Abstract
Unlike in human research, infants are poorly represented in the literature on nonhuman primate laterality. Studies have traditionally measured adults, a trend captured by prior reviews. The extent of the knowledge gaps related to laterality measured early in the lifespan is unknown. As a starting point, this systematic review examined the evidence on behavioral laterality across the first year of life in nonhuman primates using the PRISMA guidelines. The inclusion criteria were at least one measure of behavioral laterality in at least one subject < 1 year old. Database searches were conducted in PsycINFO, PubMed, and OVID Medline using the filterNHP search builder tool, and additional records were identified through citation searching. Two independent reviewers screened abstracts and full texts; 47 articles were retained (0 prosimian, 6 platyrrhine, 27 catarrhine, and 14 ape studies). Macaca and Pan were overrepresented. Nipple preference was the most-studied behavior, followed by hand preference. Modifying how data are collected and analyzed will increase developmental rigor in primate studies. To facilitate comparisons with the human infant literature, we suggest measuring a behavior more than once to test for change or continuity in preference over time and measuring different behaviors at different timepoints to test for potential developmental cascades.
1. Introduction
Reports of behavioral biases across the first year of life are ubiquitous in humans, but, for nonhuman primates, what is known about laterality largely stems from adult studies. The most well-known example of behavioral laterality is handedness, which is a marked preference for using one hand over the other. Reviews of primate handedness, e.g., [1,2,3,4,5,6,7], have focused on the search for population-level preferences in the context of revealing the evolutionary origins of handedness and the origins of laterality in general. This quest has largely been driven by the distinctive pattern in human handedness (9:1 ratio of right-handers to left-handers; [8]). Yet the human adult ratio does not reflect the pattern seen in human infants. Large-scale longitudinal studies have found that there are multiple trajectories in the development of handedness—some infants exhibit a clear right-hand preference, others exhibit a clear left-hand preference, and some exhibit no hand preference [9,10]. Studying early infancy is important for elucidating the origins of handedness because evidence supports that handedness is not innate but rather emerges in human infants from cascading developmental events beginning prenatally [11,12,13]. Thus, it is not sufficient to ask questions about the origins of handedness in primates using only adult data [14]. This review prioritized characterizing how investigators have examined development rather than individual- or population-level findings typically reported in other reviews. Testing the three Cs (change, continuity, and cascades) requires developmental rigor, which will facilitate a fourth “C” that we believe is a key goal for investigators examining laterality in nonhuman samples: comparisons with human studies.
To capture potential developmental approaches to studying behavioral laterality in primates, we focused our review on studies that included subjects of less than 1 year of age. While infants have been described as poorly represented in primate handedness studies [14,15,16,17] and in primate cognitive research more broadly [18], to our knowledge, no one has quantified how many studies have measured behavioral laterality across the first year of life in nonhuman primates, nor has anyone assessed the developmental quality of this body of work. This study reviewed the literature on primate behavioral laterality to synthesize information on: (1) what species have been studied; (2) what behaviors have been examined; and (3) what types of comparisons, if any, have been made. The primary goal was to assess the developmental rigor of the included studies to determine whether the author(s) addressed how laterality develops, as evidenced by how the research question was framed, the study design and analyses used, and how data were interpreted. As a secondary goal, we described findings from the highest-rated studies on developmental quality to illustrate the kinds of information that can be gained with this research lens. We provided comparisons to human infants where relevant for readers who may be less familiar with this literature. Finally, we generated recommendations for future research.
2. Methods
This systematic review of the literature on behavioral laterality across the first year of life in primates was conducted and reported using the Preferred Reporting Items for Systematic Review and Meta-Analyses guidelines (PRISMA 2020 statement; [19]), and the review protocol was prepared with the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) statement [20,21]. The protocol was registered via OSF on 15 January 2023 and amended on 4 May 2023 [22].
Studies examining any aspect of behavioral laterality where at least one individual was a nonhuman primate <1 year old were included, and data were provided for these individuals separately or as a group within the target range. Longitudinal studies that began in the target range but continued beyond 1 year of age were included. In any cases where multiple species were measured, data were reported for species meeting our age-based criteria only. If the exact age(s) of the subject(s) could not be determined from a review of the full text (e.g., the article used the term “infants” with no numerical values), the original authors were contacted via email to determine whether the study met the inclusion criteria. If the study reported additional data beyond behavioral laterality, these findings were not considered in our review. There was no restriction on the type of study design or setting. Only studies with the full text in English were included.
PubMed, PsycINFO, and Web of Science were searched. No date restrictions were imposed for PubMed or PsycINFO. However, coverage for Web of Science started at 1977. The search strategies were developed in two steps. First, search filters were created to select all nonhuman primate species using the filterNHP tool [23]. Second, specific search terms for the topic related to behavioral laterality and early development were developed. Topic keywords included “laterality” or “handedness” or “hand preference” or “asymmetry” AND “infant” or “longitudinal” or “developmental” or “neonatal” or “neonate”. The terms from filterNHP were combined with the topic terms to increase the sensitivity and magnitude of searches. For the full search strings, see Table A1.
Records identified from database searching were entered into EndNote [24], a software program for managing references, to remove duplicates across the databases. Once duplicates were removed, the remaining results were uploaded to Abstrackr [25], which is an online tool for organizing results in a systematic review. Abstrackr was used to manually screen abstracts. Using the eligibility criteria, both authors independently screened the titles and abstracts of all search results. The full text was obtained for titles marked “yes” or “maybe” by the reviewer authors. The full text was also obtained in cases where the reviewers disagreed on classification. Some disagreements were due to a difference between reviewers in prior knowledge of specific studies rather than the application of the objective inclusion/exclusion criteria while reading abstracts. Full-text reports were screened independently by both reviewer authors to determine eligibility, and any disagreements were resolved through discussion. At the end of the screening, a spreadsheet was exported from Abstrackr. Additional studies that were identified from cited or citing articles were added manually to the spreadsheet. Figure 1 shows the study selection process. Table A2 contains a list of reports that were excluded after the screening stage along with the reason. Data extraction was performed in duplicate by reviewers working independently using a form developed by the first author. Any disagreements were resolved by discussion. The reviewers recorded characteristics about the subjects, study design, any comparators (i.e., comparisons to conspecifics, other primate species, or humans), and outcomes.
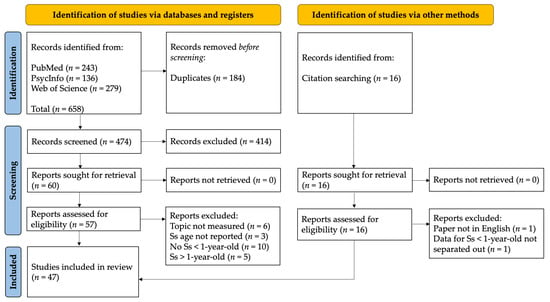
Figure 1.
PRISMA flow chart. Ss = subjects. See Table A2 for a list of reports excluded by reason.
Both authors independently assessed the developmental quality of the included studies using a tool developed by the first author for this systematic review. Articles were rated with respect to five questions, where a “0” was “no” and a “1” was “yes” (range: 0–5 points). Regarding the Introduction, the reviewers considered whether the author(s) framed a research question on behavioral laterality from a developmental perspective (e.g., ontogeny of laterality, change or continuity in lateralized behavior relative to other life stages). Regarding the Methods, there were two questions on the use of a longitudinal design. The reviewers examined whether the author(s) measured the same preference more than once and whether there was more than one timepoint to account for the possibility that different preferences were measured at different times. Any study that pooled data into a single score for analysis was rated “0”. Regarding the Results, the reviewers examined whether the author(s) reported a developmental analysis related to behavioral laterality. In other words, was continuity or change found when the data were examined across time? Regarding the Discussion, the reviewers considered whether the author(s) interpreted their laterality data from a developmental perspective. Thus, the reviewers looked for developmental rigor in examining laterality in behavior beyond simply collecting data on subjects who were not adults. It is important to note that this systematic review interpreted data through a developmental lens; however, this may not have been the goal of the original author(s). Therefore, ratings given here for developmental quality should not be interpreted as ratings of methodological quality.
3. Results and Discussion
A total of 47 studies were retained, representing 17 total primate species (Table 1), including 6 studies with platyrrhine monkeys (3 species; 13% of reports), 27 studies with catarrhine monkeys (11 species; 57% of reports), and 14 studies with apes (3 species; 30% of reports). The most-sampled genera within each of the taxonomic groups were Callithrix (67%), Macaca (74%), and Pan (93%). No prosimian study met the review criteria. Roughly one-half of the studies included a comparator that was either a conspecific (the mother in 17 out of 20 studies) or another primate species (6 studies). No studies included human data. The presence (or absence) of comparators is relevant for identifying differences in sampling. In some studies, all subjects met our age criteria, like most human infant research. In other studies, however, younger subjects were mixed with other age classes in analyses. Convenience sampling is common in primate studies but not in human studies. Most data were collected in captive settings (35 of 47 studies; 75% of reports). Four studies (8% of reports) measured behavioral laterality in free-ranging animals (i.e., island groups), while eight studies (17% of reports) were conducted on individuals in the wild. The studies were published between 1964 and 2022, representing over six decades of research.

Table 1.
Details of the included articles, including the source, species, N, setting, behavior(s), and developmental quality rating.
Behaviors examined for laterality in the included studies were classified into one of eight categories (Figure 2). The most-studied asymmetry was nipple preference, followed by hand preference. Within hand preference, unimanual measures were most common and included behaviors like food reaching, nonfood reaching, self-touch, and tool use. Four studies measured bimanual hand preference with the tube task, which is the gold standard in adult primate handedness (for a discussion, see [16]). Nipple preference and hand preference accounted for approximately two-thirds of the laterality categories that were studied. The remaining one-third comprised the other six categories: head orientation preference; neonatal assessment (e.g., adapted Brazelton); leading limb in stepping, crawling, or walking; ear preference; foot preference; or another asymmetry, such as laterality in facial or vocal expressions. Most studies reported data within one laterality category (34 of 47; 72%). This type of design can be used to test continuity or change in a single behavior if multiple timepoints are used with appropriate statistics. There were some studies that reported data for two (7 of 47; 15%) or three (5 of 47; 11%) of the categories, and only one study reported data in four of the categories. Designs with multiple behaviors can be used to test cascades (e.g., early asymmetry predicting a later asymmetry), in addition to change or continuity within individual behaviors, if multiple timepoints are used with appropriate statistics. Only nine studies provided complete raw data for use in future meta-analyses.
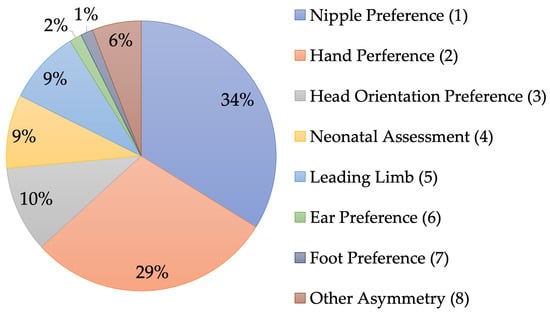
Figure 2.
The proportion of studies that assessed each type of behavioral laterality category. Some studies measured preferences in multiple behaviors within or across categories (see Table 1).
Rather than reviewing the patterns of laterality found in the included papers (i.e., individual- and/or population-level effects for the direction and strength of preferences, which could be the focus of a future meta-analysis), we first summarized outcomes from studies that reported on the top two most-studied behaviors, nipple preference and hand preference, from a developmental perspective. Regardless of the study design, the findings were categorized into patterns to determine how often the authors reported continuity, or a period of stable preference; change, or a period of variable or no preference; or cascades, where different variables were measured at different timepoints and relation(s) between them were examined. The cascades category did not include any findings from analyses between variables collected at the same timepoint. Since several studies measured preference at a single timepoint or pooled data for reporting, we created a final category called snapshot to denote when authors captured a moment in time—in other words, the authors collected data on subjects under 1 year of age but did not address any of our markers for developmental rigor (i.e., continuity, change, or cascades). We provide methodological considerations for collecting nipple preference and hand preference data in future research since these topics were the most studied before turning our focus to discussing the individual articles that received the highest developmental quality scores.
3.1. Considerations for Measuring Nipple Preference Across the First Year of Life
A summary of developmental outcomes by species in studies that measured nipple preference across the first year of life can be found in Table 2. A general trend in these studies is a period where preference was not observed, followed by a period of stability. This pattern change was often used to determine when nipple preference is established. Within species that have received more research attention, there were conflicting reports regarding timing. However, these discrepancies are confounded by differences in how the data were collected and analyzed—a point addressed by Damerose and Hopkins [53], who argued for focal-dyad sampling with continuous recording to measure nipple preference and maternal cradling preference.

Table 2.
Summary of developmental outcomes by species in studies that measured nipple preference across the first year of life.
While several investigators have speculated on whether nipple preferences are driven by the infant or the mother (or a bidirectional link—a third explanation), the studies thus far have not consistently collected maternal data, which is needed to fully evaluate these possibilities. Current evidence is mixed regarding whether nipple preference and maternal behaviors like cradling or carrying are linked. To preserve independence in data points, some investigators have measured mothers’ infant-directed behaviors separately from observations of suckling, e.g., [28]. Collecting in-the-moment behaviors for both members of the dyad is needed to characterize behavioral sequences that may shape the emergence of laterality. For example, Regaiolli, Spiezio and Hopkins [51] suggested that a nipple preference may create an opportunity for asymmetrical early hand experience where the infant clings to the mother’s fur with the hand opposite to the preferred nipple. Testing this hypothesis requires a longitudinal design, where each data point includes nipple preference and infant hand use during suckling. It is also possible that nipple preference does not create asymmetric early hand experience and therefore is not related to later measures of hand preference where data are collected outside of suckling episodes.
Another notable trend is that investigators often measured nipple preference on its own, and we know very little about whether this early bias has any connection to or is independent of handedness. Only one study in the review examined nipple preference as a predictor for later hand preference, and it did not find a link [28]. This review suggests that a nipple preference can be observed in several primate species, but the meaning of this early asymmetry has yet to be put into context. We recommend that investigators study nipple preference from birth through the period when a hand preference has been reported in the study species (if such information exists; see Table 3) or the period when a hand preference can reliably be measured based on species-typical development of manual abilities. These data, paired with rich recordings of mother–infant interactions, would facilitate a hypothesis-driven developmental approach in future studies to expand on the research on nipple preference to date, which has been largely descriptive and not well connected to other early measures of behavioral laterality like hand preference.

Table 3.
Summary of developmental outcomes by species in studies that measured hand preference across the first year of life.
3.2. Considerations for Measuring Hand Preference across the First Year of Life
A summary of developmental outcomes by species in studies that measured hand preference across the first year of life can be found in Table 3. Like nipple preference, there was no consistency in how hand preference was evaluated (i.e., manual actions varied or were combined in some studies), nor was there any consistency in how hand preferences were determined by investigators (i.e., cutoffs for making categories). For these reasons, our summary focused on outcomes related to time (e.g., when preferences were observed or how preferences changed or stayed the same across observations). Two general trends we could identify are that it is possible to observe hand preferences during the first year of life and that fluctuations by task and/or time are possible when individuals are followed longitudinally. Rather than evidence against a population-level preference, we encourage investigators to consider that this variability is a measurement issue. For example, simple reaching is considered a poor measure of hand preference in adult primates because it is a relatively easy and practiced task and reaching is sensitive to postural demands [16]. In human infants, bimanual tasks are the preferred measure once the child can perform this type of manual skill [73]. Finally, reaching is not used to measure hand preference in human adults; rather, questionnaires on how the hands are used to perform specific types of object manipulation are the norm. In this view, reaching may be an appropriate measure early in life, as in studies on human infants, but investigators should consider the tube task for subjects capable of coordinating the hands together. This shift in selecting what to measure would also facilitate comparisons to other age classes, such as juveniles/sub-adults and adults, especially in species where tube data already exist for older individuals. Furthermore, the focus on identifying when and for whom population-level preferences exist has obscured the possibility that there may be multiple patterns in the development of hand preference. We encourage investigators to consider this possibility as an explanation when group-level patterns are not found. This suggestion requires a larger number of subjects for statistical analyses, which would be facilitated by the adoption of a standard assessment (i.e., the tube task).
Investigators who measured hand preference early in the life course were more likely to test cascades relative to investigators who measured nipple preference, and they did so in one of two ways: (a) examining a behavior that temporally preceded the hand preference assessment as a predictor for later hand preference or (b) using hand preference as a predictor for a variable collected later in development. Both approaches increase the developmental rigor of a study since they utilize a longitudinal design. By highlighting ways in which analyses can be performed, we hope to inspire more investigators to adopt a cascades framework.
3.3. Increasing Developmental Rigor in Primate Behavioral Laterality Studies
The remainder of this review focuses on the ratings for developmental quality. Scores spanned the full range from 0 to 5 (M = 3.09 ± 1.52). In total, only 13 studies (28% of articles reviewed) received the max score of “5” for developmental quality. This statistic suggests that increasing developmental rigor should be a priority for future research. Studies rated “5” are discussed in detail in the following subsections. The discussion was delineated here between platyrrhine monkeys, catarrhine monkeys, and apes in consideration of their differences in evolutionary closeness to humans for comparisons. Moreover, an extended juvenile period is more pronounced in great apes (like humans) relative to monkeys.
3.3.1. Developmental Rigor in Platyrrhine Studies
Six studies on platyrrhine monkeys (marmosets or capuchin monkeys; Table 1) were included in the review. All studies measured hand preference in captive subjects. One study also reported on nipple preference, and another study additionally measured head orientation preference. No other types of behavioral laterality were assessed, and no study measured more than two types of behavior. The number of animals in each study varied from 2 to 23. On the developmental quality scale, platyrrhine studies ranged from 2 to 5 (M = 4.17 ± 1.33). No study was rated “0”, “1”, or “4”. One study was rated “2”, and one study was rated “3”. Two-thirds of the studies (4 of 6) were rated “5”, including two papers on marmosets and two papers on capuchin monkeys.
Marmosets have a distinctive social structure that involves cooperative infant care by the mother, father, and older siblings; this sociality differs from that of most other primates and allows for testing unique hypotheses regarding the development of laterality. Rogers and Kaplan [28] capitalized on this species-typical behavior to examine early life factors that may contribute to the development of hand preference. These authors tracked hand preference for reaching at four timepoints—0–2, 5–8, 10–12, and 22 months—and measured nipple preference alongside carrying preferences in the family group. Carrying was recorded separately from suckling. Most infants developed a preference for the left or right nipple within the first week of life, and this preference was correlated with the mother’s carrying preference but not the father’s or siblings’ carrying preferences. No individuals had a hand preference at 0–2 months of age. However, hand preference was measured from a mix of activities at the earliest timepoint relative to the later three timepoints that measured only food holding. At 5–8 months, all but two individuals had a significant hand preference, and all monkeys exhibited a significant hand preference at 10–12 and 22 months. There was no link between nipple preference and hand preference at any age. Early side biases in suckling and carrying, while present at the individual level in marmosets, may have no direct influence on the development of handedness.
Hook and Rogers [26] investigated the development of handedness in marmosets further in a longitudinal study examining reaching to food at nine timepoints from birth to 51–70 months. The amount of bimanual food holding was also measured for the first six study timepoints through 22 months of age. Human infant researchers have similarly tracked symmetric bimanual hand use relative to the emergence of hand preference [74]. In marmosets, bimanual hand use significantly decreased between 1–2 and 5–8 months. Hand preference strength was the mirror opposite of the bimanual hand use pattern, significantly increasing over the first two timepoints. There was a second increase in hand preference strength between 5–8 and 10–12 months, followed by a leveling off. This type of quadratic hand preference shape for reaching where preference rises and then asymptotes resembles that of human infants [9]. Another parallel with human-infant hand preference research is that Hook and Rogers plotted marmoset trajectories separately for left and right subgroups. Once preferences were established, they remained consistent over time. Identifying multiple trajectories in marmoset handedness development paves the way for studies to explore what factors might lead to marmosets having one hand preference trajectory over another. This approach, while not yet implemented widely in nonhuman primates, is motivated by the theoretical framework developmental cascades, whereby early lateralized experiences may have downstream cumulative effects in the emergence of handedness [14]. The open challenge for laterality researchers is to identify what these factors may be that shape preference groups, especially considering the differences in early development between primates (i.e., timing of weaning/independence)
Handedness cascades may differ based on species-typical experiences across early development, as evidenced by a study examining head orientation preference and later hand preference in capuchin monkeys. Westergaard, Byrne and Suomi [31] measured prone head orientation preference at 1–2 weeks of age when capuchin monkey infants rode dorsally on their mother’s back and reaching hand preference at two distal timepoints once the monkeys were independent of their mother. A negative correlation was found between head orientation preference and hand preference at 23–24 weeks but not hand preference at 47–48 weeks. These findings are in agreement with other studies on rhesus monkeys, chimpanzees, and human infants that have found that supine, but not prone, head orientation is lateralized and positively correlated with hand use patterns [48,68,71,75,76,77]. There may be a yet unidentified species-typical early context in which capuchin monkeys receive asymmetric experience of one hand, which in turn could predict the later hand preference bias. Exploring this possibility in future work requires a rich longitudinal design that samples posture, visual regard, and hand movements at several timepoints across early development, ideally with a sample that is large enough to test for subgroups (i.e., trajectories that differ in preference direction or strength).
A study by Westergaard, Byrne and Suomi [30] identified subgroups in capuchins based on stress reactivity, which were linked to differences in hand preference. Hand preference was measured from reaching when monkeys were 6 and 12 months of age. The scores were strongly correlated, suggesting continuity in hand preference across the first year of life in capuchin monkeys. At both study timepoints, plasma cortisol was collected at baseline and following a 2 hr separation from the social group. Stress cortisol at 6 months, but not baseline cortisol, was positively correlated with hand preference at both timepoints. Further analysis found a difference in hand preference at 6, but not 12, months between stress reactivity groups categorized as low, middle, and high reactivity. At 6 months, monkeys in the low cortisol reactive group differed in hand preference from monkeys in the middle or high cortisol reactive groups, and the middle and high groups did not differ from each other. These findings suggest that neonatal stress may play a role in the development of handedness in capuchin monkeys, although more data are needed to fully characterize developmental links and examine possible mechanisms. An alternative possibility is that a third factor influences both hand preference and stress reactivity in development, and this hypothesis could be tested in future research.
3.3.2. Developmental Rigor in Catarrhine Studies
Twenty-seven studies on catarrhine monkey species (macaques, baboons, langurs, or snub-nosed monkeys; Table 1) were included in the review. A theme related to this grouping is that most studies measured only one type of behavior. Nipple preference was measured alone in 16 of the studies, whereas hand preference was measured alone in 5 studies. One study measured ear preference as the direction of head turn in a playback experiment, and another study reported on asymmetry in facial and vocal expressions; both studies compared infants to adults but did not capture change or continuity in preference within individuals. Four studies measured laterality from a combination of two or three behaviors. Three studies used a twin design to examine nipple preference. The study Ns in non-twin papers ranged from 1 to 64. Data were collected in all three types of settings: captivity, free-ranging conditions, and in the wild. Ratings for catarrhine monkey studies ranged from 0 to 5 (M = 2.74 ± 1.56). Grouped by rating, two studies (7.4%) were rated “0”, three studies (11.1%) were rated “1”, nine studies (33.3%) were rated “2”, five studies (18.5%) were rated “3”, two studies (7.4%) were rated “4”, and six studies (22.2%) were rated “5”, including three papers on rhesus macaques, one on Taihangshan macaques, one on olive baboons, and one on Guinea baboons.
Nipple preference was widely reported in the studies on behavioral laterality in catarrhine monkeys over the first year of life, and the most comprehensive study based on the number of mother–infant pairs and the length of the observation period was the paper by Jaffe, Evans, Howell, Westergaard, Snoy and Higley [37] on free-ranging rhesus monkeys. This project examined 64 mother–infant pairs at 15 timepoints, including 48 h, 2 weeks, 4 weeks, 6 weeks, 8 weeks, and monthly intervals from 3 to 12 months. Weaning in rhesus monkeys is typically around 1 year of age. Nipple preferences emerged after 48 h between the first and second observation and increased in strength until 3 months of age, remaining consistent thereafter. A pattern was observed in mothers who had an infant in two consecutive years of the study—the second infant preferred the opposite nipple relative to its older sibling. Two limitations of this study included (a) no data on mothers’ behaviors involving the infant, which may provide asymmetric developmental experiences, and (b) no data on infants’ behaviors outside of nipple contact for testing cascades or independence in preferences; other studies included in this review address these gaps.
Tomaszycki, Cline, Griffin, Maestripieri and Hopkins [38] similarly reported on nipple preference in rhesus monkeys, but in a smaller developmental window than that of Jaffe, Evans, Howell, Westergaard, Snoy and Higley [37], and additionally measured hand use for three behaviors related to infant care in mothers. In this study, preferences were found for nipple preference at 1, 2, and 3 weeks of age but not weeks 4, 5, or 6. No preference was found for cradling measured independently of infant nipple preference or for retrieval, which was recorded as the arm used to retrieve an infant out of reach. There was a significant bias for carrying the infant with one arm while walking. There was no effect of infant age on mothers’ preferences for infant carrying, cradling, or retrieval. One interpretation of these effects, suggested by the authors, is that nipple preference is independent of maternal behavior. However, the authors looked at whether each preference (i.e., nipple, carrying, cradling, retrieval) on its own changed with age and did not examine whether the links between preferences varied over time.
Guo, Garber, Tian and Lu [49] measured nipple preference and maternal cradling in a related monkey, Taihangshan macaques, observed in the wild over the first 12 weeks of life. These authors found a bias for nipple preference and a bias for maternal cradling, but no link between the two behaviors. Like Tomaszycki, Cline, Griffin, Maestripieri and Hopkins [38], who studied rhesus monkeys, and Rogers and Kaplan [28], who studied marmosets, Guo, Garber, Tian and Lu [49] concluded that maternal cradling was unrelated to nipple preference. This interpretation of their data is supported by the fact that Taihangshan macaques can reposition their head or body to reach the nipple on their own within 48 h of birth. Taken together, the studies suggest that macaques have nipple preferences early in life, but these preferences do not seem to be influenced by mother–infant interactions or at least by the mother–infant variables collected to date. Whether nipple preferences influence later handedness in macaques is unknown.
Fagot [55] examined the emergence of nipple preference and hand preference in four Guinea baboons observed up to 36 weeks of age. In two of the baboons, nipple preference was observed at 0–2 weeks, and, after week 2, all four exhibited individual preferences. By contrast, only two of the four mothers had a significant and consistent hand preference for carrying. Given the small sample size, no conclusions were drawn regarding the link between nipple preference and maternal cradling. The data collection on the emergence of hand preference showed that a preference was first recorded in three of the infant baboons by 2 weeks and between 2 and 4 weeks for the fourth infant baboon. Fagot noted that that hand preference in young baboons showed some fluctuations—a common pattern seen in the human infant literature in small N studies that has been used as evidence for the argument that hand preference does not stabilize until later in childhood, e.g., [78]. Some human infants do have variable hand preference trajectories, but these patterns can only be captured in robust longitudinal datasets. Large samples are needed to characterize multiple trajectories (i.e., differences in preference direction or strength) in the emergence of hand preference and to examine how social environments may impact the development of laterality.
A study by Boulinguez-Ambroise, Pouydebat, Disarbois and Meguerditchian [52] in mother–infant olive baboon pairs reported that the relation between the mothers’ cradling preference and the infants’ grasping preference changed as the infants’ postural experiences changed. During the first four months of life when infants are mostly cradled, there was an association between cradling preference and infant hand preference. This link weakened when mothers shifted from cradling to carrying infants between 4 and 6 months and disappeared by 9–10 months when infants were moving independently. This effect was explained by different cradling experiences (i.e., the direction of the mother’s preference). In a prior study with data only from baboon mothers, Boulinguez-Ambroise, et al. [79] found that cradling bias was tied to social density. Mothers living in low-density groups had a strong cradling preference. However, mothers living in high-density groups, an indicator of social pressure, showed a shift to the opposite side for cradling preference. These baboon data, together with the research on stress reactivity by Westergaard and colleagues, suggest that laterality may be malleable by stress. While several investigators have shown that reaching hand use is susceptible to postural challenge in primates, e.g., [80], few have explored whether hand use is susceptible to social challenge. It would be possible to test this hypothesis across the lifespan, not merely during the first year of life.
Unlike the studies discussed so far that examined early life asymmetries in mother-reared monkeys, Nelson, Emery, Babcock, Novak, Suomi and Novak [48] reported on behavioral laterality in nursery-reared rhesus macaques. Monkeys raised in this type of environment show comparable motor development to their mother-reared counterparts [81]. Moreover, nursery rearing allows for the administration of neonatal assessments and other tests of behavioral laterality that would not be possible in mother–infant pairs. Nelson and colleagues paired measures adapted from the human infant literature with standard hand preference assessments used in primates. During the first month of life, the monkeys exhibited a greater response to tactile stimulation on one side of the body, preferentially turned their head while supine, and made more hand-to-face movements with the hand they saw while supine. The monkeys were assessed for hand preference twice, first for reaching at 14–44 days and then using the tube task at 6–9 months. A bias was found in the tube task but not in reaching. Motivated by the human literature, the authors examined whether head orientation preference predicted any measure of hand use. A link was found between head orientation and hand-to-face contacts, but head bias did not predict reaching or tube preferences. Monkeys did see one hand more than the other while supine, just like human infants [82], but, unlike human infants, rhesus monkeys do not spend much time supine outside of experimental testing. Moreover, macaques develop four times as fast as human infants [83], and their brain is more mature at birth relative to humans, which could mean that early environmental experiences could be less likely to impact lateralization [84]. Alternatively, the emergence of handedness in rhesus monkeys (and primates more generally) may involve a different cascading series of events than in humans that has not been captured by developmental studies to date.
3.3.3. Developmental Rigor in Ape Studies
Fourteen studies on apes (bonobos, chimpanzees, or orangutan; Table 1) were included in the review. One-half of the studies (7 of 14) reported one category of behavioral laterality, including nipple preference, head orientation preference, neonatal assessment, and leading limb. The other half of the studies reported a combination of two, three, or four types of behaviors. All but one of the combination studies included a measure of hand preference. The number of subjects ranged from 1 to 53, and data collection took place in captivity, except for one study conducted in the wild on nipple preference. Ratings for developmental quality ranged from 0 to 5 (M = 3.21 ± 1.25). One study was rated “1”, four studies were rated “2”, two studies were rated “3”, five studies were rated “4”, and two studies were rated “5”. The two highest-rated papers for developmental quality in apes were chimpanzee studies, with one of the papers also reporting on bonobos.
Hopkins and De Lathouwers [59] added to the growing body of work that has found a nipple preference in primates, reporting on captive chimpanzees and, for the first time, bonobos. Both species showed a nipple preference, which was consistent in a longitudinal analysis at four timepoints. While other studies have reported a cradling bias in chimpanzees, e.g., [85], this study did not capture any maternal behavior. For a summary of all published studies on nipple preference and maternal cradling in apes and catarrhine monkeys that addresses population-level and individual-level findings, see Guo, Garber, Tian and Lu [49].
Studies with chimpanzees reared in nursery settings have revealed further insights into early asymmetries. Bard, Hopkins and Fort [64] provided the first look at lateral biases in chimpanzees observed from birth to 3 months old. The infants were given a test of neurobehavioral integrity called the Neonatal Behavioral Assessment Scale, or NBAS. Laterality was not present for most of the reflexes examined, which is consistent with human infants [86]. Two notable developmental findings were reported on self-calming behavior during the test. First, chimpanzees had a bias for hand-to-mouth behavior, (i.e., thumb-sucking), which increased in strength between months 1 and 2. Second, laterality for hand-to-hand grasps similarly increased in strength between months 1 and 2 and months 2 and 3, but in the opposite direction. At first glance, these patterns appear to be contradictory. However, hand-to-mouth behavior and hand-to-hand grasps were often performed together or in a sequence, suggesting a complementary bimanual preference, where one hand puts the thumb in the mouth and the other hand clasps the thumb-sucking hand. Studying either behavior separately or at only one timepoint would not have revealed this link, which appears to strengthen developmentally in chimpanzees.
None of the other studies on behavioral laterality in nursery-reared chimpanzees received the highest rating on our developmental quality scale, primarily because the data were pooled across observations, meaning that change or continuity in the preference that was measured could not be analyzed. However, we decided to briefly summarize the other related reports that tracked different biases across time, including connections to the bias observed in hand-to-mouth behavior, as this body of work may inform how future studies are designed. Hopkins and Bard [63] reported that arousal at 2 days of life predicted hand-to-mouth laterality. These data suggest that affect may be tied to hemispheric specialization (for a discussion of current hypotheses on emotional lateralization and hand use, see [15]). Extending developmental links further, Hopkins and Parr [70] found a positive correlation between hand-to-mouth laterality at 3 months and lymphocyte counts (a global marker of immunological functioning) at 1 year of age, but no link was found between neonatal hand-to-mouth laterality and juvenile hand preference for reaching measured at 2–4 years of age. Biases have also been found for leading limb in crawling [65], grasping strength [67], and supine head orientation [62]. Of these, supine head orientation has been shown to be a predictor of later hand preference for touching and grasping objects at 9–10 months [71] and hand preference measured in the tube task when individuals were 3–5 years old [68].
Behavioral laterality has been studied extensively in chimpanzees, and this work complements the available laterality data for adult chimpanzees [84]. Yet many questions remain regarding the mechanism(s) underlying these biases and the developmental implications of laterality on other behaviors and cognition [17]. We hope that this review will inspire new interest in this topic. Next, we summarize the limitations of this project and offer suggestions to guide investigators in designing future studies with a developmental lens in mind.
4. Limitations and Future Directions
The selection of the age range for this study (birth to 1 year) was an arbitrary cutoff to facilitate the literature search for an initially unknown number of species, which have different life histories. The terms “infant”, “juvenile”, and “sub-adult” vary in how they are defined in the literature for different species and how they are used by different authors. We acknowledge that some articles excluded by these selection criteria are likely to fill gaps in our knowledge, and we encourage other authors to conduct reviews at the genus or species level, particularly in species for which there are abundant data across the lifespan, or a meta-analysis of the direction and magnitude of behavioral asymmetries for different phases of the lifespan. Moreover, research with human infants has connected prenatal asymmetries with postnatal hand preference, e.g., [87,88]. Although Previc [89] suggested that primate infants may not experience asymmetry in the uterine environment because they are carried by mothers moving quadrupedally rather than bipedally, we are not aware of any data that have been used to test this hypothesis. A future direction would be to examine fetal positioning and fetal arm movements during pregnancy, with data shared with a central resource for species that are widely used in primate research, e.g., [90].
This review confirmed a pattern that has been seen in primate studies in general—macaques and chimpanzees were oversampled [18,91]. There is a need to try to increase sampling diversity, particularly in prosimians for which there are no data. Finally, we limited our review to behavioral laterality only. An emerging trend is the inclusion of subjects of less than 1 year of age in brain imaging studies [92,93], which will supplement the behavioral data discussed in this review.
5. Conclusions
The findings of this systematic review are straightforward. As expected, a small number of papers have investigated behavioral laterality in nonhuman primates from birth to 1 year of age. The most-studied behaviors were nipple preference and hand preference, and the most-studied species were macaques and chimpanzees. Some of the behaviors reported, like nipple preference, head orientation, and behaviors considered in neonatal assessments, are specific to the period of development covered in the review, while others, like hand preference, leading limb, ear preference, and foot preference, can be studied across the lifespan. One difficulty is designing laterality assessments that are species-fair, which means they can be performed on any primate without training. Another consideration is using tasks that can also be performed by human infants or children, with slight or no modification. One candidate measure that fits these criteria is the coordinated bimanual tube task [16]. Yet we did not see that the tube task has been widely used in younger primates to date.
We did observe that laterality was framed from a developmental perspective either in the introduction and/or the discussion often, but a smaller number of studies used a longitudinal approach that captured change or continuity in at least one preference with a multiple-timepoint design that may have additionally measured different preferences at different times to examine potential cascades in laterality. Moreover, only 40% of the studies reviewed reported a developmental analysis. By far, this aspect of not analyzing data with a developmental lens was the weakest point in the studies we considered. In addition to reporting individual- and population-level effects, we encourage researchers to examine potential links between behaviors and how these relations change or stay the same over time, drawing on the studies rated “5” in this review as examples to increase developmental rigor. To better facilitate future meta-analyses, we also suggest some conventions for reporting laterality data in primates, similar to efforts that are underway for human laterality [94], including (a) providing exact ages for subjects or stating that this information is approximate or not available, (b) providing raw data in the paper or permanent link, and (c) providing details about how individual- and population-level preferences were calculated and any criteria used to make preference groups.
A final comment is on the studies we did not see in this systematic review, which should also help guide future research. Most studies examined some aspect of motor laterality rather than perceptual laterality (for a review that summarizes studies on eye preference and auditory orienting, see [7]). Expanding the range of topics covered early in the lifespan will allow researchers to examine connections (or the lack thereof) within and between individuals, as well as across time and species. A priority for future research is to further characterize interactions between individuals and the environments that they encounter that contribute to the emergence of laterality in primate behavior.
Author Contributions
Conceptualization, E.L.N. (lead) and A.K. (supporting); methodology, E.L.N.; data curation, E.L.N. and A.K.; writing—original draft preparation, E.L.N.; writing—review and editing, E.L.N. (lead) and A.K. (supporting); project administration, E.L.N. All authors have read and agreed to the published version of the manuscript.
Funding
A.K. was supported during the project by a Florida International University Presidential Fellowship.
Data Availability Statement
Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A

Table A1.
Full search strings for all three databases.
Table A1.
Full search strings for all three databases.
| Database | Search Strategy |
|---|---|
| PubMed | Search: ((“catarrhini”[mh:noexp] OR “cercopithecidae”[mh] OR “gorilla gorilla”[mh] OR “haplorhini”[mh:noexp] OR “hominidae”[mh:noexp] OR “hylobatidae”[mh] OR “pan paniscus”[mh] OR “pan troglodytes”[mh] OR “platyrrhini”[mh] OR “pongo”[mh] OR “primates”[mh:noexp] OR “strepsirhini”[mh] OR “tarsii”[mh] OR “allenopithecus”[tiab] OR “allocebus”[tiab] OR “alouatta”[tiab] OR “alouattinae”[tiab] OR “angwantibo *”[tiab] OR “anthropoid”[tiab] OR “anthropoidea”[tiab] OR “anthropoids”[tiab] OR “aotes”[tiab] OR “aotidae”[tiab] OR “aotinae”[tiab] OR “aotus”[tiab] OR “ape”[tiab] OR “apes”[tiab] OR “arctocebus”[tiab] OR “ateles”[tiab] OR “atelidae”[tiab] OR “atelinae”[tiab] OR “avahi”[tiab] OR “aye-aye *”[tiab] OR “baboon”[tiab] OR “baboons”[tiab] OR “bonobo”[tiab] OR “bonobos”[tiab] OR “brachyteles”[tiab] OR “bushbabies”[tiab] OR “bushbaby”[tiab] OR “cacajao”[tiab] OR “callibella”[tiab] OR “callicebinae”[tiab] OR “callicebus”[tiab] OR “callimico”[tiab] OR “callithrichid *”[tiab] OR “callithrichinae”[tiab] OR “callithrix”[tiab] OR “callitrichid”[tiab] OR “callitrichidae”[tiab] OR “callitrichide”[tiab] OR “callitrichids”[tiab] OR “callitrichinae”[tiab] OR “capuchin”[tiab] OR “capuchins”[tiab] OR “carlito syrichta”[tiab] OR “catarhine *”[tiab] OR “catarhini”[tiab] OR “catarrhina”[tiab] OR “catarrhine *”[tiab] OR “catarrhini”[tiab] OR “cebid”[tiab] OR “cebidae”[tiab] OR “cebids”[tiab] OR “cebinae”[tiab] OR “ceboidea”[tiab] OR “cebuella”[tiab] OR “cebus”[tiab] OR “cephalopachus”[tiab] OR “cercocebus”[tiab] OR “cercopithecid *”[tiab] OR “cercopithecinae”[tiab] OR “cercopithecine *”[tiab] OR “cercopithecini”[tiab] OR “cercopithecoid”[tiab] OR “cercopithecoidea”[tiab] OR “cercopithecoids”[tiab] OR “cercopithecus”[tiab] OR “cheirogaleidae”[tiab] OR “cheirogaleus”[tiab] OR “cheracebus”[tiab] OR “chimp”[tiab] OR “chimpanzee”[tiab] OR “chimpanzees”[tiab] OR “chimps”[tiab] OR “chiromyiformes”[tiab] OR “chiropotes”[tiab] OR “chlorocebus”[tiab] OR “colobidae”[tiab] OR “colobinae”[tiab] OR “colobine *”[tiab] OR “colobini”[tiab] OR “colobus *”[tiab] OR “cynomolgus”[tiab] OR “daubentonia”[tiab] OR “daubentoniidae”[tiab] OR “douc”[tiab] OR “doucs”[tiab] OR “erythrocebus”[tiab] OR “eulemur”[tiab] OR “euoticus”[tiab] OR “euprimate *”[tiab] OR “galagid *”[tiab] OR “galago”[tiab] OR “galagoides”[tiab] OR “galagonidae”[tiab] OR “galagos”[tiab] OR “gelada”[tiab] OR “geladas”[tiab] OR “gibbon”[tiab] OR “gibbons”[tiab] OR “gorilla”[tiab] OR “gorillas”[tiab] OR “grivet”[tiab] OR “grivets”[tiab] OR “guenon *”[tiab] OR “guereza *”[tiab] OR “hapalemur”[tiab] OR “haplorrhine *”[tiab] OR “haplorhini”[tiab] OR “haplorrhine *”[tiab] OR “haplorrhini”[tiab] OR “hominid *”[tiab] OR “hominin”[tiab] OR “homininae”[tiab] OR “hominine”[tiab] OR “hominines”[tiab] OR “hominini”[tiab] OR “hominins”[tiab] OR “hominoidea”[tiab] OR “hoolock”[tiab] OR “howler *”[tiab] OR “hylobates”[tiab] OR “hylobatidae”[tiab] OR “indri”[tiab] OR “indridae”[tiab] OR “indriid *”[tiab] OR “indris”[tiab] OR “kipunji *”[tiab] OR “lagothrix”[tiab] OR “langur”[tiab] OR “langurs”[tiab] OR “lemur”[tiab] OR “lemurid *”[tiab] OR “lemuriform”[tiab] OR “lemuriformes”[tiab] OR “lemuriforms”[tiab] OR “lemurinae”[tiab] OR “lemuroidea”[tiab] OR “lemurs”[tiab] OR “leontideus”[tiab] OR “leontocebus”[tiab] OR “leontopithecus”[tiab] OR “lepilemur”[tiab] OR “lepilemurid *”[tiab] OR “lesula *”[tiab] OR “lophocebus”[tiab] OR “loriform”[tiab] OR “loriformes”[tiab] OR “lorinae”[tiab] OR “loris”[tiab] OR “lorises”[tiab] OR “lorisid *”[tiab] OR “lorisiform *”[tiab] OR “lorisinae”[tiab] OR “lorisoid *”[tiab] OR “lutung”[tiab] OR “lutungs”[tiab] OR “macaca”[tiab] OR “macaque’s”[tiab] OR “macaque”[tiab] OR “macaques”[tiab] OR “malbrouck *”[tiab] OR “mandrill”[tiab] OR “mandrills”[tiab] OR “mandrillus”[tiab] OR “mangabey *”[tiab] OR “marmoset”[tiab] OR “marmosets”[tiab] OR “mico argentatus”[tiab] OR “mico chrysoleucos”[tiab] OR “mico emiliae”[tiab] OR “mico humilis”[tiab] OR “mico marcai”[tiab] OR “mico melanurus”[tiab] OR “mico rondoni”[tiab] OR “microcebus”[tiab] OR “miopithecus”[tiab] OR “mirza coquereli”[tiab] OR “mirza zaza”[tiab] OR “monkey”[tiab] OR “monkeys”[tiab] OR “muriqui *”[tiab] OR “nasalis larvatus”[tiab] OR “nomascus”[tiab] OR “nycticebus”[tiab] OR “oedipomidas”[tiab] OR “orang utan *”[tiab] OR “orang-utan *”[tiab] OR “orangutan *”[tiab] OR “oreonax”[tiab] OR “otolemur”[tiab] OR “pan paniscus”[tiab] OR “pan troglodytes”[tiab] OR “panin”[tiab] OR “panina”[tiab] OR “panins”[tiab] OR “papio”[tiab] OR “papionini”[tiab] OR “paragalago”[tiab] OR “perodicticinae”[tiab] OR “perodicticus”[tiab] OR “phaner”[tiab] OR “piliocolobus”[tiab] OR “pithecia”[tiab] OR “pithecidae”[tiab] OR “pitheciid *”[tiab] OR “pitheciinae”[tiab] OR “pithecinae”[tiab] OR “platyrhine *”[tiab] OR “platyrhini”[tiab] OR “platyrrhina”[tiab] OR “platyrrhine *”[tiab] OR “platyrrhini”[tiab] OR “plecturocebus”[tiab] OR “pongid *”[tiab] OR “ponginae”[tiab] OR “pongo”[tiab] OR “potto”[tiab] OR “pottos”[tiab] OR “presbytini”[tiab] OR “presbytis”[tiab] OR “primate”[tiab] OR “primates”[tiab] OR “procolobus”[tiab] OR “prolemur”[tiab] OR “propithecus”[tiab] OR “prosimian *”[tiab] OR “prosimii”[tiab] OR “pseudopotto”[tiab] OR “pygathrix”[tiab] OR “rhinopithecus”[tiab] OR “rungwecebus”[tiab] OR “saguinus”[tiab] OR “saimiri”[tiab] OR “saimiriinae”[tiab] OR “sapajus”[tiab] OR “sciurocheirus”[tiab] OR “semnopithecus”[tiab] OR “siamang”[tiab] OR “siamangs”[tiab] OR “sifaka”[tiab] OR “sifakas”[tiab] OR “simians”[tiab] OR “simias”[tiab] OR “simiiform *”[tiab] OR “strepsir *”[tiab] OR “surili *”[tiab] OR “symphalangus”[tiab] OR “talapoin *”[tiab] OR “tamarin”[tiab] OR “tamarins”[tiab] OR “tamarinus”[tiab] OR “tarsier”[tiab] OR “tarsiers”[tiab] OR “tarsiid *”[tiab] OR “tarsiiform *”[tiab] OR “tarsius”[tiab] OR “theropithecus”[tiab] OR “trachypithecus”[tiab] OR “uacari *”[tiab] OR “uakari”[tiab] OR “uakaris”[tiab] OR “varecia”[tiab] OR “vervet *”[tiab]) AND (“laterality” or “handedness” or “hand preference” or “asymmetry”)) AND (“Infant” or “longitudinal” or “developmental” or “neonatal” or “neonate”) |
| PsycINFO | (DE(“baboons” OR “bonobos” OR “chimpanzees” OR “gorillas” OR “lemurs” OR “monkeys” OR “primates (nonhuman)”) OR TX(“allenopithecus” OR “allocebus” OR “alouatta” OR “alouattinae” OR “angwantibo *” OR “anthropoid” OR “anthropoidea” OR “anthropoids” OR “aotes” OR “aotidae” OR “aotinae” OR “aotus” OR “ape” OR “apes” OR “arctocebus” OR “ateles” OR “atelidae” OR “atelinae” OR “avahi” OR “aye-aye *” OR “baboon” OR “baboons” OR “bonobo” OR “bonobos” OR “brachyteles” OR “bushbabies” OR “bushbaby” OR “cacajao” OR “callibella” OR “callicebinae” OR “callicebus” OR “callimico” OR “callithrichid *” OR “callithrichinae” OR “callithrix” OR “callitrichid” OR “callitrichidae” OR “callitrichide” OR “callitrichids” OR “callitrichinae” OR “capuchin” OR “capuchins” OR “carlito syrichta” OR “catarhine *” OR “catarhini” OR “catarrhina” OR “catarrhine *” OR “catarrhini” OR “cebid” OR “cebidae” OR “cebids” OR “cebinae” OR “ceboidea” OR “cebuella” OR “cebus” OR “cephalopachus” OR “cercocebus” OR “cercopithecid *” OR “cercopithecinae” OR “cercopithecine *” OR “cercopithecini” OR “cercopithecoid” OR “cercopithecoidea” OR “cercopithecoids” OR “cercopithecus” OR “cheirogaleidae” OR “cheirogaleus” OR “cheracebus” OR “chimp” OR “chimpanzee” OR “chimpanzees” OR “chimps” OR “chiromyiformes” OR “chiropotes” OR “chlorocebus” OR “colobidae” OR “colobinae” OR “colobine *” OR “colobini” OR “colobus *” OR “cynomolgus” OR “daubentonia” OR “daubentoniidae” OR “douc” OR “doucs” OR “erythrocebus” OR “eulemur” OR “euoticus” OR “euprimate *” OR “galagid *” OR “galago” OR “galagoides” OR “galagonidae” OR “galagos” OR “gelada” OR “geladas” OR “gibbon” OR “gibbons” OR “gorilla” OR “gorillas” OR “grivet” OR “grivets” OR “guenon *” OR “guereza *” OR “hapalemur” OR “haplorhine *” OR “haplorhini” OR “haplorrhine *” OR “haplorrhini” OR “hominid *” OR “hominin” OR “homininae” OR “hominine” OR “hminines” OR “hominini” OR “hominins” OR “hominoidea” OR “hoolock” OR “howler *” OR “hylobates” OR “hylobatidae” OR “indri” OR “indridae” OR “indriid *” OR “indris” OR “kipunji *” OR “lagothrix” OR “langur” OR “langurs” OR “lemur” OR “lemurid *” OR “lemuriform” OR “lemuriformes” OR “lemuriforms” OR “lemurinae” OR “lemuroidea” OR “lemurs” OR “leontideus” OR “leontocebus” OR “leontopithecus” OR “lepilemur” OR “lepilemurid *” OR “lesula *” OR “lophocebus” OR “loriform” OR “loriformes” OR “lorinae” OR “loris” OR “lorises” OR “lorisid *” OR “lorisiform *” OR “lorisinae” OR “lorisoid *” OR “lutung” OR “lutungs” OR “macaca” OR “macaque’s” OR “macaque” OR “macaques” OR “malbrouck *” OR “mandrill” OR “mandrills” OR “mandrillus” OR “mangabey *” OR “marmoset” OR “marmosets” OR “mico argentatus” OR “mico chrysoleucos” OR “mico emiliae” OR “mico humilis” OR “mico marcai” OR “mico melanurus” OR “mico rondoni” OR “microcebus” OR “miopithecus” OR “mirza coquereli” OR “mirza zaza” OR “monkey” OR “monkeys” OR “muriqui *” OR “nasalis larvatus” OR “nomascus” OR “nycticebus” OR “oedipomidas” OR “orang utan *” OR “orang-utan *” OR “orangutan *” OR “oreonax” OR “otolemur” OR “pan paniscus” OR “pan troglodytes” OR “panin” OR “panina” OR “panins” OR “papio” OR “papionini” OR “paragalago” OR “perodicticinae” OR “perodicticus” OR “phaner” OR “piliocolobus” OR “pithecia” OR “pithecidae” OR “pitheciid *” OR “pitheciinae” OR “pithecinae” OR “platyrhine *” OR “platyrhini” OR “platyrrhina” OR “platyrrhine *” OR “platyrrhini” OR “plecturocebus” OR “pongid *” OR “ponginae” OR “pongo” OR “potto” OR “pottos” OR “presbytini” OR “presbytis” OR “primate” OR “primates” OR “procolobus” OR “prolemur” OR “propithecus” OR “prosimian *” OR “prosimii” OR “pseudopotto” OR “pygathrix” OR “rhinopithecus” OR “rungwecebus” OR “saguinus” OR “saimiri” OR “saimiriinae” OR “sapajus” OR “sciurocheirus” OR “semnopithecus” OR “siamang” OR “siamangs” OR “sifaka” OR “sifakas” OR “simians” OR “simias” OR “simiiform *” OR “strepsir *” OR “surili *” OR “symphalangus” OR “talapoin *” OR “tamarin” OR “tamarins” OR “tamarinus” OR “tarsier” OR “tarsiers” OR “tarsiid *” OR “tarsiiform *” OR “tarsius” OR “theropithecus” OR “trachypithecus” OR “uacari *” OR “uakari” OR “uakaris” OR “varecia” OR “vervet *”)) AND (“laterality” or “handedness” or “hand preference” or “asymmetry”) AND (“Infant” or “longitudinal” or “developmental” or “neonatal” or “neonate”) |
| Web of Science | ((TS = (“allenopithecus” OR “allocebus” OR “alouatta” OR “alouattinae” OR “angwantibo *” OR “anthropoid” OR “anthropoidea” OR “anthropoids” OR “aotes” OR “aotidae” OR “aotinae” OR “aotus” OR “ape” OR “apes” OR “arctocebus” OR “ateles” OR “atelidae” OR “atelinae” OR “avahi” OR “aye-aye *” OR “baboon” OR “baboons” OR “bonobo” OR “bonobos” OR “brachyteles” OR “bushbabies” OR “bushbaby” OR “cacajao” OR “callibella” OR “callicebinae” OR “callicebus” OR “callimico” OR “callithrichid *” OR “callithrichinae” OR “callithrix” OR “callitrichid” OR “callitrichidae” OR “callitrichide” OR “callitrichids” OR “callitrichinae” OR “capuchin” OR “capuchins” OR “carlito syrichta” OR “catarhine *” OR “catarhini” OR “catarrhina” OR “catarrhine *” OR “catarrhini” OR “cebid” OR “cebidae” OR “cebids” OR “cebinae” OR “ceboidea” OR “cebuella” OR “cebus” OR “cephalopachus” OR “cercocebus” OR “cercopithecid *” OR “cercopithecinae” OR “cercopithecine *” OR “cercopithecini” OR “cercopithecoid” OR “cercopithecoidea” OR “cercopithecoids” OR “cercopithecus” OR “cheirogaleidae” OR “cheirogaleus” OR “cheracebus” OR “chimp” OR “chimpanzee” OR “chimpanzees” OR “chimps” OR “chiromyiformes” OR “chiropotes” OR “chlorocebus” OR “colobidae” OR “colobinae” OR “colobine *” OR “colobini” OR “colobus *” OR “cynomolgus” OR “daubentonia” OR “daubentoniidae” OR “douc” OR “doucs” OR “erythrocebus” OR “eulemur” OR “euoticus” OR “euprimate *” OR “galagid *” OR “galago” OR “galagoides” OR “galagonidae” OR “galagos” OR “gelada” OR “geladas” OR “gibbon” OR “gibbons” OR “gorilla” OR “gorillas” OR “grivet” OR “grivets” OR “guenon *” OR “guereza *” OR “hapalemur” OR “haplorhine *” OR “haplorhini” OR “haplorrhine *” OR “haplorrhini” OR “hominid *” OR “hominin” OR “homininae” OR “hominine” OR “hominines” OR “hominini” OR “hominins” OR “hominoidea” OR “hoolock” OR “howler *” OR “hylobates” OR “hylobatidae” OR “indri” OR “indridae” OR “indriid *” OR “indris” OR “kipunji *” OR “lagothrix” OR “langur” OR “langurs” OR “lemur” OR “lemurid *” OR “lemuriform” OR “lemuriformes” OR “lemuriforms” OR “lemurinae” OR “lemuroidea” OR “lemurs” OR “leontideus” OR “leontocebus” OR “leontopithecus” OR “lepilemur” OR “lepilemurid *” OR “lesula *” OR “lophocebus” OR “loriform” OR “loriformes” OR “lorinae” OR “loris” OR “lorises” OR “lorisid *” OR “lorisiform *” OR “lorisinae” OR “lorisoid *” OR “lutung” OR “lutungs” OR “macaca” OR “macaque’s” OR “macaque” OR “macaques” OR “malbrouck *” OR “mandrill” OR “mandrills” OR “mandrillus” OR “mangabey *” OR “marmoset” OR “marmosets” OR “mico argentatus” OR “mico chrysoleucos” OR “mico emiliae” OR “mico humilis” OR “mico marcai” OR “mico melanurus” OR “mico rondoni” OR “microcebus” OR “miopithecus” OR “mirza coquereli” OR “mirza zaza” OR “monkey” OR “monkeys” OR “muriqui *” OR “nasalis larvatus” OR “nomascus” OR “nycticebus” OR “oedipomidas” OR “orang utan *” OR “orang-utan *” OR “orangutan *” OR “oreonax” OR “otolemur” OR “pan paniscus” OR “pan troglodytes” OR “panin” OR “panina” OR “panins” OR “papio” OR “papionini” OR “paragalago” OR “perodicticinae” OR “perodicticus” OR “phaner” OR “piliocolobus” OR “pithecia” OR “pithecidae” OR “pitheciid *” OR “pitheciinae” OR “pithecinae” OR “platyrhine *” OR “platyrhini” OR “platyrrhina” OR “platyrrhine *” OR “platyrrhini” OR “plecturocebus” OR “pongid *” OR “ponginae” OR “pongo” OR “potto” OR “pottos” OR “presbytini” OR “presbytis” OR “primate” OR “primates” OR “procolobus” OR “prolemur” OR “propithecus” OR “prosimian *” OR “prosimii” OR “pseudopotto” OR “pygathrix” OR “rhinopithecus” OR “rungwecebus” OR “saguinus” OR “saimiri” OR “saimiriinae” OR “sapajus” OR “sciurocheirus” OR “semnopithecus” OR “siamang” OR “siamangs” OR “sifaka” OR “sifakas” OR “simians” OR “simias” OR “simiiform *” OR “strepsir *” OR “surili *” OR “symphalangus” OR “talapoin *” OR “tamarin” OR “tamarins” OR “tamarinus” OR “tarsier” OR “tarsiers” OR “tarsiid *” OR “tarsiiform *” OR “tarsius” OR “theropithecus” OR “trachypithecus” OR “uacari *” OR “uakari” OR “uakaris” OR “varecia” OR “vervet *”)) AND ALL = (“laterality” or “handedness” or “hand preference” or “asymmetryy”)) AND ALL = (“Infant” or “longitudinal” or “developmental” or “neonatal” or “neonate”) |

Table A2.
List of the reports excluded at the full-text screening stage with the reason.
Table A2.
List of the reports excluded at the full-text screening stage with the reason.
| Identification of Studies via Databases and Registers |
|---|
Topic not measured (n = 6)
|
Ss’ ages not reported (n = 3)
|
No Ss < 1 year old (n = 10)
|
Ss > 1 year old (n = 5)
|
| Identification of Studies via Other Methods |
Data for Ss < 1 year old not separated out (n = 1)
|
Paper not in English (n = 1)
|
Notes. Full citations can be found in the References section. Ss = Subjects.
References
- MacNeilage, P.F.; Studdert-Kennedy, M.G.; Lindblom, B. Primate handedness reconsidered. Behav. Brain Sci. 1987, 10, 247–263. [Google Scholar] [CrossRef]
- Fagot, J.; Vauclair, J. Manual laterality in nonhuman primates: A distinction between handedness and manual specialization. Psychol. Bull. 1991, 109, 76. [Google Scholar] [CrossRef] [PubMed]
- Papademetriou, E.; Sheu, C.F.; Michel, G.F. A meta-analysis of primate hand preferences, particularly for reaching. J. Comp. Psychol. 2005, 119, 33–48. [Google Scholar] [CrossRef] [PubMed]
- McGrew, W.; Marchant, L. On the other hand: Current issues in and meta-analysis of the behavioral laterality of hand function in nonhuman primates. Am. J. Phys. Anthropol. 1997, 104, 201–232. [Google Scholar] [CrossRef]
- Caspar, K.R.; Pallasdies, F.; Mader, L.; Sartorelli, H.; Begall, S. The evolution and biological correlates of hand preferences in anthropoid primates. Elife 2022, 11, e77875. [Google Scholar] [CrossRef]
- Soto, C.; Gázquez, J.M.; Llorente, M. Hand preferences in coordinated bimanual tasks in non-human primates: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2022, 141, 104822. [Google Scholar] [CrossRef]
- Fitch, W.T.; Braccini, S.N. Primate laterality and the biology and evolution of human handedness: A review and synthesis. Ann. N. Y. Acad. Sci. 2013, 1288, 70–85. [Google Scholar] [CrossRef]
- Papadatou-Pastou, M.; Ntolka, E.; Schmitz, J.; Martin, M.; Munafo, M.R.; Ocklenburg, S.; Paracchini, S. Human handedness: A meta-analysis. Psychol. Bull. 2020, 146, 481–524. [Google Scholar] [CrossRef] [PubMed]
- Michel, G.F.; Babik, I.; Sheu, C.F.; Campbell, J.M. Latent classes in the developmental trajectories of infant handedness. Dev. Psychol. 2014, 50, 349–359. [Google Scholar] [CrossRef]
- Campbell, J.M.; Marcinowski, E.C.; Michel, G.F. The development of neuromotor skills and hand preference during infancy. Dev. Psychobiol. 2018, 60, 165–175. [Google Scholar] [CrossRef]
- Michel, G.F. Development of infant handedness. In Conceptions of Development: Lessons from the Laboratory; Lewkowicz, D.J., Lickliter, R., Eds.; Psychology Press: New York, NY, USA, 2002; pp. 165–186. [Google Scholar]
- Michel, G.F. Handedness Development: A Model for Investigating the Development of Hemispheric Specialization and Interhemispheric Coordination. Symmetry 2021, 13, 992. [Google Scholar] [CrossRef]
- Michel, G.F.; Nelson, E.L.; Babik, I.; Campbell, J.M.; Marcinowski, E.C. Multiple trajectories in the developmental psychobiology of human handedness. Adv. Child Dev. Behav. 2013, 45, 227–260. [Google Scholar] [CrossRef]
- Nelson, E.L. Developmental cascades as a framework for primate handedness. Front. Behav. Neurosci. 2022, 16, 1063348. [Google Scholar] [CrossRef] [PubMed]
- Boulinguez-Ambroise, G.; Aychet, J.; Pouydebat, E. Limb Preference in Animals: New Insights into the Evolution of Manual Laterality in Hominids. Symmetry 2022, 14, 96. [Google Scholar] [CrossRef]
- Nelson, E.L. Insights Into Human and Nonhuman Primate Handedness From Measuring Both Hands. Curr. Dir. Psychol. Sci. 2022, 31, 154–161. [Google Scholar] [CrossRef]
- Rogers, L.J. Development of Hand and Paw Preferences and Their Association with Other Patterns of Behaviour and Cognition. Symmetry 2023, 15, 926. [Google Scholar] [CrossRef]
- Nelson, E.; Alvarez, J.; Jimenez, B.; Padron, K. State of the Field. In Primate Cognitive Studies; Schwartz, B.L., Beran, M.J., Eds.; Cambridge University Press: Cambridge, UK, 2022; pp. 88–114. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 1–11. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1–9. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. Bmj 2015, 349, g7647. [Google Scholar] [CrossRef]
- Nelson, E.L.; Karimi, A. Behavioral Laterality across the First Year of Life in Nonhuman Primates [Systematic Review Protocol]; OSF: Galesburg, IL, USA, 2023. [Google Scholar] [CrossRef]
- Cassidy, L.C.; Leenaars, C.H.C.; Rincon, A.V.; Pfefferle, D. Comprehensive search filters for retrieving publications on nonhuman primates for literature reviews (filterNHP). Am. J. Primatol. 2021, 83, e23287. [Google Scholar] [CrossRef]
- The EndNote Team. EndNote; EndNote 20; Clarivate: Philadelphia, PA, USA, 2013. [Google Scholar]
- Wallace, B.C.; Small, K.; Brodley, C.E.; Lau, J.; Trikalinos, T.A. Deploying an interactive machine learning system in an evidence-based practice center: Abstrackr. In Proceedings of the 2nd ACM SIGHIT International Health Informatics Symposium, Miami, FL, USA, 28–30 January 2012; pp. 819–824. [Google Scholar] [CrossRef]
- Hook, M.A.; Rogers, L.J. Development of hand preferences in marmosets (Callithrix jacchus) and effects of aging. J. Comp. Psychol. 2000, 114, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Matoba, M.; Masataka, N.; Tanioka, Y. Cross-generational continuity of hand-use preferences in marmosets. Behaviour 1991, 117, 281–286. [Google Scholar] [CrossRef]
- Rogers, L.J.; Kaplan, G. Teat preference for suckling in common marmosets: Relationship to side of being carried and hand preference. Laterality Asymmetries Body Brain Cogn. 1998, 3, 269–281. [Google Scholar] [CrossRef]
- Guerra, R.F.; da Silveira, N.; Bernardi, N.; Legal, E.J. Hand preference during behavioral tests and spontaneous activity in two species of common marmoset (Callithrix jacchus and Callithrix penicillata). Rev. Bras. Biol. 1997, 57, 563–570. [Google Scholar]
- Westergaard, G.C.; Byrne, G.; Suomi, S.J. Handedness and cortisol in tufted capuchin monkey infants. Dev. Psychobiol. J. Int. Soc. Dev. Psychobiol. 2000, 36, 213–217. [Google Scholar] [CrossRef]
- Westergaard, G.C.; Byrne, G.; Suomi, S.J. Early lateral bias in tufted capuchins (Cebus apella). Dev. Psychobiol. 1998, 32, 45–50. [Google Scholar] [CrossRef]
- Tanaka, I. Parity-related differences in suckling behavior and nipple preference among free-ranging Japanese macaques. Am. J. Primatol. 1997, 42, 331–339. [Google Scholar] [CrossRef]
- Ôta, K.; Makino, Y.; Kimura, M.; Suzuki, J. Lactation in the Japanese monkey (Macaca fuscata): Yield and composition of milk and nipple preference of young. Primates 1991, 32, 35–48. [Google Scholar] [CrossRef]
- Tanaka, I. Change of nipple preference between successive offspring in Japanese macaques. Am. J. Primatol. 1989, 18, 321–325. [Google Scholar] [CrossRef]
- Nakamichi, M. Development of infant twin Japanese monkeys (Macaca fuscata) in a free-ranging group. Primates 1983, 24, 576–583. [Google Scholar] [CrossRef]
- Hiraiwa, M. Maternal and alloparental care in a troop of free-ranging Japanese monkeys. Primates 1981, 22, 309–329. [Google Scholar] [CrossRef]
- Jaffe, B.D.; Evans, T.A.; Howell, S.; Westergaard, G.C.; Snoy, P.J.; Higley, J.D. Left versus right nipple preference in free-ranging infant rhesus macaques (Macaca mulatta). Dev. Psychobiol. 2006, 48, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Tomaszycki, M.; Cline, C.; Griffin, B.; Maestripieri, D.; Hopkins, W.D. Maternal cradling and infant nipple preferences in rhesus monkeys (Macaca mulatta). Dev. Psychobiol. 1998, 32, 305–312. [Google Scholar] [CrossRef]
- Lindburg, D.G. The rhesus monkey in North India: An ecological and behavioral study. In Primate Behavior: Developments in Field and Laboratory Research; Rosenblum, L., Ed.; Academic Press: New York, NY, USA, 1971; Volume 2, pp. 1–106. [Google Scholar]
- Deets, A.C.; Harlow, H.F. Nipple preferences in nursing singleton-and twin-reared rhesus monkey infants. Dev. Psychol. 1970, 2, 159. [Google Scholar] [CrossRef]
- Spencer-Booth, Y. The behaviour of twin rhesus monkeys and comparisons with the behaviour of single infants. Primates 1968, 9, 75–84. [Google Scholar] [CrossRef]
- Hinde, R.; Bowell, T.; Spencer-Booth, Y. Behaviour of socially living rhesus monkeys in their first six months. Proc. Zool. Soc. Lond. 1964, 143, 609–649. [Google Scholar]
- Nelson, E.L.; Konidaris, G.D.; Berthier, N.E.; Braun, M.C.; Novak, M.F.; Suomi, S.J.; Novak, M.A. Kinematics of reaching and implications for handedness in rhesus monkey infants. Dev. Psychobiol. 2012, 54, 460–467. [Google Scholar] [CrossRef]
- Westergaard, G.; Champoux, M.; Suomi, S. Plasma cortisol is associated with handedness in infant rhesus monkeys. Dev. Psychobiol. J. Int. Soc. Dev. Psychobiol. 2001, 38, 116–122. [Google Scholar] [CrossRef]
- Westergaard, G.C.; Champoux, M.; Suomi, S.J. Hand preference in infant rhesus macaques (Macaca mulatta). Child. Dev. 1997, 68, 387–393. [Google Scholar] [CrossRef]
- Hauser, M.D.; Andersson, K. Left hemisphere dominance for processing vocalizations in adult, but not infant, rhesus monkeys: Field experiments. Proc. Natl. Acad. Sci. USA 1994, 91, 3946–3948. [Google Scholar] [CrossRef] [PubMed]
- Hauser, M.D.; Akre, K. Asymmetries in the timing of facial and vocal expressions by rhesus monkeys: Implications for hemispheric specialization. Anim. Behav. 2001, 61, 391–400. [Google Scholar] [CrossRef]
- Nelson, E.L.; Emery, M.S.; Babcock, S.M.; Novak, M.F.; Suomi, S.J.; Novak, M.A. Head orientation and handedness trajectory in rhesus monkey infants (Macaca mulatta). Dev. Psychobiol. 2011, 53, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Garber, P.A.; Tian, J.; Lu, J. Neonatal nipple preference and maternal cradling laterality in wild Taihangshan macaques (Macaca mulatta tcheliensis). Am. J. Primatol. 2020, 82, e23197. [Google Scholar] [CrossRef] [PubMed]
- Erwin, J.; Anderson, B.; Bunger, D. Nursing behavior of infant pigtail monkeys (Macaca nemestrina): Preferences for nipples. Percept. Mot. Ski. 1975, 40, 592–594. [Google Scholar] [CrossRef]
- Regaiolli, B.; Spiezio, C.; Hopkins, W.D. Asymmetries in mother-infant behaviour in Barbary macaques (Macaca sylvanus). PeerJ 2018, 6, e4736. [Google Scholar] [CrossRef] [PubMed]
- Boulinguez-Ambroise, G.; Pouydebat, E.; Disarbois, E.; Meguerditchian, A. Maternal cradling bias in baboons: The first environmental factor affecting early infant handedness development? Dev. Sci. 2022, 25, e13179. [Google Scholar] [CrossRef]
- Damerose, E.; Hopkins, W.D. Scan and focal sampling: Reliability in the laterality for maternal cradling and infant nipple preferences in olive baboons, Papio anubis. Anim. Behav. 2002, 63, 511–518. [Google Scholar] [CrossRef]
- Westergaard, G.C. Hand preference in the use of tools by infant baboons (Papio cynocephalus anubis). Percept. Mot. Ski. 1993, 76, 447–450. [Google Scholar] [CrossRef]
- Fagot, J. Ontogeny of object manipulation and manual lateralization in the Guinea baboon: Preliminary observations. In Primate Laterality Current Behavioral Evidence of Primate Asymmetries; Springer: Berlin, Germany, 1993; pp. 235–250. [Google Scholar]
- Winkler, P.; Wrogemann, D.; Prestel, H. Twins in free-ranging Hanuman langurs (Presbytis entellus). Primates 1989, 30, 255–259. [Google Scholar] [CrossRef]
- Horwich, R.H. Development of behaviors in a male spectacled langur (Presbytis obscurus). Primates 1974, 15, 151–178. [Google Scholar] [CrossRef]
- Zhao, D.; Gao, X.; Li, B.; Watanabe, K. First wild evidence of neonate nipple preference and maternal cradling laterality in Old World monkeys: A preliminary study from Rhinopithecus roxellana. Behav. Process. 2008, 77, 364–368. [Google Scholar] [CrossRef]
- Hopkins, W.D.; De Lathouwers, M. Left nipple preferences in infant Pan paniscus and P. troglodytes. Int. J. Primatol. 2006, 27, 1653–1662. [Google Scholar] [CrossRef]
- Dienske, H.; Hopkins, B.; Reid, A.K. Lateralisation of infant holding in chimpanzees: New data do not confirm previous findings. Behaviour 1995, 132, 801–809. [Google Scholar] [CrossRef]
- Nishida, T. Left nipple suckling preference in wild chimpanzees. Ethol. Sociobiol. 1993, 14, 45–51. [Google Scholar] [CrossRef]
- Hopkins, W.D.; Bard, K.A. Asymmetries in spontaneous head orientation in infant chimpanzees (Pan troglodytes). Behav. Neurosci. 1995, 109, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.D.; Bard, K.A. Hemispheric specialization in infant chimpanzees (Pan troglodytes): Evidence for a relation with gender and arousal. Dev. Psychobiol. J. Int. Soc. Dev. Psychobiol. 1993, 26, 219–235. [Google Scholar] [CrossRef]
- Bard, K.A.; Hopkins, W.D.; Fort, C.L. Lateral bias in infant chimpanzees (Pan troglodytes). J. Comp. Psychol. 1990, 104, 309. [Google Scholar] [CrossRef]
- Hopkins, W.D.; Bard, K.A.; Griner, K.M. Locomotor adaptation and leading limb asymmetries in neonatal chimpanzees (Pan troglodytes). Int. J. Primatol. 1997, 18, 105–114. [Google Scholar] [CrossRef]
- Brésard, B.; Bresson, F. Handedness in Pongo pygmaeus and Pan troglodytes. J. Hum. Evol. 1983, 12, 659–666. [Google Scholar] [CrossRef]
- Fagot, J.; Bard, K.A. Asymmetric grasping response in neonate chimpanzees (Pan troglodytes). Infant Behav. Dev. 1995, 18, 253–255. [Google Scholar] [CrossRef]
- Hopkins, W.D.; Bard, K.A. A longitudinal study of hand preference in chimpanzees (Pan troglodytes). Dev. Psychobiol. 2000, 36, 292–300. [Google Scholar] [CrossRef]
- Chorazyna, H. Shifts in laterality in a baby chimpanzee. Neuropsychologia 1976, 14, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.D.; Parr, L.A. Lateralized behavior and lymphocyte counts in chimpanzees (Pan troglodytes): A cross-sectional and longitudinal assessment. Dev. Neuropsychol. 1998, 14, 519–533. [Google Scholar] [CrossRef]
- Bard, K.A. Neonatal neurobehavioral correlates of lateral bias and affect in infant chimpanzees (Pan troglodytes). Dev. Neuropsychol. 1998, 14, 471–494. [Google Scholar] [CrossRef]
- Cunningham, D.; Forsythe, C.; Ward, J.P. A report of behavioral lateralization in an infant orang-utan (Pongo pygmaeus). Primates 1989, 30, 249–253. [Google Scholar] [CrossRef]
- Needham, A.W.; Nelson, E.L. How babies use their hands to learn about objects: Exploration, reach-to-grasp, manipulation, and tool use. WIREs Cogn. Sci. 2023, e1661. [Google Scholar] [CrossRef] [PubMed]
- Fagard, J.; Pezé, A. Age Changes in Interlimb Coupling and the Development of Bimanual Coordination. J. Mot. Behav. 1997, 29, 199–208. [Google Scholar] [CrossRef]
- Michel, G.F. Right-handedness: A consequence of infant supine head-orientation preference? Science 1981, 212, 685–687. [Google Scholar] [CrossRef]
- Michel, G.F.; Harkins, D.A. Postural and lateral asymmetries in the ontogeny of handedness during infancy. Dev. Psychobiol. 1986, 19, 247–258. [Google Scholar] [CrossRef]
- Konishi, Y.; Kuriyama, M.; Mikawa, H.; Suzuki, J. Effect of body position on later postural and functional lateralities of preterm infants. Dev. Med. Child Neurol. 1987, 29, 751–756. [Google Scholar] [CrossRef]
- Corbetta, D.; Thelen, E. Lateral biases and fluctuations in infants’ spontaneous arm movements and reaching. Dev. Psychobiol. 1999, 34, 237–255. [Google Scholar] [CrossRef]
- Boulinguez-Ambroise, G.; Pouydebat, E.; Disarbois, É.; Meguerditchian, A. Human-like maternal left-cradling bias in monkeys is altered by social pressure. Sci. Rep. 2020, 10, 11036. [Google Scholar] [CrossRef] [PubMed]
- Bailoo, J.D.; Quinius, B.; Schapiro, S.J.; Hopkins, W.D.; Bennett, A.J. Freestanding bipedal posture and coordinated bimanual manipulation significantly influence lateralized hand use in rhesus monkeys (Macaca mulatta) and chimpanzees (Pan troglodytes). J. Comp. Psychol. 2019, 133, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Dettmer, A.M.; Ruggiero, A.M.; Novak, M.A.; Meyer, J.S.; Suomi, S.J. Surrogate mobility and orientation affect the early neurobehavioral development of infant rhesus macaques (Macaca mulatta). Dev. Psychobiol. 2008, 50, 418–422. [Google Scholar] [CrossRef] [PubMed]
- van der Meer, A.L. Keeping the arm in the limelight: Advanced visual control of arm movements in neonates. Eur. J. Paediatr. Neurol. 1997, 1, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Gunderson, V.M.; Sackett, G.P. Development of pattern recognition in infant pigtailed macaques (Macaca nemestrina). Dev. Psychol. 1984, 20, 418–426. [Google Scholar] [CrossRef]
- Hopkins, W.D. Neuroanatomical asymmetries in nonhuman primates in the homologs to Broca's and Wernicke’s areas: A mini-review. Emerg. Top. Life Sci. 2022, 6, 271–284. [Google Scholar] [CrossRef]
- Manning, J.; Heaton, R.; Chamberlain, A. Left-side cradling: Similarities and differences between apes and humans. J. Hum. Evol. 1994, 26, 77–83. [Google Scholar] [CrossRef]
- Trehub, S.E.; Corter, C.M.; Shosenberg, N. Neonatal reflexes: A search for lateral asymmetries. In Manual Specialization and the Developing Brain; Young, G., Segalowitz, S.J., Corter, C.M., Trehub, S.E., Eds.; Academic Press: San Diego, CA, USA, 1983; pp. 257–274. [Google Scholar]
- Michel, G.F.; Goodwin, R. Intrauterine birth position predicts newborn supine head position preferences. Infant Behav. Dev. 1979, 2, 29–38. [Google Scholar] [CrossRef]
- Hepper, P.G.; Wells, D.L.; Lynch, C. Prenatal thumb sucking is related to postnatal handedness. Neuropsychologia 2005, 43, 313–315. [Google Scholar] [CrossRef]
- Previc, F.H. A general theory concerning the prenatal origins of cerebral lateralization in humans. Psychol. Rev. 1991, 98, 299–334. [Google Scholar] [CrossRef]
- Roberts, V.H.; Castro, J.N.; Wessel, B.M.; Conrad, D.F.; Lewis, A.D.; Lo, J.O. Rhesus macaque fetal and placental growth demographics: A resource for laboratory animal researchers. Am. J. Primatol. 2023, e23526. [Google Scholar] [CrossRef] [PubMed]
- ManyPrimates; Altschul, D.M.; Beran, M.J.; Bohn, M.; Caspar, K.R.; Fichtel, C.; Försterling, M.; Grebe, N.; Hernandez-Aguilar, R.; Kwok, S.C.; et al. Collaborative open science as a way to reproducibility and new insights in primate cognition research. Jpn. Psychol. Rev. 2019, 62, 205–220. [Google Scholar] [CrossRef]
- Becker, Y.; Sein, J.; Velly, L.; Giacomino, L.; Renaud, L.; Lacoste, R.; Anton, J.-L.; Nazarian, B.; Berne, C.; Meguerditchian, A. Early Left-Planum Temporale Asymmetry in newborn monkeys (Papio anubis): A longitudinal structural MRI study at two stages of development. NeuroImage 2021, 227, 117575. [Google Scholar] [CrossRef] [PubMed]
- Becker, Y.; Phelipon, R.; Sein, J.; Velly, L.; Renaud, L.; Meguerditchian, A. Planum temporale grey matter volume asymmetries in newborn monkeys (Papio anubis). Brain Struct. Funct. 2022, 227, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Vingerhoets, G.; Verhelst, H.; Gerrits, R.; Badcock, N.; Bishop, D.V.; Carey, D.; Flindall, J.; Grimshaw, G.; Harris, L.J.; Hausmann, M. Laterality indices consensus initiative (LICI): A Delphi expert survey report on recommendations to record, assess, and report asymmetry in human behavioural and brain research. Laterality 2023, 28, 122–191. [Google Scholar] [CrossRef]
- Van Lawick-Goodall, J. Some aspects of mother-infant relationships in a group of wild chimpanzees. In The Origins of Human Social Relations; Academic Press: Oxford, UK, 1971; p. 297. [Google Scholar]
- Kubota, K. Learning of a hiding task and a delayed response task in infant rhesus monkeys. Neurosci. Res. 1994, 18, 301–313. [Google Scholar] [CrossRef]
- Corp, N.; Byrne, R.W. The ontogeny of manual skill in wild chimpanzees: Evidence from feeding on the fruit of Saba florida. Behaviour 2002, 139, 137–168. [Google Scholar] [CrossRef]
- Rossano, F. Sequence organization and timing of bonobo mother-infant interactions. Interact. Stud. Soc. Behav. Commun. Biol. Artif. Syst. 2013, 14, 160–189. [Google Scholar] [CrossRef]
- Sakamoto, K.; Sawada, K.; Fukunishi, K.; Noritaka, I.; Sakata-Haga, H.; Yoshihiro, F. Postnatal change in sulcal length asymmetry in cerebrum of cynomolgus monkeys (Macaca fascicularis). Anat. Rec. 2014, 297, 200–207. [Google Scholar] [CrossRef]
- Scott, J.A.; Grayson, D.; Fletcher, E.; Lee, A.; Bauman, M.D.; Schumann, C.M.; Buonocore, M.H.; Amaral, D.G. Longitudinal analysis of the developing rhesus monkey brain using magnetic resonance imaging: Birth to adulthood. Brain Struct. Funct. 2016, 221, 2847–2871. [Google Scholar] [CrossRef]
- Lehman, R.A. Distribution and changes in strength of hand preference of cynomolgus monkeys. Brain Behav. Evol. 1980, 17, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Brooker, R.J.; Lehman, R.A.; Heimbuch, R.C.; Kidd, K.K. Hand usage in a colony of bonnet monkeys, Macaca radiata. Behav. Genet. 1981, 11, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.D.; Wesley, M.J.; Russell, J.L.; Schapiro, S.J. Parental and perinatal factors influencing the development of handedness in captive chimpanzees. Dev. Psychobiol. 2006, 48, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.D.; Bard, K.A.; Jones, A.; Bales, S.L. Chimpanzee Hand Preference in Throwing and Infant Cradling: Implications for the Origin of Human Handedness. Curr. Anthropol. 1993, 34, 786–790. [Google Scholar] [CrossRef]
- Manning, J.T.; Denman, J. Lateral cradling preferences in humans (Homo sapiens): Similarities within families. J. Comp. Psychol. 1994, 108, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.D.; Leavens, D.A. Hand use and gestural communication in chimpanzees (Pan troglodytes). J. Comp. Psychol. 1998, 112, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Carriba, S.; Loeches, A.; Morcillo, A.; Hopkins, W.D. Asymmetry in facial expression of emotions by chimpanzees. Neuropsychologia 2002, 40, 1523–1533. [Google Scholar] [CrossRef]
- Fletcher, A.W.; Weghorst, J.A. Laterality of hand function in naturalistically housed chimpanzees (Pan troglodytes). Laterality 2005, 10, 219–242. [Google Scholar] [CrossRef]
- Lyn, H.; Pierre, P.; Bennett, A.J.; Fears, S.; Woods, R.; Hopkins, W.D. Planum temporale grey matter asymmetries in chimpanzees (Pan troglodytes), vervet (Chlorocebus aethiops sabaeus), rhesus (Macaca mulatta) and bonnet (Macaca radiata) monkeys. Neuropsychologia 2011, 49, 2004–2012. [Google Scholar] [CrossRef]
- Bourjade, M.; Meunier, H.; Blois-Heulin, C.; Vauclair, J. Baboons’ hand preference resists to spatial factors for a communicative gesture but not for a simple manipulative action. Dev. Psychobiol. 2013, 55, 651–661. [Google Scholar] [CrossRef]
- Hopkins, W.D.; Reamer, L.; Mareno, M.C.; Schapiro, S.J. Genetic basis in motor skill and hand preference for tool use in chimpanzees (Pan troglodytes). Proc. Biol. Sci. 2015, 282, 20141223. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.D.; Meguerditchian, A.; Coulon, O.; Misiura, M.; Pope, S.; Mareno, M.C.; Schapiro, S.J. Motor skill for tool-use is associated with asymmetries in Broca’s area and the motor hand area of the precentral gyrus in chimpanzees (Pan troglodytes). Behav. Brain Res. 2017, 318, 71–81. [Google Scholar] [CrossRef]
- Rogers, L.J. Differential Ageing of the Brain Hemispheres: Evidence from a Longitudinal Study of Hand Preferences in Common Marmosets. Symmetry 2021, 13, 2349. [Google Scholar] [CrossRef]
- Drea, C.M.; Wallen, K.; Akinbami, M.A.; Mann, D.R. Neonatal testosterone and handedness in yearling rhesus monkeys (Macaca mulatta). Physiol. Behav. 1995, 58, 1257–1262. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.D. Hand preferences for simple reaching in juvenile chimpanzees (Pan troglodytes): Continuity in development. Dev. Psychol. 1995, 31, 619–625. [Google Scholar] [CrossRef]
- Westergaard, G.C.; Suomi, S.J. Lateral bias in capuchin monkeys (Cebus apella): Concordance between parents and offspring. Dev. Psychobiol. 1997, 31, 143–147. [Google Scholar] [CrossRef]
- Morino, L.; Uchikoshi, M.; Bercovitch, F.; Hopkins, W.D.; Matsuzawa, T. Tube task hand preference in captive hylobatids. Primates 2017, 58, 403–412. [Google Scholar] [CrossRef]
- Fu, W.-W.; Ren, Y.; Wang, C.-L.; Wang, X.-W.; Li, B.-G. Hand Preference in Rhinopithecus roxellana Infants: Is It Influenced by Familial Inheritance? Symmetry 2020, 12, 1905. [Google Scholar] [CrossRef]
- Vauclair, J.; Fagot, J. Spontaneous hand usage and handedness in a troop of baboons. Cortex 1987, 23, 265–274. [Google Scholar] [CrossRef]
- Gao, X.; Guo, S.; Hu, Y.; Li, B.; Qi, X. Maternal cradling laterality and neonate nipple preference in the Sichuan snub-nosed monkey (Rhinopithecus roxellana) in Qinling Mountains. Acta Theriol. Sinica 2010, 30, 133–138. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).