Movement Coordination during Functional Single-Leg Squat Tests in Healthy, Recreational Athletes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subject Information and Ethics
2.2. Data Collection
2.3. Data Processing
2.4. Statistical Analysis
3. Results
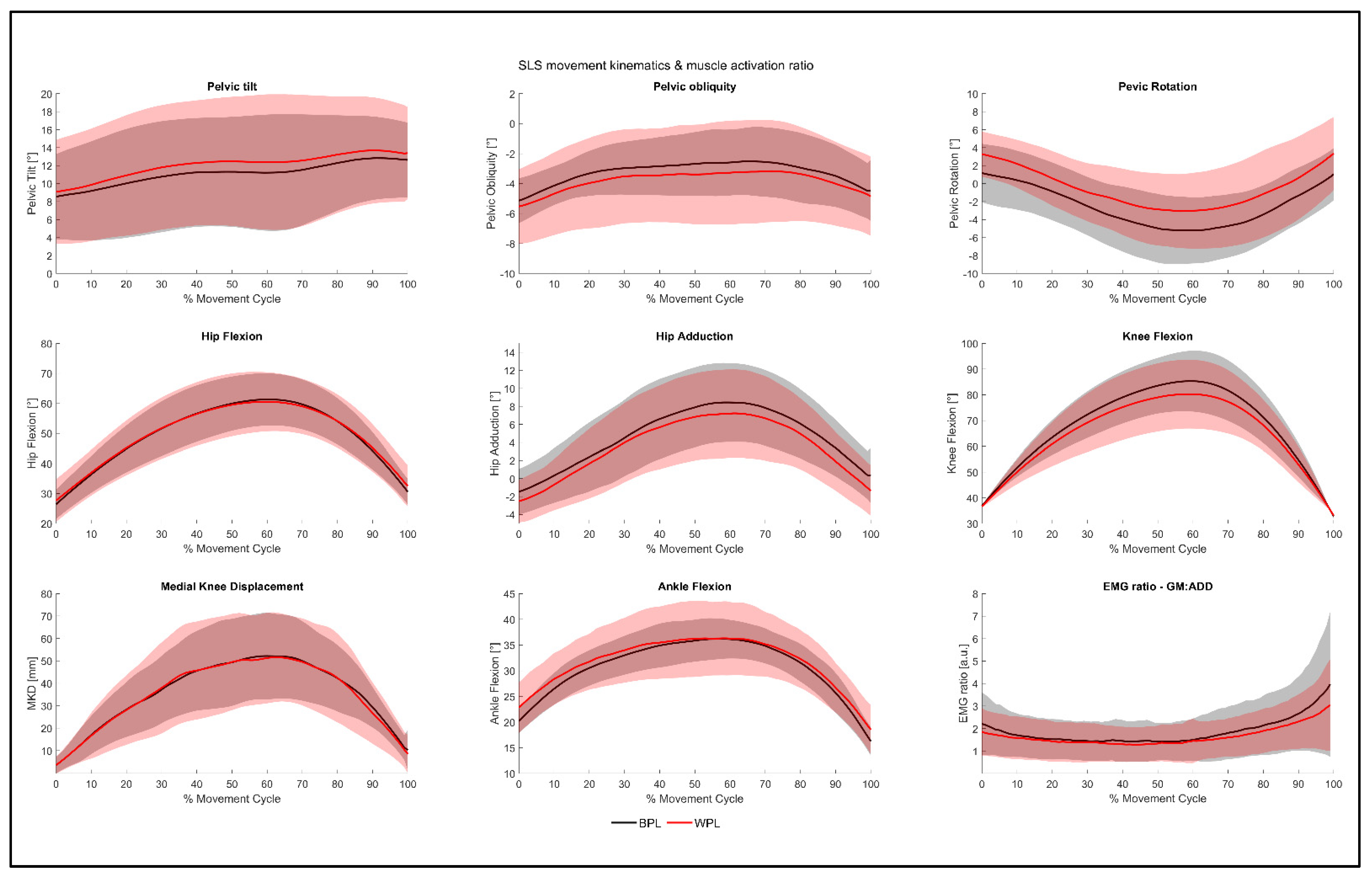
3.1. Inter-Limb Symmetry
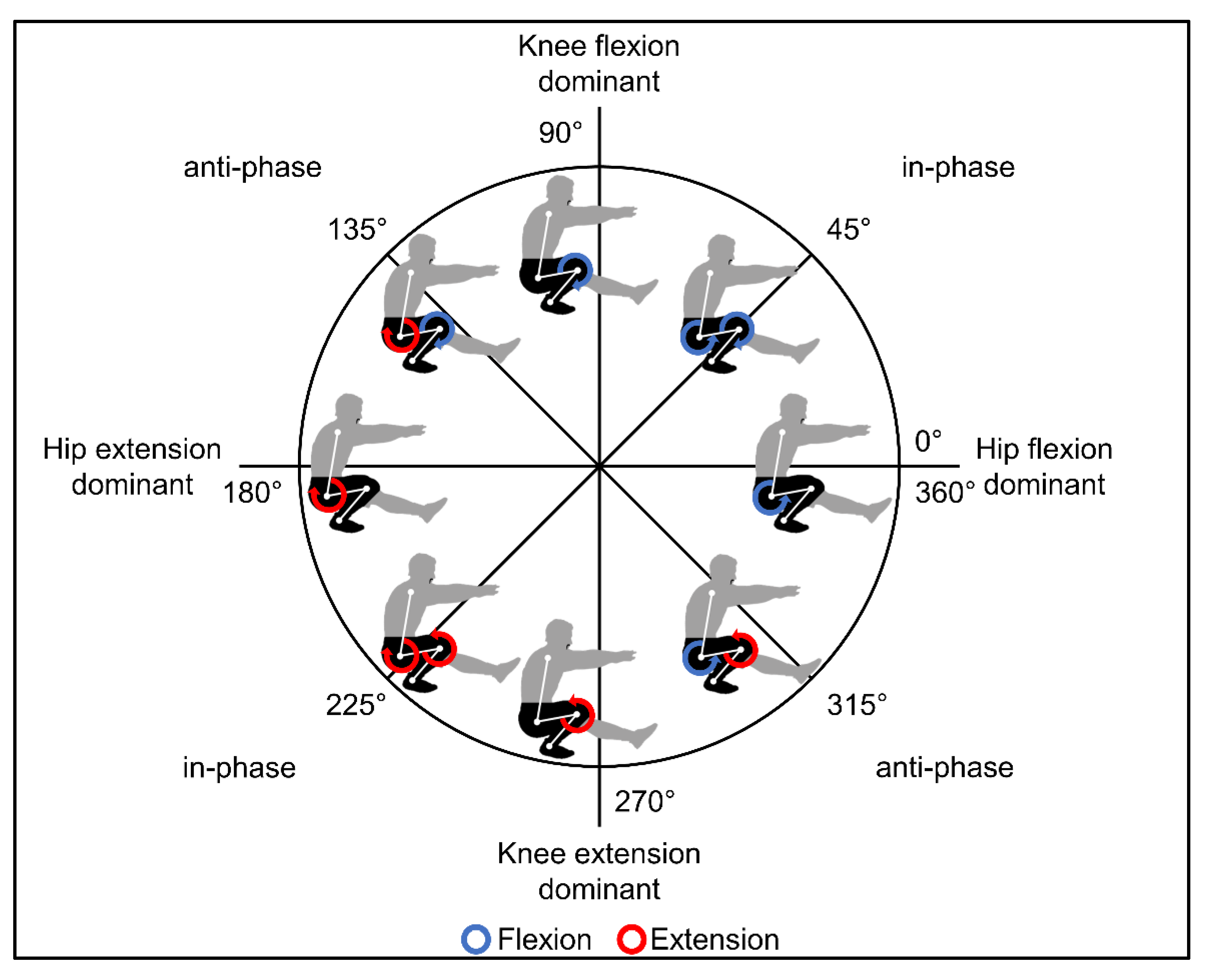
3.2. Movement Coordination
4. Discussion
4.1. Inter-Limb Symmetry, Movement Kinematics, and Hip Muscle Activity
4.2. Movement Coordination
4.3. Clinical Relevance
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davies, G.J.; McCarty, E.; Provencher, M.; Manske, R.C. ACL Return to Sport Guidelines and Criteria. Curr. Rev. Musculoskelet. Med. 2017, 10, 307–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minthorn, L.M.; Fayson, S.D.; Stobierski, L.M.; Welch, C.E.; Anderson, B.E. The Functional Movement Screen’s Ability to Detect Changes in Movement Patterns After a Training Intervention. J. Sport Rehabil. 2015, 24, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Fox, A.S.; Bonacci, J.; McLean, S.G.; Spittle, M.; Saunders, N. A Systematic Evaluation of Field-Based Screening Methods for the Assessment of Anterior Cruciate Ligament (ACL) Injury Risk. Sports Med. Auckl. N. Z. 2016, 46, 715–735. [Google Scholar] [CrossRef] [PubMed]
- Batty, L.M.; Feller, J.A.; Hartwig, T.; Devitt, B.M.; Webster, K.E. Single-Leg Squat Performance and Its Relationship to Extensor Mechanism Strength After Anterior Cruciate Ligament Reconstruction. Am. J. Sports Med. 2019, 47, 3423–3428. [Google Scholar] [CrossRef]
- Crossley, K.M.; Zhang, W.-J.; Schache, A.G.; Bryant, A.; Cowan, S.M. Performance on the Single-Leg Squat Task Indicates Hip Abductor Muscle Function. Am. J. Sports Med. 2011, 39, 866–873. [Google Scholar] [CrossRef]
- Begalle, R.L.; Distefano, L.J.; Blackburn, T.; Padua, D.A. Quadriceps and Hamstrings Coactivation during Common Therapeutic Exercises. J. Athl. Train. 2012, 47, 396–405. [Google Scholar] [CrossRef] [Green Version]
- Warner, M.B.; Wilson, D.A.; Herrington, L.; Dixon, S.; Power, C.; Jones, R.; Heller, M.O.; Carden, P.; Lewis, C.L. A Systematic Review of the Discriminating Biomechanical Parameters during the Single Leg Squat. Phys. Sport 2019, 36, 78–91. [Google Scholar] [CrossRef]
- Zeller, B.L.; McCrory, J.L.; Kibler, W.B.; Uhl, T.L. Differences in Kinematics and Electromyographic Activity between Men and Women during the Single-Legged Squat. Am. J. Sports Med. 2003, 31, 449–456. [Google Scholar] [CrossRef]
- Gianola, S.; Castellini, G.; Stucovitz, E.; Nardo, A.; Banfi, G. Single Leg Squat Performance in Physically and Non-Physically Active Individuals: A Cross-Sectional Study. BMC 2017, 18, 299. [Google Scholar] [CrossRef]
- Petersen, W.; Rembitzki, I.; Liebau, C. Patellofemoral Pain in Athletes. Open Access J. Sports Med. 2017, 8, 143–154. [Google Scholar] [CrossRef] [Green Version]
- Wilczyński, B.; Zorena, K.; Ślęzak, D. Dynamic Knee Valgus in Single-Leg Movement Tasks. Potentially Modifiable Factors and Exercise Training Options. A Literature Review. Int. J. Environ. Res. Public Health 2020, 17, 8208. [Google Scholar] [CrossRef] [PubMed]
- Petersen, W.; Ellermann, A.; Gösele-Koppenburg, A.; Best, R.; Rembitzki, I.V.; Brüggemann, G.-P.; Liebau, C. Patellofemoral Pain Syndrome. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 2264–2274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza, R.B.; Draper, C.E.; Fredericson, M.; Powers, C.M. Femur Rotation and Patellofemoral Joint Kinematics: A Weight-Bearing Magnetic Resonance Imaging Analysis. J. Orthop. Sport Phys. 2010, 40, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Hewett, T.E.; Myer, G.D.; Ford, K.R.; Heidt, R.S., Jr.; Colosimo, A.J.; McLean, S.G.; Van Den Bogert, A.J.; Paterno, M.V.; Succop, P. Biomechanical Measures of Neuromuscular Control and Valgus Loading of the Knee Predict Anterior Cruciate Ligament Injury Risk in Female Athletes: A Prospective Study. Am. J. Sports Med. 2005, 33, 492–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koga, H.; Nakamae, A.; Shima, Y.; Iwasa, J.; Myklebust, G.; Engebretsen, L.; Bahr, R.; Krosshaug, T. Mechanisms for Noncontact Anterior Cruciate Ligament Injuries. Am. J. Sports Med. 2010, 38, 2218–2225. [Google Scholar] [CrossRef] [PubMed]
- Carroll, L.A.; Kivlan, B.R.; Martin, R.L.; Phelps, A.L.; Carcia, C.R. The Single Leg Squat Test: A “Top-Down” or “Bottom-Up” Functional Performance Test? Int. J. Sports Phys. 2021, 16, 360–370. [Google Scholar] [CrossRef]
- Ageberg, E.; Bennell, K.L.; Hunt, M.A.; Simic, M.; Roos, E.M.; Creaby, M.W. Validity and Inter-Rater Reliability of Medio-Lateral Knee Motion Observed during a Single-Limb Mini Squat. BMC Musculoskelet. 2010, 11, 265. [Google Scholar] [CrossRef] [Green Version]
- Ellenberger, L.; Oberle, F.; Lorenzetti, S.; Frey, W.O.; Snedeker, J.G.; Spörri, J. Dynamic Knee Valgus in Competitive Alpine Skiers: Observation from Youth to Elite and Influence of Biological Maturation. Scand. J. Med. Sci. Sports 2020, 30, 1212–1220. [Google Scholar] [CrossRef]
- Hewett, T.E.; Ford, K.R.; Myer, G.D. Anterior Cruciate Ligament Injuries in Female Athletes. Am. J. Sports Med. 2006, 34, 490–498. [Google Scholar] [CrossRef]
- Steidl-Müller, L.; Hildebrandt, C.; Müller, E.; Fink, C.; Raschner, C. Limb Symmetry Index in Competitive Alpine Ski Racers: Reference Values and Injury Risk Identification According to Age-Related Performance Levels. J. Sport Health Sci. 2018, 7, 405–415. [Google Scholar] [CrossRef]
- Exell, T.A.; Irwin, G.; Gittoes, M.J.R.; Kerwin, D.G. Implications of Intra-Limb Variability on Asymmetry Analyses. J. Sport Sci. 2012, 30, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Bishop, C. Interlimb Asymmetries: Are Thresholds a Usable Concept? Strength Cond. J. 2020, 43, 32–36. [Google Scholar] [CrossRef]
- Bishop, C.; Turner, A.; Read, P. Effects of Inter-Limb Asymmetries on Physical and Sports Performance: A Systematic Review. J. Sport Sci. 2018, 36, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Hamill, J.; Palmer, C.; Emmerik, R.E.A.V. Coordinative Variability and Overuse Injury. Sports Med. Arthrosc. Rehabil. Ther. Technol. 2012, 4, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weir, G.; van Emmerik, R.; Jewell, C.; Hamill, J. Coordination and Variability during Anticipated and Unanticipated Sidestepping. Gait Posture 2019, 67, 1–8. [Google Scholar] [CrossRef]
- Weeks, B.K.; Carty, C.P.; Horan, S.A. Kinematic Predictors of Single-Leg Squat Performance: A Comparison of Experienced Physiotherapists and Student Physiotherapists. BMC Musculoskelet. 2012, 13, 207. [Google Scholar] [CrossRef] [Green Version]
- Tegner, Y.; Lysholm, J. Rating Systems in the Evaluation of Knee Ligament Injuries. Clin. Orthop. Relat. Res. 1985, 198, 42–49. [Google Scholar] [CrossRef]
- Gould, D.; Kelly, D.; Goldstone, L.; Gammon, J. Examining the Validity of Pressure Ulcer Risk Assessment Scales: Developing and Using Illustrated Patient Simulations to Collect the Data POINT: Visual Analogue Scale. J. Clin. Nurs. 2001, 10, 697–706. [Google Scholar] [CrossRef]
- Association, W.M. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [Green Version]
- Horan, S.A.; Watson, S.L.; Carty, C.P.; Sartori, M.; Weeks, B.K. Lower-Limb Kinematics of Single-Leg Squat Performance in Young Adults. Physiother. Can. 2014, 66, 228–233. [Google Scholar] [CrossRef] [Green Version]
- Davis, R.B.; Õunpuu, S.; Tyburski, D.; Gage, J.R. A Gait Analysis Data Collection and Reduction Technique. Hum. Mov. Sci. 1991, 10, 575–587. [Google Scholar] [CrossRef]
- Kadaba, M.; Ramakrishnan, H.; Wootten, M. Measurement of Lower Extremity Kinematics during Level Walking. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 1990, 8, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of Recommendations for SEMG Sensors and Sensor Placement Procedures. J. Electromyogr. Kines 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Krosshaug, T.; Steffen, K.; Kristianslund, E.; Nilstad, A.; Mok, K.-M.; Myklebust, G.; Andersen, T.E.; Holme, I.; Engebretsen, L.; Bahr, R. The Vertical Drop Jump Is a Poor Screening Test for ACL Injuries in Female Elite Soccer and Handball Players. Am. J. Sports Med. 2016, 44, 874–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, R.; Emmerik, R.V.; Hamill, J. Quantifying Rearfoot–Forefoot Coordination in Human Walking. J. Biomech. 2008, 41, 3101–3105. [Google Scholar] [CrossRef]
- Needham, R.A.; Naemi, R.; Chockalingam, N. A New Coordination Pattern Classification to Assess Gait Kinematics When Utilising a Modified Vector Coding Technique. J. Biomech. 2015, 48, 3506–3511. [Google Scholar] [CrossRef] [PubMed]
- Luca, C.J.D. The Use of Surface Electromyography in Biomechanics. J. Appl. Biomech. 1997, 13, 135–163. [Google Scholar] [CrossRef] [Green Version]
- Merletti, R.; Cerone, G.L. Tutorial. Surface EMG Detection, Conditioning and Pre-Processing: Best Practices. J. Electromyogr. Kines 2020, 54, 102440. [Google Scholar] [CrossRef]
- Pataky, T.C.; Vanrenterghem, J.; Robinson, M.A. Zero- vs. One-Dimensional, Parametric vs. Non-Parametric, and Confidence Interval vs. Hypothesis Testing Procedures in One-Dimensional Biomechanical Trajectory Analysis. J. Biomech. 2015, 48, 1277–1285. [Google Scholar] [CrossRef] [Green Version]
- Bohannon, R.W. Reference Values for Extremity Muscle Strength Obtained by Hand-Held Dynamometry from Adults Aged 20 to 79 Years. Arch. Phys. Med. Rehabil. 1997, 78, 26–32. [Google Scholar] [CrossRef]
- Douma, R.K.; Soer, R.; Krijnen, W.P.; Reneman, M.; Schans, C.P. van der Reference Values for Isometric Muscle Force among Workers for the Netherlands: A Comparison of Reference Values. BMC Sports Sci. Med. Rehabil. 2014, 6, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadeghi, H.; Allard, P.; Prince, F.; Labelle, H. Symmetry and Limb Dominance in Able-Bodied Gait: A Review. Gait Posture 2000, 12, 34–45. [Google Scholar] [CrossRef]
- Hall, M.P.; Paik, R.S.; Ware, A.J.; Mohr, K.J.; Limpisvasti, O. Neuromuscular Evaluation with Single-Leg Squat Test at 6 Months After Anterior Cruciate Ligament Reconstruction. Orthop. J. Sports Med. 2015, 3, 2325967115575900. [Google Scholar] [CrossRef] [Green Version]
- Lubahn, A.J.; Kernozek, T.W.; Tyson, T.L.; Merkitch, K.W.; Reutemann, P.; Chestnut, J.M. Hip Muscle Activation and Knee Frontal Plane Motion during Weight Bearing Therapeutic Exercises. Int. J. Sports Phys. 2011, 6, 92–103. [Google Scholar]
- Khuu, A.; Foch, E.; Lewis, C.L. Not All Single Leg Squats Are Equal: A Biomechanical Comparison of Three Variations. Int. J. Sports Phys. 2016, 11, 201–211. [Google Scholar]
- Khuu, A.; Lewis, C.L. Position of the Non-Stance Leg during the Single Leg Squat Affects Females and Males Differently. Hum. Mov. Sci. 2019, 67, 102506. [Google Scholar] [CrossRef] [PubMed]
- Willson, J.D.; Ireland, M.L.; Davis, I. Core Strength and Lower Extremity Alignment during Single Leg Squats. Med. Sci. Sports Exerc. 2006, 38, 945–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauntel, T.C.; Begalle, R.L.; Cram, T.R.; Frank, B.S.; Hirth, C.J.; Blackburn, T.; Padua, D.A. The Effects of Lower Extremity Muscle Activation and Passive Range of Motion on Single Leg Squat Performance. J. Strength Cond. Res. 2013, 27, 1813–1823. [Google Scholar] [CrossRef] [Green Version]
- Duchateau, J.; Enoka, R.M. Neural Control of Lengthening Contractions. J. Exp. Biol. 2016, 219, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Mehl, J.; Diermeier, T.; Herbst, E.; Imhoff, A.B.; Stoffels, T.; Zantop, T.; Petersen, W.; Achtnich, A. Evidence-Based Concepts for Prevention of Knee and ACL Injuries. 2017 Guidelines of the Ligament Committee of the German Knee Society (DKG). Arch. Orthop. Trauma Surg. 2017, 138, 51–61. [Google Scholar] [CrossRef]
- Scott, S.H. Inconvenient Truths about Neural Processing in Primary Motor Cortex. J. Physiol. 2008, 586, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, N. The Co-Ordination and Regulation of Movements; Pergamon Press Ltd.: London, UK, 1967. [Google Scholar]
- King, A.; Hannan, K. Segment Coordination Variability During Double Leg Bodyweight Squats at Different Tempos. Int. J. Sports Med. 2019, 40, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Mok, K.-M.; Kristianslund, E.K.; Krosshaug, T. The Effect of Thigh Marker Placement on Knee Valgus Angles in Vertical Drop Jumps and Sidestep Cutting. J. Appl. Biomech. 2015, 31, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Piazza, S.J.; Cavanagh, P.R. Measurement of the Screw-Home Motion of the Knee Is Sensitive to Errors in Axis Alignment. J. Biomech. 2000, 33, 1029–1034. [Google Scholar] [CrossRef]
- Cappello, A.; Cappozzo, A.; Palombara, P.F.L.; Lucchetti, L.; Leardini, A. Multiple Anatomical Landmark Calibration for Optimal Bone Pose Estimation. Hum. Mov. Sci. 1997, 16, 259–274. [Google Scholar] [CrossRef]
- Ehrig, R.M.; Taylor, W.R.; Duda, G.N.; Heller, M.O. A Survey of Formal Methods for Determining Functional Joint Axes. J. Biomech. 2007, 40, 2150–2157. [Google Scholar] [CrossRef]
- Nilstad, A.; Petushek, E.; Mok, K.-M.; Bahr, R.; Krosshaug, T. Kiss Goodbye to the ‘Kissing Knees’: No Association between Frontal Plane Inward Knee Motion and Risk of Future Non-Contact ACL Injury in Elite Female Athletes. Sport Biomech. 2021, 1–15. [Google Scholar] [CrossRef]
- Hamill, J.; Haddad, J.M.; McDermott, W.J. Issues in Quantifying Variability from a Dynamical Systems Perspective. J. Appl. Biomech. 2000, 16, 407–418. [Google Scholar] [CrossRef] [Green Version]

| Subject Characteristics | Male (n = 8) | Female (n = 9) | |
|---|---|---|---|
| Age [years] | 23 ± 2 | 26 ± 4 | |
| Mass [kg] | 79 ± 5 | 68 ± 7 | |
| Height [cm] | 181 ± 11 | 170 ± 6 | |
| BMI [kg/cm2] | 24.0 ± 2.2 | 23.4 ± 2.5 | |
| MVC [N] | Hip abductors (BPL) | 474 ± 93 | 369 ± 32 |
| Hip abductors (WPL) | 473 ± 88 | 373 ± 40 | |
| Hip adductors (BPL) | 385 ± 56 | 320 ± 66 | |
| Hip adductors (WPL) | 404 ± 43 | 309 ± 52 | |
| VAS | Before activity | 0 ± 0 | 0 ± 0 |
| During activity | 0 ± 0 | 0 ± 1 | |
| After activity | 0 ± 0 | 0 ± 0 | |
| KOOS | Overall | 100 ± 0 | 98 ± 3 |
| Pain | 100 ± 0 | 99 ± 2 | |
| Symptoms | 99 ± 1 | 97 ± 6 | |
| Activities of daily living | 100 ± 0 | 100 ± 0 | |
| Sporting activities | 100 ± 0 | 100 ± 0 | |
| Quality of daily living | 100 ± 0 | 97 ± 8 | |
| Tegner Activity Scale | 6 ± 1 | 7 ± 2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ksoll, K.S.H.; Cotic, M.; Schmalzl, K.; Beitzel, K.; Achtnich, A.; Imhoff, A.; Schwirtz, A.; Kreuzpointner, F.; Seiberl, W. Movement Coordination during Functional Single-Leg Squat Tests in Healthy, Recreational Athletes. Symmetry 2022, 14, 388. https://doi.org/10.3390/sym14020388
Ksoll KSH, Cotic M, Schmalzl K, Beitzel K, Achtnich A, Imhoff A, Schwirtz A, Kreuzpointner F, Seiberl W. Movement Coordination during Functional Single-Leg Squat Tests in Healthy, Recreational Athletes. Symmetry. 2022; 14(2):388. https://doi.org/10.3390/sym14020388
Chicago/Turabian StyleKsoll, Korbinian Sebastian Hermann, Matthias Cotic, Kathrin Schmalzl, Knut Beitzel, Andrea Achtnich, Andreas Imhoff, Ansgar Schwirtz, Florian Kreuzpointner, and Wolfgang Seiberl. 2022. "Movement Coordination during Functional Single-Leg Squat Tests in Healthy, Recreational Athletes" Symmetry 14, no. 2: 388. https://doi.org/10.3390/sym14020388
APA StyleKsoll, K. S. H., Cotic, M., Schmalzl, K., Beitzel, K., Achtnich, A., Imhoff, A., Schwirtz, A., Kreuzpointner, F., & Seiberl, W. (2022). Movement Coordination during Functional Single-Leg Squat Tests in Healthy, Recreational Athletes. Symmetry, 14(2), 388. https://doi.org/10.3390/sym14020388






