2d, or Not 2d: An Almost Perfect Mock of Symmetry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthetic Procedure
2.1.1. Chemical Materials
2.1.2. Chemical Synthesis
2.2. X-ray Diffraction Structure Studies
2.3. X-ray Powder Diffractometry
2.4. Electron Microscopy and EDX
2.5. 3D-DFT
3. Results
3.1. Discussion of the Chemical Syntheses
3.2. Crystal Structures Estimation
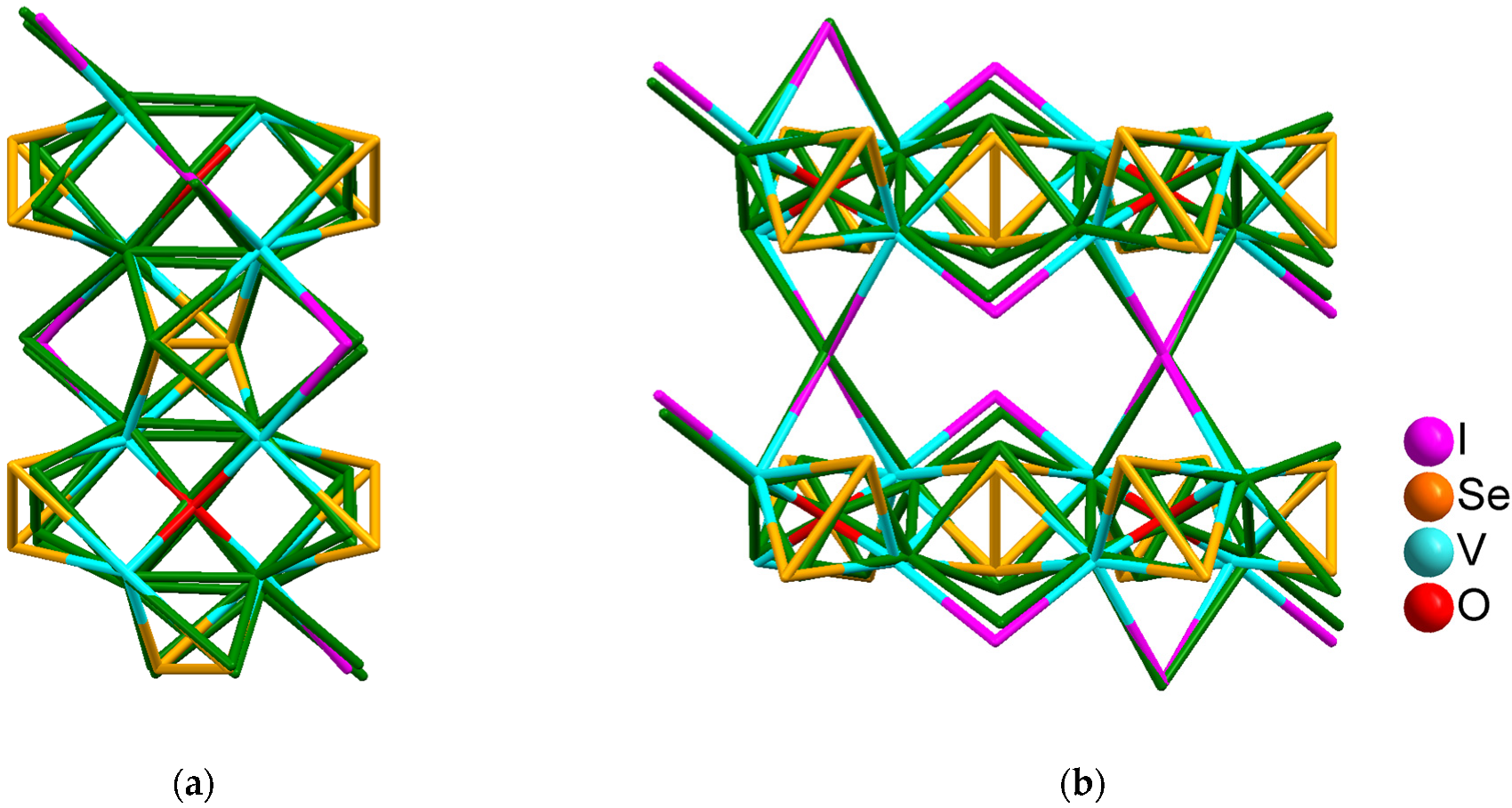
3.2.1. The Crystal Structure of V4OSe8I6·2I2 (M2)
3.2.2. The Crystal Structure of V4OSe8I5 (C)
3.2.3. The Crystal Structure of V4OSe6I3 (L)
3.3. Powder X-ray Diffraction
3.4. Computational Simulations
3.4.1. Oxidation States and Spin Multiplicities Assumptions
3.4.2. Geometry Optimization for V4OSe8I6·2I2 (M2)
3.4.3. Geometry Optimization for V4OSe6I3 (L)
3.4.4. Symmetry Consideration of M2 and L Crystal Structures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gibbs, J.W. On the Equilibrium of Heterogeneous Substances. Trans. Connect. Acad. Arts Sci. 1879, 3, 343–524. [Google Scholar] [CrossRef]
- Kuznetsov, N.T.; Novotortsev, V.M. Nikolai Semenovich Kurnakov (to the 150th Anniversary of His Birthday). Russ. J. Inorg. Chem. 2010, 55, 1668–1679. [Google Scholar] [CrossRef]
- Zhao, J.-C. Phase Diagram Determination Using Diffusion Multiples. In Methods for Phase Diagram Determination; Elsevier: Amsterdam, The Netherlands, 2007; pp. 246–272. ISBN 978-0-08-044629-5. [Google Scholar]
- Egami, T.; Billinge, S.J.L. Underneath the Bragg Peaks: Structural Analysis of Complex Materials; T. Egami, S.J.L.B., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 0080426980. [Google Scholar]
- Welberry, T.R. Diffuse X-Ray Scattering and Models of Disorder; IUCr Monog.; Oxford University Press: Oxford, UK, 2022; ISBN 9780198862482. [Google Scholar]
- Nespolo, M. Lattice versus Structure, Dimensionality versus Periodicity: A Crystallographic Babel? J. Appl. Crystallogr. 2019, 52, 451–456. [Google Scholar] [CrossRef]
- Urusov, V.S. Theoretical Analysis and Empirical Manifestation of the Distortion Theorem. Zeitschrift für Krist. Mater. 2003, 218, 709–719. [Google Scholar] [CrossRef]
- Brown, I.D. Recent Developments in the Methods and Applications of the Bond Valence Model. Chem. Rev. 2009, 109, 6858–6919. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Adams, S. Bond Softness Sensitive Bond-Valence Parameters for Crystal Structure Plausibility Tests. IUCrJ 2017, 4, 614–625. [Google Scholar] [CrossRef]
- Zwart, P.H.; Grosse-Kunstleve, R.W.; Lebedev, A.A.; Murshudov, G.N.; Adams, P.D. Surprises and Pitfalls Arising from (Pseudo)Symmetry. Acta Crystallogr. Sect. D 2008, 64, 99–107. [Google Scholar] [CrossRef]
- Lombardo, G.M.; Punzo, F. False Asymmetry, Pseudosymmetry, Disorder, Polymorphism and Atomic Displacement Parameters. J. Mol. Struct. 2014, 1078, 158–164. [Google Scholar] [CrossRef]
- Clegg, W. Some Reflections on Symmetry: Pitfalls of Automation and Some Illustrative Examples. Acta Crystallogr. Sect. E 2019, 75, 1812–1819. [Google Scholar] [CrossRef] [PubMed]
- Dornberger-Schiff, K. On Order-Disorder Structures (OD-Structures). Acta Crystallogr. 1956, 9, 593–601. [Google Scholar] [CrossRef]
- Belokoneva, E.L. Borate Crystal Chemistry in Terms of the Extended OD Theory: Topology and Symmetry Analysis. Crystallogr. Rev. 2005, 11, 151–198. [Google Scholar] [CrossRef]
- Friedrichs, O.D.; Dress, A.W.M.; Huson, D.H.; Klinowski, J.; Mackay, A.L. Systematic Enumeration of Crystalline Networks. Nature 1999, 400, 644–647. [Google Scholar] [CrossRef]
- Komarov, V.Y.; Solodovnikov, S.F.; Grachev, E.V.; Kosyakov, V.I.; Manakov, A.Y.; Kurnosov, A.V.; Shestakov, V.A. Phase Formation and Structure of High-Pressure Gas Hydrates and Modeling of Tetrahedral Frameworks with Uniform Polyhedral Cavities. Crystallogr. Rev. 2007, 13, 257–297. [Google Scholar] [CrossRef]
- Nespolo, M. Does Mathematical Crystallography Still Have a Role in the XXI Century? Acta Crystallogr. Sect. A 2008, 64, 96–111. [Google Scholar] [CrossRef] [PubMed]
- Tiekink, E. 16th Conferense of the Asian Crystallographic Association (AsCA2019). 2020. Available online: https://www.iucr.org/news/newsletter/volume-28/number-3/16th-conference-of-the-asian-crystallographic-association-asca2019 (accessed on 28 August 2020).
- Kuznetsov, V.G.; Koz’min, P.A. A Study of the Structure of (PyH) HReCl4. J. Struct. Chem. 1963, 4, 49–55. [Google Scholar] [CrossRef]
- Cotton, A.F.; Matonic, J.H.; Silva, D.d.O. Crystallographic Disorder of the M2 Units in [M2Cl8]N− (M = Mo, Re) Compounds. Inorg. Chim. Acta 1995, 234, 115–125. [Google Scholar] [CrossRef]
- Cotton, F.A.; Murillo, C.A.; Walton, R.A. Multiple Bonds between Metal Atoms, 3rd ed.; Cotton, F.A., Murillo, C.A., Walton, R.A., Eds.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2005; ISBN 0387250840. [Google Scholar]
- Fedorov, V.E.; Mishchenko, A.V.; Fedin, V.P. Cluster Transition Metal Chalcogenide Halides. Russ. Chem. Rev. 1985, 54, 408. [Google Scholar] [CrossRef]
- Gabriel, J.-C.P.; Boubekeur, K.; Uriel, S.; Batail, P. Chemistry of Hexanuclear Rhenium Chalcohalide Clusters. Chem. Rev. 2001, 101, 2037–2066. [Google Scholar] [CrossRef] [PubMed]
- Gushchin, A.L.; Laricheva, Y.A.; Sokolov, M.N.; Llusar, R. Tri- and Tetranuclear Molybdenum and Tungsten Chalcogenide Clusters: On the Way to New Materials and Catalysts. Russ. Chem. Rev. 2018, 87, 670. [Google Scholar] [CrossRef]
- Lee, S.C.; Holm, R.H. Nonmolecular Metal Chalcogenide/Halide Solids and Their Molecular Cluster Analogues. Angew. Chemie Int. Ed. English 1990, 29, 840–856. [Google Scholar] [CrossRef]
- Artemkina, S.B.; Grayfer, E.D.; Ivanova, M.N.; Ledneva, A.Y.; Poltarak, A.A.; Poltarak, P.A.; Yarovoi, S.S.; Kozlova, S.G.; Fedorov, V.E. Structural and Chemical Features of Chalcogenides of Early Transition Metals. J. Struct. Chem. 2022, 63, 1079–1100. [Google Scholar] [CrossRef]
- Fedorov, V.E.; Mironov, Y.V.; Naumov, N.G.; Sokolov, M.N.; Fedin, V.P. Chalcogenide Clusters of Group 5–7 Metals. Russ. Chem. Rev. 2007, 76, 529–552. [Google Scholar] [CrossRef]
- Feliz, M.; Guillamón, E.; Llusar, R.; Vicent, C.; Stiriba, S.; Pérez-Prieto, J.; Barberis, M. Unprecedented Stereoselective Synthesis of Catalytically Active Chiral Mo3CuS4 Clusters. Chem. Eur. J. 2006, 12, 1486–1492. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-P.; Hector, A.L.; Levason, W.; Reid, G. Chalcogenoether Complexes of Nb(v) Thio- and Seleno-Halides as Single Source Precursors for Low Pressure Chemical Vapour Deposition of NbS2 and NbSe2 Thin Films. Dalt. Trans. 2017, 46, 9824–9832. [Google Scholar] [CrossRef]
- Cramer, C.J.; Truhlar, D.G. Density Functional Theory for Transition Metals and Transition Metal Chemistry. Phys. Chem. Chem. Phys. 2009, 11, 10757–10816. [Google Scholar] [CrossRef]
- Gimferrer, M.; Van der Mynsbrugge, J.; Bell, A.T.; Salvador, P.; Head-Gordon, M. Facing the Challenges of Borderline Oxidation State Assignments Using State-of-the-Art Computational Methods. Inorg. Chem. 2020, 59, 15410–15420. [Google Scholar] [CrossRef]
- Bartashevich, E.; Yushina, I.; Kropotina, K.; Muhitdinova, S.; Tsirelson, V. Testing the Tools for Revealing and Characterizing the Iodine-Iodine Halogen Bond in Crystals. Acta Crystallogr. Sect. B 2017, 73, 217–226. [Google Scholar] [CrossRef]
- Artemkina, S.; Galiev, R.; Poltarak, P.; Komarov, V.; Gayfulin, Y.; Lavrov, A.; Fedorov, V. Vanadium O-Centered Selenoiodide Complex: Synthesis and Structure of V4O(Se2)4I6·I2. Inorg. Chem. 2021, 60, 17627–17634. [Google Scholar] [CrossRef]
- Artemkina, S.B.; Galiev, R.R.; Komarov, V.Y.; Fedorov, V.E.; Ledneva, A.Y.; Khisamov, R. Characterization of the O-Centered Vanadium Selenoiodides V4OSe8I6· X (X = I2, Dmp). Inorganica Chim. Acta, 2022; 121366, in press. [Google Scholar] [CrossRef]
- Cotton, F.A.; Feng, X.; Kibala, P.A.; Sandor, R.B.W. An Oxygen-Centered Tetranuclear Titanium Compound Ti4O(S2)4Cl6. J. Am. Chem. Soc. 1989, 111, 2148–2151. [Google Scholar] [CrossRef]
- Cotton, F.A.; Kibala, P.A.; Sandor, R.B.W. Synthesis and Crystal Structures of Ti4O(S2)4Br6. Eur. J. Solid State Inorg. Chem. 1988, 25, 631–636. [Google Scholar]
- Liu, S.X.; Huang, D.P.; Huang, C.C.; Xu, H.D.; Huang, J.L. A New Extended Tetranuclear Titanium Cluster: Ti4O(Te2)4Tel4. J. Solid State Chem. 1996, 123, 273–276. [Google Scholar] [CrossRef]
- Tremel, W. Nb4OTe9I4: A One-Dimensional Chain Compound Containing Tetranuclear Oxygen-Centred Niobium Clusters. J. Chem. Soc. Chem. Commun. 1992, 709–710. [Google Scholar] [CrossRef]
- Huang, D.P.; Huang, C.C.; Liu, S.X.; Xu, H.D.; Huang, J.L. An Oxygen-Centred Tetranuclear Tantalum Cluster: Ta4O(Te2)4TeI4. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1998, 54, 893–895. [Google Scholar] [CrossRef]
- Bruker APEX2 Software Suite, APEX2 v.2013.6-2, SADABS v. 2012/1, SAINT v. 8.32b; Bruker AXS Inc.: Madison, WI, USA, 2006.
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of Silver and Molybdenum Microfocus X-Ray Sources for Single-Crystal Structure Determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Coelho, A. TOPAS and TOPAS-Academic: An Optimization Program Integrating Computer Algebra and Crystallographic Objects Written in C++. J. Appl. Crystallogr. 2018, 51, 210–218. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- Kühne, T.D.; Iannuzzi, M.; Del Ben, M.; Rybkin, V.V.; Seewald, P.; Stein, F.; Laino, T.; Khaliullin, R.Z.; Schütt, O.; Schiffmann, F. CP2K: An Electronic Structure and Molecular Dynamics Software Package-Quickstep: Efficient and Accurate Electronic Structure Calculations. J. Chem. Phys. 2020, 152, 194103. [Google Scholar] [CrossRef] [PubMed]
- Lippert, G.; Parrinello, M.; Jurg, H. A Hybrid Gaussian and Plane Wave Density Functional Scheme. Mol. Phys. 1997, 92, 477–488. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- VandeVondele, J.; Hutter, J. Gaussian Basis Sets for Accurate Calculations on Molecular Systems in Gas and Condensed Phases. J. Chem. Phys. 2007, 127, 114105. [Google Scholar] [CrossRef]
- Goedecker, S.; Teter, M.; Hutter, J. Separable Dual-Space Gaussian Pseudopotentials. Phys. Rev. B 1996, 54, 1703. [Google Scholar] [CrossRef]
- Hartwigsen, C.; Gœdecker, S.; Hutter, J. Relativistic Separable Dual-Space Gaussian Pseudopotentials from H to Rn. Phys. Rev. B 1998, 58, 3641. [Google Scholar] [CrossRef]
- Krack, M. Pseudopotentials for H to Kr Optimized for Gradient-Corrected Exchange-Correlation Functionals. Theor. Chem. Acc. 2005, 114, 145–152. [Google Scholar] [CrossRef]
- Poltarak, P.A.; Komarov, V.Y.; Kozlova, S.G.; Sukhikh, A.S.; Artemkina, S.B.; Fedorov, V.E. First Titanium Square Fragment {Ti4(Μ4-Se)(Μ2-Se2)4} in Its Selenoiodide: Synthesis and Structure of Ti4Se9I6. Inorganica Chim. Acta 2019, 488, 285–291. [Google Scholar] [CrossRef]
- Mironov, Y.V.; Yarovoi, S.S.; Naumov, D.Y.; Kozlova, S.G.; Ikorsky, V.N.; Kremer, R.K.; Simon, A.; Fedorov, V.E. V4S9Br4: A Novel High-Spin Vanadium Cluster Thiobromide with Square-Planar Metal Core. J. Phys. Chem. B 2005, 109, 23804–23807. [Google Scholar] [CrossRef] [PubMed]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Krivovichev, S.V.; Mentré, O.; Siidra, O.I.; Colmont, M.; Filatov, S.K. Anion-Centered Tetrahedra in Inorganic Compounds. Chem. Rev. 2013, 113, 6459–6535. [Google Scholar] [CrossRef] [PubMed]
- Poltarak, P.A.; Komarov, V.Y.; Gayfulin, Y.M.; Artemkina, S.B.; Fedorov, V.E. New O-Centered Titanium Chalcohalide: Synthesis and Structure of Ti4O(Se2)4Br6. Z. Fuer Anorg. Und Allg. Chem. 2021, 647, 1729–1734. [Google Scholar] [CrossRef]
- Rodionova, T.V.; Komarov, V.Y.; Villevald, G.V.; Karpova, T.D.; Kuratieva, N.V.; Manakov, A.Y. Calorimetric and Structural Studies of Tetrabutylammonium Bromide Ionic Clathrate Hydrates. J. Phys. Chem. B 2013, 117, 10677–10685. [Google Scholar] [CrossRef]
- Dybtsev, D.N.; Yutkin, M.P.; Samsonenko, D.G.; Fedin, V.P.; Nuzhdin, A.L.; Bezrukov, A.A.; Bryliakov, K.P.; Talsi, E.P.; Belosludov, R.V.; Mizuseki, H. Modular, Homochiral, Porous Coordination Polymers: Rational Design, Enantioselective Guest Exchange Sorption and Ab Initio Calculations of Host–Guest Interactions. Chem. Eur. J. 2010, 16, 10348–10356. [Google Scholar] [CrossRef] [PubMed]
- Lysova, A.A.; Samsonenko, D.G.; Dorovatovskii, P.V.; Lazarenko, V.A.; Khrustalev, V.N.; Kovalenko, K.A.; Dybtsev, D.N.; Fedin, V.P. Tuning the Molecular and Cationic Affinity in a Series of Multifunctional Metal–Organic Frameworks Based on Dodecanuclear Zn(II) Carboxylate Wheels. J. Am. Chem. Soc. 2019, 141, 17260–17269. [Google Scholar] [CrossRef]
- Lysova, A.A.; Samsonenko, D.G.; Kovalenko, K.A.; Nizovtsev, A.S.; Dybtsev, D.N.; Fedin, V.P. A Series of Mesoporous Metal-Organic Frameworks with Tunable Windows Sizes and Exceptionally High Ethane over Ethylene Adsorption Selectivity. Angew. Chemie Int. Ed. 2020, 59, 20561–20567. [Google Scholar] [CrossRef]
- Mikhaylov, M.A.; Abramov, P.A.; Komarov, V.Y.; Sokolov, M.N. Cluster Aqua/Hydroxocomplexes Supporting Extended Hydrogen Bonding Networks. Preparation and Structure of a Unique Series of Cluster Hydrates [Mo6I8(OH)4(H2O)2]·nH2O (N = 2, 12, 14). Polyhedron 2017, 122, 241–246. [Google Scholar] [CrossRef]
- Yarovoy, S.S.; Ivanova, M.; Sukhikh, T.S.; Ryzhikov, M.R.; Fedorov, V.E.; Naumov, N.G. Replenishment in the Family of Rhenium Chalcobromides; Synthesis and Structure of Molecular {Re4S4}Br8(TeBr2)4, Dimeric [{Re4S4}Br8(TeBr2)3]2, and Polymeric {Re4S4}Br8 Compounds Based on the {Re4S4}8+ Tetrahedral Cluster Core. Inorg. Chem. 2022, 61, 20472–20479. [Google Scholar] [CrossRef]
- Dyadin, Y.A.; Yakovlev, I.I.; Bondaryuk, I.V.; Zelenina, L.S. Water—Tetra n-Butylammonium Bromide System. Clathrate Hydrates. Dokl. Akad. Nauk SSSR 1972, 203, 1068–1071. [Google Scholar]
- Lipkowski, J.; Komarov, V.Y.; Rodionova, T.V.; Dyadin, Y.A.; Aladko, L.S. The Structure of Tetrabutylammonium Bromide Hydrate (C4H9)4NBr·21/3H2O. J. Supramol. Chem. 2002, 2, 435–439. [Google Scholar] [CrossRef]
- Vasilyev, E.S.; Bizyaev, S.N.; Komarov, V.Y.; Tkachev, A. V Syntheses of Chiral Fused 4, 5-Diazafluorene–Bis (Nopinane) Derivatives. Mendeleev Commun. 2019, 29, 584–586. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komarov, V.; Galiev, R.; Artemkina, S. 2d, or Not 2d: An Almost Perfect Mock of Symmetry. Symmetry 2023, 15, 508. https://doi.org/10.3390/sym15020508
Komarov V, Galiev R, Artemkina S. 2d, or Not 2d: An Almost Perfect Mock of Symmetry. Symmetry. 2023; 15(2):508. https://doi.org/10.3390/sym15020508
Chicago/Turabian StyleKomarov, Vladislav, Ruslan Galiev, and Sofya Artemkina. 2023. "2d, or Not 2d: An Almost Perfect Mock of Symmetry" Symmetry 15, no. 2: 508. https://doi.org/10.3390/sym15020508
APA StyleKomarov, V., Galiev, R., & Artemkina, S. (2023). 2d, or Not 2d: An Almost Perfect Mock of Symmetry. Symmetry, 15(2), 508. https://doi.org/10.3390/sym15020508





