AgGaGeSe4: An Infrared Nonlinear Quaternary Selenide with Good Performance
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthetic Samples
2.2. Single Crystal Growth
2.3. Determination of Crystal Structure
2.4. Energy Dispersive X-ray Spectrometer
2.5. UV-Vis Diffuse Reflectance Spectroscopy Analysis
2.6. Second Harmonic Generation Measurement
2.7. X-ray Photoelectron Spectroscopy (XPS)
2.8. Theoretical Calculation
3. Results and Discussion
3.1. Crystal Structure
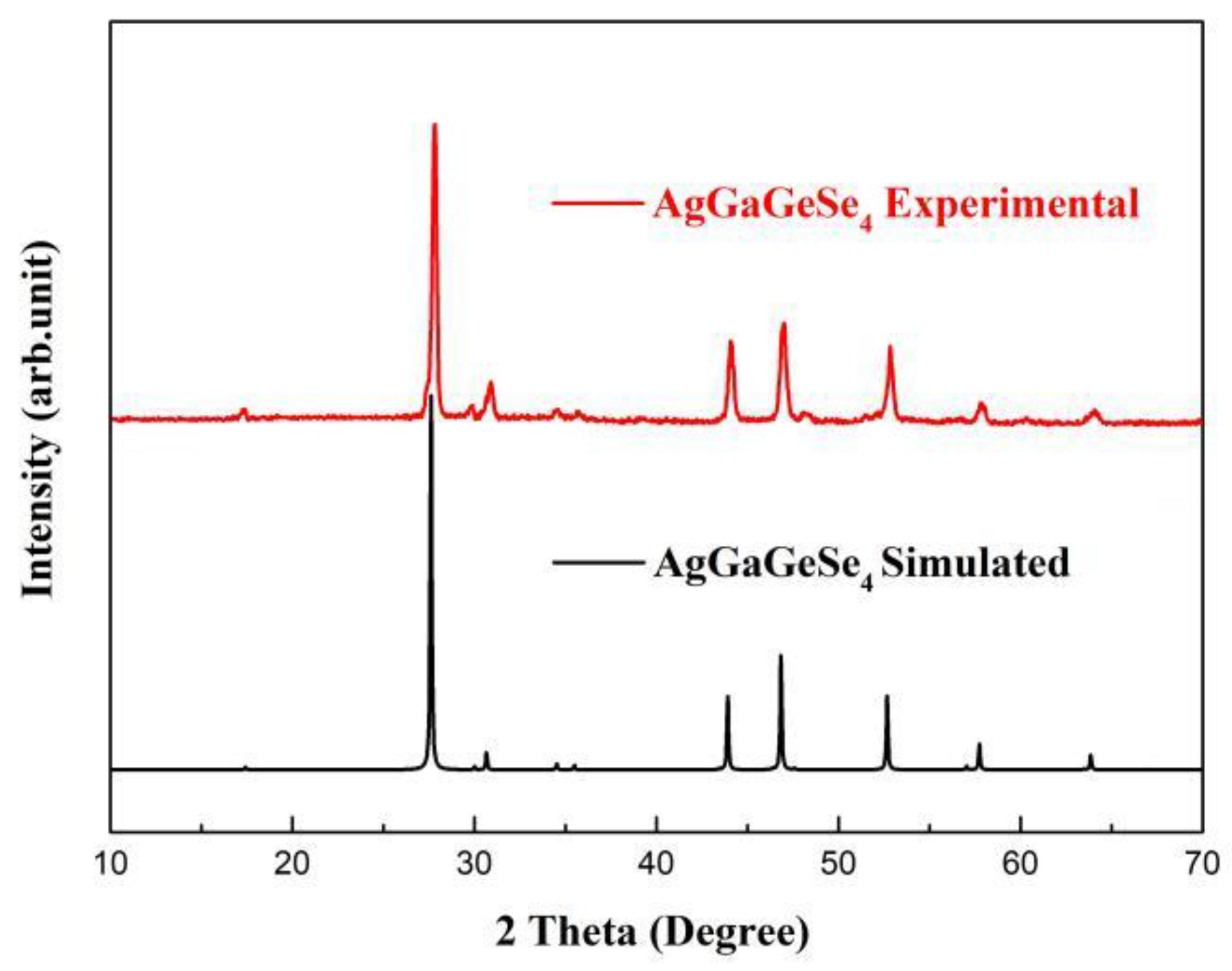
3.2. Powder XRD Pattern
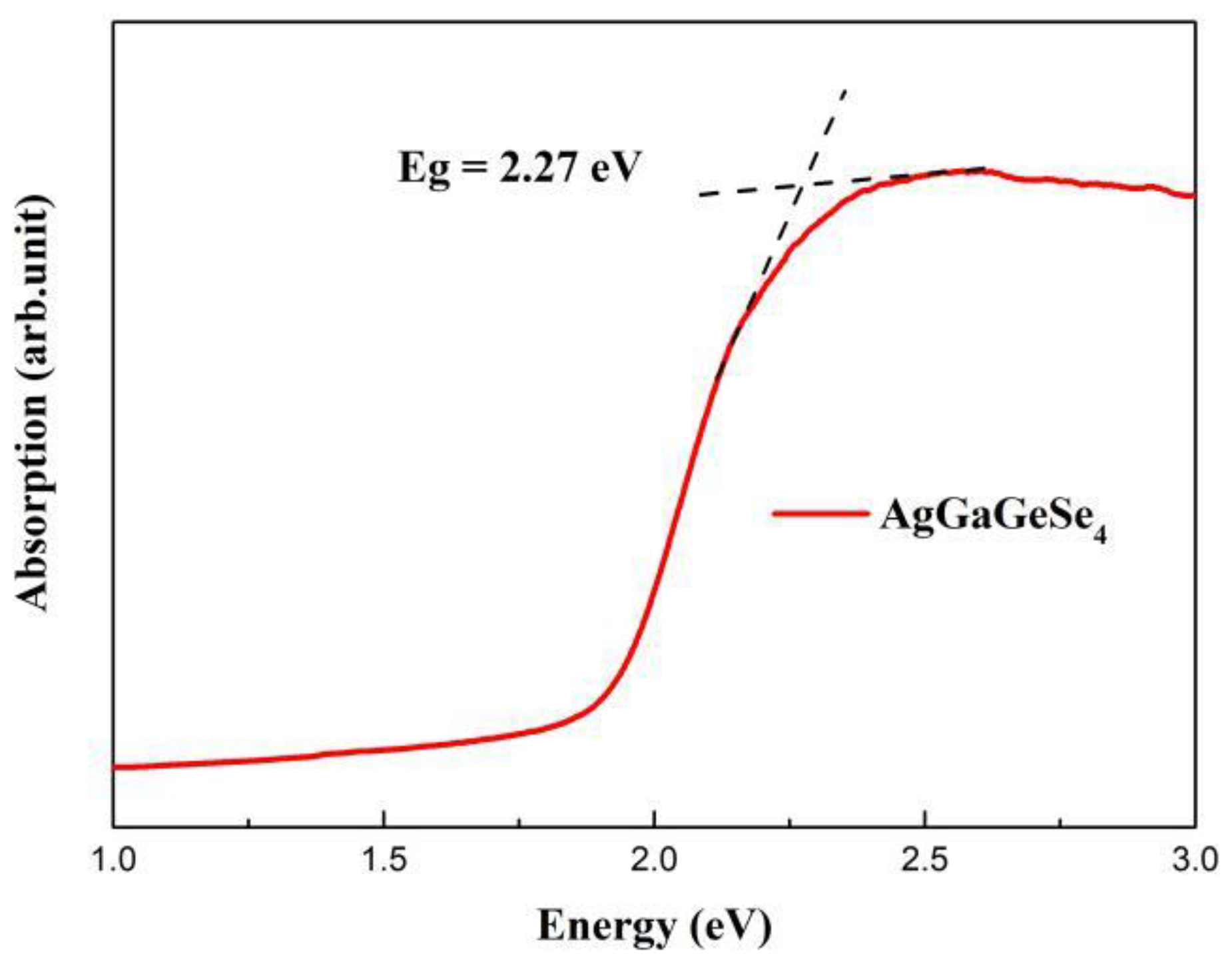
3.3. Optical Properties
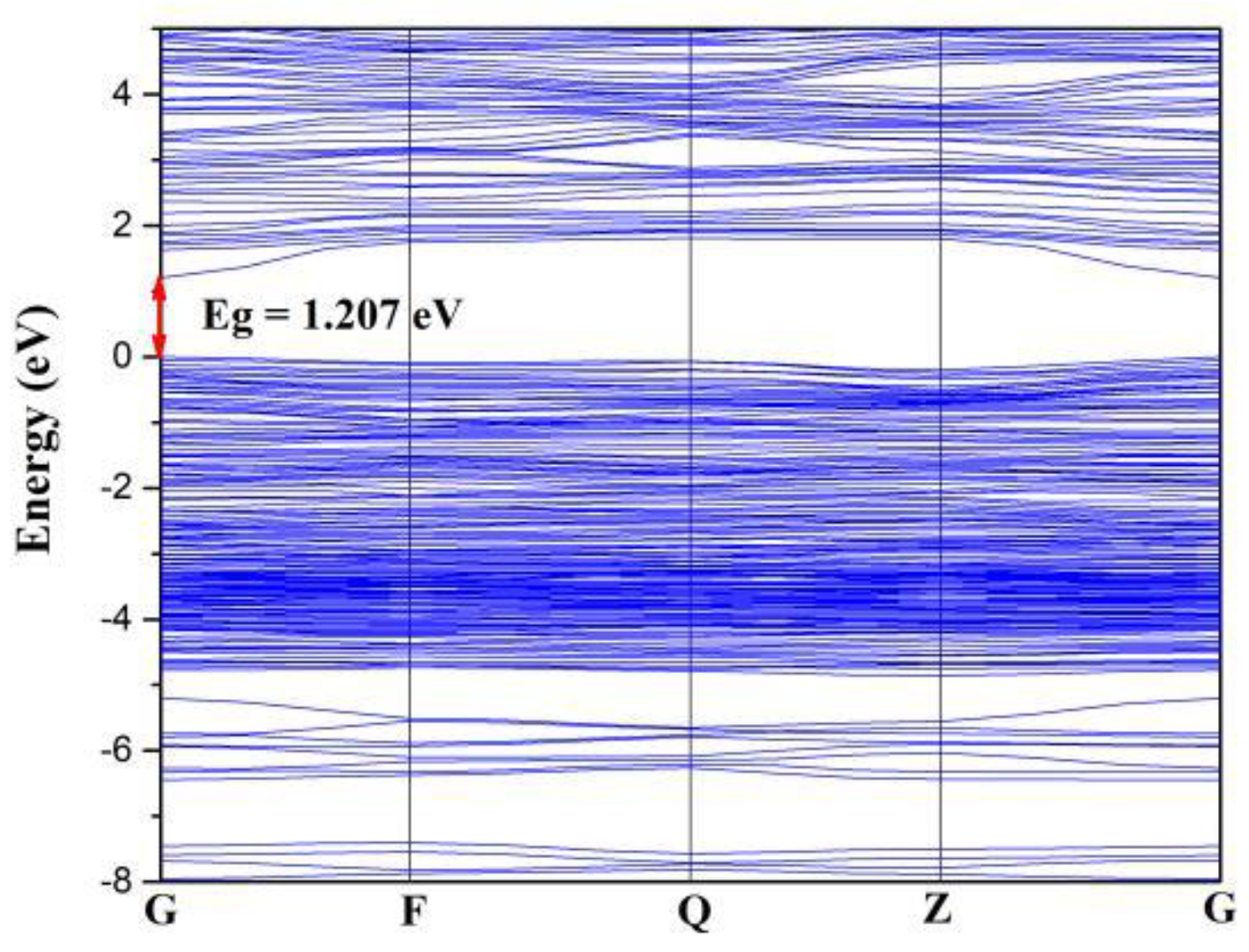
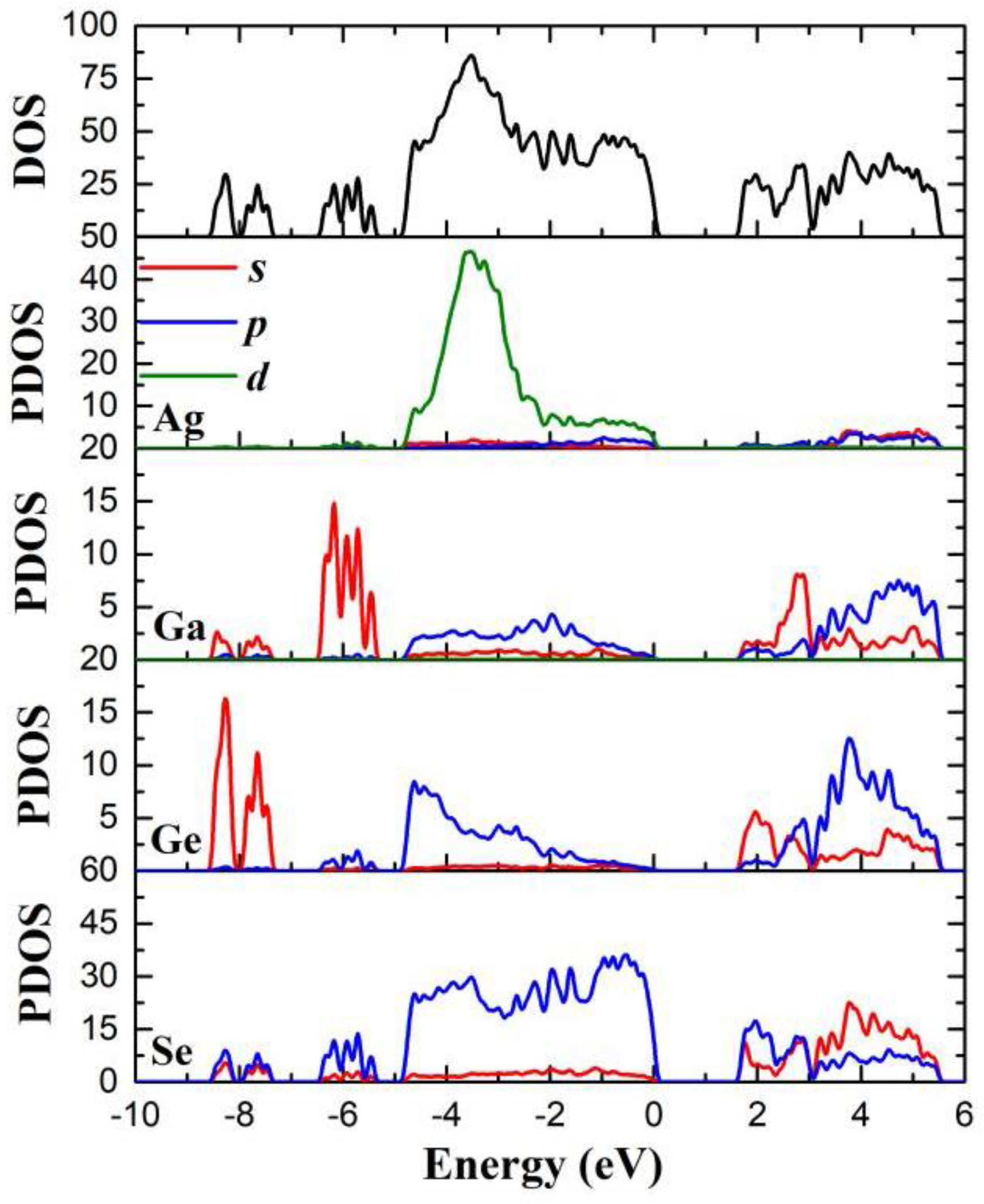
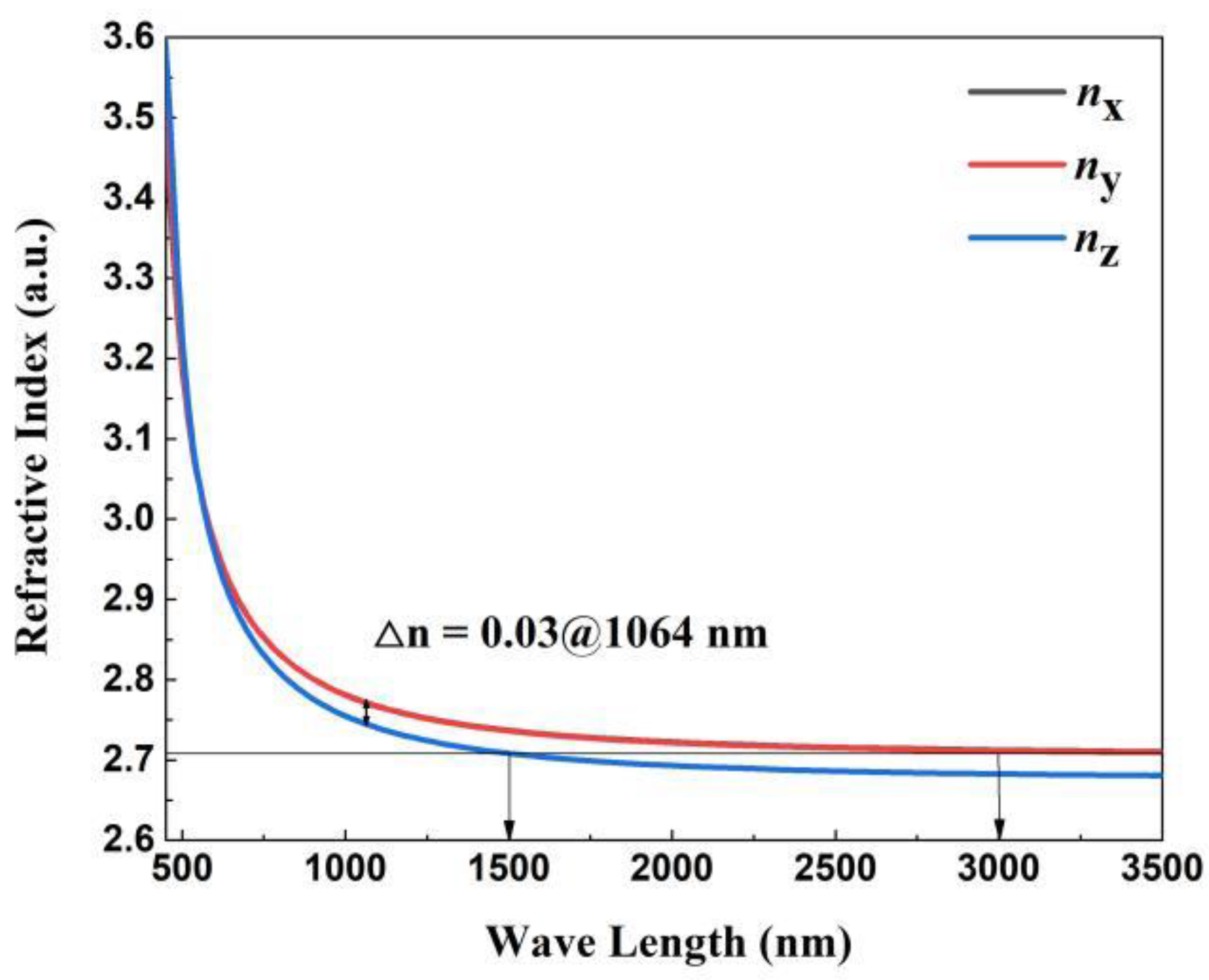
3.4. Theoretical Calculation and Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, S.P.; Chi, Y.; Guo, G.C. Recent achievements on middle and far-infrared second-order nonlinear optical materials. Coord. Chem. Rev. 2017, 335, 44–57. [Google Scholar] [CrossRef]
- Rosemann, N.W.; Eubner, J.P.; Beyer, A.; Koch, S.W.; Volz, K.; Dehnen, S.; Chatterjee, S. A highly efficient directional molecular white-light emitter driven by a continuous-wave laser diode. Science 2016, 352, 1301–1304. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Yang, C.; Liu, X.; Ma, J.; Liang, F.; Du, Y.S. Dimensionality reduction made high-performance mid-infrared nonlinear halide crystal. Mater. Today Phys. 2021, 21, 100569. [Google Scholar] [CrossRef]
- Lin, H.; Wei, W.B.; Chen, H.; Wu, X.T.; Zhu, Q.L. Rational design of infrared nonlinear optical chalcogenides by chemical substitution. Coord. Chem. Rev. 2020, 406, 213150. [Google Scholar] [CrossRef]
- Wang, W.K.; Mei, D.J.; Liang, F.; Zhao, J.; Wu, Y.D.; Lin, Z.S. Inherent laws between tetrahedral arrangement pattern and optical performance in tetrahedron-based mid-infrared nonlinear optical materials. Coord. Chem. Rev. 2020, 421, 213444. [Google Scholar] [CrossRef]
- Wang, W.K.; Mei, D.J.; Wen, S.G.; Wang, J.; Wu, Y.D. Complex coordinated functional groups: A great genes for nonlinear optical materials. Chin. Chem. Lett. 2021, 33, 2301–2315. [Google Scholar] [CrossRef]
- Boyd, G.; Kasper, H.; McFee, J. Linear and nonlinear optical properties of AgGaS2, CuGaS2, and CuInS2, and theory of the wedge technique for the measurement of nonlinear coefficients. IEEE J. Quantum Electron. 1971, 7, 563–573. [Google Scholar] [CrossRef]
- Zhu, S.; Liu, J.; Zhao, B.; Jiang, H.; Li, Z. Crystal Growth and Differential Thermal Analysis of AgGaSe2. Cryst. Res. Technol. 1995, 30, 1165–1168. [Google Scholar] [CrossRef]
- Boyd, G.D.; Buehler, E.; Storz, F.G. Linear and nonlinear optical properties of ZnGeP2 and CdSe. Appl. Phys. Lett. 1971, 18, 301–304. [Google Scholar] [CrossRef]
- Li, W.K.; Li, Y.Y.; Xu, Y.; Lu, J.; Wang, P.F.; Du, J.; Leng, Y.X. Measurements of nonlinear refraction in the mid-infrared materials ZnGeP2 and AgGaS2. Appl. Phys. B 2017, 123, 82. [Google Scholar] [CrossRef]
- Tell, B.; Kasper, H.M. Optical and Electrical Properties of AgGaS2 and AgGaSe2. Phys. Rev. B 1971, 4, 4455–4459. [Google Scholar] [CrossRef]
- Cao, W.Z.; Mei, D.J.; Yang, Y.; Wu, Y.W.; Zhang, L.Y.; Wu, Y.D.; He, X.; Lin, Z.S.; Huang, F.Q. From CuFeS2 to Ba6Cu2FeGe4S16: Rational band gap engineering achieves large second-harmonicgeneration together with high laser damage threshold. Chem. Commun. 2019, 55, 14510–14513. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Zhou, M.L.; Yao, J.Y.; Lin, Z.S.; Wu, Y.C.; Chen, C.T. Metal thiophosphates with good mid-infrared nonlinear optical performances: A first-principles prediction and analysis. J. Am. Chem. Soc. 2015, 137, 13049–13059. [Google Scholar] [CrossRef]
- Chung, I.; Kim, M.G.; Jang, I.; He, J.Q.; Ketterson, J.B.; Kanatzidis, M.G. Strongly nonlinear optical chalcogenide thin films of APSe6 (A = K, Rb) from spin-coating. Angew. Chem. Int. Ed. 2011, 50, 10867–10870. [Google Scholar] [CrossRef]
- Yu, H.W.; Young, J.S.; Wu, H.P.; Zhang, W.G.; Rondinelli, J.M.; Halasyamani, P.S. Electronic, crystal chemistry, and nonlinear optical property relationships in the dugganite A3B3CD2O14 Family. J. Am. Chem. Soc. 2016, 138, 4984–4989. [Google Scholar] [CrossRef]
- Yin, W.L.; Iyer, A.K.; Li, C.; Yao, J.Y.; Mar, A. Noncentrosymmetric chalcogenides BaZnSiSe4 and BaZnGeSe4 featuring one-dimensional structures. J. Alloys Compd. 2017, 708, 414–421. [Google Scholar] [CrossRef]
- Hou, F.J.; Mei, D.J.; Zhang, Y.L.; Liang, F.; Wang, J.; Lu, J.; Lin, Z.S.; Wu, Y.D. SrZnSnSe4: A quaternary selenide with large second harmonic generation and birefringence. J. Alloys Compd. 2022, 904, 163944. [Google Scholar] [CrossRef]
- Singh, N.B.; Knuteson, D.J.; Kanner, G.; Berghmans, A.; Green, K.; Wagner, B.; Kahler, D.; King, M.; McLaughlin, S. Ternary and quaternary selenide crystals for nonlinear optical applications. Photon. Fib. Cryst. Dev. 2011, 8120, 812002. [Google Scholar] [CrossRef]
- Mei, D.J.; Cao, W.Z.; Wang, N.Z.; Jiang, X.X.; Zhao, J.; Wang, W.K.; Dang, J.H.; Zhang, S.Y.; Wu, Y.D.; Rao, P.H.; et al. Breaking through the “3.0 eV wall” of energy band gap in mid-infrared nonlinear optical rare earth chalcogenides by charge-transfer engineering. Mater. Horiz. 2021, 8, 2330–2334. [Google Scholar] [CrossRef]
- Wu, J.; Huang, W.; Liu, H.G.; He, Z.Y.; Chen, B.J.; Zhu, S.F.; Zhao, B.J.; Lei, Y.X.; Zhou, X.N. Investigation on Thermal Properties and Crystal Growth of Nonlinear Optical Crystal AgGaS2 and AgGaGeS4. Cryst. Growth Des. 2020, 20, 3140–3153. [Google Scholar] [CrossRef]
- Huang, W.; He, Z.Y.; Zhao, B.J.; Zhu, S.F.; Chen, B.J.; Wu, Y. Effect of Thermal Annealing Treatment and Defect Analysis on AgGaGeS4 Single Crystals. Inorg. Chem. 2019, 58, 10846–10855. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.P.; Chi, Y.; Xue, H.G. SnI4(S8)2: A novel adduct-type infrared second-order nonlinear optical crystal. Angew. Chem. Int. Ed. 2018, 57, 11540–11543. [Google Scholar] [CrossRef]
- Liu, B.W.; Jiang, X.M.; Zeng, H.Y.; Guo, G.C. [ABa2Cl][Ga4S8] (A = Rb, Cs): Wide-spectrum nonlinear optical materials obtained by polycation-substitution-induced nonlinear optical (NLO)-functional motif ordering. J. Am. Chem. Soc. 2020, 142, 10641–10645. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Liu, X.; Teng, C.; Cheng, X.; Liang, F.; Wu, Q. Hierarchical molecular design of high-performance infrared nonlinear Ag2HgI4 material by defect engineering strategy. Mater. Today Phys. 2021, 19, 100432. [Google Scholar] [CrossRef]
- Zhou, H.M.; Xiong, L.; Chen, L.; Wu, L.M. Dislocations that decrease size mismatch within the lattice leading to ultrawide band gap, large second-order susceptibility, and high nonlinear optical performance of AgGaS2. Angew. Chem. Int. Ed. 2019, 58, 9979–9983. [Google Scholar] [CrossRef]
- Kim, W.T. Optical properties of undoped and cobalt-doped IB-Ga-IVA-Se4 single crystals. Phys. Rev. B 1991, 44, 8667–8671. [Google Scholar] [CrossRef]
- Goodchild, R.G.; Hughes, O.H.; Lopez-Rivera, S.A.; Woolle, J.C. Energy gap values by optical absorption in I III IV Se4 compounds. Can. J. Phys. 1982, 60, 1096–1100. [Google Scholar] [CrossRef]
- Guo, S.P.; Cheng, X.Y.; Sun, Z.D.; Chi, Y.; Liu, B.W.; Jiang, X.M.; Li, S.F.; Xue, H.G.; Deng, S.Q.; Duppel, V.; et al. Large second harmonic generation (SHG) effect and high laser-induced damage threshold (LIDT) observed coexisting in gallium selenide. Angew. Chem. Int. Ed. 2019, 131, 8171–8175. [Google Scholar] [CrossRef]
- Abudurusuli, A.L.J.; Huang, J.B.; Wang, P.; Yang, Z.H.; Pan, S.L.; Li, J.J. Li4MgGe2S7: The First Alkali and Alkaline-Earth Diamond-Like Infrared Nonlinear Optical Material with Exceptional Large Band Gap. Angew. Chem. Int. Ed. 2021, 60, 24131–24136. [Google Scholar] [CrossRef]
- Jiang, X.M.; Lin, S.J.; He, C.; Liu, B.W.; Guo, G.C. Uncovering functional motif of nonlinear optical material by insitu electron density and wavefunction studies under laser irradiation. Angew. Chem. Int. Ed. 2021, 60, 11799–11803. [Google Scholar] [CrossRef]
- Wu, K.; Zhang, B.B.; Yang, Z.H. New Compressed Chalcopyrite-like Li2BaMIVQ4 (MIV = Ge, Sn; Q = S, Se): Promising Infrared Nonlinear Optical Materials. J. Am. Chem. Soc. 2017, 139, 14885–14888. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Pan, S.L. A review on structure-performance relationship toward the optimal design of infrared nonlinear optical materials with balanced performances. Coord. Chem. Rev. 2018, 377, 191–208. [Google Scholar] [CrossRef]
- Mei, D.J.; Yin, W.L.; Feng, K.; Lin, Z.S.; Bai, L.; Yao, J.Y.; Wu, Y.C. LiGaGe2Se6: A new IR nonlinear optical material with low melting point. Inorg. Chem. 2012, 51, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Mei, D.J.; Wang, W.K.; Wu, Y.D.; Xue, D.F. Recent advances in nonlinear optical rare earth structures. J. Rare Earths 2021, 39, 1455–1466. [Google Scholar] [CrossRef]
- Dang, J.H.; Mei, D.J.; Wu, Y.D.; Lin, Z.S. A comprehensive survey on nonlinear optical phosphates: Role of multicoordinate groups. Coord. Chem. Rev. 2021, 431, 213692. [Google Scholar] [CrossRef]
- Myronchuk, G.L.; Lakshminarayana, G.; Kityk, I.V.; Fedorchuk, A.O.; Vlokh, R.O.; Kozer, V.R.; Parasyuk, O.V.; Piasecki, M. AgGaGeS4 crystal as promising optoelectronic material. Chalcogenide Lett. 2018, 15, 151–156. Available online: https://www.researchgate.net/publication/324132099 (accessed on 18 January 2022).
- Krymus, A.S.; Kityk, I.V.; Demchenko, P.; Parasyuk, O.V.; Myronchuk, G.L.; Khyzhun, O.Y.; Piasecki, M. AgGaSiSe4: Growth, crystal and band electronic structure, optoelectronic and piezoelectric properties. Mater. Res. Bull. 2017, 95, 177–184. [Google Scholar] [CrossRef]
- Mei, D.J.; Gong, P.F.; Lin, Z.S.; Feng, K.; Yao, J.Y.; Huang, F.Q.; Wu, Y.C. Ag3Ga3SiSe8: A new infrared nonlinear optical material with a chalcopyrite structure. CrystEngComm 2014, 16, 6836–6840. [Google Scholar] [CrossRef]
- Petrov, V.; Noack, F.; Badikov, V.; Shevyrdyaeva, G.; Panyutin, V.; Chizhikov, V. Phase-matching and femtosecond difference-frequency generation in the quaternary semiconductor AgGaGe5Se12. Appl. Opt. 2004, 43, 4590–4597. [Google Scholar] [CrossRef]
- Huang, W.; Wu, J.; Chen, B.J.; Li, J.P.; He, Z.Y. Crystal Growth and Thermal Annealing of AgGaGe5Se12 Crystal. J. Alloys Compd. 2021, 862, 158002–158022. [Google Scholar] [CrossRef]
- Hughes, O.H.; Woolley, J.C.; Lopez-Rivera, S.A.; Pamplin, B.R. Quaternary Adamantine Selenides and Tellurides of the Form I III IV VI4. Solid State Commun. 1980, 35, 573–575. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.I.J.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Z. Kristallogr. 2005, 220, 567–570. [Google Scholar] [CrossRef] [Green Version]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef] [Green Version]
- Payne, M.C.; Teter, M.P.; Allan, D.C.; Arias, T.A.; Joannopoulos, J.D. Iterative minimization techniques forab initiototal-energy calculations: Molecular dynamics and conjugate gradients. Rev. Mod. Phys. 1992, 64, 1045–1097. [Google Scholar] [CrossRef] [Green Version]
- Reshak, A.H.; Parasyuk, O.V.; Fedorchuk, A.O.; Kamarudin, H.; Auluck, S.; Chyský, J. Optical Spectra and Band Structure of AgxGaxGe1−xSe2 (x = 0.333, 0.250, 0.200, 0.167) Single Crystals: Experiment and Theory. J. Phys. Chem. B 2013, 117, 15220–15231. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Let. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B 1992, 45, 13244–13249. [Google Scholar] [CrossRef]
- Hamann, D.R.; Schlüter, M.; Chiang, C. Norm-Conserving Pseudopotentials. Phys. Rev. Lett. 1979, 43, 1494–1497. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Zhang, Y.; Islam, Z.; Ren, Y.; Parilla, P.A.; Ahrenkiel, S.P.; Lee, P.L. Zero thermal expansion in a nanostructured inorganic-organic hybrid crystal. Phy. Rev. Lett. 2007, 99, 215901. [Google Scholar] [CrossRef] [PubMed]
- Badikov, V.V.; Tyulyupa, A.G.; Shevyrdyaeva, G.S.; Sheina, S.G. Solid solutions in the AgGaS2-GeS2 and AgGaSe2-GeSe2 systems. Inorg. Mater. 1991, 27, 177–180. [Google Scholar]
- Cheng, W.D.; Lin, C.S.; Luo, Z.Z.; Zhang, H. Designing the syntheses and photophysical simulations of noncentrosymmetric compounds. Inorg. Chem. Front. 2015, 2, 95–107. [Google Scholar] [CrossRef]
- BullSchevciw, O.; White, W.B. The optical absorption edge of rare earth sesquisulfides and alkaline earth-rare earth sulfides. Mater. Res. Bull. 1983, 18, 1059–1068. [Google Scholar] [CrossRef]
- Zhao, J.; Mei, D.J.; Yang, Y.; Cao, W.Z.; Liu, C.; Wu, Y.D.; Lin, Z.S. Rb10Zn4Sn4S17: A Chalcogenide with Large Laser Damage Threshold Improved from the Mn-Based Analogue. Inorg. Chem. 2019, 52, 15029–15033. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dang, J.; Wang, N.; Yao, J.; Wu, Y.; Lin, Z.; Mei, D. AgGaGeSe4: An Infrared Nonlinear Quaternary Selenide with Good Performance. Symmetry 2022, 14, 1426. https://doi.org/10.3390/sym14071426
Dang J, Wang N, Yao J, Wu Y, Lin Z, Mei D. AgGaGeSe4: An Infrared Nonlinear Quaternary Selenide with Good Performance. Symmetry. 2022; 14(7):1426. https://doi.org/10.3390/sym14071426
Chicago/Turabian StyleDang, Junhui, Naizheng Wang, Jiyong Yao, Yuandong Wu, Zheshuai Lin, and Dajiang Mei. 2022. "AgGaGeSe4: An Infrared Nonlinear Quaternary Selenide with Good Performance" Symmetry 14, no. 7: 1426. https://doi.org/10.3390/sym14071426
APA StyleDang, J., Wang, N., Yao, J., Wu, Y., Lin, Z., & Mei, D. (2022). AgGaGeSe4: An Infrared Nonlinear Quaternary Selenide with Good Performance. Symmetry, 14(7), 1426. https://doi.org/10.3390/sym14071426




