Identification of Retained Austenite in 9Cr-1.4W-0.06Ta-0.12C Reduced Activation Ferritic Martensitic Steel
Abstract
1. Introduction
2. Materials and Methods
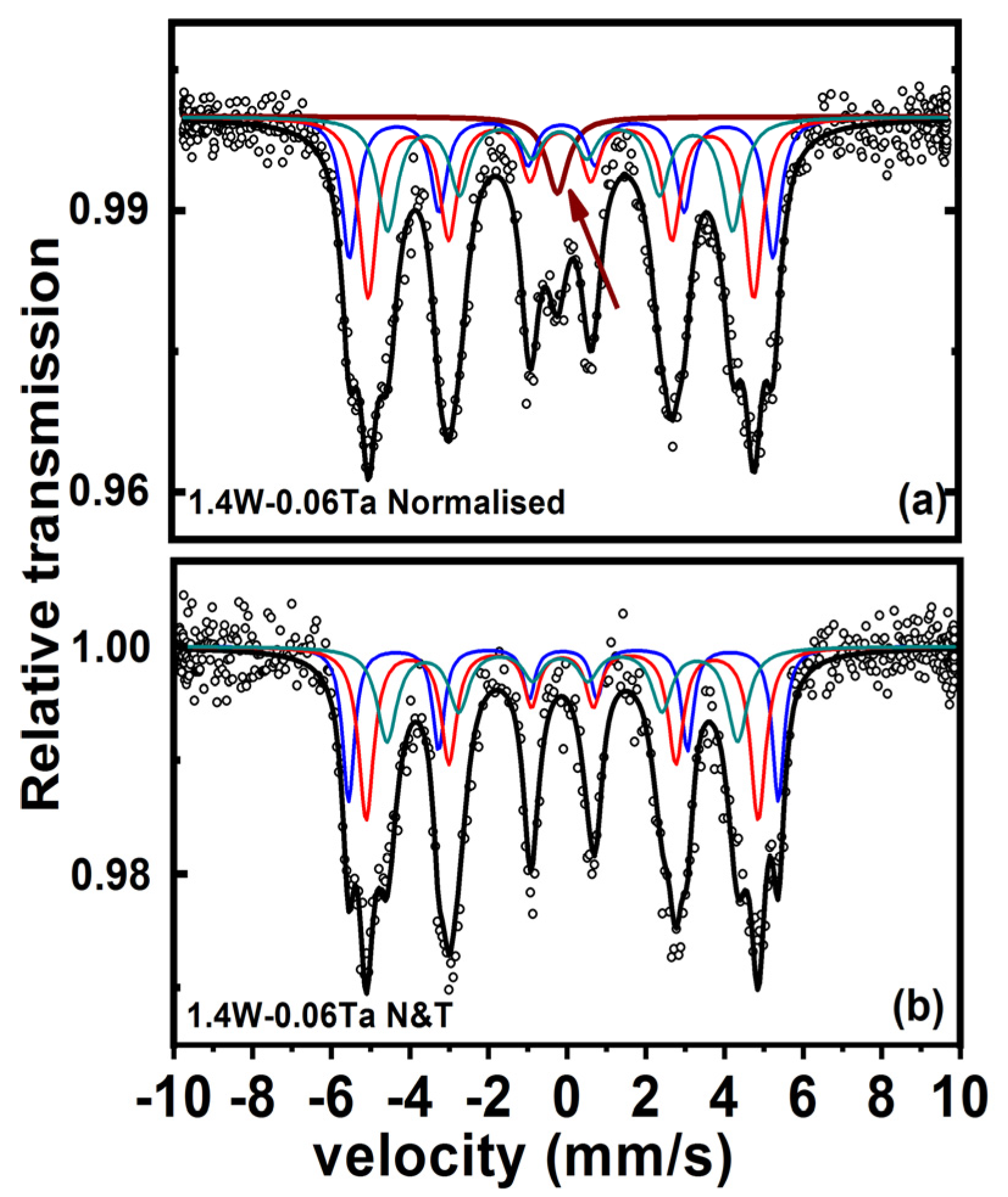
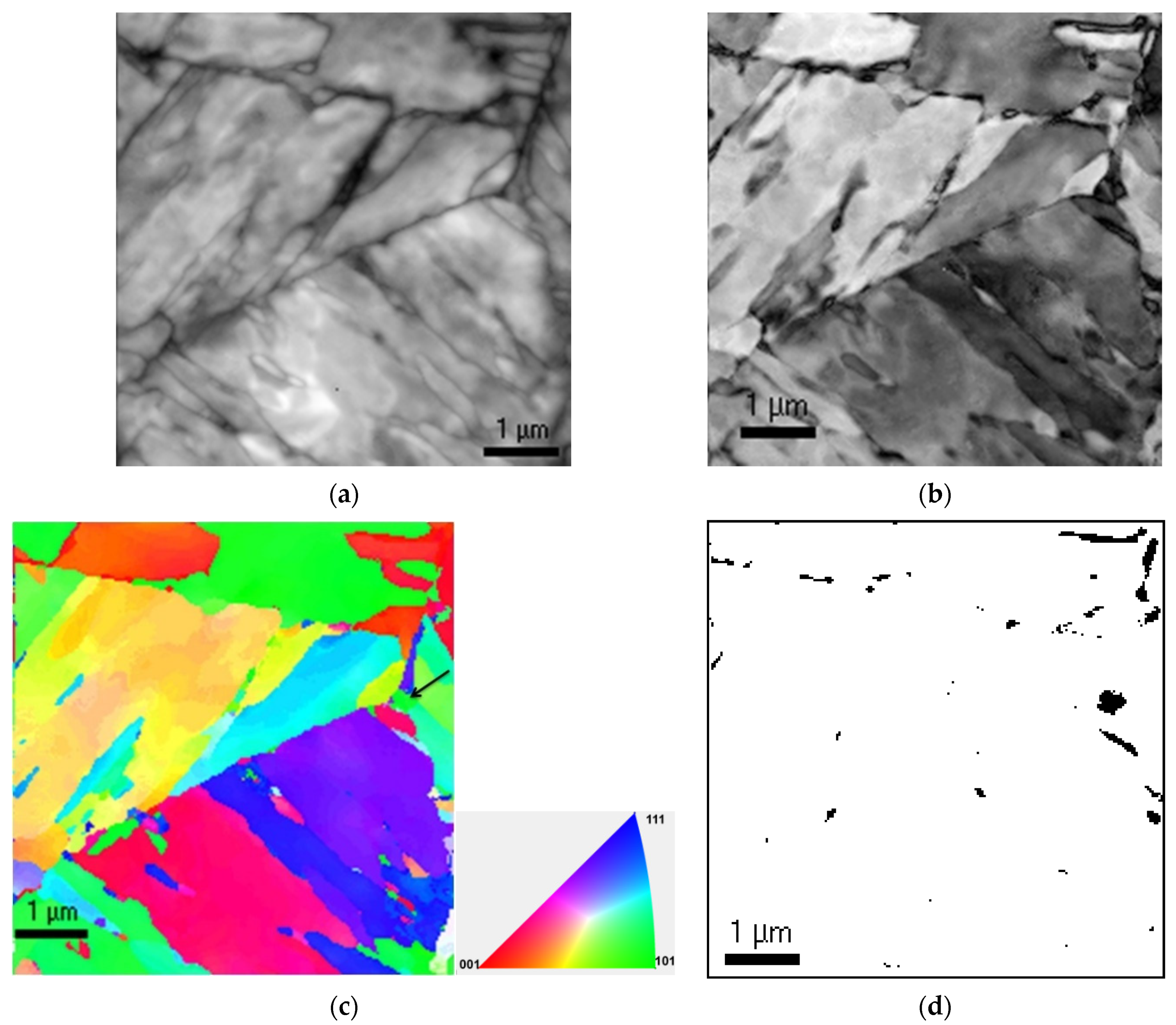
3. Results
4. Discussion
5. Conclusions
- Conventional TEM analysis of normalized steel shows a martensitic structure with few wide laths containing Fe rich M3C carbides;
- Detailed analysis of ADXRD, orientation imaging in TEM and Mossbauer studies proved the presence of retained austenite in normalised steel;
- The presence of M3C and retained austenite in the normalized steel with a martensitic microstructure is understood to be due to the inhomogeneity of austenite with incomplete dissolution of primary carbides during normalizing treatment;
- Absence of retained austenite and M3C in the tempered steel suggested their metastable nature, which could have transformed to stable M23C6 and MX precipitates on tempering.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klueh, R.L. Elevated temperature ferritic and martensitic steels and their application to future nuclear reactors. Int. Mater. Rev. 2005, 50, 287–310. [Google Scholar] [CrossRef]
- Harries, D.R. High-Chromium Ferritic and Martensitic Steels for Nuclear Applications; ASTM: West Conshohocken, PA, USA, 2001. [Google Scholar]
- Abe, F. Precipitate design for creep strengthening of 9% Cr tempered martensitic steel for ultra-supercritical power plants. Sci. Technol. Adv. Mater. 2008, 9. [Google Scholar] [CrossRef]
- Asada, Y.; Dozaki, K.; Ueta, M.; Ichimiya, M.; Mori, K.; Taguchi, K.; Kitagawa, M.; Nishida, T.; Sakon, T.; Sukekawa, M. Exploratory research on creep and fatigue properties of 9Cr-steels for the steam generator of an FBR. Nucl. Eng. Des. 1993, 139, 269–275. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, X.; Fan, P.; Liu, J. A Study of Microstructure Evolution During Creep of 9Cr-1Mo Steel Using Ultrasonic and Hardness Measurements. J. Mater. Eng. Perform. 2019, 28, 2348–2355. [Google Scholar] [CrossRef]
- Li, X.; Yan, Q.; Ma, R.; Wang, H.; Ge, C. First results of characterization of 9Cr-3WVTiTaN low activation ferritic/martensitic steel. J. Iron Steel Res. Int. 2010, 17, 57–62. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Z.; Liu, Y.; Li, W.; Liu, C.; Li, H. Diffusion Bonding of 9Cr Martensitic/Ferritic Heat-Resistant Steels with an Electrodeposited Ni Interlayer. Metals 2018, 8, 1012. [Google Scholar] [CrossRef]
- Kim, T.K.; Kim, S.H.; Lee, B. Effects of an intermediate heat treatment during a cold rolling on the tensile strength of a 9Cr-2W steel. Ann. Nucl. Energy 2009, 36, 1103–1107. [Google Scholar] [CrossRef]
- Besoky, J.I.; Danon, C.A.; Ramos, C.P. Retained austenite phase detected by Mössbauer spectroscopy in ASTM A335 P91 steel submitted to continuous cooling cycles. J. Mater. Res. Technol. 2019, 8, 1888–1896. [Google Scholar] [CrossRef]
- Wu, Q.S.; Zheng, S.H.; Huang, Q.Y.; Liu, S.J.; Han, Y.Y. Continuous cooling transformation behaviors of CLAM steel. J. Nucl. Mater. 2013, 442, S67–S70. [Google Scholar] [CrossRef]
- Ni, M.; Wang, J.; Liu, J.; Wang, X.; Zhang, K.; Du, C. Microstructure and Mechanical Properties of P91 Steel during Heat Treatment: The Effect of the Cooling Speed during the Normalization Stage. J. Mater. Eng. Perform. 2012, 30, 2329–2340. [Google Scholar] [CrossRef]
- Ravikirana Mythili, R.; Raju, S.; Saroja, S.; Jayakumar, T.; Rajendra Kumar, E. Decomposition modes of austenite in 9Cr-W-V-Ta reduced activation ferritic-martensitic steels. Mater. Sci. Technol. 2015, 31, 448–459. [Google Scholar] [CrossRef]
- Tan, L.; Hoelzer, D.T.; Busby, J.T.; Sokolov, M.A.; Klueh, R.L. Microstructure control for high strength 9Cr ferritic—Martensitic steels. J. Nucl. Mater. 2012, 422, 45–50. [Google Scholar] [CrossRef]
- Haarmann, K.; Vaillant, J.; Bendick, W.; Arbab, A. The T91/P91 Book; Vallourec Mannesmann Tubes: Duesseldorf, Germany, 2002; p. 69. [Google Scholar]
- Shiue, R.K.; Lan, K.C.; Chen, C. Toughness and Austenite Stability Of Modified 9Cr-1Mo welds after tempering. Mater. Sci. Eng. A 2000, 287, 10–16. [Google Scholar] [CrossRef]
- Santella, M.L.; Babu, S.S.; Swindeman, R.W.; Specht, E.D. In-situ characterization of austenite to martensite decomposition in 9Cr-1Mo-V steel welds. In Proceedings of the Materials Science and Technology 2003 Meeting, Chicago, IL, USA, 9–12 November 2003; pp. 247–256. [Google Scholar]
- Ning, B.; Zhou, X.; Shi, Q.; Liu, Y.; Zhao, J.; Zhang, Z. Relationship between austenite stability and martensite formation in modified 9Cr-1Mo steel. Int. J. Mater. Res. 2014, 105, 232–239. [Google Scholar] [CrossRef]
- Fedorova, I.; Kostka, A.; Tkachev, E.; Belyakov, A.; Kaibyshev, R. Tempering behavior of a low nitrogen boron-added 9%Cr steel. Mater. Sci. Eng. A 2016, 662, 443–455. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, J.; Han, L.; Shen, G.; Li, C. Thermal decomposition characteristics of retained austenite and its influence on impact toughness of B-containing 9Cr1Mo1Co(FB2) steel during the two-step tempering. J. Mater. Res. Technol. 2021, 12, 2462–2475. [Google Scholar] [CrossRef]
- Gao, Q.; Zhang, Y.; Zhang, H.; Li, H.; Qu, F.; Han, J.; Lu, C.; Wu, B.; Lu, Y.; Ma, Y. Precipitates and Particles Coarsening of 9Cr-1.7W-0.4Mo-Co Ferritic Heat-Resistant Steel after Isothermal Aging. Sci. Rep. 2017, 7, 5859. [Google Scholar] [CrossRef] [PubMed]
- Suwanpinij, P. The Synchrotron Radiation for Steel Research. Adv. Mater. Sci. Eng. 2016, 2016, 2479345. [Google Scholar] [CrossRef]
- Carrizo, D.A.; Besoky, J.I.; Luppo, M.; Danon, C.; Ramos, C.P. Characterization of an ASTM A335 P91 ferritic-martensitic steel after continuous cooling cycles at moderate rates. J. Mater. Res. Technol. 2019, 8, 923–934. [Google Scholar] [CrossRef]
- Saroja, S.; Vijayalakshmi, M.; Raghunathan, V.S. Influence of solution treatment on the microstructure of a 9wt.%Cr-lwt.%Mo-0.07wt.%C steel. Mater. Sci. Eng. A 1992, 154, 59–67. [Google Scholar] [CrossRef]
- Suikkanen, P.P.; Cayron, C.; DeArdo, A.J.; Karjalainen, L.P. Crystallographic analysis of martensite in 0.2C-2.0Mn-1.5Si-0.6Cr steel using EBSD. J. Mater. Sci. Technol. 2011, 27, 920–930. [Google Scholar] [CrossRef]
- Kwon, E.P.; Fujieda, S.; Shinoda, K.; Suzuki, S. Martensitic transformation and texture in novel bcc Fe-Mn-Al-Ni-Cr alloys. Procedia Eng. 2011, 10, 2214–2219. [Google Scholar] [CrossRef][Green Version]
- Dash, M.K.; Karthikeyan, T.; Saroja, S.; Vijayalakshmi, M. Restitution of prior-austenite grain orientation by microtexture analysis of tempered martensite structure in 9Cr-1Mo ferritic steel. Mater. Sci. Forum 2012, 702–703, 880–883. [Google Scholar] [CrossRef]
- Kitahara, H.; Ueji, R.; Tsuji, N.; Minamino, Y. Crystallographic features of lath martensite in low-carbon steel. Acta Mater. 2006, 54, 1279–1288. [Google Scholar] [CrossRef]
- Sun, D.; Li, C.; Xue, X.; Liu, Y.; Guo, Z.; Gu, J. Optimization scheme of the orientation relationship from crystallographic statistics of variants and its application to lath martensite. Mater. Des. 2020, 195, 109022. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Theory and Significance of Retained Austenite in Steels. Ph.D. Thesis, University of Cambridge, Cambridge, UK, March 1980. [Google Scholar]
- Kirana, R.; Raju, S.; Mythili, R.; Saibaba, S.; Jayakumar, T.; Rajendra Kumar, E. High-Temperature Phase Stability of 9Cr-W-Ta-V-C Based Reduced Activation Ferritic-Martensitic (RAFM) Steels: Effect of W and Ta Additions. Steel Res. Int. 2015, 86, 825–840. [Google Scholar] [CrossRef]
- Speer, J.; Matlock, D.K.; De Cooman, B.C.; Schroth, J.G. Carbon partitioning into austenite after martensite transformation. Acta Mater. 2003, 51, 2611–2622. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, D.; Liu, Y.; Wang, Q.; Yan, Z. Investigation on the precipitation behavior of M3C phase in T91 ferritic steels. Nucl. Eng. Des. 2011, 241, 2411–2415. [Google Scholar] [CrossRef]
- Shirane, T.; Tsukamoto, S.; Tsuzaki, K.; Adachi, Y.; Hanamura, T.; Shimizu, M.; Abe, F. Ferrite to austenite reverse transformation process in B containing 9%Cr heat resistant steel HAZ. Sci. Technol. Weld. Join. 2009, 14, 698–707. [Google Scholar] [CrossRef]
- Kipelova, A.Y.; Belyakov, A.N.; Skorobogatykh, V.N.; Shchenkova, I.A.; Kaibyshev, R.O. Tempering induced structual changes in steel 10Kh9K3V1M1FBR and their effect on the mechanical properties. Met. Sci. Heat Treat. 2010, 52, 100–110. [Google Scholar] [CrossRef]
- Kim, H.K.; Lee, J.W.; Moon, J.; Lee, C.H.; Hong, H.U. Effects of Ti and Ta addition on microstructure stability and tensile properties of reduced activation ferritic/martensitic steel for nuclear fusion reactors. J. Nucl. Mater. 2018, 500, 327–336. [Google Scholar] [CrossRef]
- Mythili, R.; Saroja, S. Influence of Tungsten and Tantalum Content on Evolution of Secondary Phases in 9Cr RAFM Steels: An Experimental and Computational Study. Metall. Mater. Trans. A 2017, 48, 3880–3891. [Google Scholar] [CrossRef]
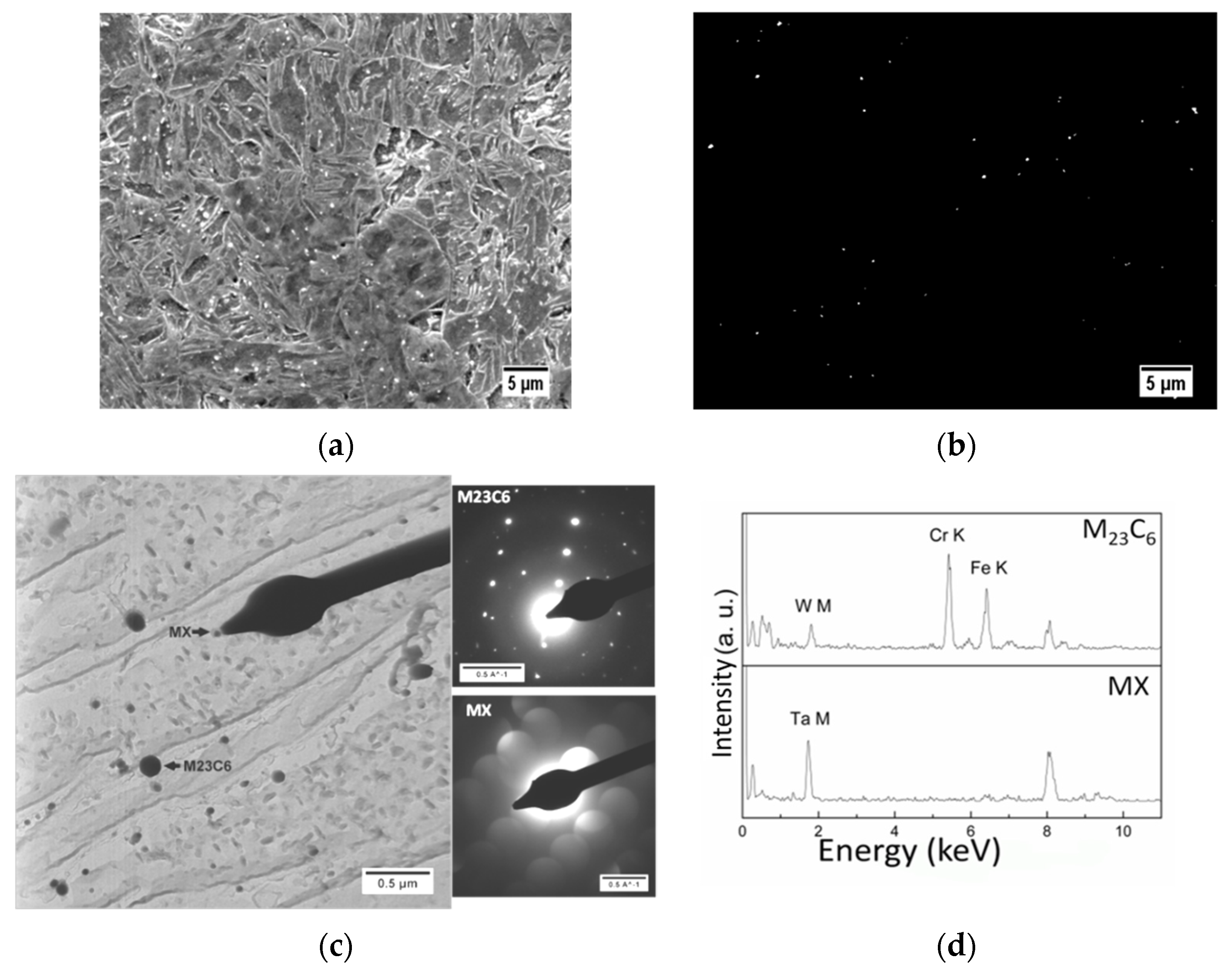
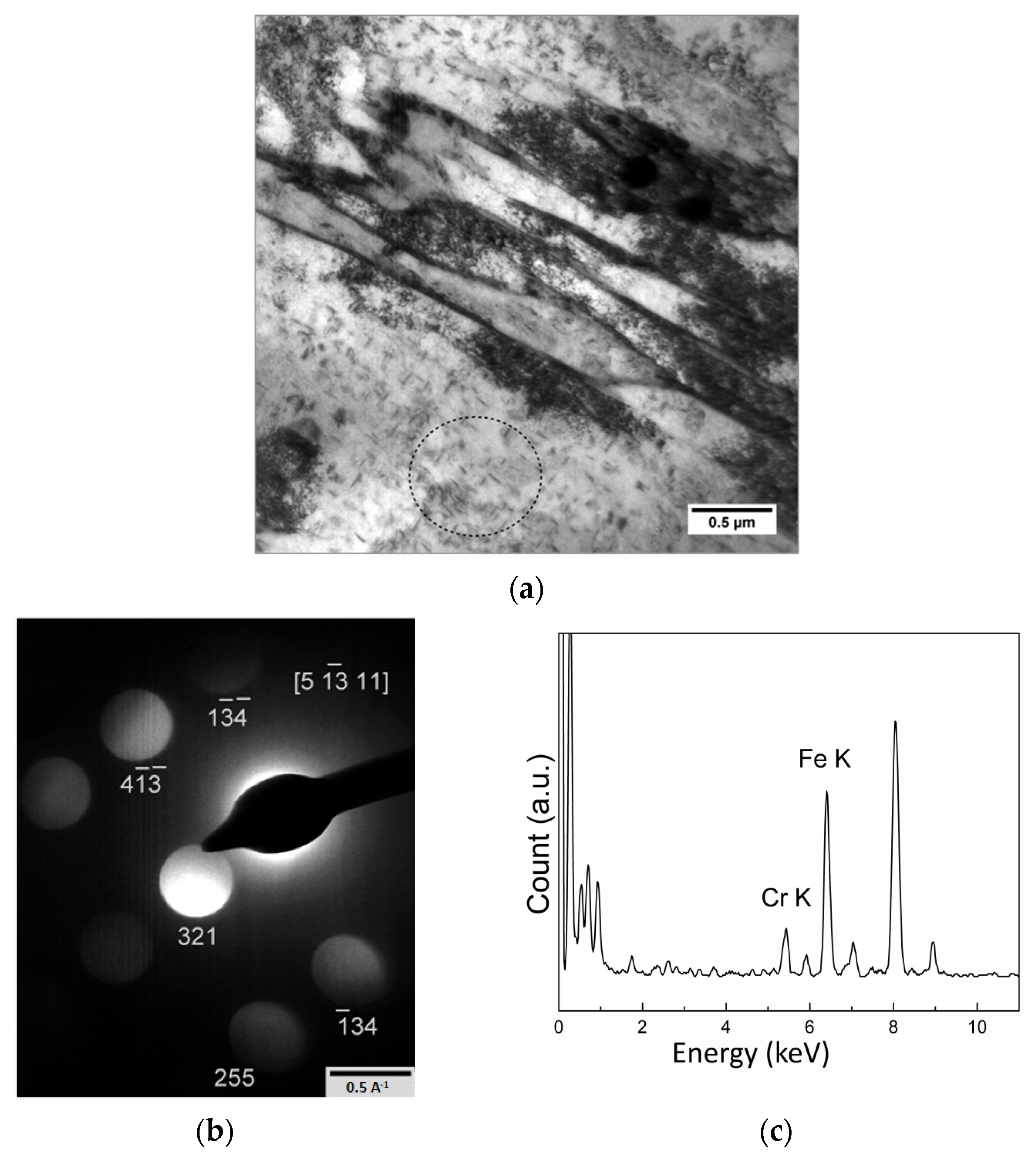
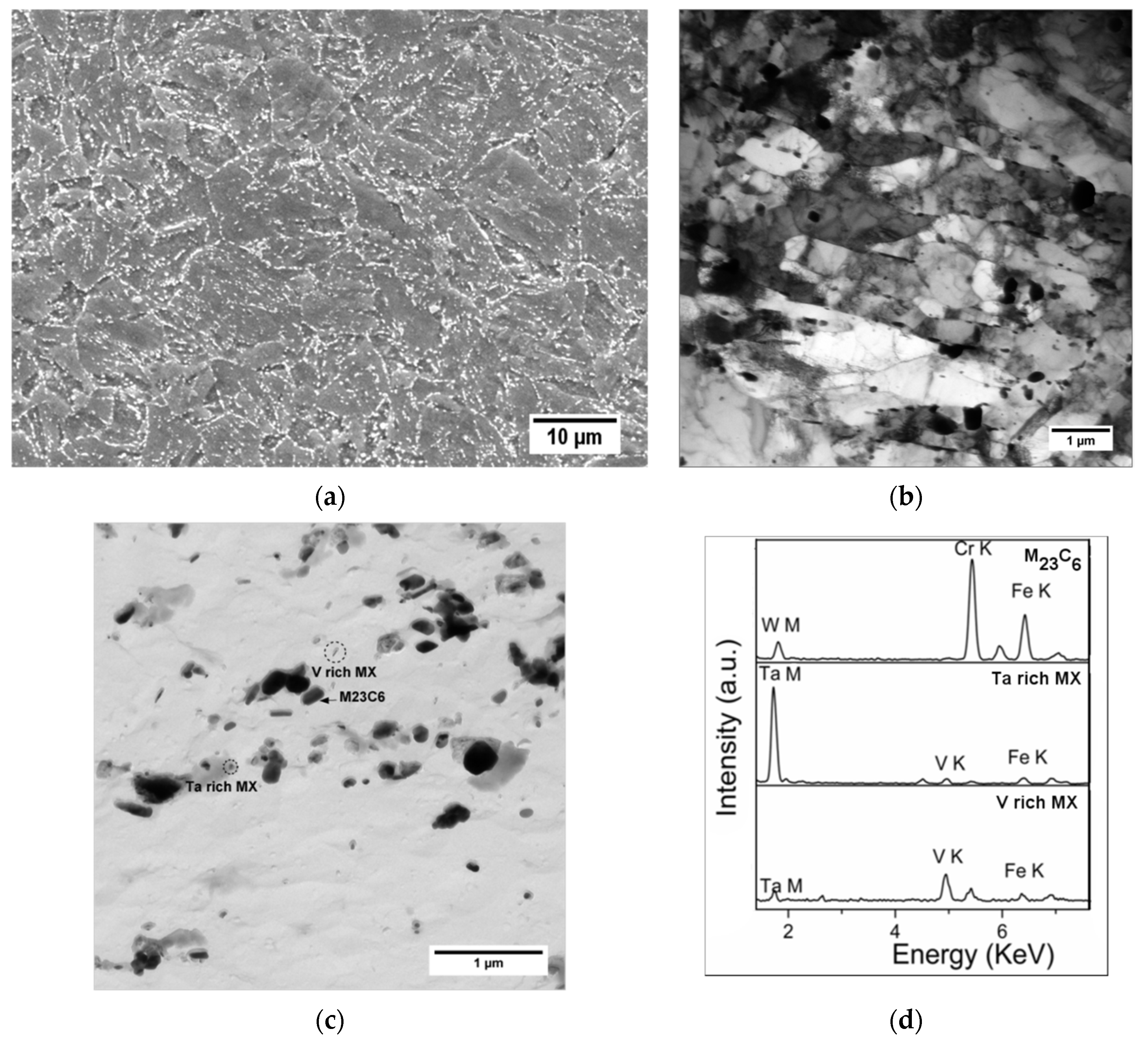
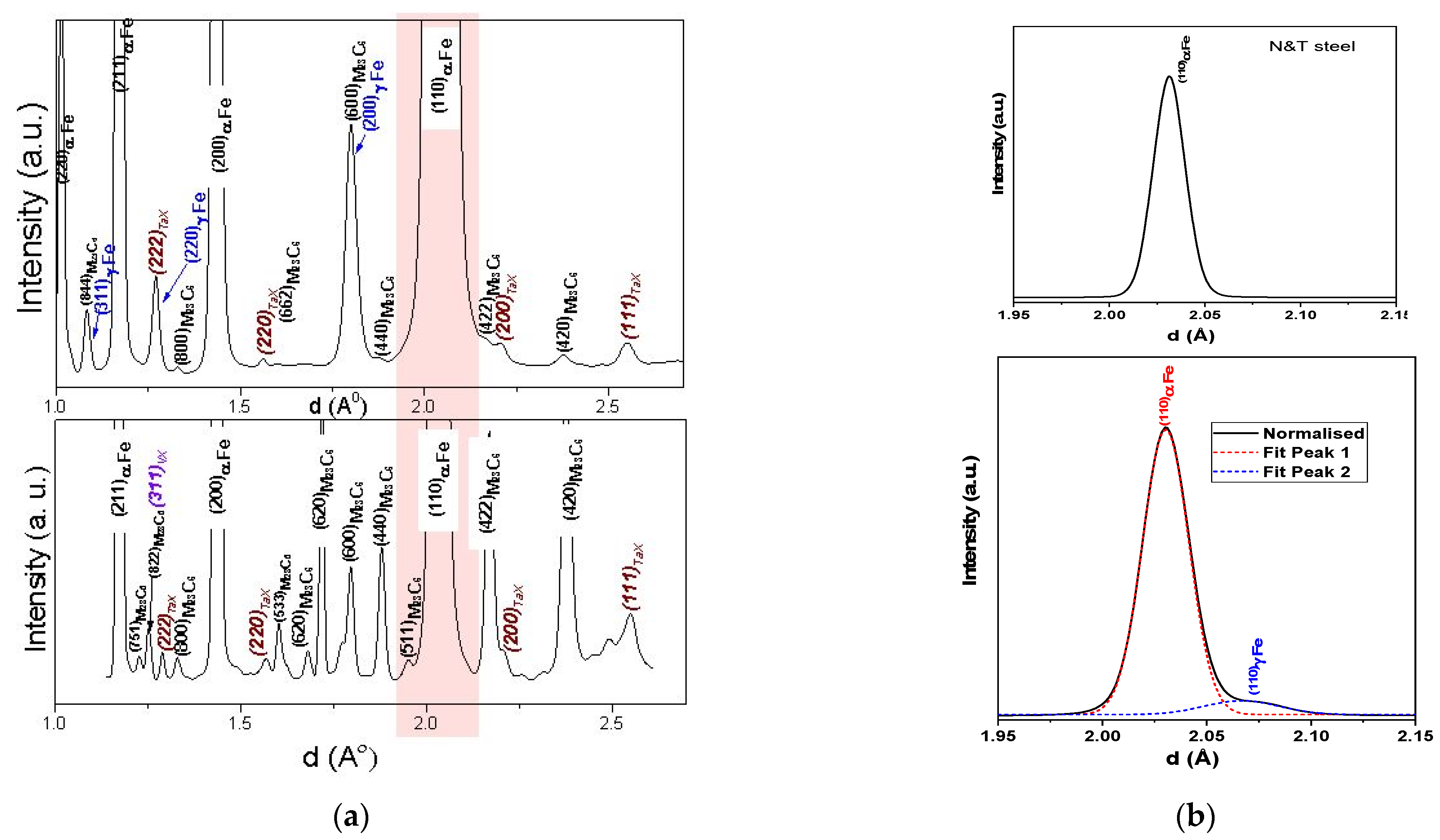
| Element | Concentration | Element | Concentration |
|---|---|---|---|
| Cr | 9.03 | Ta | 0.06 |
| C | 0.126 | N | 0.03 |
| Mn | 0.56 | O | 0.002 |
| V | 0.24 | P | <0.002 |
| W | 1.38 | S | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mythili, R.; Kirana, R.; Singh, L.H.; Govindaraj, R.; Sinha, A.K.; Singh, M.N.; Saroja, S.; Vijayalakshmi, M.; Deb, S.K. Identification of Retained Austenite in 9Cr-1.4W-0.06Ta-0.12C Reduced Activation Ferritic Martensitic Steel. Symmetry 2022, 14, 196. https://doi.org/10.3390/sym14020196
Mythili R, Kirana R, Singh LH, Govindaraj R, Sinha AK, Singh MN, Saroja S, Vijayalakshmi M, Deb SK. Identification of Retained Austenite in 9Cr-1.4W-0.06Ta-0.12C Reduced Activation Ferritic Martensitic Steel. Symmetry. 2022; 14(2):196. https://doi.org/10.3390/sym14020196
Chicago/Turabian StyleMythili, Rengachari, Ravi Kirana, Loushambam Herojit Singh, Ramanujam Govindaraj, Anil K. Sinha, Manvendra N. Singh, Saibaba Saroja, Muraleedharan Vijayalakshmi, and Sudip K. Deb. 2022. "Identification of Retained Austenite in 9Cr-1.4W-0.06Ta-0.12C Reduced Activation Ferritic Martensitic Steel" Symmetry 14, no. 2: 196. https://doi.org/10.3390/sym14020196
APA StyleMythili, R., Kirana, R., Singh, L. H., Govindaraj, R., Sinha, A. K., Singh, M. N., Saroja, S., Vijayalakshmi, M., & Deb, S. K. (2022). Identification of Retained Austenite in 9Cr-1.4W-0.06Ta-0.12C Reduced Activation Ferritic Martensitic Steel. Symmetry, 14(2), 196. https://doi.org/10.3390/sym14020196






