Revisiting the Absolute Configuration of Peyssonnoside A Using Vibrational Circular Dichroism Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Computational
2.2. Experimental
3. Results and Discussion
3.1. Vibrational Circular Dichroism
3.2. Optical Rotatory Dispersion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Berova, N.; Polavarapu, P.L.; Nakanishi, K.; Woody, R. (Eds.) Comprehensive Chiroptical Spectroscopy, Volume 1: Instrumentation, Methodologies, and Theoretical Simulations; Wiley: New York, NY, USA, 2012. [Google Scholar]
- Berova, N.; Polavarapu, P.L.; Nakanishi, K.; Woody, R. (Eds.) Comprehensive Chiroptical Spectroscopy. Volume 2: Applications in Stereochemical Analysis of Synthetic Compounds, Natural Products, and Biomolecules; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Nafie, L.A. Vibrational Optical Activity: Principles and Applications; John Wiley and Sons Inc.: New York, NY, USA, 2011. [Google Scholar]
- Polavarapu, P.L. Chiroptical Spectroscopy: Fundamentals and Applications; Taylor & Francis: Boca Raton, FL, USA, 2017. [Google Scholar]
- Barron, L.D. Molecular Light Scattering and Optical Activity; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Stephens, P.J.; Devlin, F.J.; Cheeseman, J.R. VCD Spectroscopy for Organic Chemists; CRC Press LLC: Boca Raton, FL, USA, 2012. [Google Scholar]
- Keiderling, T.A. Structure of Condensed Phase Peptides: Insights from Vibrational Circular Dichroism and Raman Optical Activity Techniques. Chem. Rev. 2020, 120, 3381–3419. [Google Scholar] [CrossRef]
- Nafie, L.A. Vibrational Optical Activity: From Discovery and Development to Future Challenges. Chirality 2020, 32, 667–692. [Google Scholar] [CrossRef]
- Pescitelli, G.; Bruhn, T. Good Computational Practice in the Assignment of Absolute Configurations by TDDFT Calculations of ECD Spectra. Chirality 2016, 28, 466–474. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, Y.; Nafie, L.A. Computational Methods and Points for Attention in Absolute Configuration Determination. Front. Nat. Prod. 2023, 1, 1086897. [Google Scholar] [CrossRef]
- Vang, Z.P.; Sonstrom, R.E.; Scolati, H.N.; Clark, J.R.; Pate, B.H. Assignment of the Absolute Configuration of Molecules That Are Chiral by Virtue of Deuterium Substitution Using Chiral Tag Molecular Rotational Resonance Spectroscopy. Chirality 2023, 35, 856–883. [Google Scholar] [CrossRef]
- Sun, W.; Schnell, M. Microwave Three-Wave Mixing Spectroscopy of Chiral Molecules in Weakly Bound Complexes. J. Phys. Chem. Lett. 2023, 14, 7389–7394. [Google Scholar] [CrossRef]
- de Albuquerque, A.C.F.; Martorano, L.H.; dos Santos, F.M. Are We Still Chasing Molecules That Were Never There? The Role of Quantum Chemical Simulations of NMR Parameters in Structural Reassignment of Natural Products. Front. Nat. Prod. 2024, 2, 1321043. [Google Scholar] [CrossRef]
- Mándi, A.; Kurtán, T. Applications of OR/ECD/VCD to the Structure Elucidation of Natural Products. Nat. Prod. Rep. 2019, 36, 889. [Google Scholar] [CrossRef]
- Batista, J.M.; Da, V.; Bolzani, S.; Ao, J.; Batista, M.; Blanch, E.W. Recent Advances in the Use of Vibrational Chiroptical Spectroscopic Methods for Stereochemical Characterization of Natural Products. Nat. Prod. Rep. 2015, 32, 1269–1358. [Google Scholar] [CrossRef]
- Polavarapu, P.L.; Santoro, E. Vibrational Optical Activity for Structural Characterization of Natural Products. Nat. Prod. Rep. 2020, 37, 1661–1699. [Google Scholar] [CrossRef]
- Grauso, L.; Teta, R.; Esposito, G.; Menna, M.; Mangoni, A. Computational Prediction of Chiroptical Properties in Structure Elucidation of Natural Products. Nat. Prod. Rep. 2019, 36, 1005. [Google Scholar] [CrossRef] [PubMed]
- Batista, A.N.L.; Angrisani, B.R.P.; Lima, M.E.D.; da Silva, S.M.P.; Schettini, V.H.; Chagas, H.A.; dos Santos, F.M.; Batista, J.M.; Valverde, A.L. Absolute Configuration Reassignment of Natural Products: An Overview of the Last Decade. J. Braz. Chem. Soc. 2021, 32, 1499–1518. [Google Scholar] [CrossRef]
- Del Río, R.E.; Joseph-Nathan, P. Vibrational Circular Dichroism Absolute Configuration of Natural Products From 2015 to 2019. Nat. Prod. Commun. 2021, 16, 1934578X21996166. [Google Scholar] [CrossRef]
- Merten, C.; Golub, T.P.; Kreienborg, N.M. Absolute Configurations of Synthetic Molecular Scaffolds from Vibrational CD Spectroscopy. J. Org. Chem. 2019, 84, 8797–8814. [Google Scholar] [CrossRef] [PubMed]
- Polavarapu, P.L. Why Is It Important to Simultaneously Use More than One Chiroptical Spectroscopic Method for Determining the Structures of Chiral Molecules? Chirality 2008, 20, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Rehman, N.U.; Hussain, H.; Al-Shidhani, S.; Kumar Avula, S.; Abbas, G.; Muhammad, A.; Anwar, U.; Orecki, D.G.; Pescitelli, M.G.; Al-Harrasi, A. Incensfuran: Isolation, X-Ray Crystal Structure and Absolute Configuration by Means of Chiroptical Studies in Solution and Solid State. RSC Adv. 2017, 7, 42357–42362. [Google Scholar] [CrossRef]
- Smyrniotopoulos, V.; Merten, C.; Kaiser, M.; Tasdemir, D. Bifurcatriol, a New Antiprotozoal Acyclic Diterpene from the Brown Alga Bifurcaria Bifurcata. Mar. Drugs 2017, 15, 245. [Google Scholar] [CrossRef]
- A Kadir, M.F.; Wibowo, A.; Salim, F.; Anouar, E.H.; Awang, K.; Langat, M.K.; Ahmad, R. Conformational Analysis of Diterpene Lactone Andrographolide towards Reestablishment of Its Absolute Configuration via Theoretical and Experimental ECD and VCD Methods. Indones. J. Chem. 2020, 21, 148. [Google Scholar] [CrossRef]
- Zhang, P.; Xue, S.; Tang, P.; Cui, Z.; Wang, Z.; Luo, J.; Kong, L. Aphamines A-C, Dimeric Acyclic Diterpene Enantiomers from Aphanamixis Polystachya. Chin. Chem. Lett. 2021, 32, 1480–1484. [Google Scholar] [CrossRef]
- Wang, W.-L.; Liu, X.-Q.; Zhu, D.-R.; Chen, C.; Gong, L.-J.; Zhu, J.-M.; Zhu, T.-Y.; Luo, J.-G.; Kong, L.-Y. Taxodinoids A-D, Four Heptacyclic C 40 Diterpene Dimers from the Seeds of Taxodium Distichum. Tetrahedron 2021, 83, 131952. [Google Scholar] [CrossRef]
- Khatri Chhetri, B.; Lavoie, S.; Marie Sweeney-Jones, A.; Mojib, N.; Raghavan, V.; Gagaring, K.; Dale, B.; McNamara, C.W.; Soapi, K.; Quave, C.L.; et al. Peyssonnosides A−B, Unusual Diterpene Glycosides with a Sterically Encumbered Cyclopropane Motif: Structure Elucidation Using an Integrated Spectroscopic and Computational Workflow. J. Org. Chem. 2019, 84, 8431–8541. [Google Scholar] [CrossRef]
- Chini, M.G.; Jones, C.R.; Zampella, A.; D’Auria, M.V.; Renga, B.; Fiorucci, S.; Butts, C.P.; Bifulco, G. Quantitative NMR-Derived Interproton Distances Combined with Quantum Mechanical Calculations of 13C Chemical Shifts in the Stereochemical Determination of Conicasterol F, a Nuclear Receptor Ligand from Theonella swinhoei. J. Org. Chem. 2012, 77, 1489–1496. [Google Scholar] [CrossRef]
- Laskowski, T.; Szwarc, K.; Szczeblewski, P.; Sowiński, P.; Borowski, E.; Pawlak, J. Monosaccharides as Potential Chiral Probes for the Determination of the Absolute Configuration of Secondary Alcohols. J. Nat. Prod. 2016, 79, 2797–2804. [Google Scholar] [CrossRef]
- Grimblat, N.; Zanardi, M.M.; Sarotti, A.M. Beyond DP4: An Improved Probability for the Stereochemical Assignment of Isomeric Compounds Using Quantum Chemical Calculations of NMR Shifts. J. Org. Chem. 2015, 80, 12526–12534. [Google Scholar] [CrossRef]
- Chesnokov, G.A.; Gademann, K. Concise Total Synthesis of Peyssonnoside A. J. Am. Chem. Soc. 2021, 143, 14083–14088. [Google Scholar] [CrossRef]
- Xu, B.; Liu, C.; Dai, M. Catalysis-Enabled 13-Step Total Synthesis of (−)-Peyssonnoside A. J. Am. Chem. Soc. 2022, 144, 19700–19703. [Google Scholar] [CrossRef]
- Batista, A.N.L.; Santos, C.H.T.; de Albuquerque, A.C.F.; Santos, F.M., Jr.; Garcez, F.R.; Batista, J.M., Jr. Absolute Configuration Reassignment of Nectamazin A: Implications to Related Neolignans. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 304, 123283. [Google Scholar] [CrossRef]
- Scholten, K.; Engelage, E.; Merten, C. Basis Set Dependence of S=O Stretching Frequencies and Its Consequences for IR and VCD Spectra Predictions. Phys. Chem. Chem. Phys. 2020, 22, 27979–27986. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Perdew, J.P.; Wang, Y. Accurate and Simple Analytic Representation of the Electron-Gas Correlation Energy. Phys. Rev. B 1992, 45, 13244–13249. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Ashvar, C.S.; Chabalowski, C.F.; Frisch, M.J. Theoretical Calculation of Vibrational Circular Dichroism Spectra. Faraday Discuss. 1994, 99, 103–119. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Gilbert, K.E. Pcmodel 10.0; Serena Software: Bloomington, IN, USA, 2013. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Varone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Hariharan, P.C.; Pople, J.A. Accuracy of AHn Equilibrium Geometries by Single Determinant Molecular Orbital Theory. Mol. Phys. 1974, 27, 209–214. [Google Scholar] [CrossRef]
- Hariharan, P.C.; Pople, J.A. The Influence of Polarization Functions on Molecular Orbital Hydrogenation Energies. Theor. Chim. Acta 1973, 28, 213–222. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef] [PubMed]
- Scalmani, G.; Frisch, M.J. Continuous Surface Charge Polarizable Continuum Models of Solvation. I. General Formalism. J. Chem. Phys. 2010, 132, 6158. [Google Scholar] [CrossRef] [PubMed]
- Mclean, A.D.; Chandler, G.S. Contracted Gaussian Basis Sets for Molecular Calculations. I. Second Row Atoms, Z = 11–18. J. Chem. Phys. 1980, 72, 5639. [Google Scholar] [CrossRef]
- Frisch, M.J.; Pople, J.A.; Binkley, J.S. Self-Consistent Molecular Orbital Methods 25. Supplementary Functions for Gaussian Basis Sets. J. Chem. Phys. 1984, 80, 3265. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-Consistent Molecular Orbital Methods. XX. A Basis Set for Correlated Wave Functions. J. Chem. Phys 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Polavarapu, P.L.; Santoro, E.; Covington, C.L.; Johnson, J.L.; Puente, A.R.; Schley, N.D.; Kallingathodi, Z.; Prakasan, P.C.; Haleema, S.; Thomas, A.A.; et al. How Important Are the Intermolecular Hydrogen Bonding Interactions in Methanol Solvent for Interpreting the Chiroptical Properties? Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 247, 119094. [Google Scholar] [CrossRef] [PubMed]
- Covington, C.L.; Polavarapu, P.L. Similarity in Dissymmetry Factor Spectra: A Quantitative Measure of Comparison between Experimental and Predicted Vibrational Circular Dichroism. J. Phys. Chem. A 2013, 117, 3377–3386. [Google Scholar] [CrossRef] [PubMed]
- Covington, C.L.; Polavarapu, P.L. CDSpecTech: A Single Software Suite for Multiple Chiroptical Spectroscopic Analyses. Chirality 2017, 29, 178–192. [Google Scholar] [CrossRef] [PubMed]
- Legault, C.Y. CYLview20; Université de Sherbrooke: Sherbrooke, QC, Canada, 2020. [Google Scholar]
- Eikås, K.D.R.; Beerepoot, M.T.P.; Ruud, K. A Computational Protocol for Vibrational Circular Dichroism Spectra of Cyclic Oligopeptides. J. Phys. Chem. A 2022, 2022, 5471. [Google Scholar] [CrossRef] [PubMed]
- Eikås, K.D.R.; Krupová, M.; Kristoffersen, T.; Beerepoot, M.T.P.; Ruud, K. Can the Absolute Configuration of Cyclic Peptides Be Determined with Vibrational Circular Dichroism? Phys. Chem. Chem. Phys. 2023, 25, 14520. [Google Scholar] [CrossRef] [PubMed]
- Vermeyen, T.; Merten, C. Solvation and the Secondary Structure of a Proline-Containing Dipeptide: Insights from VCD Spectroscopy. Phys. Chem. Chem. Phys. 2020, 22, 15640–15648. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.J.P. Optimization of Parameters for Semiempirical Methods V: Modification of NDDO Approximations and Application to 70 Elements. J. Mol. Model. 2007, 13, 1173–1213. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132. [Google Scholar] [CrossRef]
- Bünnemann, K.; Merten, C. Solvation of a Chiral Carboxylic Acid: Effects of Hydrogen Bonding on the IR and VCD Spectra of α-Methoxyphenylacetic Acid. Phys. Chem. Chem. Phys. 2017, 19, 18948–18956. [Google Scholar] [CrossRef]
- Scholten, K.; Merten, C. Solvation of the Boc-Val-Phe-n Pr Peptide Characterized by VCD Spectroscopy and DFT Calculations. Phys. Chem. Chem. Phys. 2022, 24, 3611–3617. [Google Scholar] [CrossRef]
- Merten, C.; Li, F.; Bravo-Rodriguez, K.; Sanchez-Garcia, E.; Xu, Y.; Sander, W. Solvent-Induced Conformational Changes in Cyclic Peptides: A Vibrational Circular Dichroism Study. Phys. Chem. Chem. Phys. 2014, 16, 5627–5633. [Google Scholar] [CrossRef] [PubMed]
- Perera, A.S.; Thomas, J.; Poopari, M.R.; Xu, Y. The Clusters-in-a-Liquid Approach for Solvation: New Insights from the Conformer Specific Gas Phase Spectroscopy and Vibrational Optical Activity Spectroscopy. Front. Chem. 2016, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Weirich, L.; Blanke, K.; Merten, C. More Complex, Less Complicated? Explicit Solvation of Hydroxyl Groups for the Analysis of VCD Spectra. Phys. Chem. Chem. Phys. 2020, 22, 12515–12523. [Google Scholar] [CrossRef] [PubMed]
- Puente, A.R.; Polavarapu, P.L. Influence of Microsolvation on Vibrational Circular Dichroism Spectra in Dimethyl Sulfoxide Solvent: A Bottom-Up Approach Using Quantum Cluster Growth. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 303, 123231. [Google Scholar] [CrossRef]

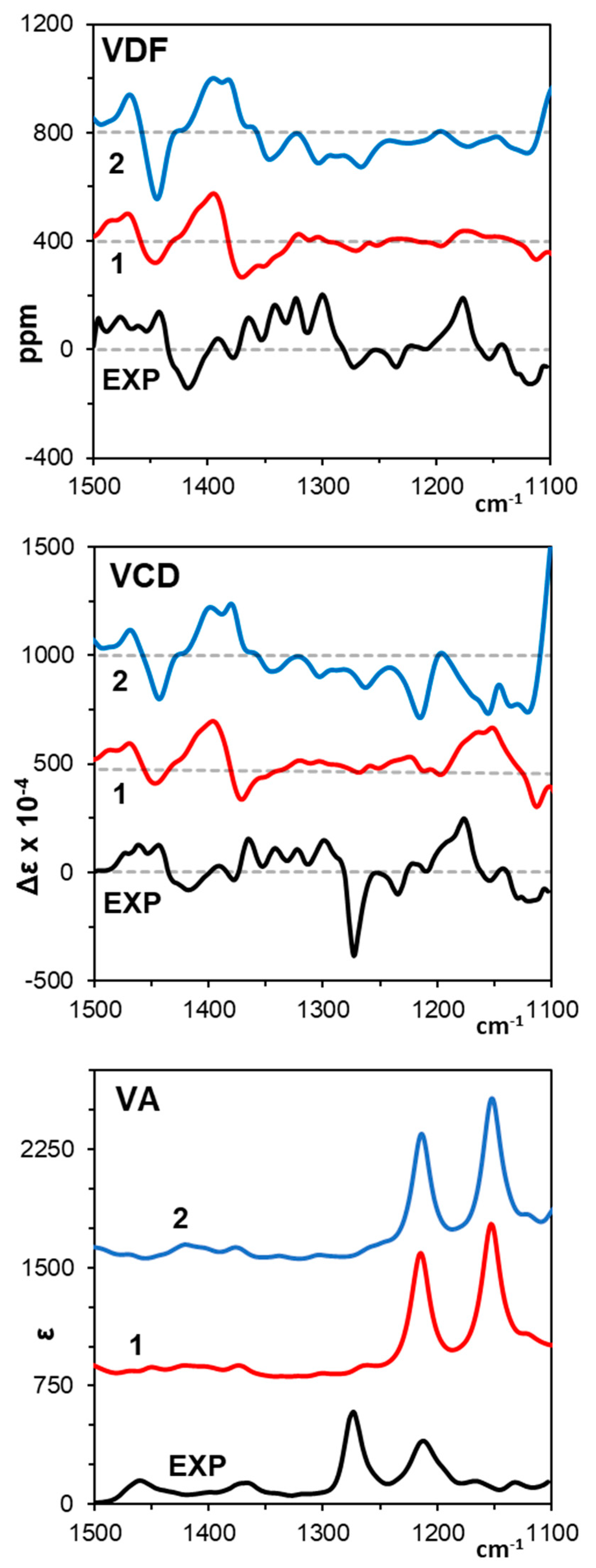

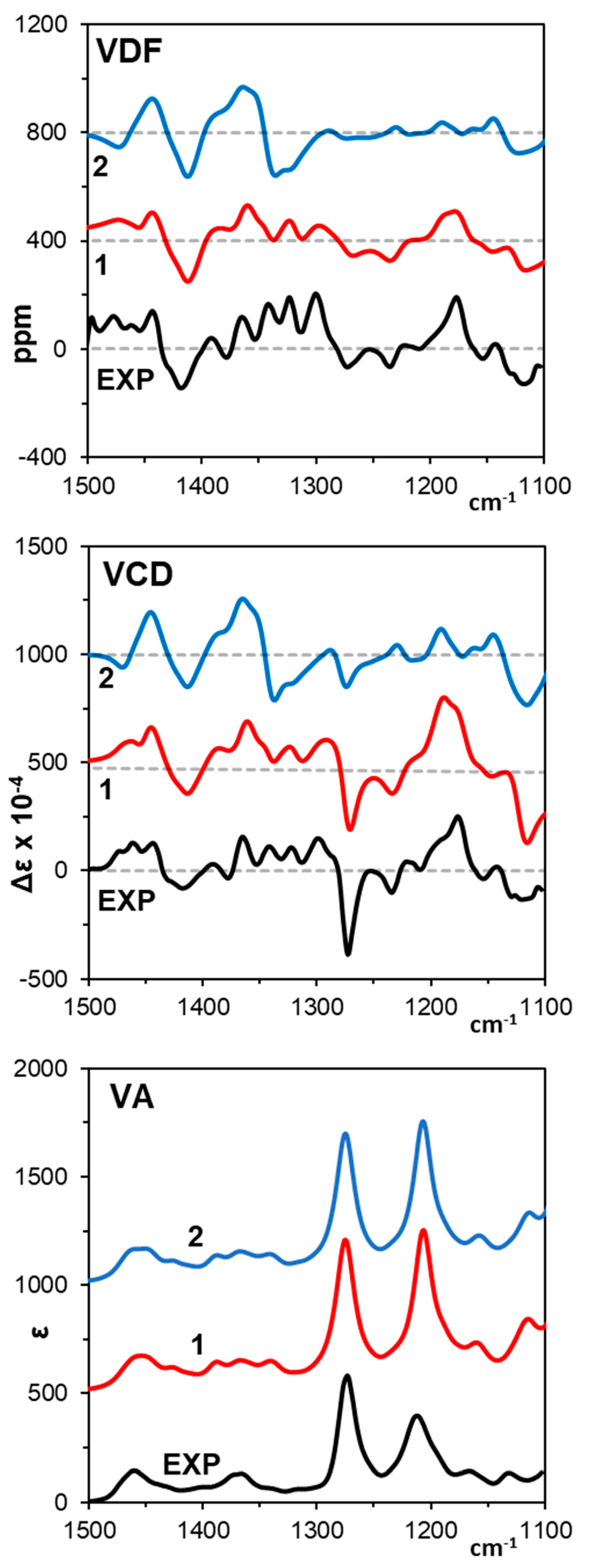

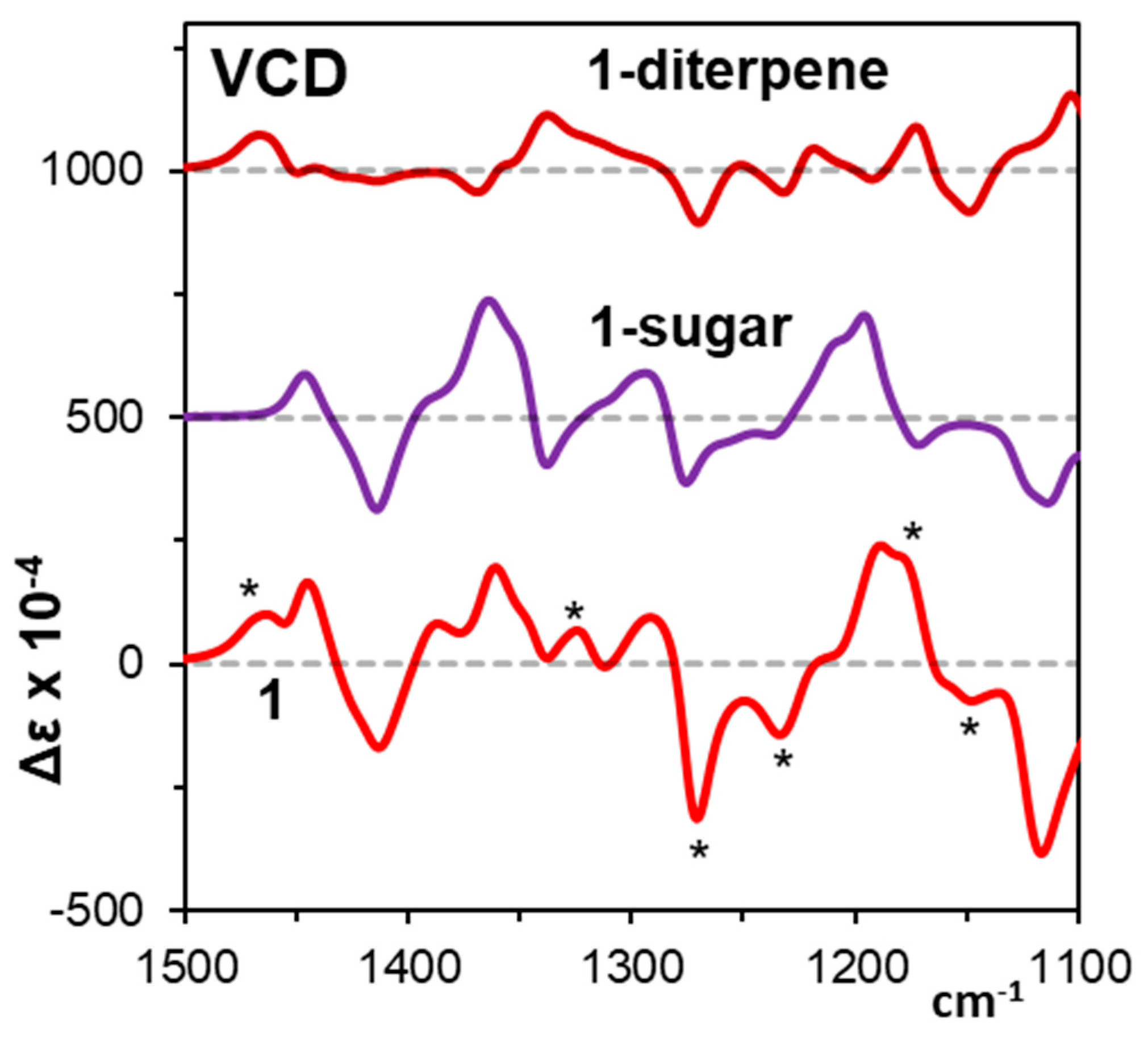
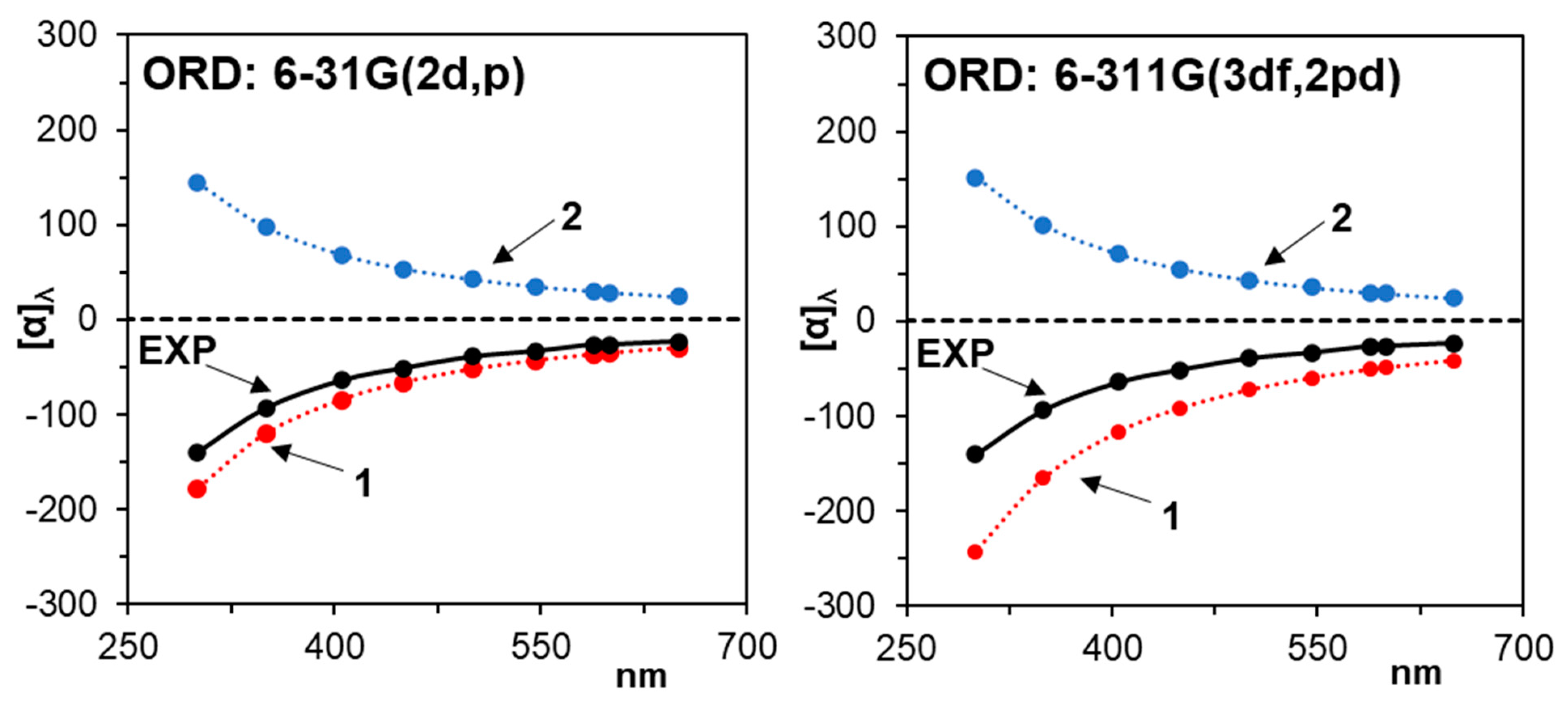
| B3LYP/6-311++G(2d, 2p) | ||||
|---|---|---|---|---|
| Compared Spectra | σ | SimVA | MaxSimVCD | SimVDF |
| 1 and Exp. | 1.035 | 0.62 | +0.30 | +0.27 |
| 2 and Exp. | 0.981 | 0.40 | +0.14 | +0.23 |
| B3PW91/6-31G(2d, p)/PCM | ||||
| 1 and Exp. | 0.980 | 0.54 | +0.47 | +0.42 |
| 2 and Exp. | 0.989 | 0.70 | +0.24 | –0.01 |
| B3PW91/6-311G(3df, 2pd)/PCM | ||||
| 1 and Exp. | 0.981 | 0.94 | +0.76 | +0.67 |
| 2 and Exp. | 0.980 | 0.94 | +0.27 | +0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puente, A.R.; Chhetri, B.K.; Kubanek, J.; Polavarapu, P.L. Revisiting the Absolute Configuration of Peyssonnoside A Using Vibrational Circular Dichroism Spectroscopy. Symmetry 2024, 16, 133. https://doi.org/10.3390/sym16020133
Puente AR, Chhetri BK, Kubanek J, Polavarapu PL. Revisiting the Absolute Configuration of Peyssonnoside A Using Vibrational Circular Dichroism Spectroscopy. Symmetry. 2024; 16(2):133. https://doi.org/10.3390/sym16020133
Chicago/Turabian StylePuente, Andrew R., Bhuwan Khatri Chhetri, Julia Kubanek, and Prasad L. Polavarapu. 2024. "Revisiting the Absolute Configuration of Peyssonnoside A Using Vibrational Circular Dichroism Spectroscopy" Symmetry 16, no. 2: 133. https://doi.org/10.3390/sym16020133
APA StylePuente, A. R., Chhetri, B. K., Kubanek, J., & Polavarapu, P. L. (2024). Revisiting the Absolute Configuration of Peyssonnoside A Using Vibrational Circular Dichroism Spectroscopy. Symmetry, 16(2), 133. https://doi.org/10.3390/sym16020133






