Evolution of Microstructure, Texture and Corrosion Properties of Additively Manufactured AlSi10Mg Alloy Subjected to Equal Channel Angular Pressing (ECAP)
Abstract
:1. Introduction
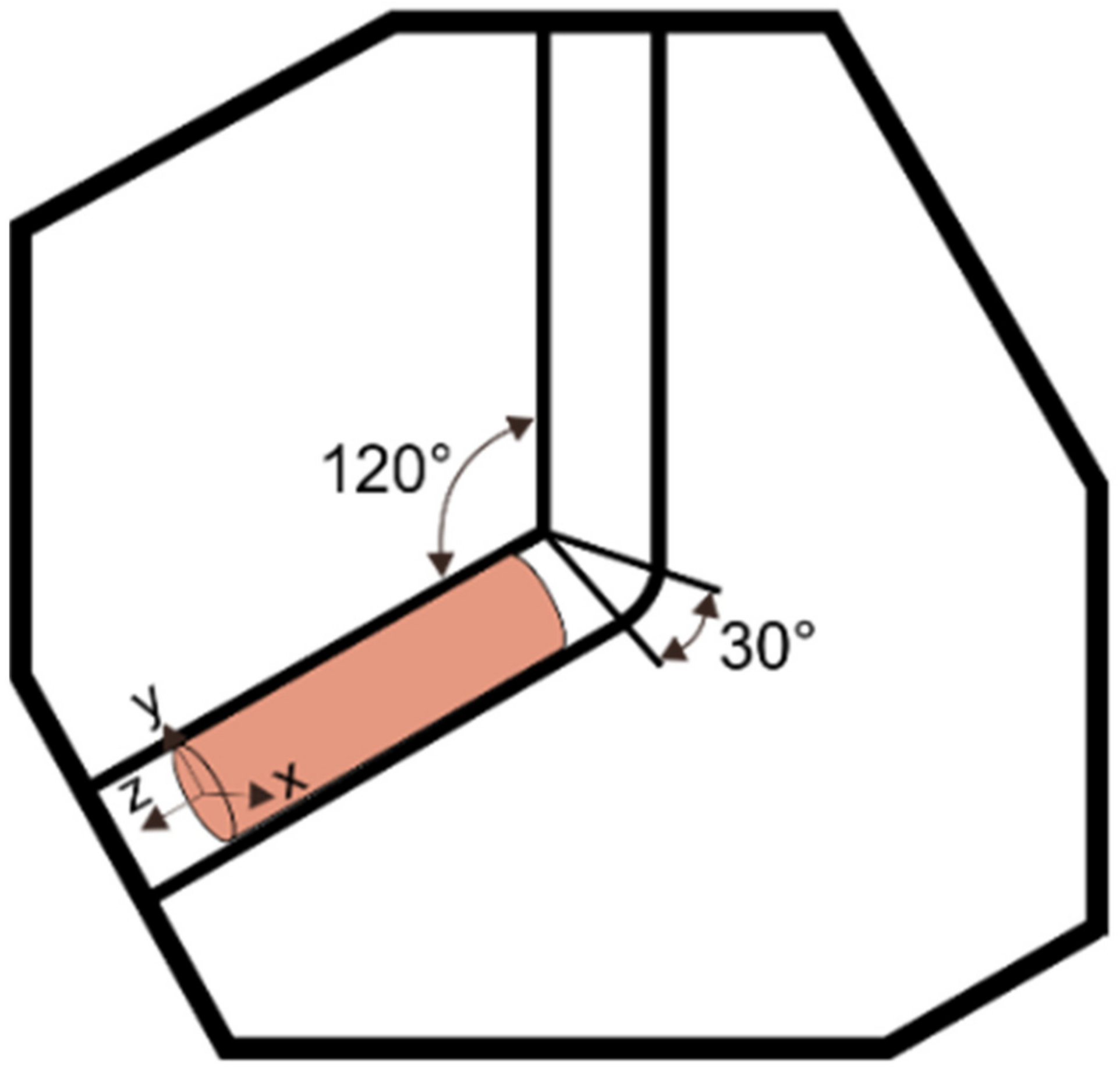
2. Materials and Methods
- Scanning speed 1400 mm/s;
- Laser beam diameter 55 μm;
- Layer thickness build-up of 20 μm.
3. Results
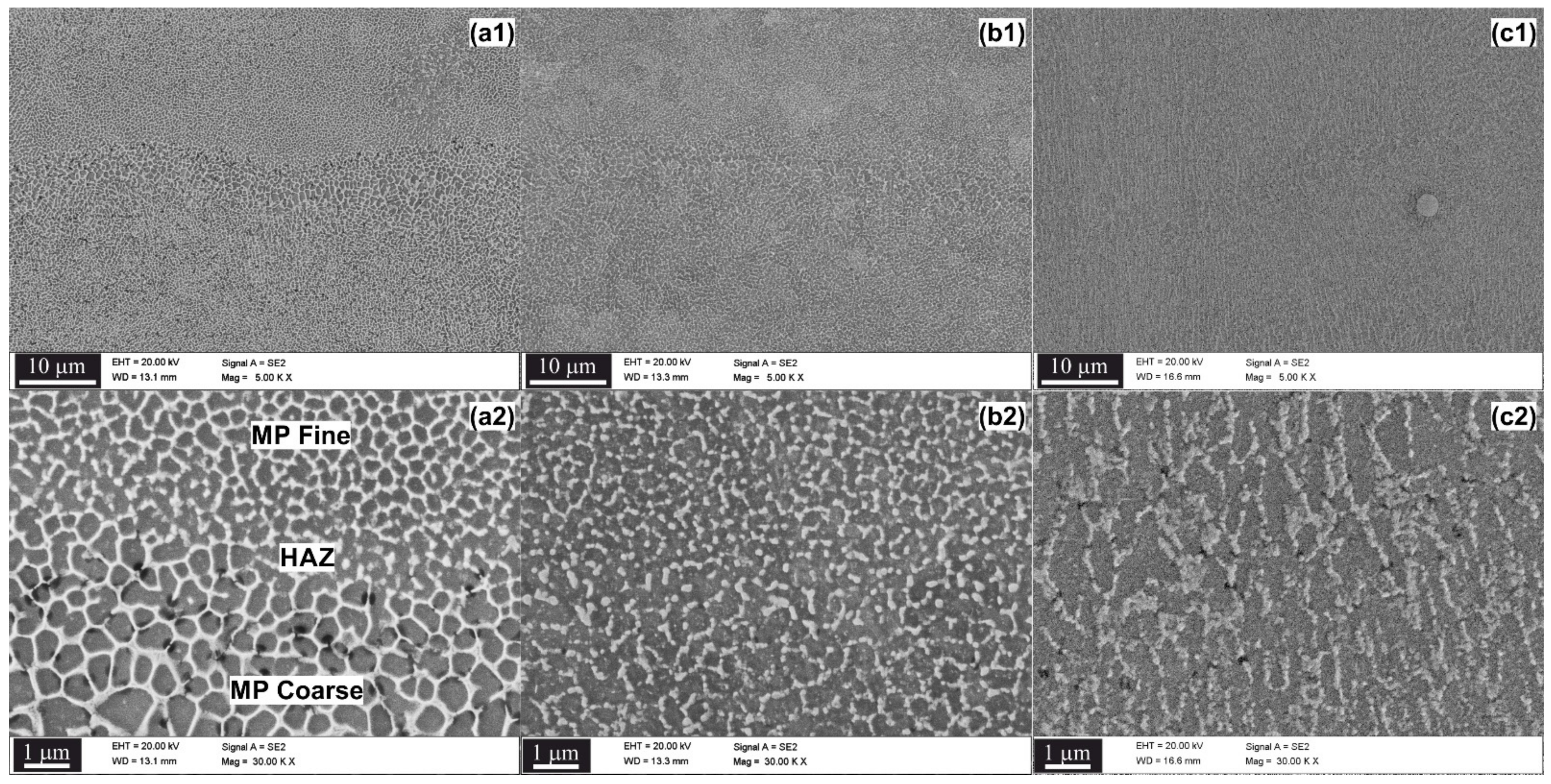
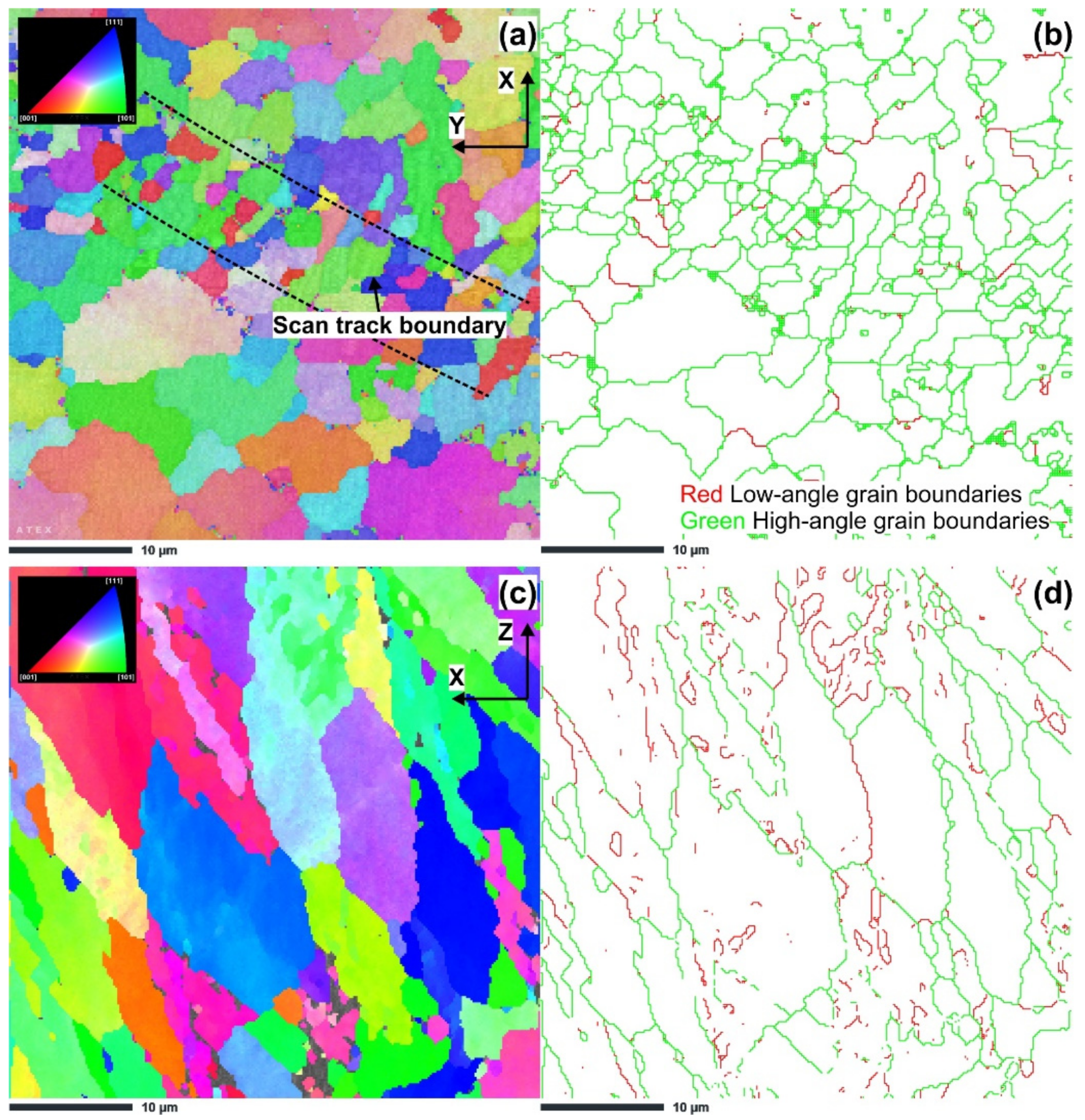
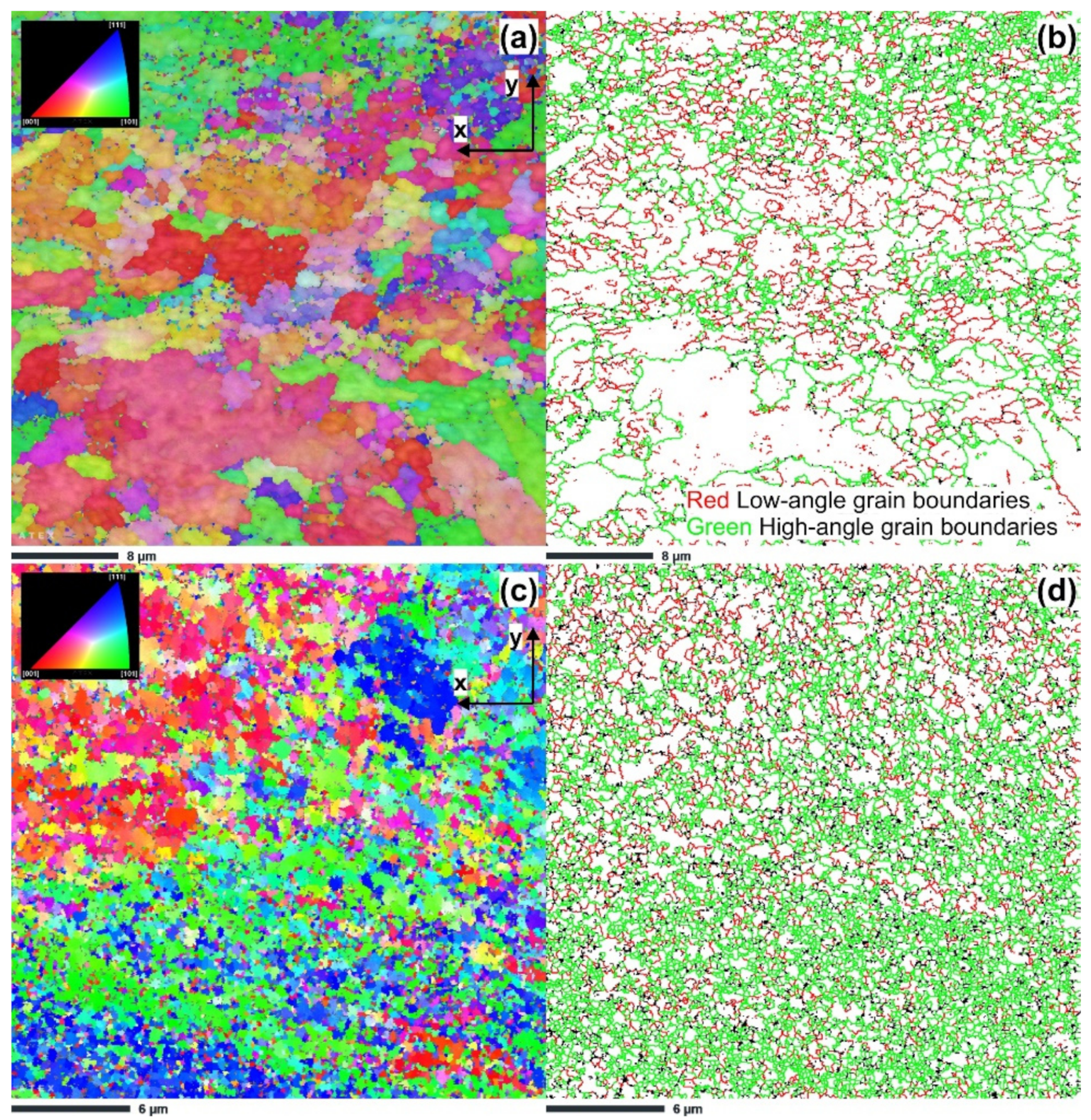
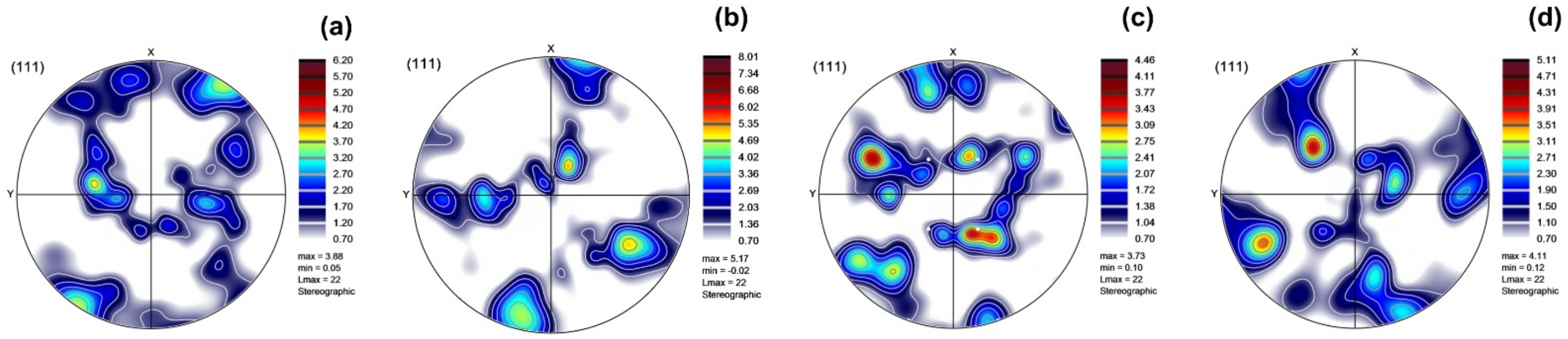
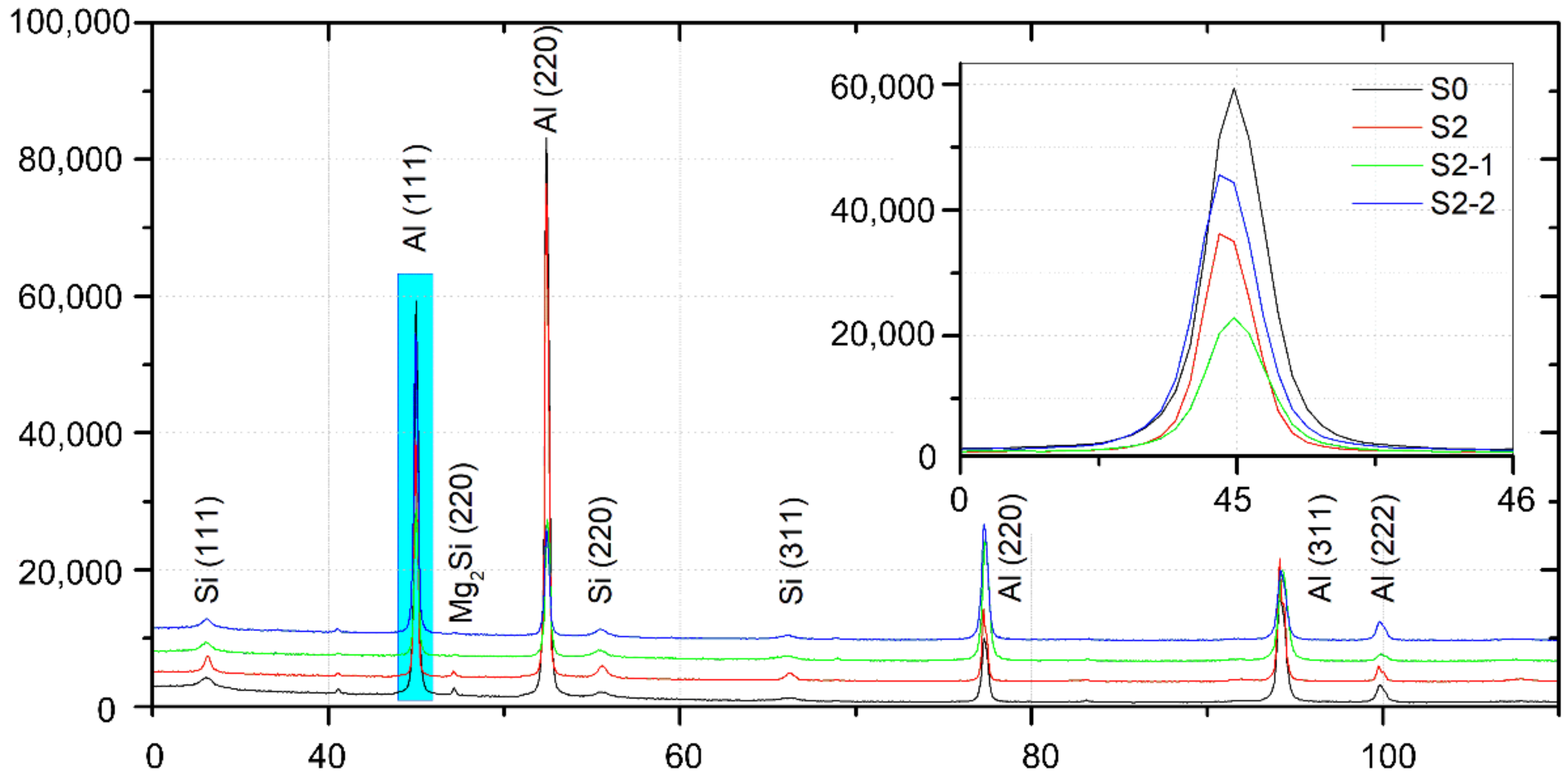
3.1. Microstructure Characterization
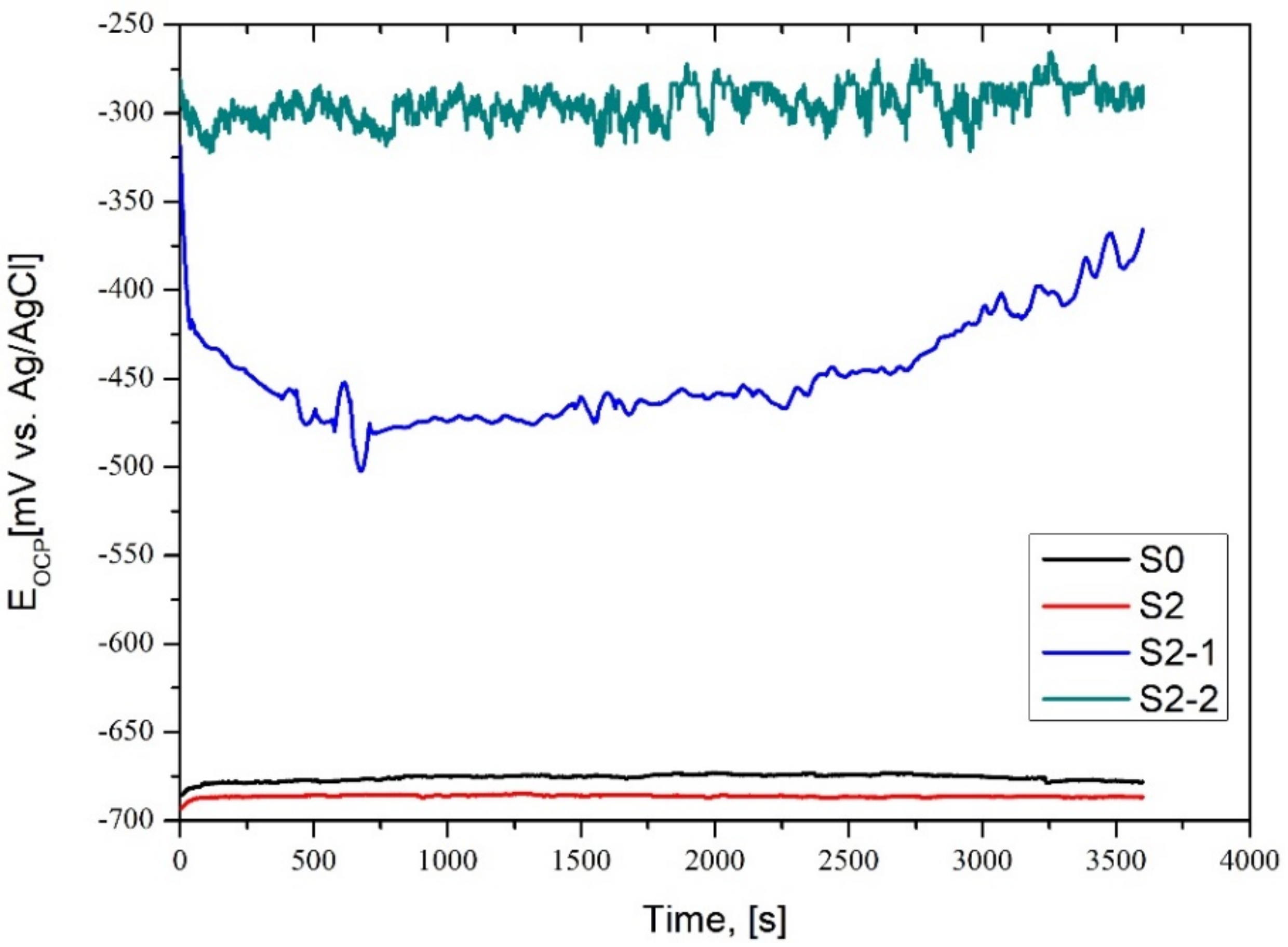
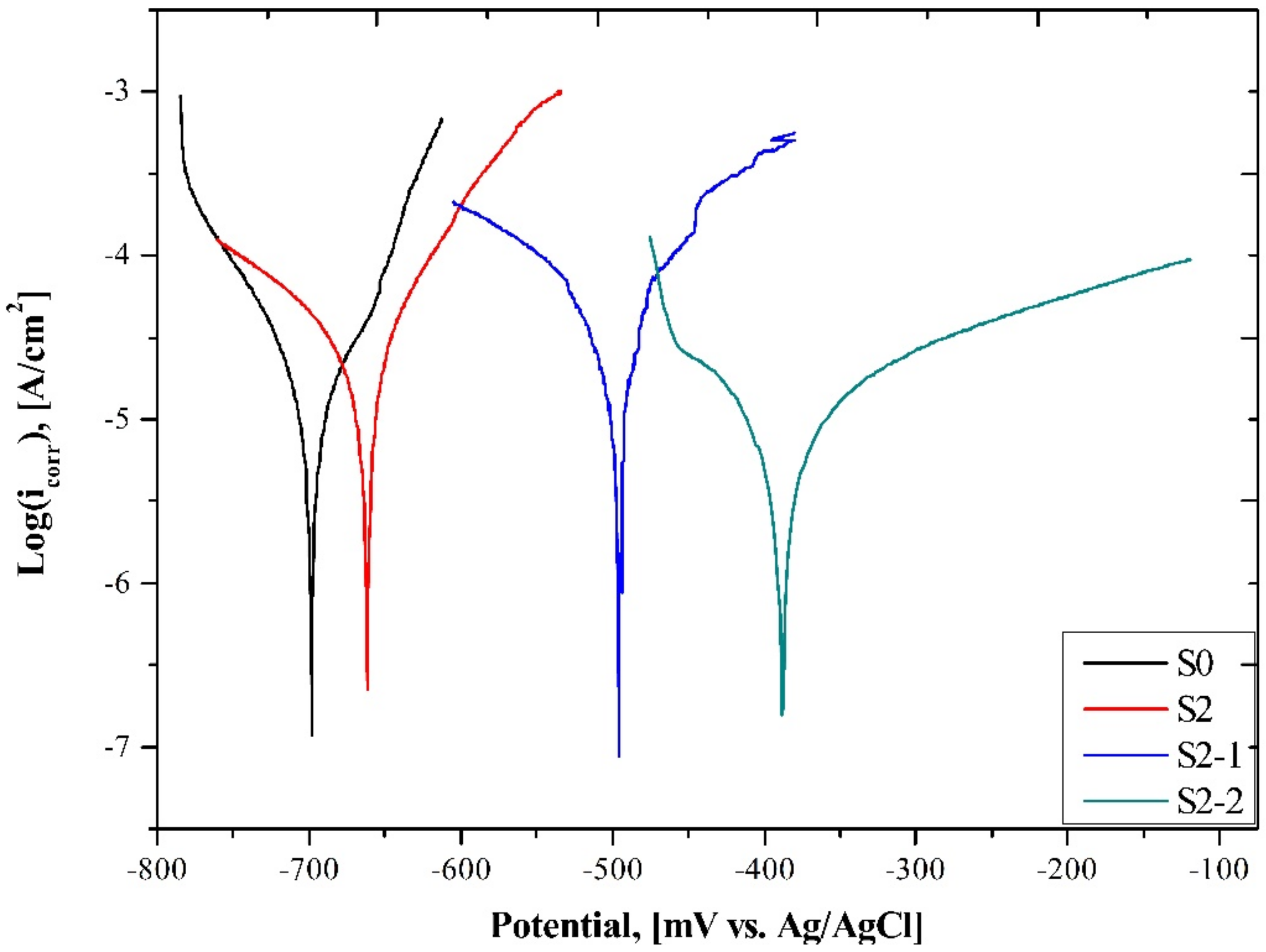
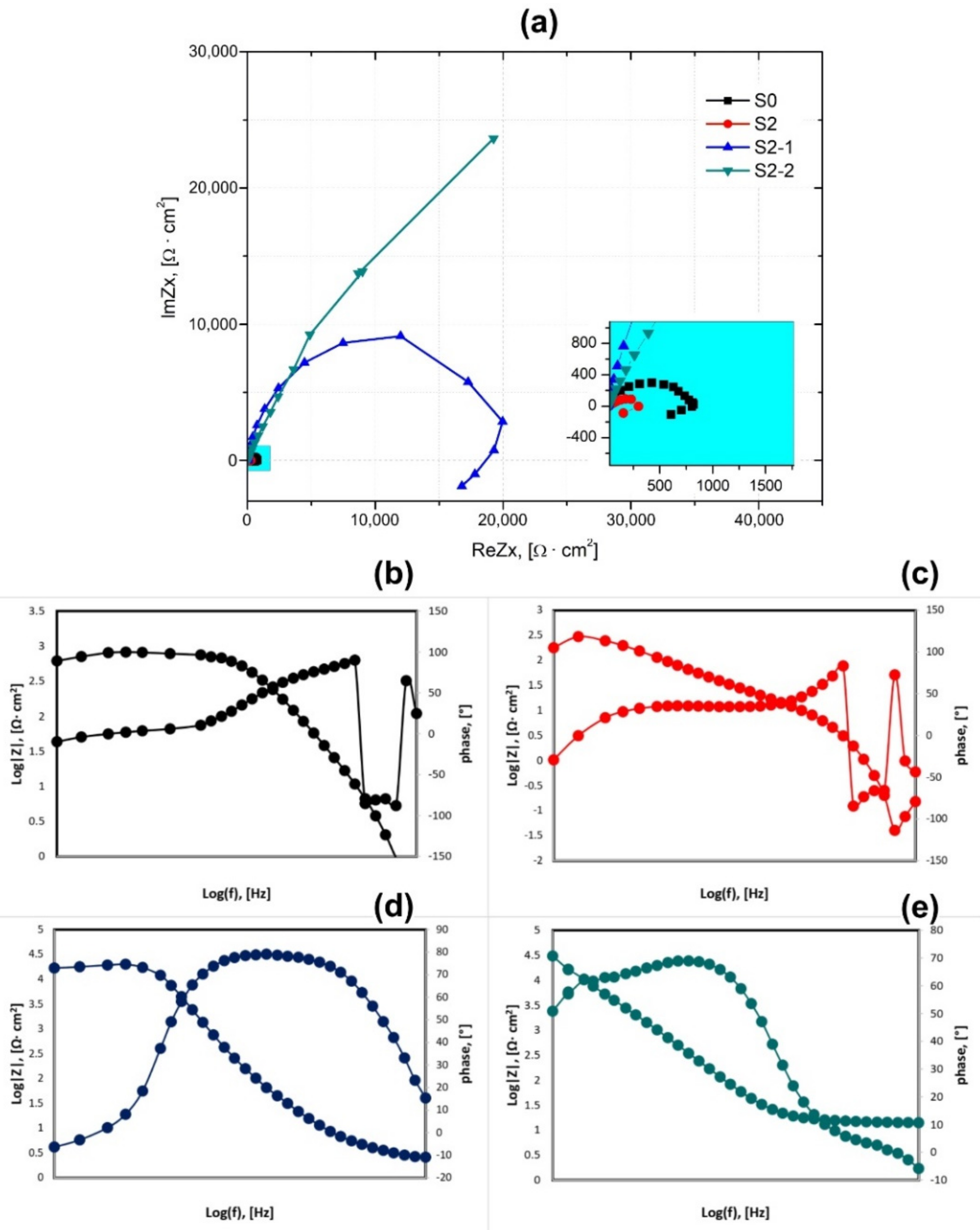
3.2. Corrosion Properties Analysis
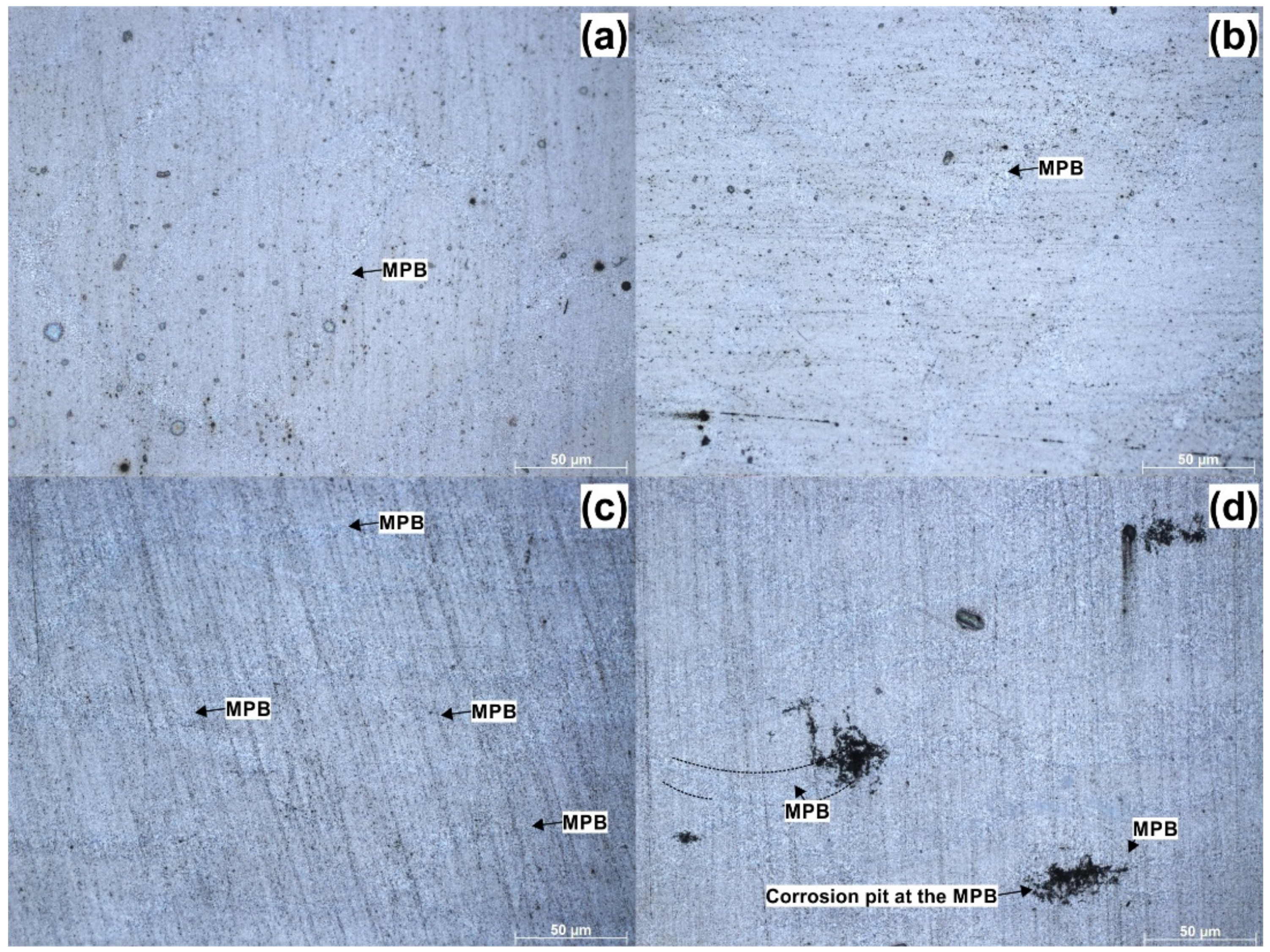
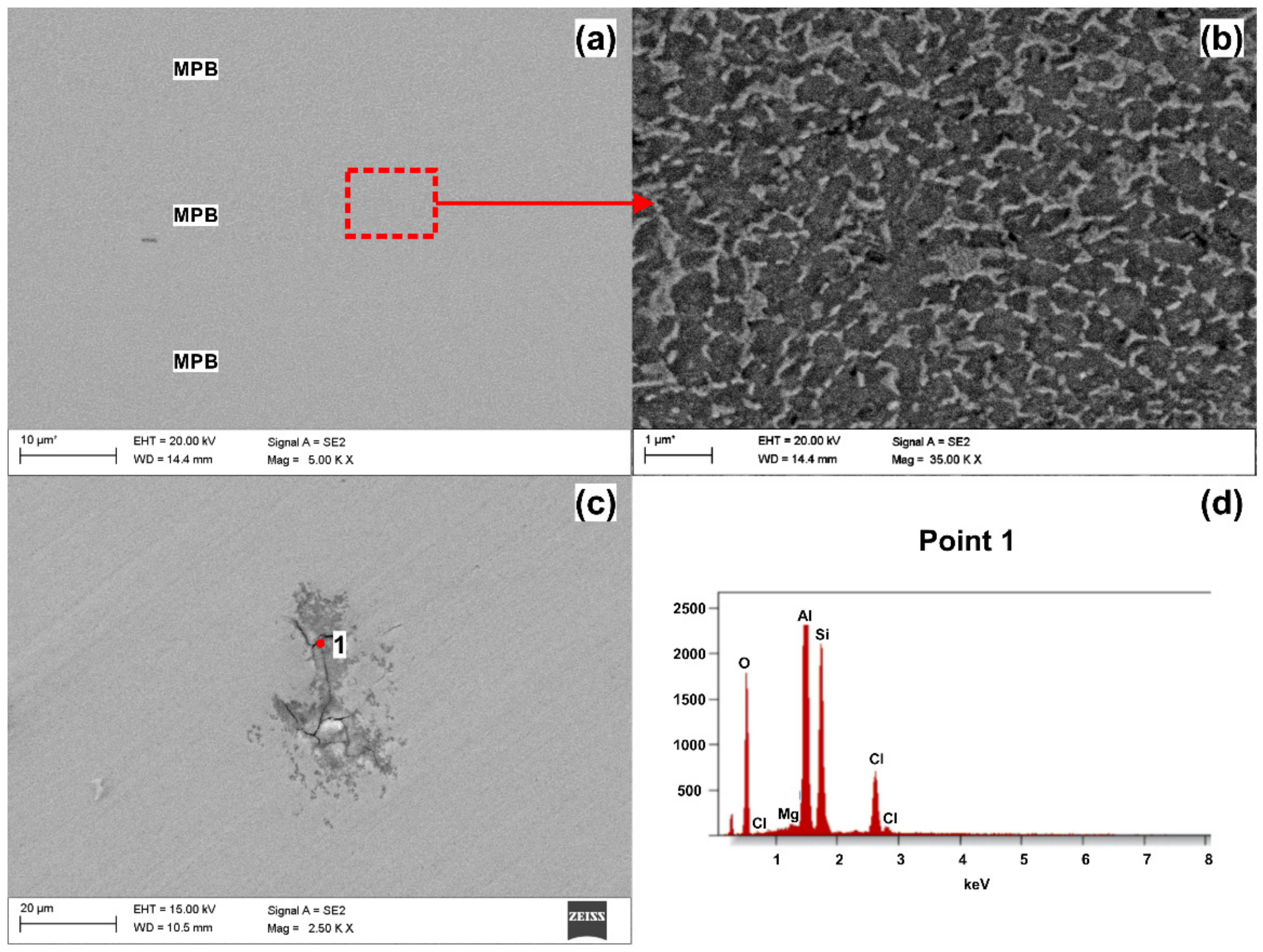
3.3. Corrosion Morphology
4. Conclusions
- The ECAP-induced microstructural modifications promoted the formation of a layered structure that comprised semi-circular patterns and melt pool boundaries, whose fraction increased with additional ECAP pressings.
- The results of the microstructural study via EBSD revealed that ECAP up to two passes led to significant grain size reduction to 1.42 μm after the first pass and 0.24 μm after the second pass.
- Different corrosion behaviors occurred between the as-built/heat-treated and ECAP processed samples. Potentiodynamic polarization test results revealed the lowest corrosion current density accompanied by higher Rp parameter for ECAP-processed samples, confirming their superior corrosion resistance.
- Microstructural examination of corroded surfaces revealed differences in corrosion attacks depending on the processing history. The as-built and heat-treated, which had microstructures composed of laser scan tracks, showed almost uniform pitting with the formation of slightly larger pits along the laser scan track boundaries (melt pool boundaries). The samples subjected to ECAP, having a microstructure composed of semi-circular patterns, showed superior corrosion resistance; however, in the sample subjected to two ECAP passes, the melt pool boundaries showed a more deeply penetrating corrosion that preferentially initiated in the areas where multiple melt-pool boundaries overlap. Nevertheless, globally, for ECAP samples, improved corrosion resistance was observed.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, Y.T.; Langdon, T.G. The fundamentals of nanostructured materials processed by severe plastic deformation. JOM 2004, 56, 58–63. [Google Scholar] [CrossRef]
- Beura, V.K.; Kale, C.; Srinivasan, S.; Williams, C.L.; Solanki, K.N. Corrosion behavior of a dynamically deformed Al–Mg alloy. Electrochim. Acta 2020, 354, 136695. [Google Scholar] [CrossRef]
- Mohd Yusuf, S.; Nie, M.; Chen, Y.; Yang, S.; Gao, N. Microstructure and corrosion performance of 316L stainless steel fabricated by Selective Laser Melting and processed through high-pressure torsion. J. Alloys Compd. 2018, 763, 360–375. [Google Scholar] [CrossRef]
- Tański, T.; Snopiński, P.; Pakieła, W.; Borek, W.; Prusik, K.; Rusz, S. Structure and properties of AlMg alloy after combination of ECAP and post-ECAP ageing. Arch. Civ. Mech. Eng. 2016, 16, 325–334. [Google Scholar]
- Reza Toroghinejad, M.; Ashrafizadeh, F.; Jamaati, R. On the use of accumulative roll bonding process to develop nanostructured aluminum alloy 5083. Mater. Sci. Eng. A 2013, 561, 145–151. [Google Scholar] [CrossRef]
- Beygelzimer, Y.; Varyukhin, V.; Synkov, S.; Orlov, D. Useful properties of twist extrusion. Mater. Sci. Eng. A 2009, 503, 14–17. [Google Scholar] [CrossRef] [Green Version]
- Snopiński, P.; Tański, T.; Gołombek, K.; Rusz, S.; Hilser, O.; Donič, T.; Nuckowski, P.M.; Benedyk, M. Strengthening of AA5754 Aluminum Alloy by DRECE Process Followed by Annealing Response Investigation. Materials 2020, 13, 301. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Zheng, R.; Xiao, W.; Li, J.; Li, G.; Peng, Q.; Ma, C. Bulk nanostructured Al-Si alloy with remarkable improvement in strength and ductility. Scr. Mater. 2021, 201, 113970. [Google Scholar] [CrossRef]
- Mungole, T.; Nadammal, N.; Dawra, K.; Kumar, P.; Kawasaki, M.; Langdon, T.G. Evolution of microhardness and microstructure in a cast Al–7% Si alloy during high-pressure torsion. J. Mater. Sci. 2013, 48, 4671–4680. [Google Scholar] [CrossRef]
- Moradi, M.; Nili-Ahmadabadi, M.; Heidarian, B. Improvement of mechanical properties of AL (A356) cast alloy processed by ECAP with different heat treatments. Int. J. Mater. Form. 2009, 2, 85. [Google Scholar] [CrossRef]
- Song, D.; Wang, G.; Zhou, Z.; Klu, E.E.; Gao, B.; Ma, A.; Wu, Y.; Sun, J.; Jiang, J.; Ma, X. Developing a high-strength Al–11Si alloy with improved ductility by combining ECAP and cryorolling. Mater. Sci. Eng. A 2020, 773, 138880. [Google Scholar] [CrossRef]
- Damavandi, E.; Nourouzi, S.; Rabiee, S.M.; Jamaati, R. Effect of ECAP on microstructure and tensile properties of A390 aluminum alloy. Trans. Nonferrous Met. Soc. China 2019, 29, 931–940. [Google Scholar] [CrossRef]
- Zhang, Z.; Hosoda, S.; Kim, I.S.; Watanabe, Y. Grain refining performance for Al and Al-Si alloy casts by addition of equal-channel angular pressed Al-5 mass% Ti alloy. Mater. Sci. Eng. A 2006, 425, 55–63. [Google Scholar] [CrossRef]
- Ma, A.; Suzuki, K.; Saito, N.; Nishida, Y.; Takagi, M.; Shigematsu, I.; Iwata, H. Impact toughness of an ingot hypereutectic Al-23 mass% Si alloy improved by rotary-die equal-channel angular pressing. Mater. Sci. Eng. A 2005, 399, 181–189. [Google Scholar] [CrossRef]
- Jiang, J.; Ma, A.; Song, D.; Yang, D.; Shi, J.; Wang, K.; Zhang, L.; Chen, J. Anticorrosion behavior of ultrafine-grained Al-26 wt% Si alloy fabricated by ECAP. J. Mater. Sci. 2012, 47, 7744–7750. [Google Scholar] [CrossRef]
- Gebril, M.A.; Omar, M.Z.; Mohamed, I.F.; Othman, N.K.; Abdelgnei, M.A.H. Corrosion Improvement and Microstructure Evaluation of SEM-Solid A356 Alloy by Ecap Process. J. Phys. Conf. Ser. 2018, 1082, 12110. [Google Scholar] [CrossRef]
- Wang, X.; Nie, M.; Wang, C.T.; Wang, S.C.; Gao, N. Microhardness and corrosion properties of hypoeutectic Al-7Si alloy processed by high-pressure torsion. Mater. Des. 2015, 83, 193–202. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Lin, C.K.; Tan, A.H.; Lin, J.C.; Lee, S.L. Effect of Spinning Deformation Processing on the Wear and Corrosion Properties of Al-7Si-0.3Mg Alloys. Mater. Manuf. Process. 2010, 25, 689–695. [Google Scholar] [CrossRef]
- Li, W.; Li, S.; Liu, J.; Zhang, A.; Zhou, Y.; Wei, Q.; Yan, C.; Shi, Y. Effect of heat treatment on AlSi10Mg alloy fabricated by selective laser melting: Microstructure evolution, mechanical properties and fracture mechanism. Mater. Sci. Eng. A 2016, 663, 116–125. [Google Scholar] [CrossRef]
- Rosenthal, I.; Stern, A.; Frage, N. Microstructure and Mechanical Properties of AlSi10Mg Parts Produced by the Laser Beam Additive Manufacturing (AM) Technology. Metallogr. Microstruct. Anal. 2014, 3, 448–453. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.Q.; Wang, W.; Attallah, M.M.; Loretto, M.H. Microstructure and strength of selectively laser melted AlSi10Mg. Acta Mater. 2016, 117, 311–320. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Li, Z.; Tan, Z.; Xiong, D.B.; Guo, Q. Stress relaxation and the cellular structure-dependence of plastic deformation in additively manufactured AlSi10Mg alloys. Int. J. Plast. 2020, 127, 102640. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, C.; Jia, D.; Wellmann, D.; Liu, W. Corrosion Behaviors of Selective Laser Melted Aluminum Alloys: A Review. Metals 2020, 10, 102. [Google Scholar] [CrossRef] [Green Version]
- Cabrini, M.; Calignano, F.; Fino, P.; Lorenzi, S.; Lorusso, M.; Manfredi, D.; Testa, C.; Pastore, T. Corrosion behavior of heat-treated AlSi10Mg manufactured by laser powder bed fusion. Materials 2018, 11, 1051. [Google Scholar] [CrossRef] [Green Version]
- Zakay, A.; Aghion, E. Effect of Post-heat Treatment on the Corrosion Behavior of AlSi10Mg Alloy Produced by Additive Manufacturing. JOM 2019, 71, 1150–1157. [Google Scholar] [CrossRef]
- Santos Macías, J.G.; Zhao, L.; Tingaud, D.; Bacroix, B.; Pyka, G.; van der Rest, C.; Ryelandt, L.; Simar, A. Hot isostatic pressing of laser powder bed fusion AlSi10Mg: Parameter identification and mechanical properties. J. Mater. Sci. 2022. [Google Scholar] [CrossRef]
- Maleki, E.; Unal, O.; Bandini, M.; Guagliano, M.; Bagherifard, S. Individual and synergistic effects of thermal and mechanical surface post-treatments on wear and corrosion behavior of laser powder bed fusion AlSi10Mg. J. Mater. Process. Technol. 2022, 302, 117479. [Google Scholar] [CrossRef]
- Mohd Yusuf, S.; Chen, Y.; Yang, S.; Gao, N. Microstructural evolution and strengthening of selective laser melted 316L stainless steel processed by high-pressure torsion. Mater. Charact. 2020, 159, 110012. [Google Scholar] [CrossRef]
- Finfrock, C.B.; Exil, A.; Carroll, J.D.; Deibler, L. Effect of Hot Isostatic Pressing and Powder Feedstock on Porosity, Microstructure, and Mechanical Properties of Selective Laser Melted AlSi10Mg. Metallogr. Microstruct. Anal. 2018, 7, 443–456. [Google Scholar] [CrossRef] [Green Version]
- Geenen, K.; Röttger, A.; Theisen, W. Corrosion behavior of 316L austenitic steel processed by selective laser melting, hot-isostatic pressing, and casting. Mater. Corros. 2017, 68, 764–775. [Google Scholar] [CrossRef]
- Lien, H.H.; Mazumder, J.; Wang, J.; Misra, A. Ultrahigh strength and plasticity in laser rapid solidified Al–Si nanoscale eutectics. Mater. Res. Lett. 2020, 8, 291–298. [Google Scholar] [CrossRef]
- Kim, D.K.; Hwang, J.H.; Kim, E.Y.; Heo, Y.U.; Woo, W.; Choi, S.H. Evaluation of the stress-strain relationship of constituent phases in AlSi10Mg alloy produced by selective laser melting using crystal plasticity FEM. J. Alloys Compd. 2017, 714, 687–697. [Google Scholar] [CrossRef]
- Snopiński, P.; Król, M.; Pagáč, M.; Petrů, J.; Hajnyš, J.; Mikuszewski, T.; Tański, T. Effects of equal channel angular pressing and heat treatments on the microstructures and mechanical properties of selective laser melted and cast AlSi10Mg alloys. Arch. Civ. Mech. Eng. 2021, 21, 92. [Google Scholar] [CrossRef]
- Snopiński, P.; Woźniak, A.; Pagáč, M. Microstructural Evolution, Hardness, and Strengthening Mechanisms in SLM AlSi10Mg Alloy Subjected to Equal-Channel Angular Pressing (ECAP). Materials 2021, 14, 7598. [Google Scholar] [CrossRef] [PubMed]
- PN-EN ISO 17475:2010; Corrosion of Metal and Alloys—Electrochemical Test Methods—Guidelines for Conducting Potentiostatic and Potentiodynamic, Polarization Measurements. ISO: Geneva, Switzerland, 2010.
- Liu, Y.J.; Liu, Z.; Jiang, Y.; Wang, G.W.; Yang, Y.; Zhang, L.C. Gradient in microstructure and mechanical property of selective laser melted AlSi10Mg. J. Alloys Compd. 2018, 735, 1414–1421. [Google Scholar] [CrossRef]
- Cheng, W.; Liu, Y.; Xiao, X.; Huang, B.; Zhou, Z.; Liu, X. Microstructure and mechanical properties of a novel (TiB2+TiC)/AlSi10Mg composite prepared by selective laser melting. Mater. Sci. Eng. A 2022, 834, 142435. [Google Scholar] [CrossRef]
- Tóth, L.S. Texture Evolution in Severe Plastic Deformation by Equal Channel Angular Extrusion. Adv. Eng. Mater. 2003, 5, 308–316. [Google Scholar] [CrossRef]
- Bahshwan, M.; Gee, M.; Nunn, J.; Myant, C.W.; Reddyhoff, T. In situ observation of anisotropic tribological contact evolution in 316L steel formed by selective laser melting. Wear 2022, 490–491, 204193. [Google Scholar] [CrossRef]
- Marola, S.; Manfredi, D.; Fiore, G.; Poletti, M.G.; Lombardi, M.; Fino, P.; Battezzati, L. A comparison of Selective Laser Melting with bulk rapid solidification of AlSi10Mg alloy. J. Alloys Compd. 2018, 742, 271–279. [Google Scholar] [CrossRef]
- Casati, R.; Coduri, M.; Checchia, S.; Vedani, M. Insight into the effect of different thermal treatment routes on the microstructure of AlSi7Mg produced by laser powder bed fusion. Mater. Charact. 2021, 172, 110881. [Google Scholar] [CrossRef]
- Immanuel, R.J.; Panigrahi, S.K. Influence of cryorolling on microstructure and mechanical properties of a cast hypoeutectic Al–Si alloy. Mater. Sci. Eng. A 2015, 640, 424–435. [Google Scholar] [CrossRef]
- Zorn, C.; Kaminski, N. Temperature-humidity-bias testing on insulated-gate bipolartransistor modules—Failure modes and acceleration due to high voltage. IET Power Electron. 2015, 8, 2329–2335. [Google Scholar] [CrossRef]
- Cabrini, M.; Lorenzi, S.; Testa, C.; Pastore, T.; Manfredi, D.; Lorusso, M.; Calignano, F.; Fino, P. Statistical approach for electrochemical evaluation of the effect of heat treatments on the corrosion resistance of AlSi10Mg alloy by laser powder bed fusion. Electrochim. Acta 2019, 305, 459–466. [Google Scholar] [CrossRef]
- Rafieazad, M.; Chatterjee, A.; Nasiri, A.M. Effects of Recycled Powder on Solidification Defects, Microstructure, and Corrosion Properties of DMLS Fabricated AlSi10Mg. JOM 2019, 71, 3241–3252. [Google Scholar] [CrossRef]
- Rafieazad, M.; Mohammadi, M.; Nasiri, A.M. On microstructure and early stage corrosion performance of heat treated direct metal laser sintered AlSi10Mg. Addit. Manuf. 2019, 28, 107–119. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Y.; Zhang, J.; Gu, X.; Qin, P.; Dai, N.; Li, X.; Kruth, J.-P.; Zhang, L.-C. Improved corrosion behavior of ultrafine-grained eutectic Al-12Si alloy produced by selective laser melting. Mater. Des. 2018, 146, 239–248. [Google Scholar] [CrossRef]
- Pezzato, L.; Dabalà, M.; Gross, S.; Brunelli, K. Effect of microstructure and porosity of AlSi10Mg alloy produced by selective laser melting on the corrosion properties of plasma electrolytic oxidation coatings. Surf. Coat. Technol. 2020, 404, 126477. [Google Scholar] [CrossRef]
- Dan, S.; Jiang, J.-h.; Lin, P.-h.; Yang, D.-h. Corrosion behavior of ultra-fine grained industrial pure Al fabricated by ECAP. Trans. Nonferrous Met. Soc. China 2009, 19, 1065–1070. [Google Scholar] [CrossRef]
- Song, D.; Ma, A.B.; Jiang, J.H.; Lin, P.H.; Shi, J. Improving corrosion resistance of pure Al through ECAP. Corros. Eng. Sci. Technol. 2011, 46, 505–512. [Google Scholar] [CrossRef]
- Revilla, R.I.; Rojas, Y.; De Graeve, I. On the Impact of Si Content and Porosity Artifacts on the Anodizing Behavior of Additive Manufactured Al-Si Alloys. J. Electrochem. Soc. 2019, 166, C530–C537. [Google Scholar] [CrossRef]
- Ji, Y.; Dong, C.; Kong, D.; Li, X. Design materials based on simulation results of silicon induced segregation at AlSi10Mg interface fabricated by selective laser melting. J. Mater. Sci. Technol. 2020, 46, 145–155. [Google Scholar] [CrossRef]
- Birbilis, N.; Ralston, K.D.; Virtanen, S.; Fraser, H.L.; Davies, C.H.J. Grain character influences on corrosion of ECAPed pure magnesium. Corros. Eng. Sci. Technol. 2010, 45, 224–230. [Google Scholar] [CrossRef]
- Zhang, H.; Gu, D.; Dai, D.; Ma, C.; Li, Y.; Cao, M.; Li, S. Influence of heat treatment on corrosion behavior of rare earth element Sc modified Al-Mg alloy processed by selective laser melting. Appl. Surf. Sci. 2020, 509, 145330. [Google Scholar] [CrossRef]
- Revilla, R.I.; Liang, J.; Godet, S.; De Graeve, I. Local Corrosion Behavior of Additive Manufactured AlSiMg Alloy Assessed by SEM and SKPFM. J. Electrochem. Soc. 2017, 164, C27. [Google Scholar] [CrossRef]
- Cabrini, M.; Lorenzi, S.; Pastore, T.; Pellegrini, S.; Manfredi, D.; Fino, P.; Biamino, S.; Badini, C. Evaluation of corrosion resistance of Al-10Si-Mg alloy obtained by means of Direct Metal Laser Sintering. J. Mater. Process. Technol. 2016, 231, 326–335. [Google Scholar] [CrossRef]
- Leon, A.; Aghion, E. Effect of surface roughness on corrosion fatigue performance of AlSi10Mg alloy produced by Selective Laser Melting (SLM). Mater. Charact. 2017, 131, 188–194. [Google Scholar] [CrossRef]
- Revilla, R.I.; Verkens, D.; Couturiaux, G.; Malet, L.; Thijs, L.; Godet, S.; De Graeve, I. Galvanostatic Anodizing of Additive Manufactured Al-Si10-Mg Alloy. J. Electrochem. Soc. 2017, 164, C1027. [Google Scholar] [CrossRef]



| Si | Mg | Fe | Ti | Zn | Mn | Ni | Co | Al |
|---|---|---|---|---|---|---|---|---|
| 9–11 | 0.25–0.45 | <0.25 | <0.15 | <0.10 | <0.10 | <0.05 | <0.05 | Balance |
| Label | Condition | Heat Treatment/ECAP Procedure |
|---|---|---|
| S0 | As-built | none |
| S2 | Heat-treated | Annealing at 300 °C for 8 min |
| S2-1 | Heat-treated and ECAP processed | Annealing at 300 °C for 8 min + 1 ECAP pass |
| S2-2 | Heat-treated and ECAP processed | Annealing at 300 °C for 8 min + 2 ECAP passes |
| Sample | Average Grain Size, [µm] | Fraction of Low-Angle Grain Boundaries, % | Fraction of High-Angle Grain Boundaries, % |
|---|---|---|---|
| S2 (XY plane) | 3.24 | 8.45 | 91.55 |
| S2 (XZ plane) | 10.11 | 26.71 | 74.29 |
| S2-1 | 1.42 | 29.24 | 70.76 |
| S2-2 | 0.25 | 19.32 | 80.68 |
| Sample | Ecorr, [mV] | icorr, [µA/cm2] | Rp, [kΩ∙cm2] |
|---|---|---|---|
| S0 | −663 ± 11 | 7.3 ± 0.1 | 1.1 ± 0.1 |
| S2 | −693 ± 21 | 18.5 ± 0.4 | 0.9 ± 0.1 |
| S2-1 | −550 ± 17 | 0.3 ± 0.05 | 60.6 ± 2 |
| S2-2 | −440 ± 14 | 0.2 ± 0.04 | 75.5 ± 4 |
| Sample | Rs, [Ω] | Rct, [kΩ·cm2] | CPEdl, [mV] | Zw | |
|---|---|---|---|---|---|
| Y, [Ω·cm−ms−n] | n2 | ||||
| S0 | 4 | 0.8 | 3.6 × 10−6 | 0.84 | 28 |
| S2 | 4 | 0.7 | 4.2 × 10−6 | 1 | 10 |
| S2-1 | 4 | 22.4 | 9.2× 10−5 | 0.80 | 75 |
| S2-2 | 4 | 24.9 | 9.7 × 10−5 | 0.84 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Snopiński, P.; Woźniak, A.; Łukowiec, D.; Matus, K.; Tański, T.; Rusz, S.; Hilšer, O. Evolution of Microstructure, Texture and Corrosion Properties of Additively Manufactured AlSi10Mg Alloy Subjected to Equal Channel Angular Pressing (ECAP). Symmetry 2022, 14, 674. https://doi.org/10.3390/sym14040674
Snopiński P, Woźniak A, Łukowiec D, Matus K, Tański T, Rusz S, Hilšer O. Evolution of Microstructure, Texture and Corrosion Properties of Additively Manufactured AlSi10Mg Alloy Subjected to Equal Channel Angular Pressing (ECAP). Symmetry. 2022; 14(4):674. https://doi.org/10.3390/sym14040674
Chicago/Turabian StyleSnopiński, Przemysław, Anna Woźniak, Dariusz Łukowiec, Krzysztof Matus, Tomasz Tański, Stanislav Rusz, and Ondřej Hilšer. 2022. "Evolution of Microstructure, Texture and Corrosion Properties of Additively Manufactured AlSi10Mg Alloy Subjected to Equal Channel Angular Pressing (ECAP)" Symmetry 14, no. 4: 674. https://doi.org/10.3390/sym14040674
APA StyleSnopiński, P., Woźniak, A., Łukowiec, D., Matus, K., Tański, T., Rusz, S., & Hilšer, O. (2022). Evolution of Microstructure, Texture and Corrosion Properties of Additively Manufactured AlSi10Mg Alloy Subjected to Equal Channel Angular Pressing (ECAP). Symmetry, 14(4), 674. https://doi.org/10.3390/sym14040674








