Abstract
This study investigates the enantiomeric composition and possibilities of analysis of 1-octen-3-yl acetate (OcAc), a chiral compound found in various samples of lavender essential oils (EOs). Essential oils are complex mixtures exhibiting diverse biological activities and are often subject to sophisticated adulteration methods, necessitating advanced analytical techniques for authenticity verification. Using gas chromatography-mass spectrometry and chiral gas chromatography, the analysis of lavender EOs samples determines the presence and enantiomeric ratios of OcAc. The results showed significant variations in the oils; however, there were minimal differences in enantiomeric purity among samples sourced from France, Bulgaria, and Slovakia. Major components such as linalool and linalyl acetate were predominant. The high enantiomeric purity of OcAc observed across all samples indicated there was no adulteration by synthetic products. The study underscores the importance of rigorous analytical methods to ensure the quality and authenticity of EOs, highlighting the influence of geographical origin on their chemical composition. These findings provided valuable insights into the factors affecting the authenticity and possible therapeutic efficacy of lavender essential oil.
1. Introduction
Essential oils (EOs) are generally complex matrices composed of terpenes, terpenoids, sesquiterpenes, epoxides, aldehydes, esters, amines, and sulfides that form a concentrated and distinct aroma [1]. EOs have a wide range of uses because of their biological activities, such as their antibacterial, antioxidant, antifungal, virucidal, and antiparasitic properties.
Lavender extract has shown antimicrobial activity against three species of medically important yeasts belonging to the genus Candida and can also evaluate the microbiological safety of the creams inoculated with C. albicinas, Staphylococcus aureus, Pseudomonas aeroginosa, and Escherichia coli over a nine-month storage period [2,3,4,5]. Recent studies have expanded on the therapeutic properties of lavender oil, highlighting its potential in managing anxiety, depression, and other neurological disorders, making it a valuable component in complementary and alternative medicine [6].
Additionally, EOs of Zataria multiflora chemotypes containing valuable phenolic compounds, thymol, and carvacrol exhibited strong antifungal activities against a broad spectrum of important agricultural and food pathogens [7]. Research also shows that EOs from Thymus vulgaris are effective against several RNA viruses in vitro [8]. Furthermore, lavender oil has been studied for its anti-inflammatory properties, which are crucial in treating chronic inflammatory conditions [9].
Natural EOs, which are produced as a secondary metabolites in various parts of aromatic plants such as flowers, roots, leaves, and stems are very popular. To satisfy the high commercial demand and to reduce costs, there is a temptation to adulterate EOs using sophisticated methods. Common techniques instead of natural cold-press EOs include the addition of distilled oils or non-volatile ingredient dilution with cheaper EOs (or their terpenes) and the addition of synthetic compounds [5]. The increasing use of EOs in the pharmaceutical and cosmetic industries has raised concerns about their quality and authenticity, necessitating more stringent regulatory standards and advanced analytical techniques [10].
Nowadays, the adulteration of EOs is more ingenious, making it harder to determine their authenticity; therefore, the tools employed to assess the quality of EOs have to be more reliable [11]. In general, analytical separation techniques such as gas chromatography (GC), liquid chromatography, high-performance thin layer chromatography, and spectroscopic techniques are a key techniques for determining authenticity of EOs. Additionally, chiral GC is a powerful technique for determining authenticity and is crucial for detecting adulterants [5,12]. The development of new analytical methods, such as multidimensional gas chromatography and tandem mass spectrometry, has further enhanced the ability to detect EO adulteration with greater precision [13].
Compounds from EOs are mostly chiral, occurring in specific enantiomer ratios, often unique to the EOs. Chiral analysis allows the detection of synthetic substituents, usually in racemic form added to natural EOs, by using values of enantiomeric purity and excess [12]. Despite having the same physical properties, enantiomers can differ in odor quality, odor properties, taste, and biological activity. For example, (R)-citronellol has a typical citronella odor, while (S)-citronellol has a geranium-type odor. Additionally, (R)-2-heptyl acetate is described as green, fatty, and banana like and (S)-2-heptyl acetate as mushroom, earthy, and wild berry like [13]. This differentiation is critical in the fragrance industry, where the specific odor profiles of enantiomers can significantly impact the marketability of EO-based products [14].
Lavender essential oil, in particular, has been widely studied due to its rich and diverse chemical composition. Studies have identified major components such as linalool and linalyl acetate, which are crucial for assessing oil quality and therapeutic benefits. These findings underscore the importance of rigorous analytical methods to ensure the quality and authenticity of lavender EOs [14]. Recent advancements in metabolomics and chemometric techniques have enabled a more comprehensive profiling of lavender oil constituents, providing deeper insights into its therapeutic potential and geographical origin [15].
1-octen-3-yl acetate (OcAc) is usually a colorless liquid with a fresh-herbaceous fruity, minty, lavender odor [15]. It belongs to the class of carboxylic fatty acid esters and, according to the database of Volatile Compounds in Food, is reported to occur in some types of foods and is also found in some natural complex substances like Agastache species, melon, cormint oils, mentha oils, certain type of thymus, and other varieties of mushrooms and anise hyssop [16]. Due to the lavender-like odor of OcAc, it could also be present in lavender oils. Overall, its volume of use is 1–10 metric tons per year [10]. OcAc is also a chiral compound. The enantiomers of OcAc, like many other chiral compounds, can have distinct odors due to their different interactions with olfactory receptors, although specific odor profiles for these enantiomers might not be extensively documented. The (R)-enantiomer of OcAc is likely to have a fresh, herbaceous, fruity, and minty lavender-like aroma. This profile aligns with the general perception of OcAc in nature, which is associated with pleasant, fresh scents. It would be preferred in applications where a cleaner, more vibrant scent is desired, such as in high-quality perfumes, personal care products, and aromatherapy. The (S)-enantiomer of OcAc might exhibit a different odor profile, potentially with earthier, mushroom-like, or more subdued undertones compared to the (R)-enantiomer [16]. In lavender essential oils, OcAc enhances both the fragrance and potential therapeutic properties, such as antimicrobial and antioxidant effects. Extracted through methods like steam distillation or solvent extraction, it is widely used in perfumes, cosmetics, and flavorings. Obtaining natural OcAc is challenging, but essential oils, particularly lavender oil, are promising natural sources, highlighting the importance of rigorous analytical methods to ensure the quality and authenticity of these oils [4,12].
The aim of this work was to characterize lavender essential oils from different sources, focusing on the enantiomeric composition of OcAc, determined by gas chromatotography as an indicator of authenticity.
2. Materials and Methods
2.1. Chemicals and Standards
OcAc, with a purity of 99% as a natural standard was purchased commercially from Axxence Slovakia s.r.o. (Bratislava, Slovakia). Racemic OcAc standard, with a 95% purity, was purchased from BLD Pharm ltd. (Shanghai, China). Samples of lavender essential oils were commercially available in stores and were obtained from various producers. All samples of levander EOs were derived from the same species of lavender plant Lavandula augustifolia from southeastern France, northeastern Bulgaria, and western Slovakia. All lavender essential oil samples were purchased from stores in Slovakia.
2.2. GC-MS Analysis of EOs
An amount of 20 µL of each essential oil was added to 1.5 mL standard chromatographic vials with glass inserts; 0.01 µL of each sample without dilution with solvent was directly injected via an autosampler using a 0.5 µL syringe (SGE, Trajan, Australia) into the GC column in split mode with a ratio of 1:20. The inlet temperature was set at 280 °C. Analysis was conducted using an 8890 gas chromatograph coupled to a 5977B single-Quadrupole Mass Spectrometer (Agilent Technologies, Palo Alto, CA, USA). The column was HP-5, 30 m in length, with a non-polar stationary phase and 0.25 mm internal diameter and 0.25 µm film thickness, and was purchased from Agilent Technologies. The temperature program started at 40 °C and was then ramped to 310 °C at 2 °C/min and held for 2 min. The carrier gas was helium (5.0, Messer, Slovakia), with a starting flow rate of 3.514 mL/min and a constant pressure of 180 kPa during the whole GC separation. An MS detector with electron ionization of 70 eV was operated in scanning mode in a mass range of m/z 33 to 330 amu. The temperature of the MS detector quadrupole was set at 150 °C and the ion source temperature was set at 230 °C. After analysis, compounds were identified (minimal match at 80%) by comparing each mass spectrum with the spectra from reference library NIST 20 (Gaithersburg, MD, USA). Volatile compounds were quantified and are reported as a relative area percent (rel. GC%). Samples were analyzed in randomized order in one run. Chromatographic data were processed by MSD ChemStation F01 (Agilent Technologies).
2.3. Chiral GC-MS Analysis of Selected EOs
For determining the enantiomer ratio, lavender essential oils, in which OcAc was identified, were analyzed by an Agilent 6890N gas chromatograph coupled to a chiral GC column and MS detector 5793 (Agilent Technologies, Palo Alto, CA, USA). Based on an artificial (racemic) standard of OcAc from BLD Pharm, the retention times of both (R- and S-) enantiomers were determined. The enantiomer ratio was calculated by the relative peak area of each enantiomer. An amount of 0.1 µL of each sample was directly injected with split mode 50:1. Inlet temperature was set to 280 °C. The column was CYCLOSIL-B, 30 m in length, with a chiral stationary phase, 0.25 mm internal diameter, and 0.25 µm film thickness, purchased from Agilent Technologies. The temperature program started at 100 °C for 1 min and was then ramped to 200 °C at 7 °C/min and held for 2 min. Helium (5.0) was used as the mobile phase, with a constant flow of 1.3 mL/min. The MS detector was working with an electron ionization of 70 eV in selected ion-monitoring mode with selected ions m/z 99 and 128 (for quantifying). The dwell time was 80 ms. The temperature of the MS detector ion source was set at 230 °C and the temperature of the quadrupole was set at 150 °C. Chromatographic data were processed by MSD ChemStation F01 (Agilent Technologies).
3. Results
The variety and location of sources of the lavender essential oil samples analyzed in this study are presented in Table 1. The samples were sourced from different geographical regions, including France, Bulgaria, and Slovakia. All samples were derived from Lavandula angustifolia. The analysis of essential oil samples revealed the presence of OcAc in varying concentrations across the different lavender oil samples. The results from qualitative analyses are shown in Table 2.

Table 1.
Producer, variety, and location for lavender EOs.

Table 2.
Composition of lavender essential oils: all compounds with rel. GC% over 0.1.
The chiral analysis using GC-MS revealed small differences in the enantiomeric ratios of OcAc in the lavender essential oils. Enantiomeric separation of racemic and natural OcAc compared with lavender EO 1 is shown in Figure 1. The results are shown in Table 3.
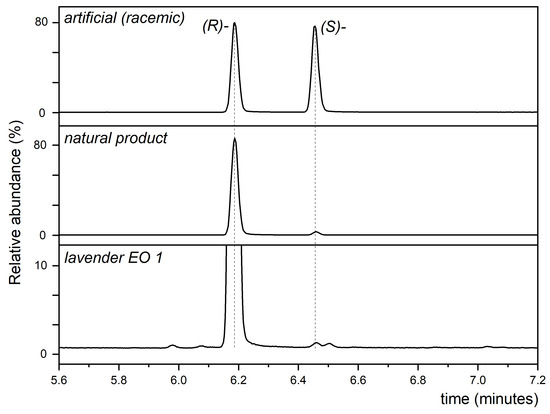
Figure 1.
GC-MS-SIM (m/z 128) chromatograms from chiral analysis 1-octen-3-yl acetate. EO—essential oil.

Table 3.
Ratio of OcAc enantiomers (rel. GC%) in lavender essential oil samples.
4. Discussion
4.1. GC-MS Qualitative Analysis
Numerous essential oils, including those from basil, bergamot, cardamom, coriander, eucalyptus, geranium, grapefruit, clove, lemon, lavender, cinnamon, wild orange, oregano, peppermint, Siberian fir, spearmint, tea tree, and thyme, were analyzed using GC-MS (unpublished results). Among the various oils tested, 1-octen-3-yl acetate was found only in lavender oil. Consequently, our research focused on lavender oil, conducting further chiral analysis to determine the enantiomer ratios for verifying the authenticity of lavender oils from different producers.
GC-MS analysis of lavender essential oils from various sources (EO1 to EO5) reveals a rich and diverse chemical composition. Major components such as linalool (18.8% in EO5 to 38.9% in EO3) and linalyl acetate (17.1% in EO4 to 39.3% in EO2) dominate the profiles, and are essential markers of high-quality lavender oil. Sesquiterpenes like caryophyllene (2.11% in EO4 to 5.19% in EO2) and monoterpenes including -pinene (0.17% in EO4 to 0.20% in EO1 and EO2) and camphene (0.12% in EO2 to 0.31% in EO3) contribute to the oils’ characteristic aroma and potential therapeutic properties. Similarly, the study by Beale et al. (2017) used GC-MS to analyze lavender oils, identifying linalool and linalyl acetate, which are crucial for assessing oil quality, as dominant components. Their findings underscore the importance of these compounds in authenticating and evaluating the therapeutic benefits of lavender oils. Additionally, Shellie et al. (2002) characterized lavender essential oils using GC-MS, highlighting the correlation of linear retention indices with compound profiles, further supporting the relevance of detailed chemical analysis for quality assessment. These studies collectively emphasize the significance of advanced analytical techniques in ensuring the authenticity and therapeutic efficacy of lavender essential oils [17,18].
Oxygenated compounds, such as eucalyptol (0.70% in EO5 to 1.42% in EO4), lavandulol acetate (0.27% in EO2 to 5.50% in EO1), and terpinen-4-ol (0.21% in EO3 to 4.43% in EO2), enhance the antimicrobial efficacy of the EO. Esters and alcohols, including butyl butanoate (0.18% in EO2 to 0.52% in EO4), hexyl acetate (0.32% in EO5 to 0.97% in EO4), 1-octen-3-ol (0.18% in EO4 to 0.31% in EO3), and 3-octanol (0.23% in EO5 to 1.04% in EO4), though only present in moderate amounts, significantly influence the fragrance profile with their fruity and floral scents. A characteristic compound with content between 0.53% in EO3 to 1.26% in EO5 was OcAc, with a levander and mushroom-like scent [16].
Trace compounds like -terpinene (0.11% in EO2) and -terpinolen (0.63% in EO4), and unique components such as ethyl-2-(5-methyl-5-vinyltetrahydrofuran-2-yl) propan-2-yl carbonate (0.18% in EO2 to 2.85% in EO5) and cis--bergamotene (0.13% in EO5 to 0.26% in EO3), despite their lower concentrations, contribute to the complexity and authenticity of the oils.
The identified compounds underscore the geographical and botanical influences on lavender EOs and highlight the importance of GC-MS in ensuring their quality, authenticity, and therapeutic efficacy [12].
4.2. GC-MS Chiral Analysis
Table 3 provides information on the producer variety and location for each lavender essential oil sample, which are crucial for understanding the geographical influence on the chemical profiles. EO1 is sourced from France, and EO2 and EO3 are also sourced from France, suggesting that these samples might share similar characteristics due to being produced in the same country, though slight regional variations could still be present. EO4 is sourced from Bulgaria, potentially showcasing a different chemical profile influenced by the distinct climatic and geographical conditions of this region. EO5 is sourced from Slovakia, adding another layer of geographic diversity.
Table 2 explores the enantiomer ratios of OcAc in the lavender EO samples, emphasizing the chiral purity of these compounds. The natural product standard exhibits 97.0% (R)-OcAc and 3.0% (S)-OcAc, serving as a baseline for comparison. EO1 shows an enantiomeric purity of 99.5% (R)-OcAc and 0.5% (S)-OcAc, indicating a high level of natural composition. EO2 and EO3 exhibit 99.8% and 99.8% (R)-OcAc, with slight variations in (S)-OcAc at 0.2% and 0.1%, respectively, indicating no synthetic material addition. EO4 also has a high purity of 99.7% (R)-OcAc and 0.3% (S)-OcAc, aligning closely with the natural standard. EO5 stands out with the highest enantiomeric purity of 99.8% (R)-OcAc and 0.2% (S)-OcAc, indicating exceptional quality.
5. Conclusions
This study provided a detailed analysis of lavender essential oils from various regions using advanced techniques such as GC-MS and chiral analysis. We focused on 1-octen-3-yl acetate (OcAc), a key chiral compound in lavender oil. The results revealed a diverse chemical composition across different lavender oil samples, with linalool and linalyl acetate as predominant components.
OcAc was found only in lavender oil among the various essential oils tested, highlighting its significance in lavender’s scent. Chiral analysis showed the high enantiomeric purity of OcAc, with (R)-OcAc percentages ranging from 99.7% to 99.9%, confirming no synthetic adulteration and high authenticity.
These findings underscore the importance of rigorous analytical methods in ensuring essential oil quality and authenticity. They also highlight the influence of geographical origin on lavender oil composition, providing valuable insights for producers, consumers, and regulators. Understanding compounds like OcAc is crucial for sourcing high-quality, natural products that meet commercial and therapeutic standards.
In summary, this research enhances the scientific understanding of lavender essential oils and emphasizes the importance of maintaining high standards in production and authenticity verification. Future research should explore essential oil composition variability and its implications for quality control and therapeutic efficacy.
Author Contributions
Conceptualization, J.Č., P.F. and J.B.; methodology J.Č and J.B.; investigation J.Č. and J.P.; writing-original draft preparation J.Č. and J.B.; writing-review and editing P.F.; visualization J.P. All authors have read and agreed to the published version of the manuscript.
Funding
This publication was supported by the Slovak Research and Development Agency under contract numbers APVV-18-0282 and APVV-20-0317. The work was also supported of the project “Advancing University Capacity and Competence in Research, Development and Innovation” (ITMS project code: 313021X329) supported by Operational Programme Integrated Infrastructure and funded by the European Regional Development Fund.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Meenu, M. Antibacterial activity of essential oils from different parts of plants against. Food Chem. 2023, 404, 142–149. [Google Scholar] [CrossRef]
- Atanasova, T.; Gochev, V.; Nenov, N.; Djurkov, T.; Girova, T.; Merdzhanov, P.; Stoyanov, A. Lavender extract with tetraflouroethane—Chemical composition, antimicrobial activity and application in cosmetics. World Sci. 2016, 1, 10–15. [Google Scholar]
- Tomicic, Z.; Cabarkapa, I.; Saric, L.; Dzomba, M.; Tomicic, R. Germicidal efficancy of disinfectant based on sodium hypochlorite and essential oils. J. Food Nutr. Res. 2023, 62, 346–353. [Google Scholar]
- Capetti, F.; Marengo, A.; Cagliero, C.; Liberto, E.; Bicchi, C.; Rubiolo, P.; Sgorbini, B. Adulteration of Essential Oils: A Multitask Issue for Quality Control. Three Case Studies: Lavandula angustifolia Mill., Citrus limon (L.) Osbeck and Melaleuca alternifolia (Maiden & Betche) Cheel. Molecules 2021, 26, 5610. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.M.; Poulson, A.; Murphy, B.J.; Nebeker, B.; Cuchet, A.; Schiets, F.; Casabianca, H.; Carlson, R.E. Authentication of Lavandula angustifolia Mill. (Lamiaceae) essential oil using physical property, gas chromatography, enantiomeric selectivity, and stable isotope analyses. J. Essent. Oil Res. 2022, 35, 529–541. [Google Scholar] [CrossRef]
- Koulivand, P.H.; Khaleghi Ghadiri, M.; Gorji, A. Lavender and the nervous system. Evid.-Based Complement. Altern. Med. 2013, 2013, 681304. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Torsten, M. Antifungal activity of Zataria multiflora Boiss. essential oils and changes in volatile compound composition under abiotic stress conditions. Ind. Crop. Prod. 2021, 171, 113888. [Google Scholar] [CrossRef]
- Catella, C.; Camero, M.; Lucente, M.S.; Fracchiolla, G.; Sblano, S.; Tempesta, M.; Martella, V.; Buonavoglia, C.; Lanave, G. Virucidal and antiviral effects of Thymus vulgaris essential oil on feline coronavirus. Res. Vet. Sci. 2021, 137, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, H.M.A.; Wilkinson, J.M. Biological activities of lavender essential oil. Phytother. Res. 2002, 16, 301–308. [Google Scholar] [CrossRef]
- Boelens, M.H.; Jimenez, R. Chemical composition of the essential oils from lavender and other linalool-rich plants. J. Chromatogr. A 2003, 1000, 325–356. [Google Scholar]
- Schipilliti, L.; Dugo, P.; Bonaccorsi, I.; Mondello, L. Authenticity control on lemon essential oils employing Gas Chromatography– Combustion-Isotope Ratio Mass Spectrometry (GC–C-IRMS). Food Chem. 2012, 131, 1523–1530. [Google Scholar] [CrossRef]
- Do, K.T.; Hadji-Minaglou, F.; Antoniotti, S.; Fernandez, X. Authenticity of essential oils. TrAC 2015, 66, 146–157. [Google Scholar] [CrossRef]
- Brenna, E.; Fuganti, C.; Serra, S. Enantioselective perception of chiral odorants. Tetrahedron Asymmetry 2003, 14, 1–42. [Google Scholar] [CrossRef]
- Aimila, A.; Aisa, H.A.; Maiwulanjiang, M. Study on Lavender Essential Oil Chemical Compositions by GC-MS and Improved pGC. Molecules 2020, 25, 3166. [Google Scholar] [CrossRef] [PubMed]
- Api, A.M.; Belmonte, F.; Belsito, D.; Biserta, S.; Botelho, D.; Bruze, M.; Burton, G.A., Jr.; Buschmann, J.; Cancellieri, M.A.; Dagli, M.L.; et al. RIFM fragrance ingredient safety assessment, 1-octen-3-yl acetate, CAS Registry Number 2442-10-6. Food Chem. Toxicol. 2019, 134, 110638. [Google Scholar] [CrossRef] [PubMed]
- Burdock, G.A. 1-octen-3-yl acetate. In Fenaroli’s Handbook of Flavor Ingredients; Burdock, G. Press: Boca Raton, FL, USA, 2005; p. 752. [Google Scholar]
- Beale, D.J.; Morrison, P.D.; Karpe, A.V.; Dunn, M.S. Chemometric Analysis of Lavender Essential Oils Using Targeted and Untargeted GC-MS Acquired Data for the Rapid Identification and Characterization of Oil Quality. Molecules 2017, 22, 1339. [Google Scholar] [CrossRef] [PubMed]
- Shellie, R.; Mondello, L.; Marriott, P.; Dugo, G. Characterisation of lavender essential oils by using gas chromatography–mass spectrometry with correlation of linear retention indices and comparison with comprehensive two-dimensional gas chromatography. J. Chromatogr. A 2002, 970, 225–234. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).