Synthesis of a New Ag(I)-Azine Complex via Ag(I)-Mediated Hydrolysis of 2-(((1-(Pyridin-2-yl)ethylidene)hydrazineylidene) Methyl)phenol with AgClO4; X-ray Crystal Structure and Biological Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Instrumentations
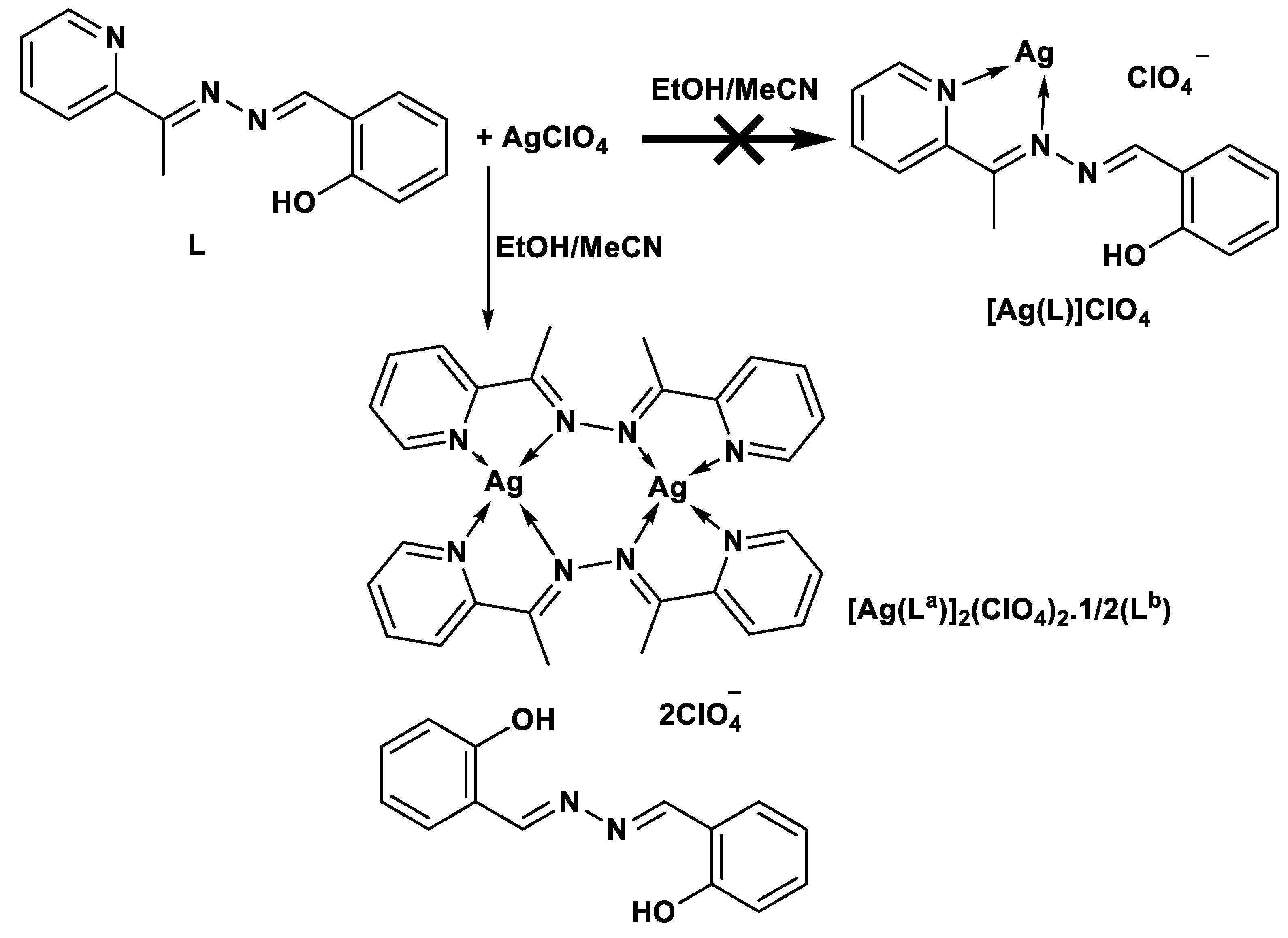
2.2. Synthesis of [Ag(La)]2(ClO4)2.1/2(Lb)
2.3. Hirshfeld Calculations
2.4. Biological Studies
3. Results and Discussion
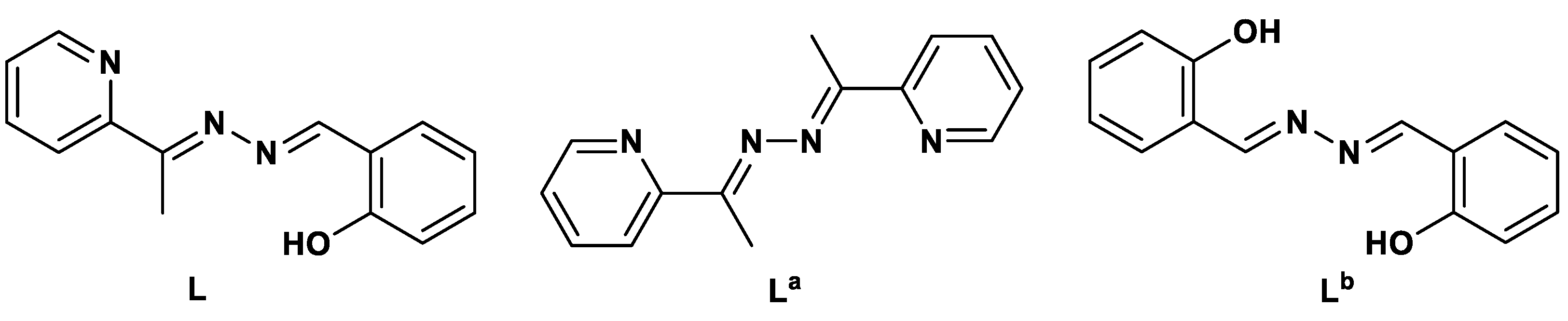
3.1. Synthesis and Characterizations
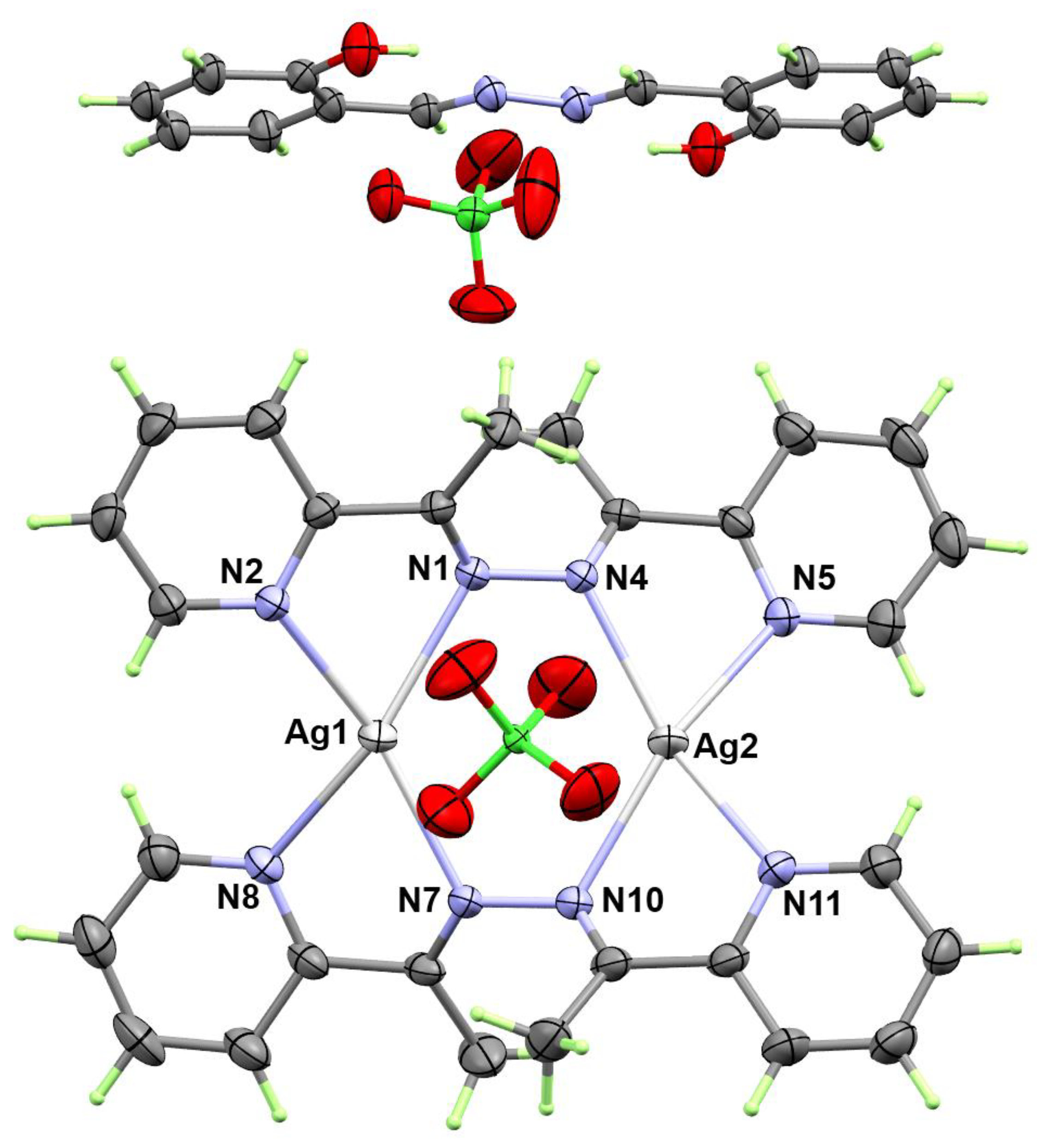
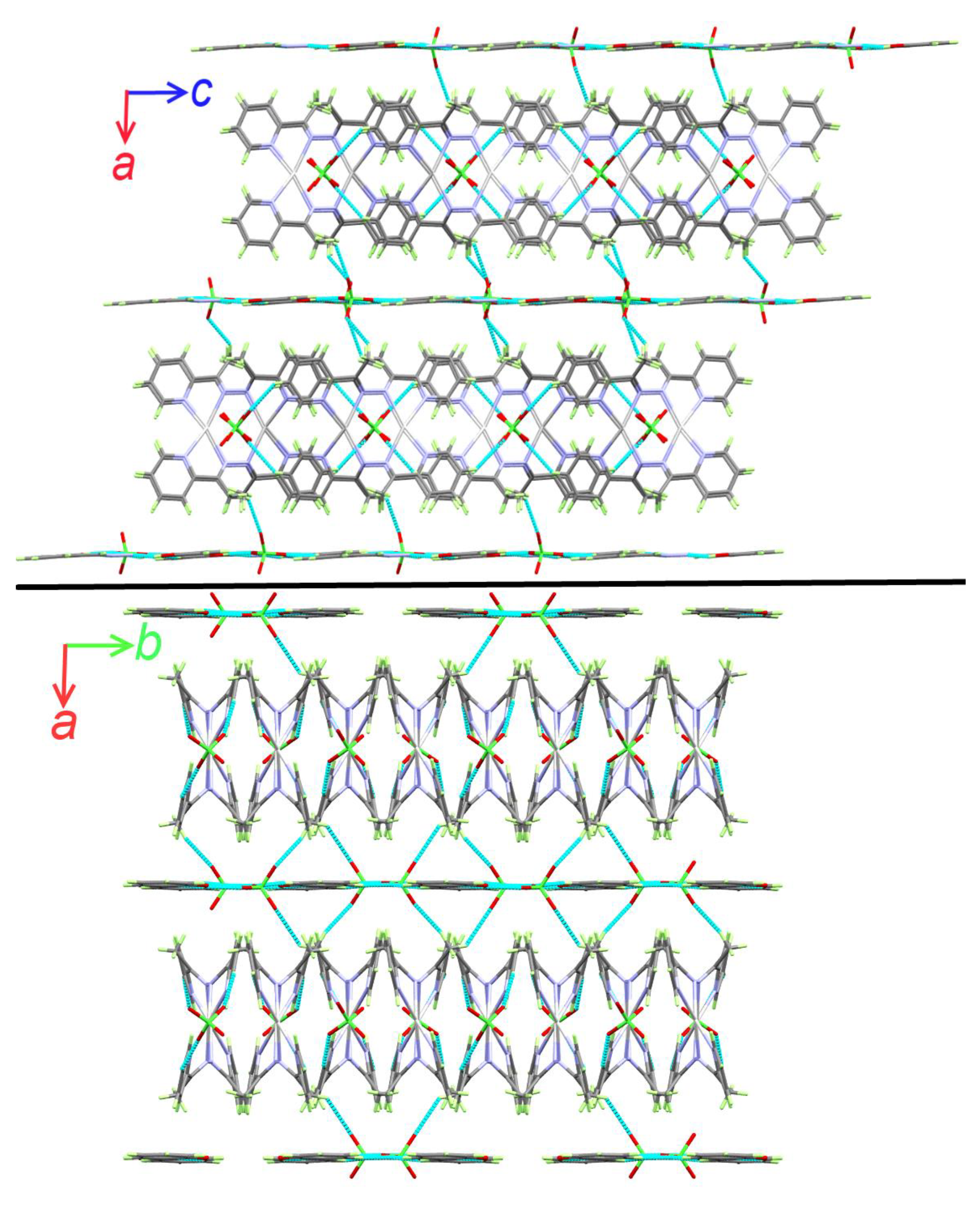
3.2. X-ray Structure Description of [Ag(La)]2(ClO4)2.1/2(Lb) Complex
3.3. Hirshfeld Analysis
3.4. Biological Studies
3.4.1. Antimicrobial Activity
3.4.2. Anticancer and Antioxidant Activities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, Y.; Lu, Z.; Zheng, L.; Cao, Q.-E. Silver-Driven Coordination Self-Assembly of Tetraphenylethene Stereoisomer: Construct Charming Topologies and Their Mechanochromic Behaviors. Inorg. Chem. 2020, 59, 6508–6517. [Google Scholar] [CrossRef] [PubMed]
- Lehn, J.M. Supramolecular chemistry—Scope and perspectives molecules, supermolecules, and molecular devices (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 1988, 27, 89–112. [Google Scholar] [CrossRef]
- Garrett, T.M.; Koert, U.; Lehn, J.-M.; Rigault, A.; Meyer, D.; Fischer, J. Self-assembly of silver (I) helicates. J. Chem. Soc. Chem. Commun. 1990, 7, 557–558. [Google Scholar] [CrossRef]
- Hung-Low, F.; Klausmeyer, K.K. Silver coordination complexes of 2-(diphenylphosphinomethyl) pyridine and their bipyridine derivatives. Inorg. Chim. Acta 2008, 361, 1298–1310. [Google Scholar] [CrossRef]
- Pyykkö, P. Strong closed-shell interactions in inorganic chemistry. Chem. Rev. 1997, 97, 597–636. [Google Scholar] [CrossRef] [PubMed]
- Janiak, C. A critical account on π–π stacking in metal complexes with aromatic nitrogen-containing ligands. J. Chem. Soc. Dalton Trans. 2000, 3885–3896. [Google Scholar] [CrossRef]
- Patroniak, V.; Lehn, J.-M.; Kubicki, M.; Ciesielski, A.; Wałęsa, M. Chameleonic ligand in self-assembly and synthesis of polymeric manganese(II), and grid-type copper(I) and silver(I) complexes. Polyhedron 2006, 25, 2643–2649. [Google Scholar] [CrossRef]
- Nachon, F.; Brazzolotto, X.; Dias, J.; Courageux, C.; Drożdż, W.; Cao, X.-Y.; Stefankiewicz, A.R.; Lehn, J.-M. Grid-type Quaternary Metallosupramolecular Compounds Inhibit Human Cholinesterases through Dynamic Multivalent Interactions. ChemBioChem 2022. [Google Scholar] [CrossRef]
- Fromm, K.M. Silver coordination compounds with antimicrobial properties. Appl. Organomet. Chem. 2013, 27, 683–687. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Umar, K.; Ibrahim, M.N.M. Silver nanoparticles: Various methods of synthesis, size affecting factors and their potential applications—A review. Appl. Nanosci. 2020, 10, 1369–1378. [Google Scholar] [CrossRef]
- Medici, S.; Peana, M.; Nurchi, V.M.; Zoroddu, M.A. Medical uses of silver: History, myths, and scientific evidence. J. Med. Chem. 2019, 62, 5923–5943. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Luan, S.; Yin, Z.; He, M.; He, C.; Yin, L.; Zou, Y.; Yuan, Z.; Li, L.; Song, X. Recent advances in the medical use of silver complex. Eur. J. Med. Chem. 2018, 157, 62–80. [Google Scholar] [CrossRef] [PubMed]
- Sierra, M.A.; Casarrubios, L.; de la Torre, M.C. Bio-Organometallic Derivatives of Antibacterial Drugs. Chem. A Eur. J. 2019, 25, 7232–7242. [Google Scholar] [CrossRef] [PubMed]
- Aguzzi, C.; Sandri, G.; Bonferoni, C.; Cerezo, P.; Rossi, S.; Ferrari, F.; Caramella, C.; Viseras, C. Solid state characterisation of silver sulfadiazine loaded on montmorillonite/chitosan nanocomposite for wound healing. Colloids Surf. B Biointerfaces 2014, 113, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Batarseh, K.I. Anomaly and correlation of killing in the therapeutic properties of silver (I) chelation with glutamic and tartaric acids. J. Antimicrob. Chemother. 2004, 54, 546–548. [Google Scholar] [CrossRef]
- Chastre, J. Preventing ventilator-associated pneumonia: Could silver-coated endotracheal tubes be the answer? JAMA 2008, 300, 842–844. [Google Scholar] [CrossRef]
- Mahmodiyeh, B.; Kamali, A.; Zarinfar, N.; Joushani, M.M. The Effect of Silver-Coated Endotracheal Tube on the Incidence of Ventilator-Induced Pneumonia in Intubated Patients Admitted to the Intensive Care Unit (ICU). Syst. Rev. Pharm. 2021, 12, 2499–2503. [Google Scholar]
- Baxter, P.N.; Lehn, J.M.; Fischer, J.; Youinou, M.T. Self-Assembly and Structure of a 3× 3 Inorganic Grid from Nine Silver Ions and Six Ligand Components. Angew. Chem. Int. Ed. Engl. 1994, 33, 2284–2287. [Google Scholar] [CrossRef]
- Marquis, A.; Kintzinger, J.P.; Graff, R.; Baxter, P.N.; Lehn, J.M. Mechanistic Features, Cooperativity, and Robustness in the Self-Assembly of Multicomponent Silver (i) Grid-Type Metalloarchitectures. Angew. Chem. 2002, 114, 2884–2888. [Google Scholar] [CrossRef]
- Nimia, H.H.; Carvalho, V.F.; Isaac, C.; Souza, F.A.; Gemperli, R.; Paggiaro, A.O. Comparative study of Silver Sulfadiazine with other materials for healing and infection prevention in burns: A systematic review and meta-analysis. Burns 2019, 45, 282–292. [Google Scholar] [CrossRef]
- Ahmadian, S.; Ghorbani, M.; Mahmoodzadeh, F. Silver sulfadiazine-loaded electrospun ethyl cellulose/polylactic acid/collagen nanofibrous mats with antibacterial properties for wound healing. Int. J. Biol. Macromol. 2020, 162, 1555–1565. [Google Scholar] [CrossRef] [PubMed]
- Aziz, Z.; Hassan, B.A.R. The effects of honey compared to silver sulfadiazine for the treatment of burns: A systematic review of randomized controlled trials. Burns 2017, 43, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Miyazaki, M.; Mashima, K.; Takagi, S.; Hara, S.; Kamimura, H.; Jimi, S. The effects of silver sulfadiazine on methicillin-resistant staphylococcus aureus biofilms. Microorganisms 2020, 8, 1551. [Google Scholar] [CrossRef]
- Möhler, J.S.; Sim, W.; Blaskovich, M.A.; Cooper, M.A.; Ziora, Z.M. Silver bullets: A new lustre on an old antimicrobial agent. Biotechnol. Adv. 2018, 36, 1391–1411. [Google Scholar] [CrossRef] [PubMed]
- Banti, C.N.; Hadjikakou, S.K. Anti-proliferative and anti-tumor activity of silver (I) compounds. Metallomics 2013, 5, 569–596. [Google Scholar] [CrossRef] [PubMed]
- Bharathi, S.; Mahendiran, D.; Kumar, R.S.; Choi, H.J.; Gajendiran, M.; Kim, K.; Rahiman, A.K. Silver (I) metallodrugs of thiosemicarbazones and naproxen: Biocompatibility, in vitro anti-proliferative activity and in silico interaction studies with EGFR, VEGFR2 and LOX receptors. Toxicol. Res. 2020, 9, 28–44. [Google Scholar] [CrossRef]
- Hossain, M.S.; Zakaria, C.M.; Kudrat-E-Zahan, M. Metal complexes as potential antimicrobial agent: A review. Am. J. Heterocycl. Chem. 2018, 4, 1. [Google Scholar] [CrossRef]
- Chen, X.; Yang, Q.; Chen, J.; Zhang, P.; Huang, Q.; Zhang, X.; Yang, L.; Xu, D.; Zhao, C.; Wang, X. Inhibition of proteasomal deubiquitinase by silver complex induces apoptosis in non-small cell lung cancer cells. Cell. Physiol. Biochem. 2018, 49, 780–797. [Google Scholar] [CrossRef]
- Batten, S.R.; Robson, R. Interpenetrating nets: Ordered, periodic entanglement. Angew. Chem. Int. Ed. 1998, 37, 1460–1494. [Google Scholar] [CrossRef]
- Venkataraman, D.; Gardner, G.B.; Lee, S.; Moore, J.S. Zeolite-like behavior of a coordination network. J. Am. Chem. Soc. 1995, 117, 11600–11601. [Google Scholar] [CrossRef]
- Kitagawa, S.; Kitaura, R.; Noro, S.i. Functional porous coordination polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, S.; Noro, S.-i.; Nakamura, T. Pore surface engineering of microporous coordination polymers. Chem. Commun. 2006, 701–707. [Google Scholar] [CrossRef]
- Feazell, R.P.; Carson, C.E.; Klausmeyer, K.K. Variability in the structures of luminescent [2-(aminomethyl) pyridine] silver (i) complexes: Effect of ligand ratio, anion, hydrogen bonding, and π-stacking. Eur. J. Inorg. Chem. 2005, 3287–3297. [Google Scholar] [CrossRef]
- Wen, C.; Yin, A.; Dai, W.-L. Recent advances in silver-based heterogeneous catalysts for green chemistry processes. Appl. Catal. B Environ. 2014, 160, 730–741. [Google Scholar] [CrossRef]
- Hussain, S.; Aneggi, E.; Goi, D. Catalytic activity of metals in heterogeneous Fenton-like oxidation of wastewater contaminants: A review. Environ. Chem. Lett. 2021, 19, 2405–2424. [Google Scholar] [CrossRef]
- Hussain-Khil, N.; Ghorbani-Choghamarani, A.; Mohammadi, M. A new silver coordination polymer based on 4, 6-diamino-2-pyrimidinethiol: Synthesis, characterization and catalytic application in asymmetric Hantzsch synthesis of polyhydroquinolines. Sci. Rep. 2021, 11, 1–15. [Google Scholar]
- Lo, V.K.-Y.; Chan, A.O.-Y.; Che, C.-M. Gold and silver catalysis: From organic transformation to bioconjugation. Org. Biomol. Chem. 2015, 13, 6667–6680. [Google Scholar] [CrossRef]
- Soliman, S.M.; Albering, J.H.; Barakat, A. Unexpected formation of polymeric silver (I) complexes of azine-type ligand via self-assembly of Ag-salts with isatin oxamohydrazide. R. Soc. Open Sci. 2018, 5, 180434. [Google Scholar] [CrossRef]
- Soliman, S.M.; Barakat, A. Self-assembly of azine-based hydrolysis of pyridine and isatin oxamohydrazides with AgNO3; synthesis and structural studies of a novel four coordinated Ag (I)-azine 2D coordination polymer. Inorg. Chim. Acta 2019, 490, 227–234. [Google Scholar] [CrossRef]
- Elbadawy, H.A.; Khalil, S.M.; Al-Wahaib, D.; Barakat, A.; Soliman, S.M.; Eldissouky, A. Ag (I)-mediated hydrolysis of hydrazone to azine: Synthesis, X-ray structure, and biological investigations of two new Ag (I)-azine complexes. Appl. Organomet. Chem. 2022, 36, e6757. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT: Integrating space group determination and structure solution. Acta Crystallogr. Sect. A Found. Adv. 2014, 70, C1437. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- El-Faham, A.; Soliman, S.M.; Ghabbour, H.A.; Elnakady, Y.A.; Mohaya, T.A.; Siddiqui, M.R.; Albericio, F. Ultrasonic promoted synthesis of novel s-triazine-Schiff base derivatives; molecular structure, spectroscopic studies and their preliminary anti-proliferative activities. J. Mol. Struct. 2016, 1125, 121–135. [Google Scholar] [CrossRef]
- Banerjee, S.; Wu, B.; Lassahn, P.-G.; Janiak, C.; Ghosh, A. Synthesis, structure and bonding of cadmium (II) thiocyanate systems featuring nitrogen based ligands of different denticity. Inorg. Chim. Acta 2005, 358, 535–544. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Clsi, C. M100-S25: Performance standards for antimicrobial susceptibility testing. In Twenty-Fifth Informational Supplement; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Yen, G.C.; Duh, P.D. Scavenging effect of methanolic extracts of peanut hulls on free-radical and active-oxygen species. J. Agric. Food Chem. 1994, 42, 629–632. [Google Scholar] [CrossRef]
- Cockerill, F.; Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-First informational Supplement; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012; pp. M100–M121. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Ferraria, A.M.; Carapeto, A.P.; do Rego, A.M.B. X-ray photoelectron spectroscopy: Silver salts revisited. Vacuum 2012, 86, 1988–1991. [Google Scholar] [CrossRef]
- Volkov, I.L.; Smirnova, A.; Makarova, A.A.; Reveguk, Z.V.; Ramazanov, R.R.; Usachov, D.Y.; Adamchuk, V.K.; Kononov, A.I. DNA with ionic, atomic, and clustered silver: An XPS study. J. Phys. Chem. B 2017, 121, 2400–2406. [Google Scholar] [CrossRef]



| CCDC | 2207700 |
|---|---|
| Empirical formula | C35H34Ag2Cl2N9O9 |
| Fw | 1011.35 g/moL |
| Temp (K) | 296(2) |
| λ (Å) | 1.54178 |
| Cryst syst | Μonoclinic |
| Space group | P21/c |
| a (Å) | 15.6034(11) |
| b (Å) | 14.9707(12) |
| c (Å) | 17.3993(12) |
| β (deg) | 110.554(3) |
| V (Å3) | 3805.6(5) |
| Z | 4 |
| ρcalc (Μg/m3) | 1.765 |
| μ (Μo Kα) (mm−1) | 10.126 |
| No. reflns. | 45,309 |
| Unique reflns. | 6708 [R(int) = 0.0597] |
| Completeness to θ = 33.33 | 99.50% |
| GOOF (F2) | 1.054 |
| Final R indices [I > 2sigma(I)] | R1 = 0.0624, wR2 = 0.1938 |
| R indices (all data) | R1 = 0.0733, wR2 = 0.2099 |
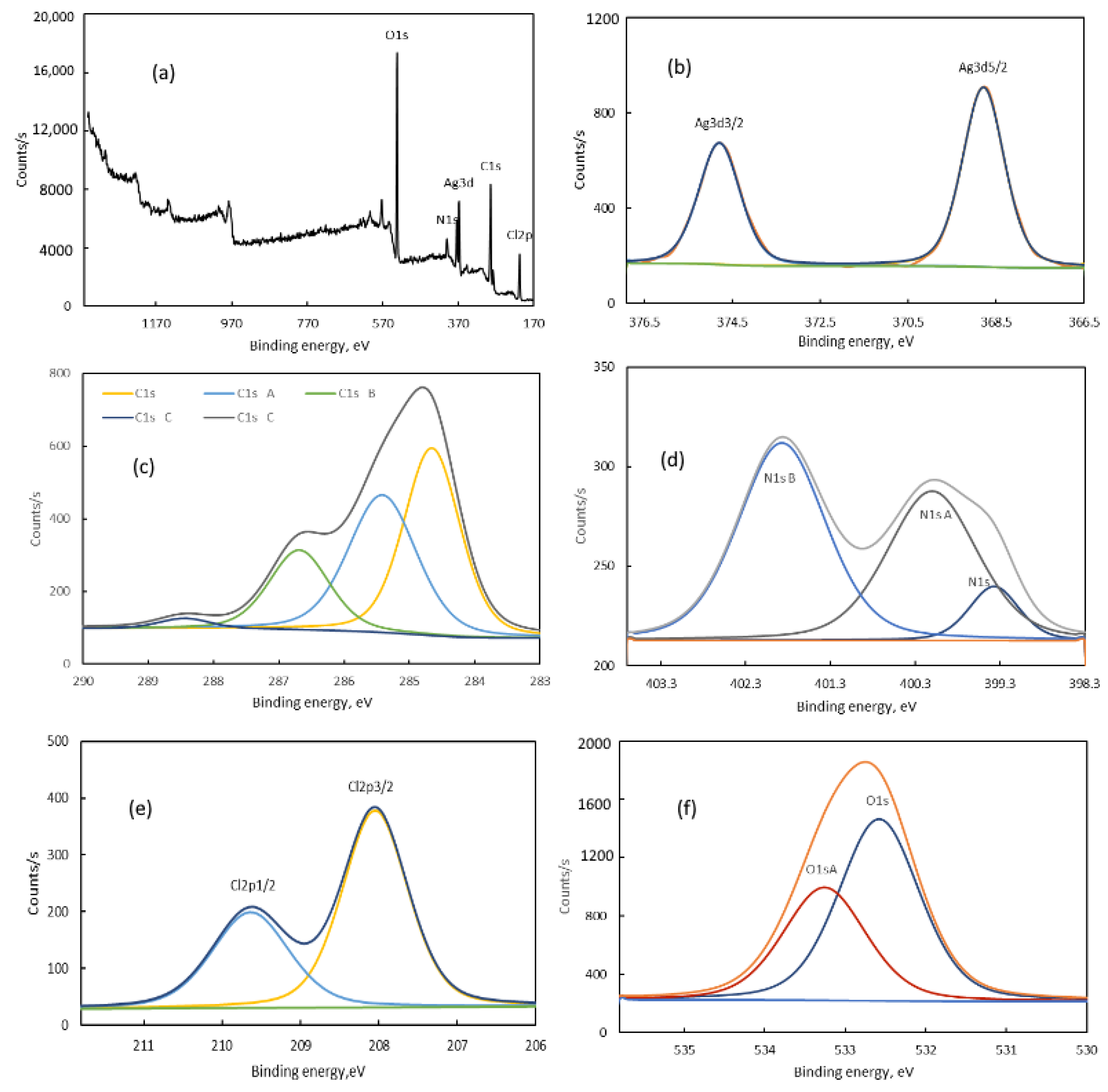
| Name | Peak BE | FWHM eV | Atomic % |
|---|---|---|---|
| C1s | 284.66 | 1.02 | 20 |
| C1s A | 285.42 | 1.24 | 17.72 |
| C1s B | 286.69 | 1.09 | 9.03 |
| C1s C | 288.44 | 0.94 | 1.02 |
| O1s | 532.58 | 1.2 | 22.48 |
| O1s A | 533.26 | 1.22 | 14.3 |
| N1s | 399.37 | 0.7 | 0.36 |
| N1s A | 400.1 | 1.26 | 1.78 |
| N1s B | 401.87 | 1.23 | 2.3 |
| Ag3d, 5/2 | 368.8 | 1.13 | 1.6 |
| Ag3d, 3/2 | 374.8 | 1.12 | 1.08 |
| Cl2p, 3/2 | 208.04 | 1 | 5.31 |
| Cl2p, 1/2 | 209.63 | 1.16 | 3.01 |
| Bond | Distance | Bond | Distance |
| Ag1-N2 | 2.347(4) | Ag2-N11 | 2.349(5) |
| Ag1-N8 | 2.356(5) | Ag2-N5 | 2.358(5) |
| Ag1-N7 | 2.368(5) | Ag2-N10 | 2.371(5) |
| Ag1-N1 | 2.383(5) | Ag2-N4 | 2.373(5) |
| Bonds | Angle | Bonds | Angle |
| N2-Ag1-N8 | 107.17(16) | N11-Ag2-N5 | 106.50(16) |
| N2-Ag1-N7 | 155.48(17) | N11-Ag2-N10 | 70.22(17) |
| N8-Ag1-N7 | 70.63(17) | N5-Ag2-N10 | 157.77(16) |
| N2-Ag1-N1 | 70.35(16) | N11-Ag2-N4 | 153.97(17) |
| N8-Ag1-N1 | 155.55(17) | N5-Ag2-N4 | 70.82(15) |
| N7-Ag1-N1 | 121.94(16) | N10-Ag2-N4 | 122.02(16) |
| D-H...A | d(D-H) | d(H...A) | d(D...A) | <(DHA) | Symm. Code |
|---|---|---|---|---|---|
| O1-H1...N13 | 0.82 | 1.99 | 2.692(7) | 144 | |
| O1-H1..O2 | 0.82 | 2.45 | 2.892(11) | 115 | |
| C5-H5...O8 | 0.93 | 2.39 | 3.112(15) | 134 | 1-x,1-y,2-z |
| C13-H13A...O4 | 0.96 | 2.53 | 3.290(12) | 136 | |
| C13-H13B...O3 | 0.96 | 2.53 | 3.345(12) | 142 | 2-x,1-y,2-z |
| C19-H19...O7 | 0.93 | 2.55 | 3.288(13) | 137 | 1-x,1-y,2-z |
| C25-H25...O9 | 0.93 | 2.48 | 3.264(16) | 142 | 1-x,1-y,2-z |
| C31-H31...O5 | 0.93 | 2.44 | 3.323(10) | 158 | 2-x,-y,2-z |
| C34-H34...O5 | 0.93 | 2.43 | 3.314(9) | 158 | x,1/2-y,-1/2+z |
| C35-H35...O2 | 0.93 | 2.6 | 3.481(11) | 158 | 2-x,-y,2-z |
| O1-H1...N13 | 0.82 | 1.99 | 2.692(7) | 144 | |
| O1-H1...O2 | 0.82 | 2.45 | 2.892(11) | 115 |
| Contact | Distance | Contact | Distance |
|---|---|---|---|
| Ag1…O6 | 3.163 | O4…H13 | 2.444 |
| Ag2…O7 | 3.165 | O2…H1 | 2.387 |
| Ag2…O9 | 3.197 | O2…H35 | 2.458 |
| O6…H11 | 2.569 | O2…H35 | 2.53 |
| O9…H25 | 2.364 | O5…H31 | 2.302 |
| O8…H5 | 2.287 | O5…H34 | 2.29 |
| O7…H19 | 2.439 | H5…H28C | 2.067 |
| O5…H14A | 2.541 | H25…H13C | 2.021 |
| O3…H13B | 2.437 |
| Microbe | Ag(I) Complex | Free Ag(I) b | Control |
|---|---|---|---|
| A. fumigatus | 26 (39) | - (-) | 17(156) c |
| C. albicans | 16 (1250) | 10 (2500) | 20(312) c |
| S. aureus | 9 (5000) | 14 (1250) | 24(10) d |
| B. subtilis | 8 (5000) | 13 (1250) | 26(5) d |
| E. coli | 10 (2500) | 15 (625) | 30(5) d |
| P. vulgaris | 11 (2500) | 20 (625) | 25(5) d |
| Sample Conc. (µg/mL) | Viability % | Inhibitory % | S.D. (±) |
|---|---|---|---|
| 500 | 1.87 | 98.13 | 0.39 |
| 250 | 4.65 | 95.35 | 0.67 |
| 125 | 9.83 | 90.17 | 1.05 |
| 62.5 | 21.74 | 78.26 | 2.08 |
| 31.25 | 38.72 | 61.28 | 1.56 |
| 15.6 | 69.40 | 30.6 | 2.13 |
| 7.8 | 85.06 | 14.94 | 1.58 |
| 3.9 | 92.38 | 7.62 | 0.64 |
| 2 | 97.65 | 2.35 | 0.31 |
| 1 | 99.23 | 0.77 | 0.49 |
| 0 | 100 | 0 | 0 |
| Sample Conc. (µg/mL) | DPPH Scavenging % | S.D. (±) |
|---|---|---|
| 1280 | 83.02 | 0.94 |
| 640 | 71.84 | 1.28 |
| 320 | 62.95 | 2.03 |
| 160 | 41.68 | 1.94 |
| 80 | 29.36 | 0.48 |
| 40 | 18.61 | 0.75 |
| 20 | 10.28 | 0.42 |
| 10 | 5.79 | 0.13 |
| 5 | 3.47 | 0.25 |
| 2.5 | 1.95 | 0.31 |
| 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altowyan, M.S.; Soliman, S.M.; Al-Wahaib, D.; Barakat, A.; Ali, A.E.; Elbadawy, H.A. Synthesis of a New Ag(I)-Azine Complex via Ag(I)-Mediated Hydrolysis of 2-(((1-(Pyridin-2-yl)ethylidene)hydrazineylidene) Methyl)phenol with AgClO4; X-ray Crystal Structure and Biological Studies. Symmetry 2022, 14, 2226. https://doi.org/10.3390/sym14112226
Altowyan MS, Soliman SM, Al-Wahaib D, Barakat A, Ali AE, Elbadawy HA. Synthesis of a New Ag(I)-Azine Complex via Ag(I)-Mediated Hydrolysis of 2-(((1-(Pyridin-2-yl)ethylidene)hydrazineylidene) Methyl)phenol with AgClO4; X-ray Crystal Structure and Biological Studies. Symmetry. 2022; 14(11):2226. https://doi.org/10.3390/sym14112226
Chicago/Turabian StyleAltowyan, Mezna Saleh, Saied M. Soliman, Dhuha Al-Wahaib, Assem Barakat, Ali Eldissouky Ali, and Hemmat A. Elbadawy. 2022. "Synthesis of a New Ag(I)-Azine Complex via Ag(I)-Mediated Hydrolysis of 2-(((1-(Pyridin-2-yl)ethylidene)hydrazineylidene) Methyl)phenol with AgClO4; X-ray Crystal Structure and Biological Studies" Symmetry 14, no. 11: 2226. https://doi.org/10.3390/sym14112226
APA StyleAltowyan, M. S., Soliman, S. M., Al-Wahaib, D., Barakat, A., Ali, A. E., & Elbadawy, H. A. (2022). Synthesis of a New Ag(I)-Azine Complex via Ag(I)-Mediated Hydrolysis of 2-(((1-(Pyridin-2-yl)ethylidene)hydrazineylidene) Methyl)phenol with AgClO4; X-ray Crystal Structure and Biological Studies. Symmetry, 14(11), 2226. https://doi.org/10.3390/sym14112226









