1. Introduction
There are extensive spectra of applications of flow and heat transfer along a stretching surface in numerous technological processes; polymer extrusion, including wire drawing; the manufacture of glass fiber; continuous casting; the manufacture of food and paper; plastic film’s stretching, etc. During the manufacture of these surfaces, melting occurs through a slit and is subsequently stretched to achieve the desired thickness. The final product’s required qualities are solely determined by the stretching rate, stretching procedure, and cooling rate during the process. Nevertheless, due to several uses of nanofluids flow, several investigators have been attracted by it, including nanofluid adhesives: transformer cooling, vehicle cooling, electronic devices cooling, electronics cooling, super powerful and small computer cooling; medical applications: safer surgery and cancer therapy by cooling and process industries; chemicals and materials: detergency, drink and food, paper, printing, and textiles. Several industrial technologies require intense and highly efficient cooling [
1,
2,
3]. Traditional heat transfer fluids, such as thermic, water, and ethylene glycol, are widely used in a variety of industrial applications, including air conditioning and refrigeration, transportation, microelectronics, and solar thermal applications. However, the limitations in the performance of these heat transfer fluids require novel strategies for improving thermal transport properties and for increasing the energy efficiency of systems. It is generally known that suspending micro-solid particles in a base fluid produces a great heat transfer potential [
4]. Despite this, the size of suspended pieces adds to precipitation, abrasion, and clogging in the fluid’s flow direction. The remarkable advancements in nanotechnology have resulted in the development of a revolutionary type of heat transmission liquid known as the nanofluid, which contains suspended pieces of less than 100 nm in size. Nanopowders such as Cu, Al, CuO, and SiC, as well as carbon nanotubes, are examples of nanomaterials (CNTs). The thermal conductivity of heat transport liquids has a substantial impact on increasing heat transfer rates, and many studies on the thermal conductivity of nanoliquids, particularly water- and ethylene glycol-based nanoliquids, have been conducted. In comparison to the base fluid, the experimental examinations of nanoliquid thermal conductivity revealed a considerable increase. Lee et al. [
5] measured the thermal conductivity of several oxide nanoliquids (Al
2O
3 in ethylene glycol, Al
2O
3 in water, CuO in ethylene glycol, and CuO in water) and found that the ethylene glycol–CuO nanoliquid increased thermal conductivity by more than 20%. However, a 40% increase in thermal conductivity in ethylene glycol–Cu nanoliquid documented by Eastman et al. [
6] resulted in an improvement. Xie et al. [
7] explored the role of base liquids on the thermal conductivity of nanofluids using a variety of base fluids. With the increased thermal conductivity of the base fluid, the thermal conductivity ratio decreased. As a result, nanofluids could be an intriguing choice for enhanced heat-transport applications in the future, specifically those in the micro-scale. Furthermore, Dogonchi et al. [
8] studied the significance of natural convective magnetic nanoliquids in an enclosure with a porous medium by taking Brownian motion phenomena. Shafiq et al. [
9] analyzed the single as well as multiple wall carbon nanotubes on magnetohydrodynamic stagnant-point nanoliquid flow towards variable thick plates towards concave and convex phenomena. Hayat et al. [
10] examined the Stratification impact on MHD Tangent hyperbolic nanofluid flow, which is induced by inclined surfaces. Shafiq et al. [
11] investigated the Casson magnetohydrodynamic axisymmetric Marangoni forced-convection nanofluid flow towards a flat surface. Rehman et al. [
12] analyzed the heat transport with nano-sized materials suspended in a magnetized rotatory flow field. Marangoni convective boundary-layer carbon-nanotube flow over a Riga surface was studied by Shafiq et al. [
13]. Shafiq et al. [
14] developed an artificial neural network (ANN) model to predict the flow of a single-walled carbon nanotube liquid over three various non-linear thin isothermal needles of cone, paraboloid, and cylinder shapes under convective conditions. The temperature profile increased according to the Biot number. Moreover, Shafiq et al. [
15] studied hydromagnetic unsteady Williamson nanoliquid flows towards a radiative plate via numerical as well as artificial neural network modeling. They found good agreements according to the numerical results with ANN results. Noteworthy developments concerning nanoliquids can be found in refs. [
16,
17,
18,
19,
20].
Porous media flows are particularly popular among engineers, mathematicians, and modelers due to its involvement in geothermal energy resources, oil reservoir modeling in isolation processes, crude oil processing, groundwater systems, water movement in reservoirs, etc. Heat transfer causes the flow in porous media to become even more important in processes involving thermal insulation materials, solar collectors and receivers, nuclear waste disposal, and energy storage systems [
21,
22,
23]. Thus, much focus has been provided in the existing literature on certain porous media problems that are developed and produced using the classical Darcy theory. Under lower velocity and small porosity conditions, the classic Darcy principle remains true. When there are inertial and boundary impacts at a high flow rate, Darcy’s rule is insufficient. Nonlinear flow is caused when the Reynolds number is greater than unity. The effects of inertia and constraints cannot be neglected in some situations. Under these conditions, the effects of inertia and boundaries cannot be overlooked. To assess inertia and boundary effects, Forchheimer [
24] added a square velocity equation to the Darcian velocity term. This word is known as the “Forchheimer term”, according to Muskat [
25], and it is always true for large Reynolds numbers. In fact, in the momentum expression, larger filtration velocities result in quadratic drag for porous materials. In [
26], the authors studied the effects of thermophoresis and viscous dissipation in Darcy–Forchheimer mixed convection flows embedded in porous medium. They considered two cases in which one corresponded to the presence of viscous dissipation and the other one corresponded to the absence of it. In [
27], the authors implemented Darcy–Forchheimer law to examine the hydromagnetic flow of variate viscosity fluid in a porous medium. The most recent achievements for further Darcy–Forchheimer laws in different geometries and effects are discussed in refs. [
28,
29,
30,
31,
32,
33].
The goal of this research study, which was inspired by Jedi et al. [
34], is to investigate the importance of the Darcy–Forchheimer flow of Casson cobalt ferrite nanofluids towards a rotating disk under Newtonian heating conditions statistically. Cobalt ferrite nanomaterials with water and ethylene glycol are considered. The porous space describing Darcy Forchheimer expression is filled by Casson fluids. The major goal here is to determine the influence of the critically considered variables. To solve a set of governing equations, the numerical shooting technique with the Runge–Kutta scheme is applied after similarity transformations. A physical–statistical model, as well as its distribution, was used to study the thermal conductivity of a Casson nanoliquid containing cobalt ferrite nanoparticles via two base fluids: water and ethylene glycol. The proposed model could also be employed in nanoliquid investigations for a wide range of practical commercial applications such as refrigeration and air conditioning, microelectronics, transportation, and solar thermal. Such an investigation is novel and unachieved, according to the finest complete evaluation to date.
2. Mathematical Modelling
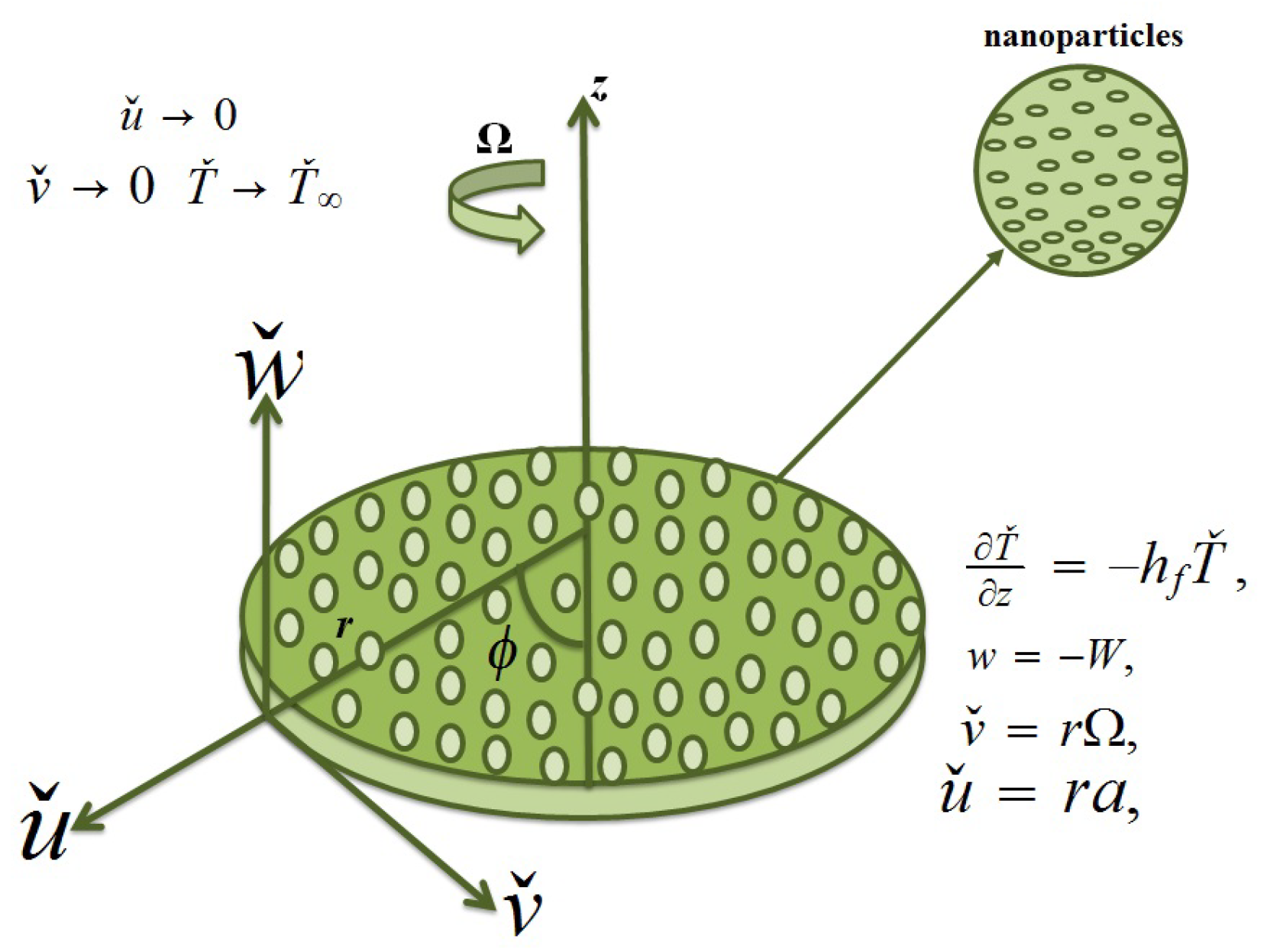
A steady Darcy–Forchheimer flow of Casson nanofluid flow towards a rotating disk is considered. The phenomenon of heat transfer is examined with nonlinear thermal radiation, Newtonian heating, and viscous dissipation. The porous space via DF expression is filled by an incompressible Casson cobalt ferrite nanoparticle liquid. The current investigation includes two kind of base fluids: One is water, and the other is ethylene glycol, while cobalt ferrite is taken as the nanomaterial. Furthermore, such nanomaterials are friendly and appropriate for soil microbes and in their involved processes. At z = 0, the disk spins with Ω as the constant angular velocity (see
Figure 1). The governing equations according to above mentioned assumptions are as follows [
18,
20]:
with
where
and
are stretched velocity and rotational speed, respectively. The effective thermophysical characteristics of the nanofluids are provided below.
The transformations into consideration are as follows.
Utilizing the above-mentioned transformations and applying them in Equations (
1)–(
6), we obtain the following:
Here, the dimensionless parameters
,
,
,
,
,
, and
are presented as the inertia coefficient, the permeability parameter, the Prandtl number, the suction parameter, the radiation parameter, the stretching-strength parameter, the Eckert number, and the conjugate number, respectively, which are presented as follows.
Physical quantities, namely, the local skin friction coefficent (LSFC) and local Nusselt number (LNN), are defined in the following forms.
The dimensionless forms are as follows.
4. Model Selection and Discussion
The influences of
,
,
, and
on the LSFC and LNN were investigated.
Table 1 lists the thermophysical properties of base fluids as well as nanomaterials. The different statistical models are mentioned in
Table 2. Here, we illustrate the superiority of the suitably fitted model compared to some other distributions using three generated data sets for LSFC when
= 0.1, 0.5, 0.9 and
= 1.7, 2.1, 2.5, under water-cobalt ferrite (H
2O-CoFe
2O
4), and ethylene glycol-cobalt ferrite (C
2H
6O
2-CoFe
2O
4). Moreover, we generated another three datasets for LNN when
= 0.1, 0.3, 0.6 and
= 0.1, 0.2, 0.3 under water-cobalt ferrite (H
2O-CoFe
2O
4), and ethylene glycol-cobalt ferrite (C
2H
6O
2-CoFe
2O
4). For each data set, we estimate the model’s parameters by using maximum likelihood estimation from the above fitted models and listed them in
Table 3,
Table 4,
Table 5 and
Table 6. The following excellent statistical benchmarks have been used to compare these models: Anderson–Darling (
A *), Cramer–von Mises (
W *), and Kolmogorov–Smirnov (K-S) with
p-values. The best model for the real data set might be the one with the lowest values of the above-mentioned goodness-of-fit (GoF) measures. These tests are used to determine the model’s goodness of fit and to identify which model best fits the data. The statistics
A * and
W * for each model are calculated using the algorithm in the R package [
35], while the K-S is calculated using the algorithm in the R package GLDEX [
36]. The statistics
A * and
W * were provided in detail by Chen and Balakrishnan [
37]. The data are better represented by the model with the minimum value of these metrics and a large
p-value (PV) than the other models. The values of goodness-of-fit (GOF) measures for the fitted models can be found in
Table 7,
Table 8,
Table 9 and
Table 10.
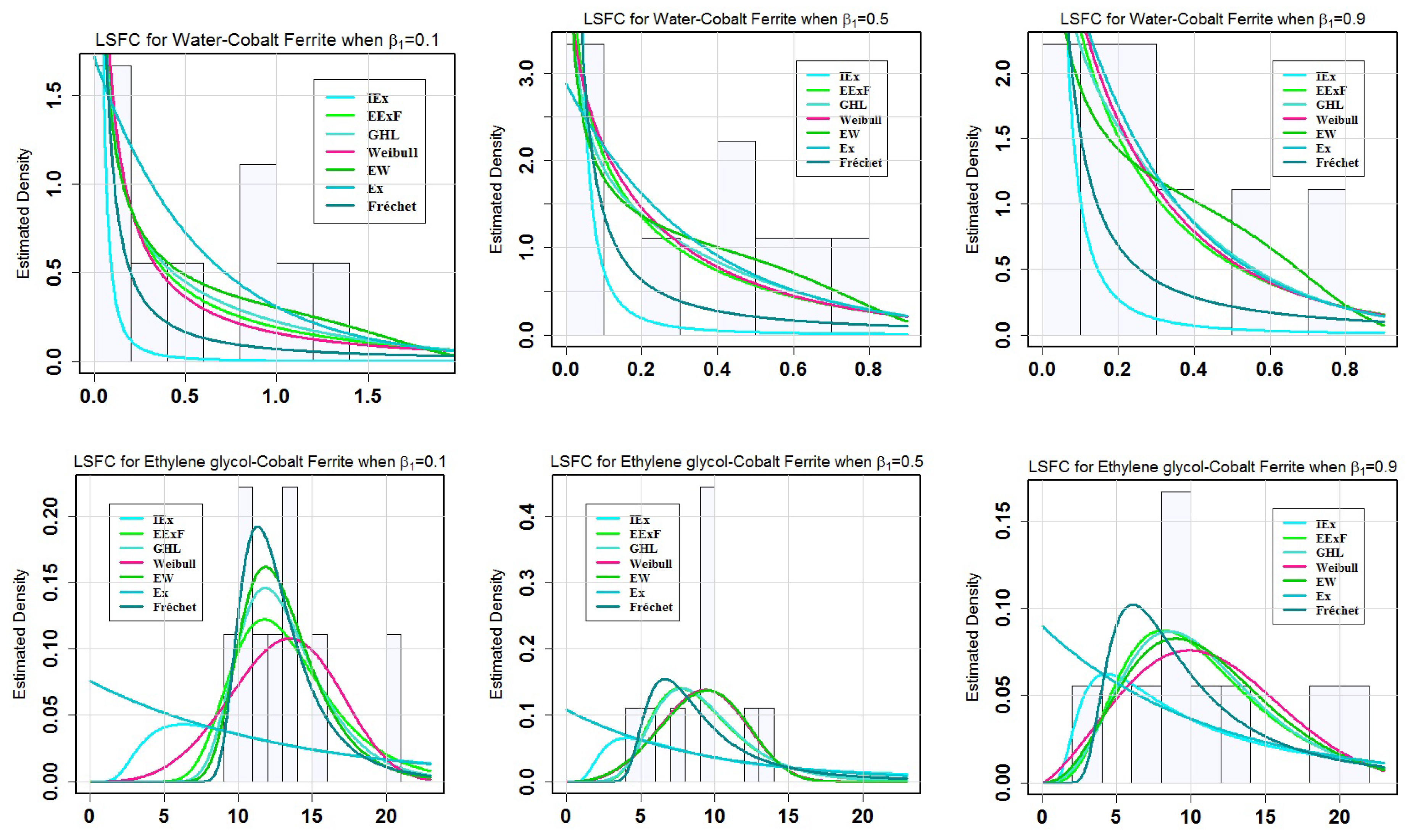
Table 7 provides the values of GoF Statistics for the LSFC when
= 0.1, 0.5, 0.9 under water-cobalt ferrite (H
2O-CoFe
2O
4) and ethylene glycol-cobalt ferrite (C
2H
6O
2-CoFe
2O
4). It is revealed from
Table 7 that for the LSFC under water-cobalt ferrite (H
2O-CoFe
2O
4), the EW model could be chosen as a best model among the other fitted models since it has the lowest values of the
A * and
W * and K-S statistics. It also has the largest PV of the KS test. For the LSFC of C
2H
6O
2-CoFe
2O
4, three models could be chosen as the best model among the fitted models. As it can be seen, the results indicate that the Frechet model has the smallest values of Gof among the fitted models at
= 0.1; therefore, it can be considered as the best model. It is noted that, the outcomes at
= 0.5 indicates that the Weibull model has the smallest values of Gof among the fitted models. As a consequence, it may be called the best model. On the other hand, for the case of
= 0.9, the EExF model has the smallest values of Gof among the fitted models; therefore, it can be considered as the best model.
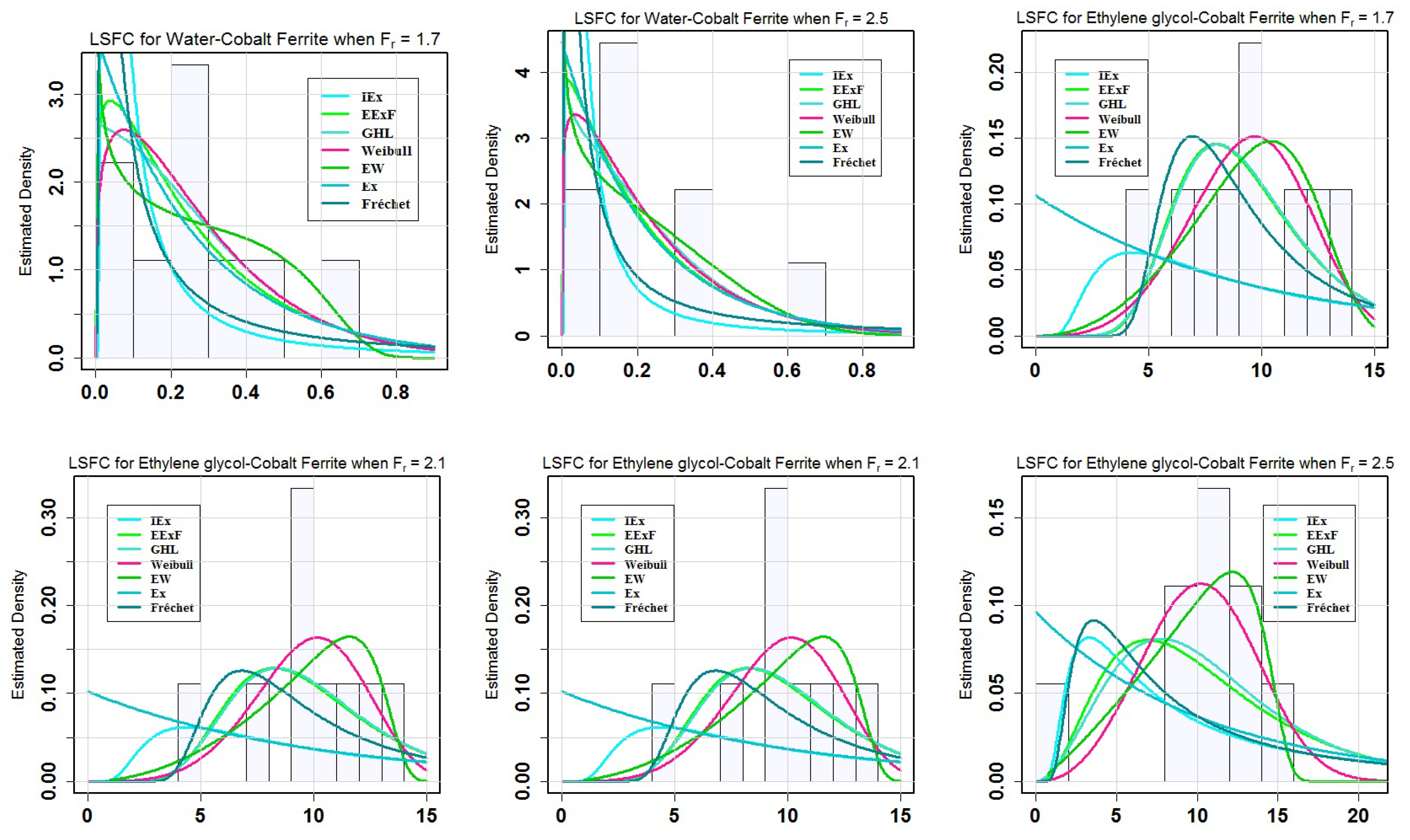
Table 8 demonstrates that the EW model, which has the lowest values of the
A * and
W *, and K-S statistics is the best model among the fitted models for the LSFC of water-cobalt ferrite (H
2O-CoFe
2O
4) at
= 1.7, 2.1 and 2.5. The PV of the KS test is likewise the highest. Three different models could perhaps be considered as the best among the fitted models for the LSFC of C
2H
6O
2-CoFe
2O
4, at
= 1.7, 2.1 and 2.5 because the goodness-of-fit criterion is met by three models, namely, GHL, Weibull, and EW, at
= 1.7, 2.1 and 2.5 respectively.
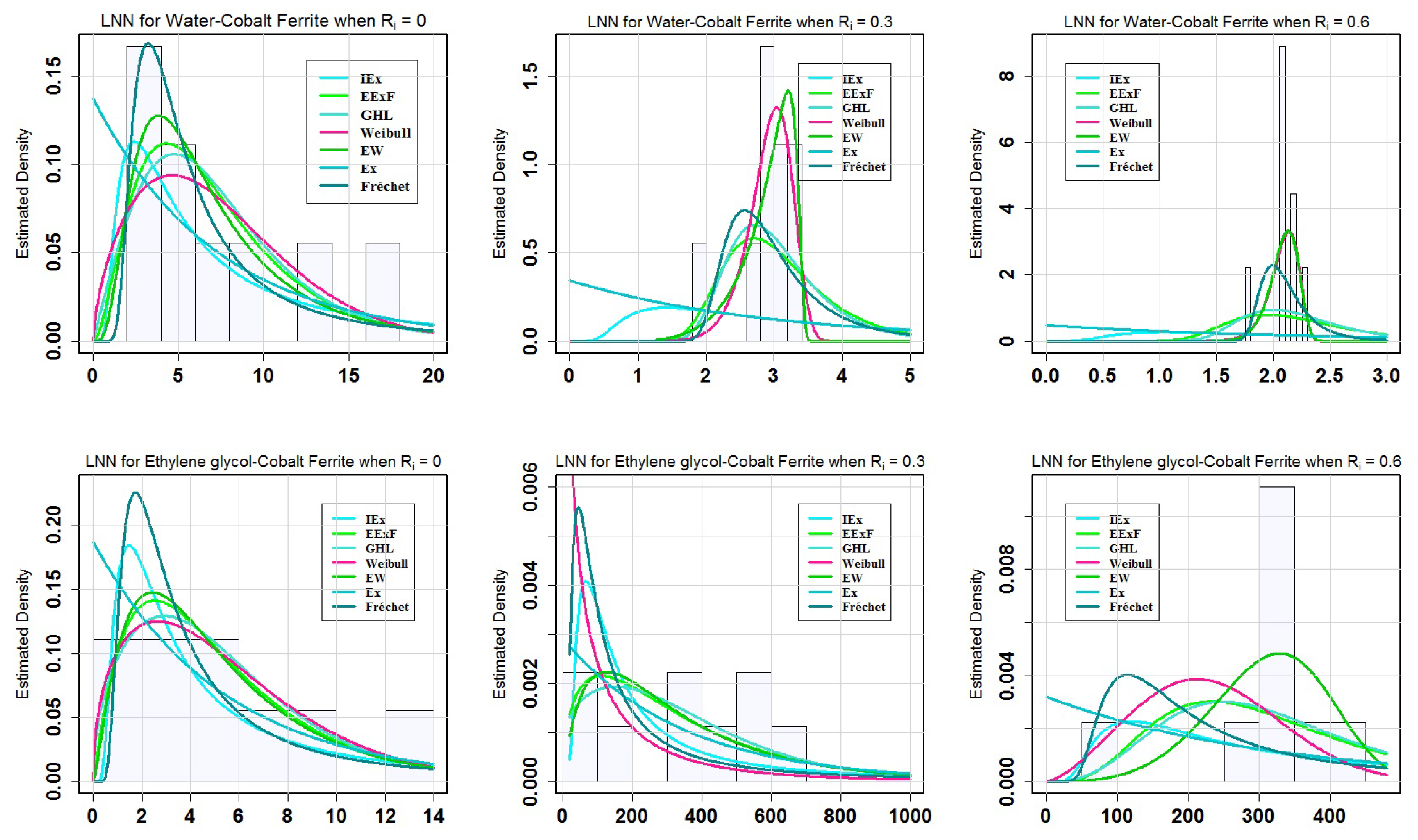
Table 9 shows that the EW model, with the exception of
= 0.3, is the best model among the fitted models for the LNN of water-cobalt ferrite (H
2O-CoFe
2O
4) and ethylene glycol-cobalt ferrite (C
2H
6O
2-CoFe
2O
4) for
= 0, 0.3 and 0.6. Whereas for
= 0.3, the best model among the fitted models for the LNN of water-cobalt ferrite (H
2O-CoFe
2O
4) is the Weibull model with the lowest values of the above-mentioned goodness-of fit (GoF) measures.
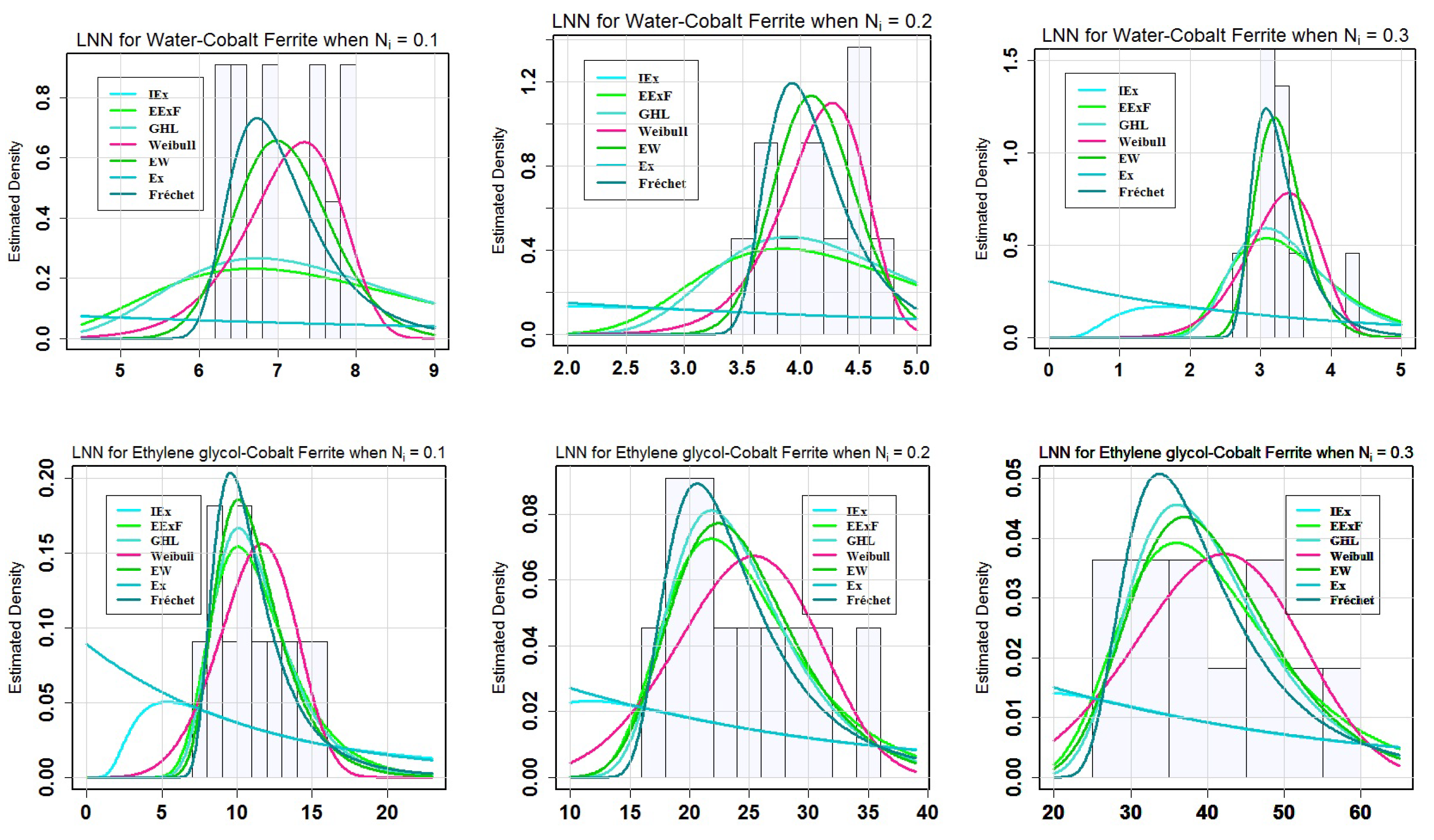
Table 10 reveals the following findings. For the LNN of water-cobalt ferrite (H
2O-CoFe
2O
4), two models (Weibull and EW) could be chosen as the best model among the fitted models. At
= 0.1, the Weibull model is the best since it has the lowest values of the above-mentioned goodness-of fit (GoF) measures; on the other hand, for
= 0.2 and 0.3, Ew showed its suitability because of having the minimum values of GoF metrics. On the other hand, among the fitted models for the LNN of ethylene glycol-cobalt ferrite, the two models EW and EExF may be considered as the most appropriate ethylene glycol-cobalt ferrite (C
2H
6O
2-CoFe
2O
4) for
= 0.1 and
= 0.2, 0.3, respectively, because the lowest values of GoF metrics for Ew and EExF demonstrated suitable suitability.
Figure 2,
Figure 3,
Figure 4 and
Figure 5 show the fitted models that support the findings of
Table 7,
Table 8,
Table 9 and
Table 10.
Figure 2,
Figure 3,
Figure 4 and
Figure 5 show the fitted models that support the findings of
Table 7,
Table 8,
Table 9 and
Table 10.
Figure 2 is plotted to observe the behaviour of the estimated models under water-cobalt ferrite (H
2O-CoFe
2O
4), and ethylene glycol-cobalt ferrite (C
2H
6O
2-CoFe
2O
4) at various levels of
. From the upper panel of
Figure 2, it is noted that the EW model is a suitable candidate for the data sets generated for water-cobalt ferrite (H
2O-CoFe
2O
4) at all considered levels of
. On the other hand, from the lower panel of
Figure 2, the Frechet model showed good suitability with the data behavior generated for ethylene glycol-cobalt ferrite (C
2H
6O
2-CoFe
2O
4) at
= 0.1, whereas Weibull and EExF models provide the correct fit to the data generated at
= 0.5, 0.9 compared to the other models. The behavior of estimated models under water-cobalt ferrite (H
2O-CoFe
2O
4) and ethylene glycol-cobalt ferrite (C
2H
6O
2-CoFe
2O
4) is plotted in
Figure 3 at various levels of
. It can be seen in the upper panel of
Figure 3 that the EW model is a good fit for the data obtained for water-cobalt ferrite (H
2O-CoFe
2O
4) at all values of
. The GHL model, on the other hand, exhibited good appropriateness with the data behavior obtained for ethylene glycol-cobalt ferrite (C
2H
6O
2-CoFe
2O
4) at
= 1.7, besides the Weibull and EW models, when compared to the other models and provides the correct match to the data generated at
= 2.1, 2.5.
Figure 4 presents the characteristics of the estimated models for water-cobalt ferrite (H
2O-CoFe
2O
4) and ethylene glycol-cobalt ferrite (C
2H
6O
2-CoFe
2O
4) at different levels of
. This is visible in the top panel of
Figure 4 in which the EW model fits the data obtained for the water-cobalt ferrite (H
2O-CoFe
2O
4) at
= 0.1 and 0.6 values. The Weibull model, on the other hand, exhibited good appropriateness with the data at
= 0.3. On the other hand, the EW model when compared to the other models, provided the best match for the data generated for ethylene glycol-cobalt ferrite (C
2H
6O
2-CoFe
2O
4) at all values of
. At varying quantities of
,
Figure 5 shows the pattern of estimated models for water-cobalt ferrite (H
2O-CoFe
2O
4) (top panel) and ethylene glycol-cobalt ferrite (C
2H
6O
2-CoFe
2O
4) (bottom panel). The EW model fits the data observed for the water-cobalt ferrite (H
2O-CoFe
2O
4) for
= 0.2 and 0.3, as shown in the top panel of
Figure 5. At
= 0.1, the Weibull model, on the other hand, showed good adequacy with the data. When compared to other models, the EW model provided the best fit to the data generated for the ethylene glycol-cobalt ferrite (C
2H
6O
2-CoFe
2O
4 at
= 0.1 and EExF model at
= 0.2 and 0.3, respectively.