A Hypothesis for Self-Organization and Symmetry Reduction in the Synchronization of Organ-Level Contractions in the Human Uterus during Labor
Abstract
:1. Introduction
2. Myocytes—the Smallest Contractile Units of the Uterus
3. From Quiescence to Critical Point—the Role of Sex Hormones and Contraction-Associated Proteins
4. The Emergence of Synchronized Contractions—the Appearance and Synchronization of Syncytia

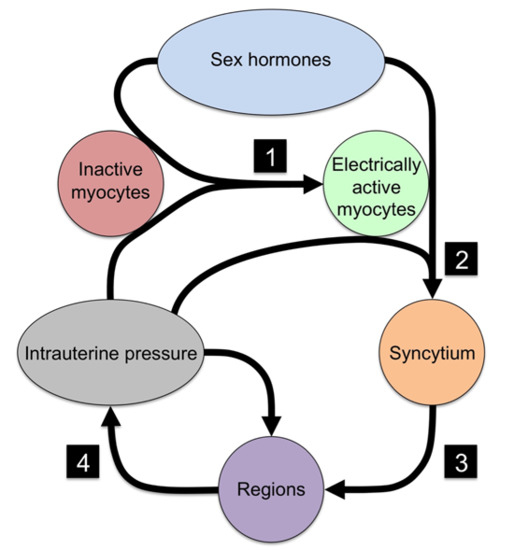
| No. | Event | Necessary Component | Mechanism |
|---|---|---|---|
| 1 | Quiescent myocytes to electrical active myocytes | Ion channels | Electrical excitability |
| 2 | Isolated myocytes to syncytial organization | Cx43 | Electrical and metabolic sharing |
| 3 | Syncytial grouping to regions | Cx43 | Phase coupling of oscillators |
| 4 | Regional signaling to organ-level organization | Intrauterine pressure | Mechanotransduction |
5. Conclusions
“The maintenance of organization in nature is not—and cannot be—achieved by central management. Order can only be maintained by self-organization. Self-organizing systems allow adaptation to the prevailing environment, i.e., they react to changes in the environment with a thermodynamic response which makes the systems extraordinarily flexible and robust against perturbations from outside conditions.”[22]
Author Contributions
Conflicts of Interest
References and Notes
- Depolarization results from an influx of anions across the membrane, initiated by mechanisms including the hyperpolarization-activated inward current and the low-threshold T-type calcium ion (Ca2+) currents. With increasing depolarization, sodium currents are activated, causing further rapid depolarization, which in turn activates high-threshold L-type Ca2+ currents that sustain the plateau phase of the action potential. Subsequently voltage- and calcium-activated potassium currents, and the deactivation of depolarizing currents, drive the membrane potential towards resting potential and terminate the action potential.
- Note: In this sequence, Ca2+ binds to Calmodulin, producing a complex that activates myosin light chains. This leads to phosphorylation of myosin light chains, which in turn enables interaction between myosin and actin heavy chains. It is this interaction between myosin and actin heavy chains that causes cell shortening [20]
- Butler, T.; Paul, J.; Europe-Finner, N.; Smith, R.; Chan, E. Role of serine-threonine phosphoprotein phosphatases in smooth muscle contractility. Am. J. Physiol. Cell Physiol. 2013, 304, C485–C504. [Google Scholar] [CrossRef] [PubMed]
- Wray, S.; Jones, K.; Kupittayanant, S.; Li, Y.; Matthew, A.; Monir-Bishty, E.; Noble, K.; Pierce, S.J.; Quenby, S.; Shmygol, A.V. Calcium signaling and uterine contractility. J. Soc. Gynecol. Investig. 2003, 10, 252–264. [Google Scholar] [CrossRef]
- Parkington, H.C.; Tonta, M.A.; Brennecke, S.P.; Coleman, H.A. Contractile activity, membrane potential, and cytoplasmic calcium in human uterine smooth muscle in the third trimester of pregnancy and during labor. Am. J. Obstet. Gynecol. 1999, 181, 1445–1451. [Google Scholar] [CrossRef]
- Miller, F.; Yeh, S.Y.; Schifrin, B.S.; Paul, R.H.; Hon, E.H. Quantitation of uterine activity in 100 primiparous patients. Am. J. Obstet. Gynecol. 1976, 124, 398–405. [Google Scholar] [PubMed]
- Young, R.C.; Barendse, P. Linking myometrial physiology to intrauterine pressure; how tissue-level contractions create uterine contractions of labor. PLoS Comput. Biol. 2014, 10. [Google Scholar] [CrossRef] [PubMed]
- Csapo, A. Progesterone “block”. Am. J. Anat. 1956, 98, 273–291. [Google Scholar] [CrossRef] [PubMed]
- Mesiano, S.; Chan, E.-C.; Fitter, J.T.; Kwek, K.; Yeo, G.; Smith, R. Progesterone withdrawal and estrogen activation in human parturition are coordinated by progesterone receptor A expression in the myometrium. J. Clin. Endocrinol. Metab. 2002, 87, 2924–2930. [Google Scholar] [CrossRef] [PubMed]
- Smith, R. Parturition. N. Engl. J. Med. 2007, 356, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Two forms of estrogen are produced during pregnancy-estriol, produced in the placenta from fetal steroid precursors, and estradiol, produced from precursors made in the maternal adrenal gland. While both estriol and estradiol act on estrogen receptors, in eqimolar concentrations they form heterodimers which block estrogenic actions. Late in pregnancy, the growth of the fetal adrenal causes the rate of increase in estriol production to outstrip that of estradiol production, causing estriol to become dominant over estradiol, and through the production of estriol homodimers the uterine environment becomes increasingly estrogenic. An extraordinary consequence of this is that, while in a healthy pregnancy it is the maturation of the fetus that stimulates the estrogenic signals that lead to the emergence of labor, in the event that the baby dies, maternal estrogen becomes the signal, ensuring that the mother is not left with a dead fetus in-utero [23].
- Sakai, N.; Tabb, T.; Garfield, R.E. Studies of connexin 43 and cell-to-cell coupling in cultured human uterine smooth muscle. Am. J. Obstet. Gynecol. 1992, 167, 1267–1277. [Google Scholar] [CrossRef]
- Sims, S.M.; Daniel, E.E.; Garfield, R.E. Improved electrical coupling in uterine smooth muscle is associated with increased numbers of gap junctions at parturition. J. Gen. Physiol. 1982, 80, 353–375. [Google Scholar] [CrossRef] [PubMed]
- Garfield, R.E.; Blennerhassett, M.G.; Miller, S.M. Control of myometrial contractility: Role and regulation of gap junctions. Oxf. Rev. Reprod. Biol. 1988, 10, 463–490. [Google Scholar]
- Young, R.C.; Goloman, G. Phasic oscillations of extracellular potassium (K(o)) in pregnant rat myometrium. PLoS ONE 2013, 8, e65110. [Google Scholar] [CrossRef] [PubMed]
- Ramon, C.; Preissl, H.; Murphy, P.; Wilson, J.D.; Lowery, C.; Eswaran, H. Synchronization analysis of the uterine magnetic activity during contractions. Biomed. Eng. Online 2005, 4. [Google Scholar] [CrossRef] [PubMed]
- Csapo, A. The diagnostic significance of the intrauterine pressure. II. Clinical considerations and trials. Obstet. Gynecol. Surv. 1970, 25, 515–543. [Google Scholar] [CrossRef] [PubMed]
- Gibb, W.; Challis, J.R. Mechanisms of term and preterm birth. J. Obstet. Gynaecol. Can. 2002, 24, 874–883. [Google Scholar] [PubMed]
- Caglioti, G. The Dynamics of Ambiguity; Springer Verlag: Berlin, Germany, 1992. [Google Scholar]
- Golubitsky, M.; Stewart, I.; Buono, P.-L.; Collins, J.J. Symmetry in locomotor central pattern generators and animal gaits. Nature 1999, 401, 693–695. [Google Scholar] [CrossRef] [PubMed]
- Stewart, I.; Golubitsky, M. Fearful Symmetry: Is God a Geometer? Dover Publications: Mineola, NY, USA, 2011; p. 320. [Google Scholar]
- Prigogine, I. The End of Certainty: Time, Chaos, and the New Laws of Nature; Free Press: New York, NY, USA, 1997. [Google Scholar]
- Melamed, M.; Castano, E.; Notides, A.C.; Sasson, S. Molecular and kinetic basis for the mixed agonist/antagonist activity of estriol. Mol. Endocrinol. Baltim. Md. 1997, 11, 1868–1878. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banney, D.; Young, R.; Paul, J.W.; Imtiaz, M.; Smith, R. A Hypothesis for Self-Organization and Symmetry Reduction in the Synchronization of Organ-Level Contractions in the Human Uterus during Labor. Symmetry 2015, 7, 1981-1988. https://doi.org/10.3390/sym7041981
Banney D, Young R, Paul JW, Imtiaz M, Smith R. A Hypothesis for Self-Organization and Symmetry Reduction in the Synchronization of Organ-Level Contractions in the Human Uterus during Labor. Symmetry. 2015; 7(4):1981-1988. https://doi.org/10.3390/sym7041981
Chicago/Turabian StyleBanney, David, Roger Young, Jonathan W. Paul, Mohammad Imtiaz, and Roger Smith. 2015. "A Hypothesis for Self-Organization and Symmetry Reduction in the Synchronization of Organ-Level Contractions in the Human Uterus during Labor" Symmetry 7, no. 4: 1981-1988. https://doi.org/10.3390/sym7041981
APA StyleBanney, D., Young, R., Paul, J. W., Imtiaz, M., & Smith, R. (2015). A Hypothesis for Self-Organization and Symmetry Reduction in the Synchronization of Organ-Level Contractions in the Human Uterus during Labor. Symmetry, 7(4), 1981-1988. https://doi.org/10.3390/sym7041981