Perfusion, Stance and Plantar Pressure Asymmetries on the Human Foot in the Absence of Disease—A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental
- -
- Phase I, baseline register for 5 min in the orthostatic position;
- -
- Phase II, register during continuous bipodal squatting for 2 min (25 to 30 complete movements per minute);
- -
- Phase III, recovery register for 5 min in the standing position.
- -
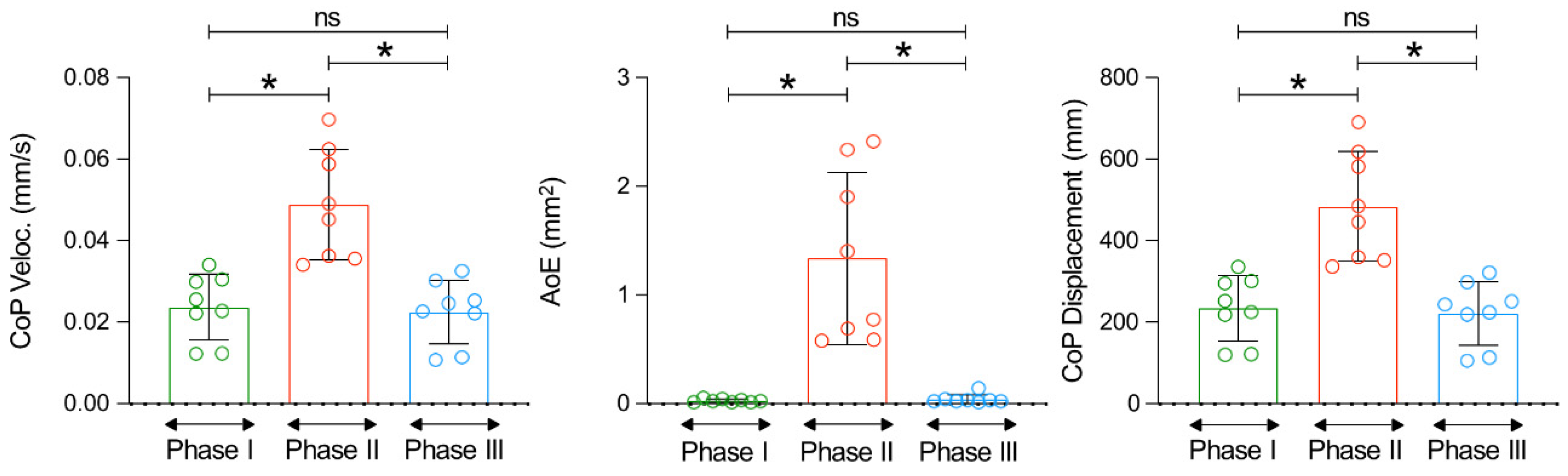
- the displacement oscillation of the centre of pressure (CoP) defining the total length of the path marked by the CoP, expressed in mm;
- -
- the average velocity of the CoP, referring to the average speed at which the CoP moves. This parameter indicates the speed of changes in the CoP location, which reflects the speed of postural reactions on standing, expressed in mm/s.
- -
- the area of the ellipse (AoE) representing the size of the area marked by the CoP. The area of the ellipse includes 95% of the CoP measurement points, and this parameter allows us to evaluate the size of the area of CoP movement (bipodal) on the support surface expressed in mm2.
2.3. Statistical Analysis
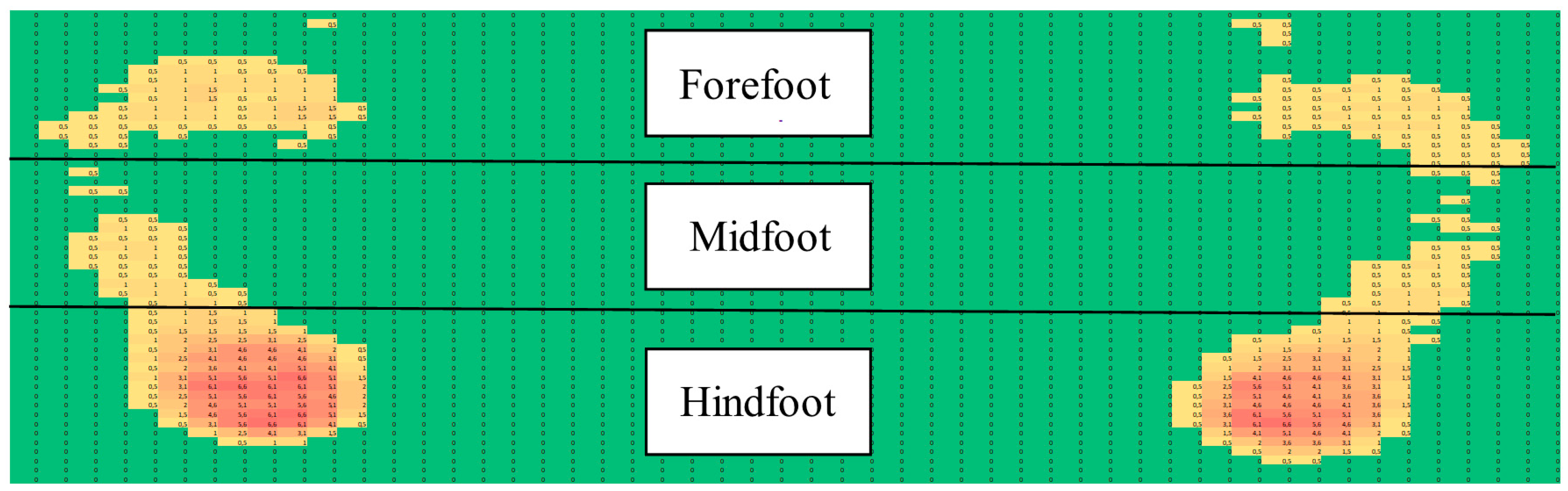
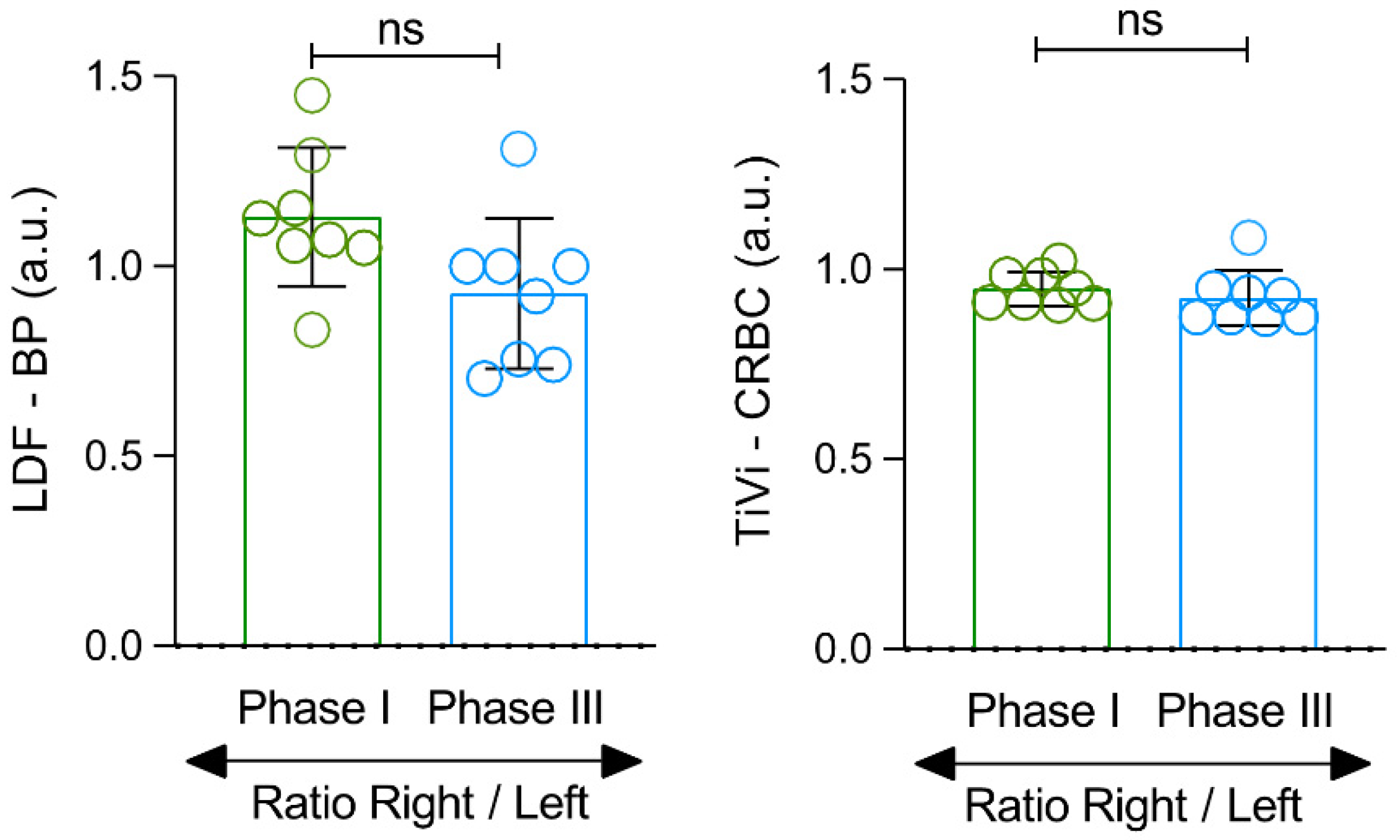
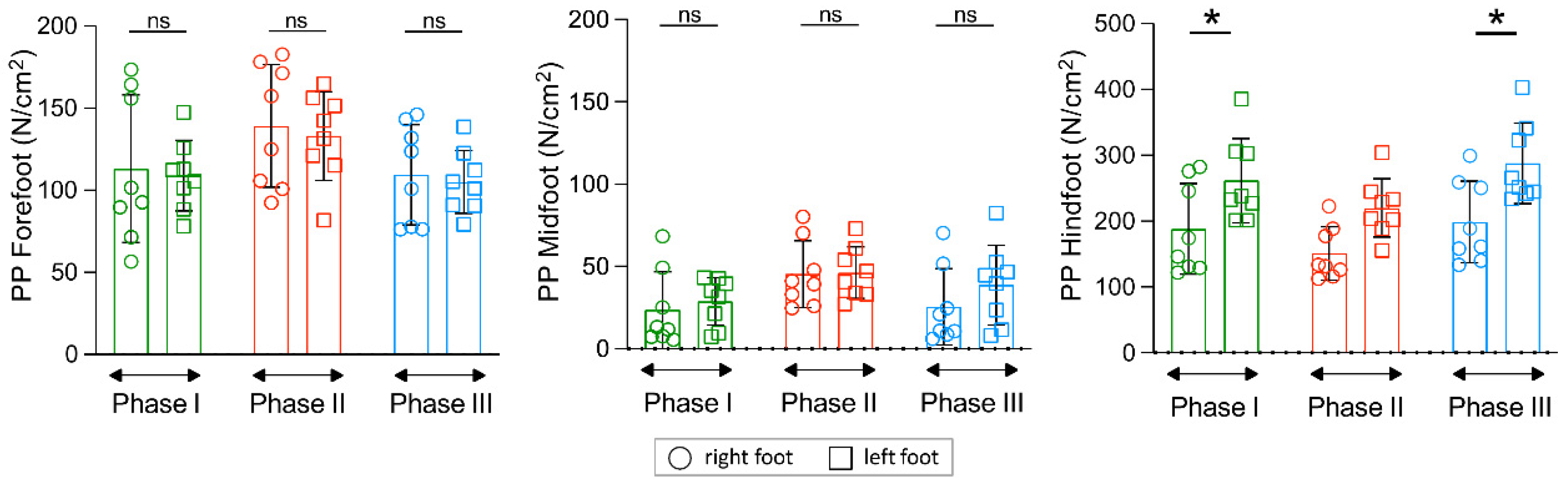
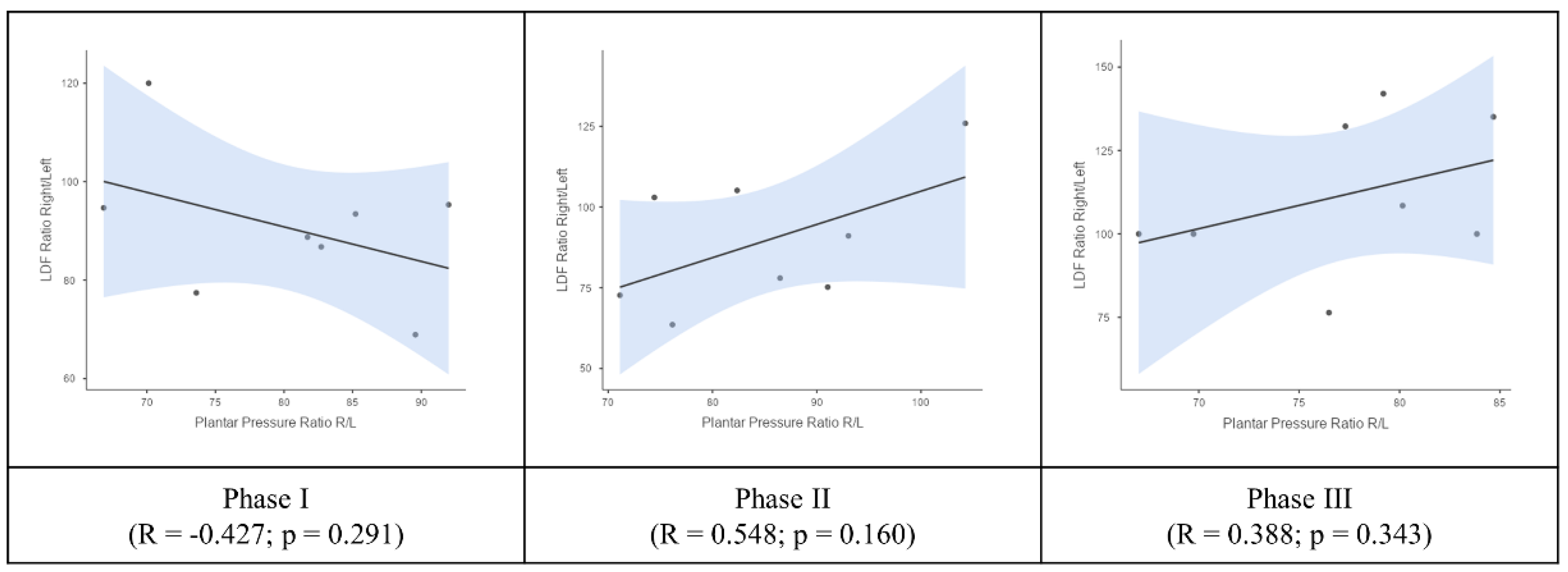
3. Results
4. Discussion
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bouley, J. Claudication Intermittent des Membres Posterieurs, determinee par L’obliteration des Arteres Femorales. Rec. Med. Vet. Ec. Alfort. 1831, 8, 517–527. [Google Scholar]
- Zusmanovich, F.; Elizarova, S. Perfusion pressure dynamics in lower extremities at rest and after exercise. Fiziol. Cheloveka 2002, 28, 133. (In Russian) [Google Scholar] [PubMed]
- Siegel, M.E.; Siemsen, J.K. A new noninvasive approach to peripheral vascular disease: Thallium-201 leg scans. AJR 1978, 131, 827–830. [Google Scholar] [CrossRef][Green Version]
- Seder, J.S.; Botvinick, E.H.; Rahimtoola, S.H.; Goldstone, J.; Price, D.C. Detecting and localizing peripheral arterial disease: Assessment of 201Tl scintigraphy. AJR 1981, 137, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Collins, R.; Burch, J.; Cranny, G.; Aguiar-Ibáñez, R.; Craig, D.; Wright, K.; Berry, E.; Gough, M.; Kleijnen, J.; Westwood, M. Duplex ultrasonography, magnetic resonance angiography, and computed tomography angiography for diagnosis and assessment of symptomatic, lower limb peripheral arterial disease: Systematic review. BMJ Clin. Res. 2007, 334, 1257. [Google Scholar] [CrossRef]
- Mayrovitz, H.N.; Larsen, P.B. Pulsatile blood flow asymmetry in paired human legs. Clin. Physiol. 1996, 16, 495–505. [Google Scholar] [CrossRef]
- Nuno, S.; Atalaia, T.; Gregório, J.; Florindo, M.; Granja, T.; Abrantes, J.; Rodrigues, L.M. Influence of posture on the foot perfusion in the upright position. Physiol. 2021 Proc. Physiol. Soc. 2021, 48, PC013. Available online: https://www.physoc.org/abstracts/influence-of-posture-on-the-foot-perfusion-in-the-upright-position/ (accessed on 21 December 2021).
- Gregório, J.; Florindo, M.; Nuno, S.; Rodrigues, L.M. Insights into human healthy. Physiol. 2021 Proc. Physiol. Soc. 2021, 48, OC04. Available online: https://www.physoc.org/abstracts/insights-into-human-healthy-lower-limb-perfusion-asymmetry-during-rest/ (accessed on 21 December 2021).
- Rocha, C.; Silva, H.; Ferreira, H.; Rodrigues, L.M. Evidence of a Physiological Perfusion Balance Between Human Limb Pairs. Europhysiology 2018. Abstract Book 2018. Available online: https://www.europhysiology2018.org/sites/default/files/files/Europhysiology%202018_ABSTRACTS_ONLINE.pdf (accessed on 21 December 2021).
- Rodrigues, L.M.; Rocha, C.; Ferreira, H.T.; Silva, H.M. Lower limb massage in humans increases local perfusion and impacts systemic hemodynamics. J. Appl. Physiol. 2020, 128, 1217–1226. [Google Scholar] [CrossRef]
- Gregório, J.; Silva, H.; Rocha, C.; Rodrigues, L.M. Perfusion is sex related but response to massage evokes the same hemodynamic adaptation in both sexes—Results from an exploratory factor analysis. Proceed Physioma 2019—1st Int Meeting Portuguese Physiological Society. Biomed. Biopharm. Res. 2019, 16, 31. [Google Scholar] [CrossRef]
- Rodrigues, L.M.; Rocha, C.G.; Florindo, M.E.; Gregório, J. Lower Limb Perfusion Asymmetries in Humans at Rest and Following Activity—A Collective View. Symmetry 2021, 13, 2348. [Google Scholar] [CrossRef]
- Keeley, D.W.; Plummer, H.A.; Oliver, G.D. Predicting Asymmetrical Lower Extremity Strength Deficits in College-Aged Men and Women Using Common Horizontal and Vertical Power Field Tests: A Possible Screening Mechanism. J. Strength Cond. Res. 2011, 25, 1632–1637. [Google Scholar] [CrossRef]
- Bishop, C.; Read, P.; Chavda, S.; Turner, A. Asymmetries of the Lower Limb: The Calculation Conundrum in Strength Training and Conditioning. Strength Cond. J. 2016, 38, 27–32. [Google Scholar] [CrossRef]
- Lanshammar, K.; Ribom, E.L. Differences in muscle strength in dominant and non-dominant leg in females aged 20–39 years—A population-based study. Phys. Ther. Sport 2011, 12, 76–79. [Google Scholar] [CrossRef]
- Manevska, N.; Gjorceva, D.P.; Ahmeti, I.; Todorovska, L.; Stojanoski, S.; Kocovska, M.Z. Tissue-Muscle Perfusion Scintigraphy of the Lower Limbs in a Patient with Type 2 Diabetes Mellitus and Peripheral Arterial Disease. Mol. Imaging Radionucl. Ther. 2016, 25, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.R.; Sanfilippo, J.L.; Binkley, N.; Heiderscheit, B.C. Lean mass asymmetry influences force and power asymmetry during jumping in collegiate athletes. J. Strength Cond. 2014, 28, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.R.; Ratamess, N.A.; Klatt, M.; Faigenbaum, A.D.; Kang, J. Do bilateral power deficits influence direction-specific movement patterns? Res. Sports Med. 2007, 15, 125–132. [Google Scholar] [CrossRef]
- Maloney, S.J. The Relationship Between Asymmetry and Athletic Performance: A Critical Review. J. Strength Cond. 2019, 33, 2579–2593. [Google Scholar] [CrossRef]
- Heil, J.; Loffing, F.; Büsch, D. The Influence of Exercise-Induced Fatigue on Inter-Limb Asymmetries: A Systematic Review. Sports Med. 2020, 6, 39. [Google Scholar] [CrossRef]
- Kadoguchi, T.; Horiuchi, M.; Kinugawa, S.; Okita, K. Heterogeneity in the vasodilatory function of individual extremities. Vascular 2020, 28, 87–95. [Google Scholar] [CrossRef]
- Jungmann, P.M.; Pfirrmann, C.; Federau, C. Characterization of lower limb muscle activation patterns during walking and running with Intravoxel Incoherent Motion (IVIM) MR perfusion imaging. Magn. Reson. Imaging 2019, 63, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Wafai, L.; Zayegh, A.; Woulfe, J.; Aziz, S.M.; Begg, R. Identification of Foot Pathologies Based on Plantar Pressure Asymmetry. Sensors 2015, 15, 20392–20408. [Google Scholar] [CrossRef] [PubMed]
- Menz, H.B.; Morris, M.E. Clinical determinants of plantar forces and pressures during walking in older people. Gait Posture 2006, 24, 229–236. [Google Scholar] [CrossRef]
- Erdoğanoğlu, Y.; Sayaca, Ç.; Çalık, M.; Noyan, C.O.; Çetin, A.; Yertutanol, D.K.; Taşcılar, L.N.; Kaya, D. Evaluation of Plantar Foot Sensation, Balance, Physical Performance, and Fear of Movement in Substance Use Disorders. J. Am. Podiat. Med. Assoc. 2020, 110, 5. [Google Scholar] [CrossRef] [PubMed]
- de Cock, A.; Vanrenterghem, J.; Willems, T.; Witvrouw, E.; de Clercq, D. The trajectory of the centre of pressure during barefoot running as a potential measure for foot function. Gait Posture 2008, 27, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Florindo, M.; Silva, H.; Rodrigues, L.M. Impact of the isometric contraction of the calf on the local microcirculation. Biomed. Biopharm. Res. 2017, 14, 179–186. [Google Scholar] [CrossRef]
- Nuno, S.; Florindo, M.; Silva, H.; Rodrigues, L.M. Studying the impact of different body positioning, squatting, and unipodal flexion on perfusion in the lower limb—An exploratory approach complemented with optical spectroscopy (TiVi). Biomed. Biopharm. Res. 2020, 17, 1–10. [Google Scholar] [CrossRef]
- Florindo, M.; Nuno, S.L.; Rodrigues, L.M. Lower limb dynamic activity significantly reduces foot skin perfusion—Exploring data with different optical sensors in age-grouped healthy adults. Skin Pharmacol. Physiol. 2022, 35, 13–22. [Google Scholar] [CrossRef]
- Aboyans, V.; Criqui, M.H.; Abraham, P.; Allison, M.A.; Creager, M.A.; Diehm, C.; Fowkes, F.G.R.; Hiatt, W.R.; Jönsson, B.; Lacroix, P.; et al. Measurement and interpretation of the ankle-brachial index: A scientific statement from the American Heart Association. Circulation 2012, 126, 2890–2909. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Rocha, C.; Silva, H.; Ferreira, H.; Rodrigues, L.M. About the in vivo discriminatory capacity of photoplethysmography versus laser Doppler flowmetry. Biomed. Biopharm. Res. 2017, 14, 37–44. [Google Scholar] [CrossRef]
- Srivaratharajah, K.; Abramson, B.L. Women and Peripheral Arterial Disease: A Review of Sex Differences in Epidemiology, Clinical Manifestations, and Outcomes. Can. J. Cardiol. 2018, 34, 356–361. [Google Scholar] [CrossRef]
- Xu, X.; Wang, B.; Ren, C.; Hu, J.; Greenberg, D.A.; Chen, T.; Xie, L.; Jin, K. Age-related Impairment of Vascular Structure and Functions. Aging Dis. 2017, 8, 590–610. [Google Scholar] [CrossRef] [PubMed]
- Abiri, B.; Vafa, M. Dietary Restriction, Cardiovascular Aging and Age-Related Cardiovascular Diseases: A Review of the Evidence. Adv. Exp. Med. Biol. 2019, 1178, 113–127. [Google Scholar] [CrossRef]
- Bergstrand, S.; Lindberg, L.G.; Ek, A.C.; Lindén, M.; Lindgren, M. Blood flow measurements at different depths using photoplethysmography and laser Doppler techniques. Ski. Res. Technol. ISBS ISDIS ISSI 2009, 15, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, G.E.; Tenland, T.; Oberg, P.A. Evaluation of a laser Doppler flowmeter for measurement of tissue blood flow. IEEE Trans. Bio-Med. Eng. 1980, 27, 597–604. [Google Scholar] [CrossRef]
- Nilsson, G.E.; Salerud, E.G.; Stromberg, N.O.T.; Wardell, K. Laser Doppler perfusion monitoring and imaging. In Biomedical Photonics Handbook; Vo-Dinh, I.T., Ed.; CRC Press: Boca Raton, FL, USA, 2003; pp. 1–24. [Google Scholar]
- Rodrigues, L.M.; Rocha, C.; Ferreira, H.; Silva, H. Different lasers reveal different skin microcirculatory flowmotion—Data from the wavelet transform analysis of human hindlimb perfusion. Sci. Rep. 2019, 9, 16951. [Google Scholar] [CrossRef]
- O’Doherty, J.; Henricson, J.; Anderson, C.; Leahy, M.J.; Nilsson, G.E.; Sjöberg, F. Sub-epidermal imaging using polarized light spectroscopy for assessment of skin microcirculation. Skin Res. Technol. 2007, 13, 472–484. [Google Scholar] [CrossRef]
- Raffetto, J.D.; Khalil, R.A. Mechanisms of varicose vein formation: Valve dysfunction and wall dilation. Phlebology 2008, 23, 85–98. [Google Scholar] [CrossRef]
- Antle, D.M.; Cormier, L.; Findlay, M.; Miller, L.L.; Côté, J.N. Lower limb blood flow and mean arterial pressure during standing and seated work: Implications for workplace posture recommendations. Prev. Med. Rep. 2018, 10, 117–122. [Google Scholar] [CrossRef]
- Thorne, C.S.; Bartolo, E.; Gatt, A.; Formosa, C. The Impact of Peripheral Artery Disease (PAD) on Lower Limb Kinematics in Type 2 Diabetes Mellitus. Rev. Diabet. Stud. RDS 2021, 17, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Myers, S.A.; Applequist, B.C.; Huisinga, J.M.; Pipinos, I.I.; Johanning, J.M. Gait kinematics and kinetics are affected more by peripheral arterial disease than by age. JRRD J. Rehabil. Res. Dev. 2016, 53, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Boulton, A.J.; Hardisty, C.A.; Betts, R.P.; Franks, C.I.; Worth, R.C.; Ward, J.D.; Duckworth, T. Dynamic foot pressure and other studies as diagnostic and management aids in diabetic neuropathy. Diabetes Care 1983, 6, 26–33. [Google Scholar] [CrossRef] [PubMed]


| MEN | WOMEN | p-Value | |
|---|---|---|---|
| N (%) | 4 (50) | 4 (50) | _ |
| Smokers (%) | 0 (100) | 0 (100) | _ |
| Age, years (Q1–Q3) | 28.8(20–32) | 21.8(21–22) | 0.098 |
| Body mass, kg (Q1–Q3) | 74.5 (68.0–85.0) | 61.5 (58.0–68.0) | 0.201 |
| Height, m (Q1–Q3) | 1.8 (1.7–1.8) | 1.6 (1.6–1.7) | <0.001 * |
| BMI, kg/m2 (Q1–Q3) | 23.9 (22.9–24.9) | 22.8 (22.1–24.9) | 0.546 |
| SYSTP, mmHg (Q1–Q3) | 122.0 (113.7–129.0) | 120.9 (111.7–135.3) | 0.670 |
| DIASP, mmHg (Q1–Q3) | 82.4 (74.7–88.0) | 78.1 (75.0–78.7) | 0.424 |
| ABI (Q1–Q3) | 1.0 (1.0–1.1) | 1.0 (1.0–1.1) | 0.062 |
| PR, bpm (Q1–Q3) | 68.8 (61.5–77.3) | 65.5 (59.0–69.5) | 0.088 |
| SpO2 (%), bpm (Q1–Q3) | 98.5 (98–99) | 98.3 (98–99) | 0.951 |
| Phase 1 | Phase 2 | Phase 3 | ||||
|---|---|---|---|---|---|---|
| Right Foot | Left Foot | Right Foot | Left Foot | Right Foot | Left Foot | |
| LDF_BPU (AU) ⸸ | 6.0 ± 1.3 | 6.7 ± 1.4 | 12.1 ± 4.3 | 13.9 ± 4.8 | 7.7 ± 2.1 | 6.9 ± 1.3 |
| p-value | 0.007 * | 0.571 | 0.015 * | |||
| PSp_CRBC (AU) ¥ | 217.2 ± 14.8 | 206.0 ± 18.0 | 227.2 ± 12.0 | 222.9 ± 14.8 | 217.1 ± 13.7 | 200.0 ± 11.0 |
| p-value | 0.094 | 0.691 | 0.125 | |||
| PR (p-value 🢗) ¥ | 63.1 ± 9.8 | 73.6 ± 8.9 (0.002) * | 65.5 ± 9.0 (0.020) * | |||
| sAP (p-value 🢗) ¥ | 123.0 ± 7.0 | 130.5 ± 5.8 (0.004) * | 124.9 ± 7.8 (0.495) | |||
| dAP (p-value 🢗) ¥ | 65.0 ± 7.1 | 70.3 ± 4.8 (0.049) * | 67.3 ± 7.0 (0.079) | |||
| LDF ⸸ | ||
| right foot Phase I 6.0 ± 1.3 | right foot Phase II 12.1 ± 4.3 | <0.001 * |
| right foot Phase I 6.0 ± 1.3 | right foot Phase III 7.7 ± 2.1 | <0.001 * |
| left foot Phase I 6.7 ± 1.4 | left foot Phase II 13.9 ± 4.8 | <0.001 * |
| left foot Phase I 6.7 ± 1.4 | left foot Phase III 6.9 ± 1.3 | 0.207 |
| PSp ¥ | ||
| right foot Phase I 217.2 ± 14.8 | right foot Phase II 227.2 ± 12.0 | 0.080 |
| right foot Phase I 217.2 ± 14.8 | right foot Phase III 217.1 ± 13.7 | 1.000 |
| left foot Phase I 206.0 ± 18.0 | left foot Phase II 222.9 ± 14.8 | 0.016 * |
| left foot Phase I 206.0 ± 18.0 | left foot Phase III 200.0 ± 11.0 | 0.613 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, L.M.; Nuno, S.L.; Granja, T.; Florindo, M.E.; Gregório, J.; Atalaia, T. Perfusion, Stance and Plantar Pressure Asymmetries on the Human Foot in the Absence of Disease—A Pilot Study. Symmetry 2022, 14, 441. https://doi.org/10.3390/sym14030441
Rodrigues LM, Nuno SL, Granja T, Florindo ME, Gregório J, Atalaia T. Perfusion, Stance and Plantar Pressure Asymmetries on the Human Foot in the Absence of Disease—A Pilot Study. Symmetry. 2022; 14(3):441. https://doi.org/10.3390/sym14030441
Chicago/Turabian StyleRodrigues, Luis Monteiro, Sérgio Loureiro Nuno, Tiago Granja, Margarida Esteves Florindo, João Gregório, and Tiago Atalaia. 2022. "Perfusion, Stance and Plantar Pressure Asymmetries on the Human Foot in the Absence of Disease—A Pilot Study" Symmetry 14, no. 3: 441. https://doi.org/10.3390/sym14030441
APA StyleRodrigues, L. M., Nuno, S. L., Granja, T., Florindo, M. E., Gregório, J., & Atalaia, T. (2022). Perfusion, Stance and Plantar Pressure Asymmetries on the Human Foot in the Absence of Disease—A Pilot Study. Symmetry, 14(3), 441. https://doi.org/10.3390/sym14030441









