Structural Stability of γ-Boron under High Pressure up to 126 GPa with Fine Pressure Increments
Abstract
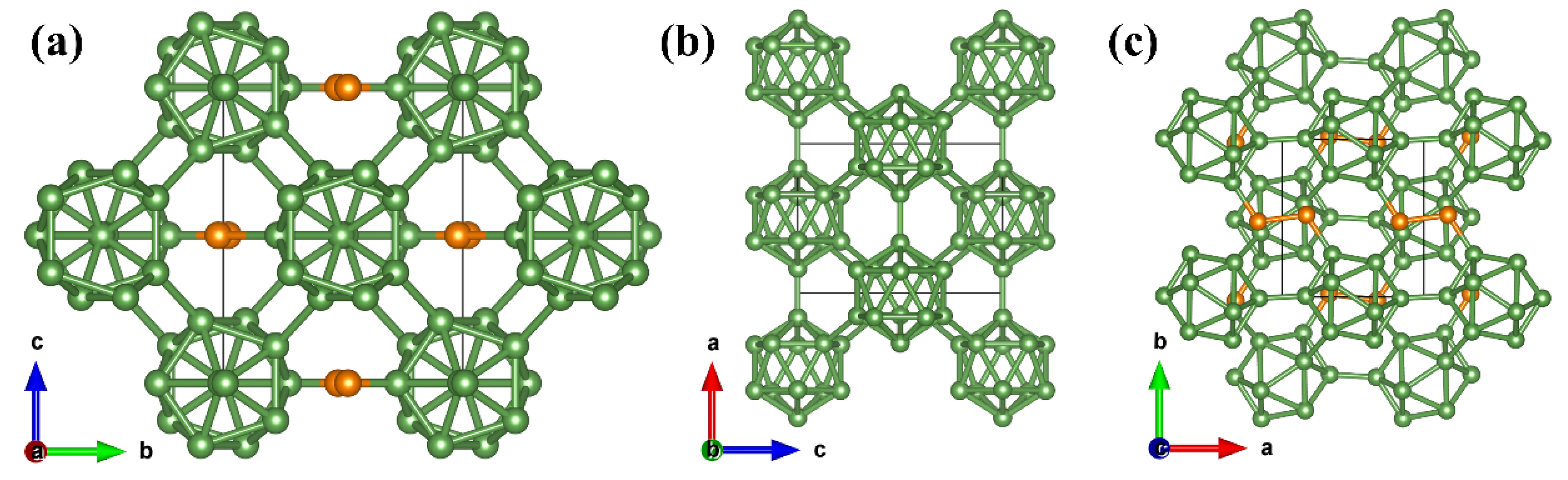
1. Introduction
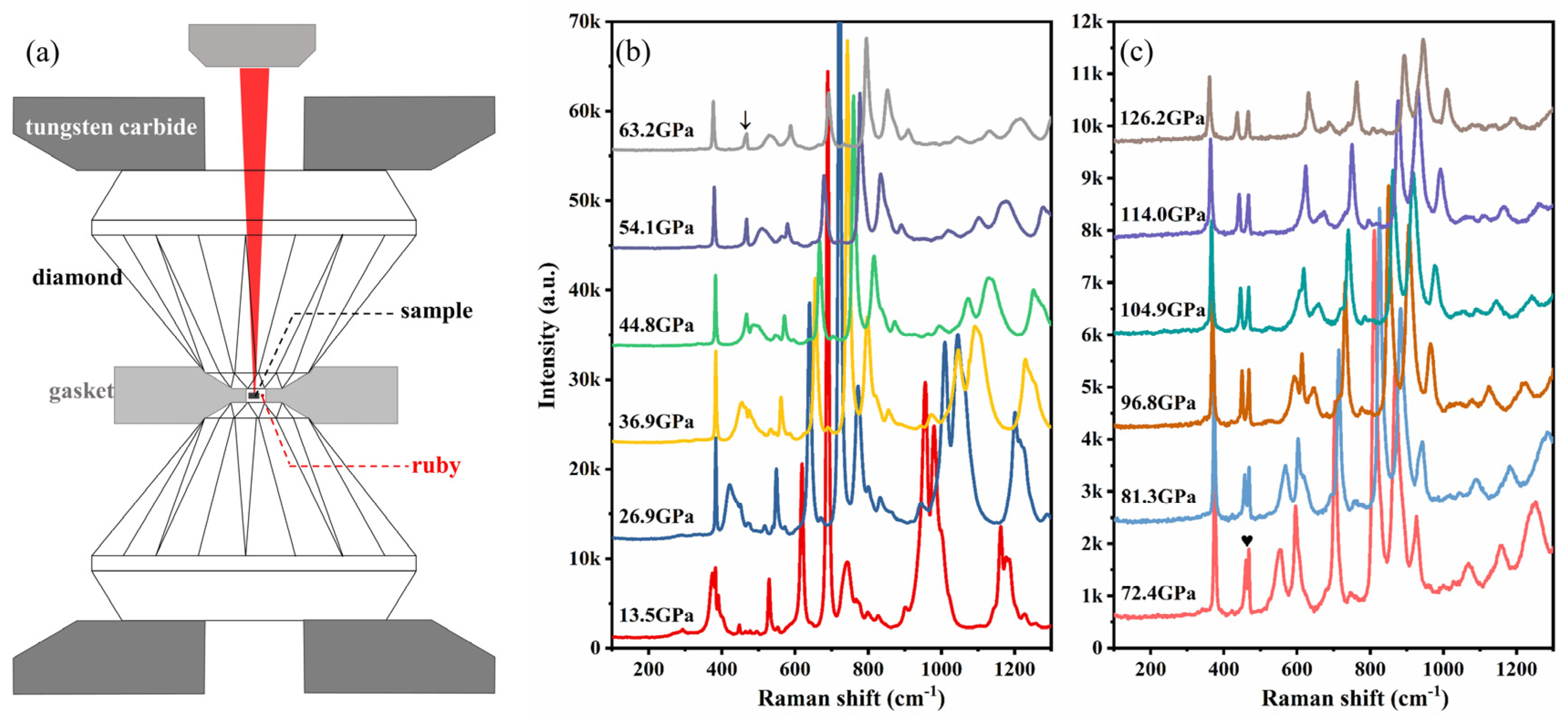
2. Experimental Details
3. First Principle Calculations
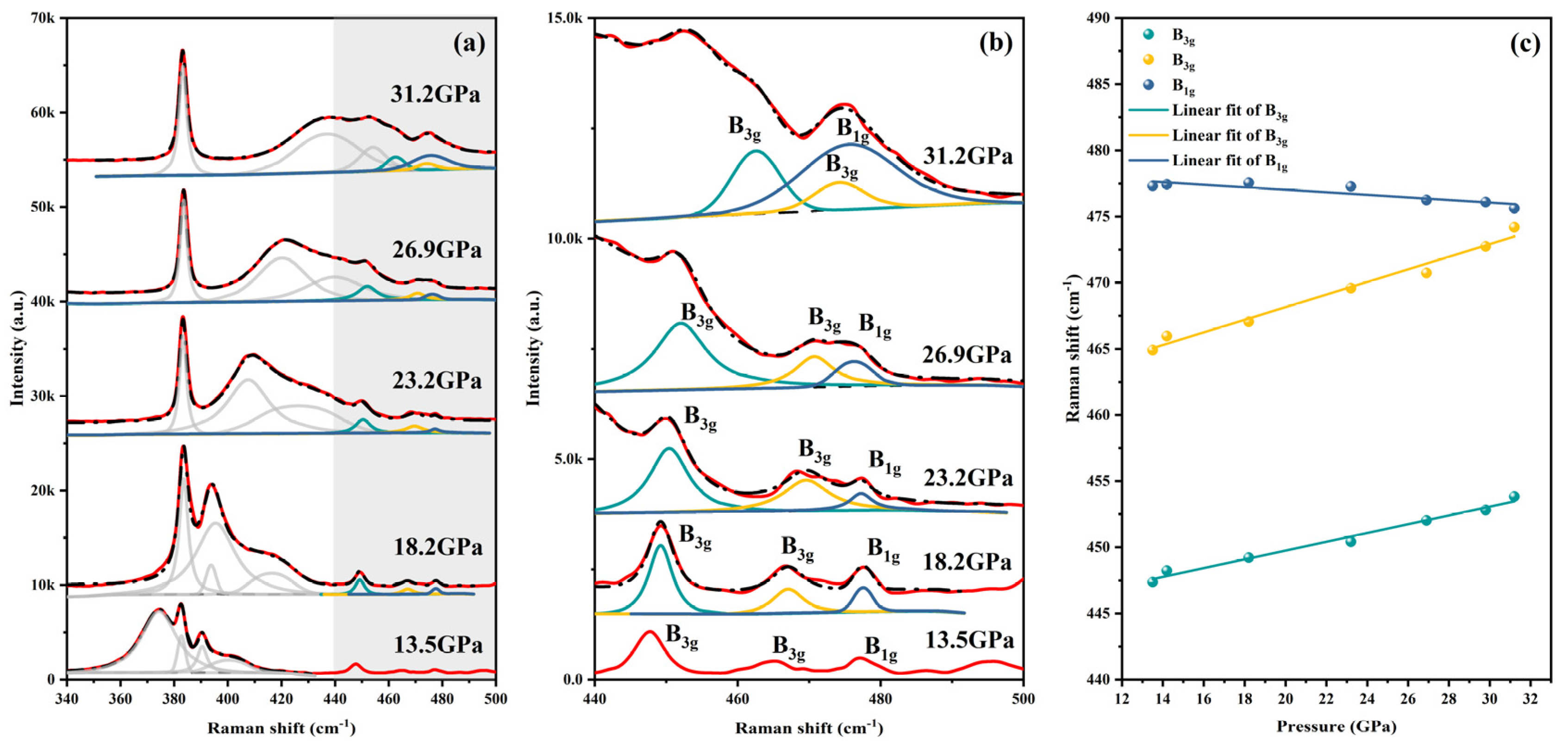
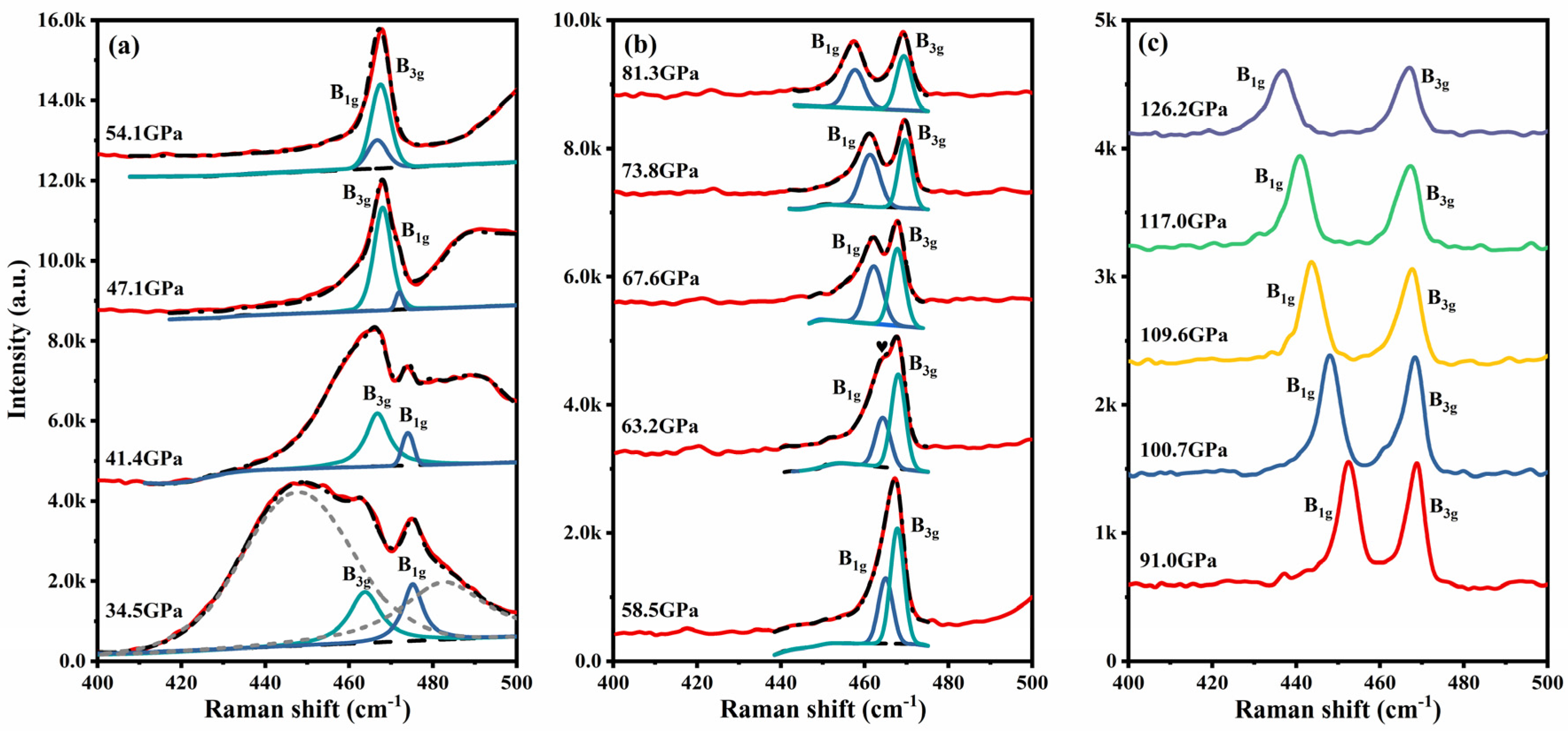
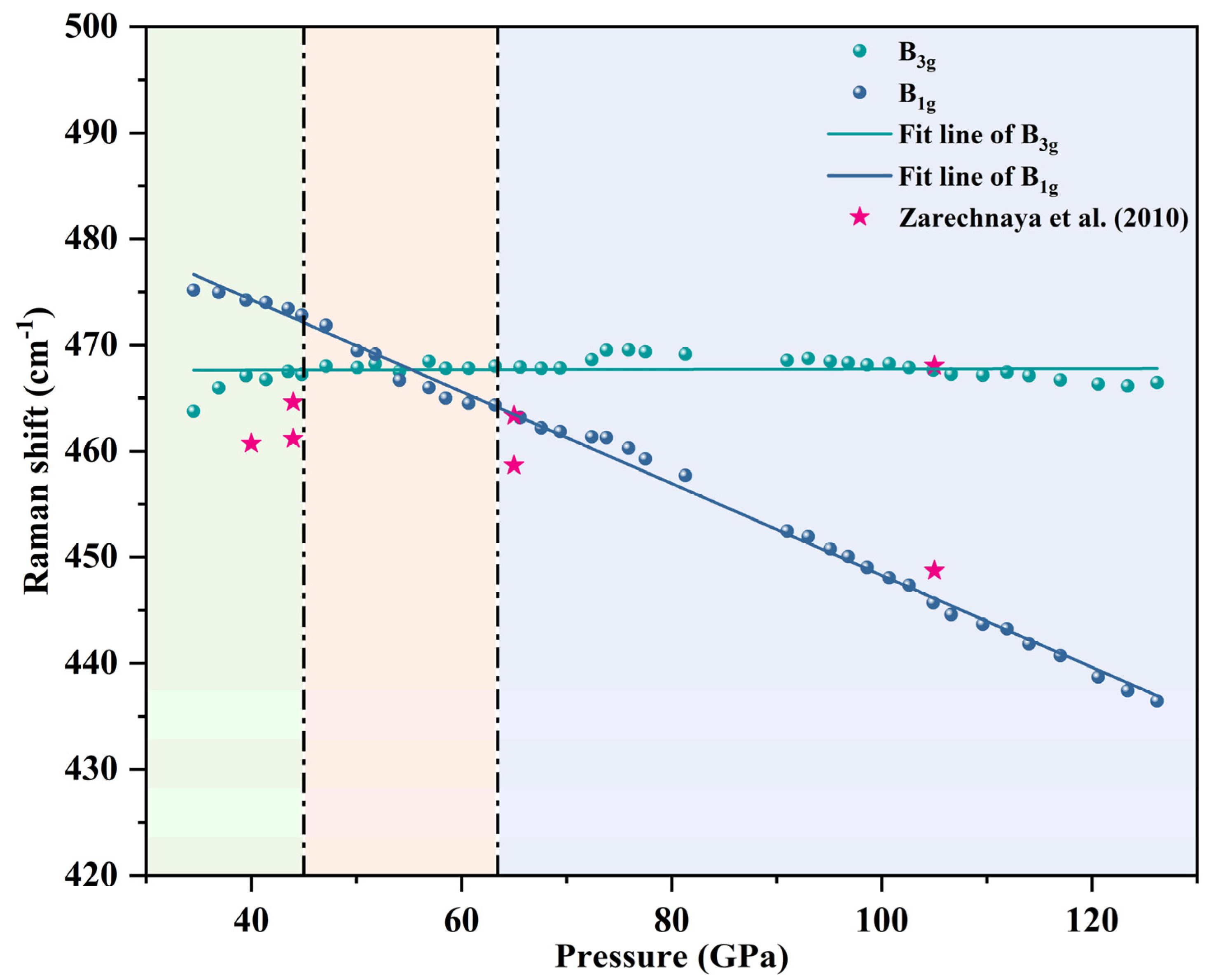
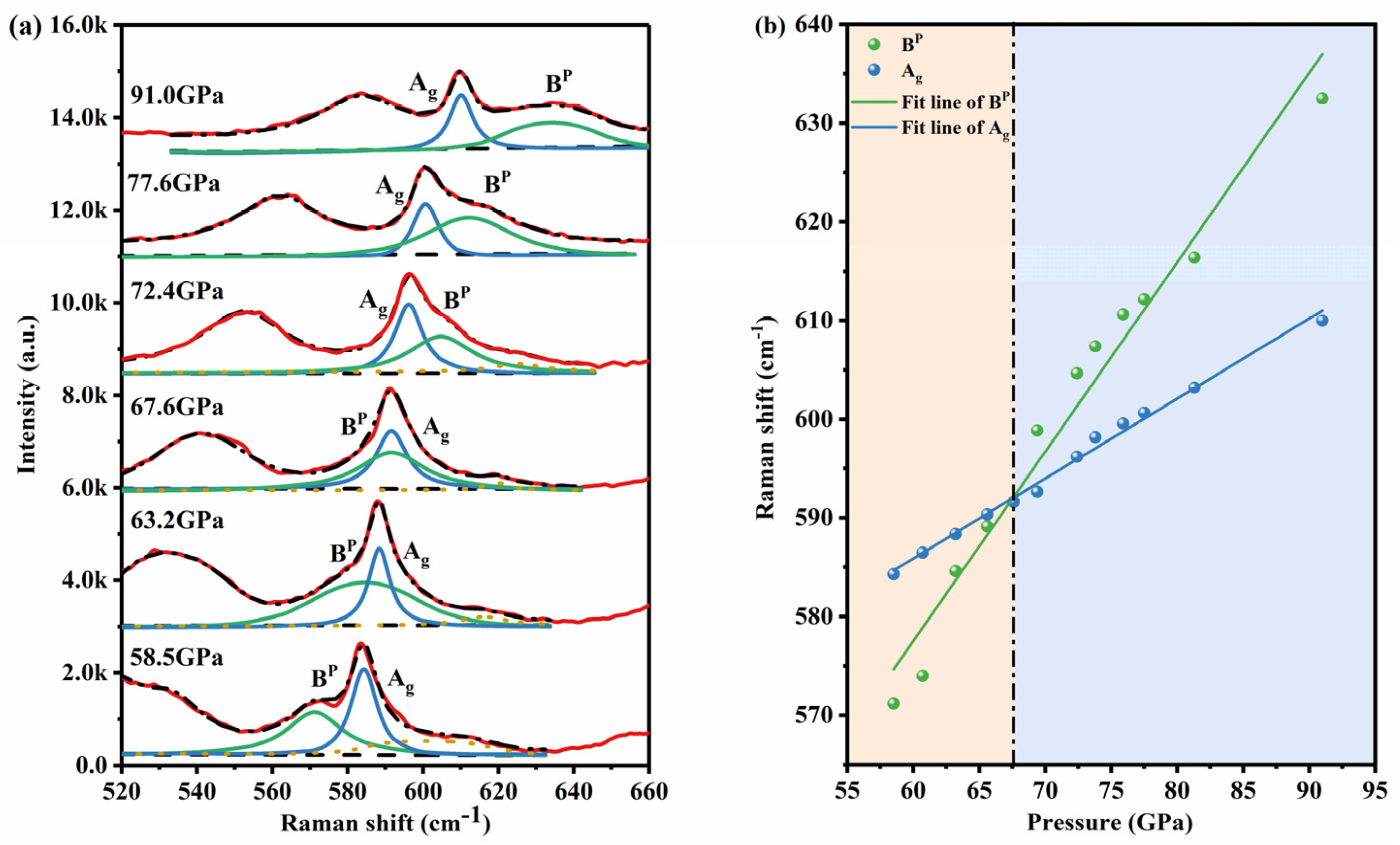
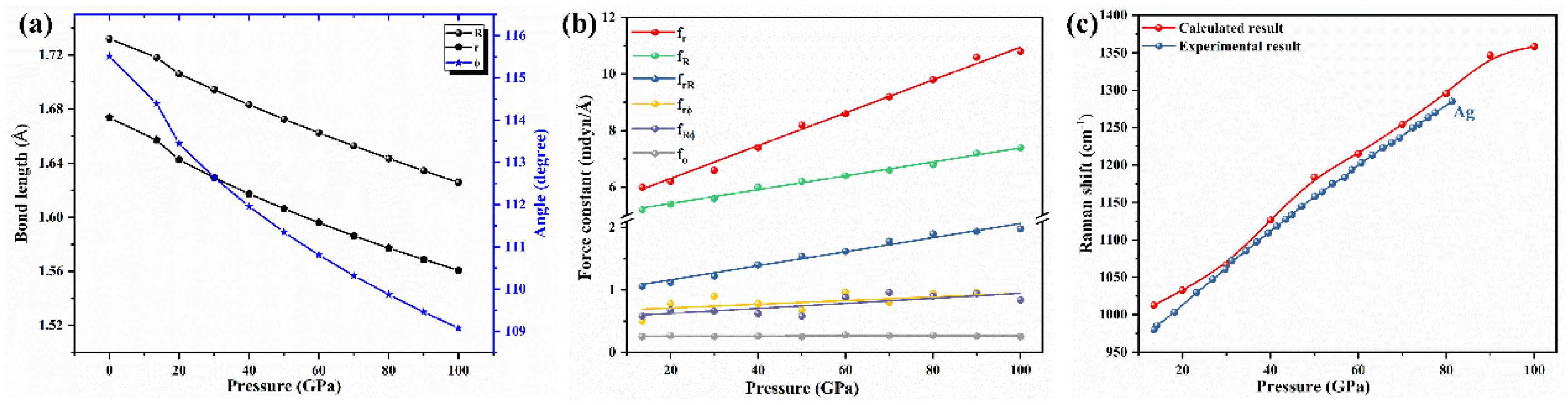
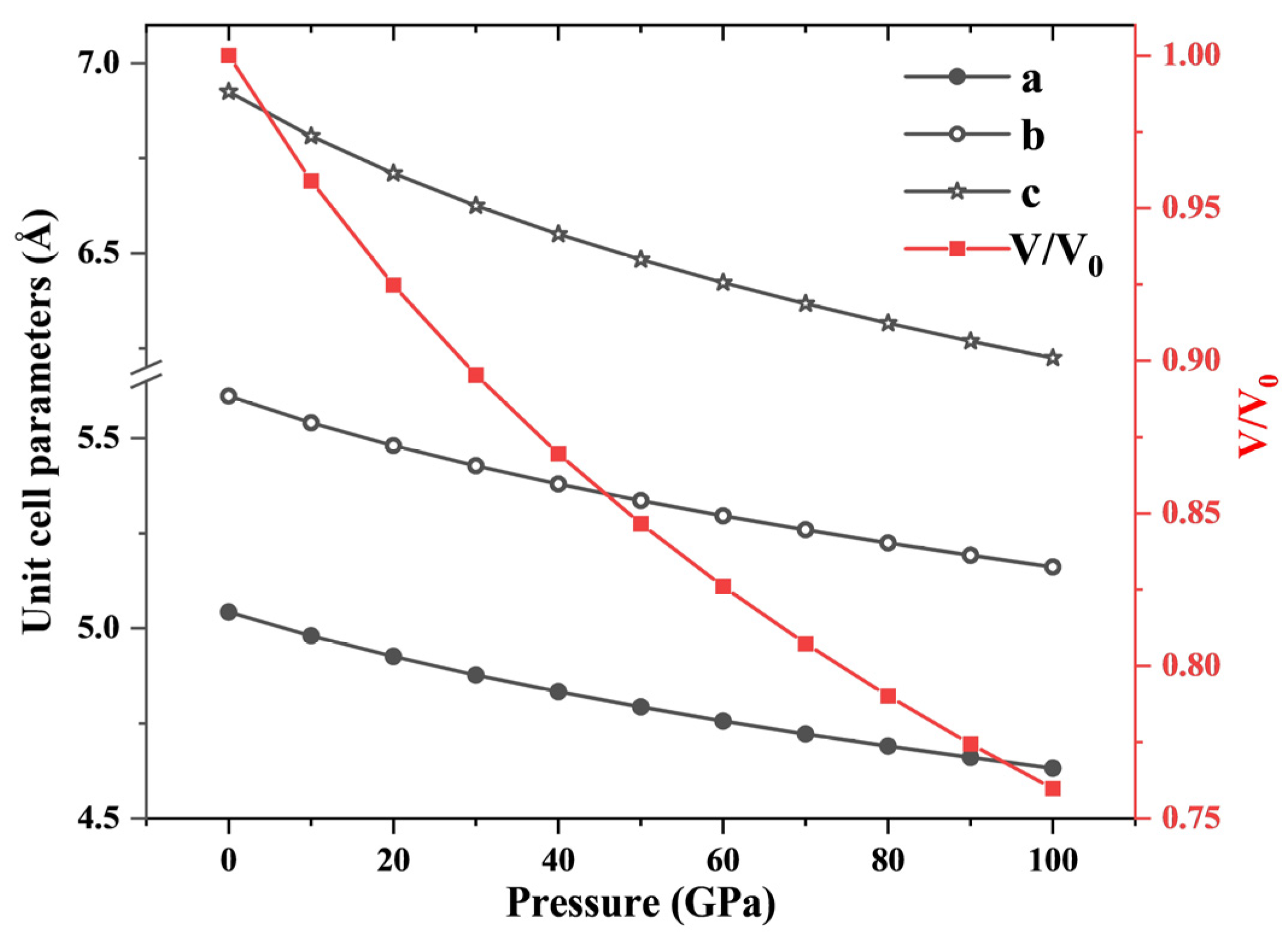
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Douglas, B.; Ho, S.-M. Structure and Chemistry of Crystalline Solids; Springer: New York, NY, USA, 2006. [Google Scholar]
- Hughes, R.E.; Kennard, C.H.L.; Sullenger, D.B.; Weakliem, H.A.; Sands, D.E.; Hoard, J.L. The Structure of Β-Rhombohedral Boron. J. Am. Chem. Soc. 1963, 85, 361–362. [Google Scholar] [CrossRef]
- Oganov, A.R.; Chen, J.; Gatti, C.; Ma, Y.; Ma, Y.; Glass, C.W.; Liu, Z.; Yu, T.; Kurakevych, O.O.; Solozhenko, V.L. Ionic High-Pressure Form of Elemental Boron. Nature 2009, 457, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lu, J.P. Pressure-Induced Metallization in Solid Boron. Phys. Rev. B 2002, 66, 092101. [Google Scholar] [CrossRef]
- Haussermann, U.; Simak, S.I.; Ahuja, R.; Johansson, B. Metal-Nonmetal Transition in the Boron Group Elements. Phys. Rev. Lett. 2003, 90, 065701. [Google Scholar] [CrossRef]
- Shirai, K.; Dekura, H.; Yanase, A. Electronic Structure and Electrical Resistivity of A-Boron under High Pressure. J. Phys. Soc. Jpn. 2009, 78, 084714. [Google Scholar] [CrossRef]
- Eremets, M.I.; Struzhkin, V.V.; Mao, H.; Hemley, R.J. Superconductivity in Boron. Science 2001, 293, 272–274. [Google Scholar] [CrossRef]
- Kaneshige, M.; Hirayama, S.; Yabuuchi, T.; Matsuoka, T.; Shimizu, K.; Mita, Y.; Hyoudo, H.; Kimura, K. Measurement of Electrical Resistance and Raman Spectrum of A-Boron under High Pressure. J. Phys. Soc. Jpn. 2007, 76, 19–20. [Google Scholar] [CrossRef]
- Shimizu, K.; Kaneshige, M.; Hashimoto, Y.; Nagatochi, T.; Hyodo, H.; Kimura, K. Superconductivity in A-Boron at Mbar Pressure. Phys. C Supercond. Appl. 2010, 470, S631–S632. [Google Scholar] [CrossRef]
- Vast, N.; Baroni, S.; Zerah, G.; Besson, J.M.; Polian, A.; Grimsditch, M.; Chervin, J.C. Lattice Dynamics of Icosahedralα-Boron under Pressure. Phys. Rev. Lett. 1997, 78, 693–696. [Google Scholar] [CrossRef]
- Masago, A.; Shirai, K.; Katayama-Yoshida, H. Crystal Stability Ofα- Andβ-Boron. Phys. Rev. B 2006, 73, 104102. [Google Scholar] [CrossRef]
- Shang, S.; Wang, Y.; Arroyave, R.; Liu, Z.-K. Phase Stability Inα- Andβ-Rhombohedral Boron. Phys. Rev. B 2007, 75, 092101. [Google Scholar] [CrossRef]
- Polian, A.; Chervin, J.C.; Munsch, P.; Gauthier, M. A-Boron at Very High Pressure: Structural and Vibrational Properties. J. Phys. Conf. Ser. 2008, 121, 042017. [Google Scholar] [CrossRef]
- Parakhonskiy, G.; Vtech, V.; Dubrovinskaia, N.; Caracas, R.; Dubrovinsky, L. Raman Spectroscopy Investigation of Alpha Boron at Elevated Pressures and Temperatures. Solid State Commun. 2013, 154, 34–39. [Google Scholar] [CrossRef]
- Chuvashova, I.; Bykova, E.; Bykov, M.; Svitlyk, V.; Gasharova, B.; Mathis, Y.-L.; Caracas, R.; Dubrovinsky, L.; Dubrovinskaia, N. High-Pressure Behavior of A-Boron Studied on Single Crystals by X-Ray Diffraction, Raman and Ir Spectroscopy. J. Solid. State Chem. 2017, 245, 50–60. [Google Scholar] [CrossRef]
- Pokatashkin, P.A.; Korotaev, P.Y.; Yanilkin, A.V. Amorphization Inα-Boron: A Molecular Dynamics Study. Phys. Rev. B 2017, 95, 064113. [Google Scholar] [CrossRef]
- Sanz, D.N.; Loubeyre, P.; Mezouar, M. Equation of State and Pressure Induced Amorphization of Beta-Boron from X-Ray Measurements up to 100 Gpa. Phys. Rev. Lett. 2002, 89, 245501. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Prewitt, C.T.; Zou, G.; Mao, H.-k.; Hemley, R.J. High-Pressure High-Temperature X-Ray Diffraction of Β-Boron to 30 Gpa. Phys. Rev. B 2003, 67, 174116. [Google Scholar] [CrossRef]
- Siberchicot, B. Ab Initioequation of State Ofα- Andβ-Boron: Possible Amorphization Ofβ-Boron under High Pressure. Phys. Rev. B 2009, 79, 224101. [Google Scholar] [CrossRef]
- An, Q.; Morozov, S.I. Brittle Failure of Β- and Τ-Boron: Amorphization under High Pressure. Phys. Rev. B 2017, 95, 064108. [Google Scholar] [CrossRef]
- Shirai, K.; Masago, A.; Katayama-Yoshida, H. High-Pressure Properties and Phase Diagram of Boron. Phys. Status Solidi 2007, 244, 303–308. [Google Scholar] [CrossRef]
- Parakhonskiy, G.; Dubrovinskaia, N.; Bykova, E.; Wirth, R.; Dubrovinsky, L. Experimental Pressure-Temperature Phase Diagram of Boron: Resolving the Long-Standing Enigma. Sci. Rep. 2011, 1, 96. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Irifune, T.; Dekura, H.; Ohfuji, H.; Nishiyama, N.; Lei, L.; Shinmei, T. Phase Relations in Boron at Pressures up to 18 Gpa and Temperatures up to 2200 °C. Phys. Rev. B 2012, 85, 014107. [Google Scholar] [CrossRef]
- Solozhenko, V.L.; Kurakevych, O.O. Equilibrium P-T Phase Diagram of Boron: Experimental Study and Thermodynamic Analysis. Sci. Rep. 2013, 3, 2351. [Google Scholar] [CrossRef] [PubMed]
- Shirai, K. Phase Diagram of Boron Crystals. Jpn. J. Appl. Phys. 2017, 56, 05FA06. [Google Scholar] [CrossRef]
- Wentorf, R.H., Jr. Boron: Another Form. Science 1965, 147, 49–50. [Google Scholar] [CrossRef]
- Ma, Y.; Tse, J.S.; Klug, D.D.; Ahuja, R. Electron-Phonon Coupling Ofα−Gaboron. Phys. Rev. B 2004, 70, 214107. [Google Scholar] [CrossRef]
- Jiang, C.; Lin, Z.; Zhang, J.; Zhao, Y. First-Principles Prediction of Mechanical Properties of Gamma-Boron. Appl. Phys. Lett. 2009, 94, 191906. [Google Scholar] [CrossRef]
- Zarechnaya, E.Y.; Dubrovinsky, L.; Dubrovinskaia, N.; Filinchuk, Y.; Chernyshov, D.; Dmitriev, V.; Miyajima, N.; El Goresy, A.; Braun, H.F.; Van Smaalen, S.; et al. Superhard Semiconducting Optically Transparent High Pressure Phase of Boron. Phys. Rev. Lett. 2009, 102, 185501. [Google Scholar] [CrossRef]
- Isaev, E.I.; Simak, S.I.; Mikhaylushkin, A.S.; Vekilov, Y.K.; Zarechnaya, E.Y.; Dubrovinsky, L.; Dubrovinskaia, N.; Merlini, M.; Hanfland, M.; Abrikosov, I.A. Impact of Lattice Vibrations on Equation of State of the Hardest Boron Phase. Phys. Rev. B 2011, 83, 132106. [Google Scholar] [CrossRef]
- Godec, Y.L.; Kurakevych, O.O.; Munsch, P.; Garbarino, G.; Solozhenko, V.L. Equation of State of Orthorhombic Boron, -B28. Solid. State Commun. 2009, 149, 1356–1358. [Google Scholar] [CrossRef]
- Le Godec, Y. Comparative Review of Theoretical and Experimental Equations of State of Orthorhombic Boron Γ-B28. J. Superhard Mater. 2011, 33, 388–393. [Google Scholar] [CrossRef]
- Oganov, A.R. Discovery of Γ-B28, a Novel Boron Allotrope with Partially Ionic Bonding. MRS Online Proc. Libr. 2011, 1307, 201. [Google Scholar] [CrossRef]
- Zarechnaya, E.; Dubrovinskaia, N.; Caracas, R.; Merlini, M.; Hanfland, M.; Filinchuk, Y.; Chernyshov, D.; Dmitriev, V.; Dubrovinsky, L. Pressure-Induced Isostructural Phase Transformation Inγ-B28. Phys. Rev. B 2010, 82, 184111. [Google Scholar] [CrossRef]
- Oganov, A.R.; Solozhenko, V.L.; Gatti, C.; Kurakevych, O.O.; Le Godec, Y. The High-Pressure Phase of Boron, Γ-B28: Disputes and Conclusions of 5 Years after Discovery. J. Superhard Mater. 2011, 33, 363–379. [Google Scholar] [CrossRef]
- Yu Zarechnaya, E.; Dubrovinsky, L.; Dubrovinskaia, N.; Miyajima, N.; Filinchuk, Y.; Chernyshov, D.; Dmitriev, V. Synthesis of an Orthorhombic High Pressure Boron Phase. Sci. Technol. Adv. Mater. 2008, 9, 044209. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.K.; Xu, J.; Bell, P.M. Calibration of the Ruby Pressure Gauge to 800 Kbar under Quasi-Hydrostatic Conditions. J. Geophys. Res. 1986, 91, 4673–4676. [Google Scholar] [CrossRef]
- Akahama, Y.; Kawamura, H. High-Pressure Raman Spectroscopy of Diamond Anvils to 250 gpa: Method for Pressure Determination in the Multimegabar Pressure Range. J. Appl. Phys. 2004, 96, 3748–3751. [Google Scholar] [CrossRef]
- Dalladay-Simpson, P.; Howie, R.T.; Gregoryanz, E. Evidence for a New Phase of Dense Hydrogen above 325 Gigapascals. Nature 2016, 529, 63–67. [Google Scholar] [CrossRef]
- Zarechnaya, E.Y.; Dubrovinskaia, N.; Dubrovinsky, L. Polarized Raman Spectroscopy of High-Pressure Orthorhombic Boron Phase. High Press. Res. 2009, 29, 530–535. [Google Scholar] [CrossRef]
- Silvera, I.F.; Jeon, S.J.; Lorenzana, H.E. Pressure Dependence of the Vibron Modes in Solid Hydrogen and Deuterium. Phys. Rev. B Condens. Matter 1992, 46, 5791–5794. [Google Scholar] [CrossRef]
- Wilson, E.B. Some Mathematical Methods for the Study of Molecular Vibrations. J. Chem. Phys. 1941, 9, 76–84. [Google Scholar] [CrossRef]
- Decius, J.C. A Tabulation of General Formulas for Inverse Kinetic Energy Matrix Elements in Acyclic Molecules. J. Chem. Phys. 1948, 16, 1025–1034. [Google Scholar] [CrossRef]
- Wilson, E.B. A Method of Obtaining the Expanded Secular Equation for the Vibration Frequencies of a Molecule. J. Chem. Phys. 1939, 7, 1047–1052. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, C.; Mai, D.; Li, X.; Wang, J.; Dai, R.; Wang, Z.; Sun, X.; Zhang, Z. Structural Stability of γ-Boron under High Pressure up to 126 GPa with Fine Pressure Increments. Symmetry 2023, 15, 1308. https://doi.org/10.3390/sym15071308
Zhong C, Mai D, Li X, Wang J, Dai R, Wang Z, Sun X, Zhang Z. Structural Stability of γ-Boron under High Pressure up to 126 GPa with Fine Pressure Increments. Symmetry. 2023; 15(7):1308. https://doi.org/10.3390/sym15071308
Chicago/Turabian StyleZhong, Cheng, Di Mai, Xiangdong Li, Junke Wang, Rucheng Dai, Zhongping Wang, Xiaoyu Sun, and Zengming Zhang. 2023. "Structural Stability of γ-Boron under High Pressure up to 126 GPa with Fine Pressure Increments" Symmetry 15, no. 7: 1308. https://doi.org/10.3390/sym15071308
APA StyleZhong, C., Mai, D., Li, X., Wang, J., Dai, R., Wang, Z., Sun, X., & Zhang, Z. (2023). Structural Stability of γ-Boron under High Pressure up to 126 GPa with Fine Pressure Increments. Symmetry, 15(7), 1308. https://doi.org/10.3390/sym15071308






