A Methodology for Contrast Enhancement in Laser Speckle Imaging: Applications in Phaseolus vulgaris and Lactuca sativa Seed Bioactivity
Abstract
1. Introduction
2. Material and Methods
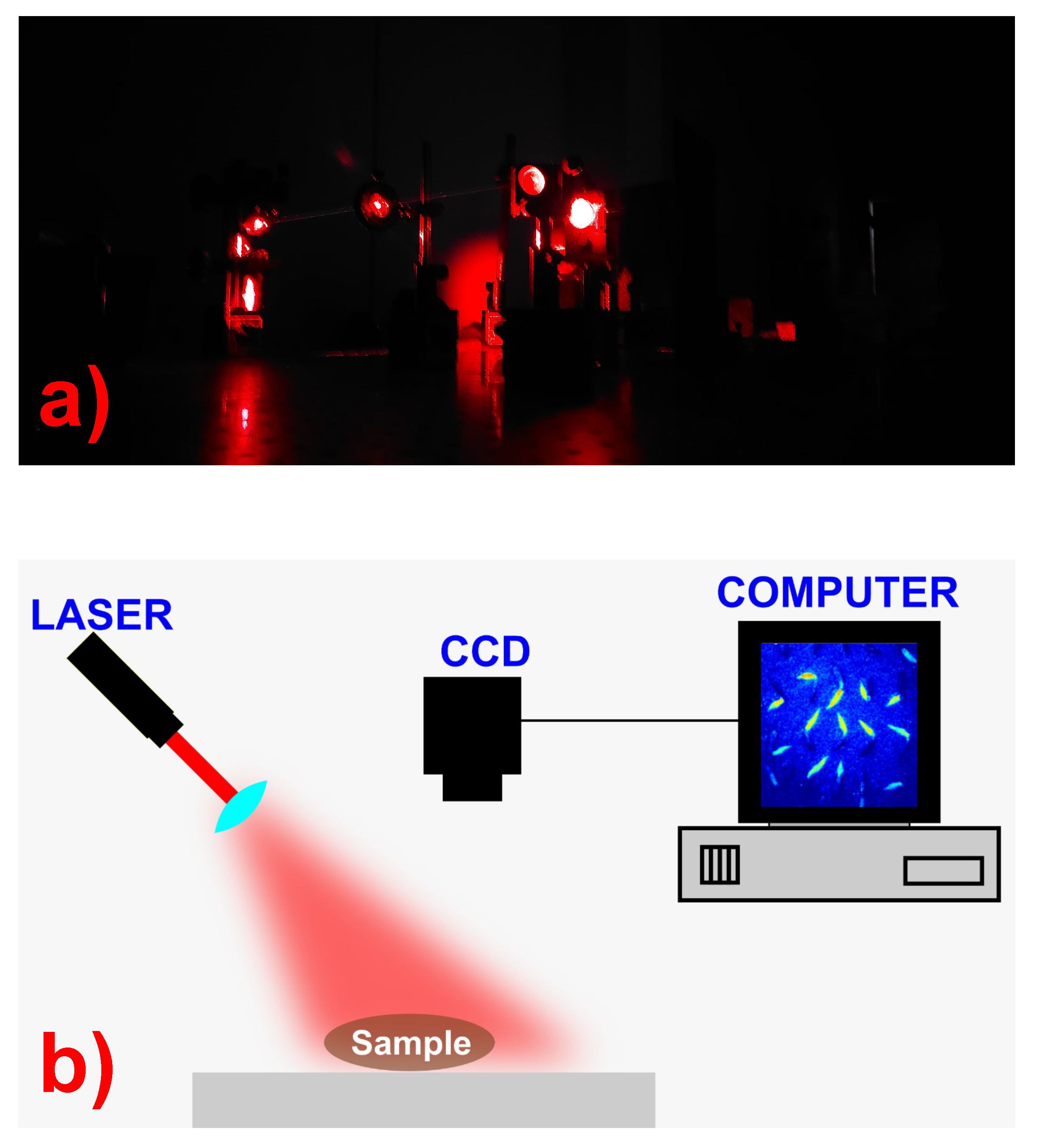
2.1. LSI Image Dataset
2.2. Image Enhancement Techniques
2.3. Applied Methodology
| Algorithm 1 Methodology for Laser Speckle Image Processing. |
|
2.4. Evaluation Metrics
- Contrast (C) [48], provides the overall contrast of the output laser speckle image. It is defined as:where are the dimensions of the image I, is the grayscale intensity at pixel , and is the mean brightness of the image, given by:where L is the number of grayscale levels (256 in this case) and is the probability of occurrence of level k in the image. The contrast value of the resulting image must be higher than that of the original image to be considered an improvement.
- Structural Similarity Index (SSIM) [50], measures structural similarity between two images. Calculated in blocks, given two image windows and , SSIM is expressed as:where and are average intensities, and are intensity variances, is covariance, and , are constants for stabilization.
- Contrast Improvement Ratio (CIR) [49,51], measures local contrast improvement in the processed image:where w is local contrast of the original image I, is local contrast of the enhanced image , and D is the domain. w is defined as:where is the central pixel and is the mean of its 3 × 3 neighborhood.
2.5. Visual Validation and Perspective
3. Results and Discussion
- Quantify the performance of the proposed methodology in terms of improving the activity map images generated by the GAVD method, based on the application of different contrast enhancement algorithms. To quantify the numerical results obtained, six objective metrics were applied to the output images .
- Analyze the visual impact of the contrast enhancement algorithms. This analysis was carried out through a visual evaluation conducted by specialists, who examined a representative sample of the obtained results.
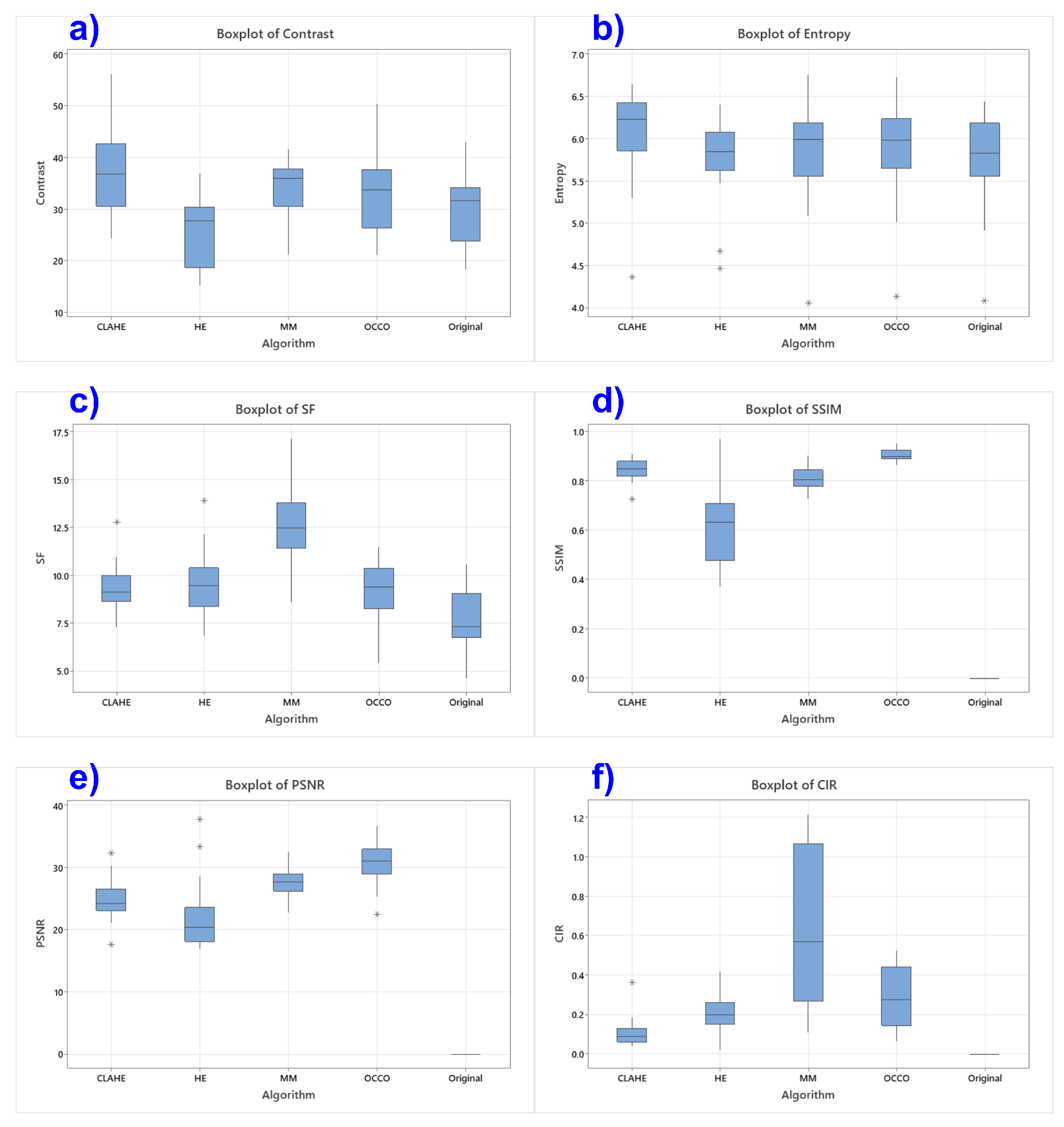
3.1. Numerical Results
- The CLAHE algorithm showed the best performance in terms of overall contrast and detail richness, reaching the highest values of 36.88 for contrast and 6.12 for entropy.
- The MMCE algorithm stood out for its ability to preserve spatial information and improve local contrast. This is reflected in its highest values for SF (12.44) and CIR (0.640). Although MMCE improved certain aspects of image contrast, it also tended to amplify background noise, which may hinder the identification of subtle bioactivity patterns. This effect suggests that future work could explore background suppression techniques or region-specific contrast control strategies to mitigate such artifacts while preserving relevant speckle structures.
- The OCCO-MTH algorithm achieved the best results in terms of structural similarity preservation and lowest introduced distortion, with the highest SSIM value (0.906) and highest PSNR (30.71) among the analyzed methods.
- The original image, without enhancement techniques applied, showed a contrast value of 29.51 and an entropy of 5.76, serving as a reference to evaluate the effects generated by the algorithms. SSIM, PSNR, and CIR metrics were not computed for this condition, as they are based on comparisons with the original image.
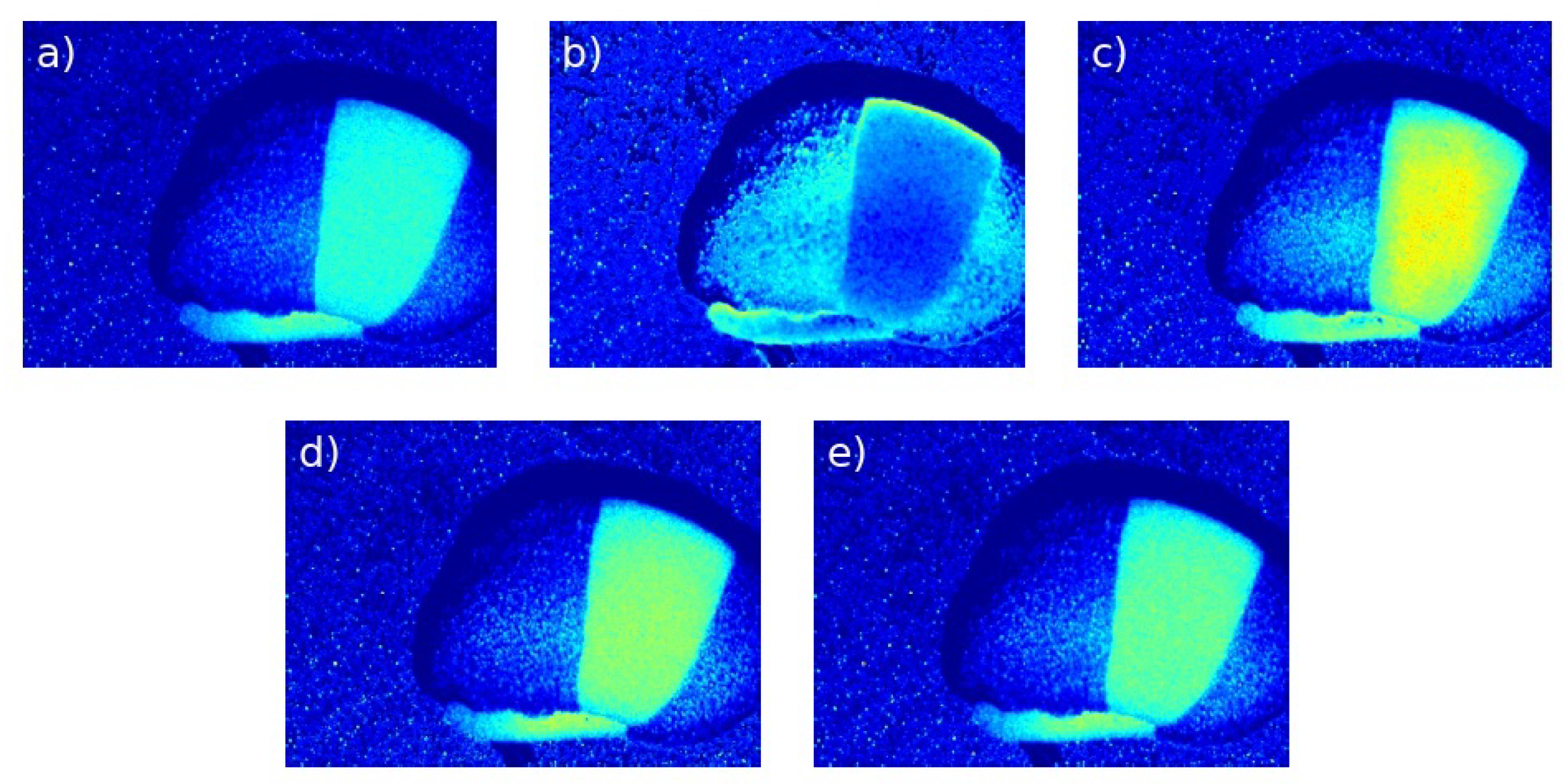
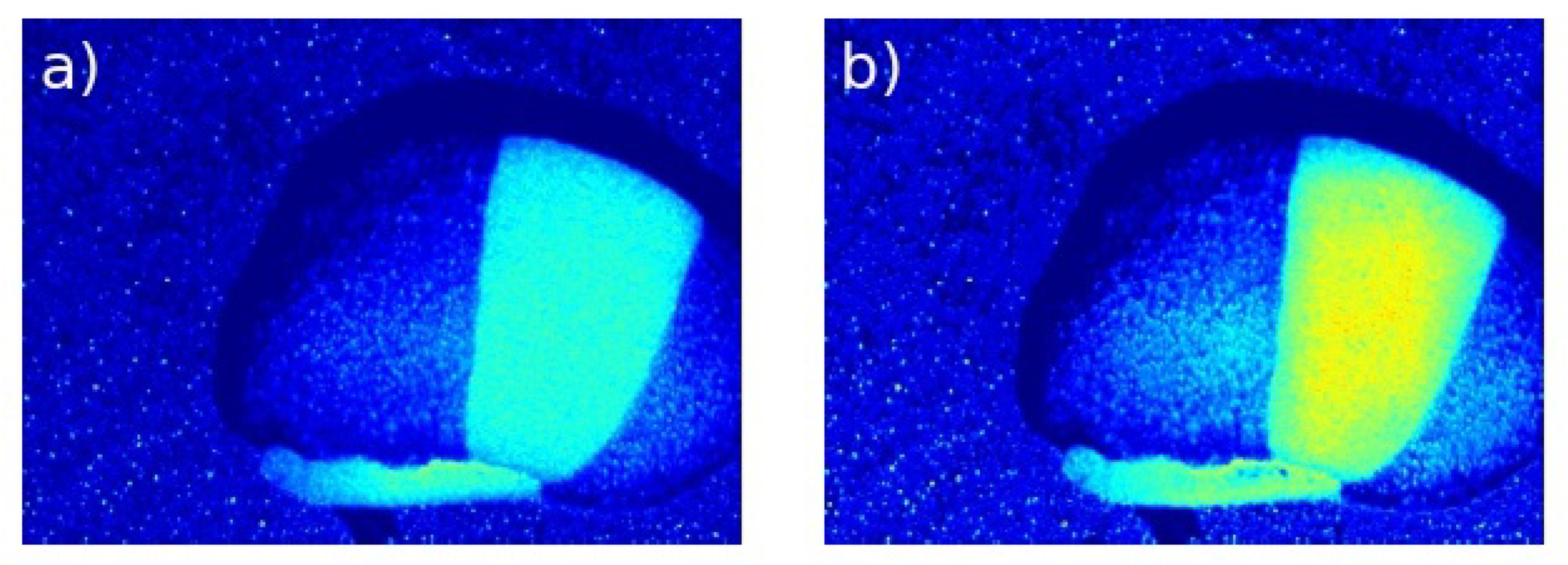
3.2. Visual Analysis
3.2.1. Statistical Analysis
3.2.2. Visual Evaluation
3.2.3. Computational Efficiency and Practical Implications
4. Conclusions
- CLAHE achieved the highest global contrast and entropy, improving the visibility of biologically active regions.
- MMCE excelled in local contrast and edge enhancement, improving the definition of active regions in .
- OCCO-MTH provided the best structural preservation and lowest distortion, maintaining the underlying speckle pattern.
5. Limitations and Future Work
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rabal, H.; Braga, R. Dynamic Laser Speckle and Applications; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar] [CrossRef]
- Zdunek, A.; Adamiak, A.; Pieczywek, P.M.; Kurenda, A. The biospeckle method for the investigation of agricultural crops: A review. Opt. Lasers Eng. 2014, 52, 276–285. [Google Scholar] [CrossRef]
- Ali, M.M.; Hashim, N.; Aziz, S.A.; Lasekan, O. Emerging non-destructive thermal imaging technique coupled with chemometrics on quality and safety inspection in food and agriculture. Trends Food Sci. Technol. 2020, 105, 176–185. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Mayookha, V.; Kothakota, A.; Ramesh, S.; Thirumdas, R.; Juvvi, P. Biospeckle laser technique—A novel non-destructive approach for food quality and safety detection. Trends Food Sci. Technol. 2020, 97, 1–13. [Google Scholar] [CrossRef]
- Pujaico, F.; Braga, R.; Moreira, J. A Practical Guide to Biospeckle Laser Analysis: Theory and Software; Editora UFLA: Lavras, Brazil, 2016. [Google Scholar]
- Briers, D.; Duncan, D.D.; Hirst, E.; Kirkpatrick, S.J.; Larsson, M.; Steenbergen, W.; Stromberg, T.; Thompson, O.B. Laser speckle contrast imaging: Theoretical and practical limitations. J. Biomed. Opt. 2013, 18, 066018. [Google Scholar] [CrossRef]
- Heeman, W.; Steenbergen, W.; van Dam, G.M.; Boerma, E.C. Clinical applications of laser speckle contrast imaging: A review. J. Biomed. Opt. 2019, 24, 080901. [Google Scholar] [CrossRef]
- Shimizu, M.; Sawano, H.; Yoshioka, H.; Shinno, H. Multi-dimensional assessment of nano/micro scale surface texture using laser speckle pattern analysis. J. Adv. Mech. Des. Syst. Manuf. 2015, 9, JAMDSM0011. [Google Scholar] [CrossRef]
- Lee, B.; Sosnovtseva, O.; Sørensen, C.M.; Postnov, D.D. Multi-scale laser speckle contrast imaging of microcirculatory vasoreactivity. Biomed. Opt. Express 2022, 13, 2312–2322. [Google Scholar] [CrossRef]
- Konovalov, A.; Gadzhiagaev, V.; Grebenev, F.; Stavtsev, D.; Piavchenko, G.; Gerasimenko, A.; Telyshev, D.; Meglinski, I.; Eliava, S. Laser speckle contrast imaging in neurosurgery: A systematic review. World Neurosurg. 2023, 171, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Golubova, N.; Potapova, E.; Seryogina, E.; Dremin, V. Time–frequency analysis of laser speckle contrast for transcranial assessment of cerebral blood flow. Biomed. Signal Process. Control 2023, 85, 104969. [Google Scholar] [CrossRef]
- Kumar, N.; Nirala, A. A novel computational method for dynamic laser speckle and its application to analyze water activity during photosynthesis in papaya leaf. Optik 2023, 274, 170518. [Google Scholar] [CrossRef]
- Xu, Z.; Joenathan, C.; Khorana, B.M. Temporal and spatial properties of the time-varying speckles of botanical specimens. Opt. Eng. 1995, 34, 1487–1502. [Google Scholar] [CrossRef]
- Singh, P.; Chatterjee, A.; Rajput, L.S.; Rana, S.; Kumar, S.; Nataraj, V.; Bhatia, V.; Prakash, S. Development of an intelligent laser biospeckle system for early detection and classification of soybean seeds infected with seed-borne fungal pathogen (Colletotrichum truncatum). Biosyst. Eng. 2021, 212, 442–457. [Google Scholar] [CrossRef]
- Sutton, D.B.; Punja, Z.K. Investigating biospeckle laser analysis as a diagnostic method to assess sprouting damage in wheat seeds. Comput. Electron. Agric. 2017, 141, 238–247. [Google Scholar] [CrossRef]
- Singh, P.; Chatterjee, A.; Bhatia, V.; Prakash, S. Application of laser biospeckle analysis for assessment of seed priming treatments. Comput. Electron. Agric. 2020, 169, 105212. [Google Scholar] [CrossRef]
- Braga, R.A.; Dal Fabbro, I.M.; Borem, F.M.; Rabelo, G.; Arizaga, R.; Rabal, H.J.; Trivi, M. Assessment of seed viability by laser speckle techniques. Biosyst. Eng. 2003, 86, 287–294. [Google Scholar] [CrossRef]
- Passoni, I.; Dai Pra, A.; Rabal, H.; Trivi, M.; Arizaga, R. Dynamic speckle processing using wavelets based entropy. Opt. Commun. 2005, 246, 219–228. [Google Scholar] [CrossRef]
- Xing, M.; Long, Y.; Wang, Q.; Tian, X.; Fan, S.; Zhang, C.; Huang, W. Physiological alterations and nondestructive test methods of crop seed vigor: A comprehensive review. Agriculture 2023, 13, 527. [Google Scholar] [CrossRef]
- Thakur, P.S.; Tiwari, B.; Kumar, A.; Gedam, B.; Bhatia, V.; Krejcar, O.; Dobrovolny, M.; Nebhen, J.; Prakash, S. Deep transfer learning based photonics sensor for assessment of seed-quality. Comput. Electron. Agric. 2022, 196, 106891. [Google Scholar] [CrossRef]
- Braga, R.A., Jr.; Contado, J.L.; Ducatti, K.R.; da Silva, E.A.A. Analysis of Seed Vigor Using the Biospeckle Laser Technique. AgriEngineering 2024, 7, 3. [Google Scholar] [CrossRef]
- Zhu, J.; Cai, J.; Sun, B.; Xu, Y.; Lu, F.; Ma, H. Inspection and classification of wheat quality using image processing. Qual. Assur. Saf. Crop. Foods 2023, 15, 43–54. [Google Scholar] [CrossRef]
- Wang, W.; Yin, B.; Li, L.; Li, L.; Liu, H. A Low Light Image Enhancement Method Based on Dehazing Physical Model. Comput. Model. Eng. Sci. (CMES) 2025, 143, 1595–1616. [Google Scholar] [CrossRef]
- Wang, J.; He, Y.; Li, K.; Li, S.; Zhao, L.; Yin, J.; Zhang, M.; Shi, T.; Wang, X. MDANet: A multi-stage domain adaptation framework for generalizable low-light image enhancement. Neurocomputing 2025, 627, 129572. [Google Scholar] [CrossRef]
- Wang, Z.; Tao, H.; Zhou, H.; Deng, Y.; Zhou, P. A content-style control network with style contrastive learning for underwater image enhancement. Multimed. Syst. 2025, 31, 60. [Google Scholar] [CrossRef]
- Herrera, E.Z.; Mereles Menesse, G.E.; Mello Román, J.C.; Vazquez Noguera, J.L.; Méndez Gaona, F.J. Biospeckle Images Dataset—SEEDS; Zenodo: Geneva, Switzerland, 2024. [Google Scholar] [CrossRef]
- Goodman, J.W. Speckle Phenomena in Optics: Theory and Applications; Roberts and Company Publishers: Greenwood Village, CO, USA, 2007. [Google Scholar]
- Gonzalez, R.C.; Woods, R.E. Intensity Transformations and Spatial Filtering. In Digital Image Processing; Pearson: London, UK, 2018. [Google Scholar]
- Menotti, D.; Najman, L.; Facon, J.; Araujo, A.D.A. Multi-Histogram Equalization Methods for Contrast Enhancement and Brightness Preserving. IEEE Trans. Consum. Electron. 2007, 53, 1186–1194. [Google Scholar] [CrossRef]
- Brizuela Pineda, I.A.; Medina Caballero, R.D.; Cáceres Silva, J.J.; Mello Román, J.C.; Vázquez Noguera, J.L. Quadri-histogram equalization using cutoff limits based on the size of each histogram with preservation of average brightness. Signal Image Video Process. 2019, 13, 843–851. [Google Scholar] [CrossRef]
- Chatterjee, A.; Bhatia, V.; Prakash, S. Anti-spoof touchless 3D fingerprint recognition system using single shot fringe projection and biospeckle analysis. Opt. Lasers Eng. 2017, 95, 1–7. [Google Scholar] [CrossRef]
- Zuiderveld, K. Contrast Limited Adaptive Histogram Equalization. In Graphics Gems; Elsevier: Amsterdam, The Netherlands, 1994; pp. 474–485. [Google Scholar] [CrossRef]
- Yeom, E.; Nam, K.H.; Paeng, D.G.; Lee, S.J. Improvement of ultrasound speckle image velocimetry using image enhancement techniques. Ultrasonics 2014, 54, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Kong, P.; Xu, H.; Li, R.; Huang, G.; Liu, W. Laser Speckle Contrast Imaging Based on a Mobile Phone Camera. IEEE Access 2021, 9, 76730–76737. [Google Scholar] [CrossRef]
- Soille, P. Morphological Image Analysis; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar] [CrossRef]
- Maragos, P. Morphological Filtering for Image Enhancement and Feature Detection. In Handbook of Image and Video Processing; Elsevier: Amsterdam, The Netherlands, 2005; pp. 135–156. [Google Scholar] [CrossRef]
- Mello Román, J.; Vázquez Noguera, J.; Legal-Ayala, H.; Pinto-Roa, D.; Gomez-Guerrero, S.; García Torres, M. Entropy and Contrast Enhancement of Infrared Thermal Images Using the Multiscale Top-Hat Transform. Entropy 2019, 21, 244. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Chanda, B. A multiscale morphological approach to local contrast enhancement. Signal Process. 2000, 80, 685–696. [Google Scholar] [CrossRef]
- Bai, X.; Zhou, F.; Xue, B. Image enhancement using multi scale image features extracted by top-hat transform. Opt. Laser Technol. 2012, 44, 328–336. [Google Scholar] [CrossRef]
- He, Y.; Kang, S.; Li, W.; Xu, H.; Liu, S. Advanced enhancement technique for infrared images of wind turbine blades utilizing adaptive difference multi-scale top-hat transformation. Sci. Rep. 2024, 14, 15604. [Google Scholar] [CrossRef]
- Román, J.C.M.; Noguera, J.L.V.; García-Torres, M.; Benítez, V.E.C.; Matto, I.C. Retinal Image Enhancement via a Multiscale Morphological Approach with OCCO Filter. In Information Technology and Systems; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; pp. 177–186. [Google Scholar] [CrossRef]
- Aptoula, E.; Lefèvre, S. A comparative study on multivariate mathematical morphology. Pattern Recognit. 2007, 40, 2914–2929. [Google Scholar] [CrossRef]
- Bai, X.; Zhou, F.; Xue, B. Noise-suppressed image enhancement using multiscale top-hat selection transform through region extraction. Appl. Opt. 2012, 51, 338. [Google Scholar] [CrossRef]
- Roman, J.C.M.; Legal-Ayala, H.; Noguera, J.L.V. Applications of Multiscale Mathematical Morphology to Contrast Enhancement and Images Fusion. In Proceedings of the 2020 15th Iberian Conference on Information Systems and Technologies (CISTI), Seville, Spain, 24–27 June 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 1–7. [Google Scholar] [CrossRef]
- Herrera-Arellano, M.; Peregrina-Barreto, H.; Terol-Villalobos, I. Visible-NIR Image Fusion Based on Top-Hat Transform. IEEE Trans. Image Process. 2021, 30, 4962–4972. [Google Scholar] [CrossRef]
- Arhami, M.; Desiani, A.; Yahdin, S.; Putri, A.I.; Primartha, R.; Husaini, H. Contrast enhancement for improved blood vessels retinal segmentation using top-hat transformation and otsu thresholding. Int. J. Adv. Intell. Inform. 2022, 8, 210. [Google Scholar] [CrossRef]
- Roy, M.; Mukhopadhyay, S. Infrared and Visible Image Fusion Using Morphological Reconstruction Filters and Refined Toggle-Contrast Edge Features. In Computer Vision and Machine Intelligence; Lecture Notes in Networks and Systems; Springer Nature: Singapore, 2023; pp. 641–654. [Google Scholar] [CrossRef]
- Moulden, B.; Kingdom, F.; Gatley, L.F. The Standard Deviation of Luminance as a Metric for Contrast in Random-Dot Images. Perception 1990, 19, 79–101. [Google Scholar] [CrossRef]
- Román, J.C.M.; Fretes, V.R.; Adorno, C.G.; Silva, R.G.; Noguera, J.L.V.; Legal-Ayala, H.; Mello-Román, J.D.; Torres, R.D.E.; Facon, J. Panoramic Dental Radiography Image Enhancement Using Multiscale Mathematical Morphology. Sensors 2021, 21, 3110. [Google Scholar] [CrossRef]
- Wang, Z.; Bovik, A.; Sheikh, H.; Simoncelli, E. Image Quality Assessment: From Error Visibility to Structural Similarity. IEEE Trans. Image Process. 2004, 13, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.P.; Wu, Q.; Castleman, K.; Xiong, Z. Chromosome image enhancement using multiscale differential operators. IEEE Trans. Med. Imaging 2003, 22, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Shapley, R.; Lennie, P. Spatial Frequency Analysis in the Visual System. Annu. Rev. Neurosci. 1985, 8, 547–581. [Google Scholar] [CrossRef] [PubMed]
- Hore, A.; Ziou, D. Image Quality Metrics: PSNR vs. SSIM. In Proceedings of the 2010 20th International Conference on Pattern Recognition, Istanbul, Turkey, 23–26 August 2010; IEEE: Piscataway, NJ, USA, 2010. [Google Scholar] [CrossRef]
- Derrac, J.; García, S.; Molina, D.; Herrera, F. A practical tutorial on the use of nonprametric statistical tests as a methodology for comparing evolutionary and swarm intelligence algorithm. Swarm Evol. Comput. 2011, 1, 3–18. [Google Scholar] [CrossRef]




| Code | Polarizer | Spectral Filter |
|---|---|---|
| P0_F0 | No | No |
| P0_F1 | No | Yes |
| P1_F0 | Yes | No |
| P1_F1 | Yes | Yes |
| Algorithm | Contrast | Entropy | SF | SSIM | PSNR | CIR |
|---|---|---|---|---|---|---|
| CLAHE | 36.88 | 6.12 | 9.35 | 0.847 | 24.69 | 0.104 |
| HE | 26.03 | 5.77 | 9.62 | 0.618 | 21.99 | 0.020 |
| MMCE | 34.32 | 5.87 | 12.44 | 0.813 | 27.45 | 0.640 |
| OCCO-MTH | 32.79 | 5.88 | 9.30 | 0.906 | 30.71 | 0.287 |
| Original | 29.51 | 5.76 | 7.82 | – | – | – |
| Algorithms | Sample | Mean | Std. Dev. | Ranking (Friedman) | p-Value (Shaffer) vs. I |
|---|---|---|---|---|---|
| I | 24 | 1.000 | 0.000 | 3.713 | - |
| HE | 24 | 0.250 | 0.504 | 4.665 | 0.101 |
| CLAHE | 24 | 1.403 | 0.742 | 2.035 | 0.042 |
| MMCE | 24 | 1.569 | 0.347 | 2.089 | 0.007 |
| OCCO-MTH | 24 | 1.417 | 0.408 | 2.497 | 0.055 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera, E.Z.; Mello-Román, J.C.; Florentin, J.; Palacios, J.; Mereles Menesse, G.E.; Jara Avalos, J.A.; Franco, M.; Méndez, F.; García-Torres, M.; Vázquez Noguera, J.L.; et al. A Methodology for Contrast Enhancement in Laser Speckle Imaging: Applications in Phaseolus vulgaris and Lactuca sativa Seed Bioactivity. Symmetry 2025, 17, 2029. https://doi.org/10.3390/sym17122029
Herrera EZ, Mello-Román JC, Florentin J, Palacios J, Mereles Menesse GE, Jara Avalos JA, Franco M, Méndez F, García-Torres M, Vázquez Noguera JL, et al. A Methodology for Contrast Enhancement in Laser Speckle Imaging: Applications in Phaseolus vulgaris and Lactuca sativa Seed Bioactivity. Symmetry. 2025; 17(12):2029. https://doi.org/10.3390/sym17122029
Chicago/Turabian StyleHerrera, Edher Zacarias, Julio César Mello-Román, Joel Florentin, José Palacios, Gustavo Eduardo Mereles Menesse, Jorge Antonio Jara Avalos, Marcos Franco, Fernando Méndez, Miguel García-Torres, José Luis Vázquez Noguera, and et al. 2025. "A Methodology for Contrast Enhancement in Laser Speckle Imaging: Applications in Phaseolus vulgaris and Lactuca sativa Seed Bioactivity" Symmetry 17, no. 12: 2029. https://doi.org/10.3390/sym17122029
APA StyleHerrera, E. Z., Mello-Román, J. C., Florentin, J., Palacios, J., Mereles Menesse, G. E., Jara Avalos, J. A., Franco, M., Méndez, F., García-Torres, M., Vázquez Noguera, J. L., Pérez-Estigarribia, P., Grillo, S., & Legal-Ayala, H. (2025). A Methodology for Contrast Enhancement in Laser Speckle Imaging: Applications in Phaseolus vulgaris and Lactuca sativa Seed Bioactivity. Symmetry, 17(12), 2029. https://doi.org/10.3390/sym17122029








