Homochiral or Heterochiral: A Systematic Study of Threonine Clusters Using a FT ICR Mass Spectrometer
Abstract
1. Introduction
2. Materials and Methods
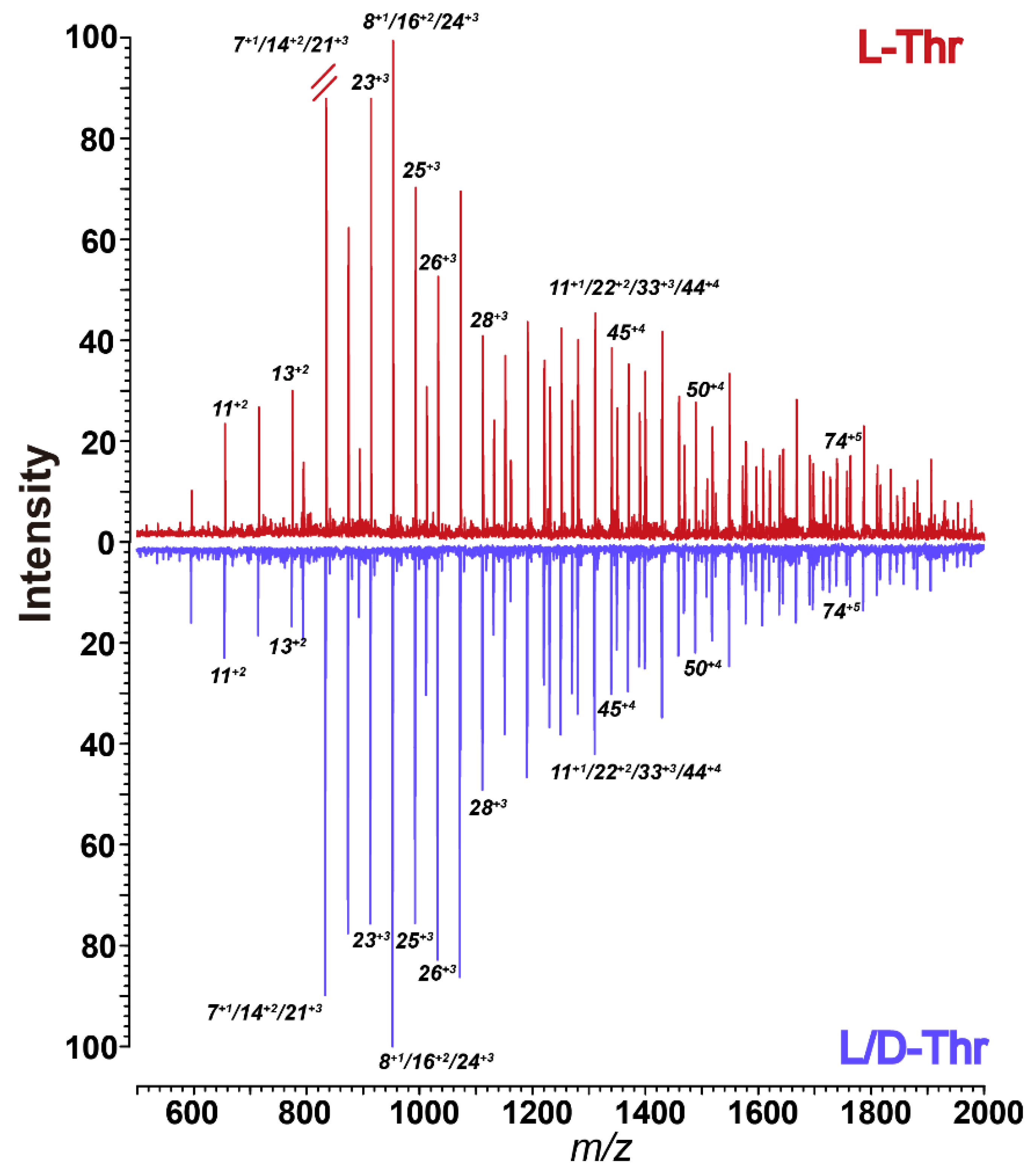
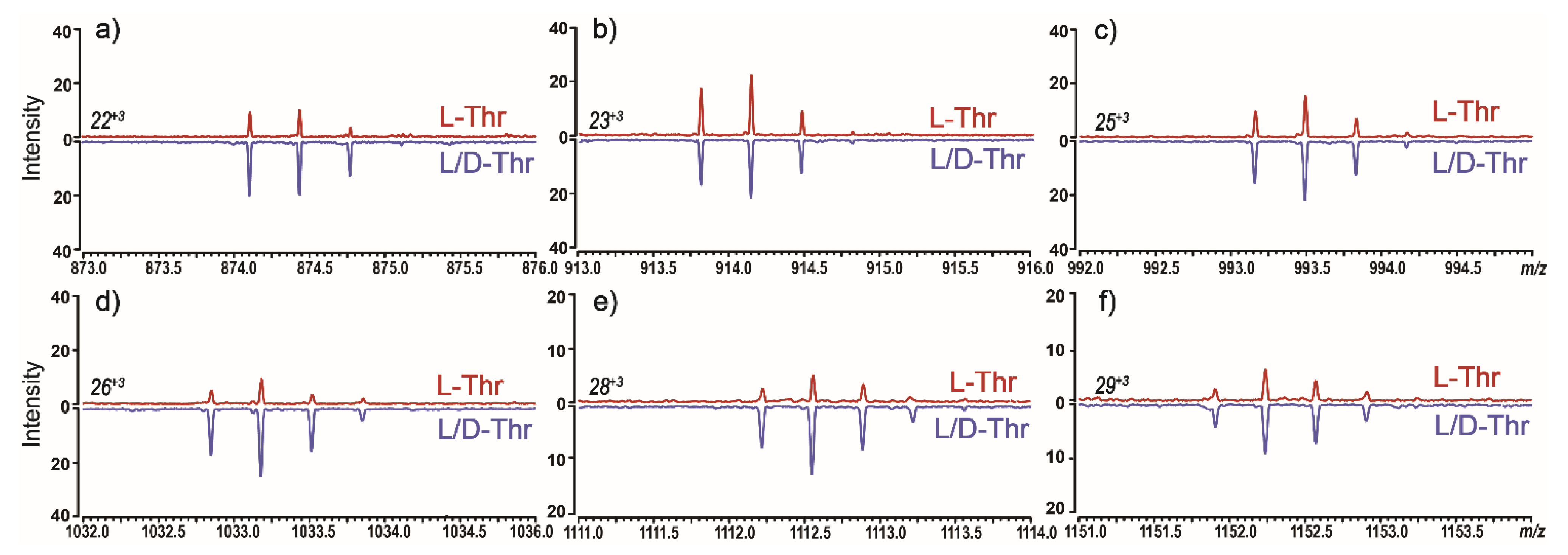
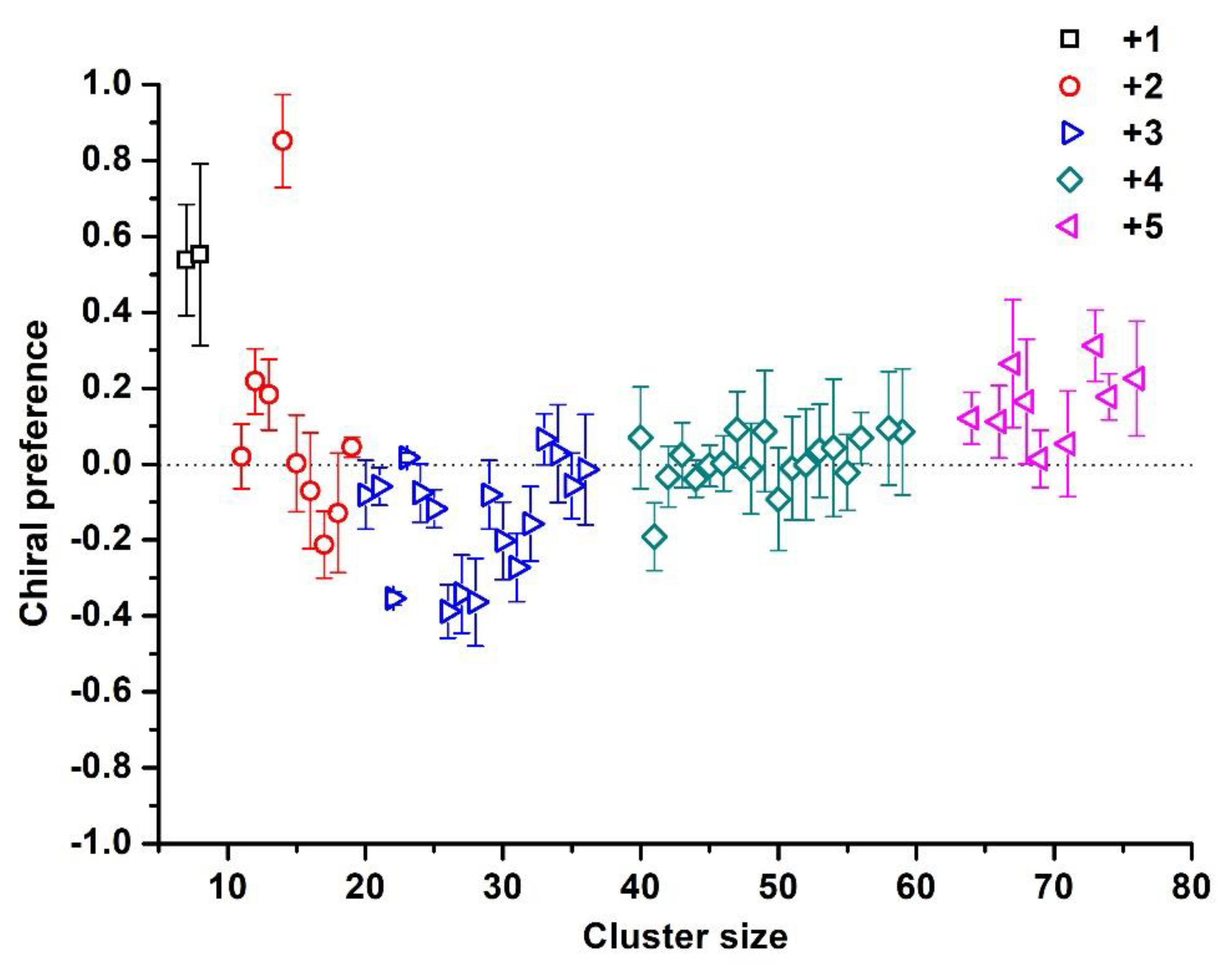
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cooks, R.G.; Zhang, D.; Koch, K.J.; Gozzo, F.C.; Eberlin, M.N. Chiroselective Self-Directed Octamerization of Serine: Implications for Homochirogenesis. Anal. Chem. 2001, 73, 3646–3655. [Google Scholar] [CrossRef] [PubMed]
- Counterman, A.E.; Clemmer, D.E. Magic Number Clusters of Serine in the Gas Phase. J. Phys. Chem. B 2001, 105, 8092–8096. [Google Scholar] [CrossRef]
- Hodyss, R.; Julian, R.R.; Beauchamp, J.L. Spontaneous chiral separation in noncovalent molecular clusters. Chirality 2001, 13, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Julian, R.R.; Hodyss, R.; Kinnear, B.; Jarrold, M.F.; Beauchamp, J.L. Nanocrystalline Aggregation of Serine Detected by Electrospray Ionization Mass Spectrometry: Origin of the Stable Homochiral Gas-Phase Serine Octamer. J. Phys. Chem. B 2002, 106, 1219–1228. [Google Scholar] [CrossRef][Green Version]
- Nemes, P.; Schlosser, G.; Vékey, K. Amino acid cluster formation studied by electrospray ionization mass spectrometry. J. Mass Spctrum. 2005, 40, 43–49. [Google Scholar] [CrossRef]
- Julian, R.R.; Myung, S.; Clemmer, D.E. Spontaneous Anti-Resolution in Heterochiral Clusters of Serine. J. Am. Chem. Soc. 2004, 126, 4110–4111. [Google Scholar] [CrossRef]
- Koch, K.J.; Gozzo, F.C.; Nanita, S.C.; Takats, Z.; Eberlin, M.N.; Cooks, R.G. Chiral Transmission between Amino Acids: Chirally Selective Amino Acid Substitution in the Serine Octamer as a Possible Step in Homochirogenesis. Angew. Chem. Int. Ed. 2002, 41, 1721–1724. [Google Scholar] [CrossRef]
- Takats, Z.; Nanita, S.C.; Cooks, R.G. Serine Octamer Reactions: Indicators of Prebiotic Relevance. Angew. Chem. Int. Ed. 2003, 42, 3521–3523. [Google Scholar] [CrossRef]
- Nanita, S.C.; Takats, Z.; Cooks, R.G.; Myung, S.; Clemmer, D.E. Chiral enrichment of serine via formation, dissociation, and soft-landing of octameric cluster ions. J. Am. Soc. Mass Spectr. 2004, 15, 1360–1365. [Google Scholar] [CrossRef]
- Myung, S.; Julian, R.R.; Nanita, S.C.; Cooks, R.G.; Clemmer, D.E. Formation of Nanometer-Scale Serine Clusters by Sonic Spray. J. Phys. Chem. B 2004, 108, 6105–6111. [Google Scholar] [CrossRef]
- Takáts, Z.; Cooks, R.G. Thermal formation of serine octamer ions. Chem. Commun. 2004, 4, 444–445. [Google Scholar] [CrossRef]
- Gronert, S.; O’Hair, R.A.J.; Fagin, A.E. Ion/molecule reactions of the protonated serine octamer. Chem. Commun. 2004, 17, 1944–1945. [Google Scholar] [CrossRef]
- Mazurek, U.; Geller, O.; Lifshitz, C.; McFarland, M.A.; Marshall, A.G.; Reuben, B.G. Protonated Serine Octamer Cluster: Structure Elucidation by Gas-Phase H/D Exchange Reactions. J. Phys. Chem. A 2005, 109, 2107–2112. [Google Scholar] [CrossRef] [PubMed]
- Nanita, S.C.; Cooks, R.G. Negatively-Charged Halide Adducts of Homochiral Serine Octamers. J. Phys. Chem. B 2005, 109, 4748–4753. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Xu, R.; Nanita, S.C.; Cooks, R.G. Thermal Formation of Homochiral Serine Clusters and Implications for the Origin of Homochirality. J. Am. Chem. Soc. 2006, 128, 17074–17086. [Google Scholar] [CrossRef] [PubMed]
- Nanita, S.C.; Cooks, R.G. Serine Octamers: Cluster Formation, Reactions, and Implications for Biomolecule Homochirality. Angew. Chem. Int. Ed. 2006, 45, 554–569. [Google Scholar] [CrossRef]
- Mazurek, U. Letter: Some More Aspects of Formation and Stability of the Protonated Serine Octamer Cluster. Eur. J. Mass Spectrom. 2006, 12, 63–69. [Google Scholar] [CrossRef]
- Nanita, S.C.; Sokol, E.; Cooks, R.G. Alkali metal-cationized serine clusters studied by sonic spray ionization tandem mass spectrometry. J. Am. Soc. Mass Spectr. 2007, 18, 856–868. [Google Scholar] [CrossRef][Green Version]
- Kong, X.; Tsai, I.A.; Sabu, S.; Han, C.-C.; Lee, Y.T.; Chang, H.-C.; Tu, S.-Y.; Kung, A.H.; Wu, C.-C. Progressive Stabilization of Zwitterionic Structures in [H(Ser)2–8]+ Studied by Infrared Photodissociation Spectroscopy. Angew. Chem. Int. Ed. 2006, 45, 4130–4134. [Google Scholar] [CrossRef]
- Kong, X.; Lin, C.; Infusini, G.; Oh, H.-B.; Jiang, H.; Breuker, K.; Wu, C.-C.; Charkin, O.P.; Chang, H.-C.; McLafferty, F.W. Numerous Isomers of Serine Octamer Ions Characterized by Infrared Photodissociation Spectroscopy. Chemphyschem A Eur. J. Chem. Phys. Phys. Chem. 2009, 10, 2603–2606. [Google Scholar] [CrossRef]
- Sunahori, F.X.; Yang, G.; Kitova, E.N.; Klassen, J.S.; Xu, Y. Chirality recognition of the protonated serine dimer and octamer by infrared multiphoton dissociation spectroscopy. Phys. Chem. Chem. Phys. 2013, 15, 1873–1886. [Google Scholar] [CrossRef]
- Seo, J.; Warnke, S.; Pagel, K.; Bowers, M.T.; von Helden, G. Infrared spectrum and structure of the homochiral serine octamer–dichloride complex. Nat. Chem. 2017, 9, 1263–1268. [Google Scholar] [CrossRef]
- Scutelnic, V.; Perez, M.A.S.; Marianski, M.; Warnke, S.; Gregor, A.; Rothlisberger, U.; Bowers, M.T.; Baldauf, C.; von Helden, G.; Rizzo, T.R.; et al. The Structure of the Protonated Serine Octamer. J. Am. Chem. Soc. 2018, 140, 7554–7560. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wei, Z.; Jiang, J.; Cooks, R.G. Nebulization Prior to Isolation, Ionization, and Dissociation of the Neutral Serine Octamer Allows Its Characterization. Angew. Chem. Int. Ed. 2018, 57, 17141–17145. [Google Scholar] [CrossRef]
- Spencer, E.A.C.; Ly, T.; Julian, R.R. Formation of the serine octamer: Ion evaporation or charge residue? Int. J. Mass Spectrom. 2008, 270, 166–172. [Google Scholar] [CrossRef]
- Liao, G.H.; Yang, Y.J.; Kong, X.L. Chirality effects on proline-substituted serine octamers revealed by infrared photodissociation spectroscopy. Phys. Chem. Chem. Phys. 2014, 16, 1554–1558. [Google Scholar] [CrossRef]
- Ren, J.; Wang, Y.Y.; Feng, R.X.; Kong, X.L. Investigation of L/D-threonine substituted L-serine octamer ions by mass spectrometry and infrared photodissociation spectroscopy. Chin. Chem. Lett. 2017, 28, 537–540. [Google Scholar] [CrossRef]
- Shi, Y.Y.; Zhou, M.; Zhang, K.L.; Ma, L.F.; Kong, X.L. Chiral Differentiation of Non-Covalent Diastereomers Based on Multichannel Dissociation Induced by 213-nm Ultraviolet Photodissociation. J. Am. Soc. Mass Spectr. 2019, 30, 2297–2305. [Google Scholar] [CrossRef]
- Chen, R.; Wei, Z.; Cooks, R.G. Collection and Characterization by Mass Spectrometry of the Neutral Serine Octamer Generated upon Sublimation. Anal. Chem. 2021, 93, 1092–1099. [Google Scholar] [CrossRef]
- Jordan, J.S.; Williams, E.R. Effects of Electrospray Droplet Size on Analyte Aggregation: Evidence for Serine Octamer in Solution. Anal. Chem. 2021, 93, 1725–1731. [Google Scholar] [CrossRef]
- Jordan, J.S.; Williams, E.R. Dissociation of large gaseous serine clusters produces abundant protonated serine octamer. Analyst 2021, 146, 2617–2625. [Google Scholar] [CrossRef] [PubMed]
- Jordan, J.S.; Williams, E.R. Homochiral preference of serine octamer in solution and formed by dissociation of large gaseous clusters. Analyst 2021, 146, 6822–6830. [Google Scholar] [CrossRef] [PubMed]
- Pietrusiewicz, K.M.; Borkowski, M.; Strzelecka, D.; Kielar, K.; Kicińska, W.; Karevych, S.; Jasiński, R.; Demchuk, O.M. A General Phenomenon of Spontaneous Amplification of Optical Purity under Achiral Chromatographic Conditions. Symmetry 2019, 11, 680. [Google Scholar] [CrossRef]
- Holliday, A.E.; Atlasevich, N.; Myung, S.; Plasencia, M.D.; Valentine, S.J.; Clemmer, D.E. Oscillations of Chiral Preference in Proline Clusters. J. Phys. Chem. A 2013, 117, 1035–1041. [Google Scholar] [CrossRef]
- Kong, X.L. Serine-phosphoric acid cluster ions studied by electrospray ionization and tandem mass spectrometry. J. Mass Spectrom. 2011, 46, 535–545. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, L.; Du, M.; Hou, Y.; Ma, Y.; Kong, X. Homochiral or Heterochiral: A Systematic Study of Threonine Clusters Using a FT ICR Mass Spectrometer. Symmetry 2022, 14, 86. https://doi.org/10.3390/sym14010086
Jiao L, Du M, Hou Y, Ma Y, Kong X. Homochiral or Heterochiral: A Systematic Study of Threonine Clusters Using a FT ICR Mass Spectrometer. Symmetry. 2022; 14(1):86. https://doi.org/10.3390/sym14010086
Chicago/Turabian StyleJiao, Luyang, Mengying Du, Yameng Hou, Yuan Ma, and Xianglei Kong. 2022. "Homochiral or Heterochiral: A Systematic Study of Threonine Clusters Using a FT ICR Mass Spectrometer" Symmetry 14, no. 1: 86. https://doi.org/10.3390/sym14010086
APA StyleJiao, L., Du, M., Hou, Y., Ma, Y., & Kong, X. (2022). Homochiral or Heterochiral: A Systematic Study of Threonine Clusters Using a FT ICR Mass Spectrometer. Symmetry, 14(1), 86. https://doi.org/10.3390/sym14010086







