Abstract
The regularities of chromatographic retention and separation enantioselectivity of the selected β-blockers (propranolol, pindolol, alprenolol, atenolol, oxprenalol, metoprolol, clenbuterol, sotalol, pronethalol, salbutamol, and labetalol) were studied with eight chiral stationary phases (CSPs) in polar ionic mode (PIM) elution system. A range of novel CSPs was prepared by immobilisation of macrocyclic glycopeptide antibiotic eremomycin (E-CSP); structurally related antibiotics chloreremomycin (Chloro-E-CSP) and semi-synthetic oritavancin (O-CSP); and five eremomycin derivatives including amide- (Amide-E-CSP), adamantyl-2-amide- (Adamantylamide-E-CSP), eremomycin aglycon (EAg-CSP), eremosaminyl eremomycin aglycon (EEA-CSP), and des-eremosamynyl eremomycin (DEE-CSP) onto microspherical silica (Kromasil, particle size 5 micron, pore size 11 nm). The effect of different functional groups in eremomycin structure on chiral recognition of β-blockers was studied. The original E-CSP revealed moderate enantioseparation for all studied β-blockers. The presence of a free carboxylic group in a chiral selector molecule is found to be critical for the general retention of enantiomers as no separation enantioselectivity was recorded for Amide-E-CSP and Adamantyl-E-CSP. Modification of the aromatic system of eremomycin by the introduction of a chloro- substituent in the aromatic ring (Chloro-E-CSP) or a hydrophobic 4’-chlorobiphenylmethyl substituent to the disaccharide sugar residue (O-CSP) resulted in decreased enantioselectivity. The best enantioseparation of β-blockers was obtained for CSPs with eremosaminyl eremomycin aglycon and des-eremosamynyl eremomycin as chiral selectors.
1. Introduction
Beta-blockers or β-adrenergic antagonists are an important class of organic substances which are widely used to reduce the blood pressure of patients with corresponding cardiovascular diseases. The major structural fragment in typical β-blockers is aryloxypropanolamine (see Table 1) containing at least one chiral centre. It is known that only one enantiomer of beta blockers exhibits pharmaceutical activity, so only half of a racemic active pharmaceutical ingredient (API) is effective and the rest is considered inactive, but not an absolutely non-harmful component. At present, commercially available β-blockers are sold as racemic mixtures except for very few, e.g., timolol [1].
Enantioseparation of APIs and preparative isolation of all possible isomers is a significant part of biomedical testing required for the medical approval of drugs. Determination of the optical purity of APIs at the production stage is also of great importance as optical isomers of one substance can produce diverse effects on humans. The application of various techniques including capillary zone electrophoresis (CZE) [2], capillary electrochromatography (CEC) [3], supercritical fluid chromatography (SFC) [4], gas chromatography (GC [5], and thin layer chromatography (TLC) [6] have been reported for enantioseparation of β-blockers. However, enantioselective or chiral high-performance liquid chromatography (HPLC) is commonly used for the separation and determination of optical isomers. The most practical separation of enantiomers includes the use of chiral stationary phases (CSPs) with immobilised substrates or chiral selectors, which can interact differently with optical isomers and provide so-called chiral recognition of enantiomers. There is a large number of CSPs designed for the enantioseparation of various groups of substances. This is due to an enormous number of APIs with different chemical structures, so there is no universal CSP allowing enantioseparation of all possible types of racemic drugs [7].
The majority of β-blockers have aryloxypropanolamine fragment in their structure with the chiral centre at the OH-substituted carbon, while maximum pharmaceutical activity is associated with the S configuration. The structures and properties of β-blockers studied in this work are summarised in Table 1. The application of various types of CSPs for the enantioseparation of beta-blockers has been recently reviewed [8]. According to the literature, good enantioselectivity and peak resolutions for beta-blockers have been reported for CSPs with immobilised polysaccharides [9], proteins [10], β-cyclodextrin [11], crown-ethers [12], Pirkle-type selectors [13], macrocyclic glycopeptide antibiotics [14,15,16], and many others.

Table 1.
Structure of beta-blockers used in this work.
Table 1.
Structure of beta-blockers used in this work.
| Analyte | logP [17] | pKa [18] | Structure |
|---|---|---|---|
| Propranolol | 3.48 | 9.45 | 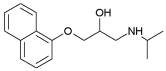 |
| Pindolol | 1.75 | 8.80; 9.54 | 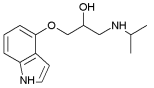 |
| Alprenolol | 3.10 | 9.60 | 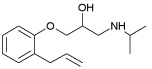 |
| Atenolol | 0.16 | 9.60 | 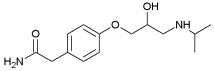 |
| Metoprolol | 1.88 | 9.70 | 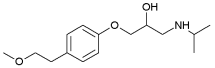 |
| Oxprenalol | 2.10 | 9.5 | 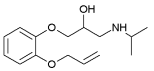 |
| Sotalol | 0.24 | 8.28; 9.72 | 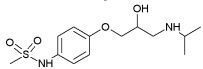 |
| Pronethalol | 3.00 | 9.42 | 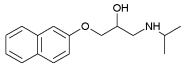 |
| Clenbuterol | 2.00 a | 9.37 | 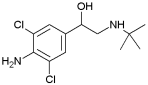 |
| Salbutamol | 0.64 a | 9.20; 10.7 | 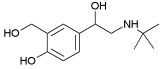 |
| Labetalol | 3.09 | 7.44; 9.38 | 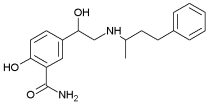 |
a These values are calculated by using KOWWIN software [17].
CSPs with immobilised macrocyclic antibiotics are well-known for their enantioselective properties towards β-blockers. Enantioseparations of β-blockers have been reported for silica-based CSPs with vancomycin [14,19], rifamycin B [20], teicoplanin [21], dalbavancin [22], eremomycin [23], ristocetin [24], and others used as chiral selectors. Elucidation of both chromatographic retention and enantioseparation mechanisms for these selectors is an important, but very complex, task due to the large numbers of chiral centres and functional groups available for various interactions. There are three options to obtain the required information. The most common option is a variation of eluent composition to suppress or increase certain interactions between enantiomers and a chiral selector. It could be a simple variation of eluent polarity to suppress hydrogen bonding [25], the addition of salts and pH regulation to change electrostatic interactions [26], blocking complexing groups with Cu2+ [27], etc. A more difficult route is the analysis of datasets on retention and enantioselectivity values obtained for a large number of structurally similar racemates with a single CSP. Of course, this approach requires a representative collection of specially designed compounds [24]. The final option is a targeted modification of the chiral selector structure in CSPs and an analysis of corresponding changes in retention and enantioselectivity. This latter option is commonly used for the modification of glycopeptide antibiotics and the preparation of new CSPs.
There have been few attempts to improve the enantioselectivity of vancomycin-based phases by modifying their chemical structure. The structure of vancomycin and its structural analogues are shown in Figure 1. The family of modified vancomycin-based CSPs includes phases with immobilised vancomycin Edman degradation product (EDP) and with immobilised crystalline degradation product (CDP). The former differs from native vancomycin by N-terminus leucine residue converted into additional primary amino- group [28], and the latter by containing a second carboxylic acid group due to rearrangement in the structure [29]. Several other CSPs were also prepared by using antibiotics closely related to the vancomycin structure as chiral selectors. Chloroeremomycin, which is also known as an A82846B antibiotic, has epimeric disaccharide amino sugar and additional epi-vancosamine residue. Chloroeremomycin-containing CSPs (Chloro-E-CSP) demonstrated a superior enantioseparation of aromatic amino acids [30] and profens [31] over eremomycin-containing chiral stationary phase (E-CSP). Eremomycin has no chloro-substituent in benzene ring 3, which promotes coordination via π-π interactions. Chloroeremomycin is a precursor for semi-synthetic antibiotic oritavancin (LY333328), which has an additional aromatic 4’-chlorobiphenylmethyl group at aminosugar. A baseline separation of enantiomers of ketoprofen, indoprofen, ibuprofen, flurbiprofen, and fenoprofen was recently obtained with an oritavancin-containing CSP (O-CSP) [31]. Amide derivatives of eremomycin have also been tested as chiral selectors in CSPs for the separation of amino acids and profens [30,31].
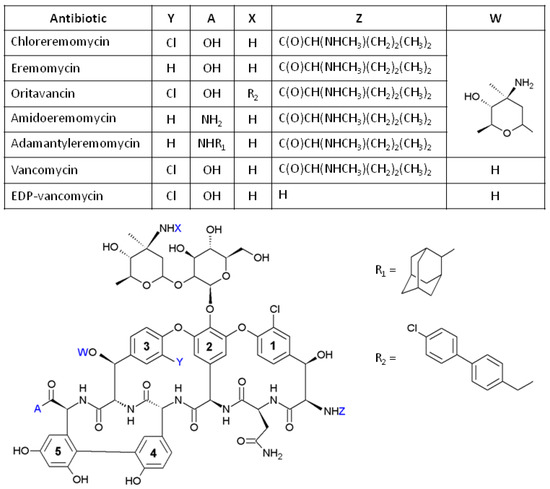
Figure 1.
Structures of eremomycin and its analogues used as chiral selectors in CSPs.
The use of aglycones of macrocyclic glycopeptide antibiotics as chiral selectors has a strong effect on the enantioselectivity and resolution of CPSs. Vancomycin aglycon containing CSP was prepared and used for enantioseparation of chiral sulfoxides [32]. DEE-CSP with immobilised eremosaminyl aglycon (see Figure 2 for corresponding structure) demonstrated better enantioselectivity and resolution for the separation of aliphatic amino acids, but not for aromatic amino acids and profens, as compared with eremomycin-containing E-CSP [33].
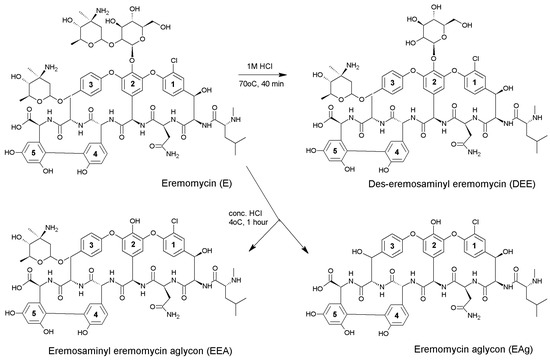
Figure 2.
The synthesis and structures of eremomycin aglycons used as chiral selectors in CSPs.
The present work is devoted to the investigation of enantioseparation of β-blockers by using seven CSPs with eremomycin and its derivatives as chiral selectors. The aim of this work is to identify key structural elements responsible for the separation of enantiomers of this group of pharmaceuticals. The CSPs with immobilised eremomycin aglycon (EAG-CSP) and des-eremosaminyl eremomycin (DEE-CSP) were prepared as originally described by Kuzhetsov et al. [33]. The structures of these aglycones are presented in Figure 2.
2. Materials and Methods
2.1. Preparation of Chiral Selectors
Eremomycin amide and (adamantyl-2) eremomycin amide were provided by Gause Institute of New Antibiotics (Moscow, Russia). Chloreremomycin was isolated from the culture liquid produced by JSC «Biohimik» (Saransk, Russia) and purified by using preparative reversed-phase HPLC. Eremosaminyl eremomycin aglycon (EEA), deseremosaminyl eremomycin (DEE), and eremomycin aglycon (EAg) were produced by eremomycin deglycosylation according to slightly modified (see Section 2.1.1 and Section 2.1.2) methods reported in [34].
2.1.1. Synthesis of Des-Eremosaminyl Eremomycin
Three grams of eremomycin were dissolved in 40 mL of 1 M HCl. The solution was heated at 70 °C for 40 min. The resultant mixture was cooled down to room temperature and neutralised with 1M NaOH solution. The obtained des-eremosaminyl eremomycin (DEE) was purified by preparative HPLC using a 250 × 24 mm ID column packed with Diasorb-130-S16T stationary phase (particle size 8 µm, JSC BioChemMack S&T, Moscow, Russia) and a 5% aqueous solution of isopropanol with 0.1% trifluoroacetic acid (TFA) as an eluent at a flow rate of 23 mL/min. The resultant product was lyophilised. The product yield was 13.3% (400 mg). The des-eremosaminyl eremomycin purity was 95% (HPLC data, see Section 2.3.2.).
2.1.2. Synthesis of Eremosaminyl Aglycon of Eremomycin and Eremomycin Aglycon
Synthesis of eremosaminyl aglycon of eremomycin (EEA) and eremomycin aglycon (EAg) was performed simultaneously. For this, 3.28 g of eremomycin was dissolved in 65 mL of concentrated HCl and cooled down to 4 °C for four hours. Following the completion of hydrolysis, the mixture was neutralised using dry sodium carbonate and diluted with deionised water to 1 L until a precipitate that was formed at the initial stage of the neutralisation process was dissolved. Purification and separation of target products was performed by preparative HPLC using a 250 × 24 mm ID column packed with Diasorb-130-S16T stationary phase (particle size 8 µm) and a 10% aqueous solution of isopropanol with 1% acetic acid as an eluent at a flow rate of 23 mL/min.
The resultant product was lyophilized and 0.85 g of eremosaminyl eremomycin aglycon (98% purity, HPLC data) and 1.2 g of eremomycin aglycon (92% purity, HPLC data) were obtained. For HPLC conditions see Section 2.3.2.
Structures of all products synthesised in Section 2.1.1 and Section 2.1.2 were confirmed using mass spectrometry. The spectra demonstrated corresponding masses of eremomycin (1557), des-eremosaminyl eremomycin (1414), eremosaminyl aglycon (1252), and eremomycin aglycon (1109). Additionally, these spectra (Supplementary Materials, Figures S1–S4) show the presence of sodium and potassium salts of these compounds, as well as products of partial degradation of the samples during the experiment. These admixtures contain mainly compounds, which cannot interfere with the immobilisation of target chiral selectors onto the silica surface.
2.2. Synthesis of Chiral Phases
All studied CSPs were prepared by using a two-step immobilisation reaction as described in detail in previous works of the authors [30,31,33]. In essence, the synthesis included activation of the silica surface with 3-glycidoxypropyltriethoxysilane followed by treatment with an aqueous solution of antibiotics under mild conditions. Upon completion of the reaction, the resultant precipitate was decanted and washed with water, ethanol, and acetone, then filtered and air dried.
Electron spectroscopy for chemical analysis (ESCA) and elemental analysis were used to prove the covalent attachment of glycopeptide antibiotics to silica and the bonding chemistry of CSPs. The corresponding data can be found in our previous publication [30].
2.3. Chromatographic Experiments
2.3.1. Columns and Equipment
The HPLC system used for separation studies comprised a gradient pump (Smartline 1000), an optical detector (Smartline 2500 UV-Vis), and an injector valve with a 10 µL loop (all Knauer GmbH, Berlin, Germany). Eurochrom software (also Knauer GmbH, Berlin, Germany) was used for data processing.
2.3.2. Materials and Consumables
All solvents were of chromatographic grade purity and included acetonitrile, methanol, isopropanol, n-propanol, and ethanol (AppliChem, Darmstadt, Germany). Amino acid standards were purchased from Sigma-Aldrich (St. Louis, MO, USA, USA). Deionised water (18 MΩ/cm) from the Milli-Q system (Millipore, Bedford, MA, USA) was used for the preparation of solutions and eluents.
Chemical purity and concentration of macrocyclic glycopeptide antibiotics in reaction mixtures (Section 2.1.1 and Section 2.1.2) were determined by reversed-phase HPLC using a 250 × 4.6 mm ID column packed with 5 µm Kromasil-100-C18 (Nouryon, Göteborg, Sweden). The column was initially equilibrated with 0.05% trifluoroacetic acid (Solvent A). Linear gradient elution from 5% to 25% of acetonitrile (Solvent B) was applied over 20 min at a flow rate of 1 mL/min and room temperature. Detection of chromatographic peaks was performed at 280 nm.
3. Results and Discussion
3.1. Sselection of the Eluent
CSPs with eremomycin and their derivatives have been widely used for enantioseparation of different substances including amino acids [15,26,30,35]; their benzyloxycarbonyl- (CBZ), benzoyl-, tert-butyloxycarbonyl- (BOC) derivatives [36]; 2-phenylpropionic acid derivatives known as profens [31,33,37]; α-hydroxy acids [33,38]; pyrroloquinolones [39], chemotherapy drug pemetrexed [40], and others.
Significantly less has been reported on the enantioseparation of β-blockers using this subclass of CSPs. Bosakova et al. [19] studied enantioseparation of propranolol, atenolol, alprenolol, oxprenolol, and pindolol (see structures in Figure 1) with Chirobiotic V and Chirobiotic V2 columns containing different amounts of bonded vancomycin. Separation of all enantiomers of studied β-blockers was obtained under conditions of reversed-phase and polar organic HPLC modes. In the reversed-phase mode, the retention of β-blockers increased with an increase of methanol concentration in the eluent for both of the above CSPs. Optimal separations were observed when 90% methanol with 0.5% triethylammonium acetate at pH 5 was used as an eluent; however, in this case, separation was achieved in 30 to 60 min. Under equal separation conditions, β-blockers were retained for a longer period on a column with a higher concentration of vancomycin in the stationary phase, but as the amount of methanol in the eluent increased this difference in retention levelled out. The higher concentration of immobilised vancomycin provided a stronger retention of beta-blockers due to an increased number of binding sites providing stronger hydrogen bonding for Chirobiotic V2, but this difference levelled out at high methanol contents in the eluent.
Additionally, under otherwise same conditions, a higher concentration of vancomycin on the surface of the stationary phase leads to higher separation enantioselectivity and improved peak shape of all β-blockers. In pure methanol (polar organic HPLC mode) β-blockers enantiomers do not elute within 70 min, but with the addition of triethylamine and acetic acid, the experiment time is significantly reduced, to 20 min. However, high concentrations of these additives result in the worsening of the separation. The best chromatograms with enantioselectivity α = 1.08–1.12 were obtained with a low concentration of the aforementioned additives to the eluent (see Table 2).

Table 2.
Retention factors (k’), enantioselectivity (α), and resolution (RS) for beta-blockers for the studied CSPs. Eluent: 100% methanol containing 0.1% TEAA, flowrate 0.7 mL/min.
Hashem et al. investigated the enantioseparation of salbutamol with Chirobiotic V column and 100% methanol containing a varied concentration of ammonium acetate [41]. The enantioselectivity increased with decreasing salt content in the eluent, and baseline separation of enantiomers was achieved in 20 min at a concentration of 2.5 mM.
The separation of albuterol enantiomers was studied on an E-CSP column (250 × 4 mm, particle size 5 μm) [23] in a polar organic mode. The authors reported an increase in enantioselectivity with an increase in acetonitrile content in methanol-based eluent, with peak resolution (RS) remaining constant. The resolution of enantiomer peaks increased with decreasing acid and amine concentrations in the eluent, while the best resolution was obtained in methanol–acetonitrile–triethylamine–acetic acid (80:20:0.075:0.025) mixture as the eluent. The Rs value improved slightly, up to 1.0, with a decrease in flow rate from 1.0 to 0.5 mL/min.
Microspherical polystyrene-divinylbenzene macroporous particles with eremomycin-stabilised gold nanoparticles (Eremo@GNP@PS-DVB) demonstrated improved enantioseparation of pindolol as compared with eremomycin bonded silica (E-CSP) [42]. The baseline separation with α = 1.5 of enantiomers was obtained in polar organic mode with 100% methanol containing 0.1% TEAA as an eluent. Probably the changes in eremomycin conformation on GNP surface are favourable for enantioseparation [43].
Thus, according to the literature, the separations of β-blockers are the most selective in a polar organic mode of HPLC. Both vancomycin and eremomycin-containing CSPs demonstrated superior enantioselectivity in methanol with added ammonium acetate or triethylammonium acetate (TEAA) at low (0.05–0.5%) concentrations. A dilution of methanol-based eluent with acetonitrile increased the retention of β-blockers. Based on this analysis of the literature 100% methanol containing 0.1% TEAA was selected as the initial eluent in this study. However, very weak or no retention was observed for β-blockers in the eluent, so 50% methanol–50% acetonitrile containing 0.1% TEAA eluent was used instead. The obtained results for E-CSP, Chloro-E-CSP, Amide-E-CSP, O-CSP, DEE-CSP, and EAg-CSP including retention factors, enantioselectivity, and resolution of enantiomer peaks are summarised in Table 3. The corresponding chromatograms for selected β-blockers are shown in Figure 3.

Table 3.
Enantioselectivity (α) and resolution (RS) for beta-blockers for the studied CSPs. (Eluent 50% methanol—50% acetonitrile containing 0.1% TEAA, 0.7 mL/min.
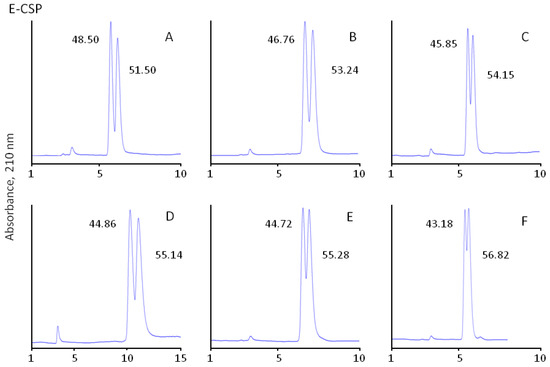
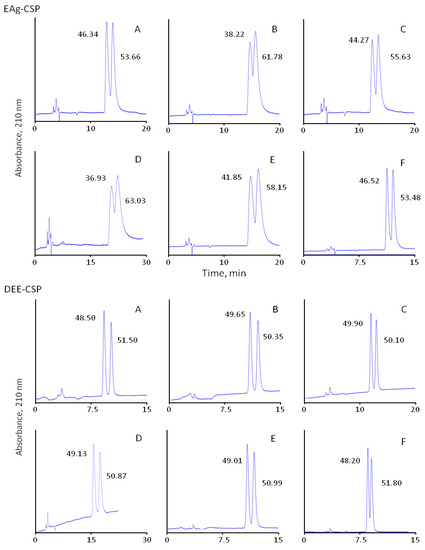
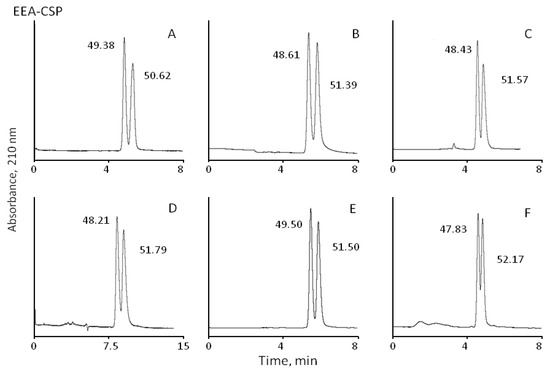
Figure 3.
Separation of β-blockers enantiomers on CSPs with immobilised eremomycin (E-CSP) and eremomycin aglycons (EAg-CSP, DEE-CSP, and EEA-CSP) chiral columns (250 × 4.0 mm I.D.); eluents: 100% methanol with 0.01% TEAA (EEA-CSP) and 50% methanol–50% acetonitrile containing 0.1% TEAA; flow rate 0.7 mL/min for other columns, temperature 25 °C; injection volume 10 µL, UV detection at 210 nm. Analytes: metoprolol (A), pindolol (B), alprenolol (C), atenolol (D), propranolol (E), and oxprenalol (F). The numbers indicate peak areas.
It should be noted that labetalol has two chiral centres in the molecule (see Table 1), so there are two racemates containing four stereoisomers that are present in equal amounts. The separation of all four enantiomers of labetalol has been reported with Chirobitic V column in polar organic mode and a mobile phase consisting of methanol, acetic acid, and diethylamine (100:0.3:0.1 v/v/v) [44]. In this work, similar separations of labetalol enantiomers were achieved with E-CSP, Chloro-E-CSP, and DEE-CSP. However, only three chromatographic peaks of labetalol isomers were recorded with the mobile phase containing 50% methanol–50% acetonitrile containing 0.1% TEAA. The first eluted peak is related to the coeluted (S,R)-labetalol and (S,S)-labetalol stereoisomers, while two other well resolved peaks belong to (R,R)-labetalol and (5) (R,S)-labetalol enantiomers. The corresponding retention data are presented in Table 3. It should be outlined that no separation of any stereoisomers of labetalol was obtained with EAg-CSP unless significantly stronger retention time of 50 min as shown in Figure 4. Therefore, the presence of glycoside residues is necessary for the chiral recognition of labetalol enantiomers.
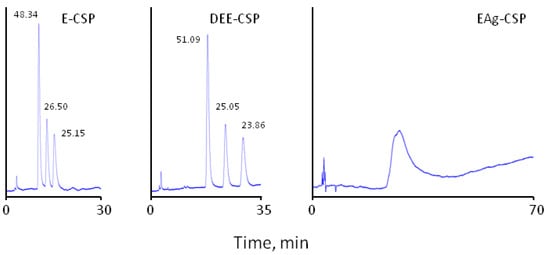
Figure 4.
Separation of labetalol enantiomers on CSPs with immobilised eremomycin (E-CSP) deseremosaminyl eremomycin (DEE-CSP) and eremomycin aglycons (EAg-CSP) chiral columns (250 × 4.0 mm I.D.); eluent: 50% methanol—50% acetonitrile containing 0.1% TEAA; flow rate 0.7 mL/min, temperature 25 °C; injection volume 10 µL, UV detection at 210 nm. The numbers indicate peak areas.
3.2. Retention of β-Blockers on CSPs
Often in chiral liquid chromatography, the retention of β-blockers and separation selectivity of enantiomers can be connected to a certain type of CSP. Typically, this occurs if one of the three interactions required for chiral recognition of enantiomers is dominant. For this reason, the dependences of retention factors (k’) on hydrophobicity measure as logP were checked for E-CSP as a standard stationary phase for comparison. As expected, no correlation was observed in polar organic mode when 100% organic solvent containing mobile phase was used in combination with a relatively hydrophilic stationary phase. EAg-CSP with removed saccharide residues from eremomycin should be the most hydrophobic among studied CSPs. However, no visible correlation between k’ and logP was observed.
All studied β-blockers are cations due to the protonation of the secondary amino- group having pKa between 9.4 and 10.7 (Table 1). This group is located in the beta-position relatively chiral centre, so it must be involved in chiral recognition via electrostatic interaction with the carboxylic group marked as A (Figure 1) in the molecule of eremomycin. In this work, Amide-E-CSP and Adamantylamide-E-CSP had carboxylic groups converted into amido-groups, which are neutral. As the result, Amide-E-CSP demonstrated the lowest ability to retain β-blockers. Adamantylamide-E-CSP provided stronger retention of analytes, but as expected neither CSPs showed any enantioselectivity for the studied racemates (Table 3).
The role of hydrogen bonding in the retention of β-blockers can be evaluated by comparison of retention factors obtained with 100% methanol and 50% methanol–50% acetonitrile as eluents. Aprotic acetonitrile is less competitive for hydrogen bonding sites in CSPs, so the retention of β-blockers should be stronger with the latter eluent. In truth, all β-blockers retained stronger on EAg-CSP with acetonitrile containing eluent. The ratio k’2(50% methanol—50% acetonitrile containing 0.1% TEAA)/k’2(100% methanol containing 0.1% TEAA) has values between 1.82 and 2.04 for the studied β-blockers.
Interestingly, O-CSP with an additional 4-chlorodiphenylmethyl- group and Chloro-E-CSP with chloro- substituent in an aromatic ring exhibited lower retention of β-blockers. Obviously, π-π interactions do not contribute strongly to the retention of these substances. A similar effect was noted for the Chirobitic V column with immobilised vancomycin as shown in Table 2.
There is no clear correlation between retention and enantioselectivity established for the studied CSPs. For example, labetalol is strongly retained by EAg-CSP without reasonable enantioseparation. At the same time, a good enantioselectivity and resolution of peaks are observed for E-CSP in less than 20 min (see Figure 4). However, according to the chromatographic theory, the retention time impacts strongly on the resolution of chromatographic peaks proportionally k’2/(k’2 +1), so, in this work, only the use of a weaker eluent 50% methanol–50% acetonitrile containing 0.1% TEAA allowed better resolution of β-blockers peaks.
3.3. Enantioselectivity of CSPs
Standard E-CSP column demonstrated weak enantioselectivity in the separation of β-blockers with an incomplete resolution of chromatographic peaks for all studied β-blockers (Figure 3). As previously mentioned, no enantioselectivity was obtained for amide type stationary phases Amide-E-CSP and Adamantylamide-E-CSP due to the conversion of a charged carboxyl group into a neutral amide. A simple introduction of the chloro- substituent in aromatic ring 3 of eremomycin resulted in absence of any enantioselectivity for the corresponding Chloro-E-CSP as compared to E-CSP. This effect has no clear explanation, but it confirms that the chiral recognition takes place in the corresponding basket of eremomycin for all blockers except clenbuterol and labetalol. It should be noted that labetalol has two asymmetric carbon atoms, so it exists as a mixture of four isomers. Therefore, the separation of all enantiomers was not achieved and two peaks could be diastereomers. No reasonable enantioselectivity in the separation of β-blockers was also obtained with O-CSP. Orivatancin is structurally related to chloreremomycin with an extra 4-chlorodiphenylmethyl- group aside from the aforementioned “basket”, so it has a similar enantioselectivity for β-blockers, except for metoprolol (Table 3).
The role of carbohydrate residues in the retention and separation of enantiomers is the most intriguing part of the current investigation. For the first time enantioselectivity of CSPs with original eremomycin (E-CSP) and three different eremomycin aglycons was compared to understand the contribution of different carbohydrate residues in chiral recognition. The standard E-CSPs with all three carbohydrate residues presented in the chiral selector shows moderate enantioselectivity with resulting resolution Rs in the range between 0.52 and 0.83 (Table 3). Surprisingly, the same Rs values of 0.52–0.85 were obtained for EAg-CSP with no carbohydrate residues in the chiral selector. Therefore, only partial resolution of enantiomers was obtained for these CSPs, as shown in Figure 3.
At the same time, EEA-CSP with immobilised eremosaminyl eremomycin aglycon (one carbohydrate residue) and DEE-CSP with immobilised des-eremosaminyl eremomycin (two carbohydrate residues) demonstrated a significantly higher resolution (Rs > 1.5) of enantiomer peaks of β-blockers (Figure 3). The baseline separation of optical isomers was obtained for all analytes except oxprenalol with an EEA-CSP column, while a different eluent was used. In this case, 100% methanol with a reduced concentration of TEAA was used.
It should be noted that the enantioselectivity does not depend on the structure of β-blockers for DEE-CSP and EAg-CSP columns and varied between 1.12 and 1.17, but not for EEA-CSP (see Table 2 and Table 3). CSP with immobilised eremosaminyl eremomycin aglycon cannot split enantiomers of oxprenalol, pronetalol, and atenolol racemates due to the absence of enantioselectivity observed with 100% methanol containing 0.01% TEAA as an eluent. At the same time, higher enantioselectivity (α = 1.17–2.00) was observed for other studied β-blockers. Where separation occurred, enantioselectivity was higher than that for a sorbent with eremomycin aglycon (EAg-CSP) for all analytes except for propranolol (Table 2). The fact that the retention and enantioselectivity of both enantiomers increase with a decrease in the number of carbohydrate substitutes from eremomycin (E-CSP) to its complete aglycon (EAg-CSP) indicates that bulky disaccharide moiety in eremomycin produces profound steric hindrance, thus restricting access of β-blockers to the enantioselective site of the molecule. As such, for β-blockers, this site is located in the left part of the eremomycin aglycon molecule, free from chlorine atoms (Figure 2). The positive effect of one (EEA-CSP) or two carbohydrate residues (DEE-CSP) on the resolution of enantiomers is an obvious fact, but it could not be easily explained. Possibly, these residues provide effective shielding of selector sites, which cause strong adsorption but non-enantioselective interactions with β-blockers. One more option relates to the possibility of obtaining an optimal configuration for carbohydrate residues, which can be available for hydrogen bonding interactions in chiral recognition (Figure 2).
4. Conclusions
The retention of ten β-adrenergic blockers on eight structurally similar chiral stationary phases was investigated with a focus on the chiral recognition mechanism. It was found that the hydroxyl- group from the β-blocker molecule interacts with the binding site via hydrogen bonding. The amino- group is protonated in a weakly acidic media according to pKa values (Table 1) and interacts electrostatically with the carboxylic group in eremomycin and its analogues.
It was found that a definitive role in this process belongs to the first “basket” or “pocket” in the molecules of eremomycin-based selectors. The addition of chloro-substituent to the structure of this basket (Chloro-E-CSP, O-CSP) prevents enantioseparation of β-blockers. Conversion of the carboxylic group in the eremomycin molecule into an amide group had a crucial effect on the chiral recognition of β-blockers. Both Amide-E-CSP and Adamantylamide-E-CSP did not show enantioselectivity towards this group of racemates. The presence of carbohydrate residues was found to be a critical factor in the separation of enantiomers, affecting both selectivity and peak resolution. CSPs with intermediate products of hydrolysis demonstrated the best resolution of β-blocker enantiomers.
Under optimum conditions, the separation of enantiomers for all studied racemates was achieved, except for labetalol, which has two chiral centres in the molecule.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/sym15020373/s1, Figures S1–S4.
Author Contributions
Conceptualisation, P.N.N.; methodology, M.A.K.; validation, N.S. and M.A.K.; formal analysis, M.A.K. and R.P.; investigation, M.A.K.; resources, S.M.S.; data curation, P.N.N.; writing—original draft preparation, P.N.N.; writing—review and editing, P.N.N.; supervision, S.M.S.; project administration, S.M.S.; and funding acquisition, S.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank Ekaterina Nesterenko for the help in manuscript preparation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vashistha, V.K.; Kumar, A. Stereochemical facets of clinical β-blockers: An overview. Chirality 2020, 32, 722–735. [Google Scholar] [CrossRef]
- Shapovalova, E.N.; Fedorova, I.A.; Anan’eva, I.A.; Shpigun, O.A. Macrocyclic Antibiotics as Chiral Selectors in High-Performance Liquid Chromatography and Capillary Electrophoresis. J. Anal. Chem. 2018, 73, 1064–1075. [Google Scholar] [CrossRef]
- Desiderio, C.; Aturki, Z.; Fanali, S. Use of vancomycin silica stationary phase in packed capillary electrochromatography I. Enantiomer separation of basic compounds. Electrophoresis 2001, 22, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Pokrovskiy, O.I.; Kayda, A.S.; Usovich, O.I.; Parenago, O.O.; Lunin, V.V. Effect of additives on eremomycin sorbent selectivity in separation of salbutamol enantiomers using supercritical fluid chromatography. Russ. J. Phys. Chem. A 2017, 91, 2288–2290. [Google Scholar] [CrossRef]
- Ribeiro, C.; Gonçalves, R.; Tiritan, M.E. Separation of Enantiomers Using Gas Chromatography: Application in Forensic Toxicology, Food and Environmental Analysis. Crit. Rev. Anal. Chem. 2021, 51, 787–811. [Google Scholar] [CrossRef]
- Malik, P.; Bhushan, R. Thin Layer Chromatographic Resolution of Some β-adrenolytics and a β2-Agonist Using Bovine Serum Albumin as Chiral Additive in Stationary Phase. J. Chromatogr. Sci. 2017, 56, 92–98. [Google Scholar] [CrossRef]
- Subramanian, G. (Ed.) Chiral Separation Techniques: A Practical Approach. Third Completely Revised and Updated Edition; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2006; p. 618. [Google Scholar]
- Yang, Y.; Wang, Y.; Bao, Z.; Yang, Q.; Zhang, Z.; Ren, Q. Progress in the enantioseparation of β-blockers by chromatographic methods. Molecules 2021, 26, 468. [Google Scholar] [CrossRef]
- Moldovan, R.C.; Dascăl, G.S.; Mirel, V.; Bodoki, E.; Oprean, R. Chiral separation of 16 beta-blockers on immobilized polysaccharide chiral stationary phases. Farmacia 2015, 63, 909–912. [Google Scholar]
- Imre, S.; Ormenişan, A.; Tero-Vescan, A.; Muntean, D.L.; Vari, C.E. HPLC Enantioseparation of β-Blockers on Ovomucoid Stationary Phase. J. Chromatogr. Sci. 2016, 54, 1578–1583. [Google Scholar] [CrossRef]
- Ahmed, M.; Ghanem, A. Chiral β-cyclodextrin functionalized polymer monolith for the direct enantioselective reversed phase nano liquid chromatographic separation of racemic pharmaceuticals. J. Chromatogr. A 2014, 1345, 115–127. [Google Scholar] [CrossRef]
- Hyun, M.H. Liquid chromatographic enantioseparations on crown ether-based chiral stationary phases. J. Chromatogr. A 2016, 1467, 19–32. [Google Scholar] [CrossRef]
- Pirkle, W.H.; Burke Iii, J.A. Chiral stationary phase designed for β-blockers. J. Chromatogr. A 1991, 557, 173–185. [Google Scholar] [CrossRef]
- Armstrong, D.W.; Tang, Y.; Chen, S.; Zhou, Y.; Bagwlll, C.; Chen, J.R. Macrocyclic Antibiotics as a New Class of Chiral Selectors for Liquid Chromatography. Anal. Chem. 1994, 66, 1473–1484. [Google Scholar] [CrossRef]
- Berthod, A.; Qiu, H.X.; Staroverov, S.M.; Kuznestov, M.A.; Armstrong, D.W. Chiral recognition with macrocyclic glycopeptides: Mechanisms and applications. In Chiral Recognition in Separation Methods: Mechanisms and Applications; Berthod, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 203–222. [Google Scholar] [CrossRef]
- Berkecz, R.; Tanács, D.; Péter, A.; Ilisz, I. Enantioselective liquid chromatographic separations using macrocyclic glycopeptide-based chiral selectors. Molecules 2021, 26, 3380. [Google Scholar] [CrossRef]
- KOWWIN ver. 1.68. EPI Suite™-Estimation Program Interface. US Environmental Protection Agency. 2010. Available online: https://www.epa.gov/tsca-screening-tools/epi-suitetm-estimation-program-interface (accessed on 10 January 2023).
- Lombardo, F.; Obach, R.S.; Shalaeva, M.Y.; Gao, F. Prediction of Human Volume of Distribution Values for Neutral and Basic Drugs. 2. Extended Data Set and Leave-Class-Out Statistics. J. Med. Chem. 2004, 47, 1242–1250. [Google Scholar] [CrossRef]
- Bosáková, Z.; Cuřínová, E.; Tesařová, E. Comparison of vancomycin-based stationary phases with different chiral selector coverage for enantioselective separation of selected drugs in high-performance liquid chromatography. J. Chromatogr. A 2005, 1088, 94–103. [Google Scholar] [CrossRef]
- Armstrong, D.W.; Rundlett, K.; Reid, G.L. Use of a Macrocyclic Antibiotic, Rifamycin B, and Indirect Detection for the Resolution of Racemic Amino Alcohols by CE. Anal. Chem. 1994, 66, 1690–1695. [Google Scholar] [CrossRef]
- Shen, B.C.; Zhang, D.T.; Xu, B.J.; Xu, X.Z. Comparative enantioseparation of seven amino alcohols on teicoplanin-based chiral stationary phases. Anal. Lett. 2007, 40, 2821–2839. [Google Scholar] [CrossRef]
- Zhang, X.; Bao, Y.; Huang, K.; Barnett-Rundlett, K.L.; Armstrong, D.W. Evaluation of dalbavancin as chiral selector for HPLC and comparison with teicoplanin-based chiral stationary phases. Chirality 2010, 22, 495–513. [Google Scholar] [CrossRef]
- Shapovalova, E.N.; Fedorova, I.A.; Priporova, A.A.; Ananieva, I.A.; Shpigun, O.A. Determination of the enantiomeric purity of albuterol on sorbents modified by macrocyclic antibiotics. Mosc. Univ. Chem. Bull. 2017, 72, 56–62. [Google Scholar] [CrossRef]
- Ekborg-Ott, K.H.; Liu, Y.; Armstrong, D.W. Highly enantioselective HPLC separations using the covalently bonded macrocyclic antibiotic, ristocetin A, chiral stationary phase. Chirality 1998, 10, 434–483. [Google Scholar] [CrossRef]
- Nesterenko, P.N.; Krotov, V.V.; Staroverov, S.M. Effect of mobile phase composition on the enantioselectivity of chromatographic separation on a quinine-bonded silica stationary phase. J. Chromatogr. A 1994, 667, 19–28. [Google Scholar] [CrossRef]
- Kuznetsov, M.A.; Nesterenko, P.N.; Vasiyarov, G.G.; Staroverov, S.M. High-performance liquid chromatography of α-amino acid enantiomers on eremomycin-modified silica. J. Anal. Chem. 2008, 63, 57–64. [Google Scholar] [CrossRef]
- Nair, U.B.; Chang, S.S.C.; Armstrong, D.W.; Rawjee, Y.Y.; Eggleston, D.S.; McArdle, J.V. Elucidation of vancomycin's enantioselective binding site using its copper complex. Chirality 1996, 8, 590–595. [Google Scholar] [CrossRef]
- Hellinghausen, G.; Lopez, D.A.; Lee, J.T.; Wang, Y.; Weatherly, C.A.; Portillo, A.E.; Berthod, A.; Armstrong, D.W. Evaluation of the Edman degradation product of vancomycin bonded to core-shell particles as a new HPLC chiral stationary phase. Chirality 2018, 30, 1067–1078. [Google Scholar] [CrossRef]
- Ghassempour, A.; Alizadeh, R.; Najafi, N.M.; Karami, A.; Römpp, A.; Spengler, B.; Aboul-Enein, H.Y. Crystalline degradation products of vancomycin as chiral stationary phase in microcolumn liquid chromatography. J. Sep. Sci. 2008, 31, 2339–2345. [Google Scholar] [CrossRef]
- Sarvin, N.; Puzankov, R.; Vasiyarov, G.; Nesterenko, P.N.; Staroverov, S.M. Silica Immobilised Chloro- and Amido-Derivatives of Eremomycine as Chiral Stationary Phases for the Enantioseparation of Amino Acids by Reversed-Phase Liquid Chromatography. Molecules 2023, 28, 85. [Google Scholar] [CrossRef]
- Sarvin, N.; Puzankov, R.; Nesterenko, P.N.; Staroverov, S.M. Enantioselectivity of liquid chromatographic separation of profens on chiral sorbents with immobilized eremomycin derivatives and oritavancin. Sorbtsionnye Khromatograficheskie Protsessy 2022, 22, 638–649. [Google Scholar] [CrossRef]
- Berthod, A.; Xiao, T.L.; Liu, Y.; Jenks, W.S.; Armstrong, D.W. Separation of chiral sulfoxides by liquid chromatography using macrocyclic glycopeptide chiral stationary phases. J. Chromatogr. A 2002, 955, 53–69. [Google Scholar] [CrossRef]
- Kuznetsov, M.A.; Nesterenko, P.N.; Vasiyarov, G.G.; Staroverov, S.M. Sorbents with immobilized glycopeptide antibiotics for separating optical isomers by high-performance liquid chromatography. Appl. Biochem. Microbiol. 2006, 42, 536–544. [Google Scholar] [CrossRef]
- Lomakina, N.N.; Berdnikova, T.F.; Tokareva, N.L.; Abramova, E.A.; Dokshina Yu, N. Structure of eremomycin, a novel antibiotic of the group of polycyclic glycopeptides. Antibiot. Khimioterapiya 1989, 34, 254–258. [Google Scholar]
- Staroverov, S.M.; Kuznetsov, M.A.; Nesterenko, P.N.; Vasiarov, G.G.; Katrukha, G.S.; Fedorova, G.B. New chiral stationary phase with macrocyclic glycopeptide antibiotic eremomycin chemically bonded to silica. J. Chromatogr. A 2006, 1108, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, I.A.; Shapovalova, E.N.; Staroverov, S.M.; Shpigun, O.A. Enantioseparation of derivatives of amino acids on silica modified by macrocyclic antibiotic eremomycin. Sorbtsionnye Khromatograficheskie Protsessy 2015, 15, 769–775. [Google Scholar]
- Reshetova, E.N.; Asnin, L.D. The chromatographic behavior and thermodynamic characteristics of adsorption of profen enantiomers on silica gel with grafted eremomycin antibiotic. Russ. J. Phys. Chem. A 2009, 83, 547–551. [Google Scholar] [CrossRef]
- Gogolishvili, O.S.; Reshetova, E.N. Chromatographic enantioseparation and adsorption thermodynamics of hydroxy acids and their derivatives on antibiotic-based chiral stationary phases as affected by eluent pH. Chromatographia 2021, 84, 53–73. [Google Scholar] [CrossRef]
- Stepanova, M.V.; Asnin, L.D.; Boteva, A.A.; Kudinov, A.V. Chromatographic behavior and adsorption thermodynamics of chiral pyrroloquinolones on silica grafted with antibiotic eremomycin. Vestn. Permsk. Naz. Issledovatel'skogo Polytekhnicheskogo Universiteta. Khimicheskaya Tekhnologiya Biotekhnologiya 2018, 4, 20–34. [Google Scholar] [CrossRef]
- Shapovalova, E.N.; Fedorova, I.A.; Proporova, A.A.; Ananieva, I.A.; Shpigun, O.A. Determination of the enantiomeric purity of pemetrexed on the macrocyclic glycopeptides bonded phases. Anal. Kontrol 2016, 20, 168–174. [Google Scholar] [CrossRef]
- Hashem, H.; Tründelberg, C.; Attef, O.; Jira, T. Effect of chromatographic conditions on liquid chromatographic chiral separation of terbutaline and salbutamol on Chirobiotic V column. J. Chromatogr. A 2011, 1218, 6727–6731. [Google Scholar] [CrossRef]
- Prosuntsova, D.S.; Ananieva, I.A.; Nesterenko, P.N.; Shpigun, O.A. Microspherical polystyrene-divinylbenzene particles hybridized with eremomycin stabilized gold nanoparticles as a stationary phase for chiral liquid chromatography. Ind. Laboratory. Diagn. Mater. 2022, 88, 14–22. [Google Scholar] [CrossRef]
- Prosuntsova, D.S.; Plodukhin, A.Y.; Ananieva, I.A.; Beloglazkina, E.K.; Nesterenko, P.N. New composite stationary phase for chiral high-performance liquid chromatography. J. Porous Mater. 2020, 28, 407–414. [Google Scholar] [CrossRef]
- Carvalho, T.M.; Cavalli Rde, C.; Marques, M.P.; Da Cunha, S.P.; Baraldi Cde, O.; Lanchote, V.L. Stereoselective analysis of labetalol in human plasma by LC-MS/MS: Application to pharmacokinetics. Chirality 2009, 21, 738–744. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).