Abstract
In order to expand the group of chiral thiourea structures, several optically active thioureas derived from the (S)-1-(2-pyridyl)ethylamine enantiomer were prepared via its reaction with achiral or optically active isothiocyanates. To show their synthetic potential as chiral auxiliaries the isolated thioureas were tested as an optically active organocatalyst in the asymmetric version of the selected aldol condensation and addition of diethylzinc to benzaldehyde. The observation of asymmetric induction in these model reactions encourages further research on the use of this group of thioureas in asymmetric versions of multicomponent reactions and cycloadditions. The mechanistic aspects of the reactions under study are also briefly discussed.
1. Introduction
The last 25 years, beginning with the independent publications of the first two publications on this topic by List [1] and McMillan [2] in 2000, brought with them an extremely rapid development of organocatalysis, both in basic and applied research, aimed at obtaining optically active compounds [3,4,5,6] that also exhibit biological activity [7,8,9,10]. Today, organocatalysis is a powerful and environmentally friendly tool for obtaining enantiomerically pure natural products [11], drugs [12,13] and substrates for use in the material chemistry [14,15,16,17,18,19,20]. One of the basic groups of organocatalysts are thiourea derivatives. Jacobsen [21], Schreiner [22] and Takemoto [23], who successfully carried out asymmetric C-C bond formation reactions, were pioneers in the synthesis and application of optically active thiourea catalysts. In recent years, such optically active thioureas have been successfully applied among other compounds in the Michael reaction [23], acylo-Pictet–Spengler reaction [24], aza-Morita–Baylis–Hillman reaction [25] and Mannich reaction [26]. The results of the latest research on this topic have been discussed in the three most recent publications [27,28,29]. After studying the above-mentioned literature describing the synthesis and application of thiourea catalysts, and having access to enantiomerically pure (S)-1-(2-pyridyl)-ethylamine 1 (now available according to a patent procedure developed in our laboratory [30]), we decided to check whether thiourea derivatives would be as effective as organocatalysts as a number of thioureas already described in the chemical literature. It is also our hope that the results discussed below will induce additional studies devoted to optically active derivatives of enantiomers of 1-(2-pyridyl)-ethylamine 1 in which the presence of a nitrogen atom in the aromatic ring should significantly modify the physicochemical properties of the entire molecule. It can therefore be expected that their enantiomers will exhibit, among other characteristics, “better” solvation and coordination properties, which should make them more effective chiral auxiliaries in asymmetric synthesis reactions than those described previously [31,32]. It should also be expected that the enantiomers of this amine can become useful building blocks in the synthesis of optically active materials with, for example, optoelectronic properties or compounds designed for pharmaceutical uses. The selected examples from the literature mentioned below fully confirm this assumption. For example, the catalytic (with a single Fe atom catalyst) amination of p-fluorophenyl methyl ketone with the dextrorotatory enantiomer of 1-(2-pyridyl)ethylamine 1 gave the secondary N-1-(2-pyridyl)ethyl-N’-1-(p-fluorophenyl)ethylamine as a diastereomeric mixture with dr 67:33 [33]. The bidentate N-coordinate aluminum complex of an imine derived from (−)-(S)- or (+)-(R)-1-(2-pyridyl)ethylamine 1 and 9-fluorenecarboxaldehyde and trimethyl aluminum was found to be a very effective catalyst for the asymmetric hydrophosphonylation of aldimines [34]. Chiral tri and tetranuclear Cd(II)clusters with luminiscent and ferroelectric properties were prepared by the reaction of Cd(ClO4)2 with imines derived from (−)-(S)-1-(2-pyridyl)ethylamine 1 and 2-hydroxy-1-naphtylaldehyde or from (+)-(R)-1-(2-pyridyl)ethylamine 1 and 5-bromo-2-hydroxy-3-methoxybenzaldehyde [35]. Similarly to other compounds containing a pyridine ring [36], the enantiomers of 1-(2-pyridino)ethylamine should exhibit biological activity that can modify the therapeutic properties of compounds in which this amine is a part of their molecular structure. This statement is supported, for example, by the use of the enantiomer of 1-(2-pyridino)ethylamine to modify the analog of 1,2,3,4-tetrahydroisoquinoline-based CXCR4 (a chemokine receptor present on the surface of many cancer cell types) antagonists [37]. Similarly, the dextrorotatory enantiomer of this amine was used to synthesize one of the intermediate products in the multigram synthesis of quillaic acid [38].
2. Results and Discussion
Our experiments began with an attempt to synthesize symmetric thiourea 2, a derivative of enantiomerically pure (S)-1-(2-pyridyl)-ethylamine 1. We planned to obtain it from the reaction of thiophosgene with two molar equivalents of an optically active amine 1 in the presence of two equivalents of triethylamine. Unfortunately, the experiments carried out in chloroform solution at 0 °C did not provide the expected thiourea 2. A spectroscopic analysis of the purified reaction product, isolated in 42% yield, showed a heterocyclic structure similar to derivative 3 (1-methyl-2H,3H-imidazo [1,5-a]pirydyne-3-tione—already described in the chemical literature) [39] (Scheme 1).
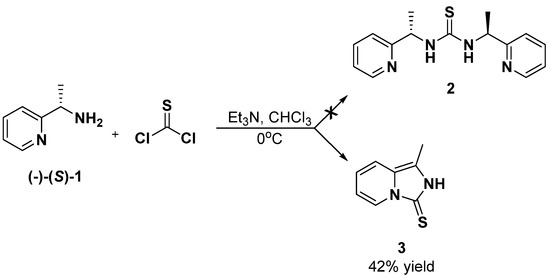
Scheme 1.
Reaction of (S)-1-(2-pyridyl)-ethylamine 1 with thiophosgene.
The unexpected formation of the heterocyclic derivative 3 can be explained on the basis of the proposed reaction mechanism described in Scheme 2. According to this proposal, in the first step of the reaction, the nucleophilic attack carried out by the aliphatic amino group of pyridylamine 1 on the thiocarbonyl carbon atom leads to the formation of a chloroamidothiocarbonate 4 as an intermediate. This intermediate is transformed into the final heterocyclic derivative 3 as a result of another nucleophilic attack carried out by the pyridine nitrogen atom on the thiocarbonyl carbon atom of chloroamidothiocarbonate 4, which generates another intermediate, 5 (Scheme 2).
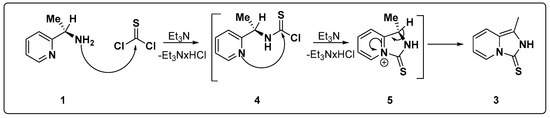
Scheme 2.
Proposed mechanism for the formation of a bicyclic heterocycle 3.
However, a series of unsymmetrical thioureas, 7a–f, containing enantiomerically pure (S)-1-(2-pyridyl)-ethylamine 1 in their structure were obtained as expected in the reaction of this pyridylamine enantiomer with corresponding isothiocyanates 6, which were commercially available (phenylisothiocyanate 6a, 1-isothiocyano-3,5-bis (trifluoromethyl) benzene 6b and naphthyl-1-isothiocyanate 6c) or obtained according to the procedure described in the literature [40], with appropriate amines using carbon disulfide and DCC (N,N’-dicyclohexylcarbodiimide)-[(1S)-1-isothiocyanoethyl)-benzene 6d, 1-[(1S)-1-isothiocyanoethyl) naphthalene 6e and (2S)-2-isothiocyanatobutane 6f (Scheme 3). All reactions carried out in diethyl ether at 0 °C provided thioureas 7a–f in good yields (77.2–92.1%) after chromatographic purification of the crude reaction products.
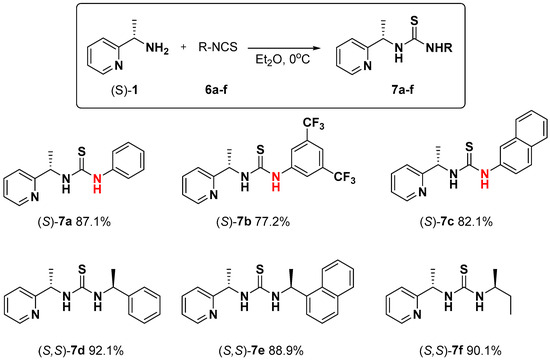
Scheme 3.
Synthesis of asymmetric thiourea catalysts 7a–f derived from the (S)-1-(2-pyridyl)-ethylamine 1.
The resulting optically active thioureas 7a–f were then used as chiral auxiliaries. The first model reaction was the addition of diethylzinc to benzaldehyde (Scheme 4). The results of the experiments carried out, which are summarized in Table 1, show that the reaction product, 1-phenylpropan-1-ol 8, was formed with low yields and was characterized by low enantiomeric excesses (ee) determined by HPLC with the use of chiral chromatographic columns. It is noteworthy that all reactions, regardless of the ligand 7 structure, led to the formation of an excess of the levorotatory enantiomer of alcohol 8 with the absolute configuration S [41].
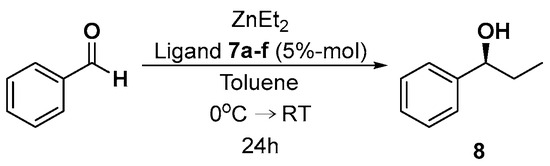
Scheme 4.
Stereoselective addition of diethylzinc to benzaldehyde, catalyzed by thioureas 7a–f.

Table 1.
The effect of catalysts 7a–f on the results of the addition reaction of diethylzinc to benzaldehyde.
In the context of these results, it will be interesting to test the use of thioureas 7 as a catalyst alongside the addition of other organometallic reagents, for example, Grignard reagents, which have been found to be added stereo-selectively in the presence of a catalytic amount of DPP-binol and excess titanium tetraisopropoxide [42,43].
The second model reaction tested was aldol condensation between acetone and 4-nitrobenzaldehyde catalyzed by ligands 7a–f and carried out in water solution in the presence of zinc trifluoroacetate for 72 h (Scheme 5, Table 2). The results summarized in the table below show that the condensation product, 4-hydroxy-4-(4-nitrophenyl)butan-2-one 9, was obtained in yields in the range of 10–42% and with enantiomeric excesses in the range of 14–35%. It is also noteworthy that all reactions, regardless of the ligand 7 structure, led to the formation of an excess of the levorotatory enantiomer of the ketoalcohol with the absolute configuration S [44]. These chemical yields and the observed enantiomeric excesses indicate that the thiourea derivatives of (S)-1-(2-pyridyl)-ethylamine 7a–f did not show as high catalytic activity as the analogs described in the literature [45]. The poor efficiency of the thiourea catalysts 7a–f based on the molecule (S)-1-(2-pyridyl)-ethylamine 1 is most likely due to the relatively low basicity of the pyridine nitrogen atom, which acts as a Brønsted’s base. This implies a less efficient interaction between the “source of chirality” and the organometallic species responsible for generating the new stereogenic center through the addition of an enol derived from acetone to 4-nitrobenzaldehyde. This assumption is supported by the fact that the addition of an external amine (triethylamine) to the reaction mixture improved the reaction yields (81%) but resulted in a simultaneous disappearance of the asymmetric induction (0% ee). In the context of these results, it is interesting to note that (S)-Pyrrolidine sulfonamide catalyzed asymmetric direct aldol reactions of aryl methyl ketones with aryl aldehydes [46].
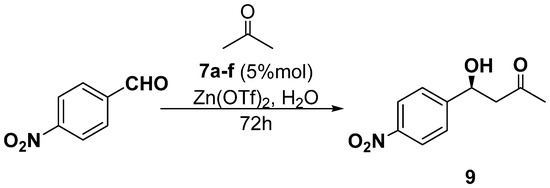
Scheme 5.
Aldol reaction between acetone and 4-nitrobenzaldehyde catalyzed by thioureas 7a–f.

Table 2.
The influence of catalysts 7a–f on the course of the aldol reaction between acetone and 4-nitrobenzaldehyde.
3. Conclusions
Optically active thioureas constitute a very useful family of organocatalysts with growing importance as chiral auxiliaries in asymmetric synthesis. In this paper we demonstrated that several unsymmetric thioureas derived from the ((S)-1-(2-pyridyl)-ethylamine 1 can be efficiently prepared by the reaction of this amine with the corresponding isothiocyanates. All reactions went smoothly and the isolation of products did not require any special procedures. The isolated thioureas were tested as a chiral catalyst in asymmetric version of two model reactions, namely the addition of diethylzinc do benzaldehyde and the aldol reaction of acetone with 4-nitrobenzaldehyde. The results obtained indicate that this new group of optically active thioureas did not show as high a catalytic activity as the analogs described in the literature. However, the observation of asymmetric induction in these model reactions encourages further research into the use of this group of thioureas in asymmetric versions of multicomponent reactions and cycloadditions.
4. Experimental Section
4.1. Materials and Methods
All reactions were carried out in standard glass apparatus and/or according to the Schlenk technique in glass vessels previously dried using a gun dryer. Solvents dried using standard procedures were degassed before use. All chemicals purchased from Aldrich or TCI were used as received. The procedures described in the literature [32] and in the patent developed in our laboratory [30] were used for the synthesis and isolation of the optically active enantiomer of the (S)-1-(2-pyridyl)-ethylamine 1. The reaction progress and purity of the product were determined by thin layer chromatography (TLC) on silica gel-coated plates [(Merck 60 F254) (40–63 or 230–400 mesh)] with the appropriately selected solvent mixture. All chromatographic purifications were carried out using a Merck 60 (40–63 or 230–400 mesh) silica gel-filled column. All NMR spectra were recorded for CDCl3 solutions (at a frequency of 200 or 500 MHz for 1H and 126 MHz for 13C NMR spectra). 1H NMR and 13C chemical shifts are given in ppm with respect to tetramethylsilane (TMS) as an internal standard. Coupling constant (J) values are reported in Hz. A Finningan MAT 95 spectrometer was used to determine all Mass Spectra (MS) measured using the chemical ionization technique. The enantiomeric excess (ee) values were determined by chiral HPLC [Lux 5µ Cellulose-1 (Phenomenex)].
1-Methyl-2H,3H-imidazo [1,5-a]pirydyne-3-tione 3
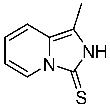
To a solution of (S)-1-(2-pyridyl)-ethylamine 1 (0.5667 g, 4.65 mmol) in CHCl3 (15 mL) which was cooled to 0 °C, thiophosgene (0.177 mL, 2.32 mmol) was added dropwise. The solution was stirred at 0 °C for 1 h and then for 24 h at room temperature. After completion of the reaction, a 5% NaHCO3 solution (20 mL) was added. The layers formed were separated, and the aqueous layer was extracted with CHCl3 (3 × 20 mL). The combined organic layers were dried over anhydrous Na2SO4, the drying agent was filtered off, and the filtrate was concentrated under reduced pressure. The residue was purified using column chromatography (CHCl3 100% → CHCl3:MeOH 50:1). A colorless oil (0.320 g, 42%) was obtained. This compound is described in the literature30.
1H NMR (500 MHz, CDCl3) δ 2.50 (s, 3H), 6.57 (t, 1H, J = 6.7 Hz), 6.71–7.20 (d, 1H, J = 9.4 Hz), 8.09 (d, 1H, J = 7.2 Hz); 13C NMR (126 MHz, CDCl3) δ 10.10, 107.04, 113.91, 126.50, 130.21, 130.63, 139.76, 161.62; MS (Cl) = 165.
4.2. General Procedure for the Synthesis of Asymmetric Thioureas 7a–f
Isothiocyanate 6 (1.0 eq.) was dissolved in Et2O (1 mmol/10 mL) and (S)-1-(2-pyridyl)-ethylamine 1 (1.0 eq.) was added dropwise to the reaction mixture at 0 °C. The resulting solution was stirred for 24 h at room temperature. After completion of the reaction, the reaction mixture was concentrated, and the residue was purified using column chromatography (Hexane:]EtOAc on gradient).
(S)-1-phenyl-3-(1-(pyridin-2-yl)ethyl)thiourea—7a
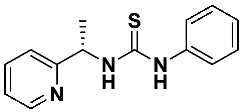
This thiourea was prepared according to the general procedure using (S)-1-(2-pyridyl)-ethylamine 1 (0.1821 g, 1.5 mmol) and phenylisothiocyanate 6a (0.18 mL, 1.5 mmol), respectively. The crude product was purified using column chromatography (Hexane: EtOAc 5:1 → 2:1). A white solid was obtained (0.336 g, 87.1%). 1H NMR (500 MHz, CD3OD) δ 1.52 (d, 3H, J = 6.9 Hz), 5.62 (q, 1H, J = 6.7 Hz), 7.09–7.49 (m, 7H), 7.78 (td, 1H, J = 7.7, 1.8 Hz), 8.47 (d, 1H, J = 4.9 Hz); 13C NMR (126 MHz, CD3OD) δ 16.98, 56.92, 120.71, 123.34, 123.50, 124.57, 128.30, 136.78, 139.30, 149.20, 163.98, 181.27; HRMS for C14H15N3S: calculated: 257.0987; determined: 257.0996; [α]D = + 40.0 (c 1.1, CHCl3).
(S)-1-(3,5-bis(trifluoromethyl)phenyl)-3-(1-(pyridin-2-yl)ethyl)thiourea—7b
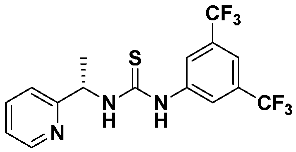
Thiourea 7b was obtained according to the general procedure, using, respectively, (S)-1-(2-pyridyl)-ethylamine 1 (0.238 g, 1.95 mmol) and 1-isothiocyano-3,5-bis (trifluoromethyl) benzene 6b (0.36 mL, 1.95 mmol). The crude product was purified using column chromatography (Hexane: EtOAc 10:1 → 1:1). A colorless oil was obtained (0.592 g, 77.2%). 1H NMR (500 MHz, CD3OD) δ 1.57 (d, 3H, J = 6.9 Hz), 5.62 (q, 1H, J = 6.8 Hz), 7.30 (dd, 1H, J = 7.5, 4.9 Hz), 7.43 (d, 1H, J = 7.9 Hz), 7.62 (s, 1H), 7.80 (td, 1H, J = 7.7, 1.7 Hz), 8.24 (s, 2H), 8.53 (d, 1H, J = 4.8 Hz).
13C NMR (126 MHz, CD3OD) δ 22.36, 56.31, 122.81, 123.50, 124.17, 126.30, 128.46, 132.76, 133.03, 133.16 (q, JC-F = 33.4 Hz, CF3), 139.14, 143.64, 150.33, 163.36, 182.46; 19F NMR (188 MHz, CD3OD) δ −63.85; HRMS for C16H13F6N3S: calculated: 393.0734; determined: 393.0724; [α]D = + 3.75 (c 1.2, CHCl3).
(S)-1-(naphthalen-1-yl)-3-(1-(pyridin-2-yl)ethyl)thiourea—7c
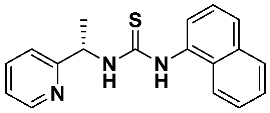
Thiourea 7c was obtained according to the general procedure, using, respectively, (S)-1-(2-pyridyl)-ethylamine 1 (0.2216 g, 1.82 mmol) and naphthyl-1-isothiocyanate 6c (0.3535 g, 1.82 mmol). The crude product was purified using column chromatography (Hexane: EtOAc 5:1 → 2:1). A colorless oil (0.459 g, 82.1%) was obtained. 1H NMR (500 MHz, CD3OD) δ 1.44 (d, 3H, J = 6.9 Hz), 5.66 (q, 1H, J = 7.0 Hz), 7.15–7.29 (m, 2H), 7.34 (d, 1H, J = 7.7 Hz), 7.44–7.62 (m, 5H), 7.75 (t, 1H, J = 7.0 Hz), 7.92 (t, 3H, J = 6.9 Hz), 8.33 (s, 1H); 13C NMR (126 MHz, CD3OD) δ 17.20, 57.81, 113.84, 120.40, 121.51, 121.64, 123.34, 125.13, 125.79, 126.00, 126.27, 128.27, 134.19, 136.31, 136.78, 149.20, 163.88, 181.19; HRMS for C18H17N3S: calculated: 307.1143; determined: 307.1156; [α]D = + 24.1 (c 1.1, CHCl3).
1-((S)-1-phenylethyl)-3-((S)-1-(pyridin-2-yl)ethyl)thiourea—7d
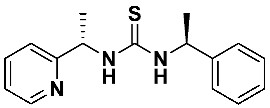
Thiourea 7d was prepared according to the general procedure using, respectively, (S)-1-(2-pyridyl)-ethylamine 1 (0.3319 g, 2.72 mmol) and (1S)-1-isothiocyanoethyl) benzene 6d (0.4435 g, 2.72 mmol). The crude product was purified using column chromatography (Hexane: EtOAc 3:1 → 1:1). A colorless oil was obtained (0.714 g, 92.1%). 1H NMR (500 MHz, C6D6) δ 1.19 (d, 3H, J = 6.7 Hz), 1.52 (d, 3H, J = 6.3 Hz), 5.19 (q, 1H, J = 6.7 Hz), 5.67 (q, 1H, J = 6.3 Hz), 6.45–6.68 (m, 2H), 6.90 (td, 1H, J = 7.7, 1.7 Hz), 6.98–7.28 (m, 6H), 7.40 (d, 1H, J = 5.2 Hz), 8.11 (d, 1H, J = 4.6 Hz); 13C NMR (126 MHz, C6D6) δ 18.01, 22.33, 55.38, 56.72, 120.78, 123.34, 126.30, 127.20, 128.56, 136.78, 143.47, 149.20, 164.08, 181.16; HRMS for C16H19N3S: calculated: 285.1300; determined: 285.1296; [α]D = + 90.1 (c 1.1, CHCl3).
1-((S)-1-(naphthalen-1-yl)ethyl)-3-((S)-1-(pyridin-2-yl)ethyl)thiourea—7e
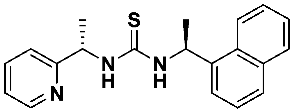
Thiourea 7e was prepared according to the general procedure using, respectively, (S)-1-(2-pyridyl)-ethylamine 1 (0.0915 g, 0.75 mmol) and 1-[(1S)-1-isothiocyanoethyl) naphthalene 6e (0.1597 g, 0.75 mmol). The crude product was purified using column chromatography (Hexane: EtOAc 5:1 → 2:1). A yellow oil was obtained (0.223 g, 88.9%). 1H NMR (200 MHz, C6D6) δ 1.42 (d, 3H, J = 6.1 Hz), 1.53 (d, 3H, J = 5.4 Hz), 5.53 (q, 1H, J = 6.1 Hz), 6.08–6.46 (m, 2H), 6.61 (s, 1H), 6.82 (t, 1H, J = 7.6 Hz), 6.99–7.37 (m, 4H), 7.56 (dt, 4H, J = 14.1, 6.6 Hz), 7.87 (d, 1H, J = 6.0 Hz, 1H), 8.24 (d, 1H, J = 5.8 Hz); 13C NMR (126 MHz, C6D6) δ 18.24, 22.54, 56.05, 56.76, 120.47, 123.34, 124.04, 125.25, 125.76, 126.86, 127.39, 128.46, 129.02, 132.02, 133.20, 136.78, 143.74, 149.20 164.02, 181.19; HRMS for C20H21N3S: calculated: 335.1456; determined: 335.1461; [α]D = + 67.8 (c 1.1, CHCl3).
1-((S)-sec-butyl)-3-((S)-1-(pyridin-2-yl)ethyl)thiourea—7f
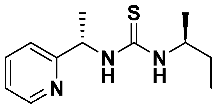
Thiourea 7f was prepared according to the general procedure using, respectively, (S)-1-(2-pyridyl)-ethylamine 1 (0.2469 g, 2.0 mmol) and (2S)-2-isothiocyanatobutane 6f (0.2327 g, 2.0 mmol). The crude product was purified using column chromatography (Hexane:EtOAc 5:1 → 2:1). A yellow oil was obtained (0.427 g, 90.1%). 1H NMR (500 MHz, C6D6) δ 0.70 (t, 3H, J = 7.4 Hz), 1.05 (d, 3H, J = 6.5 Hz), 1.23–1.51 (m, 5H), 4.34 (q, 1H, J = 6.1 Hz), 5.62 (bs, 1H), 6.63–6.79 (m, 1H), 6.98 (d, 1H, J = 7.7 Hz, 1H), 7.07–7.26 (m, 2H), 8.01 (d, J = 5.9 Hz, 1H), 8.27 (d, J = 4.5 Hz, 1H); 13C NMR (126 MHz, C6D6) δ 10.38, 17.19, 19.66, 29.76, 51.77, 57.08, 121.49, 123.34, 136.78, 149.17, 164.11, 180.63; HRMS for C12H19N3S: calculated: 237.1300; determined: 237.1312; [α]D = + 12.9 (c 1.2, CHCl3).
4.3. Asymmetric Addition of Diethylzinc to Benzaldehyde
The chiral catalysts, 7a–f (0.1 mmol), were placed in a round bottom flask and dissolved in toluene (5 mL). After cooling the reaction mixture to 0 °C, a solution of diethylzinc (3.0 mL, 3 mmol, C = 1M in hexane) was added under argon. After 30 min, benzaldehyde (0.1 mL, 1.0 mmol) was added. The resulting solution was stirred at room temperature for 24 h. After completion of the reaction, 5% aqueous HCl (10 mL) was added, the layers formed were separated, and the aqueous layer was extracted with Et2O (4 × 10 mL). The combined organic layers were dried over anhydrous MgSO4. The drying agent was removed by filtration and the filtrate was concentrated on an evaporator. The crude product 8 was purified using column chromatography (Hexane: EtOAc 20:1 → 5:1). 1 H NMR (200 MHz, CDCl3) δ 0.90 (t, 3H, J = 7.4 Hz), 1.68–1.86 (m, 2H), 2.04 (br s, 1H), 4.57 (dd, 1H, J = 6.5 Hz), 7.24–7.36 (m, 5H). The enantiomeric excess (ee) was determined by HPLC using a chiral packed column: Lux 5µ Cellulose-1 (Phenomenex), Hexane: iPrOH 70:30, tR1 = 4.9 min, t R2 = 8.6 min. Details of all experiments are summarized in Table 1.
4.4. Asymmetric Aldol Condensation
Catalyst 7a–f (0.025 mmol) and zinc triflate (0.025 mmol) were placed in a round bottom flask. After dissolving in acetone–water mixture (1.8 mL: 0.1 mL), the resulting solution was stirred for 15 min, and then 4-nitrobenzaldehyde (0.5 mmol) was added. Stirring was continued at room temperature for 24 h. After completion of the reaction, the product was purified using column chromatography (Hexane: EtOAc 20:1 → 5:1). 1H NMR (200 MHz, CDCl3) δ 2.21 (s, 3H), 2.83 (m, 2H), 3.56 (d, 1H, J = 3.2 Hz), 5.25 (br s, 1H), 7.52 (d, 2H, J = 7.0 Hz), 8.20 (d, 2H, J = 7.0 Hz). The enantiomeric excess (ee) was determined by HPLC using a chiral packed column: Lux 5µ Cellulose-1 (Phenomenex), Hexane:iPrOH 70:30, tR1 = 7.5 min, tR2 = 9.6 min). Details of all experiments are summarized in Table 2.
Author Contributions
Conceptualization, J.D and J.C., Methodology, J.D. and J.C.; Investigation, J.C., M.D., M.R. and L.S., Resources, J.D., Writing—Original Draft Preparation, J.D. and L.S., Writing—Review and Editing, J.D. and L.S., Supervision, J.D.; Project Administration, J.D. and J.C.; Funding Acquisition, J.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Center, Poland fund awarded based on the decision UMO-2012/06/A/ST5/00227 (MAESTRO to J.D.).
Data Availability Statement
Data supporting reported results can be found in Ph.D. thesis of J.C.H. (Publicly accessible from the Centre of Molecular and Macromolecular Studies, Polish Academy of Sciences, Lodz Library, (Tylna 1, 90-363 Lodz, Poland).
Acknowledgments
The authors are grateful for spectroscopic laboratories of the Center of Molecular and Macromolecular Studies PAS for technical assistance in measuring NMR spectra and mass spectra.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- List, B.; Lerner, R.A.; Barbas, C.F. Proline-Catalyzed Direct Asymmetric Aldol Reactions. J. Am. Chem. Soc. 2000, 122, 2395–2396. [Google Scholar] [CrossRef]
- Ahrendt, K.A.; Borths, C.J.; MacMillan, D.W.C. New Strategies for Organic Catalysis: The First Highly Enantioselective Organocatalytic Diels–Alder Reaction. J. Am. Chem. Soc. 2000, 122, 4243–4244. [Google Scholar] [CrossRef]
- Reetz, M.T.; List, B.; Jaroch, S.; Weinmann, H. (Eds.) Organocatalysis; Ernst Schering Foundation Symposium Proceedings; Springer: Berlin/Heidelberg, Germany, 2008; Volume 2007/2. [Google Scholar] [CrossRef]
- List, B. (Ed.) Asymmetric Organocatalysis; Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2009; Volume 291. [Google Scholar] [CrossRef]
- Dalko, P.I. (Ed.) Comprehensive Enantioselective Organocatalysis; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013. [Google Scholar] [CrossRef]
- Berkessel, A.; Gröger, H. Asymmetric Organocatalysis; Wiley: New York, NY, USA, 2005. [Google Scholar] [CrossRef]
- Pellissier, H. Asymmetric Organocatalytic Tandem/Domino Reactions to Access Bioactive Products. Curr. Org. Chem. 2021, 25, 1457–1471. [Google Scholar] [CrossRef]
- Carlone, A.; Bernardi, L. Enantioselective Organocatalytic Approaches to Active Pharmaceutical Ingredients—Selected Industrial Examples. Phys. Sci. Rev. 2019, 4, 401–434. [Google Scholar] [CrossRef]
- Hughes, D.L. Asymmetric Organocatalysis in Drug Development—Highlights of Recent Patent Literature. Org. Process Res. Dev. 2018, 22, 574–584. [Google Scholar] [CrossRef]
- Ardevines, S.; Marqués-López, E.; Herrera, R.P. Horizons in Asymmetric Organocatalysis: En Route to the Sustainability and New Applications. Catalysts 2022, 12, 101. [Google Scholar] [CrossRef]
- Grondal, C.; Jeanty, M.; Enders, D. Organocatalytic Cascade Reactions as a New Tool in Total Synthesis. Nat. Chem. 2010, 2, 167–178. [Google Scholar] [CrossRef]
- Howell, G.P. Asymmetric and Diastereoselective Conjugate Addition Reactions: C–C Bond Formation at Large Scale. Org. Process Res. Dev. 2012, 16, 1258–1272. [Google Scholar] [CrossRef]
- Simmons, E.M.; Mudryk, B.; Lee, A.G.; Qiu, Y.; Razler, T.M.; Hsiao, Y. Development of a Kilogram-Scale Process for the Enantioselective Synthesis of 3-Isopropenyl-Cyclohexan-1-One via Rh/DTBM-SEGPHOS-Catalyzed Asymmetric Hayashi Addition Enabled by 1,3-Diol Additives. Org. Process Res. Dev. 2017, 21, 1659–1667. [Google Scholar] [CrossRef]
- Dalko, P.I.; Moisan, L. Enantioselective Organocatalysis. Angew. Chem. Int. Ed. 2001, 40, 3726–3748. [Google Scholar] [CrossRef]
- Dalko, P.I.; Moisan, L. In the Golden Age of Organocatalysis. Angew. Chem. Int. Ed. 2004, 43, 5138–5175. [Google Scholar] [CrossRef] [PubMed]
- Houk, K.N.; List, B. Asymmetric Organocatalysis. Acc. Chem. Res. 2004, 37, 487. [Google Scholar] [CrossRef]
- List, B.; Yang, J.W. The Organic Approach to Asymmetric Catalysis. Science 2006, 313, 1584–1586. [Google Scholar] [CrossRef]
- Pellissier, H. Asymmetric Organocatalysis. Tetrahedron 2007, 63, 9267–9331. [Google Scholar] [CrossRef]
- Dondoni, A.; Massi, A. Asymmetric Organocatalysis: From Infancy to Adolescence. Angew. Chem. Int. Ed. 2008, 47, 4638–4660. [Google Scholar] [CrossRef] [PubMed]
- Melchiorre, P.; Marigo, M.; Carlone, A.; Bartoli, G. Asymmetric Aminocatalysis-Gold Rush in Organic Chemistry. Angew. Chem. Int. Ed. 2008, 47, 6138–6171. [Google Scholar] [CrossRef] [PubMed]
- Sigman, M.S.; Jacobsen, E.N. Schiff Base Catalysts for the Asymmetric Strecker Reaction Identified and Optimized from Parallel Synthetic Libraries. J. Am. Chem. Soc. 1998, 120, 4901–4902. [Google Scholar] [CrossRef]
- Schreiner, P.R.; Wittkopp, A. H-Bonding Additives Act Like Lewis Acid Catalysts. Org. Lett. 2002, 4, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Okino, T.; Hoashi, Y.; Takemoto, Y. Enantioselective Michael Reaction of Malonates to Nitroolefins Catalyzed by Bifunctional Organocatalysts. J. Am. Chem. Soc. 2003, 125, 12672–12673. [Google Scholar] [CrossRef]
- Taylor, M.S.; Jacobsen, E.N. Highly Enantioselective Catalytic Acyl-Pictet–Spengler Reactions. J. Am. Chem. Soc. 2004, 126, 10558–10559. [Google Scholar] [CrossRef]
- Abermil, N.; Masson, G.; Zhu, J. Highly Enantioselective Aza Morita–Baylis–Hillman Reaction Catalyzed by Bifunctional β-Isocupreidine Derivatives. J. Am. Chem. Soc. 2008, 130, 12596–12597. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Shih, H.-W.; Deng, L. Asymmetric Mannich Reactions with in Situ Generation of Carbamate-Protected Imines by an Organic Catalyst. Org. Lett. 2007, 9, 603–606. [Google Scholar] [CrossRef]
- Vera, S.; Garcia-Urricelquui, A.; Mielgo, A. Oriabide Progress in (Thio)urea- and Squaramide-Based Brønsted Base Catalysts with Multiple H-Bond Donors. Eur. J. Org. Chem. 2023, 26, e202201254. [Google Scholar] [CrossRef]
- Steppeler, F.; Iwan, D.; Wojaczynska, E.; Wojaczynski, J. Chiral Thioureas-Preparation and Significance in Asymmetric Synthesis. Molecules 2020, 25, 401. [Google Scholar] [CrossRef]
- Gandhi, S.; Sivadas, V.; Baire, B. Thio-urea-Amine Promoted Cascade Catalysis: A Tool for Complexity generation. Eur. J. Org. Chem. 2021, 2021, 220–234. [Google Scholar] [CrossRef]
- Drabowicz, J.; Kłos, M.; Chrzanowski, J. Sposób Rozdzielania Racemicznych 1-(2 lub 3)-pirydyloetyloamin na Enancjomery. Polish Patent P-411027, 13 May 2021. [Google Scholar]
- Brunner, H.; Fisch, H. Asymmetrische Katalysen, 39. Mitt.: Monohydrosilane in der enantioselektiven katalytischen Hydrosilylierung prochiraler Ketone. Monatshefte Chem./Chem. Mon. 1988, 119, 525–532. [Google Scholar] [CrossRef]
- Brunner, H.; Fisch, H. Asymmetrische katalysen XXXVI. Neue mehrzähnige liganden mit dem (S)-(α)-(2-Pyridyl)ethylrest; Rh-komplexe und enantioselektive hydrosilylierungen. J. Organomet. Chem. 1987, 335, 1–14. [Google Scholar] [CrossRef]
- Ma, Z.; Kuloor, C.; Kreyenschulte, C.; Bartling, S.; Malina, O.; Haumann, M.; Menezes, P.W.; Zboril, R.; Beller, M.; Jagadeesh, R.V. Development of iron- based single atom materials for general a nd efficient synthesis of amines. Angew. Chem. Int. Ed. 2024, 63, e20240785. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Wang, C.; Miao, H.; Qin, Z.; Huang, M.; Xu, Y.; Xue, W.; Yang, S.; Liu, C.; Bai, C.; et al. Novel bidentate N-coordinated alkylaluminum complexes: Synthesis, characterization, andefficient catalysis for hydrophosphonylation. Dalton Trans. 2024, 53, 4186–4193. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.-R.; Qi, T.-T.; Liu, S.-J.; Liu, C.-M.; Tang, Y.-Z.; Chen, J.-L. Syntheses and structures of chiral tri- and tetranuclear Cd(II) clusters with luminescent and ferroelectric properties. Polyhedron 2015, 85, 894–899. [Google Scholar] [CrossRef]
- Marinescu, M.; Popa, C.-V. Pyridine compounds with antimicrobial and antivarius activity. Int. J. Mol. Sci. 2022, 23, 5650. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.J.; Jecs, E.; Miller, E.J.; Nguyen, H.H.; Tahirovic, Y.A.; Truax, V.M.; Kim, M.B.; Kuo, K.M.; Wang, T.; Sum, C.S.; et al. Synthesis and SAR of 1,2,3,4-Tetrahydroisoquinoline-Based CXCR4 Antagonists. ACS Med. Chem. Lett. 2018, 9, 17–22. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Chen, C.-R.; Chen, C.Y.; Liang, P.-H. Synthesis of Quillaic Acid through Sustainable C–H Bond Activations. J. Org. Chem. 2024, 89, 5491–5497. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.Y.; Lee, G.H.; No, Z.S. Direct Access to Various 1-Substituted-Imidazo[1,5-a]Pyridine-3(2H)-Thione Derivatives. Bull. Korean Chem. Soc. 2007, 28, 685–688. [Google Scholar] [CrossRef]
- Ferreira, R.B.; Tormena, C.F.; Almeida, W.P. Synthesis and Spectroscopic Analysis of Substituted 2-Aminothiazolines. J. Mol. Struct. 2013, 1037, 186–190. [Google Scholar] [CrossRef]
- Leśniak, S.; Rachwalski, M.; Sznajder, E.; Kiełbasiński, P. New highly efficient aziridine-functionalized tridentate sulfinyl catalysts for enantioselective diethylzinc addition to carbonyl compounds. Tetrahedron Asymmetry 2009, 20, 2311–2314. [Google Scholar] [CrossRef]
- Muramatsu, Y.; Harada, T. Catalytic Asymmetric Alkylation of Aldehydes with Grignard Reagents. Angew. Chem. Int. Ed. 2008, 47, 1088. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Tomihama, M.; Yamamoto, K.; Matsubara, N.; Harada, T.J. Method for Catalytic Enantioselective Alkylation of Aldehydes Using Grignard Reagents as Alkyl Sources. J. Org. Chem. 2018, 83, 6127. [Google Scholar] [CrossRef] [PubMed]
- Rachwalski, M.; Leśniak, S.; Kiełbasiński, P. Highly enantioselective asymmetric direct aldol reaction catalyzed by amine-functionalized tridentate sulfinyl ligands. Tetrahedron Asymmetry 2011, 22, 1325–1327. [Google Scholar] [CrossRef]
- Fotaras, S.; Kokotos, C.G.; Kokotos, G. A Tripeptide-like Prolinamide-Thiourea as an Aldol Reaction Catalyst. Org. Biomol. Chem. 2012, 10, 5613. [Google Scholar] [CrossRef]
- Mei, K.; Zhang, S.; He, S.; Li, P.; Jin, M.; Xue, F.; Luo, G.; Zhang, H.; Song, L.; Duan, W.; et al. (S)-Pyrrolidine sulfonamide catalyzed asymmetric direct aldol reactions of aryl methyl ketones with aryl aldehydes. Tetrahedron Lett. 2008, 49, 2681–2685. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).