Homochirality Emergence: A Scientific Enigma with Profound Implications in Origins of Life Studies
Abstract
1. Introduction
What Is Meant by Chirality?
2. Possible Chiral Influence Form Space?
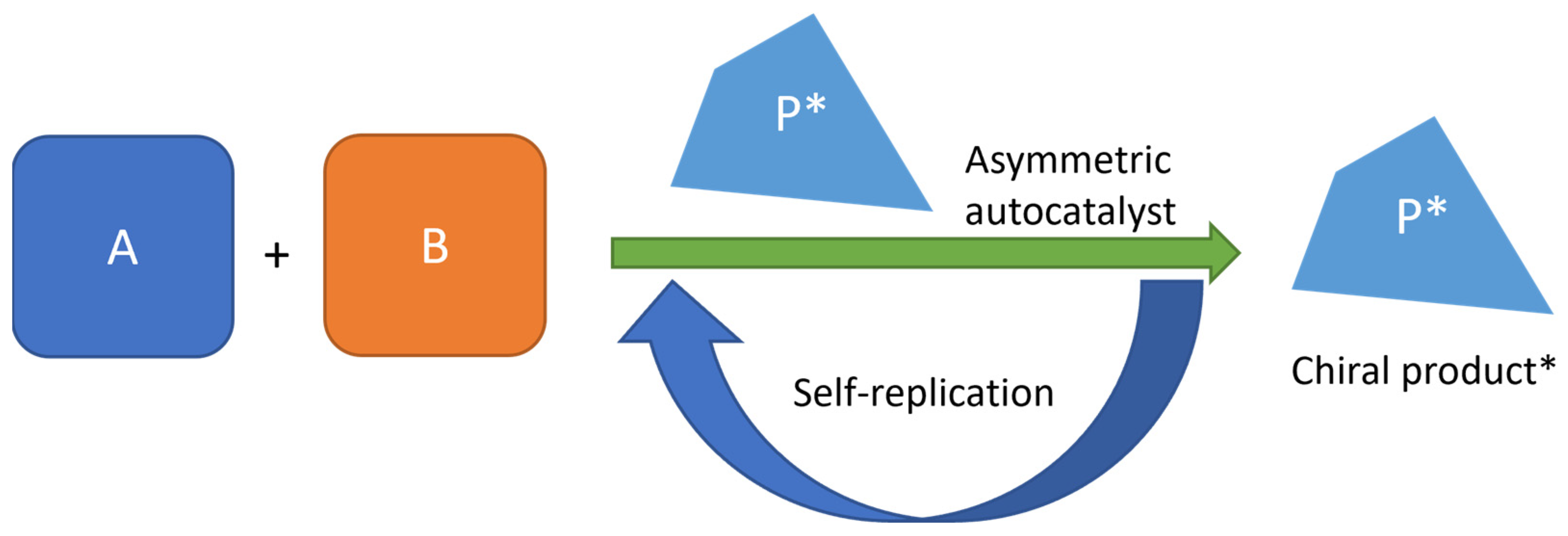
3. Autocatalytic Processes
- Amplifying Reaction Mechanisms: Certain reaction pathways exhibit a characteristic where the products generated serve to increase the rate or extent of the reaction itself. This self-reinforcing dynamic can result in a rapid escalation of product formation, potentially leading to a state of uncontrolled progression under specific environmental parameters.
- Regulatory Reaction Mechanisms: Conversely, other reaction pathways are characterized by a product-mediated deceleration. In these systems, the accumulation of reaction products acts to diminish the reaction rate, effectively establishing a self-limiting process. This type of regulatory control is frequently observed in complex systems, where it functions to maintain a stable internal state by preventing excessive product accumulation and ensuring system equilibrium.
3.1. Thermodinamic Limits
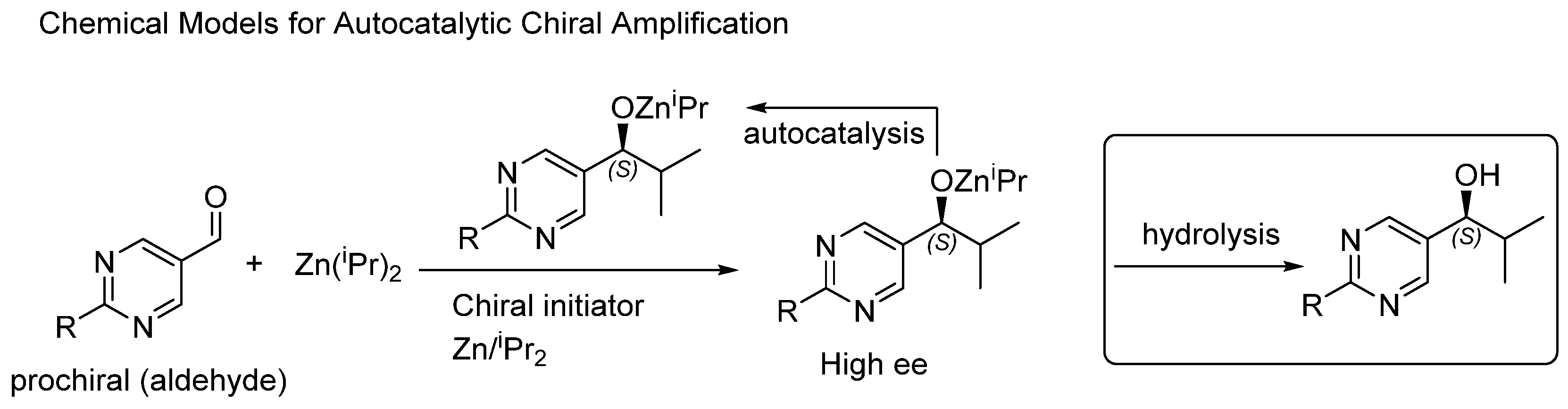
3.2. Models: One Mathematical and One Experimental
3.3. The Role of Autocatalysis in Evolution
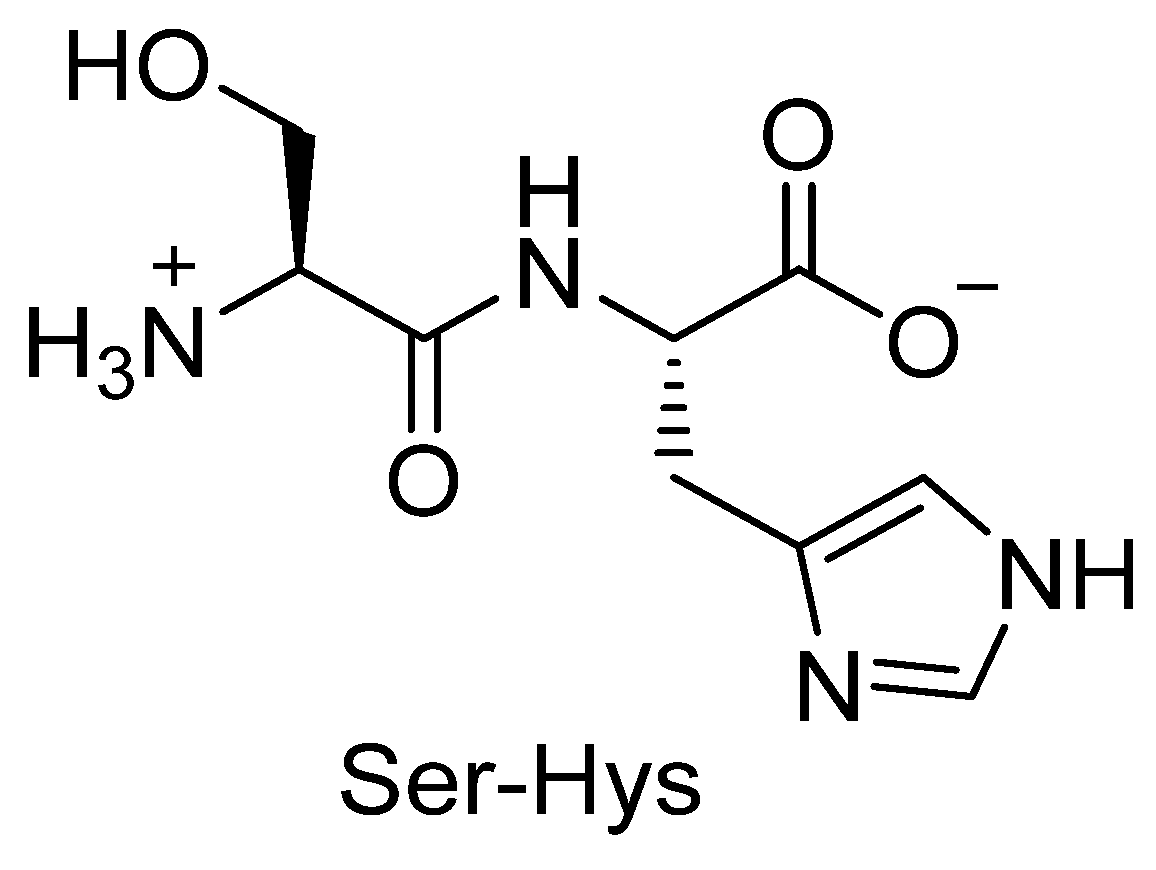
3.4. The Case of Seryl-Histidine
3.5. Biological Amplification: From Randomness to Selection
3.6. The Power of Chirality in Modern Science
4. Conclusions: The Continuing Mystery
Funding
Acknowledgments
Conflicts of Interest
References
- Chieffo, C.; Shvetsova, A.; Skorda, F.; Lopez, A.; Fiore, M. The Origin and Early Evolution of Life: Homochirality Emergence in Prebiotic Environments. Astrobiology 2023, 23, 1368–1382. [Google Scholar] [CrossRef]
- Bailey, J. Chirality and the Origin of Life. Acta Astronaut. 2000, 46, 627–631. [Google Scholar] [CrossRef]
- Byrne, S. Prebiotic Chemistry: Common Origins of Glycerol, Amino Acids, and Pyrimidines, and Cosmic Origin of Nature’s Enantiomeric Excess of Amino Acids. Trinity Student Scientific Review. Chemistry 2016, 2, 174–183. [Google Scholar]
- Hein, J.E.; Blackmond, D.G. On the Origin of Single Chirality of Amino Acids and Sugars in Biogenesis. Acc. Chem. Res. 2012, 45, 2045–2054. [Google Scholar] [CrossRef]
- Skorda, F.; Chieffo, C.; Fiore, M. Chemical Models for Understanding the Emergence of Homo-Chirality of Phospholipids for Origin of Life Studies. Symmetry 2022, 14, 2109. [Google Scholar] [CrossRef]
- Blackmond, D.G. The Origin of Biological Homochirality. Cold Spring Harb. Perspect. Biol. 2010, 2, a002147. [Google Scholar] [CrossRef]
- Skolnick, J.; Zhou, H.; Gao, M. On the Possible Origin of Protein Homochirality, Structure, and Biochemical Function. Proc. Natl. Acad. Sci. USA 2019, 116, 26571–26579. [Google Scholar] [CrossRef]
- Ozturk, S.F.; Sasselov, D.D. On the Origins of Life’s Homochirality: Inducing Enantiomeric Excess with Spin-Polarized Electrons. Proc. Natl. Acad. Sci. USA 2022, 119, e2204765119. [Google Scholar] [CrossRef]
- Kawasaki, T.; Soai, K. Amplification of Chirality as a Pathway to Biological Homochirality. J. Fluor. Chem. 2010, 131, 525–534. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, W. The Origin of Biological Homochirality along with the Origin of Life. PLoS Comput. Biol. 2020, 16, e1007592. [Google Scholar] [CrossRef]
- Soai, K.; Kawasaki, T.; Matsumoto, A. Role of Asymmetric Autocatalysis in the Elucidation of Origins of Homochirality of Organic Compounds. Symmetry 2019, 11, 694. [Google Scholar] [CrossRef]
- Fiore, M.; Buchet, R. Symmetry Breaking of Phospholipids. Symmetry 2020, 12, 1488. [Google Scholar] [CrossRef]
- Altamura, E.; Comte, A.; D’Onofrio, A.; Roussillon, C.; Fayolle, D.; Buchet, R.; Mavelli, F.; Stano, P.; Fiore, M.; Strazewski, P. Racemic Phospholipids for Origin of Life Studies. Symmetry 2020, 12, 1108. [Google Scholar] [CrossRef]
- Strazewski, P. The Relationship between Difference and Ratio and a Proposal: Equivalence of Temperature and Time, and the First Spontaneous Symmetry Breaking. J. Syst. Chem. 2013, 1, 11. [Google Scholar] [CrossRef]
- Strazewski, P. Omne Vivum Ex Vivo ⋯ Omne? How to Feed an Inanimate Evolvable Chemical System so as to Let It Self-Evolve into Increased Complexity and Life-like Behaviour. Isr. J. Chem. 2015, 55, 851–864. [Google Scholar] [CrossRef]
- Bocková, J.; Jones, N.C.; Hoffmann, S.V.; Meinert, C. The Astrochemical Evolutionary Traits of Phospholipid Membrane Homochirality. Nat. Rev. Chem. 2024, 8, 652–664. [Google Scholar] [CrossRef]
- Cahn, R.S. An Introduction to the Sequence Rule: A System for the Specification of Absolute Configuration. J. Chem. Educ. 1964, 41, 116. [Google Scholar] [CrossRef]
- Suh, I.-H.; Park, K.H.; Jensen, W.P.; Lewis, D.E. Molecules, Crystals, and Chirality. J. Chem. Educ. 1997, 74, 800. [Google Scholar] [CrossRef]
- Newman, M.S. A Notation for the Study of Certain Stereochemical Problems. J. Chem. Educ. 1955, 32, 344–347. [Google Scholar] [CrossRef]
- Cody, J.A.; Craig, P.A.; Loudermilk, A.D.; Yacci, P.M.; Frisco, S.L.; Milillo, J.R. Design and Implementation of a Self-Directed Stereochemistry Lesson Using Embedded Virtual Three-Dimensional Images in a Portable Document Format. J. Chem. Educ. 2012, 89, 29–33. [Google Scholar] [CrossRef]
- Mistry, N.; Singh, R.; Ridley, J. A Web-Based Stereochemistry Tool to Improve Students’ Ability to Draw Newman Projections and Chair Conformations and Assign R/S Labels. J. Chem. Educ. 2020, 97, 1157–1161. [Google Scholar] [CrossRef]
- Beauchamp, P.S. “Absolutely” Simple Stereochemistry. J. Chem. Educ. 1984, 61, 666. [Google Scholar] [CrossRef]
- Burrmann, N.J.; Moore, J.W. Implementation and Student Testing of a Web-Based, Student-Centered Stereochemistry Tutorial. J. Chem. Educ. 2015, 92, 1178–1187. [Google Scholar] [CrossRef]
- Strazewski, P. Low-Digit and High-Digit Polymers in the Origin of Life. Life 2019, 9, 17. [Google Scholar] [CrossRef]
- Boon, G.; Van Alsenoy, C.; De Proft, F.; Bultinck, P.; Geerlings, P. Study of Molecular Quantum Similarity of Enantiomers of Amino Acids. J. Phys. Chem. A 2006, 110, 5114–5120. [Google Scholar] [CrossRef]
- Garcia, A.D.; Meinert, C.; Finger, F.; Meierhenrich, U.J.; Hejl, E. Racemate Resolution of Alanine and Leucine on Homochiral Quartz, and Its Alteration by Strong Radiation Damage. Life 2021, 11, 1222. [Google Scholar] [CrossRef]
- Myrgorodska, I.; Meinert, C.; Martins, Z.; Le Sergeant D’Hendecourt, L.; Meierhenrich, U.J. Molecular Chirality in Meteorites and Interstellar Ices, and the Chirality Experiment on Board the ESA Cometary Rosetta Mission. Angew. Chem. Int. Ed. 2015, 54, 1402–1412. [Google Scholar] [CrossRef]
- Meinert, C.; Hoffmann, S.V.; Cassam-Chenaï, P.; Evans, A.C.; Giri, C.; Nahon, L.; Meierhenrich, U.J. Photonenergy-Controlled Symmetry Breaking with Circularly Polarized Light. Angew. Chem.—Int. Ed. 2014, 53, 210–214. [Google Scholar] [CrossRef]
- Janoschek, R. Chirality: From the Weak Boson to the a-Helix; Janoschek, R., Ed.; Springer: New York, NY, USA, 1991; ISBN 978-3-642-76569-8. [Google Scholar]
- Dadon, Z.; Wagner, N.; Ashkenasy, G. The Road to Non-Enzymatic Molecular Networks. Angew. Chem. Int. Ed. 2008, 47, 6128–6136. [Google Scholar] [CrossRef]
- Hordijk, W.; Steel, M. Autocatalytic Networks at the Basis of Life’s Origin and Organization. Life 2018, 8, 62. [Google Scholar] [CrossRef]
- Lancet, D.; Zidovetzki, R.; Markovitch, O. Systems Protobiology: Origin of Life in Lipid Catalytic Networks. J. R. Soc. Interface 2018, 15, 20180159. [Google Scholar] [CrossRef] [PubMed]
- Peretó, J. Out of Fuzzy Chemistry: From Prebiotic Chemistry to Metabolic Networks. Chem. Soc. Rev. 2012, 41, 5394–5403. [Google Scholar] [CrossRef] [PubMed]
- de la Escosura, A. The Informational Substrate of Chemical Evolution: Implications for Abiogenesis. Life 2019, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.W. An Alternative to the RNA World. Nat. Hist. 2016, 125, 28–33. [Google Scholar]
- Carter, C.W. What RNA World? Why a Peptide/Rna Partnership Merits Renewed Experimental Attention. Life 2015, 5, 294–320. [Google Scholar] [CrossRef]
- Gilbert, W. Origin of Life: The RNA World. Nature 1986, 319, 618. [Google Scholar] [CrossRef]
- Szilágyi, A.; Zachar, I.; Scheuring, I.; Kun, Á.; Könnyű, B.; Czárán, T. Ecology and Evolution in the RNA World Dynamics and Stability of Prebiotic Replicator Systems. Life 2017, 7, 48. [Google Scholar] [CrossRef]
- Lopez, A.; Fiore, M. Investigating Prebiotic Protocells for a Comprehensive Understanding of the Origins of Life: A Prebiotic Systems Chemistry Perspective. Life 2019, 9, 49. [Google Scholar] [CrossRef]
- Gaeta, G. Bifurcation and Symmetry Breaking. Phys. Rep. 1990, 189, 1–87. [Google Scholar] [CrossRef]
- Li, P.; Li, R.; Dai, C. Existence, Symmetry Breaking Bifurcation and Stability of Two-Dimensional Optical Solitons Supported by Fractional Diffraction. Opt. Express 2021, 29, 3193. [Google Scholar] [CrossRef]
- Frank, F.C. On Spontaneous Asymmetric Synthesis. Biochim. Biophys. Acta 1953, 11, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Plasson, R.; Bersini, H.; Commeyras, A. Recycling Frank: Spontaneous Emergence of Homochirality in Noncatalytic Systems. Proc. Natl. Acad. Sci. USA 2004, 101, 16733–16738. [Google Scholar] [CrossRef] [PubMed]
- Hud, N.V.; Anet, F.A.L. Intercalation-Mediated Synthesis and Replication: A New Approach to the Origin of Life. J. Theor. Biol. 2000, 205, 543–562. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Morioka, H.; Hayase, T.; Choji, K.; Soai, K. Highly Enantioselective Catalytic Asymmetric Automultiplication of Chiral Pyrimidyl Alcohol. J. Am. Chem. Soc. 1996, 118, 471–472. [Google Scholar] [CrossRef]
- Shibata, T.; Yamamoto, J.; Matsumoto, N.; Yonekubo, S.; Osanai, S.; Soai, K. Amplification of a Slight Enantiomeric Imbalance in Molecules Based on Asymmetric Autocatalysis: The First Correlation between High Enantiomeric Enrichment in a Chiral Molecule and Circularly Polarized Light. J. Am. Chem. Soc. 1998, 120, 12157–12158. [Google Scholar] [CrossRef]
- Micheau, J.-C.; Coudret, C.; Cruz, J.-M.; Buhse, T. Amplification of Enantiomeric Excess, Mirror-Image Symmetry Breaking and Kinetic Proofreading in Soai Reaction Models with Different Oligomeric Orders. Phys. Chem. Chem. Phys. 2012, 14, 13239. [Google Scholar] [CrossRef]
- Athavale, S.V.; Simon, A.; Houk, K.N.; Denmark, S.E. Demystifying the Asymmetry-Amplifying, Autocatalytic Behaviour of the Soai Reaction through Structural, Mechanistic and Computational Studies. Nat. Chem. 2020, 12, 412–423. [Google Scholar] [CrossRef]
- Blackmond, D.G. Mechanistic Study of the Soai Autocatalytic Reaction Informed by Kinetic Analysis. Tetrahedron Asymmetry 2006, 17, 584–589. [Google Scholar] [CrossRef]
- Geiger, Y. One Soai Reaction, Two Mechanisms? Chem. Soc. Rev. 2022, 51, 1206–1211. [Google Scholar] [CrossRef]
- Hawbaker, N.A.; Blackmond, D.G. Energy Threshold for Chiral Symmetry Breaking in Molecular Self-Replication. Nat. Chem. 2019, 11, 957–962. [Google Scholar] [CrossRef]
- Bonner, W.A. Enantioselective Autocatalysis. Spontaneous Resolution and the Prebiotic Generation of Chirality. Orig. Life Evol. Biosph. 1994, 24, 63–78. [Google Scholar] [CrossRef]
- Vasas, V.; Fernando, C.; Santos, M.; Kauffman, S.; Szathmáry, E. Evolution before Genes. Biol. Direct 2012, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Gorlero, M.; Wieczorek, R.; Adamala, K.; Giorgi, A.; Schininà, M.E.; Stano, P.; Luisi, P.L. Ser-His Catalyses the Formation of Peptides and PNAs. FEBS Lett. 2009, 583, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, R.; Adamala, K.; Gasperi, T.; Polticelli, F.; Stano, P. Small and Random Peptides: An Unexplored Reservoir of Potentially Functional Primitive Organocatalysts. The Case of Seryl-Histidine. Life 2017, 7, 19. [Google Scholar] [CrossRef]
- Foden, C.S.; Islam, S.; Fernández-García, C.; Maugeri, L.; Sheppard, T.D.; Powner, M.W. Prebiotic Synthesis of Cysteine Peptides That Catalyze Peptide Ligation in Neutral Water. Science 2020, 370, 865–869. [Google Scholar] [CrossRef]
- MacDonald, M.J.; Lavis, L.D.; Hilvert, D.; Gellman, S.H. Evaluation of the Ser-His Dipeptide, a Putative Catalyst of Amide and Ester Hydrolysis. Org. Lett. 2016, 18, 3518–3521. [Google Scholar] [CrossRef]
- Anet, F. AL The Place of Metabolism in the Origin of Life. Curr. Opin. Chem. Biol. 2004, 8, 654–659. [Google Scholar] [CrossRef]
- Bélières, M.; Chouini-Lalanne, N.; Déjugnat, C. Synthesis, Self-Assembly, and Catalytic Activity of Histidine-Based Structured Lipopeptides for Hydrolysis Reactions in Water. RSC Adv. 2015, 5, 35830–35842. [Google Scholar] [CrossRef]
- Blackmond, D.G. An Examination of the Role of Autocatalytic Cycles in the Chemistry of Proposed Primordial Reactions. Angew. Chem.—Int. Ed. 2009, 48, 386–390. [Google Scholar] [CrossRef]
- Joyce, G.F.; Schwartzt, A.W.; Millers, S.L.; Orgel, L.E. The Case for an Ancestral Genetic System Involving Simple Analogues of the Nucleotides (Chemical Evolution/Origin of Life/Prebiotic Chemistry/RNA Catalysis). Proc. Natl. Acad. Sci. USA 1987, 84, 4398–4402. [Google Scholar] [CrossRef]
- Ameta, S.; Matsubara, Y.J.; Chakraborty, N.; Krishna, S.; Thutupalli, S. Self-Reproduction and Darwinian Evolution in Autocatalytic Chemical Reaction Systems. Life 2021, 11, 308. [Google Scholar] [CrossRef] [PubMed]
- Pross, A. Toward a General Theory of Evolution: Extending Darwinian Theory to Inanimate Matter. J. Syst. Chem. 2011, 2, 1. [Google Scholar] [CrossRef]
- Meléndez-Hevia, E.; Montero-Gómez, N.; Montero, F. From Prebiotic Chemistry to Cellular Metabolism-The Chemical Evolution of Metabolism before Darwinian Natural Selection. J. Theor. Biol. 2008, 252, 505–519. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, J.; Wang, Y.; Chen, H. Emerging Chirality in Nanoscience. Chem. Soc. Rev. 2013, 42, 2930–2962. [Google Scholar] [CrossRef]
- Agrawal, Y.; Bhatt, H.; Raval, H.; Oza, P.; Gogoi, P. Chirality—A New Era of Therapeutics. Mini-Rev. Med. Chem. 2007, 7, 451–460. [Google Scholar] [CrossRef]
- Sung, Y.K.; Kim, S.W. Recent Advances in Polymeric Drug Delivery Systems. Biomater. Res. 2020, 24, 12. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiore, M. Homochirality Emergence: A Scientific Enigma with Profound Implications in Origins of Life Studies. Symmetry 2025, 17, 473. https://doi.org/10.3390/sym17030473
Fiore M. Homochirality Emergence: A Scientific Enigma with Profound Implications in Origins of Life Studies. Symmetry. 2025; 17(3):473. https://doi.org/10.3390/sym17030473
Chicago/Turabian StyleFiore, Michele. 2025. "Homochirality Emergence: A Scientific Enigma with Profound Implications in Origins of Life Studies" Symmetry 17, no. 3: 473. https://doi.org/10.3390/sym17030473
APA StyleFiore, M. (2025). Homochirality Emergence: A Scientific Enigma with Profound Implications in Origins of Life Studies. Symmetry, 17(3), 473. https://doi.org/10.3390/sym17030473





