Enantiomeric Separation of Tramadol and Its Metabolites: Method Validation and Application to Environmental Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Standards and Reagents
2.2. Instrumental Conditions
2.3. Chromatographic Conditions
2.4. Sample Preparation Procedure
2.5. Method Validation
2.6. Application of Developed HPLC-FD Method in WWTP Samples
3. Results and Discussion
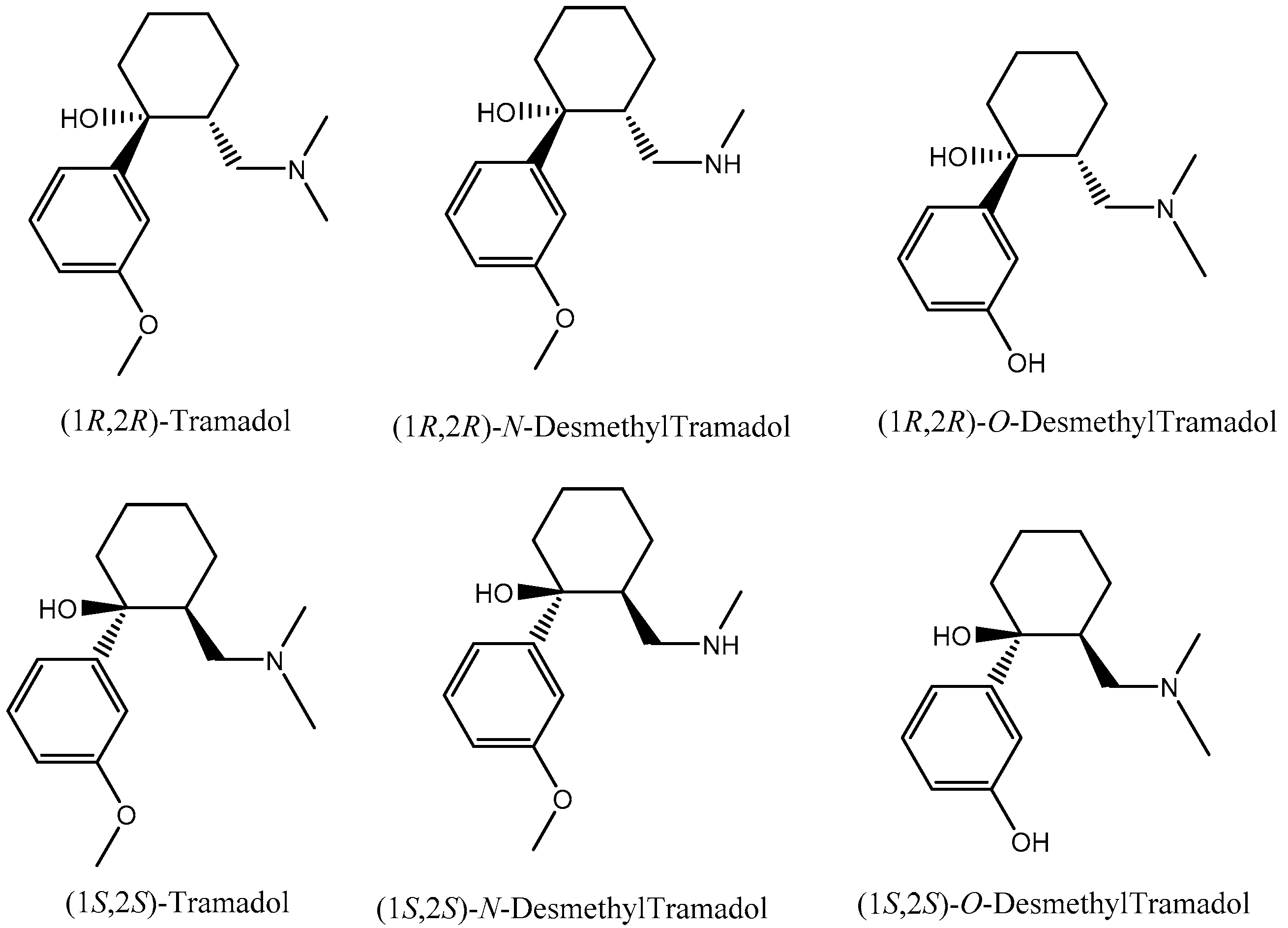
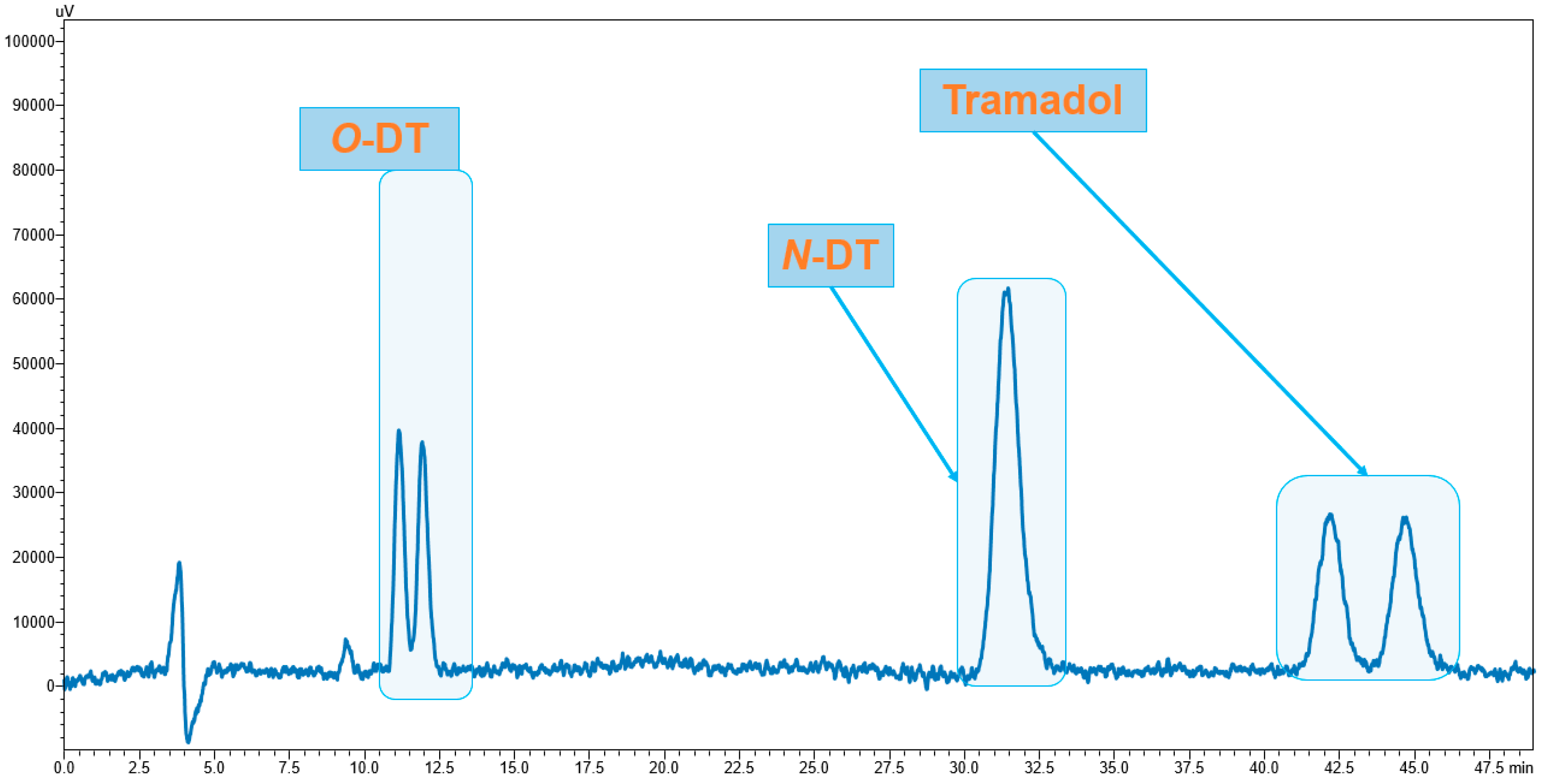
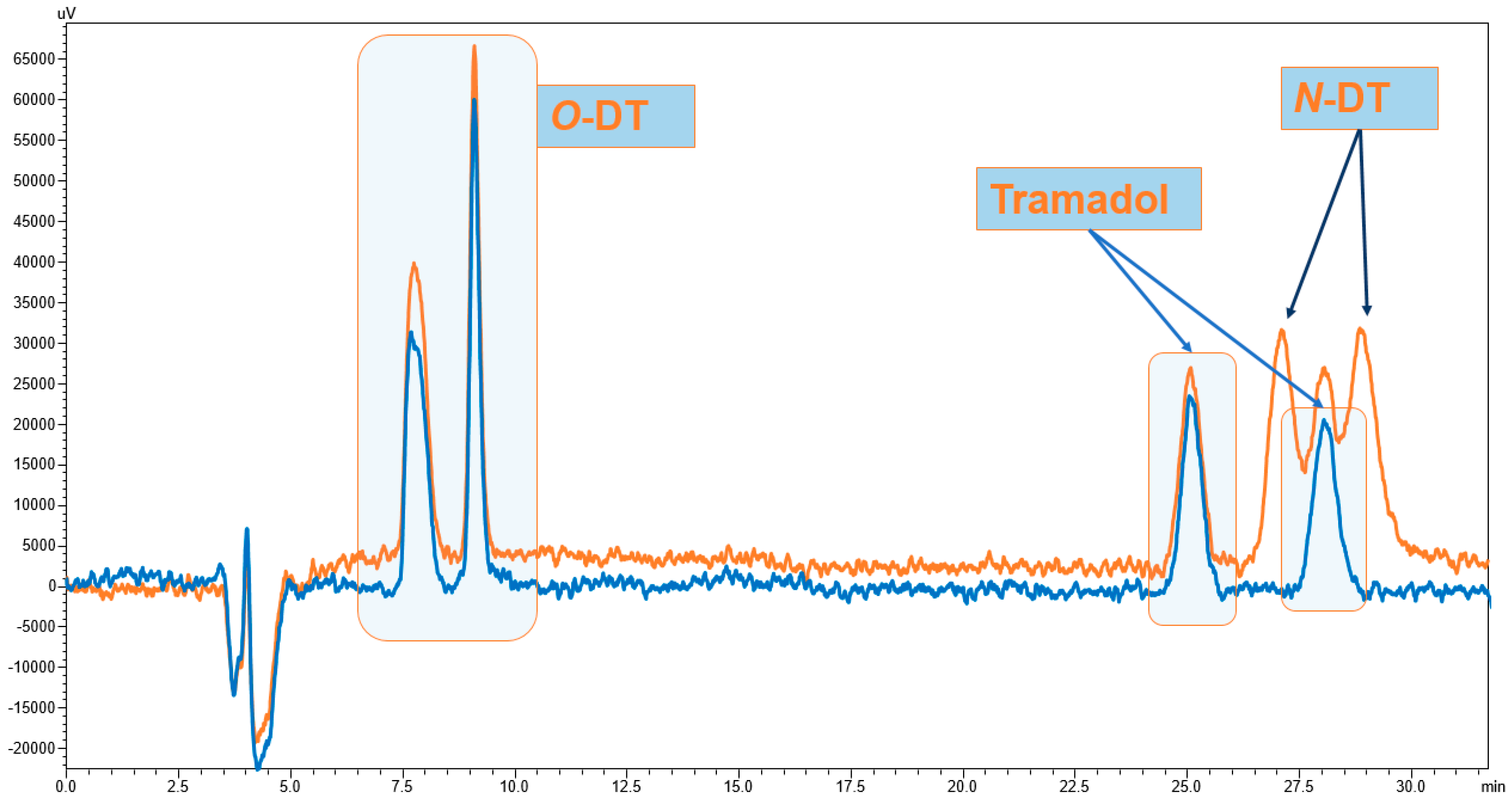
3.1. Enantiomeric Separation
3.2. Optimization of the Sample Preparation Procedure
3.3. Method Validation
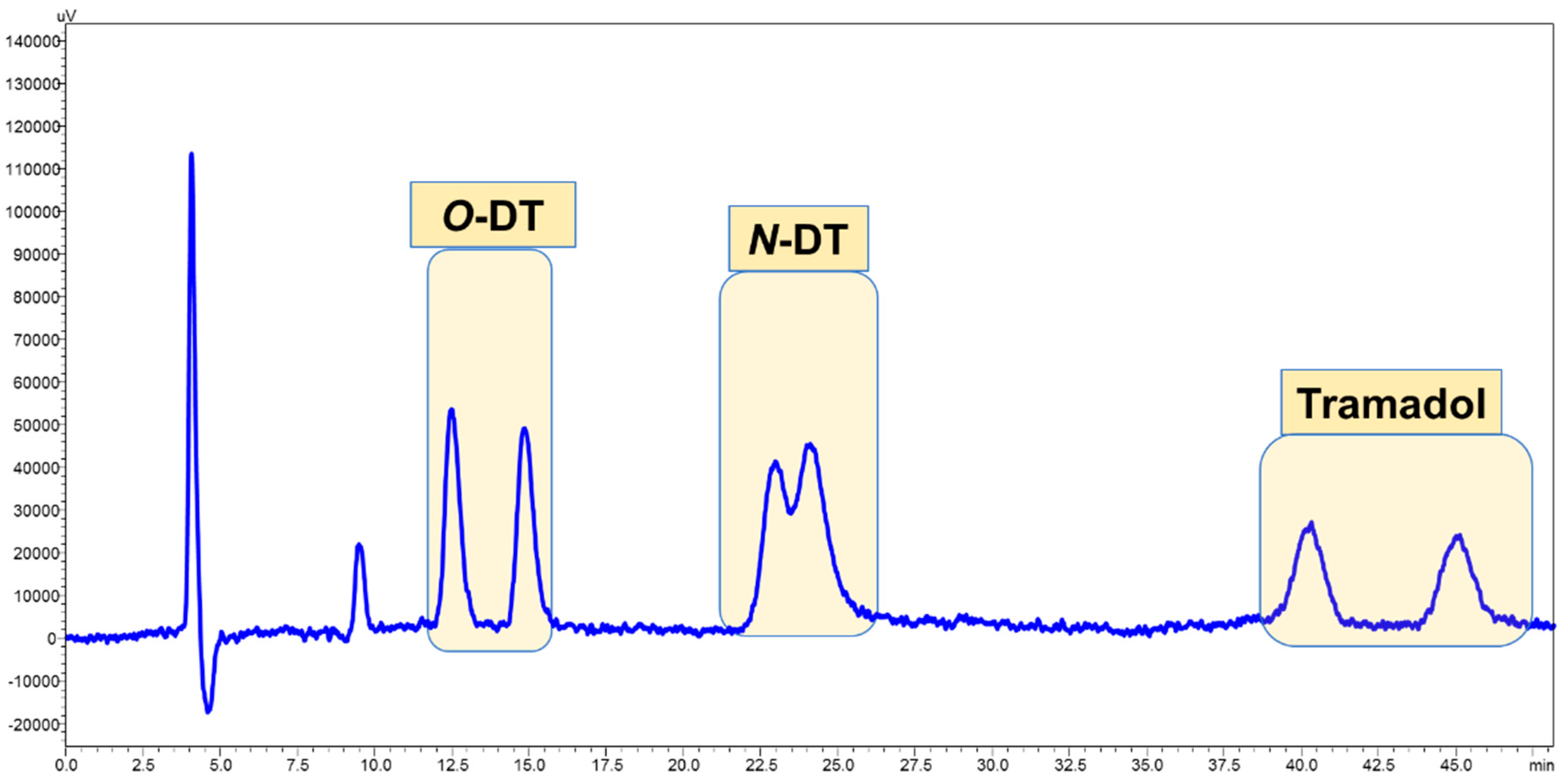
3.4. Application of Developed LC-FD Method in WWTP Samples
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Herrmann, M.; Olsson, O.; Fiehn, R.; Herrel, M.; Kümmerer, K. The significance of different health institutions and their respective contributions of active pharmaceutical ingredients to wastewater. Environ. Int. 2015, 85, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Cizmas, L.; Sharma, V.; Gray, C.; McDonald, T. Pharmaceuticals and personal care products in waters: Occurrence, toxicity, and risk. Environ. Chem. Lett. 2015, 13, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.R.; Maia, A.S.; Cass, Q.B.; Tiritan, M.E. Enantioseparation of chiral pharmaceuticals in biomedical and environmental analyses by liquid chromatography: An overview. J. Chromatogr. B 2014, 968, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.; Castro, P.L.; Tiritan, M. Environmental fate of chiral pharmaceuticals: Determination, degradation and toxicity. In Environmental Chemistry for a Sustainable World; Lichtfouse, E., Schwarzbauer, J., Robert, D., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 3–45. [Google Scholar]
- Ribeiro, A.R.; Castro, P.M.L.; Tiritan, M.E. Chiral pharmaceuticals in the environment. Environ. Chem. Lett. 2012, 10, 239–253. [Google Scholar] [CrossRef]
- Stanley, J.K.; Ramirez, A.J.; Chambliss, C.K.; Brooks, B.W. Enantiospecific sublethal effects of the antidepressant fluoxetine to a model aquatic vertebrate and invertebrate. Chemosphere 2007, 69, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Stanley, J.K.; Ramirez, A.J.; Mottaleb, M.; Chambliss, C.K.; Brooks, B.W. Enantiospecific toxicity of the beta-blocker propranolol to daphnia magna and pimephales promelas. Environ. Toxicol. Chem. 2006, 25, 1780–1786. [Google Scholar] [CrossRef] [PubMed]
- Rukhlenko, I.D.; Tepliakov, N.V.; Baimuratov, A.S.; Andronaki, S.A.; Gun’ko, Y.K.; Baranov, A.V.; Fedorov, A.V. Completely chiral optical force for enantioseparation. Sci. Rep. 2016, 6, 36884. [Google Scholar] [CrossRef] [PubMed]
- Kupai, J.; Rojik, E.; Huszthy, P.; Szekely, G. Role of chirality and macroring in imprinted polymers with enantiodiscriminative power. ACS Appl. Mater. Interfaces 2015, 7, 9516–9525. [Google Scholar] [CrossRef] [PubMed]
- Pinho, S.; Oliveira, A.; Costa, I.; Gouveia, C.A.; Carvalho, F.; Moreira, R.F.; Dinis-Oliveira, R.J. Simultaneous quantification of tramadol and O-desmethyltramadol in hair samples by gas chromatography-electron impact/mass spectrometry. Biomed. Chromatogr. 2013, 27, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Castrignano, E.; Lubben, A.; Kasprzyk-Hordern, B. Enantiomeric profiling of chiral drug biomarkers in wastewater with the usage of chiral liquid chromatography coupled with tandem mass spectrometry. J. Chromatogr. A 2016, 1438, 84–99. [Google Scholar] [CrossRef] [PubMed]
- Mohr, S.; Taschwer, M.; Schmid, M.G. Chiral separation of cathinone derivatives used as recreational drugs by HPLC-UV using a CHIRALPAK® AS-H column as stationary phase. Chirality 2012, 24, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.R.; Maia, A.S.; Moreira, I.S.; Afonso, C.M.; Castro, P.M.; Tiritan, M.E. Enantioselective quantification of fluoxetine and norfluoxetine by HPLC in wastewater effluents. Chemosphere 2014, 95, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; He, L.; Sun, W.; Cheng, Y.; Liu, J.; Ren, Z. Chiral liquid membrane for enantioselective separation of racemic ibuprofen by l-tartaric acid derivatives. RSC Adv. 2015, 5, 41729–41735. [Google Scholar] [CrossRef]
- Musshoff, F.; Madea, B. Fatality due to ingestion of tramadol alone. Forensic Sci. Int. 2001, 116, 197–199. [Google Scholar] [CrossRef]
- Grond, S.; Sablotzki, A. Clinical pharmacology of tramadol. Clin. Pharmacokinet. 2004, 43, 879–923. [Google Scholar] [CrossRef] [PubMed]
- Kusari, S.; Tatsimo, S.J.; Zuhlke, S.; Spiteller, M. Synthetic origin of tramadol in the environment. Angew. Chem. Int. Ed. 2016, 55, 240–243. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.A.; Mohamed, K.M.; Nasser, A.Y.; Button, J.; Holt, D.W. Simultaneous determination of tramadol, O-desmethyltramadol and n-desmethyltramadol in human urine by gas chromatography-mass spectrometry. J. Chromatogr. B 2013, 926, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.N.; Sharma, N.; Sanyal, M.; Shrivastav, P.S. An accurate, rapid and sensitive determination of tramadol and its active metabolite O-desmethyltramadol in human plasma by LC–MS/MS. J. Pharm. Biomed. Anal. 2009, 49, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.; El-Abhar, H.; Saleh, S. The effect of nigella sativa oil against the liver damage induced by schistosoma mansoni infection in mice. J. Ethnopharmacol. 2002, 79, 1–11. [Google Scholar] [CrossRef]
- Awadalla, E.A.; Salah-Eldin, A. Histopathological and molecular studies on tramadol mediated hepato-renal toxicity in rats. IOSR J. Pharm. Biol. Sci. 2015, 10, 90–102. [Google Scholar]
- Tao, Q.; Stone, D.J., Jr.; Borenstein, M.R.; Jean-Bart, V.; Codd, E.E.; Coogan, T.P.; Desai-Krieger, D.; Liao, S.; Raffa, R.B. Gas chromatographic method using nitrogen–phosphorus detection for the measurement of tramadol and its O-desmethyl metabolite in plasma and brain tissue of mice and rats. J. Chromatogr. B Biomed. Sci. Appl. 2001, 763, 165–171. [Google Scholar] [CrossRef]
- Ardakani, Y.H.; Rouini, M.-R. Improved liquid chromatographic method for the simultaneous determination of tramadol and its three main metabolites in human plasma, urine and saliva. J. Pharm. Biomed. Anal. 2007, 44, 1168–1173. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, R. Simultaneous analysis of tramadol, metoprolol and their metabolites in human plasma and urine by high performance liquid chromatography. Chin. Med. J. 2006, 119, 2013–2017. [Google Scholar] [PubMed]
- Zhao, L.M.; Chen, X.Y.; Cui, J.J.; Sunita, M.; Zhong, D.F. Determination of tramadol and its active metabolite O-desmethyltramadol in plasma and amniotic fluid using LC/MS/MS. Acta Pharm. Sin. 2004, 39, 458–462. [Google Scholar]
- Ceccato, A.; Chiap, P.; Hubert, P.; Crommen, J. Automated determination of tramadol enantiomers in human plasma using solid-phase extraction in combination with chiral liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 1997, 698, 161–170. [Google Scholar] [CrossRef]
- Valle, M.; Pavón, J.M.; Calvo, R.; Campanero, M.A.; Trocóniz, I.F. Simultaneous determination of tramadol and its major active metabolite O-demethyltramadol by high-performance liquid chromatography with electrochemical detection. J. Chromatogr. B Biomed. Sci. Appl. 1999, 724, 83–89. [Google Scholar] [CrossRef]
- Ceccato, A.; Vanderbist, F.; Pabst, J.Y.; Streel, B. Enantiomeric determination of tramadol and its main metabolite O-desmethyltramadol in human plasma by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 2000, 748, 65–76. [Google Scholar] [CrossRef]
- Ruda, S.; Cherkaoui, S.; Dayer, P.; Fanali, S.; Veuthey, J.L. Simultaneous stereoselective analysis of tramadol and its main phase I metabolites by on-line capillary zone electrophoresis-electrospray ionization mass spectrometry. J. Chromatogr. A 2000, 868, 295–303. [Google Scholar] [CrossRef]
- Juzwin, S.J.; Wang, D.C.; Anderson, N.J.; Wong, F.A. The determination of RWJ-38705 (tramadol N-oxide) and its metabolites in preclinical pharmacokinetic studies using LC–MS/MS. J. Pharm. Biomed. Anal. 2000, 22, 469–480. [Google Scholar] [CrossRef]
- Gan, S.H.; Ismail, R. Validation of a high-performance liquid chromatography method for tramadol and O-desmethyltramadol in human plasma using solid-phase extraction. J. Chromatogr. B Biomed. Sci. Appl. 2001, 759, 325–335. [Google Scholar] [CrossRef]
- Nobilis, M.; Kopecky, J.; Kvetina, J.; Chladek, J.; Svoboda, Z.; Vorisek, V.; Perlik, F.; Pour, M.; Kunes, J. High-performance liquid chromatographic determination of tramadol and its O-desmethylated metabolite in blood plasma: Application to a bioequivalence study in humans. J. Chromatogr. A 2002, 949, 11–22. [Google Scholar] [CrossRef]
- Campanero, M.A.; García-Quetglas, E.; Sádaba, B.; Azanza, J.R. Simultaneous stereoselective analysis of tramadol and its primary phase I metabolites in plasma by liquid chromatography: Application to a pharmacokinetic study in humans. J. Chromatogr. A 2004, 1031, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Ardakani, Y.H.; Mehvar, R.; Foroumadi, A.; Rouini, M.-R. Enantioselective determination of tramadol and its main phase I metabolites in human plasma by high-performance liquid chromatography. J. Chromatogr. B 2008, 864, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Fawcett, J.P. Improved HPLC method for the simultaneous determination of tramadol and O-desmethyltramadol in human plasma. J. Chromatogr. B 2005, 821, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Rouini, M.-R.; Ardakani, Y.H.; Soltani, F.; Aboul-Enein, H.Y.; Foroumadi, A. Development and validation of a rapid HPLC method for simultaneous determination of tramadol, and its two main metabolites in human plasma. J. Chromatogr. B 2006, 830, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Mehvar, R.; Elliott, K.; Parasrampuria, R.; Eradiri, O. Stereospecific high-performance liquid chromatographic analysis of tramadol and its O-demethylated (M1) and N,O-demethylated (M5) metabolites in human plasma. J. Chromatogr. B 2007, 852, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Curticapean, A.; Muntean, D.; Curticapean, M.; Dogaru, M.; Vari, C. Optimized HPLC method for tramadol and O-desmethyl tramadol determination in human plasma. J. Biochem. Biophys. Methods 2008, 70, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Vlase, L.; Leucuta, S.E.; Imre, S. Determination of tramadol and O-desmethyltramadol in human plasma by high-performance liquid chromatography with mass spectrometry detection. Talanta 2008, 75, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.E.; Davies, P.; Lubben, A.; Kasprzyk-Hordern, B. Determination of chiral pharmaceuticals and illicit drugs in wastewater and sludge using microwave assisted extraction, solid-phase extraction and chiral liquid chromatography coupled with tandem mass spectrometry. Anal. Chim. Acta 2015, 882, 112–126. [Google Scholar] [CrossRef] [PubMed]
- ICH Steering Committee. ICH Q2B Validation of Analytical Procedures: Methodology; (CPMP/ICH/281/95); European Agency for the Evaluation of Medicinal Products, International Commission on Harmonisation: London, UK, 1996. [Google Scholar]
- Lämmerhofer, M. Chiral recognition by enantioselective liquid chromatography: Mechanisms and modern chiral stationary phases. J. Chromatogr. A 2010, 1217, 814–856. [Google Scholar] [CrossRef] [PubMed]
- De Campos Lourenço, T.; Cassiano, N.M.; Cass, Q.B. Fases estacionárias quirais para cromatografia líquida de alta eficiência. Quim. Nova 2010, 33, 2155–2164. [Google Scholar] [CrossRef]
- Ward, T.J.; Ward, K.D. Chiral separations: Fundamental review 2010. Anal. Chem. 2010, 82, 4712–4722. [Google Scholar] [CrossRef] [PubMed]
- Kalíková, K.; Riesová, M.; Tesařová, E. Recentchiral selectors for separation in HPLC and CE. Cent. Eur. J. Chem. 2012, 10, 450–471. [Google Scholar] [CrossRef]
- Scriba, G.K.E. Chiral recognition mechanisms in analytical separation sciences. Chromatographia 2012, 75, 815–838. [Google Scholar] [CrossRef]
- Fernandes, C.; Tiritan, M.; Pinto, M. Small molecules as chromatographic tools for HPLC enantiomeric resolution: Pirkle-type chiral stationary phases evolution. Chromatographia 2013, 76, 871–897. [Google Scholar] [CrossRef]
- Haginaka, J. Recent progresses in protein-based chiral stationary phases for enantioseparations in liquid chromatography. J. Chromatogr. B 2008, 875, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Perrin, C.; Vu, V.A.; Matthijs, N.; Maftouh, M.; Massart, D.L.; Vander Heyden, Y. Screening approach for chiral separation of pharmaceuticals: Part I. Normal-phase liquid chromatography. J. Chromatogr. A 2002, 947, 69–83. [Google Scholar] [CrossRef]
- Andersson, M.E.; Aslan, D.; Clarke, A.; Roeraade, J.; Hagman, G. Evaluation of generic chiral liquid chromatography screens for pharmaceutical analysis. J. Chromatogr. A 2003, 1005, 83–101. [Google Scholar] [CrossRef]
- Cass, Q.B.; Batigalhia, F. Enantiomeric resolution of a series of chiral sulfoxides by high-performance liquid chromatography on polysaccharide-based columns with multimodal elution. J. Chromatogr. A 2003, 987, 445–452. [Google Scholar] [CrossRef]
- Fernandes, C.; Palmeira, A.; Santos, A.; Tiritan, M.E.; Afonso, C.; Pinto, M.M. Enantioresolution of chiral derivatives of xanthones on (S,S)-whelk-O1 and l-phenylglycine stationary phases and chiral recognition mechanism by docking approach for (S,S)-whelk-O1. Chirality 2013, 25, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Chytil, L.; Matouskova, O.; Cerna, O.; Pokorna, P.; Vobruba, V.; Perlik, F.; Slanar, O. Enantiomeric determination of tramadol and O-desmethyltramadol in human plasma by fast liquid chromatographic technique coupled with mass spectrometric detection. J. Chromatogr. B 2010, 878, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Musshoff, F.; Madea, B.; Stuber, F.; Stamer, U.M. Enantiomeric determination of tramadol and O-desmethyltramadol by liquid chromatography-mass spectrometry and application to postoperative patients receiving tramadol. J. Anal. Toxicol. 2006, 30, 463–467. [Google Scholar] [CrossRef] [PubMed]
- ICH, M. Q2b—Validation of analytical procedures: Methodology. In Proceedings of the International Conference on Harmonization Expert Working Group, Geneva, Switzerland, 6 Novermber 1996. [Google Scholar]
- Stamer, U.M.; Musshoff, F.; Kobilay, M.; Madea, B.; Hoeft, A.; Stuber, F. Concentrations of tramadol and O-desmethyltramadol enantiomers in different CYP2D6 genotypes. Clin. Pharmacol. Ther. 2007, 82, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Payne, K.A.; Roelofse, J.A.; Shipton, E.A. Pharmacokinetics of oral tramadol drops for postoperative pain relief in children aged 4 to 7 years—A pilot study. Anesth. Prog. 2002, 49, 109–112. [Google Scholar] [PubMed]
- Paar, W.D.; Poche, S.; Gerloff, J.; Dengler, H.J. Polymorphic CYP2D6 mediates O-demethylation of the opioid analgesic tramadol. Eur. J. Clin. Pharmacol. 1997, 53, 235–239. [Google Scholar] [CrossRef] [PubMed]

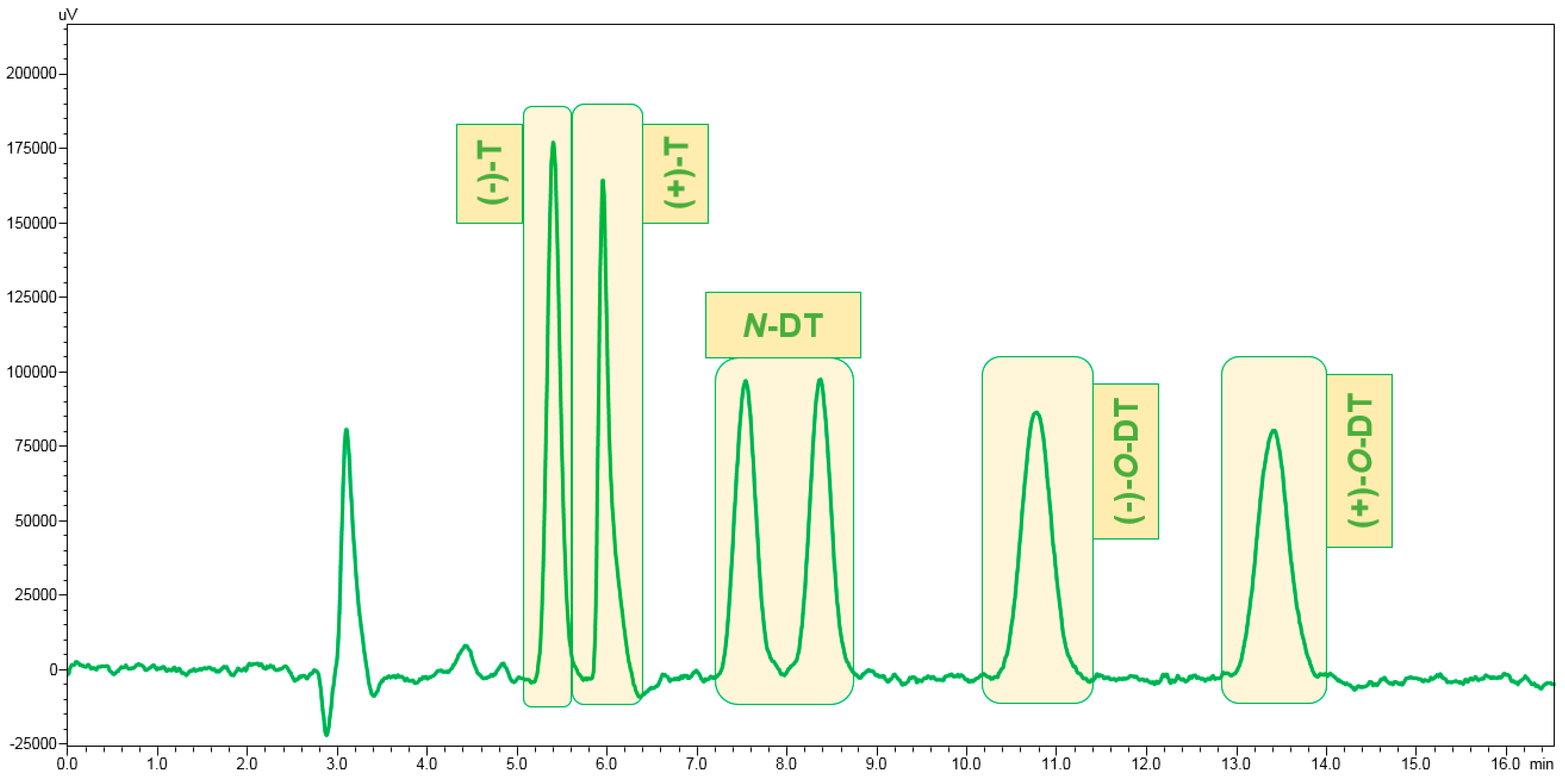

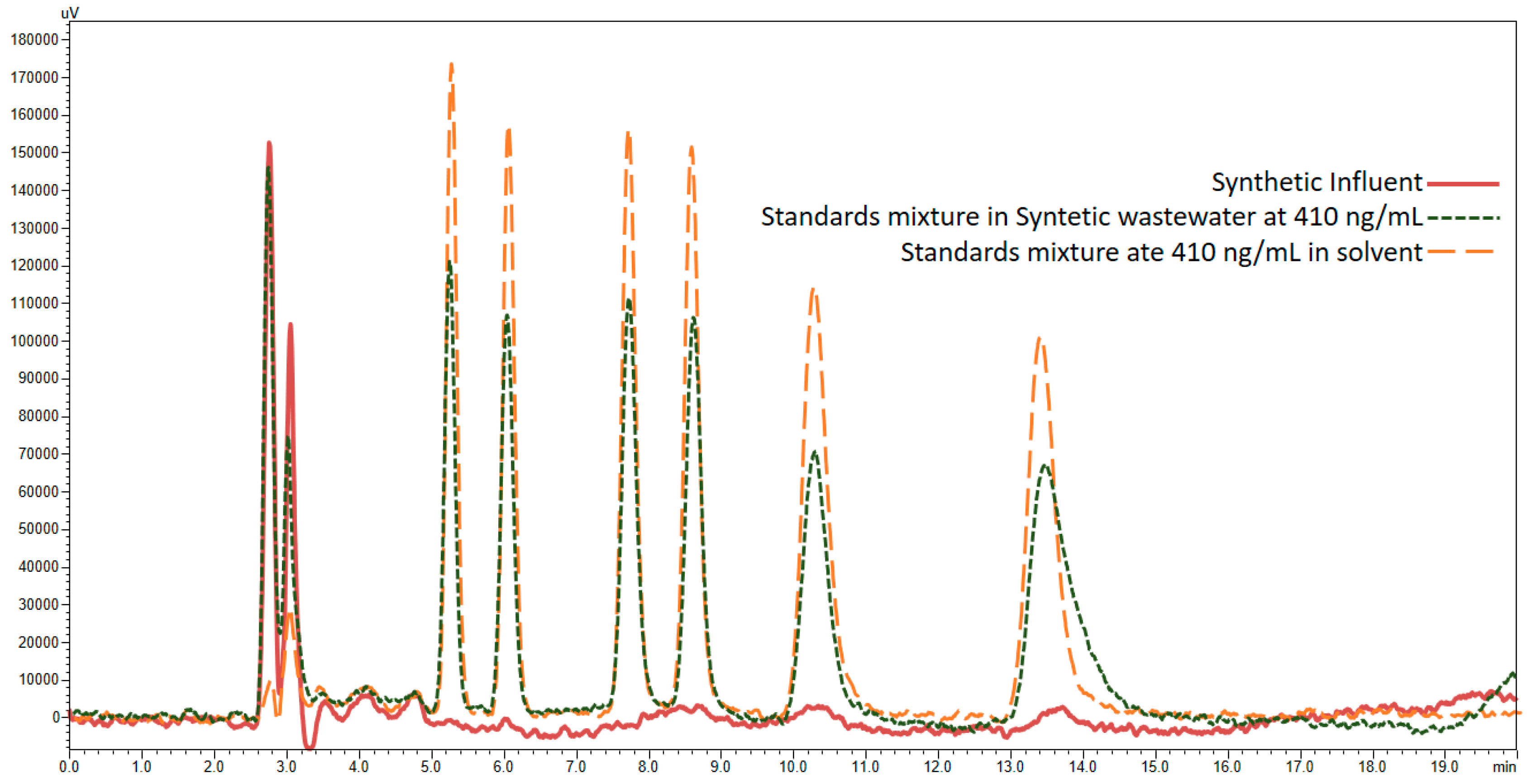
| Procedure | Cartridge | Conditioning | Washing | Elution (1st Step) | Elution (2nd Step) |
|---|---|---|---|---|---|
| 1 | MCX | 8 mL MeOH 8 mL H2O | 8 mL H2O 8 mL MeOH | 8 mL of 5% NH4OH solved in ACN/MeOH 60:40 | - |
| 2 | MCX | 8 mL MeOH 8 mL H2O | 8 mL 2% Formic Acid | 8 mL MeOH | 8 mL of 5% NH4OH solved in MeOH |
| 3 | MCX | 8 mL MeOH 8 mL H2O | 8 mL 2% Formic Acid | 12 mL of 10% NH4OH solved in MeOH | - |
| 4 | MCX | 8 mL EtOH 8 mL H2O | 8 mL 2% Formic Acid | 8 mL EtOH | 8 mL of 5% NH4OH solved in EtOH |
| 5 | MCX | 8 mL EtOH 8 mL Formic acid 2% | 8 mL 2% Formic Acid | 8 mL of 0.6% Formic Acid solved in EtOH | 8 mL of 5% NH4OH solved in EtOH |
| 6 | MCX | 8 mL EtOH 8 mL H2O (pH = 2 adjusted with HCl) | 8 mL 2% Formic Acid | 8 mL EtOH | 8 mL of 5% NH4OH solved in EtOH |
| 7 | HLB | 10 mL MetOH 10 mL H2O | 10 mL H2O | 10 mL MeOH | - |
| 8 | MCX | 8 mL MeOH 8 mL H2O | 8 mL 2% Formic Acid | 12 mL of 10% NH4OH solved in MeOH | - |
| 9 | MCX | 8 mL EtOH 8 mL H2O | 8 mL 2% Formic Acid | 8 mL EtOH | 8 mL of 5% NH4OH solved in EtOH |
| 10 | MCX | 8 mL EtOH 8 mL 2% Formic Acid | 8 mL 2% Formic Acid | 8 mL of 0.6% Formic Acid solved in EtOH | 12 mL of 5% NH4OH solved in EtOH |
| 11 | MCX | 8 mL EtOH 8 mL 2% Formic Acid | 8 mL 2% Formic Acid | 12 mL of 5% NH4OH solved in EtOH | - |
| 12 | MCX | 8 mL EtOH 8 mL 2% Formic Acid | 8 mL 2% Formic Acid | 8 mL of 0.6% Formic Acid solved in EtOH | 12 mL of 5% NH4OH solved in EtOH |
| Column | Elution Mode | Mobile Phase: Proportion (v/v) | Tramadol | N-DT | O-DT | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K1 | K2 | α | Rs | K1 | K2 | α | Rs | K1 | K2 | α | Rs | |||
| Lux Cellulose-2 | Normal | Hex/IPA/DEA: 90:10:0.1 ** | 0.73 | 0.89 | 1.22 | 1.00 | 1.13 | 1.62 | 1.44 | 3.02 | 1.55 | 3.04 | 1.96 | 5.53 |
| Hex/IPA/DEA: 90:10:0.05 ** | 4.31 | 4.66 | 1.08 | 0.92 | 4.53 | 5.57 | 1.23 | 2.75 | 9.86 | 14.7 | 1.49 | 4.10 | ||
| Hex/EtOH/DEA: 96:4: 0.1 ** | 0.98 | 1.07 | 1.11 | 1.12 | 1.91 | - | 1.00 | - | 3.56 | 4.16 | 1.17 | 2.42 | ||
| Reversed | ACN (5 mM ammonium formate/0.1% DEA)/H2O: 35:65 * | 5.75 | 6.13 | 1.07 | 1.49 | 4.29 | - | 1.00 | - | 1.29 | 1.42 | 1.11 | 0.949 | |
| ACN (5 mM ammonium formate/0.1% DEA)/H2O: 30:70 * | 9.98 | 10.6 | 1.07 | 1.89 | 7.19 | - | 1.00 | - | 1.91 | 2.11 | 1.11 | 1.31 | ||
| Lux Cellulose-4 | Reversed | ACN (5 mM ammonium formate/0.1% DEA)/H2O: 35:65 ** | 6.32 | 7.21 | 1.14 | 2.79 | 10.7 | 11.4 | 1.07 | 1.26 | 1.25 | 1.66 | 1.33 | 2.31 |
| MeOH (5 mM ammonium formate/0.05% DEA)/H2O: 55:45 ** | 10.7 | 11.9 | 1.12 | 2.18 | 4.62 | 4.83 | 1.05 | 0.236 | 2.43 | 2.85 | 1.17 | 1.43 | ||
| ACN:EtOH (10 mM ammonium formate/0.1% DEA) /H2O: 17.5:17.5:65 ** | 8.91 | 10.1 | 1.13 | 2.69 | 4.65 | 4.92 | 1.06 | 0.36 | 2.07 | 2.65 | 1.28 | 2.62 | ||
| Normal | Hex/EtOH/DEA—96:4:0.1 *** | 0.74 | 0.920 | 1.24 | 2.16 | 1.43 | 1.70 | 1.19 | 1.85 | 2.48 | 3.33 | 1.34 | 4.16 | |
| Analyte | E | Nominal Conc. Range (ng L−1) | Calibration Curve Equation | r2 | IDL (ng L−1) | IQL (ng L−1) | MDL (ng L−1) | MQL (ng L−1) |
|---|---|---|---|---|---|---|---|---|
| Tramadol | (−)-T | 28–168 | y = 6944.4x − 93525 | 0.9902 | 10 | 35 | 8 | 28 |
| (+)-T | y = 7421.2x − 113599 | 0.9901 | 10 | 35 | 8 | 28 | ||
| N-DT | (−)-N-DT | 28–168 | y = 6128.8x + 4247 | 0.9958 | 10 | 35 | 8 | 28 |
| (+)-N-DT | y = 5973.8x + 33396 | 0.9939 | 10 | 35 | 8 | 28 | ||
| O-DT | (−)-O-DT | 56–196 | y = 30420x − 2 × 106 | 0.9954 | 25 | 70 | 20 | 56 |
| (+)-O-DT | y = 44269x − 2 × 106 | 0.9948 | 25 | 70 | 20 | 56 |
| Analyte | Nominal Concentration (ng mL−1) | E | 1st Day | 2nd Day | 3rd Day | Inter-Day RSD (%) | Recovery (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Accuracy (%) | RSD (%) | Accuracy (%) | RSD (%) | Accuracy (%) | RSD (%) | |||||
| Tramadol | 75 | (−)-T | 89.6 | 7.7 | 88.5 | 4.9 | 94.3 | 3.1 | 6.5 | 87.1 |
| (+)-T | 90.6 | 8.3 | 91.4 | 3.7 | 93.3 | 5.9 | 6.4 | 88.9 | ||
| 150 | (−)-T | 80.1 | 4.6 | 80.6 | 1.6 | 82.1 | 6.5 | 4.9 | 78.4 | |
| (+)-T | 81.0 | 6.7 | 80.4 | 0.3 | 82.9 | 5.8 | 5.4 | 78.3 | ||
| 205 | (−)-T | 105.6 | 1.0 | 82.2 | 8.0 | 97.9 | 8.5 | 12.5 | 97.8 | |
| (+)-T | 70.3 | 1.1 | 84.0 | 3.8 | 90.0 | 8.4 | 11.7 | 83.2 | ||
| N-DT | 75 | (−)-N-DT | 99.1 | 8.2 | 82.0 | 8.8 | 105.7 | 3.5 | 11.7 | 99.2 |
| (+)-N-DT | 114.1 | 8.8 | 91.2 | 3.9 | 100.0 | 8.9 | 11.7 | 99.4 | ||
| 150 | (−)-N-DT | 98.3 | 1.7 | 91.9 | 6.2 | 97.5 | 6.1 | 5.8 | 97.6 | |
| (+)-N-DT | 87.8 | 5.6 | 81.3 | 1.5 | 90.6 | 6.7 | 6.8 | 88.2 | ||
| 205 | (−)-N-DT | 77.0 | 6.3 | 85.6 | 4.0 | 105.4 | 7.9 | 16.7 | 85.9 | |
| (+)-N-DT | 79.4 | 4.9 | 89.6 | 3.7 | 108.7 | 7.4 | 16.3 | 89.8 | ||
| O-DT | 75 | (−)-O-DT | 94.9 | 5.0 | 92.5 | 8.2 | 96.9 | 4.5 | 14.8 | 72.2 |
| (+)-O-DT | 96.0 | 6.8 | 97.1 | 8.9 | 97.5 | 9.2 | 9.5 | 83.6 | ||
| 150 | (−)-O-DT | 97.0 | 9.9 | 93.2 | 5.0 | 96.3 | 7.8 | 10.6 | 87.4 | |
| (+)-O-DT | 99.9 | 4.6 | 98.5 | 7.2 | 99.4 | 3.6 | 5.9 | 98.1 | ||
| 205 | (−)-O-DT | 81.7 | 7.0 | 90.0 | 4.5 | 97.9 | 3.8 | 14.9 | 82.0 | |
| (+)-O-DT | 90.2 | 5.9 | 95.6 | 6.3 | 106.3 | 8.9 | 14.5 | 92.2 | ||
| Sample nº | 1 | 2 | ||||
|---|---|---|---|---|---|---|
| Effluent | Influent | Effluent | Influent | |||
| Concentration (ng L−1) | Tramadol | (−)-T | 235.8 | 357.9 | 325.1 | 350.0 |
| (+)-T | 118.7 | 233.6 | 314.9 | 233.8 | ||
| N-DT | (−)-N-DT | <QL | <QL | <QL | <QL | |
| (+)-N-DT | 43.7 | 63.9 | 62.1 | 72.7 | ||
| O-DT | (−)-O-DT | 60.8 | 69.7 | 71.6 | 69.5 | |
| (+)-O-DT | 57.7 | 86.7 | 95.4 | 106.7 | ||
| Enantiomeric Fraction | Tramadol | EF1 | 0.67 | 0.61 | 0.51 | 0.60 |
| N-DT | EF1 | ≈0 | ≈0 | ≈0 | ≈0 | |
| O-DT | EF1 | 0.51 | 0.45 | 0.43 | 0.39 | |
| DRE (%) | Tramadol | (−)-T | 65.9 | 92.9 | ||
| (+)-T | 50.8 | 134.7 * | ||||
| N-DT | (−)-N-DT | NC | NC | |||
| (+)-N-DT | 68.4 | 85.4 | ||||
| O-DT | (−)-O-DT | 87.2 | 103.0 * | |||
| (+)-O-DT | 66.6 | 89.4 | ||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, C.; Ribeiro, C.; Maia, A.S.; Gonçalves, V.; Tiritan, M.E.; Afonso, C. Enantiomeric Separation of Tramadol and Its Metabolites: Method Validation and Application to Environmental Samples. Symmetry 2017, 9, 170. https://doi.org/10.3390/sym9090170
Silva C, Ribeiro C, Maia AS, Gonçalves V, Tiritan ME, Afonso C. Enantiomeric Separation of Tramadol and Its Metabolites: Method Validation and Application to Environmental Samples. Symmetry. 2017; 9(9):170. https://doi.org/10.3390/sym9090170
Chicago/Turabian StyleSilva, Cátia, Cláudia Ribeiro, Alexandra S. Maia, Virgínia Gonçalves, Maria Elizabeth Tiritan, and Carlos Afonso. 2017. "Enantiomeric Separation of Tramadol and Its Metabolites: Method Validation and Application to Environmental Samples" Symmetry 9, no. 9: 170. https://doi.org/10.3390/sym9090170
APA StyleSilva, C., Ribeiro, C., Maia, A. S., Gonçalves, V., Tiritan, M. E., & Afonso, C. (2017). Enantiomeric Separation of Tramadol and Its Metabolites: Method Validation and Application to Environmental Samples. Symmetry, 9(9), 170. https://doi.org/10.3390/sym9090170









