High-Field Nonresonant Response of Zundel Cations to Intense Terahertz Radiation
Abstract
:1. Introduction
2. Methods
2.1. PES Scans of Zundel Cations
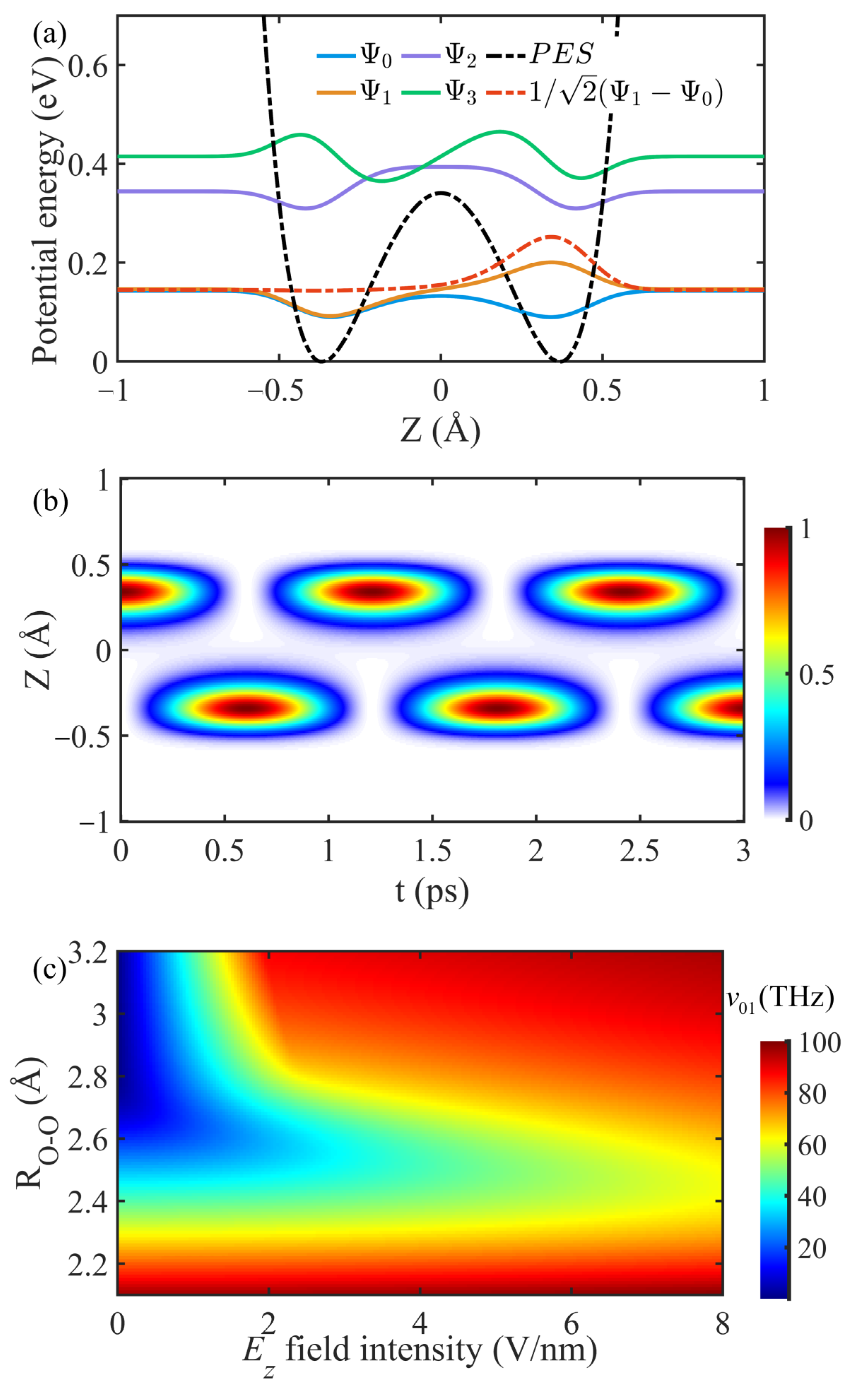
2.2. Solving the TD Schrödinger Equation via the FDTD Method
2.3. AIMD and AI-PIMD Simulations of Zundel Cations
2.4. Calculation of IR Spectrum
3. Results and Discussion
3.1. Deformation of Proton PES under Elevated Static E Fields
3.2. Resonant Interaction between THz Waves and Zundel Cations
3.3. Proton Transfer during Collisional Events
3.4. Proton Transfer Induced by High-Field Nonresonant Interaction
3.5. Vibrational Excitation Instigated by ITR’s Nonresonant Interaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ceriotti, M.; Fang, W.; Kusalik, P.G.; McKenzie, R.H.; Michaelides, A.; Morales, M.A.; Markland, T.E. Nuclear quantum effects in water and aqueous systems: Experiment, theory, and current challenges. Chem. Rev. 2016, 116, 7529–7550. [Google Scholar] [CrossRef] [PubMed]
- Janoschek, R.; Weidemann, E.-G.; Pfeiffer, H.; Zundel, G. Extremely high polarizability of hydrogen bonds. J. Am. Chem. Soc. 1972, 94, 2387–2396. [Google Scholar] [CrossRef]
- Marx, D. Proton transfer 200 years after von Grotthuss: Insights from ab initio simulations. ChemPhysChem 2006, 7, 1848–1870. [Google Scholar] [CrossRef] [PubMed]
- Marx, D.; Chandra, A.; Tuckerman, M.E. Aqueous basic solutions: Hydroxide solvation, structural diffusion, and comparison to the hydrated proton. Chem. Rev. 2010, 110, 2174–2216. [Google Scholar] [CrossRef] [PubMed]
- Sakti, A.W.; Nishimura, Y.; Nakai, H. Recent advances in quantum-mechanical molecular dynamics simulations of proton transfer mechanism in various water-based environments. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2020, 10, e1419. [Google Scholar] [CrossRef]
- Huang, X.; Braams, B.J.; Bowman, J.M. Ab initio potential energy and dipole moment surfaces for H5O2+. J. Chem. Phys. 2005, 122, 044308. [Google Scholar] [CrossRef]
- Vendrell, O.; Meyer, H.-D. A proton between two waters: Insight from full-dimensional quantum-dynamics simulations of the [H2O–H–OH2]+ cluster. Phys. Chem. Chem. Phys. 2008, 10, 4692–4703. [Google Scholar] [CrossRef]
- Ushiyama, H.; Takatsuka, K. Mechanisms of the Elementary Processes of Electron Wavepacket Dynamics Coupled with Proton Transfer and Hydrogen-Atom Migration in H2O+ H3O+. Angew. Chem. 2007, 119, 593–596. [Google Scholar] [CrossRef]
- Maupin, C.M.; Aradi, B.; Voth, G.A. The self-consistent charge density functional tight binding method applied to liquid water and the hydrated excess proton: Benchmark simulations. J. Phys. Chem. B 2010, 114, 6922–6931. [Google Scholar] [CrossRef]
- Nakai, H.; Sakti, A.W.; Nishimura, Y. Divide-and-conquer-type density-functional tight-binding molecular dynamics simulations of proton diffusion in a bulk water system. J. Phys. Chem. B 2016, 120, 217–221. [Google Scholar] [CrossRef]
- Benoit, M.; Marx, D.; Parrinello, M. Tunnelling and zero-point motion in high-pressure ice. Nature 1998, 392, 258–261. [Google Scholar] [CrossRef]
- Giberti, F.; Hassanali, A.A.; Ceriotti, M.; Parrinello, M. The role of quantum effects on structural and electronic fluctuations in neat and charged water. J. Phys. Chem. B 2014, 118, 13226–13235. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Z.; Walker, B.; Michaelides, A. Quantum nature of the hydrogen bond. Proc. Natl. Acad. Sci. USA 2011, 108, 6369–6373. [Google Scholar] [CrossRef]
- Marx, D.; Tuckerman, M.E.; Hutter, J.; Parrinello, M. The nature of the hydrated excess proton in water. Nature 1999, 397, 601–604. [Google Scholar] [CrossRef]
- Cassone, G. Nuclear quantum effects largely influence molecular dissociation and proton transfer in liquid water under an electric field. J. Phys. Chem. Lett. 2020, 11, 8983–8988. [Google Scholar] [CrossRef]
- Sakaushi, K.; Lyalin, A.; Taketsugu, T.; Uosaki, K. Quantum-to-classical transition of proton transfer in potential-induced dioxygen reduction. Phys. Rev. Lett. 2018, 121, 236001. [Google Scholar] [CrossRef] [PubMed]
- Cahlík, A.; Hellerstedt, J.; Mendieta-Moreno, J.I.; Švec, M.; Santhini, V.M.; Pascal, S.; Soler-Polo, D.; Erlingsson, S.I.; Výborný, K.; Mutombo, P. Significance of nuclear quantum effects in hydrogen bonded molecular chains. ACS Nano 2021, 15, 10357–10365. [Google Scholar] [CrossRef]
- Dahms, F.; Costard, R.; Pines, E.; Fingerhut, B.P.; Nibbering, E.T.; Elsaesser, T. The hydrated excess proton in the Zundel cation H5O2+: The role of ultrafast solvent fluctuations. Angew. Chem. Int. Ed. 2016, 55, 10600–10605. [Google Scholar] [CrossRef]
- Wolke, C.T.; Fournier, J.A.; Dzugan, L.C.; Fagiani, M.R.; Odbadrakh, T.T.; Knorke, H.; Jordan, K.D.; McCoy, A.B.; Asmis, K.R.; Johnson, M.A. Spectroscopic snapshots of the proton-transfer mechanism in water. Science 2016, 354, 1131–1135. [Google Scholar] [CrossRef]
- Dahms, F.; Fingerhut, B.P.; Nibbering, E.T.J.; Pines, E.; Elsaesser, T. Large-amplitude transfer motion of hydrated excess protons mapped by ultrafast 2D IR spectroscopy. Science 2017, 357, 491–494. [Google Scholar] [CrossRef]
- Kampfrath, T.; Tanaka, K.; Nelson, K.A. Resonant and nonresonant control over matter and light by intense terahertz transients. Nat. Photonics 2013, 7, 680–690. [Google Scholar] [CrossRef]
- Hafez, H.; Chai, X.; Ibrahim, A.; Mondal, S.; Férachou, D.; Ropagnol, X.; Ozaki, T. Intense terahertz radiation and their applications. J. Opt. 2016, 18, 093004. [Google Scholar] [CrossRef]
- Funkner, S.; Niehues, G.; Schmidt, D.A.; Heyden, M.; Schwaab, G.; Callahan, K.M.; Tobias, D.J.; Havenith, M. Watching the low-frequency motions in aqueous salt solutions: The terahertz vibrational signatures of hydrated ions. J. Am. Chem. Soc. 2012, 134, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Caldeweyher, E.; Bannwarth, C.; Grimme, S. Extension of the D3 dispersion coefficient model. J. Chem. Phys. 2017, 147, 034112. [Google Scholar] [CrossRef] [PubMed]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]
- Hellweg, A.; Hättig, C.; Höfener, S.; Klopper, W. Optimized accurate auxiliary basis sets for RI-MP2 and RI-CC2 calculations for the atoms Rb to Rn. Theor. Chem. Acc. 2007, 117, 587–597. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. Wiley Interdiscip. Rev.-Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system, version 4.0. Wiley Interdiscip. Rev.-Comput. Mol. Sci. 2018, 8, 6. [Google Scholar] [CrossRef]
- Eichkorn, K.; Weigend, F.; Treutler, O.; Ahlrichs, R. Auxiliary basis sets for main row atoms and transition metals and their use to approximate Coulomb potentials. Theor. Chem. Acc. 1997, 97, 119–124. [Google Scholar] [CrossRef]
- Neese, F. An improvement of the resolution of the identity approximation for the formation of the Coulomb matrix. J. Comput. Chem. 2003, 24, 1740–1747. [Google Scholar] [CrossRef] [PubMed]
- Goerigk, L.; Grimme, S. Efficient and Accurate Double-Hybrid-Meta-GGA Density Functionals—Evaluation with the Extended GMTKN30 Database for General Main Group Thermochemistry, Kinetics, and Noncovalent Interactions. J. Chem. Theory Comput. 2011, 7, 291–309. [Google Scholar] [CrossRef]
- Asada, T.; Haraguchi, H.; Kitaura, K. Simulation studies of proton transfer in N2H7+ cluster by classical ab initio Monte Carlo and quantum wave packet dynamics. J. Phys. Chem. A 2001, 105, 7423–7428. [Google Scholar] [CrossRef]
- Wang, K.C.; Yang, L.X.; Wang, S.M.; Guo, L.H.; Ma, J.L.; Tang, J.C.; Bo, W.F.; Wu, Z.; Zeng, B.Q.; Gong, Y.B. Transient proton transfer of base pair hydrogen bonds induced by intense terahertz radiation. Phys. Chem. Chem. Phys. 2020, 22, 9316–9321. [Google Scholar] [CrossRef] [PubMed]
- Kühne, T.D.; Iannuzzi, M.; Del Ben, M.; Rybkin, V.V.; Seewald, P.; Stein, F.; Laino, T.; Khaliullin, R.Z.; Schütt, O.; Schiffmann, F. CP2K: An electronic structure and molecular dynamics software package-Quickstep: Efficient and accurate electronic structure calculations. J. Chem. Phys. 2020, 152, 194103. [Google Scholar] [CrossRef]
- Thomas, M.; Brehm, M.; Fligg, R.; Vöhringer, P.; Kirchner, B. Computing vibrational spectra from ab initio molecular dynamics. Phys. Chem. Chem. Phys. 2013, 15, 6608–6622. [Google Scholar] [CrossRef]
- Vendrell, O.; Gatti, F.; Meyer, H.D. Dynamics and infrared spectroscopy of the protonated water dimer. Angew. Chem. Int. Ed. 2007, 46, 6918–6921. [Google Scholar] [CrossRef]
- Brünig, F.N.; Hillmann, P.; Kim, W.K.; Daldrop, J.O.; Netz, R.R. Proton-transfer spectroscopy beyond the normal-mode scenario. J. Chem. Phys. 2022, 157, 174116. [Google Scholar] [CrossRef]
- Brünig, F.N.; Rammler, M.; Adams, E.M.; Havenith, M.; Netz, R.R. Spectral signatures of excess-proton waiting and transfer-path dynamics in aqueous hydrochloric acid solutions. Nat. Commun. 2022, 13, 4210. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Guo, L.; Zhang, Q.; Ning, H.; Lu, C.; Wang, S.; Gong, Y. High-Field Nonresonant Response of Zundel Cations to Intense Terahertz Radiation. Symmetry 2023, 15, 1798. https://doi.org/10.3390/sym15091798
Wang K, Guo L, Zhang Q, Ning H, Lu C, Wang S, Gong Y. High-Field Nonresonant Response of Zundel Cations to Intense Terahertz Radiation. Symmetry. 2023; 15(9):1798. https://doi.org/10.3390/sym15091798
Chicago/Turabian StyleWang, Kaicheng, Lianghao Guo, Qin Zhang, Hui Ning, Chang Lu, Shaomeng Wang, and Yubin Gong. 2023. "High-Field Nonresonant Response of Zundel Cations to Intense Terahertz Radiation" Symmetry 15, no. 9: 1798. https://doi.org/10.3390/sym15091798
APA StyleWang, K., Guo, L., Zhang, Q., Ning, H., Lu, C., Wang, S., & Gong, Y. (2023). High-Field Nonresonant Response of Zundel Cations to Intense Terahertz Radiation. Symmetry, 15(9), 1798. https://doi.org/10.3390/sym15091798




