Symmetry Analysis of the Complex Polytypism of Layered Rare-Earth Tellurites and Related Selenites: The Case of Introducing Transition Metals
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Diagnostics
2.3. Single Crystal X-ray Analysis
3. Results
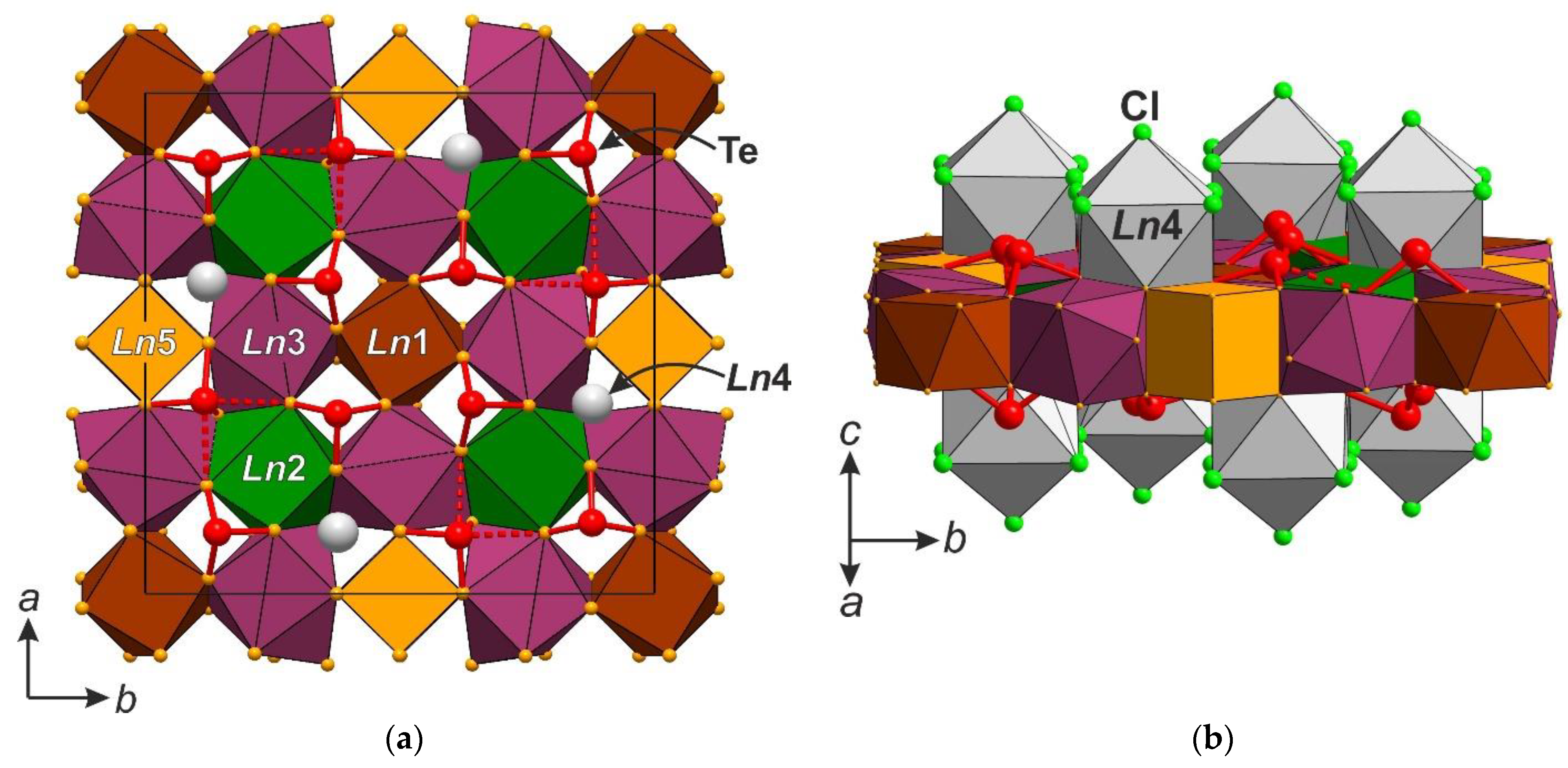
3.1. Crystal Structure Description
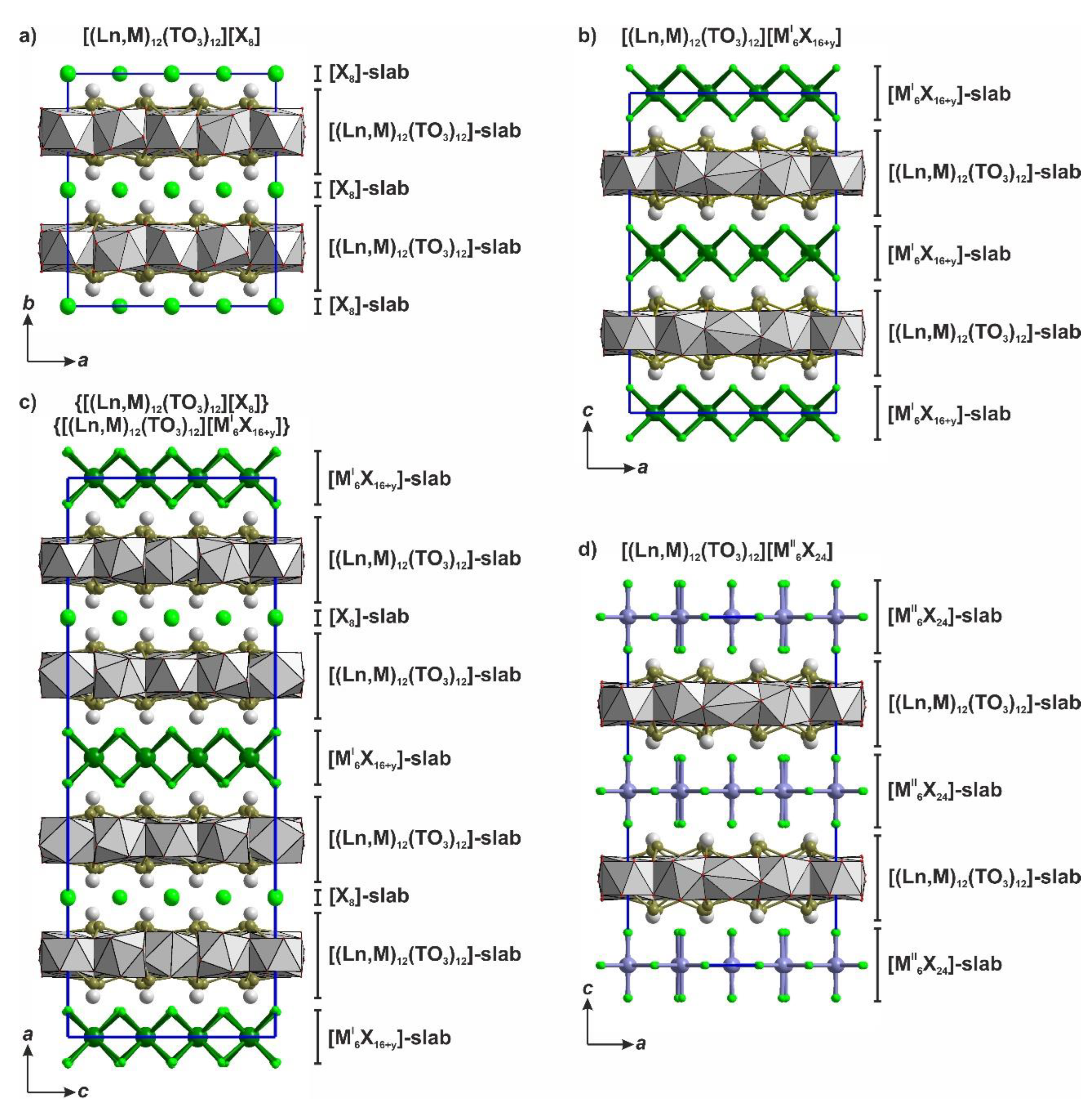
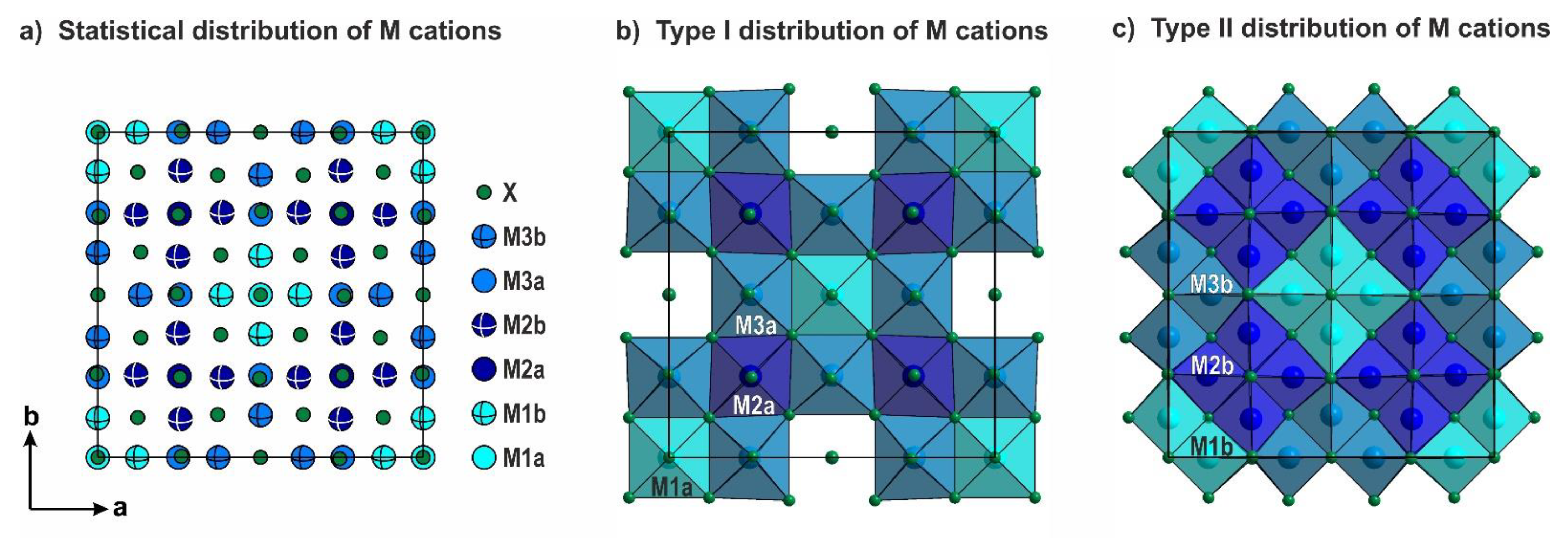
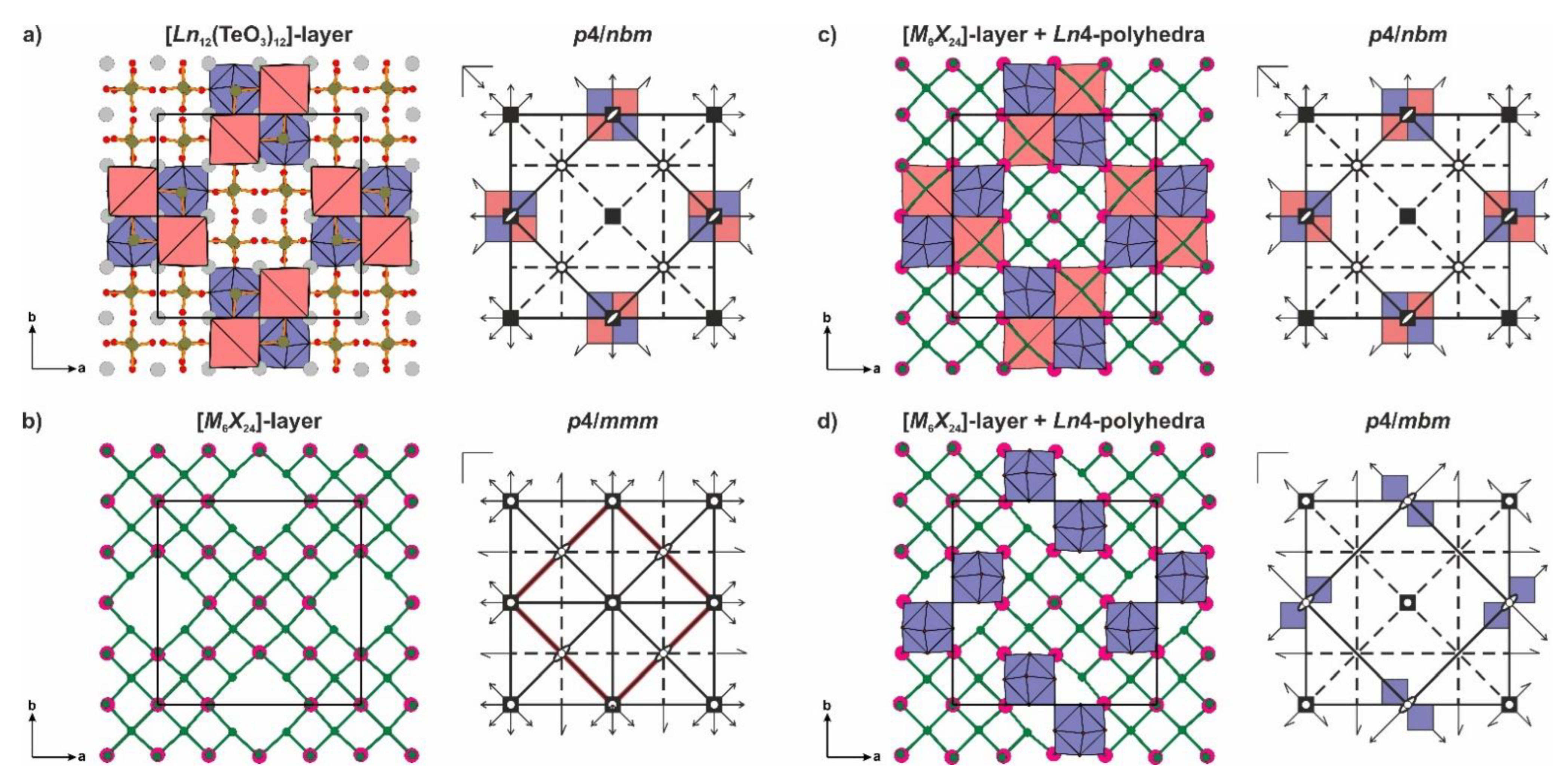
3.2. Polytypism of Tetragonal Halides Containing the [Ln12(TO3)12] Slabs
- (i)
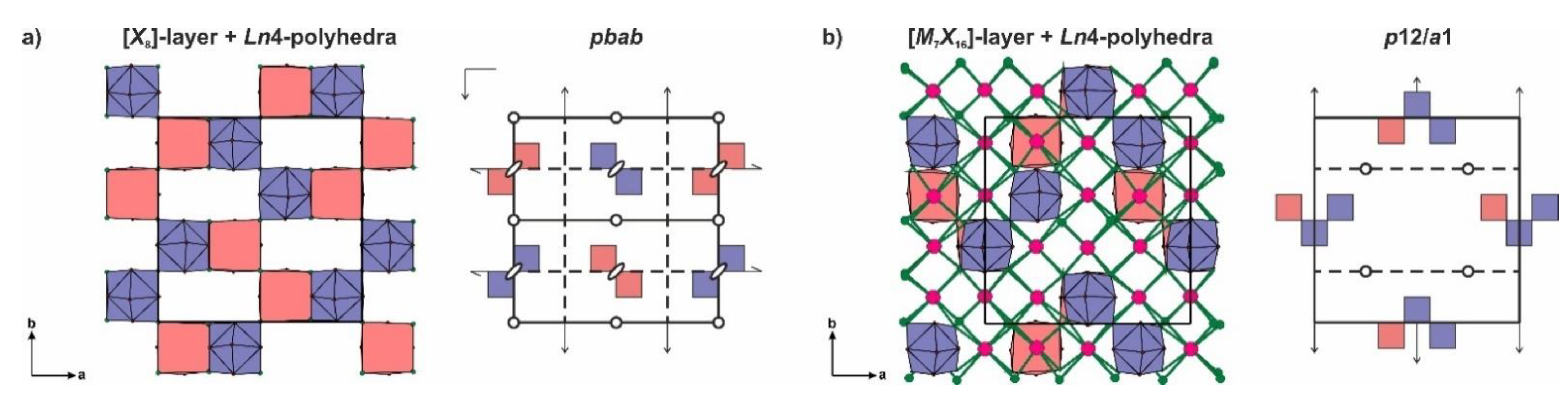
- L2n type with layer symmetry p4/nmm [or P(4/n)mm in the terms of OD notation] is formed by [M6X24], [M6X16] or [X8] layer (Figure 4b).
- (ii)
4. Discussion
4.1. Synthesis
4.2. Structural Trends
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zimmermann, I.; Johnsson, M. A Synthetic Route toward Layered Materials: Introducing Stereochemically Active Lone-Pairs into Transition Metal Oxohalides. Cryst. Growth Des. 2014, 14, 5252–5259. [Google Scholar] [CrossRef]
- Johnsson, M.; Lidin, S.; Törnroos, K.W.; Bürgi, H.-B.; Millet, P. Host–Guest Compounds in the Family of Tellurium–Nickel Oxohalogenides. Angew. Chem. Int. Ed. 2004, 43, 4292–4295. [Google Scholar] [CrossRef] [PubMed]
- Ok, K.M. Toward the Rational Design of Novel Noncentrosymmetric Materials: Factors Influencing the Framework Structures. Acc. Chem. Res. 2016, 49, 2774–2785. [Google Scholar] [CrossRef]
- Mayerová, Z.; Johnsson, M.; Lidin, S. Lone-Pair Interfaces That Divide Inorganic Materials into Ionic and Covalent Parts. Angew. Chem. Int. Ed. 2006, 45, 5602–5606. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Hu, C.-L.; Li, P.-X.; Jiang, H.-L.; Mao, J.-G. Syntheses, crystal structures and properties of new lead(ii) or bismuth(iii) selenites and tellurite. Dalt. Trans. 2012, 41, 9532. [Google Scholar] [CrossRef]
- Sun, C.; Yang, B.; Mao, J. Structures and properties of functional metal iodates. Sci. China Chem. 2011, 54, 911–922. [Google Scholar] [CrossRef]
- Zheng, T.; Wang, Q.; Ren, J.; Cao, L.; Huang, L.; Gao, D.; Bi, J.; Zou, G. Halogen regulation triggers structural transformation from centrosymmetric to noncentrosymmetric switches in tin phosphate halides Sn2PO4X (X = F, Cl). Inorg. Chem. Front. 2022, 9, 4705–4713. [Google Scholar] [CrossRef]
- Goerigk, F.C.; Schander, S.; Hamida, M.B.; Kang, D.-H.; Ledderboge, F.; Wickleder, M.S.; Schleid, T. Die monoklinen Seltenerdmetall(III)-Chlorid-Oxidoarsenate(III) mit der Zusammensetzung SE5Cl3[AsO3]4 (SE =La–Nd, Sm). Z. Naturforsch. B 2019, 74, 497–506. [Google Scholar] [CrossRef]
- Ahlers, R.; Ruschewitz, U. Non-centrosymmetric coordination polymers based on thallium and acetylenedicarboxylate. Solid State Sci. 2009, 11, 1058–1064. [Google Scholar] [CrossRef]
- Charkin, D.O.; Black, C.; Downie, L.J.; Sklovsky, D.E.; Berdonosov, P.S.; Olenev, A.V.; Zhou, W.; Lightfoot, P.; Dolgikh, V.A. Cs7Sm11[TeO3]12Cl16 and Rb7Nd11[TeO3]12Br16, the new tellurite halides of the tetragonal Rb6LiNd11[SeO3]12Cl16 structure type. J. Solid State Chem. 2015, 232, 56–61. [Google Scholar] [CrossRef]
- Hamida, M.B. Oxo-Selenate(IV) und Oxo-Arsenate(III) der Selten-Erd-Metalle und ihre Derivate. Ph.D. Thesis, Universität Oldenburg, Oldenburg, Germany, 2007. [Google Scholar]
- Lipp, C.; Schleid, T. Rb6LiNd11Cl16[SeO3]12: Ein durch zwei chloridische Flussmittel derivatisiertes Neodym(III)-Oxoselenat(IV). Z. Fuer Krist. 2005, 22, 165. [Google Scholar]
- Lipp, C.; Schleid, T. Rb6LiPr11Cl16[SeO3]12: Ein chloridisch derivatisiertes Rubidium-Lithium-Praseodym(III)-Oxoselenat(IV). Z. Für Anorg. Und Allg. Chem. 2006, 632, 2226–2231. [Google Scholar] [CrossRef]
- Zitzer, S.; Lipp, C.; Schleid, T. Two isostructural alkali-metal europium chloride oxochalcogenates: Rb6LiEu11Cl16[SeO3]12 versus Cs7Eu11Cl16[TeO3]12. In Proceedings of the European Conference on Solid State Chemistry (ECSCC), Lund, Sweden, 25–28 September 2011; p. 71. [Google Scholar]
- Charkin, D.O.; Volkov, S.N.; Dolgikh, V.A.; Aksenov, S.M. Potassium rare-earth tellurite chlorides: A new branch from the old root. Solid State Sci. 2022, 129, 106895. [Google Scholar] [CrossRef]
- Kharitonov, I.D.; Charkin, D.O.; Berdonosov, P.S.; Black, C.; Downie, L.J.; Lightfoot, P.; Dolgikh, V.A. Rare-Earth Cadmium Tellurite Chlorides with a Structural Type Exhibiting [Ln12(TeO3)12] Slabs Alternating with CdCl6 Octahedral Layers. Eur. J. Inorg. Chem. 2014, 2014, 3140–3146. [Google Scholar] [CrossRef]
- Charkin, D.O.; Grishaev, V.Y.; Volkov, S.N.; Dolgikh, V.A. Synthesis and Structure of New Rare Earth Cadmium Tellurite Halides. Russ. J. Inorg. Chem. 2020, 65, 466–471. [Google Scholar] [CrossRef]
- Ferraris, G.; Makovicky, E.; Merlino, S. Crystallography of Modular Materials; Oxford University Press: Oxford, UK, 2008. [Google Scholar]
- Zvyagin, B.B. Polytypism of crystal structures. Comput. Math. Appl. 1988, 16, 569–591. [Google Scholar] [CrossRef][Green Version]
- Fichtner, K. Order-disorder structures. Comput. Math. Appl. 1988, 16, 469–477. [Google Scholar] [CrossRef][Green Version]
- Ďurovič, S.; Hybler, J. OD structures in crystallography—basic concepts and suggestions for practice. Z. Krist. Cryst. Mater. 2006, 221, 63–76. [Google Scholar] [CrossRef]
- Dornberger-Schiff, K.; Fichtner, K. On the Symmetry of OD-Structures Consisting of Equivalent Layers. Krist. Technol. 1972, 7, 1035–1056. [Google Scholar] [CrossRef]
- Dornberger-Schiff, K. Grundzüge einer Theorie der OD-Strukturen aus Schichten. Abhandlungen der Dtsch. Akad. Der Wiss. Zu Berlin. Kl. Chem. Geol. Biol. 1964, 3, 107. [Google Scholar]
- Fichtner, K. On the Description of Symmetry of OD Structures (I) OD Groupoid Family, Parameters, Stacking. Krist. Technol. 1979, 14, 1073–1078. [Google Scholar] [CrossRef]
- Grell, J. Symmetry Description of OD Crystal Structures in Group Theoretical Terms. Acta Appl. Math. 1998, 52, 261–269. [Google Scholar] [CrossRef]
- Stöger, B. Non-Crystallographic Layer Lattice Restrictions in Order-Disorder (OD) Structures. Symmetry 2014, 6, 589–621. [Google Scholar] [CrossRef]
- Nespolo, M.; Durovic, S. Crystallographic basis of Polytypism and Twinning in Micas. Rev. Mineral. Geochem. 2002, 46, 155–279. [Google Scholar] [CrossRef]
- Topnikova, A.; Belokoneva, E.; Dimitrova, O.; Volkov, A.; Deyneko, D. Rb1.66Cs1.34Tb[Si5.43Ge0.57O15]·H2O, a New Member of the OD-Family of Natural and Synthetic Layered Silicates: Topology-Symmetry Analysis and Structure Prediction. Minerals 2021, 11, 395. [Google Scholar] [CrossRef]
- Belokoneva, E.L.; Topnikova, A.P.; Aksenov, S.M. Topolology-symmetry law of structure of natural titanosilicate micas and related heterophyllosilicates based on the extended OD theory: Structure prediction. Crystallogr. Rep. 2015, 60, 1–15. [Google Scholar] [CrossRef]
- Belokoneva, E.L. Borate crystal chemistry in terms of the extended OD theory: Topology and symmetry analysis. Crystallogr. Rev. 2005, 11, 151–198. [Google Scholar] [CrossRef]
- Reutova, O.; Belokoneva, E.; Volkov, A.; Dimitrova, O. Structure-Properties Relations in Two Iodate Families Studied by Topology-Symmetry Analysis of OD Theory. Symmetry 2021, 13, 1477. [Google Scholar] [CrossRef]
- Reutova, O.; Belokoneva, E.; Volkov, A.; Dimitrova, O.; Stefanovich, S. Two New Rb3Sc(IO3)6 Polytypes in Proposed Nonlinear Optical Family A3M(IO3)6 (A = K, Rb; M = Sc, In): Topology–Symmetry Analysis, Order–Disorder and Structure–Properties Relation. Symmetry 2022, 14, 1699. [Google Scholar] [CrossRef]
- Aksenov, S.; Antonov, A.; Deyneko, D.; Krivovichev, S.; Merlino, S. Polymorphism, polytypism and modular aspect of compounds with the general formula A2M3(TO4)4 (A = Na, Rb, Cs, Ca; M = Mg, Mn, Fe3+, Cu2+; T = S6+, P5+): Order–disorder, topological description and DFT calculations. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2022, 78, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Aksenov, S.M.; Kuznetsov, A.N.; Antonov, A.A.; Yamnova, N.A.; Krivovichev, S.V.; Merlino, S. Polytypism of Compounds with the General Formula Cs{Al2[TP6O20]} (T = B, Al): OD (Order-Disorder) Description, Topological Features, and DFT-Calculations. Minerals 2021, 11, 708. [Google Scholar] [CrossRef]
- Stöger, B.; Krüger, H.; Weil, M. Mg(H2O)2 [TeO2(OH)4]: A polytypic structure with a two-mode disordered stacking arrangement. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2021, 77, 605–623. [Google Scholar] [CrossRef]
- Stöger, B.; Weil, M.; Missen, O.P.; Mills, S.J. The Order-Disorder (OD) Polytypism of [Cu2ZnTeO4]2+[SO4 ·H2O]2−. Cryst. Res. Technol. 2020, 55, 1900182. [Google Scholar] [CrossRef]
- Eder, F.; Stöger, B.; Weil, M. Order-disorder (OD) structures of Rb2Zn(TeO3)(CO3)·H2O and Na2Zn2Te4O11. Z. Krist. Cryst. Mater. 2022, 237, 329–341. [Google Scholar] [CrossRef]
- Berdonosov, P.S.; Akselrud, L.; Prots, Y.; Abakumov, A.M.; Smet, P.F.; Poelman, D.; Van Tendeloo, G.; Dolgikh, V.A. Cs7Nd11(SeO3)12Cl16: First Noncentrosymmetric Structure among Alkaline-Metal Lanthanide Selenite Halides. Inorg. Chem. 2013, 52, 3611–3619. [Google Scholar] [CrossRef]
- Oxford Diffraction. CrysAlisPro; Oxford Diffraction Ltd: Abingdon, UK, 2009. [Google Scholar]
- Bruker. SAINT; Bruker AXS Inc.: Madison, WI, USA, 2012. [Google Scholar]
- Bruker. SADABS; Bruker AXS Inc.: Madison, WI, USA, 2001. [Google Scholar]
- Palatinus, L.; Chapuis, G. Superflip—A computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J. Appl. Crystallogr. 2007, 40, 786–790. [Google Scholar] [CrossRef]
- Petricek, V.; Dusek, M.; Palatinus, L. Crystallographic Computing System JANA2006: General features. Z. Fuer Krist. 2014, 229, 345–352. [Google Scholar]
- Brandenburg, K.; Putz, H. Diamond Version 3; Crystal Impact GbR.: Bonn, Germany, 2005. [Google Scholar]
- Prince, E. (Ed.) International Tables for Crystallography, Mathematical, Physical and Chemical Tables, 3rd ed.; Wiley: Hoboken, NJ, USA, 2004; Volume C. [Google Scholar]
- Merlino, S. (Ed.) . EMU Notes in Mineralogy. Modular Aspects of Minerals; Eötvös University Press: Budapest, Hungary, 1997; Volume 1. [Google Scholar]
- Dornberger-Schiff, K. On order–disorder structures (OD-structures). Acta Crystallogr. 1956, 9, 593–601. [Google Scholar] [CrossRef]
- Dornberger-Schiff, K. OD Structures—a Game and a Bit More. Krist. Technol. 1979, 14, 1027–1045. [Google Scholar] [CrossRef]
- Dornberger-Schiff, K.; Grell, H. Geometrical properties of MDO polytypes and procedures for their derivation. II. OD families containing OD layers of M > 1 kinds and their MDO polytypes. Acta Crystallogr. Sect. A 1982, 38, 491–498. [Google Scholar] [CrossRef]
- Dornberger-Schiff, K. On the nomenclature of the 80 plane groups in three dimensions. Acta Crystallogr. 1959, 12, 173. [Google Scholar] [CrossRef]
- Grell, H.; Dornberger-Schiff, K. Symbols for OD groupoid families referring to OD structures (polytypes) consisting of more than one kind of layer. Acta Crystallogr. Sect. A 1982, 38, 49–54. [Google Scholar] [CrossRef]
- Berdonosov, P.S.; Olenev, A.V.; Dolgikh, V.A.; Lightfoot, P. The synthesis and crystal structures of the first rare-earth alkaline-earth selenite chlorides MNd10(SeO3)12Cl8 (M=Ca and Sr). J. Solid State Chem. 2007, 180, 3019–3025. [Google Scholar] [CrossRef]
- Berdonosov, P.S.; Oleneva, O.S.; Dolgikh, V.A. Tricaesium undecalanthanum dodecaselenate(IV) dodecachloride. Acta Crystallogr. Sect. E Struct. Rep. Online 2006, 62, i29–i31. [Google Scholar] [CrossRef]
- Berdonosov, P.S.; Dolgikh, V.A.; Schmidt, P.; Ruck, M. Complex chloride-selenites of rare earthes—a family of new phases. In Proceedings of the IV National Crystal Chemical Conference, Chernogolovka, Russia, 26–30 June 2006; pp. 192–193. [Google Scholar]
- Ruck, M.; Schmidt, P. Synthesen und Kristallstrukturen der homöotypen Selenitbromide Bi8(SeO3)9Br6 und CsSm21(SeO3)24Br16. Z. Anorg. Allg. Chem. 2003, 629, 2133–2143. [Google Scholar] [CrossRef]
- Lima-de-Faria, J.; Hellner, E.; Liebau, F.; Makovicky, E.; Parthé, E. Nomenclature of inorganic structure types. Report of the International Union of Crystallography Commission on Crystallographic Nomenclature Subcommittee on the Nomenclature of Inorganic Structure Types. Acta Crystallogr. Sect. A Found. Crystallogr. 1990, 46, 1–11. [Google Scholar] [CrossRef]
- Veblen, D.R. Polysomatism and polysomatic series: A review and applications. Am. Mineral. 1991, 76, 801–826. [Google Scholar]
- Ferraris, G.; Gula, A. Polysomatic Aspects of Microporous Minerals—Heterophyllosilicates, Palysepioles and Rhodesite-Related Structures. Rev. Mineral. Geochem. 2005, 57, 69–104. [Google Scholar] [CrossRef]
- Charkin, D.O. Modular approach as applied to the description, prediction, and targeted synthesis of bismuth oxohalides with layered structures. Russ. J. Inorg. Chem. 2008, 53, 1977–1996. [Google Scholar] [CrossRef]
- Charkin, D.O.; Akinfiev, V.S.; Alekseeva, A.M.; Batuk, M.; Abakumov, A.M.; Kazakov, S.M. Synthesis and cation distribution in the new bismuth oxyhalides with the Sillén–Aurivillius intergrowth structures. Dalt. Trans. 2015, 44, 20568–20576. [Google Scholar] [CrossRef]
- Aliev, A.; Kovrugin, V.M.; Colmont, M.; Terryn, C.; Huvé, M.; Siidra, O.I.; Krivovichev, S.V.; Mentré, O. Revised Bismuth Chloroselenite System: Evidence of a Noncentrosymmmetric Structure with a Giant Unit Cell. Cryst. Growth Des. 2014, 14, 3026–3034. [Google Scholar] [CrossRef]
- Tornero, J.D.; Fayos, J. Single crystal structure refinement of MnCl2. Z. Krist. Cryst. Mater. 1990, 192, 147–148. [Google Scholar] [CrossRef]
- Wollan, E.O.; Koehler, W.C.; Wilkinson, M.K. Neutron Diffraction Study of the Magnetic Properties of MnBr2. Phys. Rev. 1958, 110, 638–646. [Google Scholar] [CrossRef]
- Ferrari, A.; Braibanti, A.; Bigliardi, G. Refinement of the crystal structure of NiCl2 and of unit-cell parameters of some anhydrous chlorides of divalent metals. Acta Crystallogr. 1963, 16, 846–847. [Google Scholar] [CrossRef]
- Wilkinson, M.K.; Cable, J.W.; Wollan, E.O.; Koehler, W.C. Neutron Diffraction Investigations of the Magnetic Ordering in FeBr2, CoBr2, FeCl2, and CoCl2. Phys. Rev. 1959, 113, 497–507. [Google Scholar] [CrossRef]
- Ketelaar, J.A.A. Die Kristallstruktur des Nickelbromids und -jodids. Z. Krist. Cryst. Mater. 1934, 88, 26–34. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
| Sample Number | [Ce12(TeO3)12] [Gd6Cl24] (1) | [Nd12(TeO3)12] [Mn6Cl24] (2) | [La12(TeO3)12] [Mn6Br24] (3) | [Eu12(TeO3)12] [Co6Cl24] (4) | [Gd12(TeO3)12] [Co6Cl24] (5) |
|---|---|---|---|---|---|
| Molecular weight (g) | 5321.5 | 5014.3 | 5978.4 | 5135.4 | 5198.4 |
| Temperature (K) | 293 | ||||
| Cell setting | Tetragonal | ||||
| Space group | P4/nbm (125) | ||||
| a (Å) | 16.3262 (1) | 16.0692 (11) | 16.4400 (49) | 15.8928 (6) | 15.8323 (7) |
| c (Å) | 13.0257 (1) | 12.6796 (9) | 13.5077 (40) | 12.4614 (13) | 12.4729 (1) |
| V (Å3) | 3471.93 (4) | 3274.12 (4) | 3650.77 (3) | 3147.5 (4) | 3126.46 (3) |
| Z | 2 | ||||
| Calculated density, Dx (g cm−3) | 5.0902 | 5.086 | 5.438 | 5.418 | 5.522 |
| Crystal size (mm) | 0.02 × 0.06 × 0.08 | 0.01 × 0.03 × 0.06 | 0.03 × 0.04 × 0.07 | 0.03 × 0.05 × 0.08 | 0.08 × 0.10 × 0.12 |
| Crystal form | Anhedral grain | Anhedral grain | Anhedral grain | Anhedral grain | Anhedral grain |
| Crystal color | yellowish | lilac | pink | deep blue | deep blue |
| Data collection | |||||
| Diffractometer | Rigaku XtaLAB Synergy (HyPix detector) | Bruker Apex II (CCD detector) | Xcalibur S Oxford Diffraction (CCD detector) | ||
| Radiation; λ | MoKα; 0.71073 | ||||
| Absorption coefficient, μ (mm−1) | 15.368 | 16.696 | 25.496 | 19.839 | 20.664 |
| F (000) | 4615 | 4377 | 5134 | 4476 | 4500 |
| Data range θ(°); h, k, l | 3.2–33.39; −18 < h < 24, −25 < k < 24, −19 < l < 20 | 2.41–30.25; −22 < h < 22, −22 < k < 13, −16 < l < 17 | 2.31–30.32; −23 < h < 22, −23< k < 23, −18 < l < 19 | 3.30–35.37; −17 < h < 25, −25 < k < 24, −19 < l < 19 | 3.31–35.39; −24 < h < 24, −24 < k < 15, −19 < l < 14 |
| No. of measured reflections | 46,388 | 16,881 | 34,403 | 32,931 | 32,476 |
| Total reflections (N2) /observed (N1) | 3194/2957 | 2314/1937 | 2625/1204 | 3432/1093 | 3411/1052 |
| Criterion for observed reflections | I > 3σ (I) | ||||
| Rσ/Rint (%) | 0.96/3.4 | 2.95/4.68 | 9.77/ 2.98 | 12.24/22.13 | 11.50/21.46 |
| Refinement | |||||
| Refinement on | Full-matrix least squares on F | Full-matrix least squares on F | Full-matrix least squares on F | Full-matrix least squares on F2 | Full-matrix least squares on F2 |
| Weight scheme | 1/(σ2|F| + 0.0004F2) | 1/(σ2|F| + 0.0004F2) | 1/(σ2|F| + 0.0004F2) | 1/(σ2|I| + 0.0036I2) | 1/(σ2|I| + 0.0064I2) |
| R1, wR2 (all reflection) (%) | 3.65/6.01 | 4.03/5.64 | 6.29/8.27 | 5.74/20.58 | 6.26/24.03 |
| GOF (Goodness of fit) (%) | 2.47 | 1.75 | 1.14 | 1.02 | 1.04 |
| Max./min. residual e density, (eÅ−3) | 9.58/−5.00 | 3.99/−5.22 | 10.27/−5.51 | 6.97/−6.44 | 9.15/−5.15 |
| CCDC Number | 2202776 | 1974365 | 2202775 | 1939966 | 1939965 |
| Compound | Sp. Gr. | Symmetry of the Triplet L2n–1,L2n,L2n + 1 | Symmetry Operation Active in L2n Layer | Unit Cell Parameters | V, Å3 | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| a, Å | b, Å | c, Å | ||||||
| MDO1-polytype | ||||||||
| [La12(TeO3)12][Cd6Cl24] | P4/nbm | P(4/n)bm | 2x, 2y | 16.401 | 16.401 | 12.918 | 3474.77 | [17] |
| [Sm12(TeO3)12][Cd6Cl24] | P4/nbm | P(4/n)bm | 2x, 2y | 15.842 | 15.842 | 13.079 | 3282.36 | [16] |
| [Eu12(TeO3)12][Cd6Cl24] | P4/nbm | P(4/n)bm | 2x, 2y | 15.774 | 15.774 | 13.113 | 3262.94 | [16] |
| [La12(TeO3)12][Cd6Br24] | P4/nbm | P(4/n)bm | 2x, 2y | 16.439 | 16.439 | 13.713 | 3705.66 | [17] |
| [Pr12(TeO3)12][Cd6Br24] | P4/nbm | P(4/n)bm | 2x, 2y | 16.231 | 16.231 | 13.771 | 3627.91 | [17] |
| MDO2-polytype | ||||||||
| [CsSm11(TeO3)12][Cs6Cl16] | I4/mcm | P(4/m)bm | 21x, 21y | 15.888 | 15.888 | 25.737 | 6496.45 | [10] |
| [RbNd11(TeO3)12][Rb6Br16] | I4/mcm | P(4/m)bm | 21x, 21y | 16.118 | 16.118 | 25.935 | 6737.51 | [10] |
| [Sm11.397(TeO3)12][K5.809Cl16] | I4/mcm | P(4/m)bm | 21x, 21y | 15.894 | 15.894 | 24.885 | 6287 | [15] |
| [Gd11.86(TeO3)12][K4.42Cl16] | I4/mcm | P(4/m)bm | 21x, 21y | 15.804 | 15.804 | 24.961 | 6234 | [15] |
| [Ho11.697(TeO3)12][K4.908Cl16] | I4/mcm | P(4/m)bm | 21x, 21y | 15.826 | 15.826 | 24.817 | 6216 | [15] |
| [Pr11.781(TeO3)12][K5.657Cl17] | I4/mcm | P(4/m)bm | 21x, 21y | 16.071 | 16.071 | 24.862 | 6421 | [15] |
| [Gd12(TeO3)12][Cd6Cl24] | I4/mcm | P(4/m)bm | 21x, 21y | 15.763 | 15.763 | 26.410 | 6561.82 | [15] |
| [LiPr11(SeO3)12][Rb6Cl16] | I4/mcm | P(4/m)bm | 21x, 21y | 15.906 | 15.906 | 24.790 | 6271.89 | [13] |
| [LiNd11(SeO3)12][Rb6Cl16] | I4/mcm | P(4/m)bm | 21x, 21y | 15.817 | 15.817 | 24.770 | 6196.90 | [12] |
| MDO3-polytype | ||||||||
| [CaNd10(SeO3)12][Cl8] * | Bbab | Pba(b) | 21x, 2y | 15.681 | 15.588 | 17.419 | 4257.82 | [52] |
| [SrNd10(SeO3)12][Cl8] * | Bbab | Pba(b) | 21x, 2y | 15.773 | 15.836 | 17.622 | 4401.64 | [52] |
| [Cs0.5Nd10.5(SeO3)12][Br8] | Bbab | Pba(b) | 21x, 2y | 15.797 | 15.797 | 17.693 | 4482.58 | [55] |
| MDO4-polytype | ||||||||
| [Nd11(SeO3)12][Cs7Cl16] | Pna21 | P12/a(1) | 2y | 15.911 | 15.951 | 25.860 | 6563.17 | [38] |
| [Sm11(SeO3)12][K7Cl16] | Pnan | P12/a(1) | 2y | 15.633 | 15.664 | 25.075 | 6140.25 | [54] |
| Non-MDO polytypes and modular structures | ||||||||
| [CsPr11(SeO3)12][Cs7Cl16] * | Aeam | n.d. | n.d. | 15.999 | 15.986 | 51.698 | 13222.28 | [54] |
| [Nd11(SeO3)12][Cs7Cl16] | Pban | n.d. | n.d. | 15.941 | 15.954 | 51.656 | 13137.29 | [38] |
| {[La11(SeO3)12][Cs6Cl16]}{[La11(SeO3)12][Cl8]} * | Aeam | P(4/m)bm | 21x, 21y | 16.073 | 16.037 | 43.176 | 11129.16 | [53] |
| Pba(b) | 21x, 2y | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charkin, D.O.; Dolgikh, V.A.; Omelchenko, T.A.; Vaitieva, Y.A.; Volkov, S.N.; Deyneko, D.V.; Aksenov, S.M. Symmetry Analysis of the Complex Polytypism of Layered Rare-Earth Tellurites and Related Selenites: The Case of Introducing Transition Metals. Symmetry 2022, 14, 2087. https://doi.org/10.3390/sym14102087
Charkin DO, Dolgikh VA, Omelchenko TA, Vaitieva YA, Volkov SN, Deyneko DV, Aksenov SM. Symmetry Analysis of the Complex Polytypism of Layered Rare-Earth Tellurites and Related Selenites: The Case of Introducing Transition Metals. Symmetry. 2022; 14(10):2087. https://doi.org/10.3390/sym14102087
Chicago/Turabian StyleCharkin, Dmitri O., Valeri A. Dolgikh, Timofey A. Omelchenko, Yulia A. Vaitieva, Sergey N. Volkov, Dina V. Deyneko, and Sergey M. Aksenov. 2022. "Symmetry Analysis of the Complex Polytypism of Layered Rare-Earth Tellurites and Related Selenites: The Case of Introducing Transition Metals" Symmetry 14, no. 10: 2087. https://doi.org/10.3390/sym14102087
APA StyleCharkin, D. O., Dolgikh, V. A., Omelchenko, T. A., Vaitieva, Y. A., Volkov, S. N., Deyneko, D. V., & Aksenov, S. M. (2022). Symmetry Analysis of the Complex Polytypism of Layered Rare-Earth Tellurites and Related Selenites: The Case of Introducing Transition Metals. Symmetry, 14(10), 2087. https://doi.org/10.3390/sym14102087








