[3 + 2] Cycloadditions in Asymmetric Synthesis of Spirooxindole Hybrids Linked to Triazole and Ferrocene Units: X-ray Crystal Structure and MEDT Study of the Reaction Mechanism
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Spirooxindole Hybrids 4a-i
2.1.1. (2′S,3R)-2′-(1-(3-Chloro-4-fluorophenyl)-5-methyl-1H-1,2,3-triazole-4-carbonyl)- 1′-(ferrocin-2-yl)-1′,2′,5′,6′,7′,7a’-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4a
2.1.2. (2′S,3R)-2′-(1-(3-Chloro-4-fluorophenyl)-5-methyl-1H-1,2,3-triazole-4-carbonyl)-5-methoxy-)- 1′-(ferrocin-2-yl)-1′,2′,5′,6′,7′,7a’-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4b
2.1.3. (2′S,3R)-2′-(1-(3-Chloro-4-fluorophenyl)-5-methyl-1H-1,2,3-triazole-4-carbonyl)-5-chloro-)- 1′-(ferrocin-2-yl)-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4c
2.1.4. (2′S,3R)-2′-(1-(3-Chloro-4-fluorophenyl)-5-methyl-1H-1,2,3-triazole-4-carbonyl)-5-bromo-)- 1′-(ferrocin-2-yl)-1′,2′,5′,6′,7′,7a’-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4d
2.1.5. (2′S,3R)-2′-(1-(3-Chloro-4-fluorophenyl)-5-methyl-1H-1,2,3-triazole-4-carbonyl)-5-fluoro-)- 1′-(ferrocin-2-yl)-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4e
2.1.6. (2′S,3R)-2′-(1-(3-Chloro-4-fluorophenyl)-5-methyl-1H-1,2,3-triazole-4-carbonyl)-5-nitro-)- 1′-(ferrocin-2-yl)-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4f
2.1.7. (2′S,3R)-2′-(1-(3-Chloro-4-fluorophenyl)-5-methyl-1H-1,2,3-triazole-4-carbonyl)-6-chloro-)- 1′-(ferrocin-2-yl)-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4g
2.1.8. (2′S,3R)-2′-(1-(3-Chloro-4-fluorophenyl)-5-methyl-1H-1,2,3-triazole-4-carbonyl)- 1-methyl-1′-(ferrocin-2-yl)-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4h
2.1.9. (2′S,3R)-1-(2-Bromoethyl)-2′-(1-(3-chloro-4-fluorophenyl)-5-methyl-1H-1,2,3-triazole-4-carbonyl) -1′-(ferrocin-2-yl)-1′,2′,5′,6′,7′,7a’-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4i
2.2. Computational Details
2.3. X-ray Structure Determinations
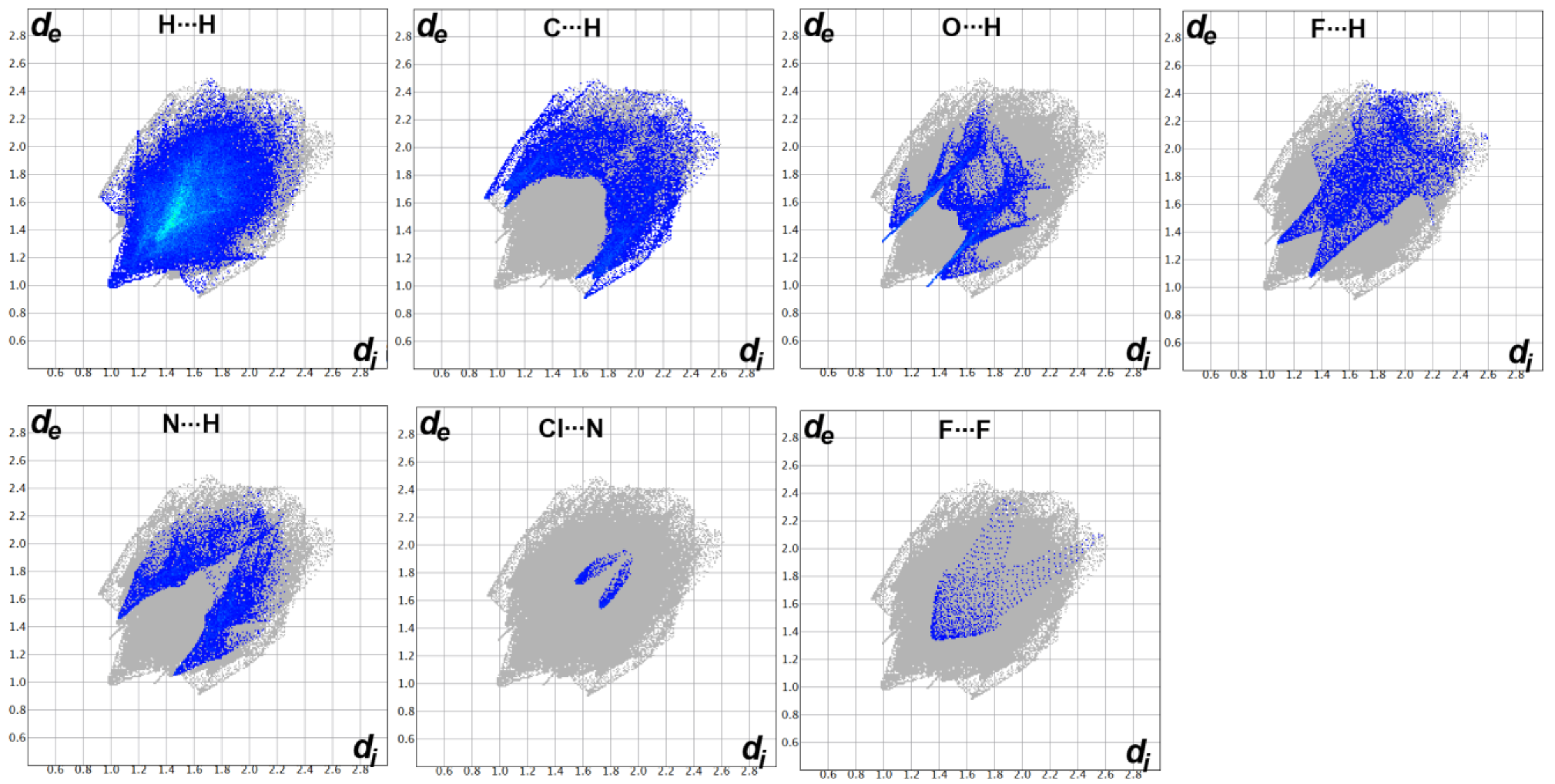
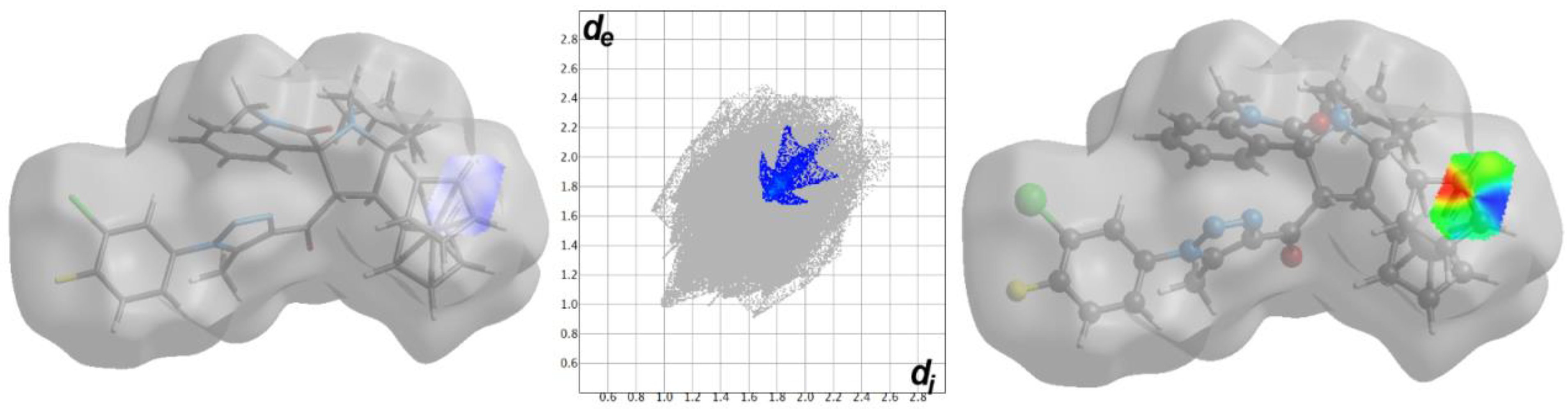
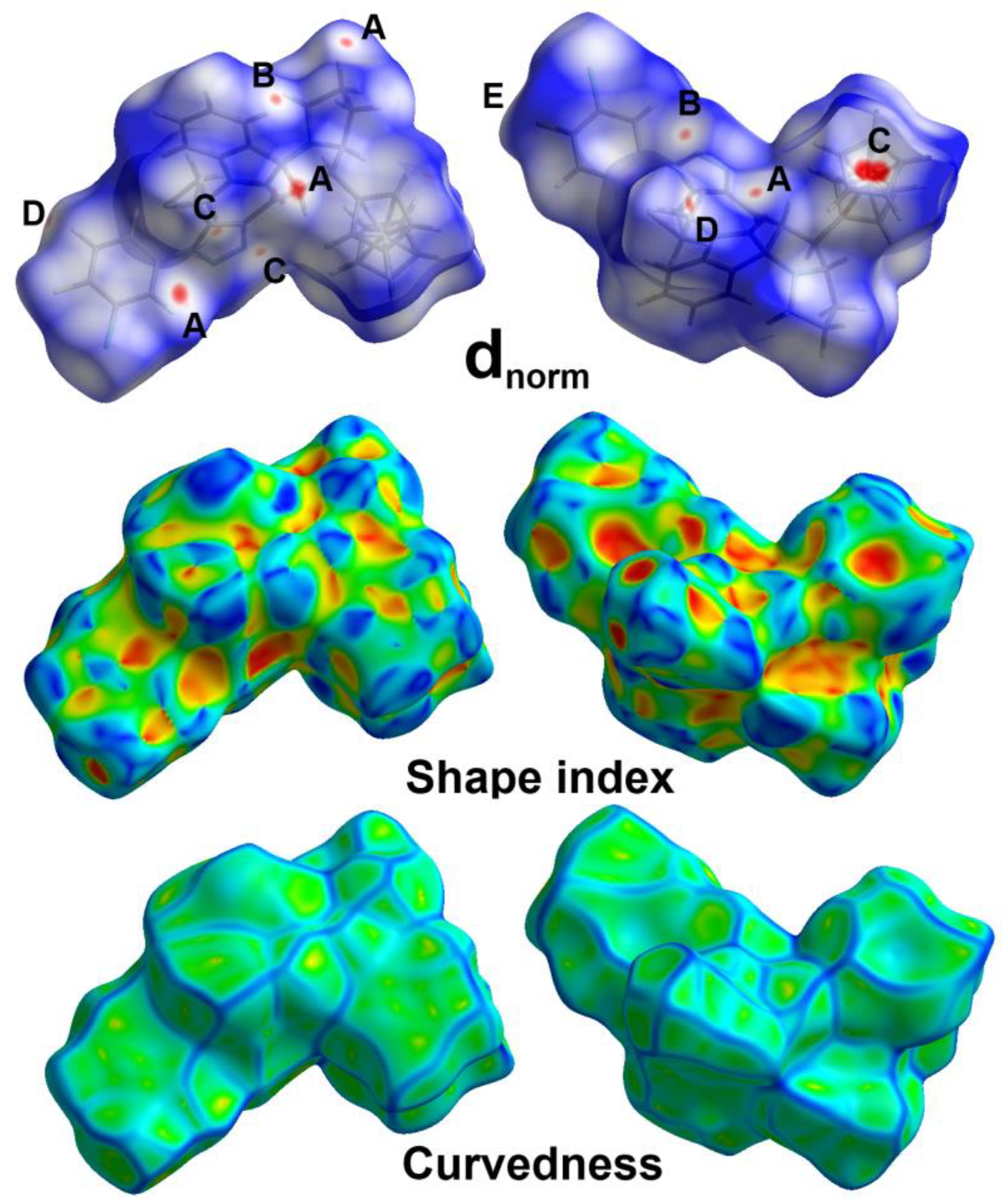
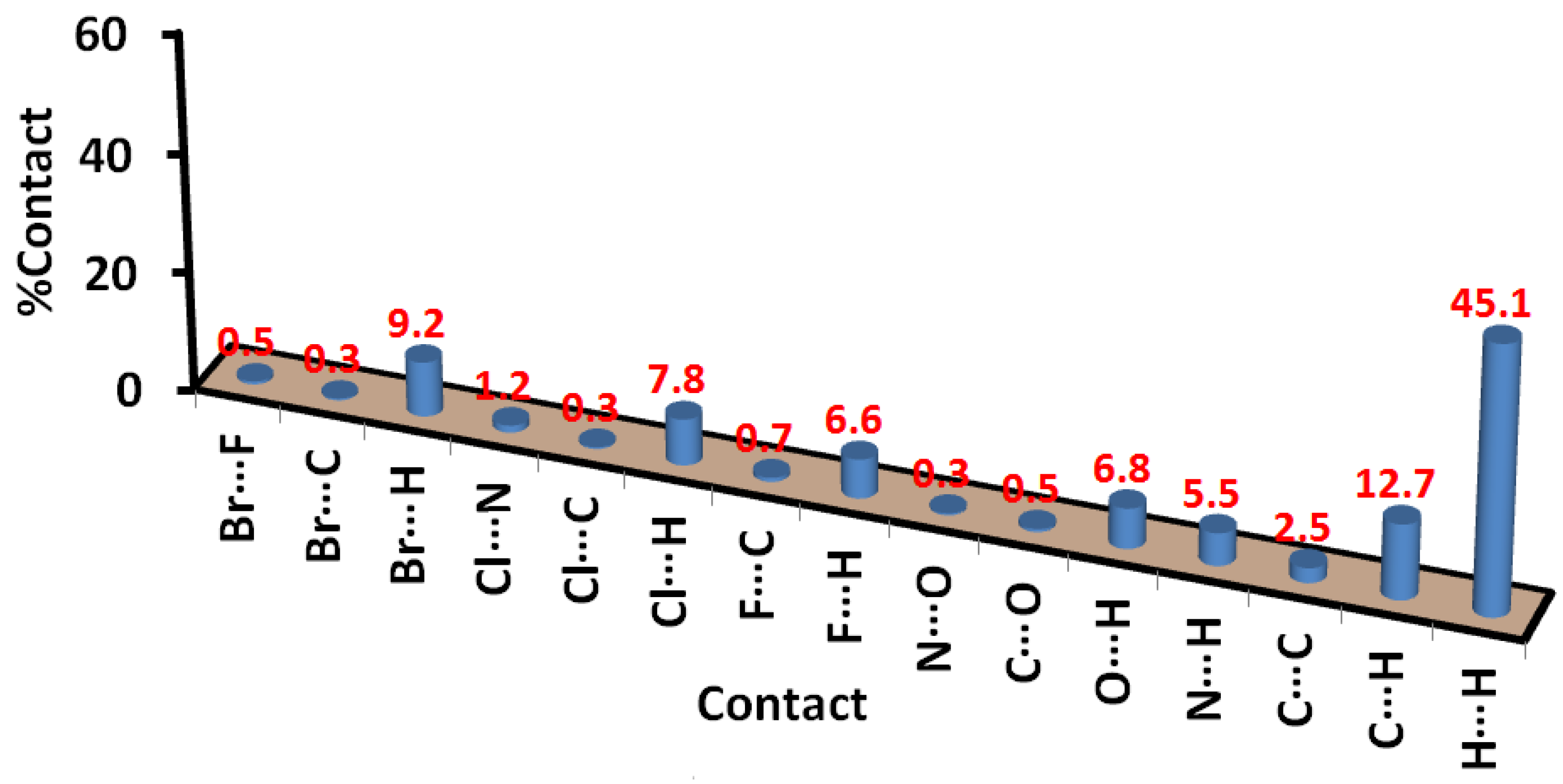
2.4. Hirshfeld Surface Analysis
3. Results and Discussion
3.1. Chemistry
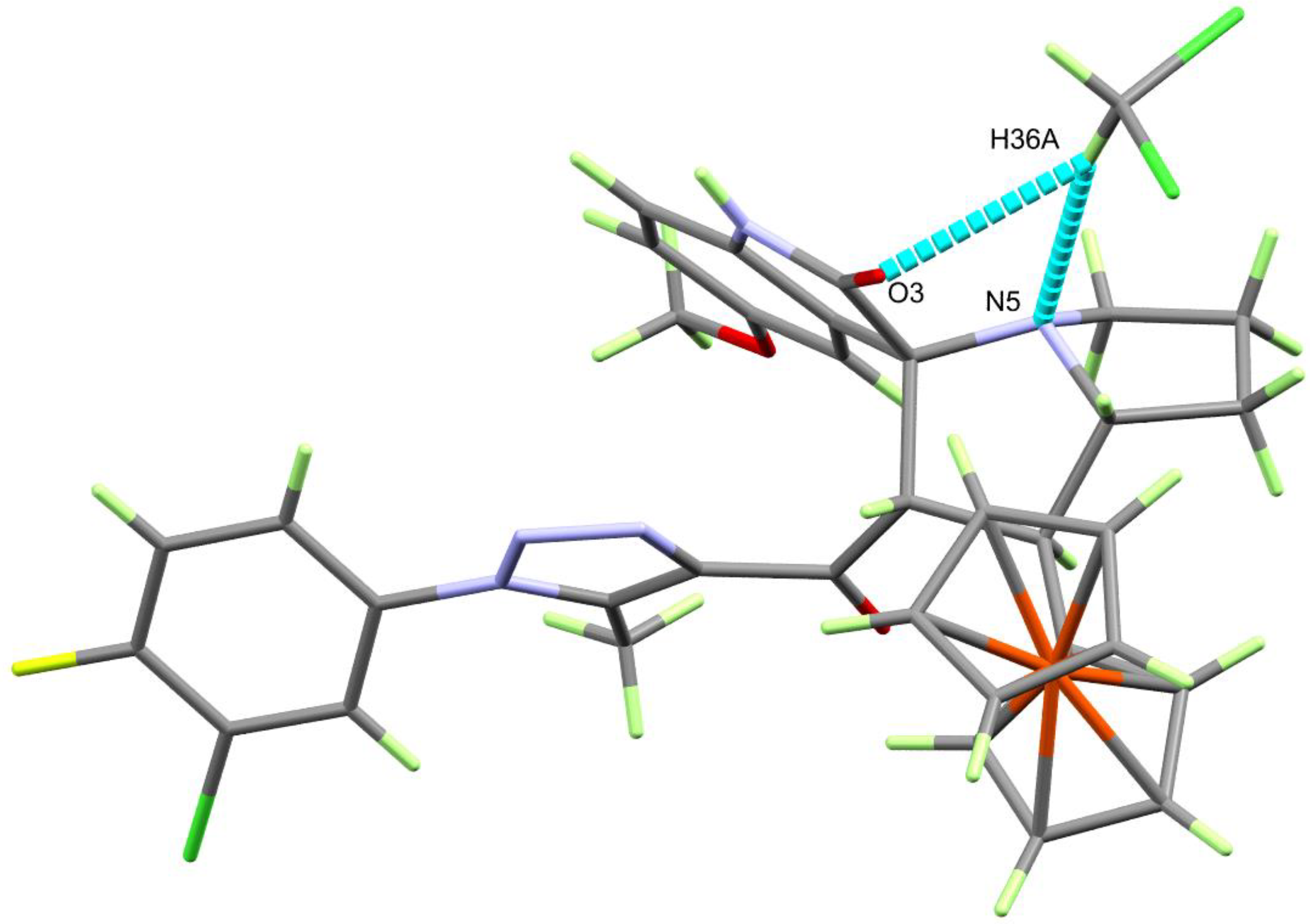
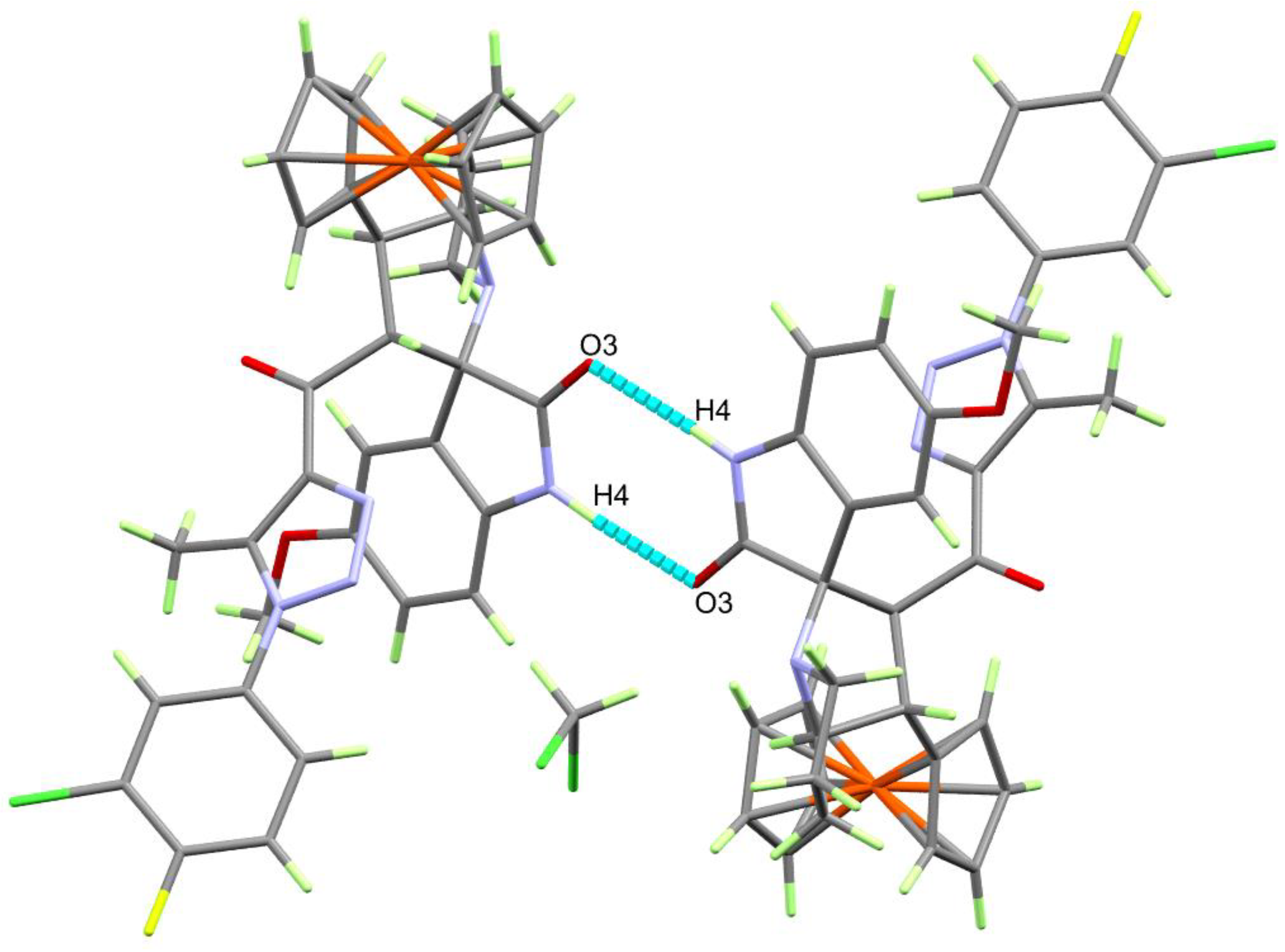
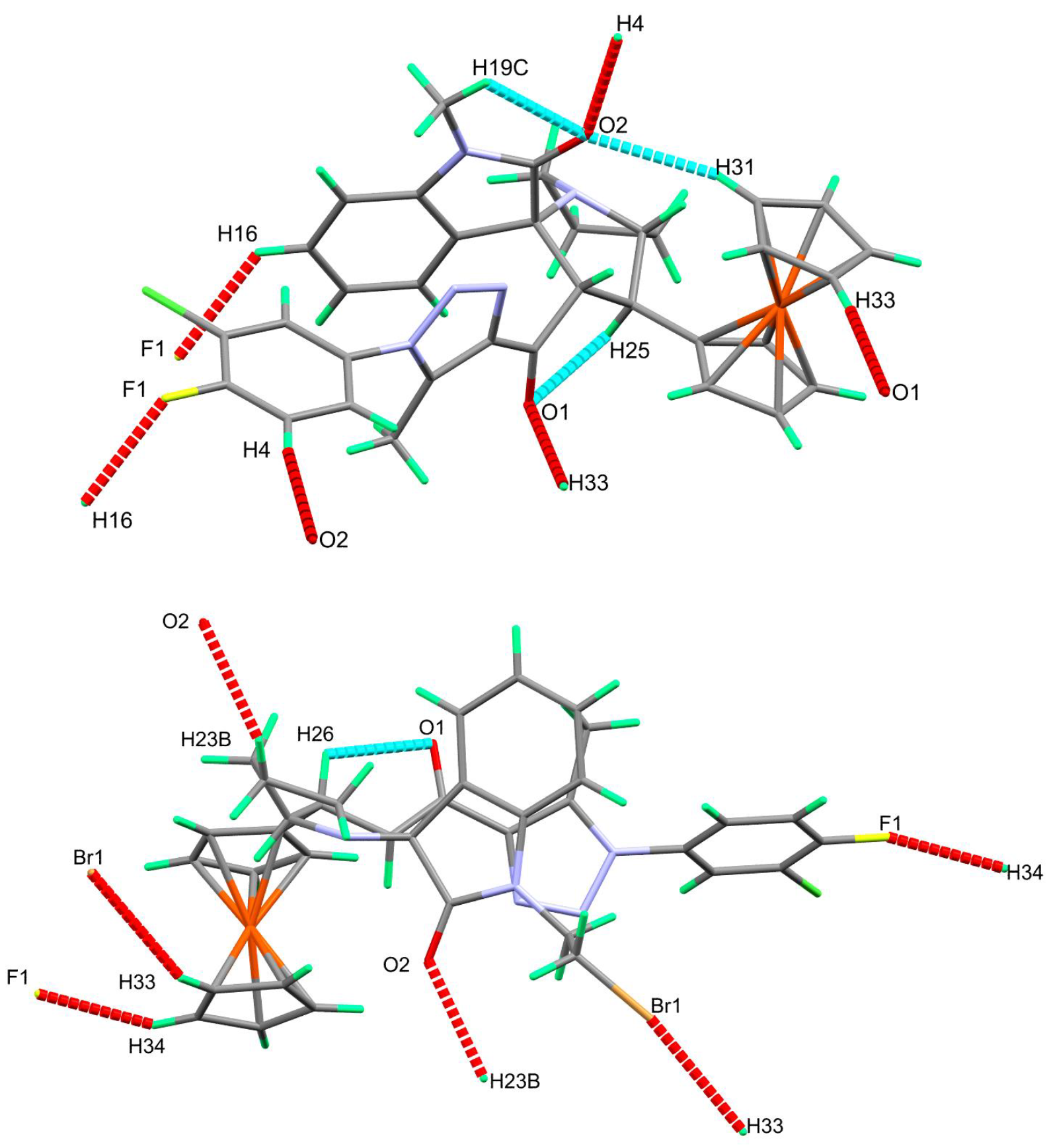
3.2. Crystal-Structure Description of 4b, 4e, 4h and 4i
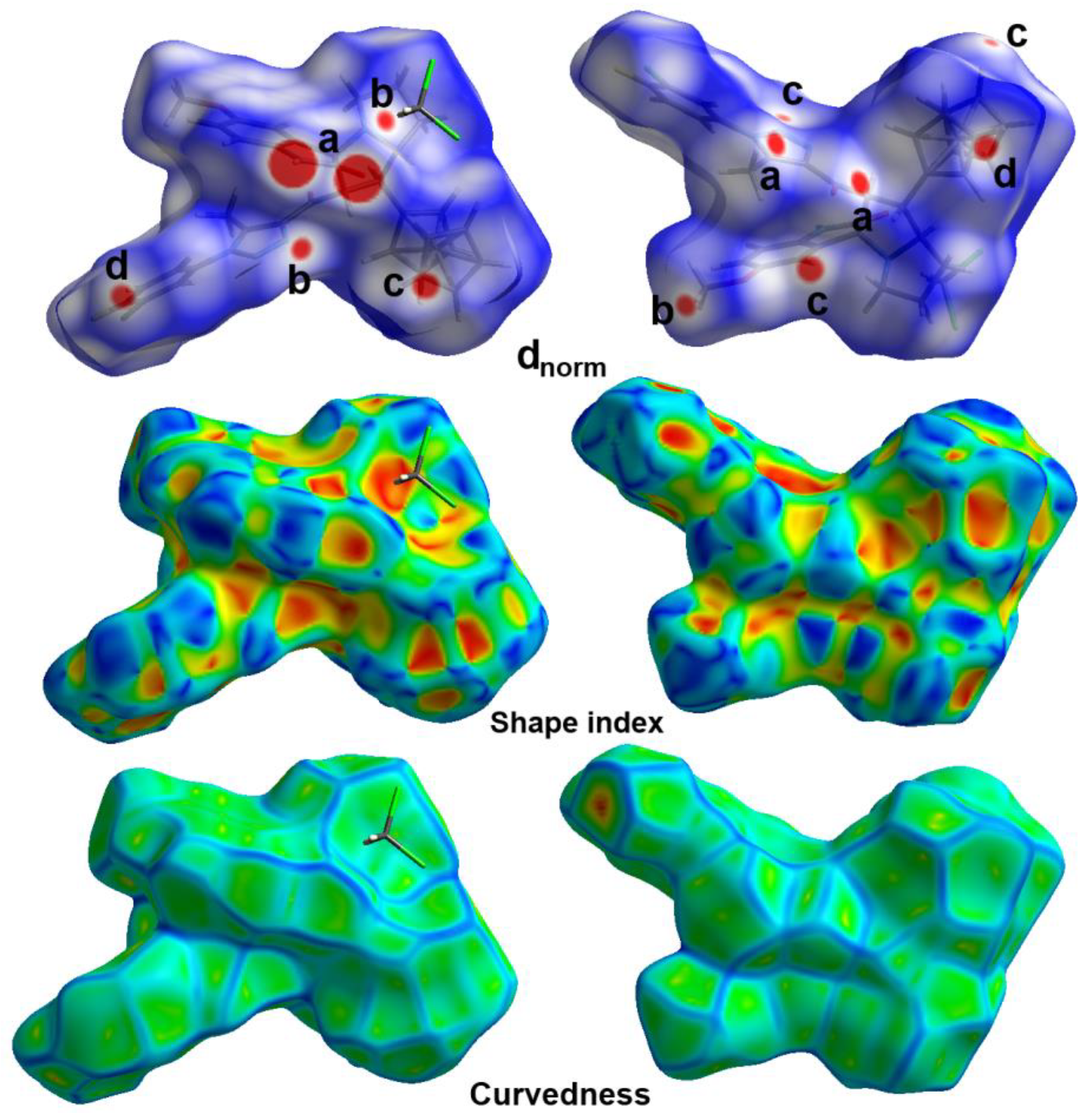
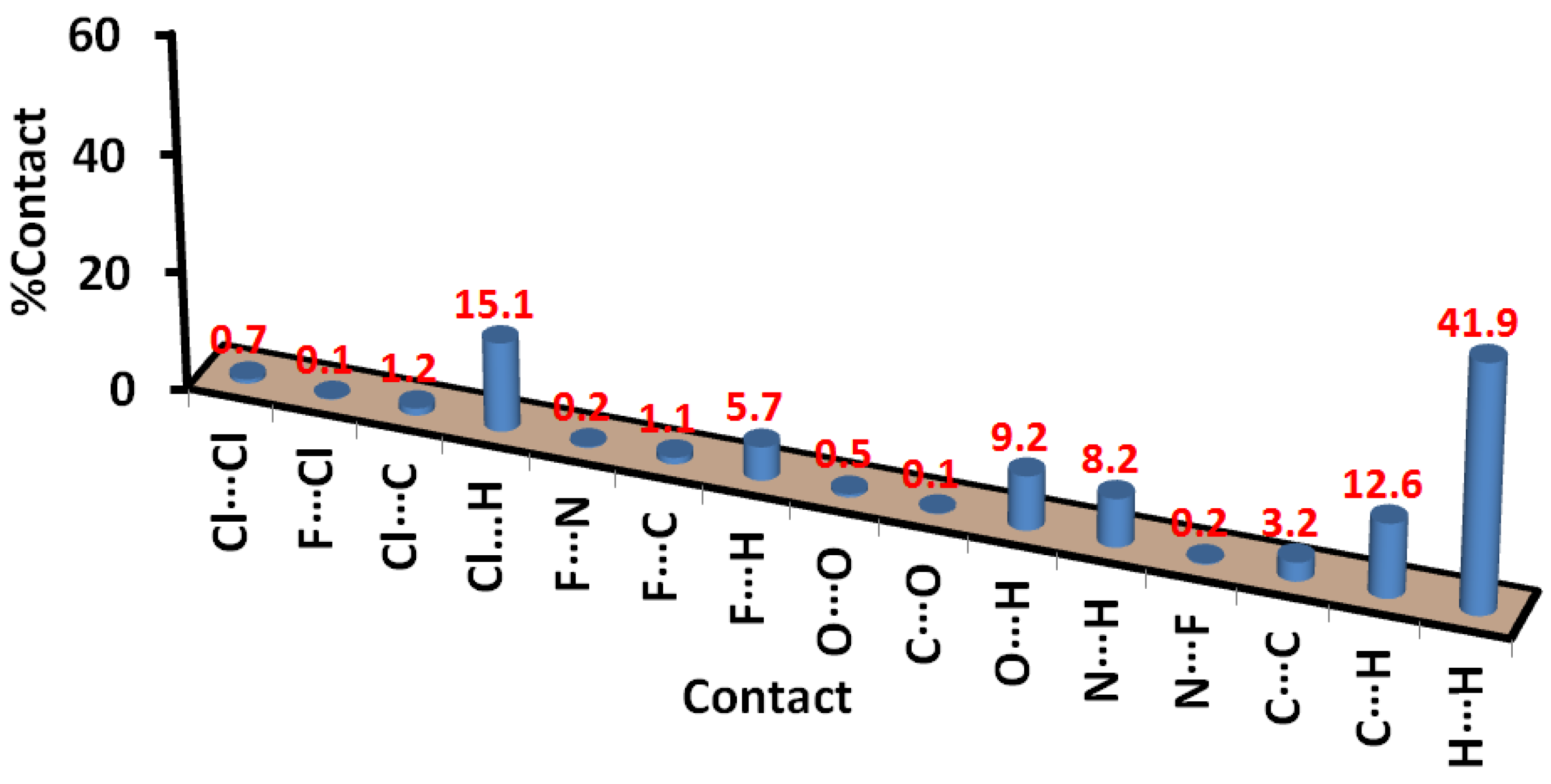
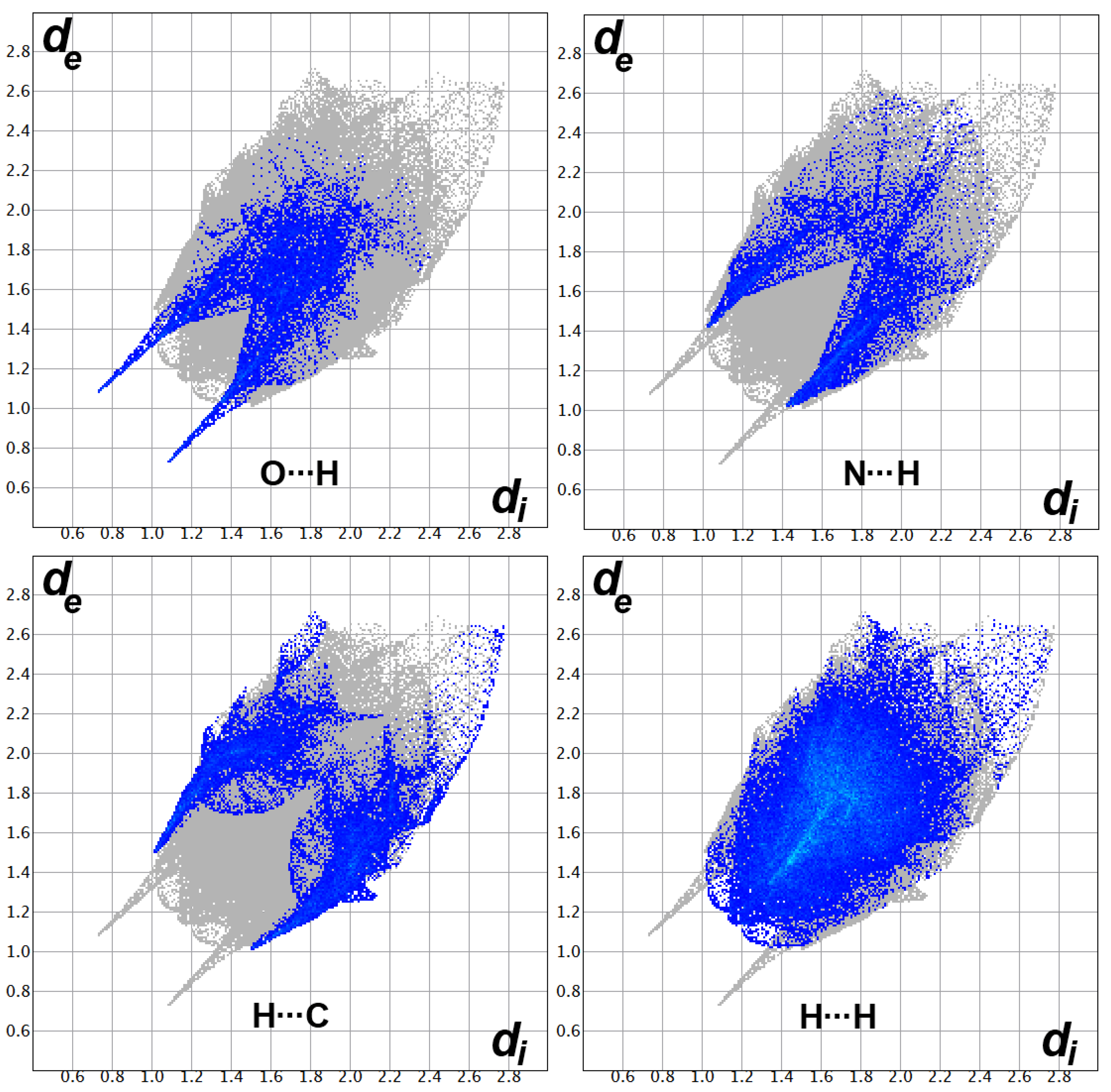
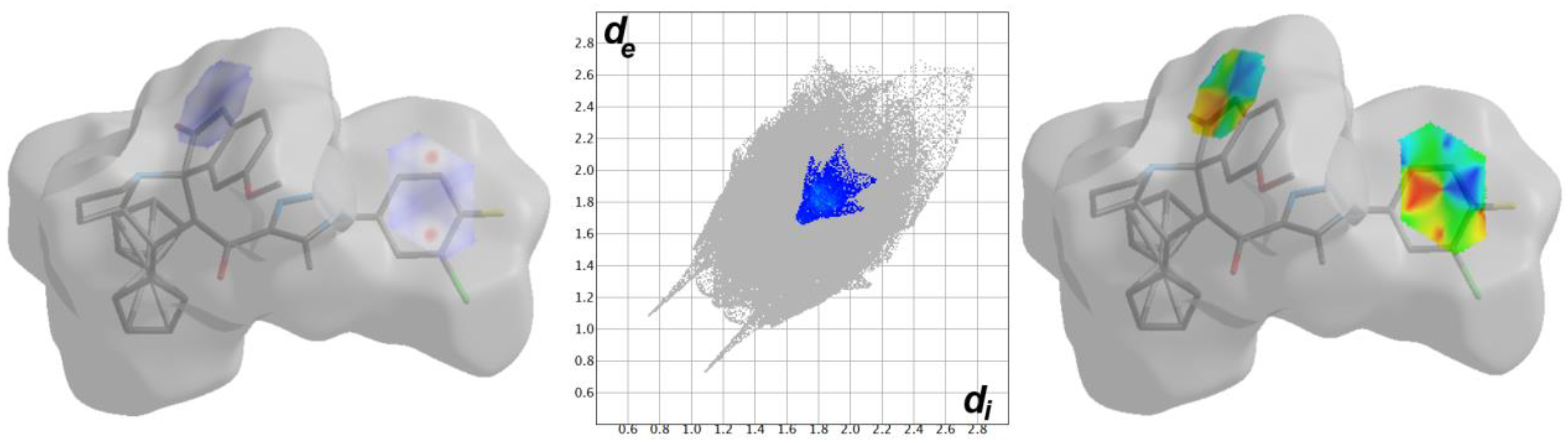
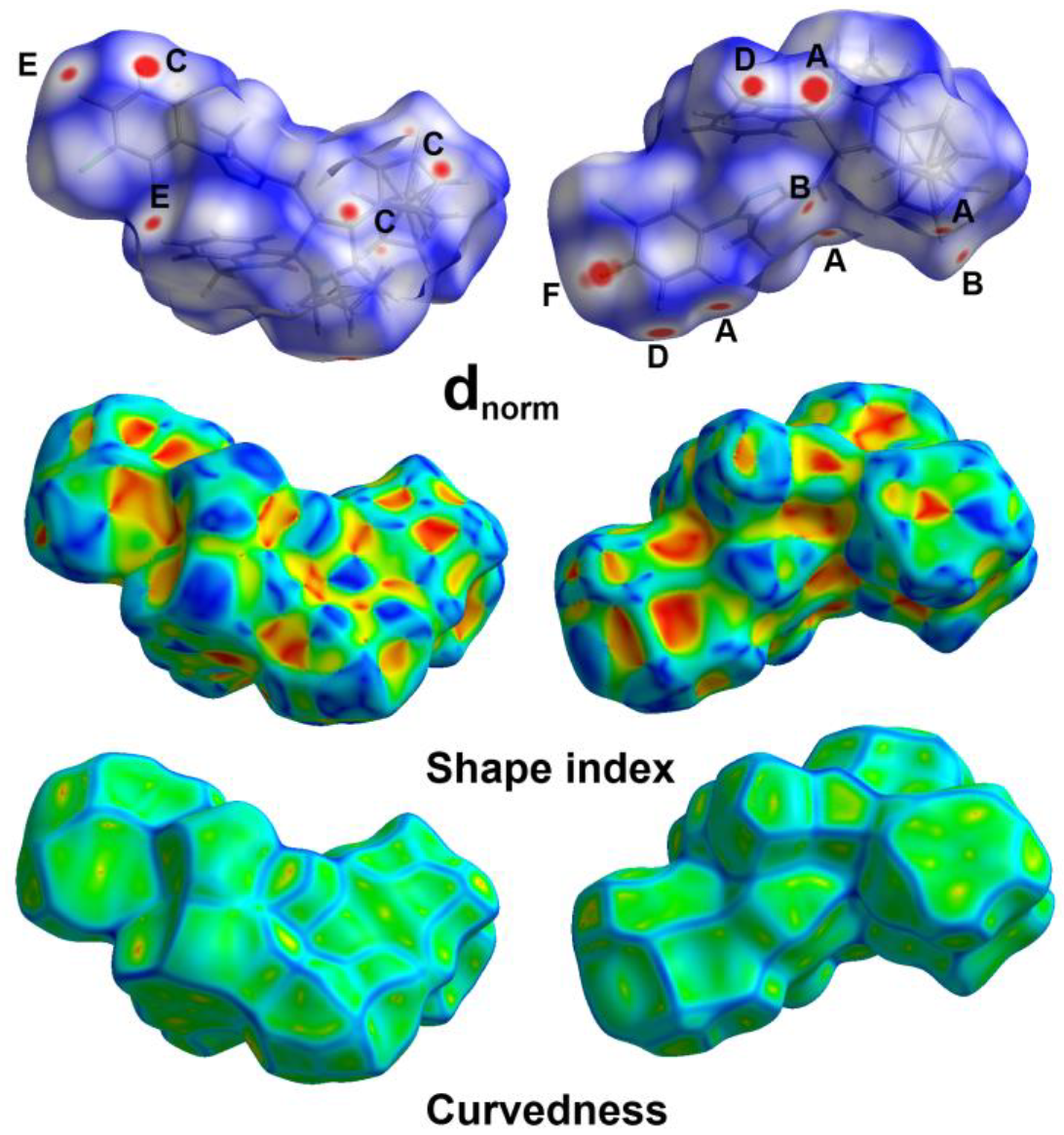
3.3. Hirshfeld Surface Analysis
3.4. MEDT Study of the 32CA Reaction of AY 5b with Ferrocene Ethylene Derivative 1
3.4.1. Conceptual DFT Analysis at the Ground State of the Reagents
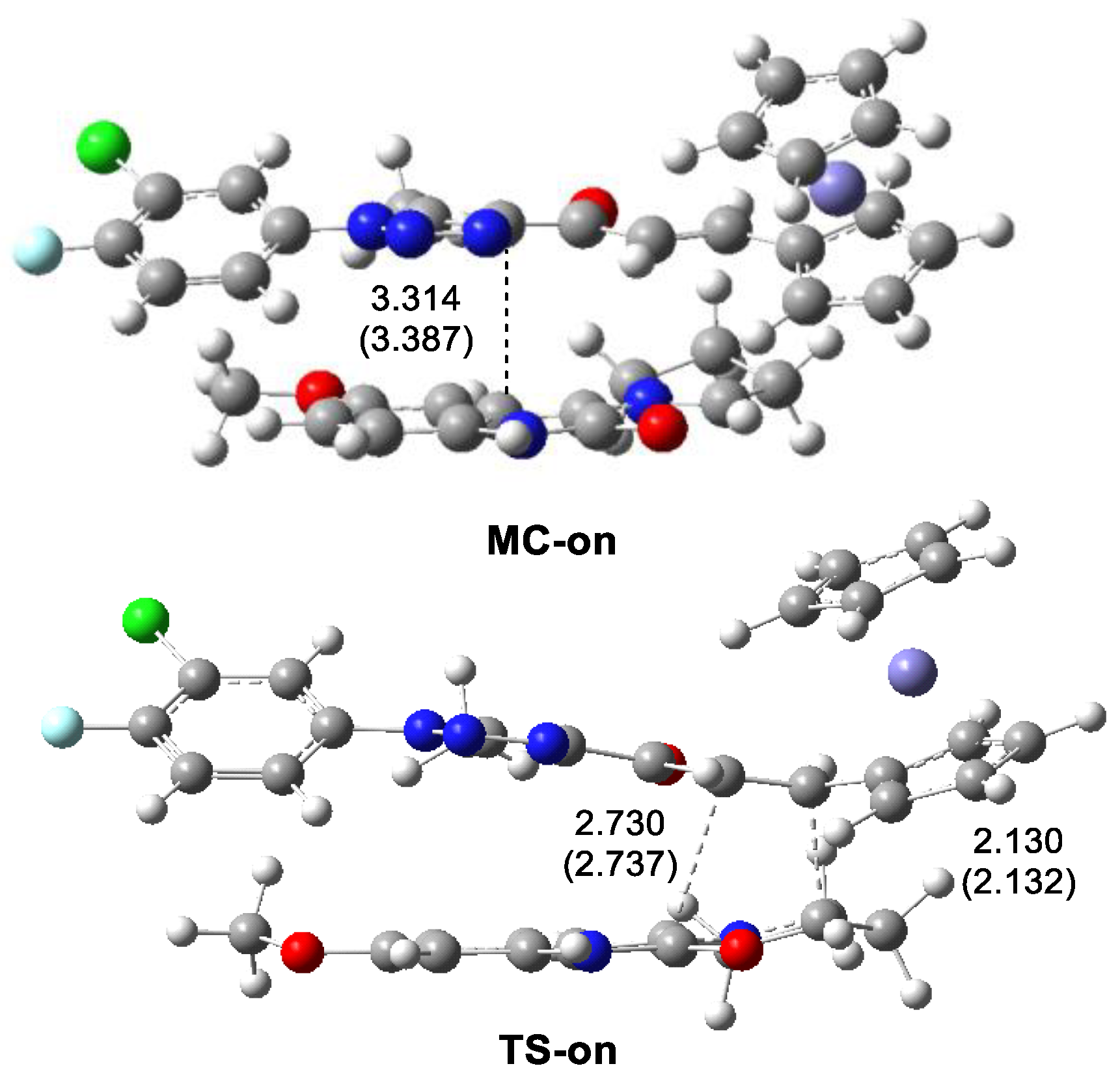
3.4.2. Study of the Reaction Mechanism of the 32CA Reaction of AY 5b with Ferrocene Ethylene 1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cerulli, V.; Banfi, L.; Basso, A.; Rocca, V.; Riva, R. Diversity oriented and chemoenzymatic synthesis of densely functionalized pyrrolidines through a highly diastereoselective Ugimulticomponent reaction. Org. Biomol. Chem. 2012, 10, 1255–1274. [Google Scholar] [CrossRef] [PubMed]
- Ganem, B. Strategies for innovation in multicomponent reaction design. Acc. Chem. Res. 2009, 42, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Domling, A. Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem. Rev. 2006, 106, 17–89. [Google Scholar] [CrossRef]
- Trost, B.M.; Brennan, M.K. Asymmetric Syntheses of Oxindole and Indole Spirocyclic Alkaloid Natural Products. Synthesis 2009, 2009, 3003–3025. [Google Scholar] [CrossRef]
- Jossang, A.; Jossang, P.; Hadi, H.A.; Sevenet, T.; Bodo, B. Horsfiline, an oxindole alkaloid from Horsfieldia superba. J. Org. Chem. 1991, 56, 6527–6530. [Google Scholar] [CrossRef]
- Cui, C.B.; Kakeya, H.; Osada, H. Novel mammalian cell cycle inhibitors, spirotryprostatins A and B, produced by Aspergillus fumigatus, which inhibit mammalian cell cycle at G2/M phase. Tetrahedron 1996, 52, 12651–12666. [Google Scholar] [CrossRef]
- Prado, E.G.; Gimenez, M.D.G.; Vazquez, R.D.L.P.; Sanchez, J.L.E.; Rodriguez, M.T.S. Antiproliferative effects of mitraphylline, a pentacyclic oxindole alkaloid of Uncaria tomentosa on human glioma and neuroblastoma cell lines. Phytomedicine 2007, 14, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Al-Majid, A.M.; Ali, M.; Islam, M.S.; Alshahrani, S.; Alamary, A.S.; Yousuf, S.; Choudhary, M.I.; Barakat, A. Stereoselective Synthesis of the Di-Spirooxindole Analogs Based Oxindole and Cyclohexanone Moieties as Potential Anticancer Agents. Molecules 2021, 26, 6305. [Google Scholar] [CrossRef] [PubMed]
- Barakat, A.; Islam, M.S.; Ali, M.; Al-Majid, A.M.; Alshahrani, S.; Alamary, A.S.; Yousuf, S.; Choudhary, M.I. Regio- and Stereoselective Synthesis of a New Series of Spirooxindole Pyrrolidine Grafted Thiochromene Scaffolds as Potential Anticancer Agents. Symmetry 2021, 13, 1426. [Google Scholar] [CrossRef]
- Kornet, M.J.; Thio, A.P. Oxindole-3-spiropyrrolidines and piperidines. Synthesis and local anesthetic activity. J. Med. Chem. 1976, 19, 892–898. [Google Scholar] [CrossRef]
- Rajanarendar, E.; Ramakrishna, S.; Reddy, K.G.; Nagaraju, D.; Reddy, Y.N. A facile synthesis, anti-inflammatory and analgesic activity of isoxazolyl-2,3- dihydrospiro[benzo[f]isoindole-1,30-indoline]-20,4,9-triones. Bioorg. Med. Chem. Lett. 2013, 23, 3954–3958. [Google Scholar] [CrossRef]
- Rajesh, M.; Perumal, S.; Menéndez, J.C.; Yogeeswari, P.; Sriram, D. Antimycobacterial activity of spirooxindolo-pyrrolidine, pyrrolizine and pyrrolothiazole hybrids obtained by a three-component regio- and stereoselective 1,3-dipolar cycloaddition. Med. Chem. Commun. 2011, 2, 626–630. [Google Scholar] [CrossRef]
- Bhaskar, G.; Arun, Y.; Balachandran, C.; Saikumar, C.; Perumal, P.T. Synthesis of novel spirooxindole derivatives by one pot multicomponent reaction and their antimicrobial activity. Eur. J. Med. Chem. 2012, 51, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Altowyan, M.S.; Barakat, A.; Al-Majid, A.M.; Al-Ghulikah, H.A. Spiroindolone Analogues as Potential Hypoglycemic with Dual Inhibitory Activity on α-Amylase and α-Glucosidase. Molecules 2019, 24, 2342. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.H.; Xu, X.G.; Li, J.; Min, X.; Yao, J.Z.; Dong, G.Q.; Zhuang, C.L.; Miao, Z.Y.; Zhang, W.N. Design, synthesis and structure–activity relationship of 4, 5-dihydropyrrolo [3, 4-c] pyrazol-6 (1H)-ones as potent p53-MDM2 inhibitors. Chin. Chem. Lett. 2017, 28, 422–425. [Google Scholar] [CrossRef]
- Barakat, A.; Alshahrani, S.; Al-Majid, A.M.; Alamary, A.S.; Haukka, M.; Abu-Serie, M.M.; Dömling, A.; Mazyed, E.A.; Badria, F.A.; El-Senduny, F.F. Novel spirooxindole based benzimidazole scaffold: In vitro, nanoformulation and in vivo studies on anticancer and antimetastatic activity of breast adenocarcinoma. Bioorg. Chem. 2022, 129, 106124. [Google Scholar] [CrossRef]
- Arumugam, N.; Almansour, A.I.; Kumar, R.S.; Dege, N. A facile ionic liquid-accelerated, four-component cascade reaction protocol for the regioselective synthesis of biologically interesting ferrocene engrafted spiropyrrolidine hybrid heterocycles. J. King Saud Univ. Sci. 2020, 32, 2500–2504. [Google Scholar] [CrossRef]
- Zhou, L.-M.; Qu, R.-Y.; Yang, G.-F. An overview of spirooxindole as a promising scaffold for novel drug discovery. Expert Opin. Drug Discov. 2020, 15, 603–625. [Google Scholar] [CrossRef]
- Barakat, A.; Alshahrani, S.; Al-Majid, A.M.; Ali, M.; Altowyan, M.S.; Islam, M.S.; Alamary, A.S.; Ashraf, S.; Ul-Haq, Z. Synthesis of a new class of spirooxindole–benzo[b]thiophene-based molecules as acetylcholinesterase inhibitors. Molecules 2020, 25, 4671. [Google Scholar] [CrossRef]
- Lotfy, G.; Aziz, Y.M.A.; Said, M.M.; El Ashry, E.S.H.; El Tamany, E.S.H.; Abu-Serie, M.M.; Teleb, M.; Dömling, A.; Barakat, A. Molecular hybridization design and synthesis of novel spirooxindole-based MDM2 inhibitors endowed with BCL2 signaling attenuation; a step towards the next generation p53 activators. Bioorg. Chem. 2021, 117, 105427. [Google Scholar] [CrossRef]
- Astruc, D. Why is Ferrocene so Exceptional? Eur. J. Inorg. Chem. 2017, 1, 6–29. [Google Scholar] [CrossRef]
- Gasser, G.; Metzler-Nolte, N. The potential of organometallic complexes in medicinal chemistry. Curr. Opin. Chem. Biol. 2012, 16, 84–91. [Google Scholar] [CrossRef]
- Santos, M.M.; Bastos, P.; Catela, I.; Zalewska, K.; Branco, L.C. Recent advances of metallocenes for medicinal chemistry. Mini-Rev. Med. Chem. 2017, 17, 771–784. [Google Scholar] [CrossRef]
- Top, S.; Vessières, A.; Leclercq, G.; Quivy, J.; Tang, J.; Vaissermann, J.; Huché, M.; Jaouen, G. Synthesis, biochemical properties and molecular modelling studies of organometallic specific estrogen receptor modulators (SERMs), the ferrocifens and hydroxyferrocifens: Evidence for an antiproliferative effect of hydroxyferrocifens on both hormone-dependent and hormone-independent breast cancer cell lines. Chem. A Eur. J. 2003, 9, 5223–5236. [Google Scholar]
- Dubar, F.; Khalife, J.; Brocard, J.; Dive, D.; Biot, C. Ferroquine, an ingenious antimalarial drug-thoughts on the mechanism of action. Molecules 2008, 13, 2900–2907. [Google Scholar] [CrossRef]
- Ferreira, V.F.; da Rocha, D.R.; da Silva, F.C.; Ferreira, P.G.; Boechat, N.A.; Magalhães, J.L. Novel 1 H-1, 2, 3-, 2 H-1, 2, 3-, 1 H-1, 2, 4-and 4 H-1, 2, 4-triazole derivatives: A patent review (2008–2011). Expert Opin. Ther. Pat. 2013, 23, 319–331. [Google Scholar] [CrossRef]
- Salmon, A.J.; Williams, M.L.; Wu, Q.K.; Morizzi, J.; Gregg, D.; Charman, S.A.; Vullo, D.; Supuran, C.T.; Poulsen, S.A. Metallocene-based inhibitors of cancerassociated carbonic anhydrase enzymes IX and XII. J. Med. Chem. 2012, 55, 5506–5517. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Carrere-Kremer, S.; Kremer, L.; Guerardel, Y.; Biot, C.; Kumar, V. 1 H-1, 2, 3-triazole-tethered isatineferrocene and isatineferrocenylchalcone conjugates: Synthesis and in vitro antitubercular evaluation. Organometallics 2013, 32, 5713–5719. [Google Scholar] [CrossRef]
- Kumar, K.; Carrère-Kremer, S.; Kremer, L.; Guérardel, Y.; Biot, C.; Kumar, V. Azide–alkyne cycloaddition en route towards 1 H-1, 2, 3-triazole-tethered β-lactam–ferrocene and β-lactam–ferrocenylchalcone conjugates: Synthesis and in vitro anti-tubercular evaluation. Dalton Trans. 2013, 42, 1492–1500. [Google Scholar] [CrossRef]
- Kumar, K.; Pradines, B.; Madamet, M.; Amalvict, R.; Benoit, N.; Kumar, V. 1H-1, 2, 3-triazole tethered isatin-ferrocene conjugates: Synthesis and in vitro antimalarial evaluation. Eur. J. Med. Chem. 2014, 87, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Van Staveren, D.R.; Metzler-Nolte, N. Bioorganometallic chemistry of ferrocene. Chem. Rev. 2004, 104, 5931–5986. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yang, Y.; Baigude, H.; Zhao, H. New ferrocene–triazole derivatives for multisignaling detection of Cu2+ in aqueous medium and their antibacterial activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 229, 117880. [Google Scholar] [CrossRef] [PubMed]
- Arivazhagan, C.; Borthakur, R.; Ghosh, S. Ferrocene and triazole-appended rhodamine based multisignaling sensors for Hg2+ and their application in live cell imaging. Organometallics 2015, 34, 1147–1155. [Google Scholar] [CrossRef]
- Fouda, M.F.; Abd-Elzaher, M.M.; Abdelsamaia, R.A.; Labib, A.A. On the medicinal chemistry of ferrocene. Appl. Organomet. Chem. 2007, 21, 613–625. [Google Scholar] [CrossRef]
- Larik, F.A.; Saeed, A.; Fattah, T.A.; Muqadar, U.; Channar, P.A. Recent advances in the synthesis, biological activities and various applications of ferrocene derivatives. Appl. Organomet. Chem. 2017, 31, e3664. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Barakat, A. A molecular electron density theory study of the [3+2] cycloaddition reaction of an azomethine ylide with an electrophilic ethylene linked to triazole and ferrocene units. Molecules 2022, 27, 6532. [Google Scholar] [CrossRef] [PubMed]
- Haque, A.; Hsieh, M.F.; Hassan, S.I.; Faizi, M.S.H.; Saha, A.; Dege, N.; Rather, J.A.; Khan, M.S. Synthesis, characterization, and pharmacological studies of ferrocene-1H-1, 2, 3-triazole hybrids. J. Mol. Struct. 2017, 1146, 536–545. [Google Scholar] [CrossRef]
- Ríos-Gutiérrez, M.; Barakat, A.; Domingo, L.R. A Molecular Electron Density Theory Study of the [3 + 2] Cycloaddition Reaction of Pseudo(mono)radical Azomethine Ylides with Phenyl Vinyl Sulphone. Organics 2022, 3, 122–136. [Google Scholar] [CrossRef]
- Barakat, A.; Haukka, M.; Soliman, S.M.; Ali, M.; Al-Majid, A.M.; El-Faham, A.; Domingo, L.R. Straightforward regio- and diastereoselective synthesis, molecular structure, intermolecular interactions and mechanistic study of spirooxindole-engrafted rhodanine analogs. Molecules 2021, 26, 7276. [Google Scholar] [CrossRef]
- Altowyan, M.S.; Soliman, S.M.; Haukka, M.; Al-Shaalan, N.H.; Alkharboush, A.A.; Barakat, A. Synthesis and Structure Elucidation of Novel Spirooxindole Linked to Ferrocene and Triazole Systems via [3 + 2] Cycloaddition Reaction. Molecules 2022, 27, 4095. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Hehre, M.J.; Radom, L.; Schleyer, P.V.R.; Pople, J. Ab Initio Molecular Orbital Theory; Wiley: New York, NY, USA, 1986. [Google Scholar]
- Domingo, L.R. Molecular electron density theory: A modern view of reactivity in organic chemistry. Molecules 2016, 21, 1319. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, J.; Persico, M. Molecular interactions in solution: And overview of methods based on continuous distributions of the solvent. Chem. Rev. 1994, 94, 2027–2094. [Google Scholar] [CrossRef]
- Simkin, B.Y.; Sheikhet, I.I. Quantum Chemical and Statistical Theory of Solutions—Computational Approach; Ellis Horwood: London, UK, 1995. [Google Scholar]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R. A new C–C bond formation model based on the quantum chemical topology of electron density. RSC Adv. 2014, 4, 32415–32428. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Natural population analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density Functional Theory of Atoms and Molecules; Oxford University Press: Oxford, UK, 1989. [Google Scholar]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. Applications of the conceptual density functional indices to organic chemistry reactivity. Molecules 2016, 21, 748. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. (Eds.) Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Otwinowski, Z.; Minor, W. Processing of X-ray Diffraction Data Collected in Oscillation Mode. In Methods in Enzymology, Macromolecular Crystallography, Part A; Carter, C.W., Sweet, J., Eds.; Academic Press: New York, NY, USA, 1997; Volume 276, pp. 307–326. [Google Scholar]
- Rikagu Oxford Diffraction. CrysAlisPro; Rikagu Oxford Diffraction Inc.: Oxfordshire, UK, 2022. [Google Scholar]
- Sheldrick, G.M. SADABS—Bruker Nonius Scaling and Absorption Correction; Bruker AXS, Inc.: Madison, WI, USA, 2012. [Google Scholar]
- Sheldrick, G.M. SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. Sect. C Struct. Chem. 2015, C71, 148–155. [Google Scholar]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. Crystal Explorer17; University of Western Australia: Crawley, WA, Australia, 2017; Available online: http://hirshfeldsurface.net (accessed on 20 July 2020).
- Domingo, L.R.; Ríos-Gutiérrez, M. Application of Reactivity Indices in the Study of Polar Diels–Alder Reactions, in Conceptual Density Functional Theory: Towards a New Chemical Reactivity Theory; Liu, S., Ed.; Wiley-VCH GmbH: Weinheim, Germany, 2022; Volume 2, pp. 481–502. [Google Scholar]
- Parr, R.G.; Pearson, R.G. Absolute hardness: Companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. A Molecular Electron Density Theory Study of the Reactivity of Tetrazines in Aza-Diels-Alder Reactions. RSC Adv. 2020, 10, 15394–15405. [Google Scholar] [CrossRef]
- Parr, R.G.; Szentpaly, L.V.; Liu, S. Electrophilicity index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Domingo, L.R.; Chamorro, E.; Pérez, P. Understanding the reactivity of captodative ethylenes in polar cycloaddition reactions. A theoretical study. J. Org. Chem. 2008, 73, 4615–4624. [Google Scholar] [CrossRef]
- Domingo, L.R.; Sáez, J.A.; Zaragozá, R.J.; Arnó, M. Understanding the Participation; f Quadricyclane as Nucleophile in Polar [2 sigma+2 sigma+2 pi] Cycloadditions toward Electrophilic pi Molecules. J. Org. Chem. 2008, 73, 8791–8799. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. How does the global electron density transfer diminish activation energies in polar cycloaddition reactions? A Molecular Electron Density Theory study. Tetrahedron 2017, 73, 1718–1724. [Google Scholar] [CrossRef]




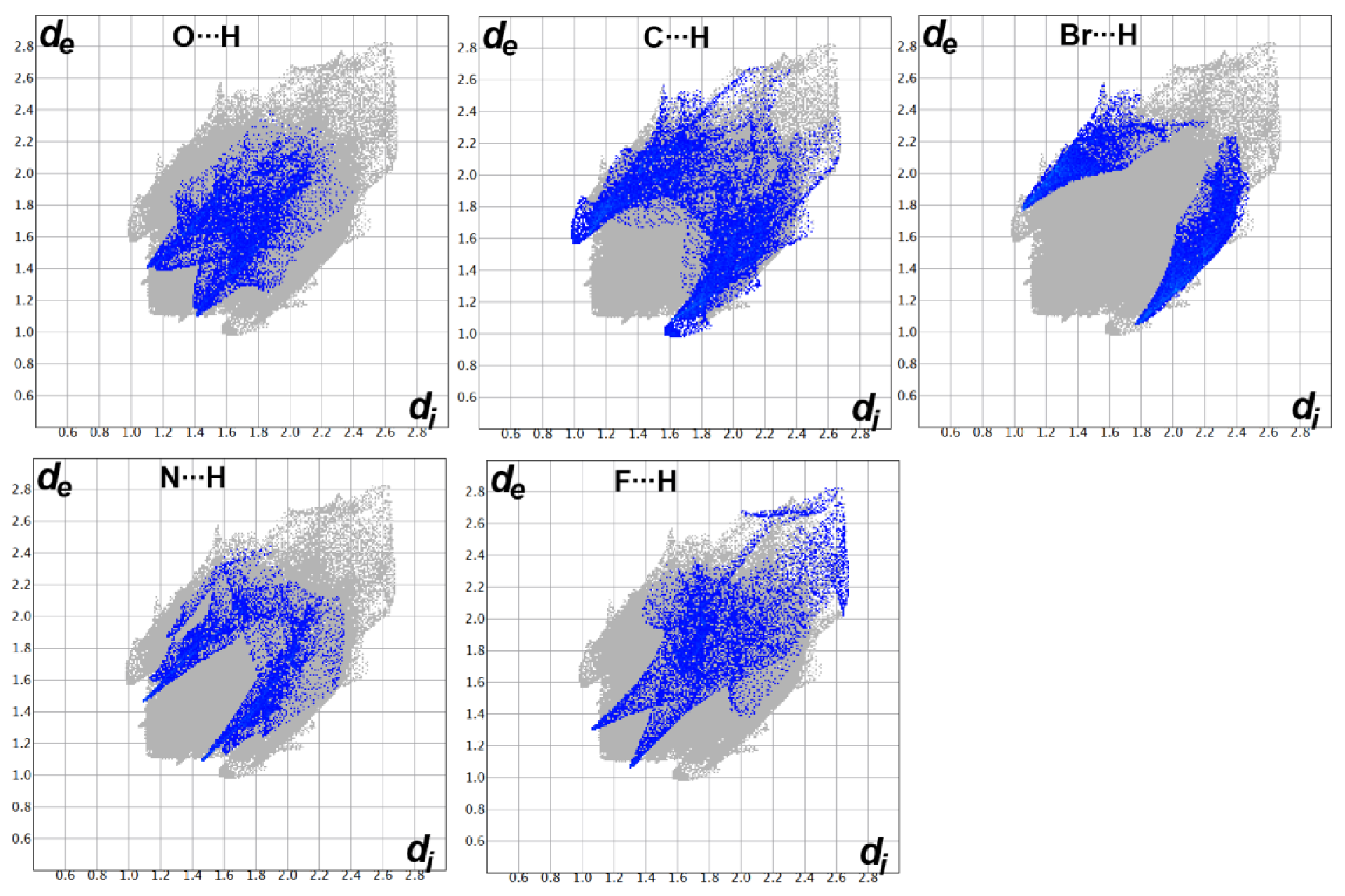



| 4b | 4e | 4h | 4i | |
|---|---|---|---|---|
| CCDC | 2203019 | 2203020 | 2203021 | 2203022 |
| empirical formula | C36H33Cl3FFeN5O3 | C34H28ClF2FeN5O2 | C35H31ClFFeN5O2 | C36H32BrClFFeN5O2 |
| fw | 764.87 | 667.91 | 663.95 | 756.87 |
| temp (K) | 170(2) | 120(2) | 120(2) | 120(2) |
| λ(Å) | 0.71073 | 1.54184 | 1.54184 | 1.54184 |
| cryst syst | Triclinic | Triclinic | Monoclinic | Monoclinic |
| space group | P | P | P21/n | P21/c |
| a (Å) | 9.5779(2) | 9.9037(2) | 8.84939(4) | 8.36150(10) |
| b (Å) | 13.8939(3) | 13.0148(3) | 16.45070(9) | 24.3249(2) |
| c (Å) | 14.2414(3) | 13.0497(2) | 20.07254(9) | 15.84530(10) |
| α(deg) | 66.0890(10) | 93.0000(10) | 90 | 90 |
| β (deg) | 85.9040(10) | 100.840(2) | 91.7059(4) | 95.8300(10) |
| γ(deg) | 81.1600(10) | 98.026(2) | 90 | 90 |
| V (Å3) | 1711.85(6) | 1630.49(6) | 2920.84(2) | 3206.15(5) |
| Z | 2 | 2 | 4 | 4 |
| ρcalc (Mg/m3) | 1.484 | 1.360 | 1.510 | 1.568 |
| μ(Mo Kα) (mm−1) | 0.725 | 4.880 | 5.393 | 6.396 |
| No. reflns. | 30,574 | 41,983 | 82,650 | 40,811 |
| Unique reflns. | 8128 | 6822 | 6154 | 6742 |
| Completeness | 99.4% c | 99.9% d | 100% d | 100% d |
| GOOF (F2) | 1.026 | 1.070 | 1.042 | 1.041 |
| Rint | 0.0281 | 0.0426 | 0.0346 | 0.0340 |
| R1a (I ≥ 2σ) | 0.0445 | 0.0411 | 0.0372 | 0.0363 |
| wR2b (I ≥ 2σ) | 0.1005 | 0.1066 | 0.1052 | 0.0949 |
| D-H⋯A | d(D-H) | d(H⋯A) | d(D⋯A) | <(DHA) | Symm. Codes |
|---|---|---|---|---|---|
| 4b | |||||
| N(4)-H(4)⋯O(3) | 0.84(3) | 1.98(3) | 2.821(2) | 176(2) | 1 −x + 2, −y, −z + 1 |
| C(16)-H(16B)⋯⋯N(3) | 0.980 | 2.547 | 3.513(3) | 168.4 | −1 + x, y, z |
| C(32)-H(32)⋯⋯O(2) | 0.950 | 2.664 | 3.244(4) | 119.89 | −1 + x, y, z |
| C(8)-H(8A)⋯⋯O(1) | 0.980 | 2.468 | 3.315(3) | 144.44 | 1 − x, 1 − y, 1 − z |
| 4h | |||||
| C4-H4⋯O2 | 0.95 | 2.44 | 3.373(3) | 169 | 3/2 − x, −1/2 + y, 3/2 − z |
| C16-H16⋯F1 | 0.95 | 2.48 | 3.192(3) | 131 | 1 − x, 1 − y, 2 − z |
| C19-H19C⋯O2 | 0.98 | 2.5 | 2.885(3) | 103 | |
| C25-H25⋯O1 | 1.00 | 2.47 | 2.831(2) | 101 | |
| C31-H31⋯O2 | 0.95 | 2.57 | 3.438(2) | 152 | |
| C33-H33⋯O1 | 0.95 | 2.6 | 3.541(2) | 172 | 1 − x, 1 − y, 1 − z |
| 4i | |||||
| C23-H23B⋯O2 | 0.99 | 2.58 | 3.403(3) | 140 | −1 + x, y, z |
| C26-H26⋯O1 | 1.00 | 2.41 | 2.816(3) | 104 | |
| C33-H33⋯Br1 | 0.95 | 2.93 | 3.759(3) | 147 | −1 + x, 3/2 − y, −1/2 + z |
| C34−H34⋯F1 | 0.95 | 2.46 | 3.207(3) | 135 | −1 + x, y, −1 + z |
| Contact | Distance | Contact | Distance |
|---|---|---|---|
| 4h | 4i | ||
| H19C⋯⋯H15 | 1.976 | Br1⋯⋯H28 | 2.927 |
| C28⋯⋯H23A | 2.746 | Br1⋯⋯H33 | 2.82 |
| C29⋯⋯H25 | 2.631 | F1⋯⋯H34 | 2.371 |
| C4⋯⋯H22D | 2.549 | O2⋯⋯H23B | 2.513 |
| C5⋯⋯H21A | 2.776 | O1⋯⋯H5 | 2.555 |
| N3⋯⋯H28 | 2.515 | N2⋯⋯H20 | 2.554 |
| O2⋯⋯H4 | 2.306 | C32⋯⋯H15B | 2.579 |
| O1⋯⋯H33 | 2.466 | C33⋯⋯H15B | 3.07 |
| F1⋯⋯H16 | 2.396 | C34⋯⋯H15B | 2.78 |
| Cl1⋯⋯N5 | 3.281 | C9⋯⋯H8C | 2.715 |
| F1⋯⋯F1 | 2.713 | ||
| μ | η | ω | N | |
|---|---|---|---|---|
| ferrocene ethylene 1 | −3.65 | 3.55 | 1.87 | 3.70 |
| AY 5b | −2.61 | 3.20 | 1.07 | 4.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Rasheed, H.H.; Al-Majid, A.M.; Ali, M.; Haukka, M.; Ramadan, S.; Soliman, S.M.; El-Faham, A.; Domingo, L.R.; Barakat, A. [3 + 2] Cycloadditions in Asymmetric Synthesis of Spirooxindole Hybrids Linked to Triazole and Ferrocene Units: X-ray Crystal Structure and MEDT Study of the Reaction Mechanism. Symmetry 2022, 14, 2071. https://doi.org/10.3390/sym14102071
Al-Rasheed HH, Al-Majid AM, Ali M, Haukka M, Ramadan S, Soliman SM, El-Faham A, Domingo LR, Barakat A. [3 + 2] Cycloadditions in Asymmetric Synthesis of Spirooxindole Hybrids Linked to Triazole and Ferrocene Units: X-ray Crystal Structure and MEDT Study of the Reaction Mechanism. Symmetry. 2022; 14(10):2071. https://doi.org/10.3390/sym14102071
Chicago/Turabian StyleAl-Rasheed, Hessa H., Abdullah Mohammed Al-Majid, M. Ali, Matti Haukka, Sherif Ramadan, Saied M. Soliman, Ayman El-Faham, Luis R. Domingo, and Assem Barakat. 2022. "[3 + 2] Cycloadditions in Asymmetric Synthesis of Spirooxindole Hybrids Linked to Triazole and Ferrocene Units: X-ray Crystal Structure and MEDT Study of the Reaction Mechanism" Symmetry 14, no. 10: 2071. https://doi.org/10.3390/sym14102071
APA StyleAl-Rasheed, H. H., Al-Majid, A. M., Ali, M., Haukka, M., Ramadan, S., Soliman, S. M., El-Faham, A., Domingo, L. R., & Barakat, A. (2022). [3 + 2] Cycloadditions in Asymmetric Synthesis of Spirooxindole Hybrids Linked to Triazole and Ferrocene Units: X-ray Crystal Structure and MEDT Study of the Reaction Mechanism. Symmetry, 14(10), 2071. https://doi.org/10.3390/sym14102071










