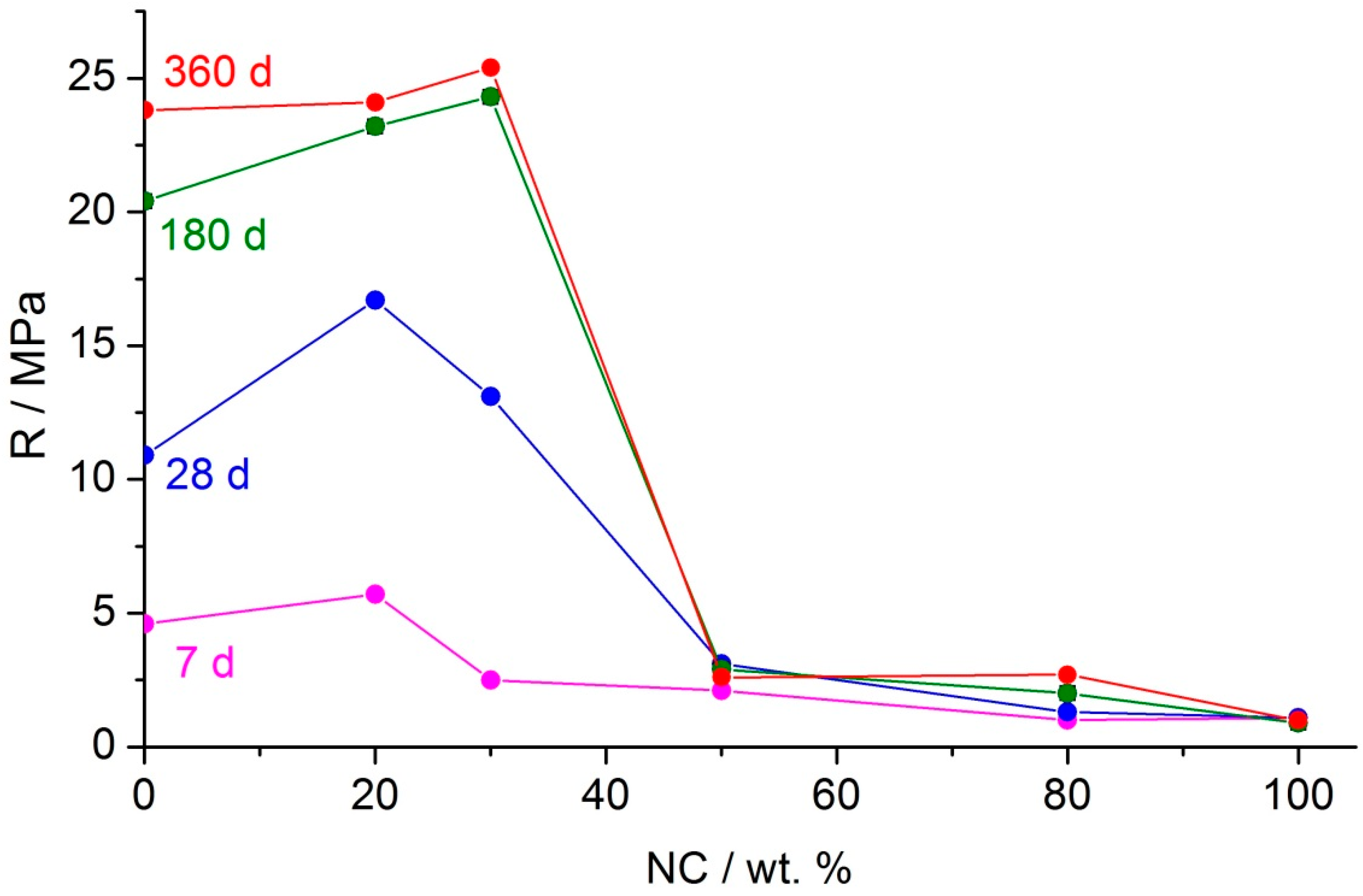
Figure 3 and
Table 3. The compressive strength of the binder based on the 100% nepheline concentrate mechanically activated in CO
2 (
Figure 3) and in air (
Table 3) in the age of 360 days was 1.5 MPa only. The corresponding values of the binders based on the 100% slag mechanically activated in air and in CO
2 were 3.1 and 23.8 MPa, respectively. As was shown in [
52,
53], mechanical activation of the slag in carbon dioxide atmosphere was accompanied by sorption of CO
2 in the form of distorted carbonate groups by the outer layers of the slag particles. This resulted in higher reactivity and faster hydration of the slag carbonized by mechanical activation in CO
2 in comparison to those of the slag mechanically activated in air.
For the nepheline concentrate–Cu–Ni slag–water binder prepared using mechanical activation in air, the compressive strength for all curing periods was in the range of 1–3 MPa (
Table 3). When CO
2 was used as an atmosphere of mechanical activation, the strength of the binders in which the slag fraction was more than 70 wt. % increased by an order of magnitude, exceeding 20 MPa after curing for 180–360 days (
Figure 3). Thus, the addition of 20–30% nepheline concentrate to the slag contributed to an increase in the strength of the nepheline concentrate–Cu–Ni slag binder compared to that of the binder based on the slag without the nepheline concentrate (
Figure 3). This means that nepheline was not an inert “diluent” in the slag–nepheline blend, but an active component.
To assess the increase in the reactivity of the nepheline concentrate due to mechanical activation, the nepheline concentrate was leached with water [
47]. Leaching degree was calculated as the amount of oxides leached to the solution from the mechanically activated nepheline concentrate to its amount contained in the nepheline concentrate. From
Table 4, it is seen that the leaching degree of sodium and potassium oxides did not exceed 5–6%. The atmosphere of mechanical activation (air or CO
2) did not significantly affect the degree of leaching. The leaching degree of silicon and aluminum was not more than 1% and the pH of the solution was 10–11. Hence, mechanical activation of the nepheline concentrate–Cu–Ni slag mixture and the subsequent addition of water led to leaching of small amount of Na, K, Si, and Al into the aqueous phase, and to the increase of the pH to some extent. However, as shown in
Figure 3, it seems likely that this was sufficient to increase strength by 10–20% (depending on the curing period), compared with that of the binder based on the slag without the nepheline concentrate. A characteristic feature of this “alkali activated” binder was that the alkaline agent was not added in the form of externally prepared alkali solution but was formed in situ upon leaching of nepheline when water was added to the mechanically activated mixture of the nepheline concentrate and the slag.
2.2. The Nepheline Concentrate–Cu–Ni Slag–Liquid Glass Binder
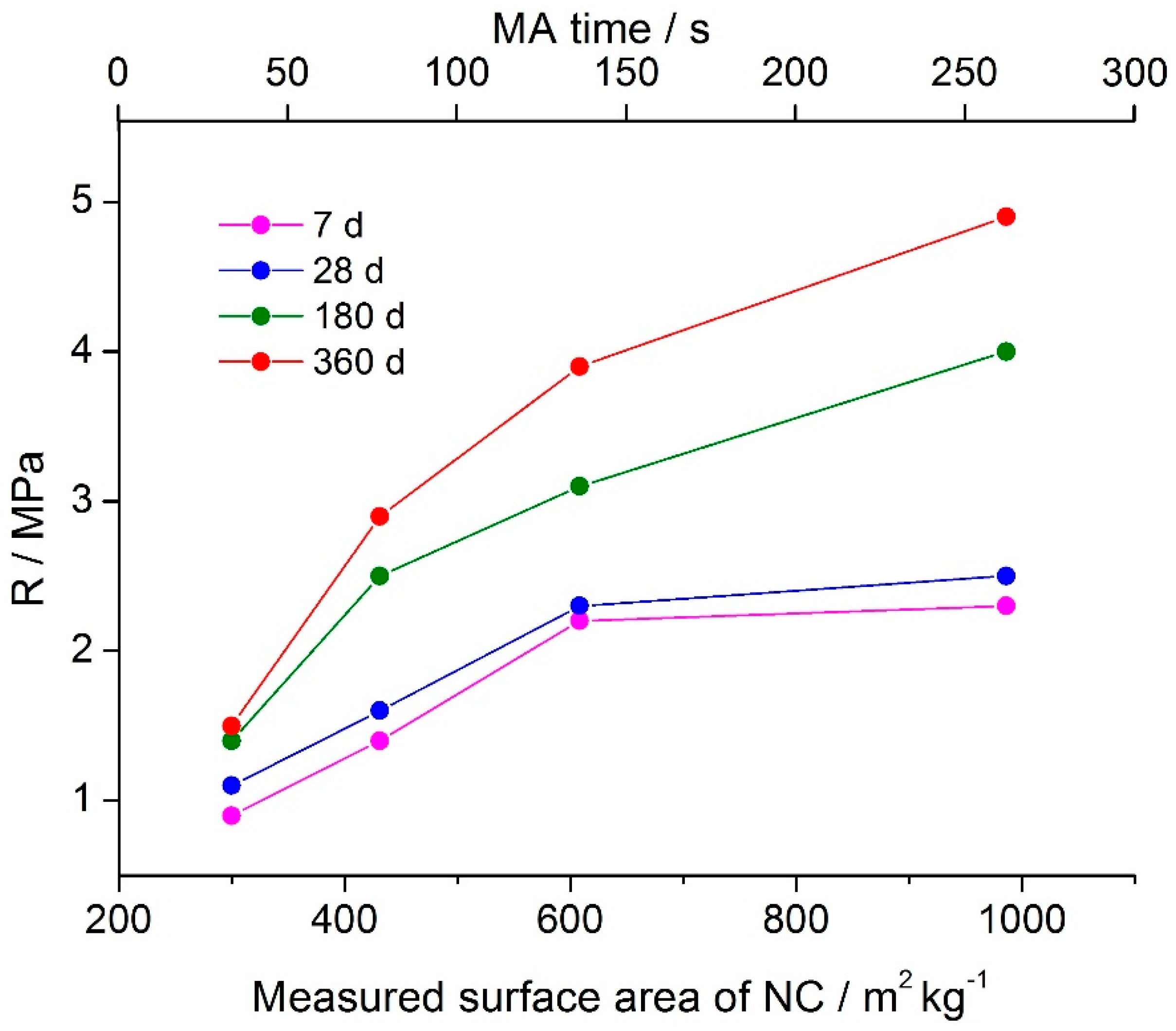
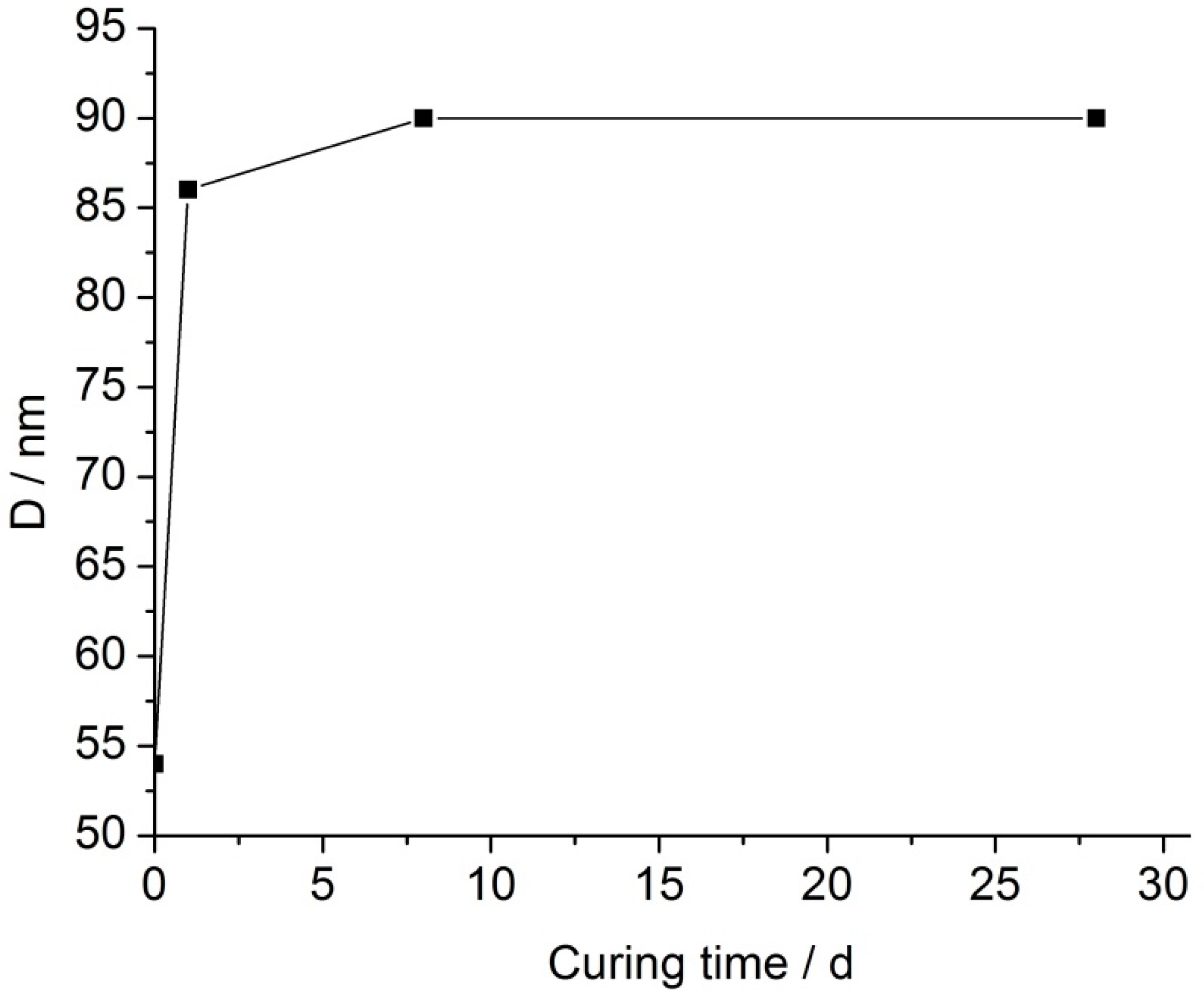
Figure 4 presents the compressive strength of the geopolymer binder prepared using the mechanically activated nepheline concentrate and liquid glass as a function of the measured surface area of the nepheline concentrate and time of mechanical activation in air. Curing was carried out under relative humidity of 95 ± 5% at temperature of 20–22 °C [
3]. As MA time increased, so did the specific surface area of the nepheline concentrate (
Figure 4) and the half-widths of the X-ray diffraction peaks of nepheline in XRD patterns of the nepheline concentrate (not shown). Thus, mechanical activation led to the accumulation of the excess energy by the nepheline concentrate and increasing its reactivity. As a result, the compressive strength of the binder continuously increased with increase in mechanical activation time for all curing periods. At 360 d of age, the compressive strength of the binder based on the nepheline concentrate mechanically activated for 270 s was 4.9 MPa. Hence, the compressive strength increased by more than 3-fold relative to that of the binder based on the nepheline concentrate mechanically activated for 30 s (1.5 MPa).
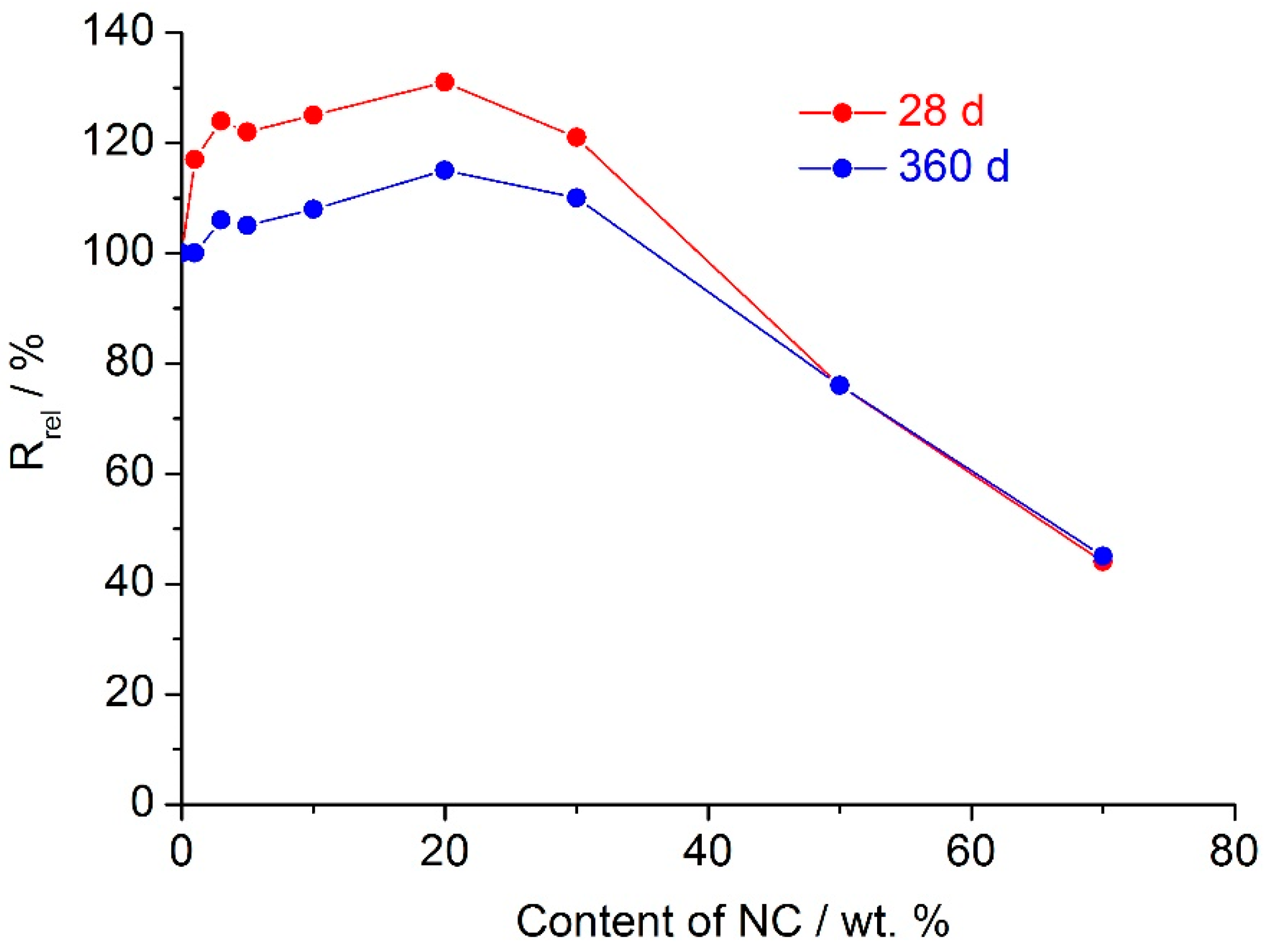
Addition of the Cu–Ni slag to the nepheline concentrate, as in case of the nepheline concentrate–Cu–Ni slag–water binder (
Figure 3), resulted in increase of the compressive strength relative to the binder based on the nepheline concentrate without the slag, regardless of the MA atmosphere (air or CO
2) [
3] (
Table 5). In this binder, the slag substituted from 1 to 30 wt. % of the nepheline concentrate. For the binder based on the 100% nepheline concentrate mechanically activated in CO
2, the compressive strength at 360 d of age was 4.9 MPa, while, for the binder with a ratio of nepheline concentrate: slag equaled to 7:3 the corresponding value was 21.9 MPa, i.e., 4.5 times larger. The advantage of carbon dioxide as an atmosphere of mechanical activation was clearly visible for the long-term curing (180 and 360 d) while at age of 28 d and less it was not the case (
Table 5).
The properties of the nepheline concentrate–Cu–Ni slag–liquid glass binder were studied also using curing in water at 20–22 °C. The water resistance coefficient designated as K
28 was determined by the ratio of the compressive strength of the binder cured in water at temperature of 20–22 °C for 28 days to the corresponding value of the binder cured in air under relative humidity of 95 ± 5% at the same temperature for the same time. It was found that, for the binder based on the mechanically activated nepheline concentrate without the slag, the K
28 value was in the range of 0.48–0.60 only. Addition of the slag to nepheline concentrate in amount of 1–5 wt. % resulted in increase of K
28 up to 0.91 [
3].
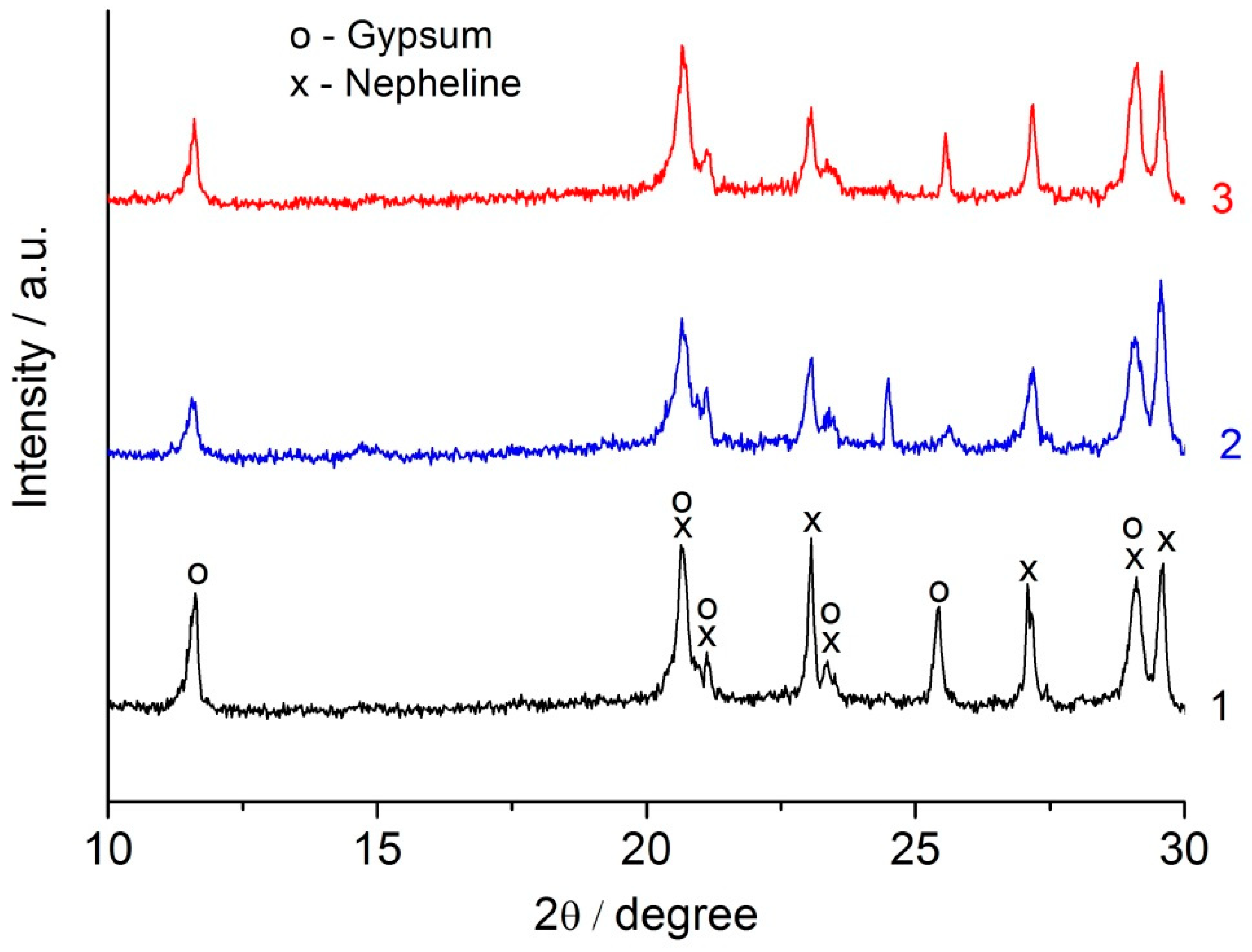
2.3. The Nepheline Containing Tailings–Alkali Agent (Liquid Glass or NaOH Solution) Binder
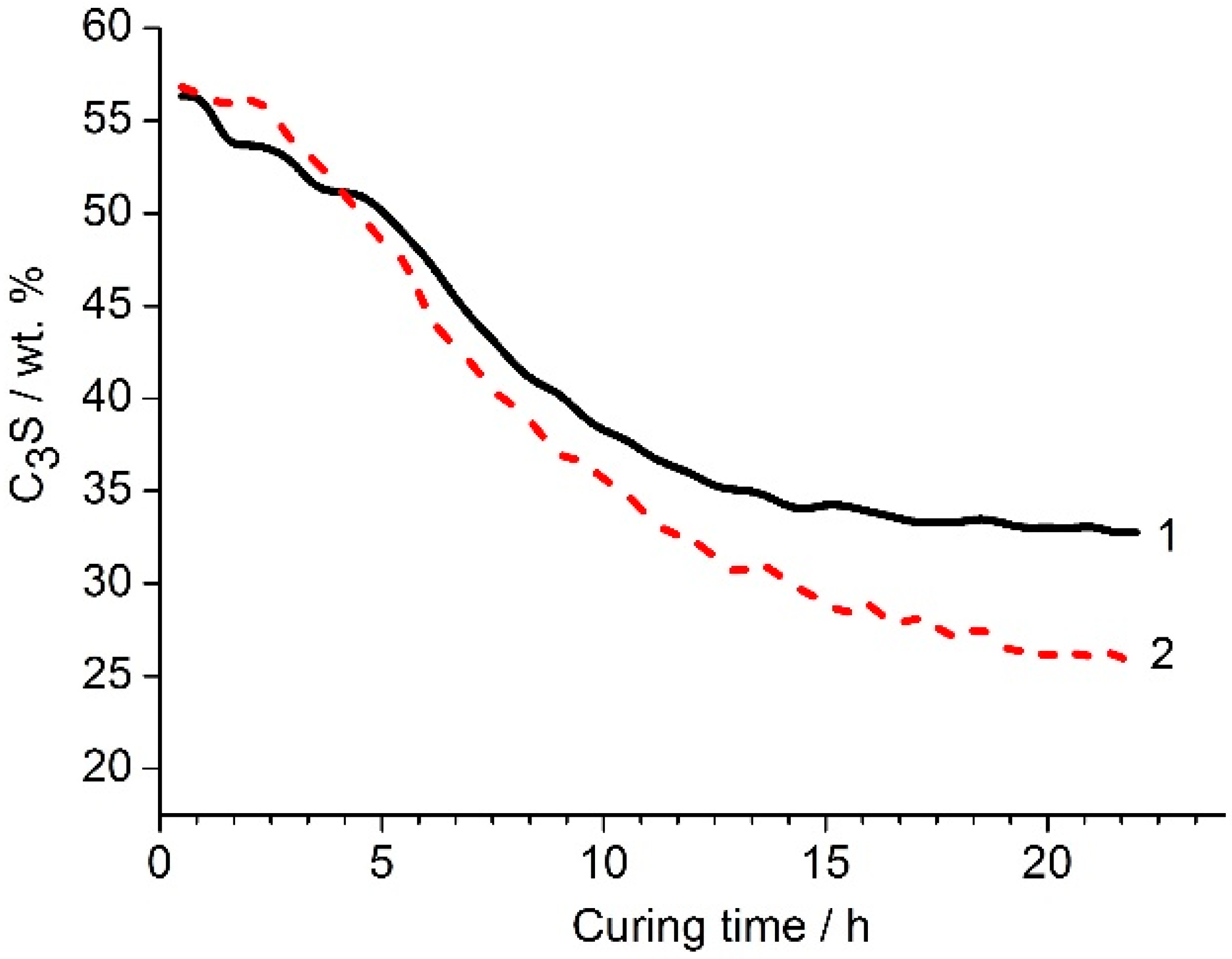
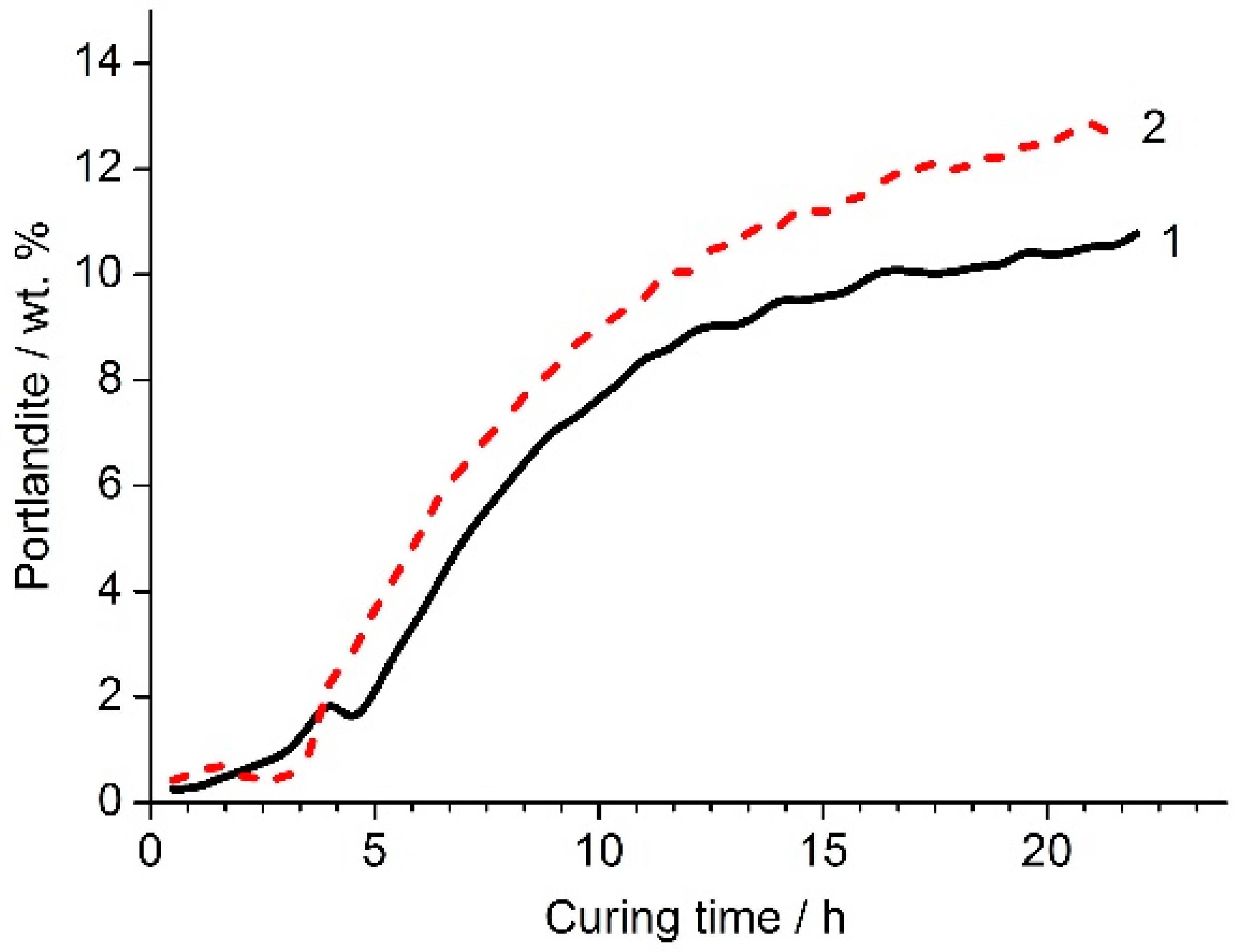
The nepheline content in the nepheline containing tailings was approximately 1.5 times lower than that in the nepheline concentrate (
Table 2). The nepheline containing tailings and the nepheline concentrate also differed in apatite content and, accordingly, in P
2O
5 content. In the nepheline concentrate, the content of P
2O
5 was at the level of hundredths of a percent, while, in the nepheline containing tailings, this value was 1.48 wt. %. To find the optimal combination of liquid glass modulus and its amount in a mixture with the mechanically activated nepheline containing tailings, the experiments were carried out according to the following scheme [
3]. The amount of liquid glass in the binder varied from 2 to 10% Na
2O (with respect to the weight of the nepheline containing tailings). For each fixed value of Na
2O, specimens were prepared using liquid glass of various silica modulus (MS) from MS = 0 (NaOH solution) to MS = 2.77. The prepared specimens were kept for 1 d in air at room temperature and then subjected to heat treatment in an oven in closed containers at 80 °C for 6 h, followed by curing in air at a relative humidity of 60–70% at 20–22 °C. According to the obtained results, the highest compressive strength was achieved for the formulations with 2–3 wt. % Na
2O using liquid glass with MS of 2.34 (
Table 6). It is worth mentioning that the compressive strength of the binders prepared using liquid glass with MS of 2.34 and contained 2% and 3% Na
2O was at 180 d age 66.7 MPa and 45.8 MPa, respectively. Sodium hydroxide solution was less effective as alkaline agent as compared to liquid glass.
Table 7 shows the effect of the silica modulus on the strength of binders based on the nepheline containing tailings under conditions of humid (relative humidity of 95–100%) and relatively dry (relative humidity of 60–70%) curing without the use of heat treatment [
3]. Under both conditions of curing, with an increase in the MS, the strength of the samples increased. Under the relatively dry curing, this tendency was more pronounced. Therefore, of the two curing conditions, the relatively dry one was preferred. The strength of the binders prepared using liquid glass with silica modulus of 2.34 at 28 d of age reached 15.1 MPa and 46.2 MPa with the humid and dry curing, respectively.
2.4. The Nepheline Containing Tailings–Cu–Ni Slag–Liquid Glass Binder
The compressive strength of the nepheline containing tailings–Cu–Ni slag–liquid glass binder is presented in
Table 8. Considering the results obtained for the nepheline concentrate–slag–water binder (
Figure 3,
Table 3), the nepheline containing tailings–slag mixture was mechanically activated in CO
2 atmosphere as described in
Section 2.1. With an increase in the content of the nepheline containing tailings in the composition, the strength decreased. However, at 28 d of age, it was not less than 15 MPa. The compressive strength of the binder based on the mechanically activated mixture of the nepheline containing tailings and the slag with a mass ratio of 1:1 at 7–360 d of age was in the range of 50–57 MPa. The corresponding value for the binder based on (20% slag + 80% nepheline containing tailings) mixture was in the range of 30–39 MPa [
3].