Abstract
The Wieland–Miescher ketone, Hajos–Parrish–Eder–Sauer–Wiechert ketone, and their analogues are bicyclic diketones essential as building blocks for the synthesis of several natural and bioactive molecules. For this reason, since 1971, when Hajos and Parrish and Eder, Sauer, and Wiechert reported the stereoselective synthesis of these compounds promoted by L-proline, numerous methodologies and organocatalysts have been studied over the years with the aim of identifying increasingly efficient asymmetrical syntheses of these bicyclic ketones. This review will outline the methodological and stereochemical features of the organocatalytic stereoselective synthesis of these bicyclic scaffolds based on the different organocatalysts employed from 1971 until today. Particular emphasis will be given to the structural features of the catalysts and to the reaction conditions.
1. Introduction
The synthesis of complex molecular architectures and building blocks towards natural products has been one of the main challenges for synthetic organic chemists. Moreover, a precise control over the generation of new stereocenters in these intermediates and final compounds is essential to target biological and pharmaceutical functions, which these molecules often possess. In the realm of organic chemistry, asymmetric organocatalysis has been widely studied in the last 21 years, and it is now considered the third pillar of asymmetric synthesis [1,2,3,4,5]. Since the seminal works of B. List [6] and D. W. Macmillan [7] in 2000, this field showed an exponential growth which culminated with the assignment of the 2021 Nobel Prize in chemistry to the two chemists. Organocatalytic procedures offer several advantages:
- ▪
- the stability of these catalysts in the presence of air and moisture compared to metal-complexes allows low-demanding reaction conditions;
- ▪
- organocatalysts are usually cheaper than enzymes or metal-based catalysts and readily available;
- ▪
- different activation modes, often simultaneous, are possible with respect to substrates, reagents, and reactions.
Within this context, organocatalysis has been employed in many strategies to achieve enantioenriched chiral compounds with potential bioactivity and optically pure building blocks for the preparation of natural products [8,9,10,11,12,13,14].
Prior to the works of List and MacMillan, which elucidated in detail the organocatalyst activation modes and proved the generality of organocatalysis, other reports on the use of small organic molecules were published in the last century. Surely the most important example was reported independently by Hajos and Parrish [15,16] and by Eder, Sauer, and Wiechert [17,18] in 1971. In these works, the authors exploited the properties of the natural amino acid L-proline to promote a stereoselective intramolecular aldol reaction for the formation of important bicyclic scaffolds: namely the Wieland–Miescher (WM) ketone 1 and the Hajos–Parrish–Eder–Sauer–Wiechert (HPESW) ketone 2.
The decalin structure 1 was firstly synthesised in 1950 by the chemists P. Wieland and K. Miescher in its racemic mixture [19], and together with its homologues bicyclo[4.3.0]nonane (HPESW, 2) and bicyclo[5.4.0]undecane (Swaminathan, 3), they represent essential building blocks in natural products synthesis (Figure 1).
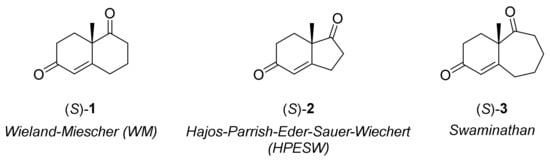
Figure 1.
Bicyclic scaffolds.
In fact, these are common scaffolds present in many biologically active compounds and natural molecules, and therefore they represent crucial building blocks in medicinal and pharmaceutical chemistry (Figure 2) [20,21,22,23]. Therefore, the possibility to achieve the stereoselective synthesis of these compounds using organocatalysis, with practically simple synthetic methods as demonstrated in 1971, represents a key point for the total synthesis of various natural products.
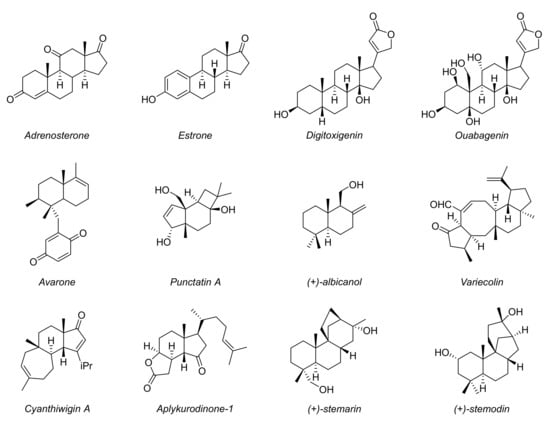
Figure 2.
Examples of natural products and bioactive compounds with bicyclic scaffolds.
Over the years, various types of strategies targeting late-stage functionalization of these intermediates have been reported, and they have been previously reviewed [20,21].
Nevertheless, a strong effort has been addressed to the synthesis of these bicyclic building blocks using amino acids and other organocatalysts to promote this annulation reaction. This review will outline the methodological and stereochemical features of the organocatalytic stereoselective synthesis of these bicyclic scaffolds reported from 1971 to the present. In Figure 3 the structures of the organocatalysts discussed in this review are depicted. Particular emphasis will be given to the structural features of the catalysts and to the reaction conditions. The discussion will be divided on the basis of the final product structure: bicyclo[4.3.0]nonane, decalin derivatives, and bicyclo[5.4.0]undecanes.
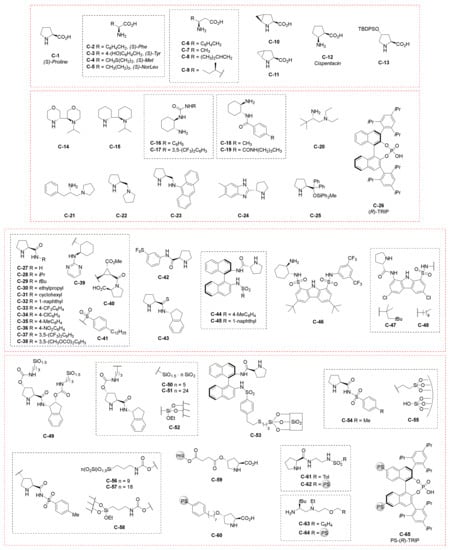
Figure 3.
Organocatalysts employed in the analysed literature in this review. PS = polystyrene, PEG = polyethylenglycol.
2. Bicyclo[4.3.0]Nonane and Decalin Derivatives
2.1. WM, HPESW and Their Analogues
In this section, the organocatalysts employed for the synthesis of WM 1, HPESW 2, and their analogues reported in Figure 4 and Figure 5 are discussed.
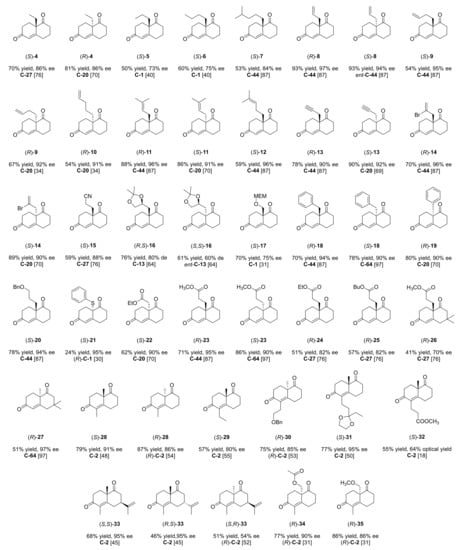
Figure 4.
WM analogues reported by the literature analysed in this review. For each compound, the best yield and ee were indicated, along with the organocatalyst used and the corresponding literature reference. MEM = CH3OCH2CH2OCH2.
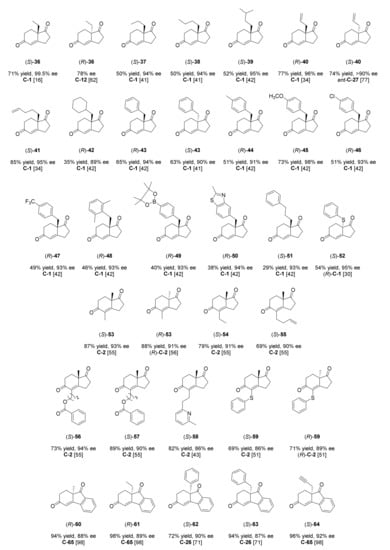
Figure 5.
HPESW analogues reported by the literature analysed in this review. For each compound, the best yield and ee were indicated, along with the organocatalyst used and the corresponding literature reference.
2.1.1. Covalent and H-Bonding Organocatalysts
L- and D-Proline
The first enantioselective intramolecular aldol reaction for the synthesis of decalin (S)-1 and bicyclo[4.3.0]nonane (S)-2 was reported in 1971 independently by Hajos and Parrish, working at Hoffmann La Roche [15,16], and Eder, Sauer, and Weichert, researchers at Schering AG [17,18]. In both works the reaction involved the opportune triketone (65 or 66), using L-Pro (C-1) as organocatalyst (Scheme 1). Under the conditions of Hajos and Parrish (i.e., L-Pro 3 mol%, DMF, room temperature, 72 h for (S)-1, 20 h for (S)-2) the reaction furnished the respective ketol products (67 or 68), which upon dehydration catalysed by p-toluensulfonic acid (PTSA) in refluxing benzene gave the condensation products (S)-1 (52% yield, 75% ee) or (S)-2 (99% yield, 88% ee). On the other hand, the contemporary presence of 47 mol% of L-Pro and 1N HClO4 in CH3CN and heating at 80 °C, as reported by Eder, Sauer, and Weichert, led directly to enone (S)-1 (83% yield, 71% ee) or (S)-2 (87% yield, 84% ee). In both cases the compounds were obtained with good chemical and optical yields, but these procedures were more efficient in terms of enantioselectivity for (S)-2 than for (S)-1. Moreover, utilizing D-Pro as an organocatalyst, the (R)-2 product was obtained, but with low yield or moderate enantioselectivity (35% of optically pure (R)-2 after crystallization [15] or 75% in 67% ee [18]). Hajos and Parrish also successfully applied their procedure (with a higher amount of L-Pro, 30 mol%) for the synthesis of (S)-36, a HPESW analogue with angular ethyl group, which was obtained with high yield (71%) and optical purity (99.5% ee).
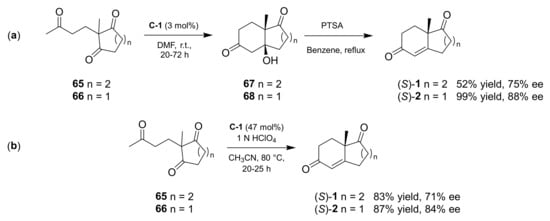
Scheme 1.
Asymmetric organocatalytic intramolecular aldol reaction towards bicyclic dicarbonyl compounds. (a) Hajos-Parrish procedure; (b) Eder-Sauer-Weichert procedure.
As regards the mechanism of the so-called Hajos–Parrish–Eder–Sauer–Weichert (HPESW) reaction catalysed by L-Pro, it has long been debated, and different alternative models have been proposed over the years [24]. However, the Houk and List model, based on a single proline enamine mechanism (Scheme 2), corroborated by both computational and experimental evidence, is currently widely accepted [25,26,27,28,29]. Based on this mechanism, proline condenses with the exocyclic carbonyl of triketone 66 to form the enamine 69; via the transition state 70, in a chair-like conformation, the enamine 69 adds to the carbonyl to form a new carbon-carbon bond, accompanied by concerted proton transfer from the carboxylic acid group to the forming alkoxide. The generated iminium 71 then undergoes hydrolysis to produce the ketol 68 and regenerate the free proline. The stereocontrol of this reaction is governed by the energy differences between the two possible chair-like transition states. The anti-transition state (70), which leads to the S,S major product, results the favoured one. Indeed, in the syn-transition state, intramolecular hydrogen bonding forces the iminium double bond out of planarity, destabilizing it; moreover, in the anti-transition state a lower +δNCH-Oδ- distance allows a major degree of electrostatic stabilization.
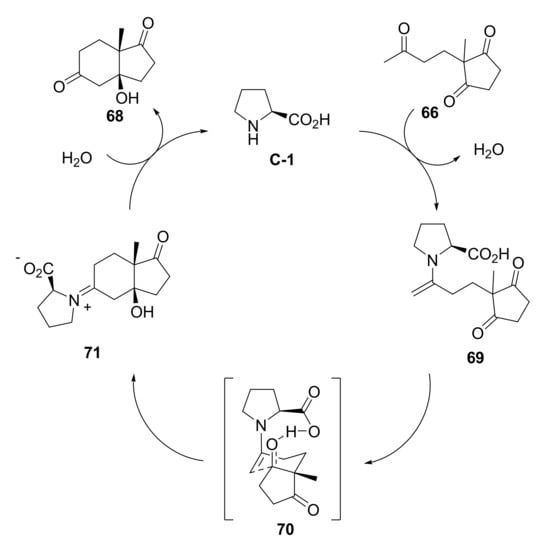
Scheme 2.
Proposed general catalytic cycle of the L-Pro mediated HPESW reaction. Adapted from [24].
Subsequently, the organocatalysts L- and D-Pro were largely employed for the synthesis of 1 and 2 and their analogues by numerous research groups, applying various changes to the original methodologies.
In 1989, Watt et al. applied the procedure described by Hajos and Parrish, with minor modifications, employing 5 or 10 mol% of D-Pro to give respectively (S)-21 (WM analogue) or (S)-52 (HPESW analogue). Both these derivatives, having an angular phenylthio group, were obtained in high enantiomeric excess (≥95%), but in low overall chemical yield (considering both steps, 54% for (S)-52, 24% for (S)-21) [30].
Uda et al. proposed a procedure for the synthesis of (S)-17, a WM analogue bearing an angular protected hydroxymethyl group, using 1 eq of L-Pro in DMSO at room temperature for 24 h to produce it with good yield and selectivity (70% yield, 75% ee) [31].
Subsequently, for the synthesis of (R)-1, in 1990 Harada and co-workers applied D-Pro (10 mol%) in DMSO at room temperature for 6 days, and this procedure led to the desired product in 82% chemical yield and 69% optical purity [32].
Similarly, in 1996 Hanselmann and Benn utilized a stoichiometric amount of L-Pro in DMSO at room temperature overnight to obtain (R)-8, a WM analogue bearing an angular allyl group, in 66% chemical yield and 80% ee [33].
More recently, in 2017 Christmann and co-workers applied 30 mol% of L-Pro in DMSO at room temperature for 17 h, followed by the dehydration step by HClO4 at 90 °C, for efficient synthesis of HPESW analogues (R)-40 (77% yield, 96% ee) and (S)-41 (85% yield, 95% ee) [34].
In 2001, Swaminathan et al. proposed a solvent-free protocol, clearly interesting for its ecofriendly aspect, for the synthesis of 1 and 2 and their analogues [35]. They tested 5–7 mol% of L- or D-Pro in neat conditions at room temperature for 70–140 h, to directly produce 1 and analogues, or followed by dehydration step by PTSA in refluxing benzene, to produce 2 and analogues. However, these compounds were obtained with moderate yields and selectivities, particularly when compared with other protocols.
Subsequently, L-Pro was also explored in different and unusual reaction conditions with the aim of optimizing the WM (S)-1 synthesis.
Srivastava tested a series of ionic liquids as alternative solvents for this reaction, comparing them with classic organic solvents or with water [36]. Ketone (S)-1 was obtained with moderate or good yield (42–88%) and selectivity (61–93% ee) employing 1 mol% of L-Pro in different ionic liquids at room temperature for 2 h. The best results were found with [pyC4]NTf2 reaction medium (88% chemical yield, 93% ee), which also proved to be better than conventional solvents or neat conditions. Carrying out the reaction at high (50–100 °C) or low (−5–0 °C) temperature was not suitable either in terms of yield or stereoselectivity. The decrease or the increase in the amount of L-Pro used were also disadvantageous. Other alternative catalysts in [pyC4]NTf2 were tested, but they resulted in lower yield and selectivity. Very importantly, the system L-Pro in [pyC4]NTf2 was recycled up to eight times with no significant loss in yield and selectivity.
Similarly, the same author described the application of polyethylene glycol 400 (PEG 400) as an alternative solvent for the synthesis of (S)-1 [37]. In this case, also, the optimized reaction conditions in PEG 400 were obtained using 1 mol% of L-Pro at room temperature for 2 h, leading to (S)-1 in excellent yield (90%) and selectivity (99% ee). Moreover, the system L-Pro in PEG 400 was successfully recycled up to eight times.
Other important modifications of the original HPESW reaction proposed in 1971 were directed towards the development of a one-pot Michael/aldol reaction, using as starting substrates not the triketone 74, but the dione 72 and the vinyl ketone 73 directly (Scheme 3). This Pro-catalysed asymmetric Robinson annulation was successfully applied for the synthesis of (S)-1, (S)-2 and their analogues.
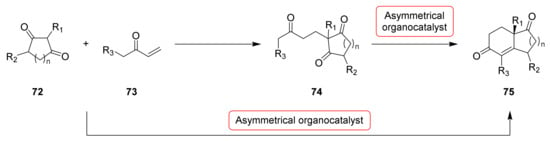
Scheme 3.
Asymmetric organocatalytic intramolecular aldol reaction towards bicyclic dicarbonyl compounds: two-steps procedure and one-pot Michael/aldol reaction. n = 1, HPESW analogues; n = 2, WM analogues; n = 3 Swaminathan analogues (see chapter 3).
Initially, in 2000 Bui and Barbas reported the one-pot synthesis of (S)-1 (49% yield, 76% ee), catalysed by 0.35 eq of L-Pro, starting from the corresponding dione and 1.5 eq of methyl vinyl ketone (MVK) in DMSO, at 35 °C for 89 h [38].
In 2001, Swaminathan et al. described a convenient one-pot synthesis of both the ketol 68, dehydrated to (S)-2 in a separate step, and the diketone (S)-1 [39]. Starting from the corresponding dione of (S)-2 in DMSO in the presence of 1 eq of L-Pro at 15–25 °C for 6 h and then adding 1 eq of MVK and stirring for additional 145 h, the reaction led to ketol 68. The latter was refluxed in benzene in the presence of PTSA to give 65–70% of (S)-2 in 77% ee. The above methodology was applied also to the synthesis of (S)-1, starting from the corresponding dione and stirring for 180 h after the addition of MVK; in this way, (S)-1 was directly obtained, without the dehydration step, in 68% chemical yield and 63% ee. Although this procedure avoids the separate synthesis step of triketone 65 or 66, using L-Pro as an organocatalyst both for the Michael and the subsequent intramolecular aldol reaction, the desired products were obtained with moderate yields and enantioselectivities. However, the optically pure compounds could be obtained after crystallization steps.
Subsequently, in 2007 Ramachary and Kishor developed an asymmetric one-pot synthesis of WM ketone analogues [40]. For the synthesis of analogue (R)-18, in the optimized conditions, the 2-benzyl-cyclohexane-1,3-dione was mixed with 3 eq of freshly distilled MVK at 25 °C in DMF in the presence of 30 mol % of L-Pro to furnish (R)-18 with 50% yield and 72% ee and the corresponding ketol in 30–45% yield. Similarly, using D-Pro as the catalyst, the opposite enantiomer (S)-18 was obtained in 50% yield with 74% ee and the corresponding ketol in 30–45% yield. The dehydration of bicyclic ketols obtained with L- or D-Pro with 1 N HClO4 in DMSO at 90 °C for 24 h furnished the corresponding ketones (R)- or (S)-18 in moderate yields (40–45%) with 71–73% ee. In the same conditions, using L-Pro, the analogues (S)-4, (S)-5, and (S)-6 were obtained in 50–75% yield and 73–75% ee. In these cases, only trace amounts of corresponding ketols were isolated.
Interestingly, in the same work the authors also investigated a one-pot double cascade asymmetric synthesis of (R)-18 from a Knoevenagel/Hydrogenation/Robinson Annulation sequence (Scheme 4a). The reaction of 5 eq of acetaldehyde 77 with 1 eq of diketone 76 and 1 eq of Hantzsch ester 78 under L-Pro catalysis (20 mol%) in CH2Cl2 at 25 °C for 3 h furnished the 2-ethyl-cyclohexane-1,3-dione 79. Removing the solvent by vacuum pump and adding DMF, 30 mol% of L-Pro, and 3 eq of MVK to the reaction mixture furnished the expected WM ketone analogue (R)-18 in 50% yield with 71% ee accompanied by the corresponding ketol 80 in 30% yield and the Michael unreacted adduct 81 in 20% yield. The same procedure was applied also for the synthesis of (S)-4, which was obtained in 35% yield and 74% ee, and for the corresponding enantiomer (R)-4 obtained in 40% yield and 52% ee, employing D-Pro as catalyst.
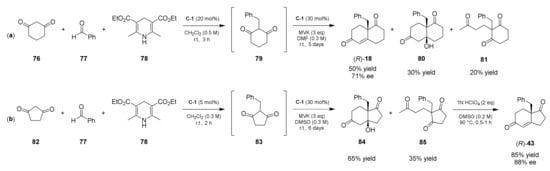
Scheme 4.
Direct organocatalytic one-pot double cascade asymmetric synthesis of WM and HPESW ketone analogues from a Knoevenagel/Hydrogenation/Robinson Annulation sequence. (a) Synthesis of WM ketone (R)-18; (b) synthesis of HPESW ketone (R)-43 [40,41].
In 2008, the same authors reported a similar work for the synthesis of HPESW ketone analogues [41]. In this case, starting from the opportune dione and MVK, applying the previously reported optimized conditions, but using DMSO as solvent, the analogues (S)-37, (S)-38, (R)-43 (using L-Pro as catalyst), and (S)-43 (with D-Pro) were obtained after the dehydration step in 50–63% overall yield and 90–94% ee. In this case, also, the one-pot double cascade asymmetric synthesis of HPESW ketone analogue (R)-43 resulted efficiently, producing the product in 55% overall yield and 88% ee after the dehydration step of ketol 84 (Scheme 4b). The same procedure was applied also for the synthesis of (S)-36, but in this case, to allow the complete conversion of the corresponding Michael adduct into the ketol, the second step was performed at 25 °C for 72 h and then at 90 °C for 12 h; in this way, after the dehydration step, (S)-36 was obtained in 85–90% yield and 86% ee.
In both works, the authors proposed a possible catalytic cycle for the double cascade reaction (Scheme 5). In the first step, the catalyst L-Pro activates aldehyde 77 by iminium ion formation, which then adds to the more reactive α-position of 76 or 82 via a formal Knoevenagel condensation (Mannich and retro-Mannich-type reaction) to generate olefins 86 or 87. In the second step, the hydrogenation of olefin 86 or 87 by Hantzsch ester 78 produces 79 or 83 through self-catalysis by decreasing the HOMO-LUMO energy gap between 78 and 86 or 87, respectively. In the third step, the Michael addition of 79 or 83 to MVK via, most likely, iminium ion activation leads to the formation of adducts 81 or 85. In the last step, L-Pro catalyses the asymmetric intramolecular aldol condensation of 81 or 85 via enamine catalysis, and subsequent dehydration releases the desired WM or HPESW ketone analogues and returns L-Pro for further cycles.
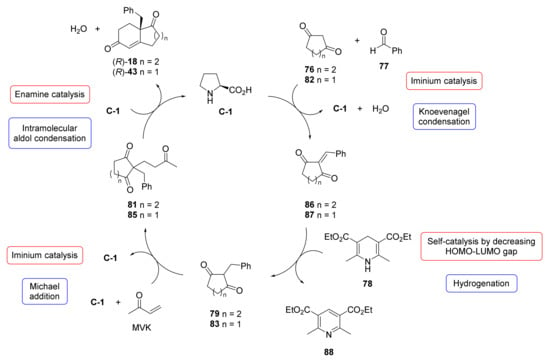
Scheme 5.
Proposed catalytic cycle for the double cascade asymmetric synthesis of WM and HPESW ketone analogues from the Knoevenagel/Hydrogenation/Robinson Annulation sequence [40,41].
In 2008, Brittain et al. reported the synthesis of (S)-2 analogues in two possible ways: by aldol cyclization of triketone 74, or by tandem Michael addition-aldol cyclization, starting directly from dione 72 and MVK [42]. In both procedures, 30 mol% of L-Pro was used as the catalyst, in DMF or DMSO, at room temperature for 5–7 days to give the corresponding ketols, which were subjected to acid-catalysed dehydration to ketones (S)-39, (R)-42—(R)-50, and (S)-51. Starting from the corresponding triketone 74, the ketones (S)-39, (R)-42—(R)-45, (R)-48, (R)-50, and (S)-51 were obtained with moderate to good yields (11–65%) in good ee (79–94%). The tandem Michael addition-aldol cyclization was successfully applied for substrates problematic with the first method ((R)-46, (R)-47, (R)-49) and for some representative diones ((S)-39, (R)-43, (R)-45, (R)-48). This procedure led to the desired products with good yields (40–73%) and excellent selectivity (93–98% ee).
L- and D-Phenylalanine
Besides L- and D-Pro, other amino acids were used as organocatalysts for this asymmetric intramolecular aldol reaction. Among these, L- or D-Phe (C-2) proved to be the catalysts of choice for the synthesis of 1 or 2 analogues substituted, respectively, in position 5 or 4. In the protocols developed over the years by different research groups, various acid co-catalysts were used in combination with L- or D-Phe in different reaction media.
In 1976, Danishefsky and Cain tried the synthesis of HPESW analogue (S)-58 by asymmetric cyclization of the corresponding trione with different amino acids, and concluded that the best conditions were with 1.2 eq of L-Phe and 0.5 eq of HClO4 in CH3CN under reflux for 40 h (82% yield, 86% ee) [43].
Subsequently, Tsuji et al. tested different amino acids for the preparation of (S)-55, an HPESW analogue used as starting material for the synthesis of (+)-19-nortestosterone. In this case, also, the best results were achieved using similar conditions to the Danishefsky procedure, reducing the amount of L-Phe and HClO4 to 1.0 and 0.4 eq, respectively, and carrying out the reaction for 72 h (85% yield, 76% ee) [44].
In addition, Agami et al. used the Danishefsky procedure to obtain WM analogues (S,S)-33 and (R,S)-33 in ≥95% ee, but in moderate yield, after 6 days [45].
Eder et al., in the work previously mentioned, applied 1.0 eq of L-Phe in acetic acid at 120 °C for 4 h to obtain the WM analogue (S)-32 in 55% chemical yield and 64% optical yield [17,18].
In 1984, Rajagopalan et al. reported the synthesis of the WM analogue bearing a methyl group in position 5 (S)-28 (80% chemical yield, 87% ee) with the same method, but allowing the reaction only for 2 h [46].
In 2008, Hanquet and co-workers described a large-scale application (>125 g batches) of the Rajagopalan et al. protocol for the synthesis of (R)-28 [47]. Using D-Phe as catalyst and starting from 216 g of the corresponding triketone, they obtained (R)-28 in 80% chemical yield and 82% ee. Interestingly, the authors demonstrated the possibility to synthesise enantiomerically pure (R)-28 after the crystallization step within one week via a one-pot preparation of the corresponding 2-methylcyclohexane-1,3-dione from 1-chloropentan-3-one, or within two days starting from the commercially available dione.
A different procedure, employing D-camphorsulfonic acid (D-CSA) as co-catalyst, was applied in 1988 by Hagiwara and Uda for the synthesis of both (S)- and (R)-28 [48]. In this case, the asymmetric cyclization of the corresponding triketone was carried out in DMF with an equimolar amount of L- or D-Phe and 0.5 eq of D-CSA, heating at 30 °C for 24 h, and then raising the temperature by 10 °C every 24 h, up to 70 °C. In this way, (S)-28 was obtained in 79% yield and 91% ee, and (R)-28 in 70% yield and 89% ee. Of course, gradual warming and the consequently long reaction time are considerable issues of this method.
In 1999, Hagiwara and co-workers applied the same method for the synthesis of (S)-53, the HPESW ketone analogue bearing a methyl group in position 4, which was obtained in 45% yield and 98% ee after recrystallization, and used as starting material for the total synthesis of the tetracyclic sesquiterpenoid (+)-cyclomyltaylan-5α-ol [49].
Previously, in 1983, Uda and co-workers reported the synthesis of WM ketone analogues (R)-34 and (R)-35, bearing an angular protected hydroxymethyl group, using D-Phe (1.0 eq) or in combination with D-CSA (0.5 eq) in DMF (room temperature to 60 °C for 120 h) ((R)-34, 77% yield, 90% ee) or with HClO4 (1.0 eq) in CH3CN (80 °C, 96 h) ((R)-35, 86% yield, 86% ee) [31].
The Hagiwara and Uda procedure was largely applied, generally with slight modifications, by numerous research groups for the synthesis of various 1 or 2 analogues.
In 1990, Corey and Virgil obtained the WM analogue (S)-31 in 77% yield and 95% ee, carrying out the reaction at room temperature for 24 days, and they used it as starting material for the synthesis of 3β,20-dihydroxyprotost-24-ene [50].
In 1999, Wicha et al., starting from the opportune triketone and raising the temperature to 55 °C, synthesized the HPESW analogues (S)-59 (69% yield, 86% ee, using L-Phe) and (R)-59 (71% yield, 89% ee, using D-Phe), useful starting material for vitamin D analogues [51].
In 2001, Li and co-workers obtained (S,R)-33 with moderate yield and selectivity (51% yield, 54% ee) employing D-Phe as catalyst, and they used this compound for the synthesis of a polyhydroxylated agarofuran [52].
In 2011, Theodorakis et al. obtained the WM analogue (R)-30, starting point for enantioselective synthesis of norzoanthamine framework, in 75% yield and 85% ee, but carried out the reaction at room temperature for 30 days [53].
In the previously mentioned work, Swaminathan et al. used 1.0 eq of L-Phe and 0.5 eq of D-CSA in solvent-free conditions with gradual heating to give both the WM and HPESW methyl analogues (S)-28 in 53% yield and 82% ee, and (S)-53 in 59% yield and 79% ee [35].
In 2007, Hagiwara et al. reinvestigated their method in order to make it more efficient and environmentally friendly using an ionic liquid as a recyclable reaction medium [54]. The reaction was carried out with an equimolar amount of D-Phe and 0.5 eq of D-CSA, with more rapid heating than the original procedure, starting from room temperature and then raising it by 10 °C per hour until reaching 70 °C. Good results were obtained using [BMim]PF6 as solvent, both for chemical yield and selectivity of (R)-28, but the system was not efficiently recyclable, probably due to loss of D-Phe during work-up. This issue was solved by employing more viscous [HMim]PF6 combined with the co-solvent N,N-dimethylpyrrolidinone or N-methyl-2-pyrrolidinone. In this way, (R)-28 was obtained in 87% yield and 86% ee, and in 79% yield and 68% ee after five reuses of the system, recycling D-Phe, D-CSA, and ionic liquid. Similarly, the method was applied for the synthesis of (R)-53 (78% yield and 84% ee after the first use, 73% yield and 58% ee after four recycles).
Another efficient procedure for the synthesis of different analogues of 1 or 2 employing L-Phe was developed by Shibasaki et al. [55]. In their optimized conditions, different to the previous procedures in which stoichiometric amount of L-Phe were required, only 30 mol% of L-Phe were used in combination with 50 mol% of pyridinium p-toluenesulfonate (PPTS) and 1.0 eq of DMSO at 50 °C for 24 h. The best chemical yield for the synthesis of HPESW analogue (S)-56, key intermediate of wortmannin synthesis, was obtained under solvent-free conditions. However, the difficulty of performing the reaction in this way, due to the high viscosity of the medium, led the authors to add DMSO, which solved this problem and produced (S)-56 with better selectivity, although in lower yield. The method was successfully tested in large scale (58 mmol), producing (S)-56 in higher yield and without loss of selectivity (73% vs. 64% yield in 0.3 mmol scale, 94% ee). The optimized conditions were successfully applied for the synthesis of various C5 or C4-substituted analogues of (S)-1 and (S)-2 ((S)-28, (S)-29, (S)-53, (S)-54, (S)-55, (S)-56, (S)-57, 57–87% yield, 80–93% ee).
Subsequently, in 2017 Kingsbury et al. successfully applied Shibasaki procedure for the efficient preparation of (R)-53 using D-Phe as catalyst, resulting in excellent yield and selectivity (88% yield, 91% ee) [56].
Other Amino Acids
Other natural and synthetic amino acids were employed as catalysts of this intramolecular aldol reaction, but generally they were less efficient than Pro and Phe, producing the reaction products with lower chemical yield and/or poorer enantioselectivity [57,58,59].
In 2006, Limbach reported a study on different β-homoamino acids as organocatalysts for the synthesis of 2, starting from the corresponding triketone [60]. Interestingly, the investigated β-amino acids (20 mol%, DMF, room temperature, 4 days, followed by acid catalysed dehydration step) gave (R)-2 with reverse stereoselectivity compared to that observed with α-amino acids (L-Pro, L-Phe). Among the studied β-amino acids, the best results were obtained with C-6–C-9. However, they required long reaction times and produced the product in moderate chemical yield (29–64%), but with good enantioselectivity (75–83% ee).
Interesting results were obtained with some amino acid derivatives of proline.
Hanessian et al. explored the use of cis- and trans-4,5-methanoproline C-10 and C-11 (3 mol%, DMF, room temperature, 125–140 h) as catalysts for the synthesis of the ketol precursor of (S)-2 [61]. Cis-4,5-methanoproline showed catalytic ability similar to L-Pro (86% yield, 93% ee vs. 98% yield, 95% ee L-Pro) and better than trans-4,5-methanoproline (67% yield, 83% ee). Computational studies carried out to explain the different catalytic ability of these two derivatives confirmed the assumption that the reaction mechanism is enamine-mediated. The enamine for trans-4,5-methanoproline in the lowest energy conformation is pyramidalized, while for cis-4,5-methanoproline is relatively planar. Therefore, the energy required to distort the enamine to the planar iminium is higher for the non-planar enamine of trans-4,5-methanoproline and lower for the planar enamine of cis-4,5-methanoproline, explaining the better catalytic activity of the latter.
Excellent catalytic activity of the β-amino acid cispentacin C-12 was revealed by Davies et al. in 2005 [62]. This catalyst (30 mol%, DMF, room temperature, 48 h) was applied for the synthesis of 1, 2, and 36, the HPESW analogue with an angular ethyl group. As reported for other β-amino acids, in this case cispentacin also showed opposite stereoselectivity compared to L-Pro, leading to the (R)-enantiomers. Starting from the opportune triketones, this catalyst promoted complete conversion to the corresponding ketols, which after acid catalysed dehydration gave (R)-1, (R)-2, and (R)-36 in 86%, 90%, and 78% ee, respectively. In the same conditions, L-Pro gave the (S)-enantiomers with lower or similar selectivity (72% ee for (S)-1, 93% ee for (S)-2, 74% ee for (S)-36). The opposite stereoselectivity of L-Pro and cispentacin was explained based on the different transition states. Indeed, if for L-Pro the favoured transition state is the anti one (in which the enamine is anti to the carboxylic acid group, see Scheme 1), for cispentacin, with a fixed cis-relative orientation of the carboxylic acid and amino functionality, the reaction proceeds preferentially via the transition state 89 that minimises steric interactions (Figure 6).
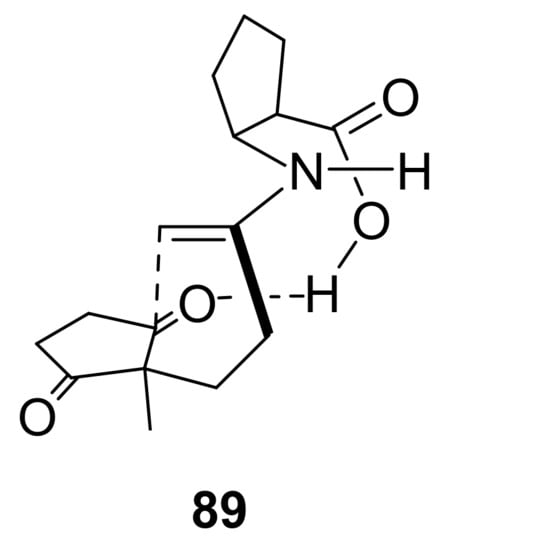
Figure 6.
Favoured transition state model for the reaction of cispentacin C-12 with triketone 66, proposed by Davies et al. [62].
Subsequently, the same authors extended their study on different β-amino acids, confirming that in cyclic α,β-disubstituted β-amino acids the fixed orientation of the carboxylic acid and amino functionalities provides high enantiocontrol in this reaction [63]. Among the studied amino acids, cispentacin promoted the highly enantioselective cyclisation of triketone 66. However, its application in the synthesis of (R)-53, HPESW bearing a methyl group on position 4, was not efficient, requiring high temperature and long reaction time (60 °C, 9 days) and producing the product with low enantioselectivity (27% ee).
Paquette et al. screened L-Pro and different proline amino acids derivatives as organocatalysts for the synthesis of both (R,S)-16 and (S,S)-16, WM analogues bearing a chiral oxygenated side chain [64]. In this case, L-Pro (0.2 eq) in DMSO at 50 °C for 21 h led to (R,S)-16 in 84% diastereomeric excess (de) and 52% conversion, while the use of proline derivatives C-13 improved the yield to 76%, keeping a similar diastereoselectivity (80% de). Lower efficiency was detected for ent-C-13, giving (S,S)-16 in 61% yield and 60% de.
Other Non-Amino Acid Organocatalysts
Although classical procedures employing proline or phenylalanine as organocatalysts produced 1, 2, and their analogues with good or excellent enantioselectivity, they suffered from several drawbacks, such as the use of high boiling point solvents (DMSO, DMF), and in some cases the extremely long reaction time or the high catalyst loading. For these reasons, over the years several research groups worked on the development of suitable catalysts with improved performance and wide applicability.
In light of these considerations, Kanger et al. investigated the use of different mono salts of bimorpholines for the synthesis of (S)-1 and (S)-2, starting from the assumption that their reactivity may be similar to proline, due to their structural similarity [65,66]. Indeed, both compounds are cyclic secondary amines with the chiral centre at the α-position of the nitrogen atom, with an acidic proton at a distance of four chemical bonds from the nucleophilic nitrogen. The best results were obtained with 5 mol% of i-Pr-bimorpholine C-14 as trifluoroacetic salt in CH3CN at reflux for almost 70 h, producing (S)-1 in 84% yield and 91% ee, and (S)-2 in 83% yield and 80% ee. The authors proposed that the acid used for the preparation of the salt has a dual role in this reaction, both promoting the enamine formation as proton donor and contributing to the generation of a fixed conformation of bimorpholine by hydrogen bonding; in this way the N atom becomes a stereogenic centre contributing to the stereoselectivity of the catalyst. This hypothesis was supported by the low catalytic activity and selectivity showed by the bimorpholines as free base. In addition, the strength of the acid used for the preparation of the salts is important for the stereoselectivity and catalytic activity. Indeed, with C-14 as triflic salt, compared to C-14 as trifluoroacetic salt, higher enantioselectivity was achieved, but with longer reaction time; this is probably due to the fact that the triflic acid (TFSA), stronger than trifluoroacetic acid (TFA), hinders the catalytic activity, reducing the amine nucleophilicity and thus retarding the enamine formation, but at the same time leads to a more strongly chelated structure of bimorpholine, favouring the enantioselectivity. In order to clarify the mechanism of bimorpholine-catalysed intramolecular aldol reaction, the authors carried out synthesis of (S)-1 in the presence of 18O enriched water. The incorporation of 18O atoms into the conjugated carbonyl moiety of the product supported the hypothesis that the reaction proceeds via enamine intermediate, which can be hydrolysed by isotopically labelled water.
Subsequently, the same authors studied bipiperidine C-15 analogue to bimorpholine C-14 with the aim to identify catalysts that could be obtained with a simpler synthetic route [67]. Unfortunately, the bipiperidine derivative was a much less efficient catalyst than the corresponding bimorpholine derivative, both for reactivity and selectivity.
In 2010, Fuentes de Arriba et al. reported the synthesis of different (R,R)-cyclohexanediamine derivatives and their application as catalysts in the preparation of (S)-1 [68]. The ureas C-16–C-17 and amides C-18–C-19 studied proved to be good organocatalysts, differently from carbamoyl and sulphonamide derivatives, and the best results were obtained with the isophtalic derivative C-19 (10 mol%, CDCl3, room temperature, 16 h), producing (S)-1 in 85% yield and 95% ee.
In 2012, Luo et al. successfully applied the primary amine C-20 for the synthesis of (R)-1, (R)-2 and their derivatives (S)-8, (S)-11, (S)-13, (S)-14, (S)-18, (R)-19, (S)-22, and (R)-60 [69]. Using 10 mol% of catalyst, accompanied by 10 mol% of TFSA, and 5 mol% of m-nitrobenzoic acid under solvent-free conditions, (R)-1 and (R)-2 were obtained in excellent yield (95%) and selectivity (92% and 96% ee, respectively) after 12 h of reaction at room temperature. The presence of the second weak acid allowed enhancement of the rate of the reaction. The same conditions, with different reaction times (24–36 h), were applied for the synthesis of WM analogues (S)-8, (S)-11, (S)-13, (S)-14, (S)-18, (R)-19, (S)-22, and HPESW analogue bearing a condensed benzo ring (R)-60, and obtained good to excellent yields (75–95%) and high enantioselectivity (79–92%). Moreover, the applicability of this catalyst was also proved in gram-scale reactions, using only 1 mol% of C-20 as TFSA and 0.5 mol% of m-nitrobenzoic acid, to afford (R)-1 (98% yield, 91% ee) after 4 days and (R)-2 (90% yield, 96% ee) after 1 week.
Subsequently, the same authors developed a one-pot protocol applying C-20 [70]. Interestingly, starting directly from the 2-methylcyclohexane-1,3-dione and the MVK, using the conditions previously reported, (R)-1 was obtained in 73% yield and 92% ee. For the scale-up the authors reduced the amount of C-20 as triflic salt and co-catalyst to 2 mol% and 1 mol%, respectively, and heated at 60 °C to give (R)-1 in 87–90% yield and 90% ee after 3 (1 g scale) or 2 days (100 g scale). The same protocol applied for (R)-2 required a longer reaction time (6 days) and resulted in moderate yield and selectivity (79% yield, 76% ee). The optimized conditions for 1 g scale were successfully applied for the synthesis of WM derivatives (R)-4, (S)-8, (S)-11, (S)-13, (S)-14, (S)-18, (R)-19, and (S)-22 (62–89% yields, 84–92% ee).
In 2017, Christmann et al. applied Luo’s catalyst and procedure, starting from the corresponding triketone, for the efficient preparation of (S)-8 (82% yield, 96% ee), (R)-9 (67% yield, 92% ee), and (R)-10 (54% yield, 91% ee) [34].
In 2009, Akiyama et al. explored, for the first time, H-bonding catalysis using the chiral phosphoric acid C-26 as organocatalyst for the synthesis of (R)-1, (R)-2, and different (R)-2 analogues bearing a condensed benzo ring ((R)-61, (S)-62, (S)-63, (S)-64) [71]. In contrast to what happens in the processes catalysed by chiral amine, which are assumed to promote the reaction through the enamine activation mode, with C-26 the stereoselectivity was controlled via non-covalent interactions, and the same catalyst promoted both the intramolecular aldol reaction and the dehydration step. In the optimized conditions, 5 mol% of C-26 in n-hexane at 70 °C for 24 h gave (R)-1 and (R)-2 in moderate yield and selectivity (64% and 86% yield, 82% and 70% ee), while generally better results were obtained for benzo-condensed derivatives of (R)-2 (72–94% yield, 87–94% ee).
Proline Related Organocatalysts
The high efficiency showed by L-Pro as organocatalyst for the intramolecular aldol reaction, both for its catalytic activity and enantioselectivity, led some researcher groups to investigate potential organocatalysts of this reaction with structures related to proline.
Inomata et al. studied some (S)-N-substituted-N-(2-pyrrolidinylmethyl)amine derivatives in combination with a stronger Brønsted acid for the synthesis of (R)-1, revealing an inversion of enantioselectivity when compared to the same reaction carried out in the absence of the Brønsted acid or mediated by L-Pro [72,73,74]. Therefore, the use of these catalysts allowed development of an alternative route to providing (R)-1 with moderate yield and good selectivity, without using unnatural D-Pro.
Initially, in 2007 they studied C-22 in combination with different Brønsted acids in DMSO at room temperature to catalyse the intramolecular aldol reaction of the triketone 65, and the highest selectivity was obtained by using 1.0 eq of C-22 with 1.5 eq of TFA (53% yield, 81% ee, after 48 h) [72]. These conditions were also applied in the one-pot reactions, starting from the corresponding dione and MVK; however, the presence of TFA suppressed the first step of Michael addition. Nevertheless, it was possible to overcome this problem by adding the acid one hour after the beginning of the reaction. The one-pot annulation required a longer reaction time, giving (R)-1 in 47% yield and 75% ee after 80 h.
The authors proposed that, in the presence of a Brønsted acid, the pyrrolidinylmethyl moiety in C-22 would be protonated to generate the ammonium salt, which could act as a hydrogen-bond donor to the β-oxygen atom originated from ketone functionality and to the nitrogen atom in the enamine moiety, stabilizing the transition state and working as the carboxylic acid group in the amino acids. Among the four possible transition states, the trans-anti 90 (Figure 7) appears the most favoured, where trans refers to the trans-fused carbocycles, while anti to the conformation between olefinic and pyrrolidinylmethyl moieties. Subsequent to the formation of the corresponding diol, the Brønsted acid promotes the dehydration step, to give (R)-1.
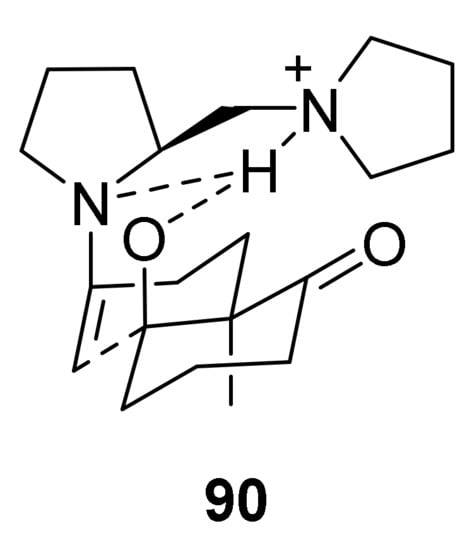
Figure 7.
Favoured trans-anti transition state model for the reaction of C-22 with triketone 65, proposed by Inomata et al. [72].
Subsequently, the same authors investigated some (S)-N-benzyl-N-(2-pyrrolidinylmethyl)amine derivatives as catalysts for the intramolecular aldol reaction of the triketone 65, applying the conditions previously reported [73]. The obtained results suggested that higher steric hindrance around the aminomethyl moiety improved the enantioselectivity; indeed, the best results were obtained with C-23, with an anthracenylmethyl group. Moreover, it was possible to use C-23 in catalytic amounts (0.3 eq) with 0.45 eq of dichloroacetic acid as co-catalyst in DMSO at room temperature for 20 h, improving both the yield and the ee of (R)-1 (68% yield, 84% ee).
In 2011, the same authors reported alternative (S)-N-substituted-N-(2-pyrrolidinylmethyl)amine derivatives as organocatalysts for this reaction [74]. However, these compounds were less efficient than the related previously reported catalysts.
The benzimidazole-pyrrolidine derivative C-24, obtained simply in 55% yield in one reaction step starting from L-Pro and 4,5-dimethyl-1,2-phenylenediamine, was applied by Vincent, Landais et al., as catalyst for the synthesis of (S)-1, (S)-2 and (R)-40 [75]. Compared to the original protocol developed by Eder, Sauer, and Weichert, C-24 combined with TFA led to (S)-1 and (S)-2 in similar or higher yield (82% and 100%, respectively) and similar enantioselectivity (68% and 86% ee, respectively), catalysing both the intramolecular aldol reaction and the dehydration step under milder conditions (THF, 0 °C, 48 h) and with a lower amount of catalyst (10 mol%). This catalyst, combined with TFA (10 mol%, THF), also produced (R)-40 in good yield and selectivity (70% yield, 87% ee), but time and temperature applied for this reaction were not specified.
Prolinamides and Prolinthioamides
An important class of organocatalysts largely investigated for the intramolecular aldol reaction is constituted by prolinamide and prolinthioamide derivatives, which have been proven to be the catalysts of choice for the synthesis of these classes of compounds. In the developed protocols, a low amount of catalyst was applied (0.5–30 mol%), in some cases accompanied by an acid as co-catalyst; especially noteworthy is that the reactions were often carried out in solvent-free conditions and at room temperature, allowing for obtaining the desired products with excellent yields and high enantioselectivity.
In 2008 Tu, Zhang et al. identified prolinamide C-27 as an effective catalyst for the enantioselective synthesis of 1 and 2 analogues, especially bearing a functionalized angular chain, useful as starting material for the synthesis of some natural products or pharmaceutical intermediates [76]. Different proline-type catalysts were tested for the synthesis of the WM analogue (R)-23, but the best results were obtained with C-27. In the optimized conditions, 30 mol% of C-27 and pyridinium p-toluenesulfonate (PPTS), in CH3CN at 50 °C for 144 h, were applied to give (S)-1 and its analogues (S)-4, (R)-8, (R)-14, (S)-15, (R)-18, (R)-23, (R)-24, (R)-25, and (R)-26 in good yield and high selectivity (41–70% yield, 70–88% ee). The lowest yield and selectivity were obtained with (R)-26, bearing two methyl groups at the C-3 position of the WM core, while the different angular groups did not have significant influence on the efficiency of C-27. Furthermore, the catalytic asymmetric activity of ent-C-27 was proved for the synthesis of (S)-40 (60% yield, 83% ee), the HPESW analogue bearing an allyl angular group, used as starting material for the construction of the tricyclic core of cylindricine-type alkaloids.
Later, Theodrakis et al. optimized the previously reported procedure for the synthesis of (S)-40, applying ent-C-27, and modifying time and temperature of the reaction [77,78]. The rise in temperature to 80 °C reduced the enantioselectivity, while the reduction of temperature to 25 °C gave (S)-40 in over 99% ee, but in 70% yield after 60 days. A good compromise between high enantioselectivity and shorter reaction time was achieved by performing this conversion at 40 °C for 14 days (74% yield, >90% ee). The authors used (S)-40 as starting material for the total synthesis of natural Illicium sp. majucin-type sesquiterpenes, such as jiadifenolide, jiadifenin, (1R,10S)-2-oxo-3,4-dehydroxyneomajucin, and related analogues.
In 2009 Morán et al. studied prolinamides C-35–C-38 for the synthesis of (S)-1 and (S)-2 [79]. Starting from triketone 66 and using 10 mol% of catalyst in CHCl3 at rt, both ketol 68 and (S)-2 were obtained in different proportions related to the NH acidity of prolinamides. The isophthalic acid derivative C-38 afforded the highest amount of (S)-2 (40%) among the prolinamides studied, while the best total yields of both 68 and (S)-2 (92%) were obtained with C-36 and C-37. Furthermore, more acidic prolinamides led to a reaction rate acceleration and an improvement in enantioselectivity; indeed, with the less acidic 4-methylphenyl prolinamide C-35, the reaction proceeded more slowly, producing (S)-2 with lower yield and selectivity (91% ee), while the 3,5-bis(methoxycarbonyl)phenyl prolinamide C-38 produced (S)-2 not only with the highest yield, but also in excellent selectivity (98% ee). These results were consistent with the proposed mechanism via enamine for the intramolecular aldol reaction and for the dehydration step (Scheme 6). Starting from triketone 65, only ketone (S)-1, with excellent selectivity (87–96% ee), was obtained. NMR studies revealed the formation preferentially of imidazolidinone intermediate 95 starting from triketone 66, while that of the enamine derivative 97 started from triketone 65, imidazolidinone intermediate 96 being present only in the initial stage of the reaction (Figure 8); this evidence underlines the different behaviour of triketones 65 and 66 during the reaction.
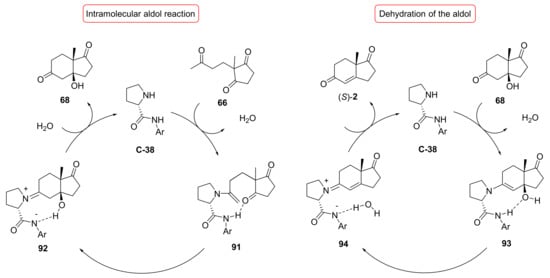
Scheme 6.
Proposed catalytic cycles for the intramolecular aldol reaction of triketone 66 and for the dehydration step catalysed by prolinamides (such as C-38) [79].
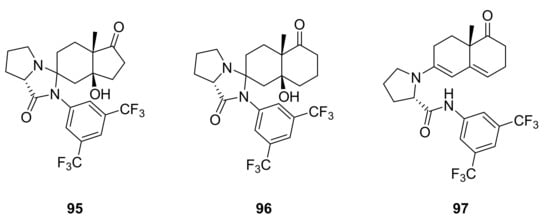
Figure 8.
Proposed structures of the intermediates formed during the synthesis of (S)-1 and (S)-2 catalysed by C-38, proposed by Morán et al. based on NMR studies [79].
More recently, in 2018 Jahn et al. reported the total synthesis of ent-pregnanolone sulfate starting from (R)-1, obtained in excellent yield and selectivity (94% yield, 95% ee) in neat conditions at room temperature for 3 days, using prolinamide C-42 (5 mol%), which proved to be the best available catalyst for the preparation of this compound [80].
An extensive study on different prolinamides, containing heterocyclic, aromatic, alkyl, acylhydrazine, or sulfonyl groups, as organocatalysts for the synthesis of (S)-1 was reported by Ding and co-workers in 2017 [81]. All reactions were carried out in neat conditions at room temperature for 5 days, in the presence of 1 mol% of the opportune prolinamide and 0.025 eq of benzoic acid as co-catalyst. Prolinamides bearing phenyl or alkyl groups (C-28–C-36) showed better catalytic activity and selectivity (79–88% yield, 77–92% ee). Among these prolinamides, the steric hindrance of naphthyl in C-32 and tert-butyl in C-29 reduced enantioselectivity. The most active and selective organocatalysts, C-28, C-33, and C-34, proved to be equally efficient in 0.5 mol%. Furthermore, performing the annulation under microwave irradiation, with power of 10 W, using 1 mol% of C-28 or C-34, the reaction time was successfully reduced from 5 days to 3 h, retaining excellent conversion and selectivity (99% conversion, 89–91% ee).
Nájera, Gómez-Bengoa et al. studied the prolinamides C-39 and ent-C-39 containing the (R,R)- or (S,S)-transcyclohexane-1,2-diamine scaffold and a 2-pyrimidinyl unit, potentially able to form extra hydrogen bonds with the carbonyl acceptor [82]. Both catalysts were tested in 10 mol%, with 10 mol% of 1,6-hexanedioic acid as co-catalyst, in solvent-free conditions at room temperature for the synthesis of (S)-1 and (S)-2; ent-C-39 resulted more enantioselective than the diastereoisomer and it was more efficient for (S)-1 (92% yield, 89% ee, after 10 h). C-39 was also applied for the synthesis of WM analogues (R)-8 and (R)-18, obtained in moderate yield and good selectivity after 16 h of reaction (77% or 53% yield, 84% or 77% ee, for (R)-8 and (R)-18, respectively). Interestingly, the authors demonstrated the possibility to recover the catalyst after the opportune workup of the reaction performed on 1 g scale for the synthesis of (S)-1.
Reiser and co-workers studied different short α/β-peptides, containing conformationally restricted cis-β-aminocyclopropylcarboxylic acid (cis-β-ACC) unit, as catalysts for the synthesis of (S)-1 and (S)-2 [83]. The tripeptide C-40, with a (+)-cis-β-ACC unit among two L-Pro units, proved to be remarkably more efficient than the corresponding catalyst with (-)-cis-β-ACC unit. Performing the reaction with 10 mol% of C-40 in CHCl3 at room temperature, without other co-catalysts and in only 1 day, (S)-1 was obtained with enantioselectivity and yield similar to that achieved with other protocols employing prolinamides (88% yield, 92% ee).
Alonso, Nájera et al. applied the L-prolinethioamide C-43 (5 mol%), containing the (R)-1-aminoindane unit, accompanied by 4-nitrobenzoic acid (5 mol%) in neat conditions at room temperature for 24 h for the synthesis of (S)-1 (99% yield, 86% ee), (S)-2 (71% yield, 88% ee) and the HPESW ethyl analogue (S)-36 (99% yield, 84% ee), proving the best efficiency of C-43 compared to L-Pro in solvent-free conditions [84]. C-43 efficiency was reduced in the absence of the co-catalyst or in the presence of water [85].
In 2008, Guillena, Nájera, and Viózquez firstly used N-Tosyl-(Sa)-binam-L-prolinamide C-44 (5 mol%) and benzoic acid (1 mol%) as catalysts for the synthesis of (S)-1 and (S)-2 in solvent-free conditions or in the presence of water [86]. The solvent-free system was more efficient for (S)-1, leading to the ketone in only 27 h at room temperature with excellent yield and high enantioselectivity (95% yield, 90% ee), while (S)-2 was obtained in lower yield (85% after 48 h), but with equal selectivity, in the presence of water.
Subsequently, Bonjoch et al. successfully applied C-44 in the same solvent-free protocol for the synthesis of different (S)-1 analogues ((S)-7, (R)-8, (S)-9, (R)-11, (S)-12, (R)-13, (R)-14, (R)-18, (S)-20, (R)-23) [87]. For the allyl analogue (R)-8 they tested different organocatalysts (C-1, C-13, C-22, C-27, C-44) under various reaction conditions, but the best results were undoubtedly obtained with C-44. In addition, L-prolinamide C-27, applied with this solvent-free protocol, was more efficient compared to the procedure previously reported by Tu, Zhang et al. [76]. Indeed, in this way the reaction occurred with a lower amount of catalyst and acid co-catalyst (5 and 1 mol%, respectively, vs. 30 mol%), more rapidly (24 h vs. 144 h), at room temperature (vs. 50 °C), and in neat conditions (vs. CH3CN), leading to (R)-8 in excellent yield and good selectivity (96% yield, 82% ee). Using C-44, a considerable improvement in the enantioselectivity (94% ee) was observed, keeping an excellent chemical yield (93%). The same efficiency was showed by ent-C-44, which gave the opposite enantiomer (S)-8. Moreover, when the reaction was carried out on a large scale (3 g), reducing the amount of C-44 to 2.5 mol%, a further increase of selectivity (97% ee) was achieved, although with longer reaction time (5 days). In this way, the catalyst could be also recovered and successfully reused for a second reaction. Additionally, for the synthesis of (S)-1 in large scale (1 or 10 g), the reduction of C-44 to 2 mol% and of benzoic acid to 0.5 mol% led to an improvement of selectivity (94% ee), keeping an excellent chemical yield (94%), although in longer reaction time (7 days). Regarding the other (S)-1 analogues: bearing different angular substituents, in general, due to the increased steric hindrance of the side chain, the reactions occurred more slowly, in some cases requiring 10 mol% of catalyst, leading to products with moderate to good yields (53–88%). However, the enantiomeric excess was uniformly excellent (84–96%) and superior to any obtained by other organocatalysts already reported in literature. The authors declared that a limitation of the procedure was found when attempting to extend it to 5-methyl derivative (R)-8.
More recently, in 2019 Bradshaw, Besora, and co-workers reported both experimental and computational studies to elucidate the catalytic mechanism of C-44 and other binam derivatives in the synthesis of WM ketone and analogues [88]. The optimal conditions using C-44 for the synthesis of (S)-1, in terms of reaction time, catalyst loading, and enantioselectivity (500 mg scale, 1 mol% C-44, 2.5 mol% benzoic acid, neat, room temperature, 5–6 days; 97% yield, 90% ee) were applied to screened alternative acid co-catalysts, covering a wide range of pKa values (0.52–4.9). With the exception of the acids at the extremes of the studied pKa range, the reaction proceeded with analogue enantioselectivity to that produced by benzoic acid. In addition, the use of chiral acids did not increase the stereoinduction. However, a significant reaction rate enhancement as the pKa decreased was observed, and formic acid was the best co-catalyst, giving (S)-1 with the same yield and ee to that obtained with benzoic acid, but after only 3 days of reaction. With this protocol, other binam catalysts were studied, modifying the electronic and steric properties of the sulfonamide group: replacing the sulfonamide by an amide or dimethylamino group, or substituting the amide hydrogen of sulfonamide by a methyl. Among the screened secondary sulphonamide, the binaphthyl catalyst C-45 led to an increase in both yield (98%) and ee (94%) with respect to C-44, while the replacement of the aromatic group with a methyl one led to decreased efficiency; in addition, the amide, amine, and tertiary sulphonamide tested showed reduced catalytic and enantiomeric performance.
The computational studies led the authors to propose a mechanism via imine and enamine formation. The role of carboxylic acid as co-catalyst is fundamental in the initial steps of the reaction for the formation of iminium and enamine intermediates, while it does not affect the enantioselectivity, being absent in the transition state 98 which leads to C-C bond formation (Figure 9). Instead, the enantioselectivity of the reaction catalysed by C-44 is due to the rigidity of the catalyst, which interacts with the substrate with the generation of the enamine intermediate, and the simultaneous formation of the two hydrogen bonds between the N-H groups and the carbonyl oxygen of the substrate; the latter has to distort to bind properly to these anchoring points, and this distortion is smaller for the favoured transition state anti/re, which leads to the major enantiomer (Figure 9). Additionally, the different enantioselectivity showed by the investigated binam derivatives is mainly related to their different rigidity.
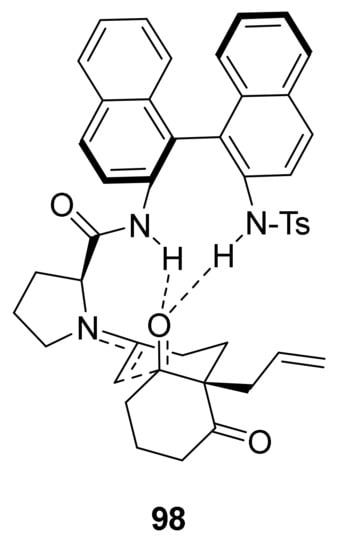
Figure 9.
Favoured anti/re transition state model for the reaction of C-44 with triketone 65, proposed by Bradshaw, Besora et al. [88].
In 2015 Fuentes de Arriba, Morán et al. reported excellent organocatalysts for the synthesis of (S)-1 and (S)-2 formed by a rigid carbazole scaffold containing a chiral amine, capable of carrying out the catalysis via enamine formation, and a synthetic oxyanion-hole, capable of donating two or more hydrogen bonds, mimicking natural aldolase [89]. Initially, different carbazoles bearing a (R,R)-cyclohexanediamine moiety were tested for the synthesis of (S)-2, and the best results were obtained with C-46 (5 mol%) using acetic acid as co-catalyst (5 mol%) in CDCl3 at room temperature for 114 h; in this way, both ketol 68 and ketone (S)-2 were obtained in almost equal quantity, with a total conversion of 94% and an ee of 96%. To further improve the results, the authors replaced the cyclohexanediamine unit with L-proline, leading to prolinamides C-47 and C-48; in this way, the synthetic oxyanion hole is formed by a prolinamide and a sulfonamide moiety, while enamine formation is assisted by the basic pyrrolidine ring. Both catalysts showed excellent enantioselectivity, but the best total conversion to ketol 68 and ketone (S)-2 (95%), with ee > 99%, was achieved with C-47 (5 mol%) using formic acid as co-catalyst (5 mol%) in CDCl3 at room temperature for 120 h. Applying the same conditions, starting from triketone 65, both ketol 67 and ketone (S)-1 were obtained after 130 h, with a total conversion of 90%, in an almost enantiomerically pure form (ee ≥ 99.9%).
2.1.2. Supported Organocatalysts
Although numerous efficient organocatalysts have been identified over the years and applied in convenient procedures for the enantioselective preparation of 1, 2, and their analogues, some limits are still present. In particular, the synthesis of complex organocatalysts usually involves several reaction steps, and this limits the advantages of their use, especially when high catalyst loadings are required. For these reasons, the possibility to recover and recycle the catalysts could represent an interesting alternative both from an environmental and an economic point of view. An approach pursued in this direction consists of immobilising the homogeneous catalyst on an insoluble support, allowing the separation of the products and the catalyst by filtration, and the consequent recovery and reuse of the latter. The materials used for the preparation of heterogeneous supported organocatalysts applied for the enantioselctive synthesis of 1, 2, and their analogues include inorganic silica matrix or organic polymers, such as polystyrene (PS) and polyethyleneglycol (PEG).
The first silica-supported organocatalyst applied for the synthesis of (S)-1 was C-49, reported by Pleixats et al. in 2011 [90]. C-49 is a bridged silsesquioxane, prepared by hydrolytic polycondensation of the bis-silylated prolinamide precursor catalysed by tetrabutylammonium fluoride (TBAF). Its structure is related to an aminoindane-derived prolinamide previously reported as an efficient organocatalyst of the direct aldol and Michael reactions. Starting from triketone 65, C-49 was used in 16 mol%, with 10 mol% of p-nitrobenzoic acid in water at room temperature, but it showed moderate catalytic activity (74% yield) and low selectivity (43% ee). The possibility to reuse C-49 was examined for five consecutive cycles, but this led to an ulterior reduction of ee (from 43% to 27%).
Slightly better results were reported by the same authors in 2012 using the silica-supported catalysts C-50, C-51, and C-52 for the synthesis of (S)-1 [91]. Starting from the mono-silylated prolinamide precursor, C-50 and C-51 were obtained by co-gelification with tetraethyl orthosilicate (TEOS) providing porous materials with high surface area, in contrast to the previously described non-porous silsesquioxane, while C-52 was prepared by anchoring the precursor to a mesostructured silica. In this case, also, the supported catalysts (10 mol%, in the same conditions previously reported) showed limited catalytic activity and selectivity, but for C-50 and C-51 the possibility to reuse them for five consecutive cycles without a significant decrease in enantioselectivity was reported.
Guillena, Nájera et al. reported the silica-gel-supported binam-prolinamide C-53 as an efficient organocatalyst for the synthesis of (S)-1, (S)-2, (R)-8, and (R)-18 under solvent-free conditions [92]. The catalyst C-53 was prepared by co-gelification of the silylated prolinamide precursor with TEOS. In the optimized conditions, 20 mol% of C-53, with 5 mol% of benzoic acid, without solvent, at room temperature for 5 days, gave (S)-1 in 88% yield and 80% ee, while slightly better results were obtained on a large scale (800 mg). Good results were also achieved for (S)-2 (85% yield, 88% ee), (R)-8 (77% yield, 92% ee), and (R)-18 (73% yield, 91% ee). Compared to the related homogeneous catalyst C-44 previously reported, the silica-supported C-53 was slightly less efficient for catalytic activity and/or selectivity; however, the possibility to recover and recycle C-53 five times, evaluated for the synthesis of (S)-1, without changes in the achieved enantioselectivity, with only a small decrease of the yield after the fifth cycle, gives an added value to this organocatalyst.
Again, in 2016 Pleixats et al. applied other silica-supported organocatalysts for the synthesis of (S)-1 and compared them with the corresponding homogeneous prolinsulphonamide C-54 [93]. Starting from the opportune silylated precursor, C-55–C-57 were obtained by sol-gel co-gelification with TEOS, while C-58 was prepared by anchoring the precursor to a mesostructured silica. Unfortunately, these catalysts (20 mol%), accompanied by p-nitrobenzoic acid (20 mol%) in water at room temperature, were even less efficient than the previously reported silica-supported organocatalysts (44–82% yield after 7–12 days, ~36% ee), while the model homogeneous catalyst C-54 presented superior activity and selectivity (89% yield after 4 days, 72% ee), although lower than other prolinamides.
Among organocatalysts supported on organic materials, in 2002 Benaglia, Cozzi, and co-workers reported PEG-supported C-59, obtained by immobilization of (2S,4R)-4-hydroxyproline on PEG5000 monomethyl ether (MeOPEG) monosuccinate [94]. The solubility profile of PEG led to the preparation of a supported organocatalyst soluble in many organic solvents, allowing operation under homogeneous catalysis conditions and to easily recover the catalyst by filtration after precipitation in Et2O. The authors proposed that this catalyst could represent a model of natural type I aldolase, with the polymer chain acting as enzyme peptide skeleton, and proline acting as the catalytic site. Interestingly, C-59 (0.35 eq) was employed in the one-pot Robinson annulation, starting directly from the 2-methylcyclohexane-1,3-dione and the MVK, carrying out the reaction in DMSO at room temperature for 90 h. In this way, (S)-1 was obtained in 55% yield and 75% ee, similarly to the one-pot Robinson annulation catalysed by L-Pro, as reported by Bui and Barbas [38].
The first examples of PS-supported organocatalysts applied for this reaction were already reported in 1985 by Takemoto et al. [95]. In these catalysts, the proline moiety was linked to polystyrene by interposition of a spacer group. However, the most efficient C-60 also showed low catalytic activity and selectivity.
More recently, in 2013 Pedrosa, Andrés et al. studied homogeneous prolinamide C-61 and the corresponding PS-supported organocatalysts C-62 and ent-C-62 in the synthesis of both 1 and 2 [96]. For the preparation of (S)-1, the best results in terms of yield and enantioselectivity using PS-supported C-62 were obtained in neat conditions using 10 mol% of catalyst accompanied by 10 mol% of acetic acid as co-catalyst at room temperature for 24 h, leading to (S)-1 in 80% yield and 78% ee. In the same conditions, the corresponding prolinamide C-61 was more efficient, producing (S)-1 in 83% yield and 90% ee. Using the supported catalyst ent-C-62, the enantiomer (R)-1 was formed. In addition, for the synthesis of (S)-2, the best results were achieved using PS-supported C-62 in the same solvent-free conditions, although the reaction proceeded more slowly, producing (S)-2 after 72 h in 70% yield and 76% ee. Differently, the prolinamide C-61 (10 mol%) with acetic acid (10 mol%), in CH3CN at −18 °C for 96 h, gave (S)-2 in 95% yield and 84% ee.
In 2017, Pericàs and co-workers reported an interesting and extensive study on the PS-supported organocatalyst C-64, revealing its excellent catalytic activity and selectivity for the efficient synthesis of different WM and HPESW derivatives, as well as the possibility to recycle it and to use it in the first reported asymmetric Robinson annulation in continuous flow [97]. This catalyst was developed starting from the diamine C-20 previously reported by Luo et al. [69] and prepared via nucleophilic substitution of the chlorine atoms of commercially available Merrifield resin by the corresponding alcohol precursor of C-20. Starting from triketone 65 and using 10 mol% of C-64, accompanied by 10 mol% of TFSA and 5 mol% of m-nitrobenzoic acid, in 2-MeTHF at room temperature for 12 h, (R)-1 was obtained in excellent yield (94%) and selectivity (93% ee). Increasing the temperature to 55 °C, the reaction was completed in only 1 h without significant erosion of enantioselectivity (99% yield, 91% ee). These optimized conditions were efficiently applied in the one-pot Robinson annulation, starting directly from the corresponding dione and the MVK, leading to (R)-1 in 88% yield and 91% ee. It was possible to reduce the amount of C-63 to 2 mol%, carrying out the reaction on a large scale and heating to 60 °C for 12 h, obtaining (R)-1 in better yield (93%) and 90% ee.
The homogeneous catalysts C-20 and C-63 were efficient in the one-pot Robinson annulation, but in different reaction conditions compared to that individuated for PS-supported C-64; indeed, in 2-MeTHF the reactions were slower than that performed under neat conditions, while the observed enhancement in the reactivity for C-64 at higher temperatures was not as pronounced for the homogeneous catalysts. Using 10 mol% of C-63 with TFSA (10 mol%) and m-nitrobenzoic acid (5 mol%) in neat conditions at room temperature, (R)-1 was obtained in 80% yield and 94% ee after 16 h. Considering the optimized conditions for each catalyst, the PS-supported C-64 showed better catalytic activity and similar enantioselectivity compared to C-20 and C-63.
The PS-supported C-64 was very efficient in the one-pot Robinson annulation for the synthesis of different (R)-1 derivatives bearing a functionalized angular chain at 8a position ((S)-8, (S)-11, (S)-13, (S)-14, (S)-18, and (S)-23, 73–86% yield, 90–93% ee), (R)-2 (79% yield, 80% ee), two (R)-2 derivatives bearing a condensed benzo ring ((R)-60 and (R)-61, 90% and 75% yield, 89% and 58% ee), C-5 and C-4 methyl substituted (R)-1 and (R)-2 ((R)-28 and (R)-53, 87% and 61% yield, 82% and 42% ee), and (R)-1 derivative bearing a gem-dimethyl group at position 3 ((R)-27, 79% yield, 55% ee, or 51% yield, 97% ee after recrystallization).
Importantly, this supported organocatalyst was efficiently reused for 10 reaction cycles starting from triketone 65, without loss of enantioselectivity and with just slight decrease in reactivity. On the other hand, recycling in the one-pot Robinson annulation was not efficient, due to the formation of mono- and dialkylated derivatives of C-64 resulting from the aza-Michael addition to MVK.
Finally, the authors developed a continuous flow method for the large-scale preparation of (R)-1 (65.5 mmol in 24 h) and for a sequential flow preparation of eight enantioenriched diketone compounds, injecting the opportune triketone prepared in batch into the catalytic reactor (60 °C, 1.3 bar) in combination with m-nitrobenzoic acid in DMF and always reusing the same column packed with C-64·TFSA.
In 2018 Almenara, Rodríguez-Escrich, and Pericàs presented the PS-supported catalyst C-65, based on the structure of the homogeneous chiral phosphoric acids C-26 previously reported by Akiyama et al. [98]. In the optimized conditions, 20 mol% of C-65 in hexane at 70 °C for 48 h gave different benzo-condensed (R)-2 derivatives ((R)-60, (R)-61, (S)-62, (S)-63, and (S)-64) in excellent yield and selectivity, with similar efficiency compared to homogeneous catalyst C-26. However, lower catalytic activity and enantioselectivity were observed in the synthesis of bicyclic compounds (R)-1 (79% yield, 59% ee), (R)-2 (60% yield, 73% ee), (S)-11 (79% yield, 71% ee), and (R)-53 (68% yield, 47% ee). Remarkably, the authors demonstrated the possibility to easily recover C-65 by simple filtration and to reuse it at least nine times without appreciable decay in activity.
2.2. Exocyclic Carbonyl Analogues to HPESW and WM
In addition to the classical structures of 1, 2, and their derivatives, some interesting bicyclo[4.3.0]nonane and decalin are characterized by an exocyclic formyl group in the original positions 5 and 4 of WM and HPESW, respectively, instead of the carbonyl in position 6 or 5. Due to the reactivity of this formyl group, which can be reduced, oxidized, or subjected to several nucleophile additions, these WM and HPESW analogues could represent attractive starting materials for the synthesis of natural compounds. However, only a few examples of their asymmetric synthesis are actually present in literature.
In 2007, Hayashi and co-workers reported the asymmetric preparation of exocyclic formyl analogues to HPESW (S)-100a–c by an intramolecular aldol reaction starting from the corresponding diketoaldehydes 99a–c, in which the aldehyde act as donor and ketone as acceptor (Scheme 7) [99]. For the synthesis of (S)-100a, they evaluated different organocatalysts including L-Pro and some of its derivatives. Among these, better results were obtained with C-22 and TFA (30 mol%) in N-methyl-2-pyrrolidinone (NMP) at 0 °C for 56 h, leading to (S)-100a in 89% yield and 89% ee. Its absolute configuration was confirmed by an alternative synthesis starting from (S)-1. In addition, (S)-100b and (S)-100c, bearing an angular propyl or benzyloxymethyl group, were obtained in good yield and enantioselectivity (81% and 79% yield, 83% and 81% ee, respectively). In the proposed mechanism, the organocatalyst reacts with the aldehyde moiety of 99a to generate the enamine 101, which then reacts with one of the carbonyl groups via the transition state 102 to give iminium 103; the hydrolysis of the iminium ion generates the aldol product 104 with regeneration of the organocatalyst (Scheme 8).
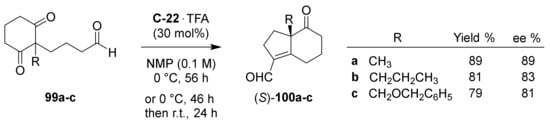
Scheme 7.
Asymmetric organocatalytic intramolecular aldol reaction towards bicyclic carbonyl compounds bearing an exocyclic formyl group ((S)-100a–c) [99].
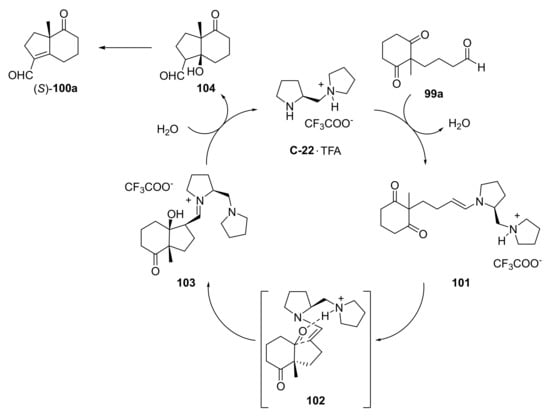
Scheme 8.
Proposed catalytic cycles for the intramolecular aldol reaction of diketoaldehyde 99a catalysed by C-22 · TFA [99].
Subsequently, in 2019 Leonelli and co-workers reported the asymmetrical synthesis of 106, proposing it as useful starting material for the preparation of different diterpenes (Scheme 9) [100]. For this purpose, they tested different amino acids as organocatalysts of the intramolecular aldol reaction of the diketoaldehyde 105, and the best results were obtained with L-tyrosine C-3 or its enantiomer (1.1 eq), in DMSO, 1M HClO4, at 10 °C for 20 h, giving (R)-106 or (S)-106, respectively (80% yield, 86% ee). The absolute configuration of (R)-106 was confirmed by X-ray analysis of a subsequent derivative proposed as potentially intermediate for the synthesis of (+)-oryzalexin S.
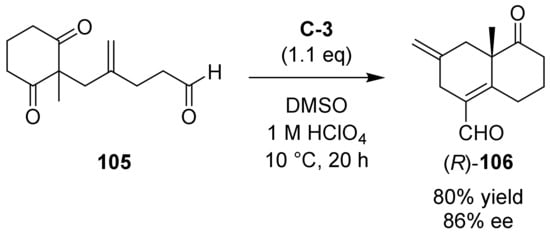
Scheme 9.
Asymmetric organocatalytic intramolecular aldol reaction towards bicyclic carbonyl compound (R)-106 bearing an exocyclic formyl group [100].
Recently, Hayashi et al. developed a domino Michael/aldol reaction of a nitroalkane with cyclohexane-1,3-dione moiety 108 and different α,β-unsaturated aldehydes 107a–k catalysed by C-25 to afford exocyclic formyl analogues to ketol 67 (Scheme 10) [101]. In the optimized conditions, starting from 3-(p-methoxyphenyl)propenal 107a, using 20 mol% of C-25, 20 mol% of p-nitrophenol, and 3 eq of water, in THF at 5 °C for 35 h, the reaction led to the formation of a diastereomeric mixture of 109a, 110a and 111a in 96% total yield and 87:7:6 dr, with almost optically pure 109a. Similar results were obtained employing other α,β-unsaturated aldehydes and carrying out the reaction at room temperature.
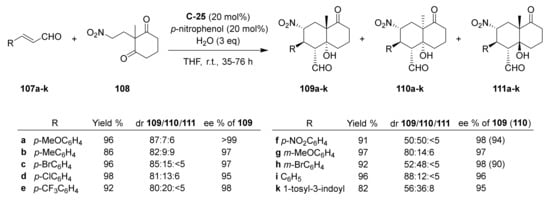
Scheme 10.
Asymmetric organocatalytic domino Michael/intramolecular aldol reaction towards bicyclic carbonyl compounds bearing an exocyclic formyl group [101].
Based on the studies carried out, the authors proposed a reaction mechanism in which, first, the Michael reaction between 107 and 108 generates the enamine 113 via the iminium ion 112; then the enamine further reacts via the intramolecular aldol reaction to afford compounds 109, 110, and 111. In a different path, the enamine 113 is hydrolyzed to afford the diketoaldehyde 115, which reacts with the organocatalyst to regenerate compound 113, or affords compounds 109, 110, and 111 by the intramolecular aldol reaction via enol 116 (Scheme 11).
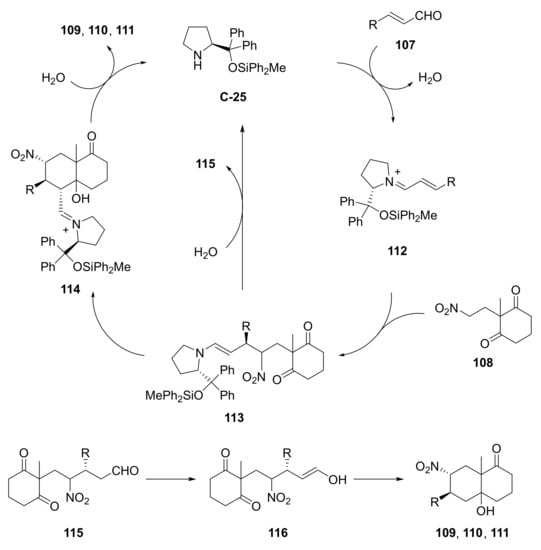
Scheme 11.
Proposed catalytic cycle for the asymmetric organocatalytic domino Michael/intramolecular aldol reaction towards bicyclic carbonyl compounds bearing an exocyclic formyl group catalysed by C-25 [101].
Very recently, Kawamoto, Ito et al. further studied the synthesis of this class of exocyclic carbonyl compounds, obtaining good results with the prolinamide catalyst C-41 under similar reaction conditions to those reported by Hayashi in 2007 (Scheme 12) [102]. Therefore, starting from the corresponding diketoaldehydes 117a–b using 0.3 eq of C-41, in NMP at rt for 5 days, they obtained ketols 118a–b as diastereomer mixture, and after the dehydration step by Burgess’ reagent, the products 119a–b, having both ketone and α,β-unsaturated aldehyde functionalities, with excellent ee (91–99%), but moderate overall yields.
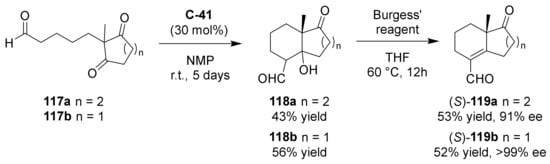
Scheme 12.
Asymmetric organocatalytic intramolecular aldol reaction towards bicyclic carbonyl compounds bearing an exocyclic formyl group ((S)-119a–b) [102].
3. Bicyclo[5.4.0]Undecane Derviatives
Although the Hajos–Parrish–Eder–Sauer–Wiechert reaction for the synthesis of ketones 1 and 2 and their derivatives was extensively studied, and different organocatalysts were efficient for the enantioselective preparation of these molecules, only a few studies to construct a bicyclic enedione containing a seven-membered ring with good enantiocontrol were reported (Figure 10).
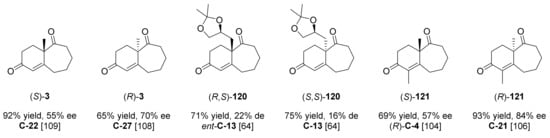
Figure 10.
Swaminathan analogues reported by the literature analysed in this review. For each compound, the best yield and ee were indicated, along with the organocatalyst used and the corresponding literature reference.
The ketone 3 was synthesised as raceme for the first time in 1966 by Swaminathan et al., starting from the corresponding trione 74 (Scheme 3, n = 3, R1 = CH3, R2 = R3 = H) by a pyrrolidine catalysed aldol reaction in the presence of acetic acid [103]. After that, subsequent attempts reported by Swaminathan et al. to obtain 3 and its derivatives using either Pro or a combination of S-Phe and D-CSA, in solvent or under neat conditions, did not give the expected optical active products [35]. Based on these results, it is clear that the HPESW reaction for the synthesis of 6–7 fused ring diverges considerably from those applied in the preparation of 5–6 or 6–6 fused compounds.
In 2005, Inomata et al. evaluated different amino acids in the synthesis of (R,S)-120 and (S,S)-120, bearing a chiral acetonide moiety in position 4a [64]. Differently from what observed for the synthesis of 16, with a 6-, low diastereoselectivity (up to 46%) was achieved with the different tested catalysts. The same diastereoselection, with (R,S)-120 as the major product, was obtained with both D- and L-amino acids, with the exception observed with the Pro derivative C-13 and its enantiomer. Indeed, C-13 (1.1 eq, MeOH, reflux, 2.5 h) gave (S,S)-120 as the major product (75% yield, 16% de), while using ent-C-13 (1.1 eq, MeOH, reflux, 1.5 h) the product (R,S)-120 was achieved (71% yield, 22% de). The absolute configuration of (S,S)-120 was confirmed by X-ray analysis of its corresponding keto alcohol.
In 2007, Inomata, Paquette et al. continued to study the application of different amino acids for the synthesis of 121, bearing a methyl group in position 1 [104]. Starting from the corresponding triketone 74 (Scheme 3, n = 3, R1 = R3 = CH3, R2 = H), using 1.0 eq of L-amino acids as catalyst, 0.5 eq of 1N HClO4 in DMSO, at 90 °C, most of the reactions gave (R)-121 as the major product, with an inversion of enantioselectivity compared to the same reaction involving the 2-methyl-2-(3-oxopentyl)cyclohexane-1,3-dione (and other triketones for the synthesis of 1, 2 and analogues). In these conditions, among the studied amino acids, the best results both for yield and enantioselectivity were obtained with L-Phe (after 100 h, 86% yield, 48% ee) and L-methionine (L-Met) C-4 (after 96 h, 99% yield, 53% ee). Although the best ee value was moderate, the authors revealed that optically pure (R)-121 could be obtained by fractional crystallization. An improvement in reaction time and in ee was achieved using as co-catalyst 0.8 eq of D-CSA (after 21 h, 91% yield, 56% ee). The reaction carried out in the presence of D-Met instead of L-Met, using as co-catalyst 0.5 eq of D-CSA, led to an inverted enantioselectivity, with formation of (S)-121 (after 40 h, 69% yield, 57% ee). The absolute configuration of (R)- and (S)-121 was confirmed by an alternative synthesis of (S)-121 starting from (S)-28 and by comparison of their relative optical rotations. Among the proposed eight transition states for the reaction in the presence of L-amino acid, the (Z)-trans-anti 122 (Figure 11) should be the most favoured due to the repulsions for steric hindrance in the others’ transition states, giving rise to the formation of (R)-121 after dehydration. The inversion of enantioselectivity with lower ee observed in the absence of Brønsted acid could be due to a dissociation of the proton from the carboxylic acid moiety and the consequent formation of a different acyclic transition state. The opposite enantioselectivity in the synthesis of 1, 2, and their derivatives is due to the fact that the (Z)-cis-anti transition state 123 is the favoured one, because in this case the smaller five- or six-membered rings do not lead to the steric hindrance present in the case of the seven-membered ring.
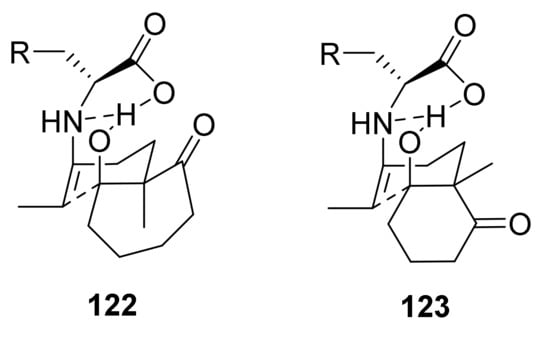
Figure 11.
Favoured (Z)-trans-anti transition state model 122 for the reaction of L-amino acid and the triketone with seven-membered ring, and favoured (Z)-cis-anti transition state model 123 for the reaction of L-amino acid and the triketone with six-membered ring, proposed by Inomata, Paquette et al. [104].
Again, Inomata and co-workers individuated other, more efficient reaction conditions for the synthesis of (R)-121 [105]. Another L-amino acid considered a good organocatalyst for this reaction was L-NorLeu C-5, which, used in equimolar amount in combination with 0.5 eq of PPTS, in DMSO at 90 °C for 24 h, gave (R)-121 in 86% yield and 63% ee. An improvement in both yield and selectivity applying L-Met, compared to the previously reported conditions, were achieved using as co-catalyst 0.5 eq of TFA or TFSA, leading to (R)-121 after 21 h in 95–96% yield and 60–61% ee. Better enantioselectivity (68% ee), but at the expense of yield and reaction time (86% in 7 days), was obtained carrying out the reaction at 50 °C instead of 90 °C.
Subsequently, the same authors investigated different chiral primary amine catalysts for the synthesis of 121 [106]. The best results were obtained employing an equimolar amount of C-21 and 2.0 eq of TFA, in DMSO at 90 °C for 138 h, giving (R)-121 in excellent yield and high selectivity (93% yield, 84% ee). When no, or a lesser amount of, TFA was used, an inversion of enantioselectiviy and lower ee were observed, while using catalytic amounts of C-21 was not effective. With this catalyst, in the favoured transition state proposed, the protonated pyrrolidinylmethyl moiety acts as the carboxylic acid in the previously reported transition state 122, forming hydrogen bonds with the β-oxygen atom originated from ketone functionality and with the nitrogen atom in the enamine moiety, stabilizing the transition state.
As regards the preparation of the Swaminathan ketone 3, in 2016 Uwai et al. proposed an asymmetric lipase-catalysed synthesis of (S)-3, as well as (S)-1 and (S)-2. However, these compounds were obtained with low enantioselectivity (8% ee for (S)-3) [107]. After that, in 2017 Pericàs and co-workers applied the PS-supported organocatalyst C-64 (in the optimized conditions discussed in the paragraph 2.2) also in the one-pot Robinson annulation for the synthesis of (R)-3, starting directly from the dione 72 (Scheme 3, n = 3, R1 = CH3, R2 = H) and the MVK [97]. In this way, (R)-3 was obtained in 77% yield and 53% ee, after 12 h of reaction. However, both Uwai et al. and Pericàs and co-workers did not provide any evidence for the determination of the absolute configuration of 3.
In 2019, Xu and co-workers reported the total asymmetric synthesis of the natural alkaloid (-)-himalensine A, using (R)-3 as starting material [108]. For this purpose, they obtained (R)-3 in 100 g scale in moderate yield and good enantioselectivity (65% yield, 70% ee), starting from the corresponding triketone and employing 1 eq of L-prolinamide C-27, accompanied with 1 eq of AcOH, in CH2Cl2 at room temperature for 24 h. Therefore, with this catalyst, the absolute configuration of 3, unambiguously confirmed by X-ray analysis and by an alternative synthesis starting from (R)-1, is opposite to that of 1 and 2 obtained in similar conditions.
Recently, in 2020 Inomata et al. applied the chiral proline amines C-22 and C-23 for the synthesis of (S)-3 [109]. In the optimized conditions, using 1 eq of C-22, in the presence of 1.5 eq of TFA, in DMSO at 50 °C for 26 h, (S)-3 was obtained in 92% yield and 55% ee; in the same conditions, C-23 showed low efficiency both for activity and selectivity. The authors also clarified the relations among absolute configurations of (S)- and (R)-3 with optical rotation and HPLC behaviour.
4. Conclusions
In summary, in this review we described the several asymmetric organocatalysts and the different procedures employed for the stereoselective synthesis of WM, HPESW, Swaminathan ketones, and their analogues, starting from 1971, when the first asymmetric syntheses of these compounds were reported, until today. As regards WM and HPESW analogues, a large number of asymmetric organocatalysts were studied, including amino acids, primary and secondary amines, prolinamides, and supported organocatalysts. Proline C-1, phenylalanine C-2, the primary amine C-20 and its supported derivatives C-64, the prolinamide C-27, and the binam prolinamide C-44 are among the best stereoselective catalysts reported, with a wide applicability for the synthesis of different WM and HPESW derivatives. Generally, the developed methodologies have allowed for obtaining these classes of compounds with an excellent level of stereocontrol and with high yields. However, some procedures suffer from important drawbacks, such as long reaction times or high catalyst loading. Therefore, there is space for further improvements and for the determination of innovative procedures to avoid time-, energy-, and cost-consuming processes. As regards WM and HPESW analogues with an exocyclic formyl group, only a few examples of their asymmetric synthesis are actually present in literature, although they may represent attractive starting materials for the synthesis of natural compounds.
The stereoselective synthesis of the Swaminathan ketone and analogues was more complex compared to the corresponding bicyclic diketones containing a six- or five-membered ring; indeed, catalysts commonly used for asymmetrical synthesis of WM and HPESW analogues showed limited efficiency for the stereoselective preparation of this bicyclic scaffold with a seven-membered ring. Moreover, only a limited number of asymmetric organocatalysts have been reported for the synthesis of these derivatives, allowing for further studies and improvements for future research.
Author Contributions
Conceptualization, M.B., F.V. and F.L.; data collection, M.B., L.P., F.P., F.V.; data analysis, M.B., L.P., F.P., F.V.; writing—original draft preparation, M.B.; writing—review and editing, F.P., L.P., F.L., F.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dondoni, A.; Massi, A. Asymmetric organocatalysis: From infancy to adolescence. Angew. Chem. Int. Ed. 2008, 47, 4638–4660. [Google Scholar] [CrossRef] [PubMed]
- Berkessel, A.; Groerger, H. Asymmetric Organocatalysis; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2005. [Google Scholar]
- Enders, D.; Grondal, C.; Huettl, M.R.M. Asymmetric organocatalytic domino reactions. Angew. Chem. Int. Ed. 2007, 46, 1570–1581. [Google Scholar] [CrossRef] [PubMed]
- Volla, C.M.R.; Atodiresei, I.; Rueping, M. Catalytic C-C bond-forming multi-component cascade or domino reactions: Pushing the boundaries of complexity in asymmetric organocatalysis. Chem. Rev. 2014, 114, 2390–2431. [Google Scholar] [CrossRef] [PubMed]
- Grondal, C.; Jeanty, M.; Enders, D. Organocatalytic cascade reactions as a new tool in total synthesis. Nat. Chem. 2010, 2, 167–178. [Google Scholar] [CrossRef]
- List, B.; Lerner, R.A.; Barbas, C.F. Proline-catalyzed direct asymmetric aldol reactions. J. Am. Chem. Soc. 2000, 122, 2395–2396. [Google Scholar] [CrossRef]
- Ahrendt, K.A.; Borths, C.J.; MacMillan, D.W.C. New Strategies for Organic Catalysis: The First Highly Enantioselective Organocatalytic Diels-Alder Reaction. J. Am. Chem. Soc. 2000, 122, 4243–4244. [Google Scholar] [CrossRef]
- Antenucci, A.; Dughera, S.; Renzi, P. Green Chemistry Meets Asymmetric Organocatalysis: A Critical Overview on Catalysts Synthesis. ChemSusChem 2021, 14, 2785–2853. [Google Scholar] [CrossRef]
- Bortolami, M.; Leonelli, F.; Feroci, M.; Vetica, F. Step economy in the Stereoselective Synthesis of Functionalized Oxindoles via Organocatalytic Domino/One-pot Reactions. Curr. Org. Chem. 2021, 25, 1321–1344. [Google Scholar] [CrossRef]
- Chen, X.Y.; Li, S.; Vetica, F.; Kumar, M.; Enders, D. N-Heterocyclic-Carbene-Catalyzed Domino Reactions via Two or More Activation Modes. iScience 2018, 2, 1–26. [Google Scholar] [CrossRef]
- Gasperi, T.; Orsini, M.; Vetica, F.; de Figueiredo, R.M. Organocatalytic Asymmetric Multicomponent Reactions. In Multicomponent Reactions. Concepts and Applications for Design and Synthesis; Herrera, R.P., Marqués-López, E., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 16–71. [Google Scholar]
- Vetica, F.; Bortolami, M.; Petrucci, R.; Rocco, D.; Feroci, M. Electrogenerated NHCs in Organic Synthesis: Ionic Liquids vs Organic Solvents Effects. Chem. Rec. 2021, 21, 2130–2147. [Google Scholar] [CrossRef]
- Vetica, F.; Chauhan, P.; Dochain, S.; Enders, D. Asymmetric organocatalytic methods for the synthesis of tetrahydropyrans and their application in total synthesis. Chem. Soc. Rev. 2017, 46, 1661–1674. [Google Scholar] [CrossRef]
- Vetica, F.; de Figueiredo, R.M.; Orsini, M.; Tofani, D.; Gasperi, T. Recent Advances in Organocatalytic Cascade Reactions toward the Formation of Quaternary Stereocenters. Synthesis 2015, 47, 2139–2184. [Google Scholar]
- Hajos, Z.G.; Parrish, D.R. Asymmetrische Synthese Polycyclischer Organischer Verbindungen. Patent DE2102623A1, 20 January 1971. [Google Scholar]
- Hajos, Z.G.; Parrish, D.R. Asymmetric synthesis of bicyclic intermediates of natural product chemistry. J. Org. Chem. 1974, 39, 1615–1621. [Google Scholar] [CrossRef]
- Eder, U.; Wiechert, R.; Sauer, G. Verfahren zur Herstellung Optisch Aktiver Bicycloalkan-Derivate. Patent DE2014757A1, 20 March 1971. [Google Scholar]
- Eder, U.; Sauer, G.; Wiechert, R. New Type of Asymmetric Cyclization to Optically Active Steroid CD Partial Structures. Angew. Chem. Int. Ed. 1971, 10, 496–497. [Google Scholar] [CrossRef]
- Wieland, P.; Miescher, K. Über die Herstellung mehrkerniger Ketone. Helv. Chim. Acta 1950, 33, 2215–2228. [Google Scholar] [CrossRef]
- Bradshaw, B.; Bonjoch, J. The Wieland-Miescher ketone: A journey from organocatalysis to natural product synthesis. Synlett 2012, 23, 337–356. [Google Scholar] [CrossRef]
- Hagiwara, H. Aspects in the Total Syntheses of Higher Terpenoids Starting from Wieland–Miescher Ketone and Its Derivative: A Review. Nat. Prod. Commun. 2020, 15, 1934578X20925340. [Google Scholar] [CrossRef]
- Leonelli, F.; Valletta, A.; Migneco, L.M.; Marini Bettolo, R. Stemarane Diterpenes and Diterpenoids. Int. J. Mol. Sci. 2019, 20, 2627. [Google Scholar] [CrossRef]
- Leonelli, F.; Migneco, L.M.; Valletta, A.; Marini Bettolo, R. Stemodane Diterpenes and Diterpenoids: Isolation, Structure Elucidation, Biogenesis, Biosynthesis, Biological Activity, Biotransformations, Metabolites and Derivatives Biological Activity, Rearrangements. Molecules 2021, 26, 2761. [Google Scholar] [CrossRef]
- Armstrong, A.; Dingwall, P. Mechanistic Understanding of Proline Analogs and Related Protic Lewis Bases (n-π*). In Lewis Base Catalysis in Organic Synthesis; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016; pp. 145–190. [Google Scholar]
- Bahmanyar, S.; Houk, K.N. Transition States of Amine-Catalyzed Aldol Reactions Involving Enamine Intermediates: Theoretical Studies of Mechanism, Reactivity, and Stereoselectivity. J. Am. Chem. Soc. 2001, 123, 11273–11283. [Google Scholar] [CrossRef]
- Bahmanyar, S.; Houk, K.N. The Origin of Stereoselectivity in Proline-Catalyzed Intramolecular Aldol Reactions. J. Am. Chem. Soc. 2001, 123, 12911–12912. [Google Scholar] [CrossRef] [PubMed]
- Hoang, L.; Bahmanyar, S.; Houk, K.N.; List, B. Kinetic and Stereochemical Evidence for the Involvement of Only One Proline Molecule in the Transition States of Proline-Catalyzed Intra- and Intermolecular Aldol Reactions. J. Am. Chem. Soc. 2003, 125, 16–17. [Google Scholar] [CrossRef] [PubMed]
- Clemente, F.R.; Houk, K.N. Computational Evidence for the Enamine Mechanism of Intramolecular Aldol Reactions Catalyzed by Proline. Angew. Chem. Int. Ed. 2004, 43, 5766–5768. [Google Scholar] [CrossRef] [PubMed]
- List, B.; Hoang, L.; Martin, H.J. New mechanistic studies on the proline-catalyzed aldol reaction. Proc. Natl. Acad. Sci. USA 2004, 101, 5839–5842. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, S.; Syed, A.; Brock, C.P.; Watt, D.S. Enantioselective Synthesis of (-)-(7aS)-2,3,7,7a-Tetrahydro-7a-phenylthio-1H-indene-1,5(6H)-dione and (+)-(8aS)-3,4,8,8a-Tetrahydro-8a-phenylthio-1,6(2H,7H)-naphthalenedione. Synthesis 1989, 1989, 818–820. [Google Scholar] [CrossRef]
- Tamai, Y.; Mizutani, Y.; Uda, H.; Harada, N. The absolute stereochemistry of Wieland–Miescher ketone analogues bearing an angular protected hydroxymethyl group. J. Chem. Soc. Chem. Commun. 1983, 3, 114–116. [Google Scholar] [CrossRef]
- Harada, N.; Sugioka, T.; Uda, H.; Kuriki, T. Efficient Preparation of Optically Pure Wieland-Miescher Ketone and Confirmation of Its Absolute Stereochemistry by the CD Exciton Chirality Method. Synthesis 1990, 1990, 53–56. [Google Scholar] [CrossRef]
- Hanselmann, R.; Benn, M. Enantioselective Synthesis of a Wieland-Miescher Ketone Bearing an Angular Hydroxymethyl Group. Synth. Commun. 1996, 26, 945–961. [Google Scholar] [CrossRef]
- Schneider, L.M.; Schmiedel, V.M.; Pecchioli, T.; Lentz, D.; Merten, C.; Christmann, M. Asymmetric Synthesis of Carbocyclic Propellanes. Org. Lett. 2017, 19, 2310–2313. [Google Scholar] [CrossRef]
- Rajagopal, D.; Narayanan, R.; Swaminathan, S. Enantioselective solvent-free Robinson annulation reactions. Proc. Indian Acad. Sci. 2001, 113, 197–213. [Google Scholar] [CrossRef]
- Srivastava, V. Recyclable L-proline organocatalyst for Wieland–Miescher ketone synthesis. J. Chem. Sci. 2013, 125, 1523–1527. [Google Scholar] [CrossRef]
- Srivastava, V. PEG-Solvent System for L-proline Catalyzed Wieland-Miescher Ketone Synthesis. Curr. Organocatal. 2014, 1, 2–6. [Google Scholar] [CrossRef]
- Bui, T.; Barbas, C.F., III. A proline-catalyzed asymmetric Robinson annulation reaction. Tetrahedron Lett. 2000, 41, 6951–6954. [Google Scholar] [CrossRef]
- Rajagopal, D.; Narayanan, R.; Swaminathan, S. Asymmetric one-pot Robinson annulations. Tetrahedron Lett. 2001, 42, 4887–4890. [Google Scholar] [CrossRef]
- Ramachary, D.B.; Kishor, M. Organocatalytic Sequential One-Pot Double Cascade Asymmetric Synthesis of Wieland−Miescher Ketone Analogues from a Knoevenagel/Hydrogenation/Robinson Annulation Sequence: Scope and Applications of Organocatalytic Biomimetic Reductions. J. Org. Chem. 2007, 72, 5056–5068. [Google Scholar] [CrossRef]
- Ramachary, D.B.; Kishor, M. Direct amino acid-catalyzed cascade biomimetic reductive alkylations: Application to the asymmetric synthesis of Hajos–Parrish ketone analogues. Org. Biomol. Chem. 2008, 6, 4176–4187. [Google Scholar] [CrossRef]
- Kennedy, J.W.J.; Vietrich, S.; Weinmann, H.; Brittain, D.E.A. Synthesis of 7a-Substituted Hajos−Wiechert Ketone Analogues. J. Org. Chem. 2008, 73, 5151–5154. [Google Scholar] [CrossRef]
- Danishefsky, S.; Cain, P. Optically specific synthesis of estrone and 19-norsteroids from 2,6-lutidine. J. Am. Chem. Soc. 1976, 98, 4975–4983. [Google Scholar] [CrossRef]
- Shimizu, I.; Naito, Y.; Tsuji, J. Synthesis of optically active (+)-19-nortestosterone by asymmetric bis-annulation reaction. Tetrahedron Lett. 1980, 21, 487–490. [Google Scholar] [CrossRef]
- Agami, C.; Meynier, F.; Puchot, C.; Guilhem, J.; Pascard, C. Stereochemistry-59: New insights into the mechanism of the proline-catalyzed asymmetric robinson cyclization; structure of two intermediates. Asymmetric dehydration. Tetrahedron 1984, 40, 1031–1038. [Google Scholar] [CrossRef]
- Uma, R.; Swaminathan, S.; Rajagopalan, K. Base catalyzed rearrangement of oxy-cope systems. Tetrahedron Lett. 1984, 25, 5825–5828. [Google Scholar] [CrossRef]
- Hanquet, G.; Lanfranchi, D.; Baldovini, N. Large-Scale Preparation of Enantiomerically Pure (4aR)-(-)-1,4a-Dimethyl-4,4a,7,8-tetrahydronaphthalene-2,5(3H,6H)-dione: A Useful Wieland-Miescher Diketone Analogue. Synthesis 2008, 2008, 3775–3778. [Google Scholar] [CrossRef]
- Hagiwara, H.; Uda, H. Optically pure (4as)-(+)- or (4ar)-(-)-1,4a-dimethyl-4,4a,7,8-tetrahydronaphthalene-2,5(3H,6H)-dione and its use in the synthesis of an inhibitor of steroid-biosynthesis. J. Org. Chem. 1988, 53, 2308–2311. [Google Scholar] [CrossRef]
- Sakai, H.; Hagiwara, H.; Ito, Y.; Hoshi, T.; Suzuki, T.; Ando, M. Total synthesis of (+)-cyclomyltaylan-5α-ol isolated from the taiwanese liverwort Reboulia hemisphaerica. Tetrahedron Lett. 1999, 40, 2965–2968. [Google Scholar] [CrossRef]
- Corey, E.J.; Virgil, S.C. Enantioselective total synthesis of a protosterol, 3β,20-dihydroxyprotost-24-ene. J. Am. Chem. Soc. 1990, 112, 6429–6431. [Google Scholar] [CrossRef]
- Przeździecka, A.; Stepanenko, W.; Wicha, J. Catalytic enantioselective annulation using phenylsulfanylmethyl vinyl ketone. An approach to trans-hydrindane building blocks for ent-vitamin D3 synthesis. Tetrahedron Asymmetry 1999, 10, 1589–1598. [Google Scholar] [CrossRef]
- Zhou, G.; Gao, X.; Li, W.Z.; Li, Y. An enantioselective synthetic strategy toward the polyhydroxylated agarofuran. Tetrahedron Lett. 2001, 42, 3101–3103. [Google Scholar] [CrossRef]
- Nguyen, T.X.; Dakanali, M.; Trzoss, L.; Theodorakis, E.A. Enantioselective Synthesis of the ABC Ring Motif of Norzoanthamine Based on Asymmetric Robinson Annulation Reactions. Org. Lett. 2011, 13, 3308–3311. [Google Scholar] [CrossRef]
- Nozawa, M.; Akita, T.; Hoshi, T.; Suzuki, T.; Hagiwara, H. Recyclable asymmetric cyclization in ionic liquid catalyzed by an amino acid, leading to a Wieland-Miescher ketone analogue. Synlett 2007, 4, 0661–0663. [Google Scholar]
- Shigehisa, H.; Mizutani, T.; Tosaki, S.Y.; Ohshima, T.; Shibasaki, M. Formal total synthesis of (+)-wortmannin using catalytic asymmetric intramolecular aldol condensation reaction. Tetrahedron 2005, 61, 5057–5065. [Google Scholar] [CrossRef]
- Kaplan, H.Z.; Rendina, V.L.; Kingsbury, J.S. General Methodologies Toward cis-Fused Quinone Sesquiterpenoids. Enantiospecific Synthesis of the epi-Ilimaquinone Core Featuring Sc-Catalyzed Ring Expansion. Molecules 2017, 22, 1041. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, T.; Watanabe, K.; Kato, Y. Asymmetric Synthesis of (S)-Homoproline and (S)-Homopipecolic Acid1. Synth. Commun. 1977, 7, 239–244. [Google Scholar] [CrossRef]
- Buchschacher, P.; Cassal, J.M.; Fürst, A.; Meier, W. Beitrag zur asymmetrischen Synthese bicyclischer Verbindungen unter Katalyse mit optisch aktiven Aminosäuren. Synthese von (S)-2-Pyrrolidin-propionsäure und (R)-4-Amino-5-phenylvaleriansäure. Helv. Chim. Acta 1977, 60, 2747–2755. [Google Scholar] [CrossRef]
- Banerjee, D.K.; Kasturi, T.R.; Sarkar, A. Chiral synthesis of optically active S(+)-2,6,7,7a-tetrahydro-1β-hydroxy-4-formyl-7aβ-methylindene. Proc. Indian Acad. Sci. 1983, 92, 181–187. [Google Scholar] [CrossRef]
- Limbach, M. β-Homoamino acids as catalysts in enantioselective intra-and intermolecular aldol reactions. Tetrahedron Lett. 2006, 47, 3843–3847. [Google Scholar] [CrossRef]
- Cheong, P.H.Y.; Houk, K.N.; Warrier, J.S.; Hanessian, S. Catalysis of the Hajos—Parrish—Eder—Sauer—Wiechert Reaction by cis-and trans-4,5-Methanoprolines: Sensitivity of Proline Catalysis to Pyrrolidine Ring Conformation. Adv. Synth. Catal. 2004, 346, 1111–1115. [Google Scholar] [CrossRef]
- Davies, S.G.; Sheppard, R.L.; Smith, A.D.; Thomson, J.E. Highly enantioselective organocatalysis of the Hajos-Parrish-Eder-Sauer-Wiechert reaction by the beta-amino acid cispentacin. Chem. Commun. 2005, 30, 3802–3804. [Google Scholar] [CrossRef]
- Davies, S.G.; Russell, A.J.; Sheppard, R.L.; Smith, A.D.; Thomson, J.E. Evaluating β-amino acids as enantioselective organocatalysts of the Hajos–Parrish–Eder–Sauer–Wiechert reaction. Org. Biomol. Chem. 2007, 5, 3190–3200. [Google Scholar] [CrossRef]
- Inomata, K.; Barragué, M.; Paquette, L.A. Diastereoselectivities realized in the amino acid catalyzed aldol cyclizations of triketo acetonides of differing ring size. J. Org. Chem. 2005, 70, 533–539. [Google Scholar] [CrossRef]
- Kanger, T.; Kriis, K.; Laars, M.; Kailas, T.; Müürisepp, A.-M.; Pehk, T.; Lopp, M. Enantioselective Synthesis of Wieland-Miescher Ketone through Bimorpholine-Catalyzed Organocatalytic Aldol Condensation. Synlett 2006, 2006, 1699–1702. [Google Scholar] [CrossRef]
- Kanger, T.; Kriis, K.; Laars, M.; Kailas, T.; Müürisepp, A.-M.; Pehk, T.; Lopp, M. Bimorpholine-mediated enantioselective intramolecular and intermolecular aldol condensation. J. Org. Chem. 2007, 72, 5168–5173. [Google Scholar] [CrossRef]
- Laars, M.; Kriis, K.; Kailas, T.; Müürisepp, A.-M.; Pehk, T.; Kanger, T.; Lopp, M. Structural constraints for C2-symmetric heterocyclic organocatalysts in asymmetric aldol reactions. Tetrahedron Asymmetry 2008, 19, 641–645. [Google Scholar] [CrossRef]
- Fuentes de Arriba, A.L.; Seisdedos, D.G.; Simon, L.; Alcazar, V.; Raposo, C.; Moran, J.R. Synthesis of Monoacylated Derivatives of 1,2-Cyclohexanediamine. Evaluation of their Catalytic Activity in the Preparation of Wieland−Miescher Ketone. J. Org. Chem. 2010, 75, 8303–8306. [Google Scholar] [CrossRef]
- Zhou, P.; Zhang, L.; Luo, S.; Cheng, J.-P. Asymmetric Synthesis of Wieland–Miescher and Hajos–Parrish Ketones Catalyzed by an Amino-Acid-Derived Chiral Primary Amine. J. Org. Chem. 2012, 77, 2526–2530. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, L.; Zhou, P.; Luo, S.; Cheng, J.P. A Practical Protocol for Asymmetric Synthesis of Wieland–Miescher and Hajos–Parrish Ketones Catalyzed by a Simple Chiral Primary Amine. Synthesis 2013, 45, 1939–1945. [Google Scholar]
- Mori, K.; Katoh, T.; Suzuki, T.; Noji, T.; Yamanaka, M.; Akiyama, T. Chiral Phosphoric Acid Catalyzed Desymmetrization of meso-1,3-Diones: Asymmetric Synthesis of Chiral Cyclohexenones. Angew. Chem. Int. Ed. 2009, 48, 9652–9654. [Google Scholar] [CrossRef]
- Akahane, Y.; Inage, N.; Nagamine, T.; Inomata, K.; Endo, Y. An alternative chiral synthesis of Wieland-Miescher ketone mediated by (S)-2-(pyrrolidinylmethyl)pyrrolidine: Remarkable effects of Brønsted acid. Heterocycles 2007, 74, 637–648. [Google Scholar] [CrossRef]
- Akahane, Y.; Inomata, K.; Endo, Y. A New Chiral Synthesis of Wieland-Miescher Ketone Catalyzed by a Combination of (S)-N-Benzyl-N-(2-pyrrolidinylmethyl)amine Derivative and Brønsted Acid. Heterocycles 2009, 77, 1065–1078. [Google Scholar] [CrossRef]
- Akahane, Y.; Inomata, K.; Endo, Y. Asymmetric intramolecular aldol reaction mediated by (S)-N-substituted-N-(2-pyrrolidinylmethyl)amine to prepare Wieland-Miescher ketone. Heterocycles 2011, 82, 1727–1737. [Google Scholar] [CrossRef]
- Lacoste, E.; Vaique, E.; Berlande, M.; Pianet, I.; Vincent, J.M.; Landais, Y. Benzimidazole-pyrrolidine/H+ (BIP/H+), a Highly Reactive Organocatalyst for Asymmetric Processes. Eur. J. Org. Chem. 2007, 2007, 167–177. [Google Scholar] [CrossRef]
- Zhang, F.-M.; Tu, Y.-Q.; Zhang, X.-M.; Wang, M.; Fan, C.-A.; Jiang, Y.-J.; Zhang, S.-Y. Prolinamide/PPTS-Catalyzed Hajos-Parrish Annulation: Efficient Approach to the Tricyclic Core of Cylindricine-Type Alkaloids. Synlett 2008, 2008, 2831–2835. [Google Scholar] [CrossRef]
- Xu, J.; Trzoss, L.; Chang, W.K.; Theodorakis, E.A. Enantioselective Total Synthesis of (−)-Jiadifenolide. Angew. Chem. Int. Ed. 2011, 50, 3672–3676. [Google Scholar] [CrossRef]
- Trzoss, L.; Xu, J.; Lacoske, M.H.; Mobley, W.C.; Theodorakis, E.A. Illicium sesquiterpenes: Divergent synthetic strategy and neurotrophic activity studies. Chemistry 2013, 19, 6398–6408. [Google Scholar] [CrossRef]
- De Arriba, Á.L.F.; Simon, L.; Raposo, C.; Alcazar, V.; Morán, J.R. Proline imidazolidinones and enamines in Hajos–Wiechert and Wieland–Miescher ketone synthesis. Tetrahedron 2009, 65, 4841–4845. [Google Scholar] [CrossRef]
- Kapras, V.; Vyklicky, V.; Budesinsky, M.; Cisarova, I.; Vyklicky, L.; Chodounska, H.; Jahn, U. Total synthesis of ent-Pregnanolone sulfate and its biological investigation at the NMDA receptor. Org. Lett. 2018, 20, 946–949. [Google Scholar] [CrossRef]
- Pan, L.; Ding, X.; Ding, J.; Li, D.; Chen, J.; Zuo, X.; An, R. Design and Synthesis of L-Proline Derivatives as Enantioselective Organocatalysts for Synthesis of the (S)-Wieland-Miescher Ketone. ChemistrySelect 2017, 2, 11999–12005. [Google Scholar] [CrossRef]
- Vizcaíno-Milla, P.; Sansano, J.M.; Nájera, C.; Fiser, B.; Gómez-Bengoa, E. Pyrimidine-Derived Prolinamides as Recoverable Bifunctional Organocatalysts for Enantioselective Inter- and Intramolecular Aldol Reactions under Solvent-Free Conditions. Eur. J. Org. Chem. 2015, 2015, 2614–2621. [Google Scholar] [CrossRef]
- D’Elia, V.; Zwicknagl, H.; Reiser, O. Short alpha/beta-peptides as catalysts for intra- and intermolecular aldol reactions. J. Org. Chem. 2008, 73, 3262–3265. [Google Scholar] [CrossRef]
- Almaşi, D.; Alonso, D.A.; Nájera, C. Prolinamides versus Prolinethioamides as Recyclable Catalysts in the Enantioselective Solvent-Free Inter- and Intramolecular Aldol Reactions. Adv. Synth. Catal. 2008, 350, 2467–2472. [Google Scholar] [CrossRef]
- Almaşi, D.; Alonso, D.A.; Balaguer, A.N.; Najera, C. Water versus Solvent-Free Conditions for the Enantioselective Inter- and Intramolecular Aldol Reaction Employing L-Prolinamides and L-Prolinethioamides as Organocatalysts. Adv. Synth. Catal. 2009, 351, 1123–1131. [Google Scholar] [CrossRef]
- Guillena, G.; Najera, C.; Viozquez, S.F. N-Tosyl-(Sa)-binam-L-prolinamide as highly efficient bifunctional organocatalyst for the general enantioselective solvent-free aldol reaction. Synlett 2008, 19, 3031–3035. [Google Scholar] [CrossRef]
- Bradshaw, B.; Etxebarría-Jardi, G.; Bonjoch, J.; Viózquez, S.F.; Guillena, G.; Nájera, C. Efficient Solvent-Free Robinson Annulation Protocols for the Highly Enantioselective Synthesis of the Wieland–Miescher Ketone and Analogues. Adv. Synth. Catal. 2009, 351, 2482–2490. [Google Scholar] [CrossRef]
- Liu, C.; Bradshaw, B.; Maseras, F.; Bonjoch, J.; Besora, M. Mechanistic Study on the Asymmetric Synthesis of the Wieland-Miescher Ketone and Analogs. ChemCatChem 2019, 11, 4064–4071. [Google Scholar] [CrossRef]
- Rubio, O.H.; Fuentes de Arriba, Á.L.; Monleón, L.M.; Sanz, F.; Simón, L.; Alcázar, V.; Morán, J.R. Bifunctional organocatalysts based on a carbazole scaffold for the synthesis of the Hajos–Wiechert and Wieland–Miescher ketones. Tetrahedron 2015, 71, 1297–1303. [Google Scholar] [CrossRef]
- Monge-Marcet, A.; Pleixats, R.; Cattoën, X.; Man, M.W.C.; Alonso, D.A.; Nájera, C. Prolinamide bridged silsesquioxane as an efficient, eco-compatible and recyclable chiral organocatalyst. New J. Chem. 2011, 35, 2766–2772. [Google Scholar] [CrossRef]
- Monge-Marcet, A.; Cattoën, X.; Alonso, D.A.; Nájera, C.; Man, M.W.C.; Pleixats, R. Recyclable silica-supported prolinamide organocatalysts for direct asymmetric Aldol reaction in water. Green Chem. 2012, 14, 1601–1610. [Google Scholar] [CrossRef]
- Bañón-Caballero, A.; Guillena, G.; Nájera, C.; Faggi, E.; Sebastián, R.M.; Vallribera, A. Recoverable silica-gel supported binam-prolinamides as organocatalysts for the enantioselective solvent-free intra- and intermolecular aldol reaction. Tetrahedron 2013, 69, 1307–1315. [Google Scholar] [CrossRef]
- Ferré, M.; Cattoën, X.; Wong Chi Man, M.; Pleixats, R. Recyclable Silica-Supported Proline Sulphonamide Organocatalysts for Asymmetric Direct Aldol Reaction. ChemistrySelect 2016, 1, 6741–6748. [Google Scholar] [CrossRef]
- Benaglia, M.; Cinquini, M.; Cozzi, F.; Puglisi, A.; Celentano, G. Poly(Ethylene Glycol)-Supported Proline: A Versatile Catalyst for the Enantioselective Aldol and Iminoaldol Reactions. Adv. Synth. Catal. 2002, 344, 533–542. [Google Scholar] [CrossRef]
- Kondo, K.; Yamano, T.; Takemoto, K. Functional monomers and polymers, 129. Asymmetric Robinson cyclization reaction catalyzed by polymer-bound L-proline. Makromol. Chem. 1985, 186, 1781–1785. [Google Scholar] [CrossRef]
- Pedrosa, R.; Andrés, J.M.; Manzano, R.; Pérez-López, C. Novel supported and unsupported prolinamides as organocatalysts for enantioselective cyclization of triketones. Tetrahedron Lett. 2013, 54, 3101–3104. [Google Scholar] [CrossRef]
- Cañellas, S.; Ayats, C.; Henseler, A.H.; Pericàs, M.A. A Highly Active Polymer-Supported Catalyst for Asymmetric Robinson Annulations in Continuous Flow. ACS Catal. 2017, 7, 1383–1391. [Google Scholar] [CrossRef]
- Clot-Almenara, L.; Rodríguez-Escrich, C.; Pericàs, M.A. Desymmetrisation of meso-diones promoted by a highly recyclable polymer-supported chiral phosphoric acid catalyst. RSC Adv. 2018, 8, 6910–6914. [Google Scholar] [CrossRef]
- Hayashi, Y.; Sekizawa, H.; Yamaguchi, J.; Gotoh, H. Organocatalyst-Mediated Enantioselective Intramolecular Aldol Reaction Featuring the Rare Combination of Aldehyde as Nucleophile and Ketone as Electrophile. J. Org. Chem. 2007, 72, 6493–6499. [Google Scholar] [CrossRef]
- Leonelli, F.; Trombetta, A.; La Bella, A.; Lucarelli, G.; Demitri, N.; Lamba, D.; Migneco, L.M.; Marini Bettolo, R. Enantioselective Synthesis and X-ray Structure of (+)((4aS,5S,8aS)-5,8a-Dimethyl-7-methyleneoctahydro-2H-spiro[naphthalene-1,2’-[1,3]dioxolan]-5-yl)methyl-4-iodobenzoate. Eur. J. Org. Chem. 2019, 2019, 1594–1599. [Google Scholar] [CrossRef]
- Hayashi, Y.; Salazar, H.A.; Koshino, S. Asymmetric Synthesis of Functionalized 9-Methyldecalins Using a Diphenylprolinol-Silyl-Ether-Mediated Domino Michael/Aldol Reaction. Org. Lett. 2021, 23, 6654–6658. [Google Scholar] [CrossRef]
- Kawamoto, Y.; Noguchi, N.; Ozone, D.; Kobayashi, T.; Ito, H. Convenient preparation of synthetically useful chiral quaternary carbon-containing bicyclic compounds with organocatalysts. Tetrahedron Lett. 2021, 85, 153495. [Google Scholar] [CrossRef]
- Selvarajan, R.; John, J.P.; Narayanan, K.V.; Swaminathan, S. Synthesis of Δ7-1-methylbicyclo[5,4,0]undecene-2,9-dione and its condensation with acetylene. Tetrahedron 1966, 22, 949–954. [Google Scholar] [CrossRef]
- Nagamine, T.; Inomata, K.; Endo, Y.; Paquette, L.A. Amino Acid Mediated Intramolecular Asymmetric Aldol Reaction to Construct a New Chiral Bicyclic Enedione Containing a Seven-Membered Ring: Remarkable Inversion of Enantioselectivity Compared to the Six-Membered Ring Example. J. Org. Chem. 2007, 72, 123–131. [Google Scholar] [CrossRef]
- Nagamine, T.; Inomata, K.; Endo, Y. Enantioselective Intramolecular Aldol Reaction Mediated by a Combination of L-Amino Acid and Brønsted Acid to Construct a Bicyclic Enedione Containing a 7-Membered Ring. Chem. Pharm. Bull. 2007, 55, 1710–1712. [Google Scholar] [CrossRef][Green Version]
- Nagamine, T.; Inomata, K.; Endo, Y. A new chiral synthesis of a bicyclic enedione containing a seven-membered ring mediated by a combination of chiral amine and Brønsted acid. Heterocycles 2008, 76, 1191–1204. [Google Scholar] [CrossRef]
- Sano, K.; Kohari, Y.; Nakano, H.; Seki, C.; Takeshita, M.; Tokiwa, M.; Hirose, Y.; Uwai, K. Lipase-catalyzed domino Michael–aldol reaction of 2-methyl-1, 3-cycloalkanedione and methyl vinyl ketone for the synthesis of bicyclic compounds. Synth. Commun. 2016, 46, 46–54. [Google Scholar] [CrossRef][Green Version]
- Chen, Y.; Hu, J.; Guo, L.-D.; Zhong, W.; Ning, C.; Xu, J. A Concise Total Synthesis of (−)-Himalensine A. Angew. Chem. Int. Ed. 2019, 58, 7390–7394. [Google Scholar] [CrossRef]
- Inomata, K.; Akahane, Y.; Miura, A. Alternative Chiral Preparations of a Swaminathan Ketone via Asymmetric Aldol Reactions Mediated by Chiral Amines Bearing a Pyrrolidine. Heterocycles 2020, 100, 12. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).