Artificial Interfacial Layers with Zwitterionic Ion Structure Improves Lithium Symmetric Battery Life and Inhibits Dendrite Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrode Preparations
2.3. Characterization
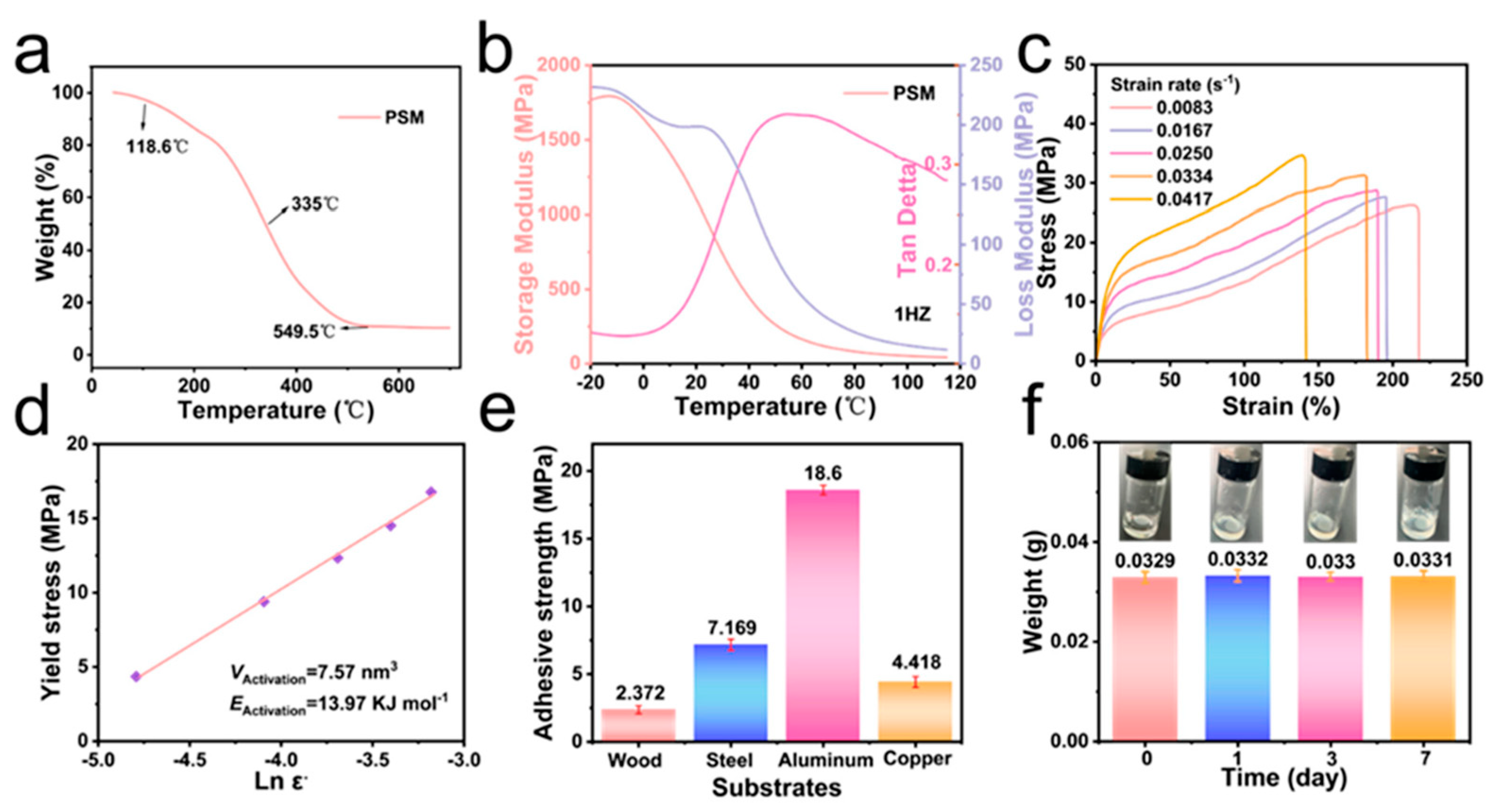
2.4. Crosslinking Density
2.5. Determination of Activation Volume and Energy
2.6. Electrochemical Measurements
3. Results and Discussion
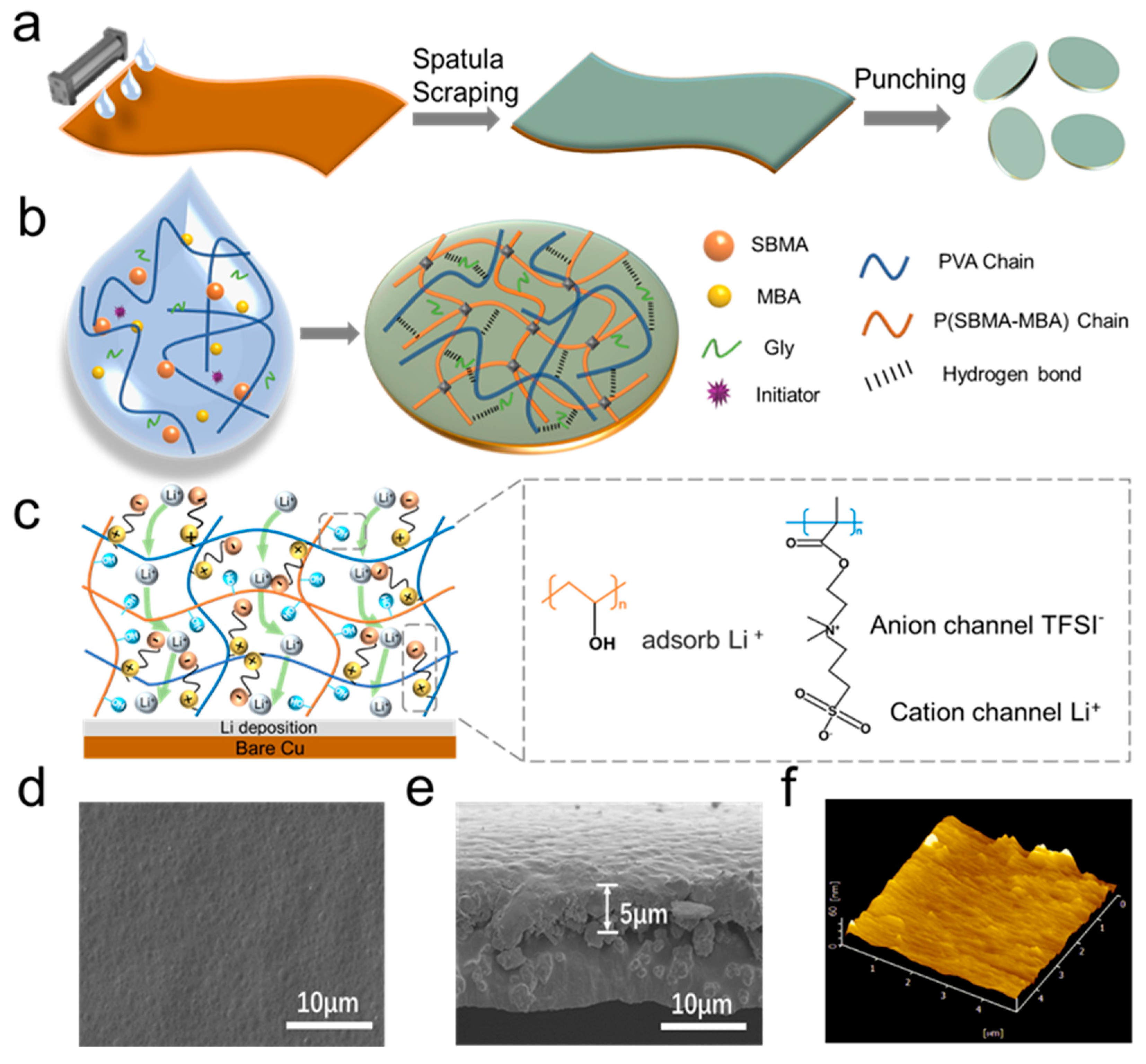
3.1. Electrode Preparation and Morphology
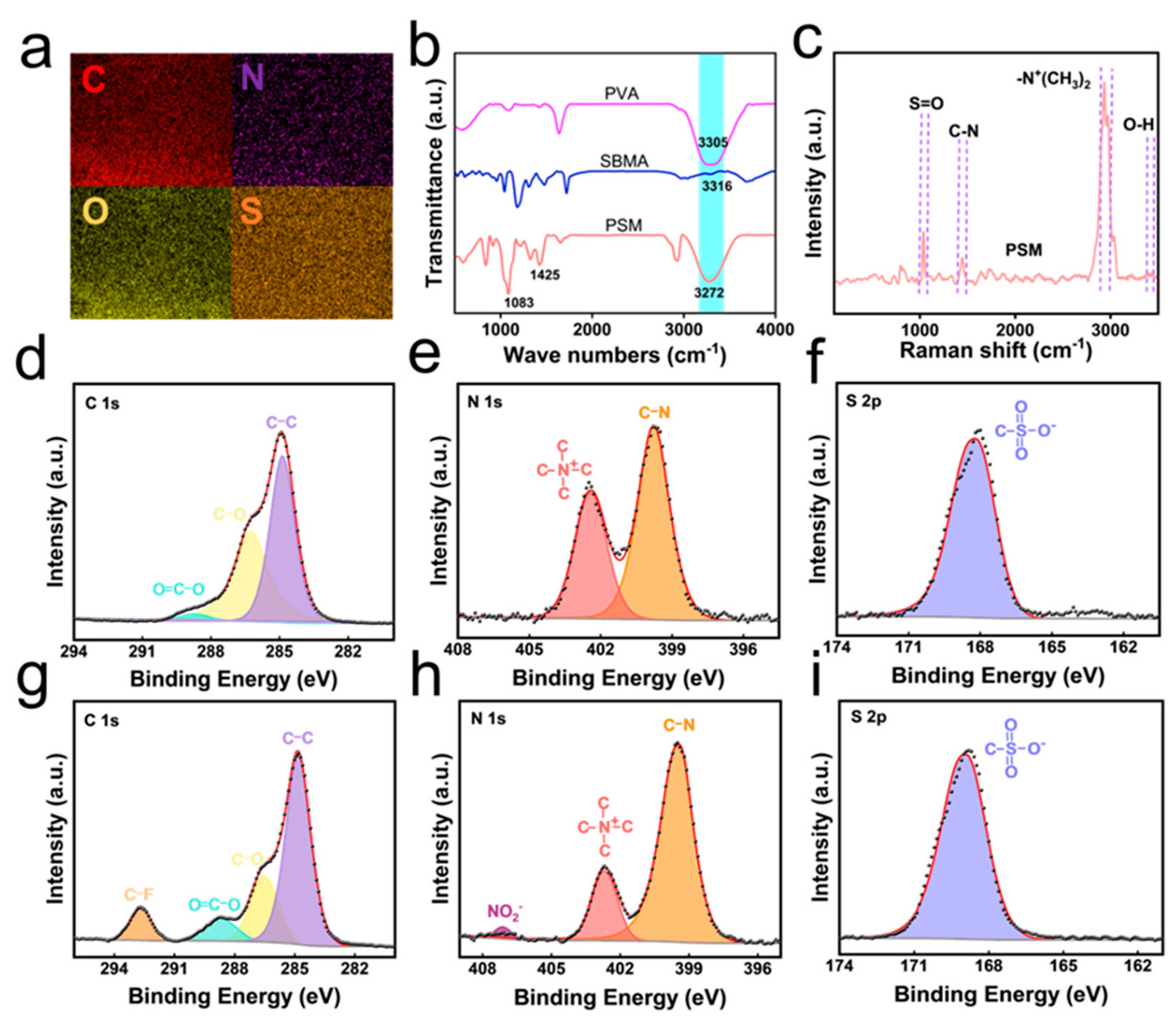
3.2. Structural Characterization
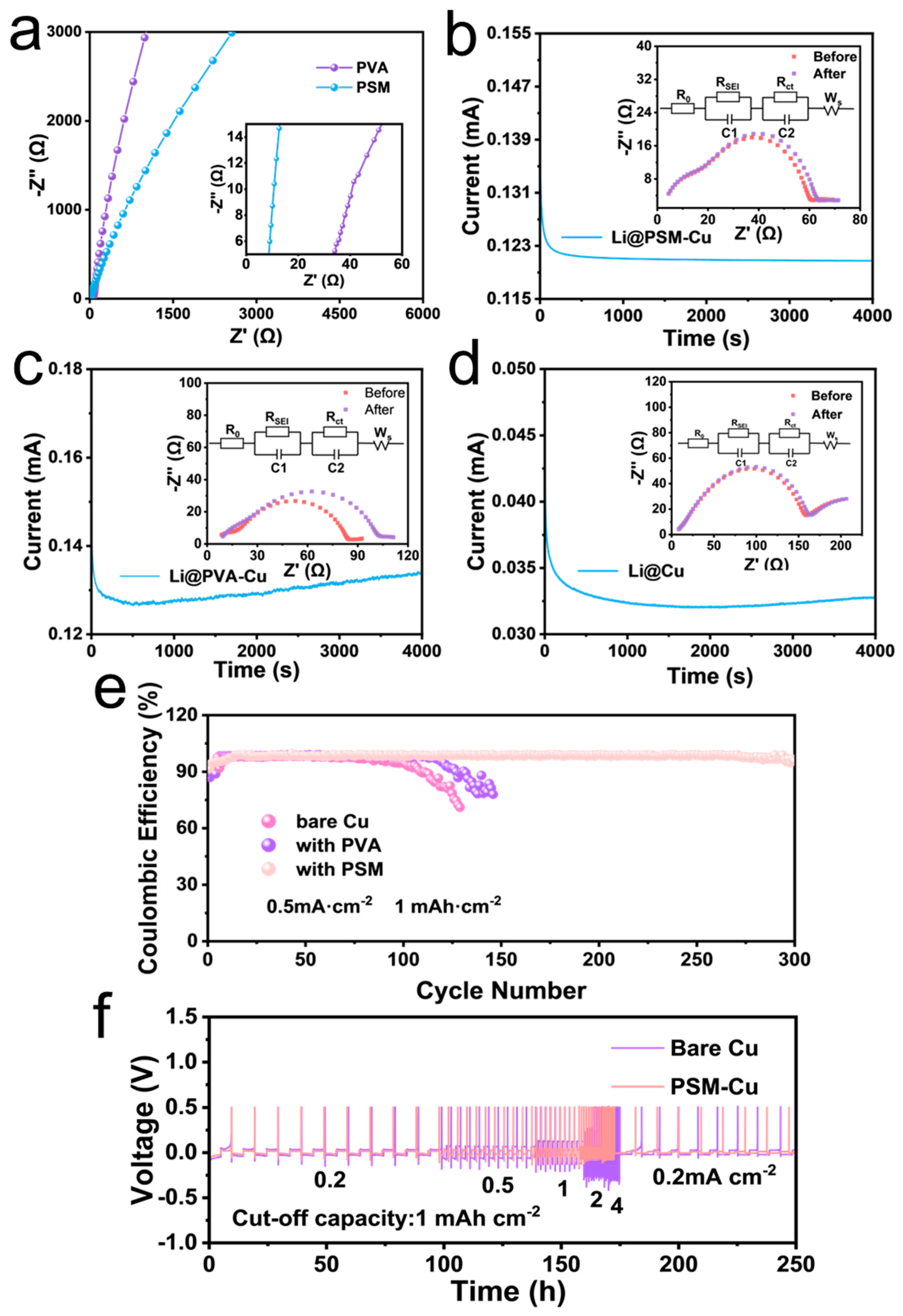
3.3. Electrochemical Performance and Coulombic Efficiency
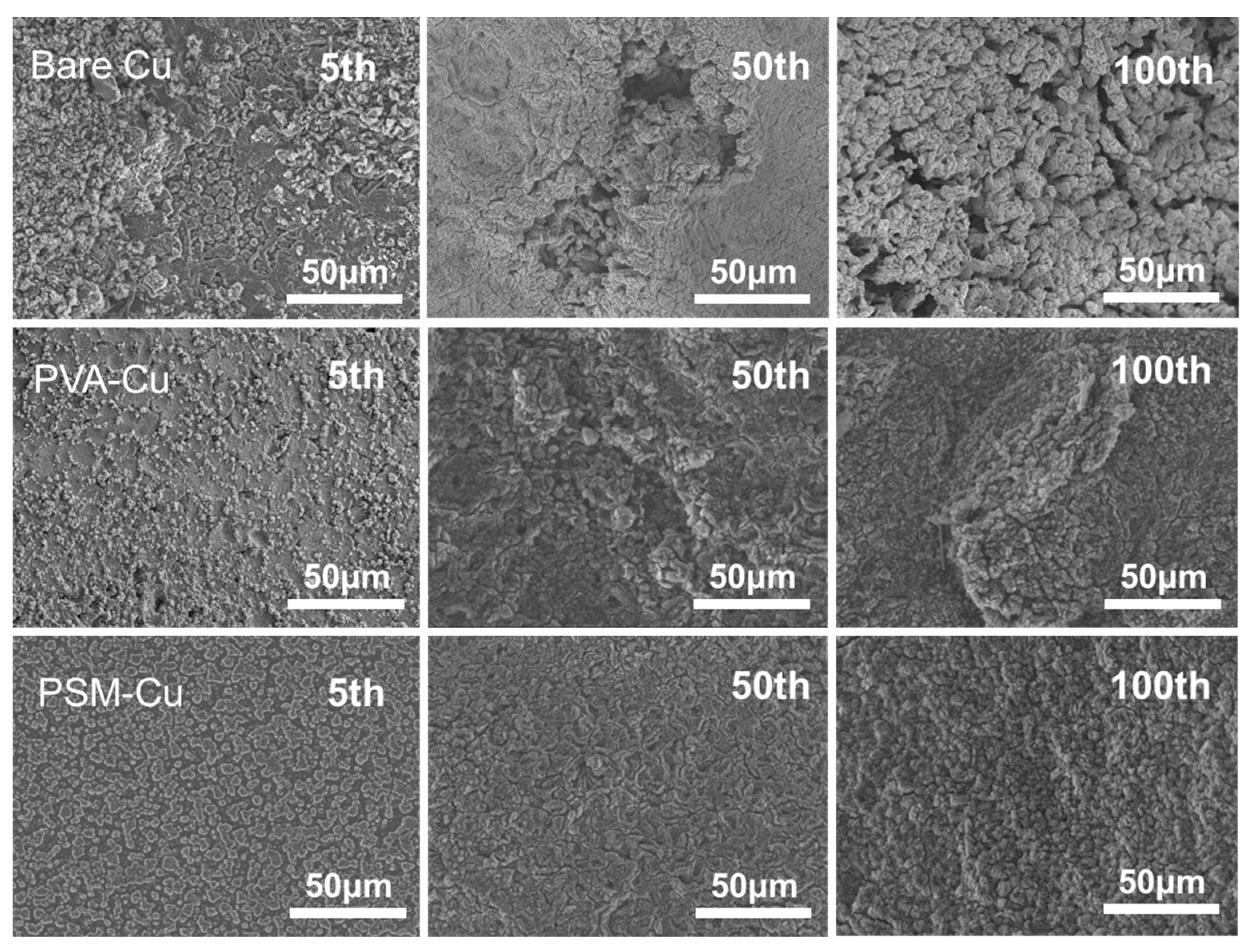
3.4. SEM Analysis
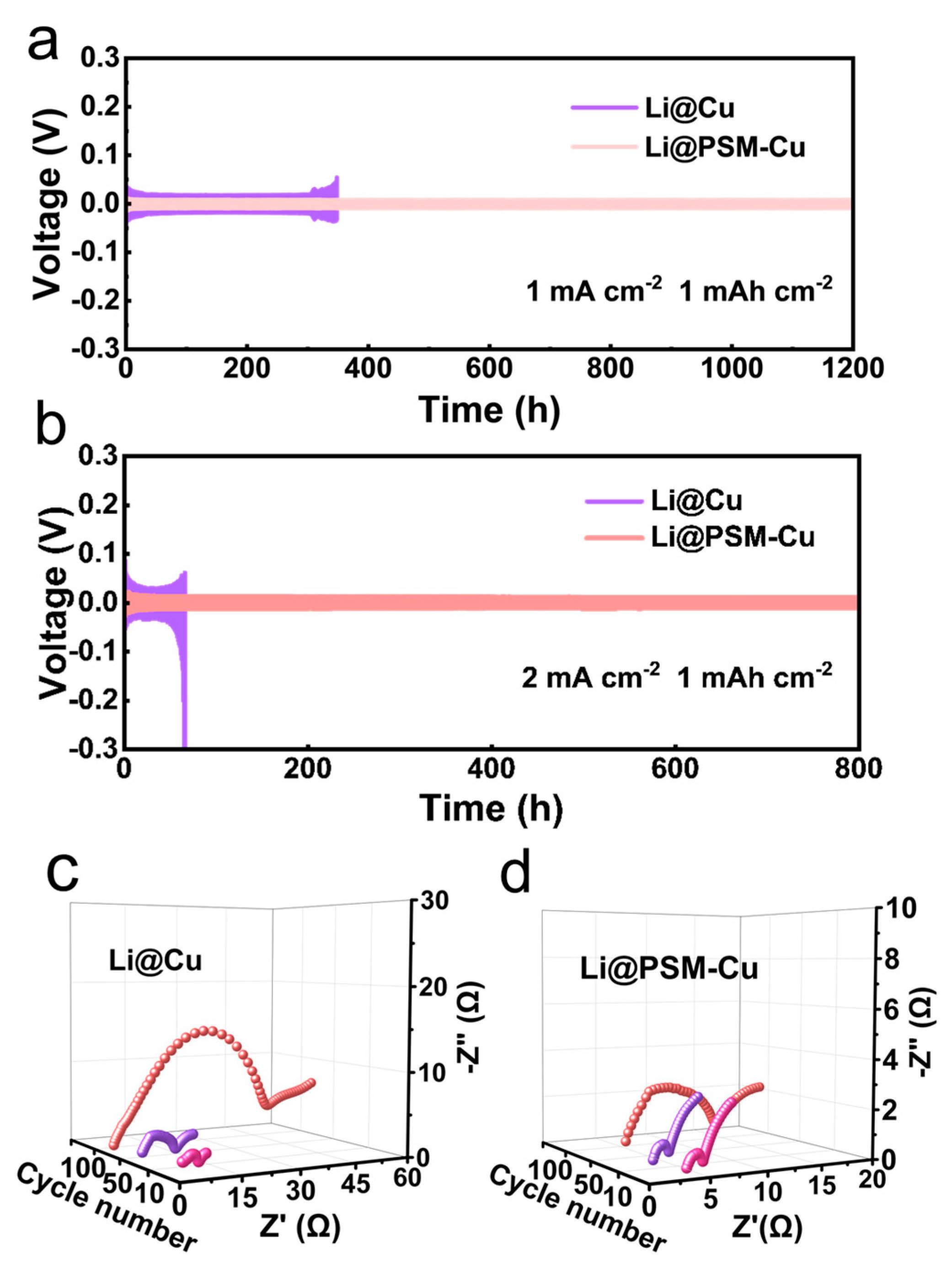
3.5. Symmetrical Battery
3.6. Asymmetric Full Battery
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SEI | Solid Electrolyte Interphase |
| LMB | Lithium Metal Battery |
| PVA | Polyvinyl Alcohol |
| SBMA | [2-(Methacryloyloxy)ethyl]dimethyl-(3-sulfopropyl)ammonium hydroxide |
| MBA | N,N′-Methylenebis(acrylamide) |
| Gly | Glycerol |
| APS | Ammonium Persulfate |
| PSM | PVA/P(SBMA-MBA) Hydrogen Bond Network Film |
| NMP | 1-Methyl-2-pyrrolidinone |
| LiTFSI | Lithium bis(trifluoromethanesulfonyl)imide |
| DOL | 1,3-Dioxolane |
| DME | 1,2-Dimethoxyethane |
| PVDF | Poly(vinylidene fluoride) |
| LFP | Lithium Iron Phosphate/LiFePO4 |
| EIS | Electrochemical Impedance Spectroscopy |
| CE | Coulombic Efficiency |
| PVA-Cu | Copper Foil Modified with PVA |
| PSM-Cu | Copper Foil Modified with PSM |
Appendix A
| Samples | Polymer Density (g/cm3) | Swelling Ratio (%) | Crosslinking Density (10−4 mol/cm3) |
|---|---|---|---|
| PVA | 1.25 ± 0.01 | 210.61 ± 0.17 | 2.71 ± 0.11 |
| PSM | 1.29 ± 0.03 | 116.70 ± 0.09 | 9.65 ± 0.02 |
References
- Lin, D.; Liu, Y.; Cui, Y. Reviving the Lithium Metal Anode for High-Energy Batteries. Nat. Nanotechnol. 2017, 12, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Liu, Y.; Xie, J. Building Better Li Metal Anodes in Liquid Electrolyte: Challenges and Progress. ACS Appl. Mater. Interfaces 2020, 13, 18–33. [Google Scholar] [CrossRef]
- Mousaei, A.; Naderi, Y.; Bayram, I.S. Advancing State of Charge Management in Electric Vehicles with Machine Learning: A Technological Review. IEEE Access 2024, 12, 43255–43283. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Yan, C.; Peng, H.-J.; Huang, J.-Q.; Yang, S.-T.; Zhang, Q. Sulfurized Solid Electrolyte Interphases with a Rapid Li+ Diffusion on Dendrite-Free Li Metal Anodes. Energy Storage Mater. 2018, 10, 199–205. [Google Scholar] [CrossRef]
- Lanjapalli, V.V.K.; Lin, F.-J.; Liou, S.; Hosseini, S.; Huang, C.-L.; Chen, Y.-S.; Li, Y.-Y. Semi-Infused Lithium Anode for Advanced Li Metal Batteries. Electrochim. Acta 2022, 410, 139976. [Google Scholar] [CrossRef]
- Yuan, H.; Nai, J.; Fang, Y.; Lu, G.; Tao, X.; Lou, X.W. Double-Shelled C@MoS2 Structures Preloaded with Sulfur: An Additive Reservoir for Stable Lithium Metal Anodes. Angew. Chem. Int. Ed. 2020, 59, 15839–15843. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, H.; Wu, H.; Li, Q.; Zhang, Y.; Fan, S.; Wang, J. Self-Standing Carbon Nanotube Aerogels with Amorphous Carbon Coating as Stable Host for Lithium Anodes. Carbon 2021, 177, 181–188. [Google Scholar] [CrossRef]
- Meyerson, M.L.; Papa, P.E.; Heller, A.; Mullins, C.B. Recent Developments in Dendrite-Free Lithium-Metal Deposition through Tailoring of Micro- and Nanoscale Artificial Coatings. ACS Nano 2020, 15, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhao, Y.; Wang, J.; Liang, J.; Wang, C.; Sun, Q.; Lin, X.; Adair, K.R.; Luo, J.; Wang, D.; et al. A Novel Organic “Polyurea” Thin Film for Ultralong-Life Lithium-Metal Anodes via Molecular-Layer Deposition. Adv. Mater. 2018, 31, 1806541. [Google Scholar] [CrossRef]
- Kim, S.; Park, G.; Lee, S.J.; Seo, S.; Ryu, K.; Kim, C.H.; Choi, J.W. Lithium Metal Batteries: From Fundamental Research to Industrialization. Adv. Mater. 2023, 35, 2206625. [Google Scholar] [CrossRef]
- Xu, C.-X.; Jiang, J.-J. Designing Electrolytes for Lithium Metal Batteries with Rational Interface Stability. Rare Met. 2020, 40, 243–245. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Q.; Wang, X.; Xu, M.; Li, W.; Lucht, B.L. LiFSI and LiDFBOP Dual-Salt Electrolyte Reinforces the Solid Electrolyte Interphase on a Lithium Metal Anode. ACS Appl. Mater. Interfaces 2020, 12, 33719–33728. [Google Scholar] [CrossRef] [PubMed]
- Le, H.T.T.; Ngo, D.T.; Ho, V.-C.; Cao, G.; Park, C.-N.; Park, C.-J. Insights into Degradation of Metallic Lithium Electrodes Protected by a Bilayer Solid Electrolyte Based on Aluminium Substituted Lithium Lanthanum Titanate in Lithium-Air Batteries. J. Mater. Chem. A 2016, 4, 11124–11138. [Google Scholar] [CrossRef]
- Ren, Y.; Cui, Z.; Bhargav, A.; He, J.; Manthiram, A. A Self Healable Sulfide/Polymer Composite Electrolyte for Long Life, Low Lithium Excess Lithium Metal Batteries. Adv. Funct. Mater. 2021, 32, 2106680. [Google Scholar] [CrossRef]
- Wang, B.; Wang, G.; He, P.; Fan, L.-Z. Rational Design of Ultrathin Composite Solid-State Electrolyte for High-Performance Lithium Metal Batteries. J. Membrane Sci. 2022, 642, 119952. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Li, J.; Tan, S.-J.; Wang, B.; Gao, Y.-B.; Xin, S.; Wang, C.-R. Fullerene-Derivative C60-(OLi)n Modified Separators toward Stable Wide-Temperature Lithium Metal Batteries. Chem. Eng. J. 2022, 446, 137207. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, C.; Zhou, J.; Yao, X.; Li, J.; Zhuang, H.; Chen, Y.; Chen, Y.; Li, S.L.; Lan, Y.Q. Anisotropically Hybridized Porous Crystalline Li-S Battery Separators. Small 2022, 19, 2206616. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, Y.; Han, Q.; Su, M.; Liu, Y.; Wang, S.; Wang, H. Vapor-Induced Phase Inversion of Poly (m-phenylene isophthalamide) Modified Polyethylene Separator for High-Performance Lithium-Ion Batteries. Chem. Eng. J. 2022, 429, 132429. [Google Scholar] [CrossRef]
- Lopez, J.; Pei, A.; Oh, J.Y.; Wang, G.J.N.; Cui, Y.; Bao, Z. Effects of Polymer Coatings on Electrodeposited Lithium Metal. J. Am. Chem. Soc. 2018, 140, 11735–11744. [Google Scholar] [CrossRef]
- Zhou, H.; Yu, S.; Liu, H.; Liu, P. Protective Coatings for Lithium Metal Anodes: Recent Progress and Future Perspectives. J. Power Sources 2020, 450, 227632. [Google Scholar] [CrossRef]
- Zheng, G.; Wang, C.; Pei, A.; Lopez, J.; Shi, F.; Chen, Z.; Sendek, A.D.; Lee, H.-W.; Lu, Z.; Schneider, H.; et al. High-Performance Lithium Metal Negative Electrode with a Soft and Flowable Polymer Coating. ACS Energy Lett. 2016, 1, 1247–1255. [Google Scholar] [CrossRef]
- Kang, D.; Sardar, S.; Zhang, R.; Noam, H.; Chen, J.; Ma, L.; Liang, W.; Shi, C.; Lemmon, J.P. In-Situ Organic SEI Layer for Dendrite-Free Lithium Metal Anode. Energy Storage Mater. 2020, 27, 69–77. [Google Scholar] [CrossRef]
- Chen, Y.; Dou, X.; Wang, K.; Han, Y. Lithium Dendrites Inhibition via Diffusion Enhancement. Adv. Energy Mater. 2019, 9, 1900019. [Google Scholar] [CrossRef]
- Shen, K.; Wang, Z.; Bi, X.; Ying, Y.; Zhang, D.; Jin, C.; Hou, G.; Cao, H.; Wu, L.; Zheng, G.; et al. Magnetic Field-Suppressed Lithium Dendrite Growth for Stable Lithium-Metal Batteries. Adv. Energy Mater. 2019, 9, 1900260. [Google Scholar] [CrossRef]
- Liu, G.; Li, Y.; Zhang, L.; Tao, H.; Yang, X. In Situ Construction of PVA/LiF Composite Artificial Protective Layer to Assist Dendrite-Free Li Metal Anode. Appl. Surf. Sci. 2023, 620, 156809. [Google Scholar] [CrossRef]
- Shi, Y.; Li, B.; Zhu, Q.; Shen, K.; Tang, W.; Xiang, Q.; Chen, W.; Liu, C.; Luo, J.; Yang, S. MXene-Based Mesoporous Nanosheets Toward Superior Lithium Ion Conductors. Adv. Energy Mater. 2020, 10, 1903534. [Google Scholar] [CrossRef]
- Liu, Y.; Tao, R.; Chen, S.; Wu, K.; Zhong, Z.; Tu, J.; Guo, P.; Liu, H.; Tang, S.; Liang, J.; et al. A Novel Polyurethane-LiF Artificial Interface Protective Membrane as a Promising Solution towards High-Performance Lithium Metal Batteries. J. Power Sources 2020, 477, 228694. [Google Scholar] [CrossRef]
- Chen, Q.; Hou, Z.; Sun, Z.; Pu, Y.; Jiang, Y.; Zhao, Y.; He, H.; Zhang, T.; Huang, L. Polymer-Inorganic Composite Protective Layer for Stable Na Metal Anodes. ACS Appl. Energy Mater. 2020, 3, 2900–2906. [Google Scholar] [CrossRef]
- Fan, L.; Guo, Z.; Zhang, Y.; Wu, X.; Zhao, C.; Sun, X.; Yang, G.; Feng, Y.; Zhang, N. Stable Artificial Solid Electrolyte Interphase Films for Lithium Metal Anode via Metal-Organic Frameworks Cemented by Polyvinyl Alcohol. J. Mater. Chem. A 2020, 8, 251–258. [Google Scholar] [CrossRef]
- Wang, R.; Han, H.-H.; Liu, F.-Q.; Jia, S.-X.; Xiang, T.-Q.; Huo, H.; Zhou, J.-J.; Li, L. Sulfonated Poly (vinyl alcohol) as an Artificial Solid Electrolyte Interfacial Layer for Li Metal Anode. Electrochim. Acta 2022, 406, 139840. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, D.; Gao, Y.; Chen, T.; Huang, Q.; Wang, D. Stable Li Metal Anode by a Polyvinyl Alcohol Protection Layer via Modifying Solid-Electrolyte Interphase Layer. Nano Energy 2019, 64, 103893. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.; Jiao, X.; Guo, L.; Song, Z.; Xiong, S.; Song, J. LiXGe Containing Ion-Conductive Hybrid Skin for High Rate Lithium Metal Anode. Chem. Eng. J. 2019, 371, 294–300. [Google Scholar] [CrossRef]
- Woo, H.-S.; Son, H.; Min, J.-Y.; Rhee, J.; Lee, H.-T.; Kim, D.-W. Ionic Liquid-Based Gel Polymer Electrolyte Containing Zwitterion for Lithium-Oxygen Batteries. Electrochim. Acta 2020, 345, 136248. [Google Scholar] [CrossRef]
- D’Angelo, A.J.; Panzer, M.J. Decoupling the Ionic Conductivity and Elastic Modulus of Gel Electrolytes: Fully Zwitterionic Copolymer Scaffolds in Lithium Salt/Ionic Liquid Solutions. Adv. Energy Mater. 2018, 8, 1801646. [Google Scholar] [CrossRef]
- Li, G.; Guan, X.; Wang, A.; Wang, C.; Luo, J. Cations and Anions Regulation through Zwitterionic Gel Electrolytes for Stable Lithium Metal Anodes. Energy Storage Mater. 2020, 24, 574–578. [Google Scholar] [CrossRef]
- Jin, T.; Liu, M.; Su, K.; Lu, Y.; Cheng, G.; Liu, Y.; Li, N.W.; Yu, L. Polymer Zwitterion-Based Artificial Interphase Layers for Stable Lithium Metal Anodes. ACS Appl. Mater. Interfaces 2021, 13, 57489–57496. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.E.; Clarkson, D.; Greenbaum, S.G.; Panzer, M.J. Examining the Impact of Polyzwitterion Chemistry on Lithium Ion Transport in Ionogel Electrolytes. ACS Appl. Polym. 2021, 3, 2635–2645. [Google Scholar] [CrossRef]
- Chen, G.; Hu, O.; Lu, J.; Gu, J.; Chen, K.; Huang, J.; Hou, L.; Jiang, X. Highly Flexible and Adhesive Poly (vinyl alcohol)/Poly (acrylic amide-co-2-acrylamido-2-methylpropane sulfonic acid)/Glycerin Hydrogel Electrolyte for Stretchable and Resumable Supercapacitor. Chem. Eng. J. 2021, 425, 131505. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, Y.; Long, X.; Bao, W.; Zhang, Y.; Fan, W.; Cheng, H. Hydroxyl-Rich Single-Ion Conductors Enable Solid Hybrid Polymer Electrolytes with Excellent Compatibility for Dendrite-Free Lithium Metal Batteries. J. Membrane Sci. 2022, 657, 120666. [Google Scholar] [CrossRef]
- ASTM D1002-10(2019); Standard Test Method for Apparent Shear Strength of Single-Lap-Joint Adhesively Bonded Metal Specimens by Tension Loading (Metal-to-Metal). ASTM: West Conshohocken, PA, USA, 2019.
- Chen, C.-X.; Chen, S.-W.; Guo, Z.-H.; Chen, Z.-P.; Wang, J.-W.; Hu, J.-S.; Guo, J.; Yang, L.-Q. Highly Efficient Self-Healing Materials with Excellent Shape Memory and Unprecedented Mechanical Properties. J. Mater. Chem. A 2020, 8, 16203–16211. [Google Scholar] [CrossRef]
- Chen, C.-X.; Hou, Z.-P.; Chen, S.-W.; Guo, J.; Chen, Z.-P.; Hu, J.-S.; Yang, L.-Q. Photothermally Responsive Smart Elastomer Composites Based on Aliphatic Polycarbonate Backbone for Biomedical Applications. Composites Part B 2022, 240, 109985. [Google Scholar] [CrossRef]
- Lv, Y.; Xiao, Y.; Xu, S.; Huo, F.; Chen, Y.; Zhao, M.; Liu, L.; Su, C.; Zhu, Y.; Chen, S. Multifunctional Polyzwitterion Ionic Liquid Coating for Long-Lifespan and Dendrite-Free Zn Metal Anodes. J. Mater. Chem. A 2022, 10, 16952–16961. [Google Scholar] [CrossRef]
- Wu, L.Q.; Wang, S.; Mao, J.; Guo, Z.Y.; Hu, Y.F. Dual Network Zwitterionic Hydrogels with Excellent Mechanical Properties, Anti-Swelling, and Shape Memory Behaviors. Eur. Polym. J. 2023, 197, 112373. [Google Scholar] [CrossRef]
- Zhao, Q.; Stalin, S.; Archer, L.A. Stabilizing Metal Battery Anodes through the Design of Solid Electrolyte Interphases. Joule 2021, 5, 1119–1142. [Google Scholar] [CrossRef]
- Chen, X.; Tang, Z. Ultra-High Thermal Conductivity FGN/PVA/MXene Composite Films with Good Electrical Insulation. Compos. Sci. Technol. 2023, 242, 110208. [Google Scholar] [CrossRef]
- Busche, M.R.; Drossel, T.; Leichtweiss, T.; Weber, D.A.; Falk, M.; Schneider, M.; Reich, M.-L.; Sommer, H.; Adelhelm, P.; Janek, J. Dynamic Formation of a Solid-Liquid Electrolyte Interphase and its Consequences for Hybrid-Battery Concepts. Nat. Chem. 2016, 8, 426–434. [Google Scholar] [CrossRef]
- Sun, X.; Yang, S.; Zhang, T.; Shi, Y.; Dong, L.; Ai, G.; Li, D.; Mao, W. Regulating Li-Ion Flux with a High-Dielectric Hybrid Artificial SEI for Stable Li Metal Anodes. Nanoscale 2022, 14, 5033–5043. [Google Scholar] [CrossRef]
- Xu, R.; Xiao, Y.; Zhang, R.; Cheng, X.B.; Zhao, C.Z.; Zhang, X.Q.; Yan, C.; Zhang, Q.; Huang, J.Q. Dual-Phase Single-Ion Pathway Interfaces for Robust Lithium Metal in Working Batteries. Adv. Mater. 2019, 31, 1808392. [Google Scholar] [CrossRef]
- Khurana, R.; Schaefer, J.L.; Archer, L.A.; Coates, G.W. Suppression of Lithium Dendrite Growth Using Cross-Linked Polyethylene/Poly(ethylene oxide) Electrolytes: A New Approach for Practical Lithium-Metal Polymer Batteries. J. Am. Chem. Soc. 2014, 136, 7395–7402. [Google Scholar] [CrossRef]
- Jeong, D.; Shim, J.; Shin, H.; Lee, J.C. Sustainable Lignin-Derived Cross-Linked Graft Polymers as Electrolyte and Binder Materials for Lithium Metal Batteries. ChemSusChem 2020, 13, 2642–2649. [Google Scholar] [CrossRef]
- Pan, Q.; Smith, D.M.; Qi, H.; Wang, S.; Li, C.Y. Hybrid Electrolytes with Controlled Network Structures for Lithium Metal Batteries. Adv. Mater. 2015, 27, 5995–6001. [Google Scholar] [CrossRef] [PubMed]
- Bilici, C.; Ide, S.; Okay, O. Yielding Behavior of Tough Semicrystalline Hydrogels. Macromolecules 2017, 50, 3647–3654. [Google Scholar] [CrossRef]
- Shi, Y.; Wu, B.; Sun, S.; Wu, P. Aqueous Spinning of Robust, Self-Healable, and Crack-Resistant Hydrogel Microfibers Enabled by Hydrogen Bond Nanoconfinement. Nat. Commun. 2023, 14, 1370. [Google Scholar] [CrossRef]
- Bao, L.-X.; Zou, X.; Luo, X.; Pu, Y.-L.; Wang, J.-L.; Lei, J.-X. Real-Time Tracking the Li-Ion Transition Behavior and Dynamics in Solid Poly(vinyl alcohol)/LiClO+4 Electrolytes. Sci. Rep. 2017, 7, 45921. [Google Scholar] [CrossRef]
- Tai, T.; Gwang, H.; Junwon, L.; Min-Sik, P.; Janghyuk, M. Electrolyte Interface Design for Regulating Li Dendrite Growth in Rechargeable Li-metal Batteries: A Theoretical Study Liu. J. Power Sources 2021, 496, 229791. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, X.Q.; Cheng, X.B.; Peng, H.J.; Zhao, C.Z.; Yan, C.; Huang, J.Q. Artificial Soft-Rigid Protective Layer for Dendrite-Free Lithium Metal Anode. Adv. Funct. Mater. 2018, 28, 1705838. [Google Scholar] [CrossRef]
- Li, S.; Fan, L.; Lu, Y. Rational Design of Robust-Flexible Protective Layer for Safe Lithium Metal Battery. Energy Storage Mater. 2019, 18, 205–212. [Google Scholar] [CrossRef]
- Yue, H.; Huang, P.; Wei, J.; Shi, Z.; Chen, J.; Li, X.; Yin, Y.; Yang, S. Rational Design of a Robust Flexible Triblock Polyurea Copolymer Protective Layer for High-Performance Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2022, 14, 55735–55744. [Google Scholar] [CrossRef]
- Han, Y.; Fang, R.; Lu, C.; Wang, K.; Zhang, J.; Xia, X.; He, X.; Gan, Y.; Huang, H.; Zhang, W.; et al. LiF-Rich Interfacial Protective Layer Enables Air-Stable Lithium Metal Anodes for Dendrite-Free Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2023, 15, 31543–31551. [Google Scholar] [CrossRef]
- Zhao, B.; Xing, C.; Shi, Y.; Duan, Q.; Shen, C.; Li, W.; Jiang, Y.; Zhang, J. Construction of High Elastic Artificial SEI for Air-Stable and Long-Life Lithium Metal Anode. J. Colloid Interf. Sci. 2023, 642, 193–203. [Google Scholar] [CrossRef]
- Macarie, L.; Pekar, M.; Simulescu, V. Properties in Aqueous Solution of Homo and Copolymers of Vinylphosphonic Acid Derivatives Obtained by UV-curing. Macromol. Res. 2017, 25, 214–221. [Google Scholar] [CrossRef]

| Electrode Structures | Current (mA cm−2) | Capacity (mAh cm−2) | Coulombic Efficiency (Cycle) | References |
|---|---|---|---|---|
| Bare Cu | 0.5 | 1 | 105 | [25] |
| 1 | 1 | 50 | ||
| With PVA | 0.5 | 1 | 115 (+10%) | |
| 1 | 1 | 65 (+30%) | ||
| Cu foil | 1 | 1 | 150 | [29] |
| 3 | 1 | 75 | ||
| Cu/PVA | 1 | 1 | 210 (+40%) | |
| 3 | 1 | 110 (+47%) | ||
| Bare Cu | 0.5 | 1 | 80 | [30] |
| SPVA-Cu | 0.5 | 1 | 100 (+25%) |
| Electrode Structures | Current (mA cm−2) | Capacity (mAh cm−2) | Time (h) | Polarization (mV) | References |
|---|---|---|---|---|---|
| CPLi-4 | 0.5 | 2 | 1000 | 25 | [22] |
| PVA/LiF | 1 2 | 1 1 | 800 600 | 40 55 | [25] |
| APM | 1 1 | 0.5 1 | 1500 1000 | 100 300 | [27] |
| Zn-MOF/PVA | 1 3 | 1 1 | 580 300 | 51.7 42.1 | [29] |
| PDMAPS +PMPC | 1 3 | 1 1 | 1400 280 | 14 25 | [35] |
| APL | 2 | 1 | 200 | 175 | [57] |
| NLI | 1 1 | 1 3 | 1000 350 | 50 100 | [58] |
| PU | 1 3 | 1 3 | 1100 800 | 26 30.4 | [59] |
| LiF | 1 | 1 | 500 | 70 | [60] |
| TPU | 1 | 1 | 800 | 50 | [61] |
| PSM | 1 2 | 1 1 | 1200 800 | 15 28 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Yuan, W.; Chen, C.; Cao, R.; Niu, H.; Song, L.; Wang, J.; Shang, X. Artificial Interfacial Layers with Zwitterionic Ion Structure Improves Lithium Symmetric Battery Life and Inhibits Dendrite Growth. Symmetry 2025, 17, 652. https://doi.org/10.3390/sym17050652
Wang H, Yuan W, Chen C, Cao R, Niu H, Song L, Wang J, Shang X. Artificial Interfacial Layers with Zwitterionic Ion Structure Improves Lithium Symmetric Battery Life and Inhibits Dendrite Growth. Symmetry. 2025; 17(5):652. https://doi.org/10.3390/sym17050652
Chicago/Turabian StyleWang, Haihua, Wei Yuan, Chaoxian Chen, Rui Cao, Huizhu Niu, Ling Song, Jie Wang, and Xinyu Shang. 2025. "Artificial Interfacial Layers with Zwitterionic Ion Structure Improves Lithium Symmetric Battery Life and Inhibits Dendrite Growth" Symmetry 17, no. 5: 652. https://doi.org/10.3390/sym17050652
APA StyleWang, H., Yuan, W., Chen, C., Cao, R., Niu, H., Song, L., Wang, J., & Shang, X. (2025). Artificial Interfacial Layers with Zwitterionic Ion Structure Improves Lithium Symmetric Battery Life and Inhibits Dendrite Growth. Symmetry, 17(5), 652. https://doi.org/10.3390/sym17050652





