Synthesis of α-Hydroxyethylphosphonates and α-Hydroxyethylphosphine Oxides: Role of Solvents During Optical Resolution
Abstract
1. Introduction
2. Materials and Methods
2.1. General
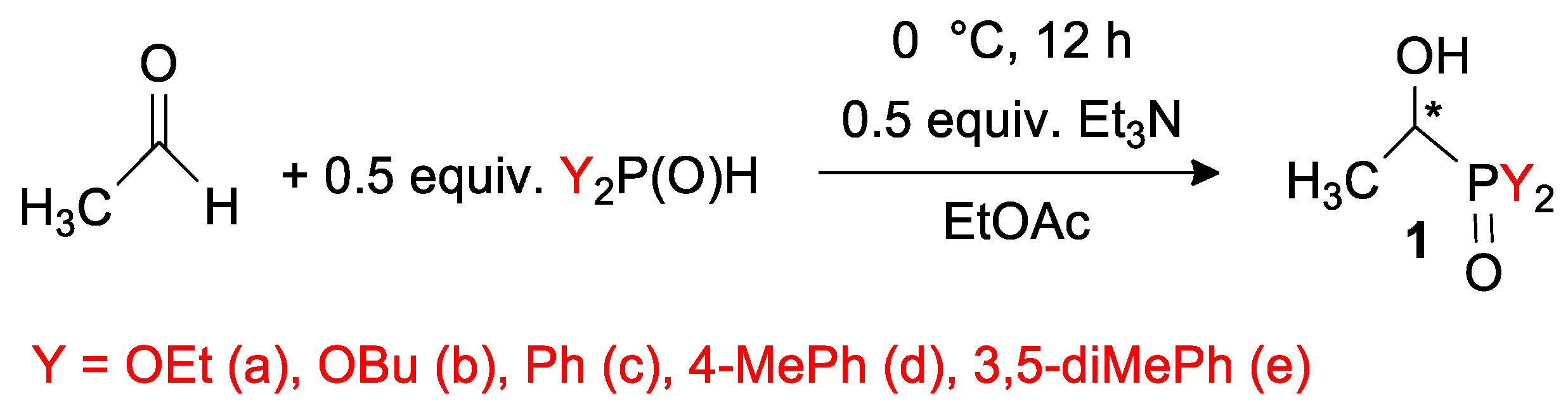
2.2. General Procedure for the Synthesis of Dialkyl α-Hydroxyethylphosphonates (1a,b) or α-Hydroxyethyl-Diarylphosphine Oxides (1c–e)
2.2.1. Diethyl α-Hydroxyethylphosphonate (1a)
2.2.2. Dibutyl α-Hydroxyethylphosphonate (1b)
2.2.3. α-Hydroxyethyl-Diphenylphosphine Oxide (1c)
2.2.4. α-Hydroxyethyl-Bis(4-Methylphenyl)phosphine Oxide (1d)
2.2.5. α-Hydroxyethyl-Bis(3,5-Dimethylphenyl)phosphine Oxide (1e)
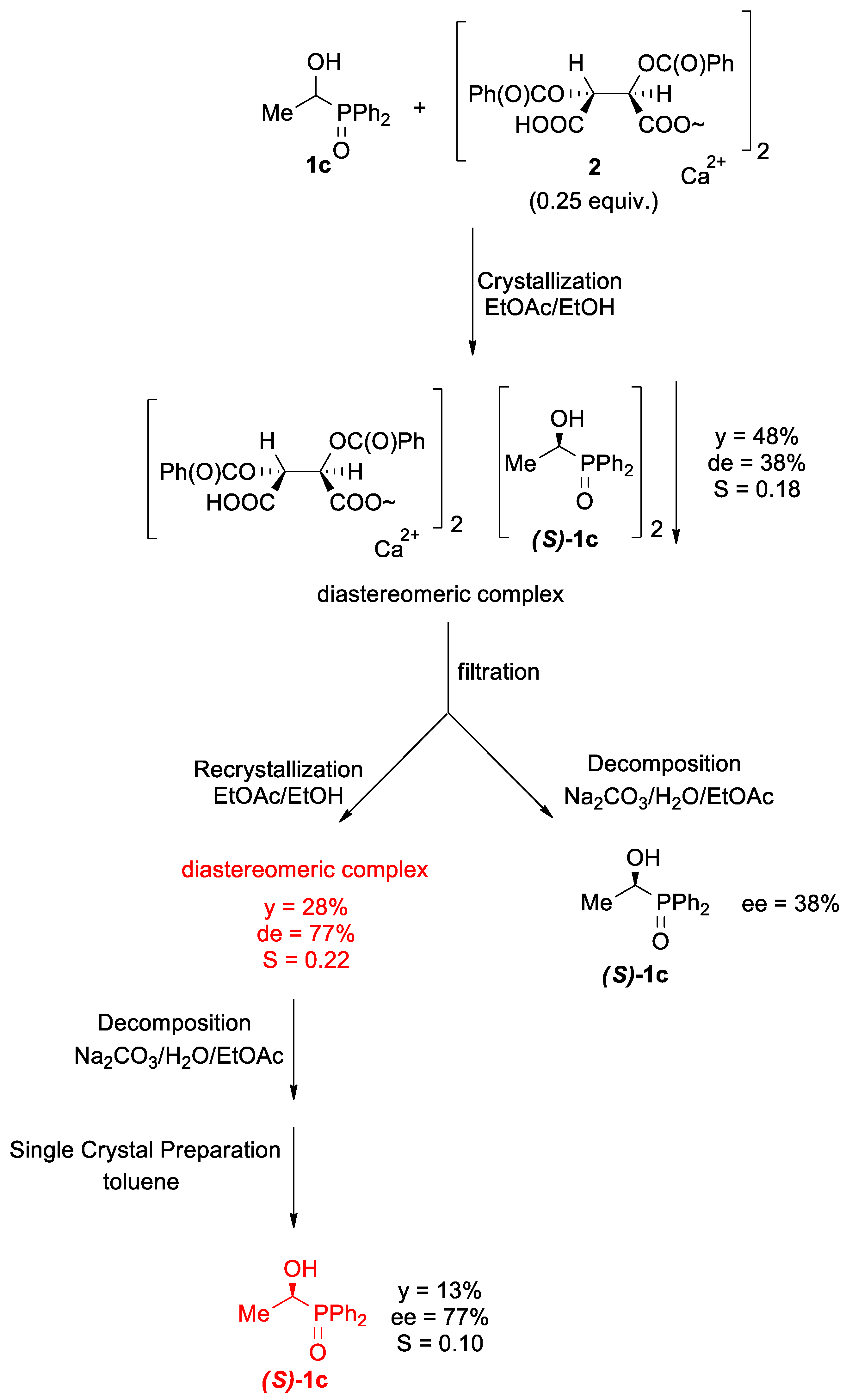
2.3. Procedure for the Preparation of Optically Active Ca[(S)-1c • H-DBTA]2 Complex
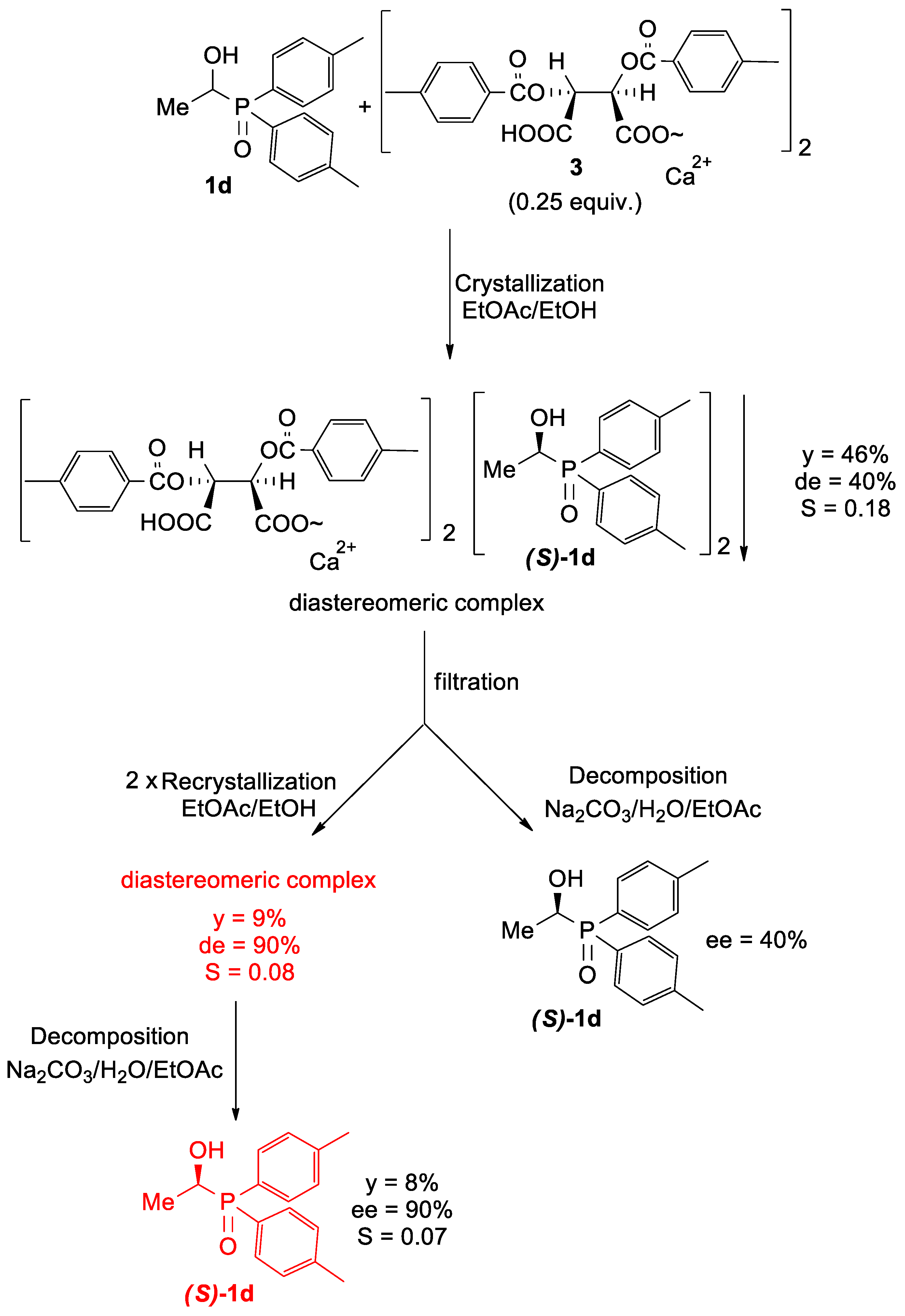
2.4. Procedure for the Preparation of Optically Active Ca[(S)-1d • H-DpTTA]2 Complex and Optically Active (S)-1d
2.5. Procedure for the Preparation of the Single Crystals of Optically Active (S)-1c
2.6. Single-Crystal X-Ray Diffraction Studies
3. Results and Discussion
3.1. Preparation of Racemic Compounds
3.2. Optical Resolution of Hydroxyphosphine Oxides 1c–e
3.3. X-Ray Analysis of the Optically Active 1c

4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Patel, D.V.; Rielly-Gauvin, K.; Ryono, D.E.; Free, C.A.; Rogers, W.L.; Smith, S.A.; DeForrest, J.M.; Oehl, R.S.; Petrillo, E.W. Alpha-hydroxy phosphinyl-based inhibitors of human renin. J. Med. Chem. 1995, 38, 4557–4569. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Mao, H.; Shi, D. Synthesis and herbicidal activity of α-hydroxy phosphonate derivatives containing pyrimidine moiety. Chin. J. Chem. 2010, 28, 2020–2024. [Google Scholar] [CrossRef]
- Pokalwar, R.U. Synthesis and antibacterial activities of α-hydroxyphosphonates and α-acetyloxyphosphonates derived from 2-chloroquinoline-3-carbaldehyde. Arkivoc 2006, 11, 196–204. [Google Scholar] [CrossRef]
- Kategaonkar, A.H.; Pokalwar, R.U.; Sonar, S.S.; Gawali, V.U.; Shingate, B.B.; Shingare, M.S. Synthesis, in vitro antibacterial and antifungal evaluations of new α-hydroxyphosphonate and new α-acetoxyphosphonate derivatives of tetrazolo [1, 5-a] quinoline. Eur. J. Med. Chem. 2010, 45, 1128–1132. [Google Scholar] [CrossRef]
- Sampath, C.; Raju, C.N.; Rao, C.V. An efficient synthesis, spectral characterization, anti-microbial and anti-oxidant activities of novel α-hydroxyphosphonates and α-hydroxyphosphinates. Phosphorus Sulfur Silicon Relat. Elem. 2015, 191, 95–99. [Google Scholar] [CrossRef]
- Rádai, Z.; Windt, T.; Nagy, V.; Füredi, A.; Kiss, N.Z.; Randelovic, I.; Tóvári, J.; Keglevich, G.; Szakács, G.; Tóth, S. Synthesis and anticancer cytotoxicity with structural context of an α-hydroxyphosphonate based compound library derived from substituted benzaldehydes. New J. Chem. 2019, 43, 14028–14035. [Google Scholar] [CrossRef]
- Kalla, R.M.N.; Lee, H.R.; Cao, J.; Yoo, J.-W.; Kim, I. Phospho sulfonic acid: An efficient and recyclable solid acid catalyst for the solvent-free synthesis of α-hydroxyphosphonates and their anticancer properties. New J. Chem. 2015, 39, 3916–3922. [Google Scholar] [CrossRef]
- Blaser, H.-U. Chirality and its implications for the pharmaceutical industry. Rend. Fis. Acc. Lincei 2013, 24, 213–216. [Google Scholar] [CrossRef]
- Nguyen, L.A.; He, H.; Pham-Huy, C. Chiral drugs: An overview. Int. J. Biomed. Sci. 2006, 2, 85–100. [Google Scholar]
- Gröger, H.; Vogl, E.M.; Shibasaki, M. New catalytic concepts for the asymmetric aldol reaction. Chem. Eur. J. 1998, 4, 1137–1141. [Google Scholar] [CrossRef]
- Kolodiazhnyi, O.I. Chiral hydroxy phosphonates: Synthesis, configuration and biological properties. Russ. Chem. Rev. 2006, 75, 227–253. [Google Scholar] [CrossRef]
- Kolodiazhnyi, O.I. Asymmetric synthesis of hydroxyphosphonates. Tetrahedron Asymmetry 2005, 16, 3295–3340. [Google Scholar] [CrossRef]
- Kozma, D. CRC Handbook of Optical Resolutions via Diastereomeric Salt Formation; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Fogassy, E.; Nógrádi, M.; Kozma, D.; Egri, G.; Pálovics, E.; Kiss, V. Optical resolution methods. Org. Biomol. Chem. 2006, 4, 3011–3030. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, R. Racemization, Optical Resolution and Crystallization-Induced Asymmetric Transformation of Amino Acids and Pharmaceutical Intermediates. In Novel Optical Resolution Technologies; Springer: Berlin/Heidelberg, Germany, 2007; pp. 83–132. [Google Scholar]
- Faigl, F.; Schindler, J.; Fogassy, E. Advantages of Structural Similarities of the Reactants in Optical Resolution Processes. In Novel Optical Resolution Technologies; Springer: Berlin/Heidelberg, Germany, 2007; pp. 133–157. [Google Scholar]
- Murakami, H. From Racemates to Single Enantiomers—Chiral Synthetic Drugs over the last 20 Years. In Novel Optical Resolution Technologies; Springer: Berlin/Heidelberg, Germany, 2007; pp. 273–299. [Google Scholar]
- Kolodyazhnaya, A.O.; Kukhar, V.P.; Kolodyazhnyi, O.I. Organic catalysis of phospha-aldol condensation. Russ. J. Gen. Chem. 2008, 78, 2043–2051. [Google Scholar] [CrossRef]
- Hirashima, S.-I.; Arai, R.; Nakashima, K.; Kawai, N.; Kondo, J.; Koseki, Y.; Miura, T. Asymmetric hydrophosphonylation of aldehydes using a cinchona-diaminomethylenemalononitrile organocatalyst. Adv. Synth. Catal. 2015, 357, 3863–3867. [Google Scholar] [CrossRef]
- Uraguchi, D.; Ito, T.; Ooi, T. Generation of chiral phosphonium dialkyl phosphite as a highly reactive P-nucleophile: Application to asymmetric hydrophosphonylation of aldehydes. J. Am. Chem. Soc. 2009, 131, 3836–3837. [Google Scholar] [CrossRef]
- Abell, J.P.; Yamamoto, H. Catalytic enantioselective pudovik reaction of aldehydes and aldimines with tethered bis (8-quinolinato) (TBOx) aluminum complex. J. Am. Chem. Soc. 2008, 130, 10521–10523. [Google Scholar] [CrossRef]
- Deng, T.; Cai, C. Bis (oxazoline)-copper catalyzed enantioselective hydrophosphonylation of aldehydes. RSC Adv. 2014, 4, 27853–27856. [Google Scholar] [CrossRef]
- Muthupandi, P.; Sekar, G. Synthesis of an unusual dinuclear chiral iron complex and its application in asymmetric hydrophosphorylation of aldehydes. Org. Biomol. Chem. 2012, 10, 5347. [Google Scholar] [CrossRef]
- Sun, L.; Guo, Q.-P.; Li, X.; Zhang, L.; Li, Y.-Y.; Da, C.-S. C2-symmetric homobimetallic zinc complexes as chiral catalysts for the highly enantioselective hydrophosphonylation of aldehydes. Asian J. Org. Chem. 2013, 2, 1031–1035. [Google Scholar] [CrossRef]
- Guin, J.; Wang, Q.; van Gemmeren, M.; List, B. The catalytic asymmetric abramov reaction. Angew. Chem. Int. Ed. 2015, 54, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Goulioukina, N.S.; Bondarenko, G.N.; Bogdanov, A.V.; Gavrilov, K.N.; Beletskaya, I.P. Asymmetric hydrogenation of α-keto phosphonates with chiral palladium catalysts. Eur. J. Org. Chem. 2009, 2009, 510–515. [Google Scholar] [CrossRef]
- Nesterov, V.; Kolodiazhnyi, O. New method for the asymmetric hydroboration of ketophosphonates and the synthesis of phospho-carnitine. Tetrahedron Asymmetry 2006, 17, 1023–1026. [Google Scholar] [CrossRef]
- Nesterov, V.; Kolodiazhnyi, O. New method for the asymmetric reduction of ketophosphonates. Phosphorus Sulfur Silicon Relat. Elem. 2008, 183, 687–688. [Google Scholar] [CrossRef]
- Skropeta, D.; Schmidt, R.R. Chiral, non-racemic α-hydroxyphosphonates and phosphonic acids via stereoselective hydroxylation of diallyl benzylphosphonates. Tetrahedron Asymmetry 2003, 14, 265–273. [Google Scholar] [CrossRef]
- Pogatchnik, D.M.; Wiemer, D.F. Enantioselective synthesis of α-hydroxy phosphonates via oxidation with (camphorsulfonyl)oxaziridines. Tetrahedron Lett. 1997, 38, 3495–3498. [Google Scholar] [CrossRef]
- Kafarski, P.; Lejczak, B. Application of bacteria and fungi as biocatalysts for the preparation of optically active hydroxyphosphonates. J. Mol. Catal. B Enzym. 2004, 29, 99–104. [Google Scholar] [CrossRef]
- Pàmies, O.; Bäckvall, J.-E. An efficient route to chiral α- and β-hydroxyalkanephosphonates. J. Org. Chem. 2003, 68, 4815–4818. [Google Scholar] [CrossRef]
- Li, Y.-F.; Hammerschmidt, F. Enzymes in organic chemistry, part 1: Enantioselective hydrolysis of α-(acyloxy)phosphonates by esterolytic enzymes. Tetrahedron Asymmetry 1993, 4, 109–120. [Google Scholar] [CrossRef]
- Demizu, Y.; Moriyama, A.; Onomura, O. Nonenzymatic kinetic resolution of racemic α-hydroxyalkanephosphonates with chiral copper catalyst. Tetrahedron Lett. 2009, 50, 5241–5244. [Google Scholar] [CrossRef]
- Kaboudin, B.; Alavi, S.; Kazemi, F.; Aoyama, H.; Yokomatsu, T. Resolution of racemic α-hydroxyphosphonates: Bi(OTF)3-catalyzed stereoselective esterification of α-hydroxyphosphonates with (+)-dibenzoyl-l-tartaric anhydride. ACS Omega 2019, 4, 15471–15478. [Google Scholar] [CrossRef] [PubMed]
- Rádai, Z.; Kiss, N.Z.; Czugler, M.; Karaghiosoff, K.; Keglevich, G. The typical crystal structures of a few representative α-aryl-α-hydroxyphosphonates. Acta Crystallogr. Sect. C Struct. Chem. 2019, 75, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Rádai, Z.; Bagi, P.; Czugler, M.; Karaghiosoff, K.; Keglevich, G. Optical Resolution of Dimethyl α-Hydroxy-Arylmethylphosphonates via Diastereomer Complex Formation Using Calcium Hydrogen O,O′-Dibenzoyl-(2R,3R)-Tartrate; X-Ray Analysis of the Complexes and Products. Symmetry 2020, 12, 758. [Google Scholar] [CrossRef]
- Bagi, P.; Ujj, V.; Czugler, M.; Fogassy, E.; Keglevich, G. Resolution of P-stereogenic P-heterocycles via the formation of diastereomeric molecular and coordination complexes (a review). Dalton Trans. 2016, 45, 1823–1842. [Google Scholar] [CrossRef] [PubMed]
- Varga, B.; Herbay, R.; Székely, G.; Holczbauer, T.; Madarász, J.; Mátravölgyi, B.; Fogassy, E.; Keglevich, G.; Bagi, P. Scalable Enantiomeric Separation of Dialkyl-Arylphosphine Oxides Based on Host–Guest Complexation with TADDOL Derivatives, and their Recovery. Eur. J. Org. Chem. 2020, 2020, 1840–1852. [Google Scholar] [CrossRef]
- Ujj, V.; Schindler, J.; Novák, T.; Czugler, M.; Fogassy, E.; Keglevich, G. Coordinative resolution of 1-phenyland 1-naphthyl-3-methyl-3-phospholene 1-oxides with calcium hydrogen O,O′-dibenzoyl-(2R,3R)-tartrate or calcium hydrogen O,O′-di-p-toluyl-(2R,3R)-tartrate. Tetrahedron Asymmetry 2008, 19, 1973–1977. [Google Scholar] [CrossRef]
- Zhao, Z.; Xue, W.; Gao, Y.; Tang, G.; Zhao, Y. Copper-Catalyzed Synthesis of α-Hydroxy Phosphonates from H-Phosphonates and Alcohols or Ethers. Chem. Asian J. 2013, 8, 713–716. [Google Scholar] [CrossRef]
- Mary, F.; Arrachart, G.; Leydier, A.; Pellet-Rostaing, P. Synthesis of organophosphorus ligands with a central oxygen atom and their applications in solvent extraction. Tetrahedron 2019, 75, 3968–3976. [Google Scholar] [CrossRef]
- Emoto, T.; Gomi, H.; Yoshifuji, M.; Okazaki, R.; Inamoto, N. Metal Phosphinylides and Phosphinothioylides. I. Formation of Metal Diphenylphosphinylides and Diphenylphosphinothioylides and the Reactions with Some Organic Halides and Aldehydes. Bull. Chem. Soc. Jpn. 1974, 47, 2449–2452. [Google Scholar] [CrossRef]
- Stankevic, M.; Pisklak, J.; Wlodarczyk, K. Aryl group—A leaving group in arylphosphine oxides. Tetrahedron 2016, 72, 810–824. [Google Scholar] [CrossRef]
- Li, B.; Liu, M.; Rehman, S.U.; Li, C. Rh-Catalyzed Regio- and Enantioselective Allylic Phosphinylation. J. Am. Chem. Soc. 2022, 144, 2893–2898. [Google Scholar] [CrossRef] [PubMed]
- Program Package. CrysAlisPro 1.171.40.82a; Rigaku Oxford Diffraction: The Woodlands, TX, USA, 2020. [Google Scholar]
- Sheldrick, G.M. SHELXS-97; Program for Crystal Structure Solution; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. SHELXL-97; Program for the Refinement of Crystal Structures; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Spek, A.L. PLATON; A Multipurpose Crystallographic Tool; Utrecht University: Utrecht, The Netherlands, 1999. [Google Scholar]
- Wang, W.; Zhang, S.S.; Zhou, Y.; Peng, H.; He, H.W.; Lu, X.T. Synthesis and Herbicidal Activity of α-(Substituted Phenoxybutyryloxy or Valeryloxy)alkylphosphonates and 2-(Substituted Phenoxybutyryloxy)alkyl-5,5-dimethyl-1,3,2-dioxaphosphinan-2-one. J. Agric. Food Chem. 2016, 64, 6911–6915. [Google Scholar] [CrossRef] [PubMed]
- Keglevich, G.; Rádai, Z.; Kiss, N.Z. To date the greenest method for the preparation of α-hydroxyphosphonates from substituted benzaldehydes and dialkyl phosphites. Green Process. Synth. 2017, 6, 197–201. [Google Scholar] [CrossRef]
- Sakai, K.; Sakurai, R.; Hirayama, N. Molecular Mechanisms of Dielectrically Controlled Resolution (DCR). In Novel Optical Resolution Technologies. Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2007; Volume 269. [Google Scholar] [CrossRef]
- Toda, F. Optical resolutions by inclusion complexation with a chiral host compound. In Enantiomer Separation; Springer: Berlin/Heidelberg, Germany, 2004; pp. 1–47. [Google Scholar]
- Tanaka, K.; Honke, S.; Urbanczyk-Lipkowska, Z.; Toda, F. New Chiral Hosts Derived from Dimeric Tartaric Acid: Efficient Optical Resolution of Aliphatic Alcohols by Inclusion Complexation. Eur. J. Org. Chem. 2000, 2000, 3171–3176. [Google Scholar] [CrossRef]
- Bálint, J.; Egri, G.; Czugler, M.; Schindler, J.; Kiss, V.; Juvancz, Z.; Fogassy, E. Resolution of α-phenylethylamine by its acidic derivatives. Tetrahedron Asymmetry 2001, 12, 1511–1518. [Google Scholar] [CrossRef]
- Bereczki, L.; Bombicz, P.; Bálint, J.; Egri, G.; Schindler, J.; Pokol, G.; Fogassy, E.; Marthi, K. Optical resolution of 1-(1-naphthyl)ethylamine by its dicarboxylic acid derivatives: Structural features of the oxalic acid derivative diastereomeric salt pair. Chirality 2009, 21, 331–338. [Google Scholar] [CrossRef]
- Bálint, J.; Schindler, J.; Egri, G.; Hánusz, M.; Marthi, K.; Juvancz, Z.; Fogassy, E. Resolution of methyl-1-phenylethylamines by acidic derivatives of 1-phenylethylamine. Tetrahedron Asymmetry 2004, 15, 3401–3405. [Google Scholar] [CrossRef]
- Tiekink, E.R.T.; Zukerman-Schpector, J. The Importance of Pi-Interactions in Crystal Engineering: Frontiers in Crystal Engineering; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Lemouzy, S.; Nguyen, D.H.; Camy, V.; Jean, M.; Gatineau, D.; Giordano, L.; Naubron, J.-V.; Vanthuyne, N.; Herault, D.; Buono, G. Stereospecific Synthesis of α- and β-Hydroxyalkyl P-Stereogenic Phosphine–Boranes and Functionalized Derivatives: Evidence of the P=O Activation in the BH3-Mediated Reduction. Chem. Eur. J. 2015, 21, 15607–15621. [Google Scholar] [CrossRef]
- Mo, X.; Xie, Y.; Wei, L.; Gu, X.; Zhang, M.; Zhang, X.; Jiang, J. Visible-Light-Induced Carbene Insertion into P–H Bonds between Acylsilanes and H-Phosphorus Oxides. Org. Lett. 2023, 25, 2338–2343. [Google Scholar] [CrossRef]
- Liu, W.-Y.; Huo, P.; Huang, T.-L.; Mei, G.-Q. (2,4-Dichlorophenyl)(diphenylphosphoryl)methanol. Acta Cryst. 2010, 66, o477. [Google Scholar] [CrossRef]
- Lan, K.; Chen, X.-L. Crystal structure of (diphenylphosphinoyl)phenylmethanol, C19H17O2P. Z. Für Krist. New Cryst. Struct. 2006, 221, 171–172. [Google Scholar] [CrossRef][Green Version]
- Priya, S.; Balakrishna, M.S.; Mague, J.T.; Mobin, S.M. Insertion of Carbon Fragments into P(III)−N Bonds in Aminophosphines and Aminobis(phosphines): Synthesis, Reactivity, and Coordination Chemistry of Resulting Phosphine Oxide Derivatives. Crystal and Molecular Structures of (Ph2P(O)CH2)2NR (R = Me, nPr, nBu), Ph2P(O)CH(OH)nPr, and cis-[MoO2Cl2{(Ph2P(O)CH2)2NEt-κO,κO}]. Inorg. Chem. 2003, 42, 1272–1281. [Google Scholar] [CrossRef]
- DIAMOND; Version 3.2i; Crystal Impact GbR: Bonn, Germany, 2014.

| (S)-1c | |
|---|---|
| Empirical formula | C14H15O2P |
| Formula mass | 246.23 |
| T [K] | 123 (2) |
| Crystal size [mm] | 0.20 × 0.10 × 0.05 |
| Crystal description | colorless rod |
| Crystal system | monoclinic |
| Space group | P21 |
| a [Ǻ] | 7.0846 (2) |
| b [Ǻ] | 10.8324 (3) |
| c [Ǻ] | 9.1135 (2) |
| α [°] | 90.0 |
| β [°] | 105.677 (3) |
| γ [°] | 90.0 |
| V [Ǻ3] | 673.38 (3) |
| Z | 2 |
| ρcalcd. [g cm−3] | 1.214 |
| μ [mm−1] | 0.192 |
| F(000) | 260 |
| Θ range [°] | 2.99–25.24 |
| Index ranges | −10 ≤ h ≤ 10 |
| −15 ≤ k ≤ 15 | |
| −13 ≤ l ≤ 13 | |
| Reflns. collected | 13284 |
| Reflns. obsd. | 3755 |
| Reflns. unique | 4112 (Rint = 0.0309) |
| R1, wR2 (2σ data) | 0.0341, 0.0730 |
| R1, wR2 (all data) | 0.0402, 0.0763 |
| GOOF on F2 | 1.042 |
| Peak/hole [e Ǻ−3] | 0.293/−0.171 |
| Compound | Solvent | Yield (%) | de (%) | S = [de (%)] × [yield (%)] | Remark | Entry |
|---|---|---|---|---|---|---|
| 1c | THF | - | - | - | no diastereomeric complex was formed | 1. |
| MeCN | - | - | - | no diastereomeric complex was formed | 2. | |
| DMSO | - | - | - | no diastereomeric complex was formed | 3. | |
| MEK | 20 | 0 | 0 | no chiral discrimination | 4. | |
| EtOAc (10×) + MeOH (2.5×) | 19 | 0 | 0 | no chiral discrimination | 5. | |
| 1,4-dioxane | 14 | 0 | 0 | no chiral discrimination | 6. | |
| EtOAc (10×) + EtOH (2.5×) | 48 (28 a) | 38 (77 a) | 0.18 (0.22 a) | - | 7. | |
| EtOAc (10×) + PrOH (2.5×) | 8 | 50 | 0.04 | - | 8. | |
| EtOAc (10×) + iPrOH (2.5×) | 16 | 45 | 0.07 | - | 9. | |
| EtOAc (10×) + BuOH (2.5×) | 15 | 50 | 0.08 | - | 10. | |
| 1d | EtOAc (10×) + EtOH (2.5×) | - | - | - | no diastereomeric complex was formed | 11. |
| 46 b (9 b,c) | 40 b (90 b,c) | 0.18 b (0.08 b,c) | 12. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szalai, Z.; Kis, A.S.; Schindler, J.; Karaghiosoff, K.; Keglevich, G. Synthesis of α-Hydroxyethylphosphonates and α-Hydroxyethylphosphine Oxides: Role of Solvents During Optical Resolution. Symmetry 2024, 16, 1557. https://doi.org/10.3390/sym16111557
Szalai Z, Kis AS, Schindler J, Karaghiosoff K, Keglevich G. Synthesis of α-Hydroxyethylphosphonates and α-Hydroxyethylphosphine Oxides: Role of Solvents During Optical Resolution. Symmetry. 2024; 16(11):1557. https://doi.org/10.3390/sym16111557
Chicago/Turabian StyleSzalai, Zsuzsanna, Anna Sára Kis, József Schindler, Konstantin Karaghiosoff, and György Keglevich. 2024. "Synthesis of α-Hydroxyethylphosphonates and α-Hydroxyethylphosphine Oxides: Role of Solvents During Optical Resolution" Symmetry 16, no. 11: 1557. https://doi.org/10.3390/sym16111557
APA StyleSzalai, Z., Kis, A. S., Schindler, J., Karaghiosoff, K., & Keglevich, G. (2024). Synthesis of α-Hydroxyethylphosphonates and α-Hydroxyethylphosphine Oxides: Role of Solvents During Optical Resolution. Symmetry, 16(11), 1557. https://doi.org/10.3390/sym16111557







