Coupling between Trigeminal-Induced Asymmetries in Locus Coeruleus Activity and Cognitive Performance
Abstract
:1. Introduction
2. Materials and Methods
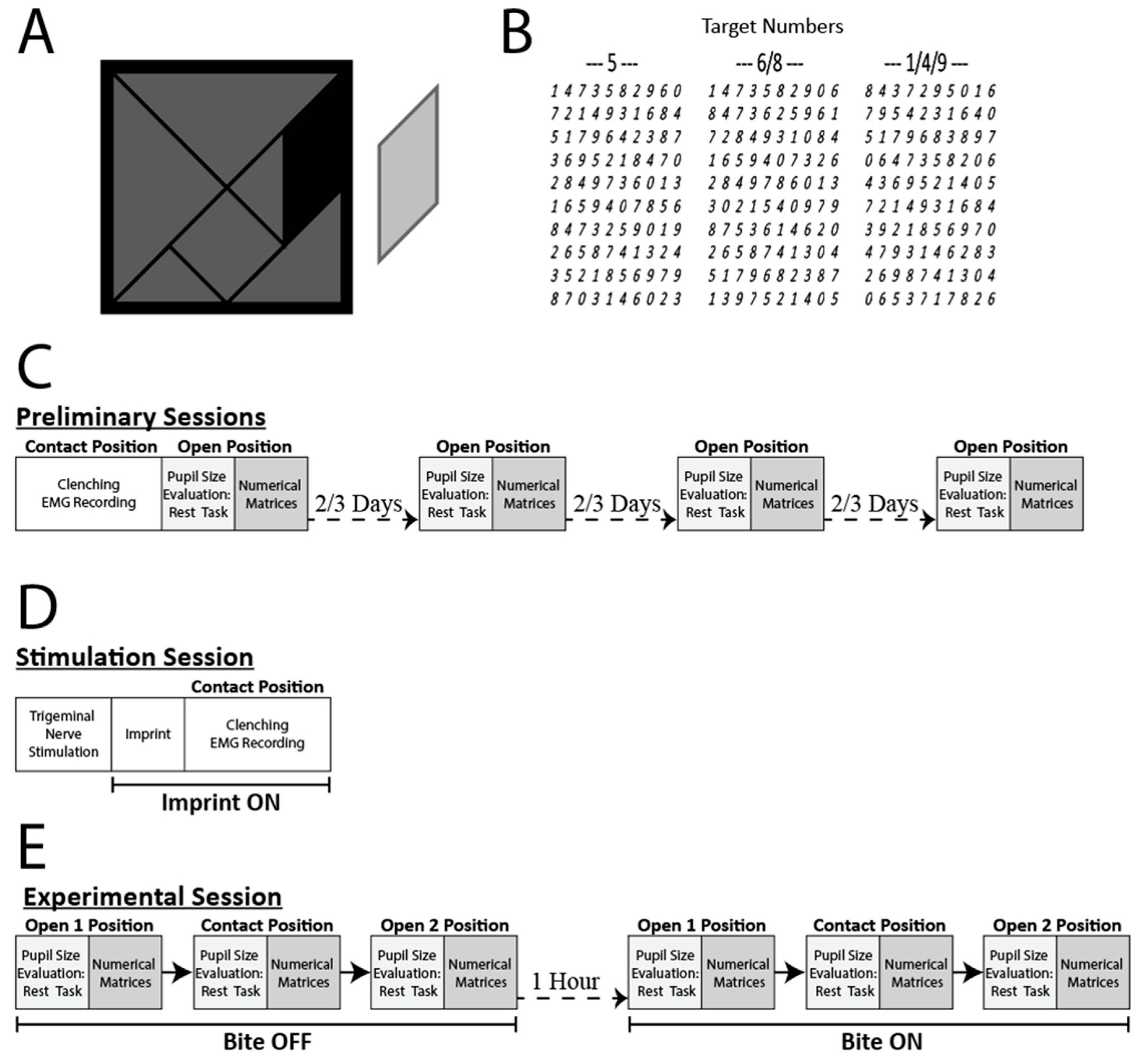
2.1. Subjects
2.2. Experimental Protocol
2.3. Trigeminal Nerve Stimulation
2.4. Haptic Task
2.5. Pupil Size and EMG Data Acquisition
2.6. Statistical Analysis
3. Results
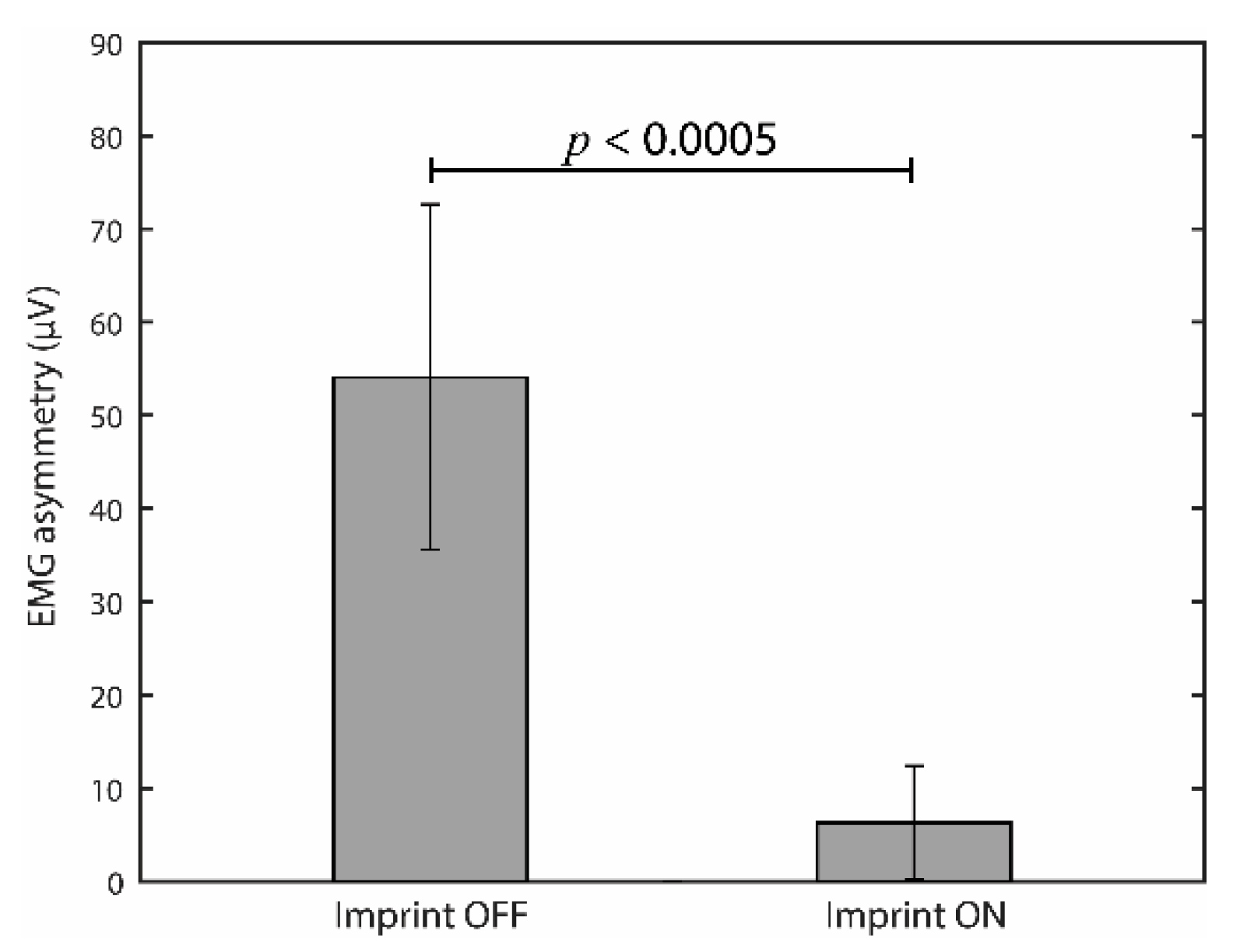
3.1. Effects of Occlusal Correction on EMG Asymmetry during Clenching
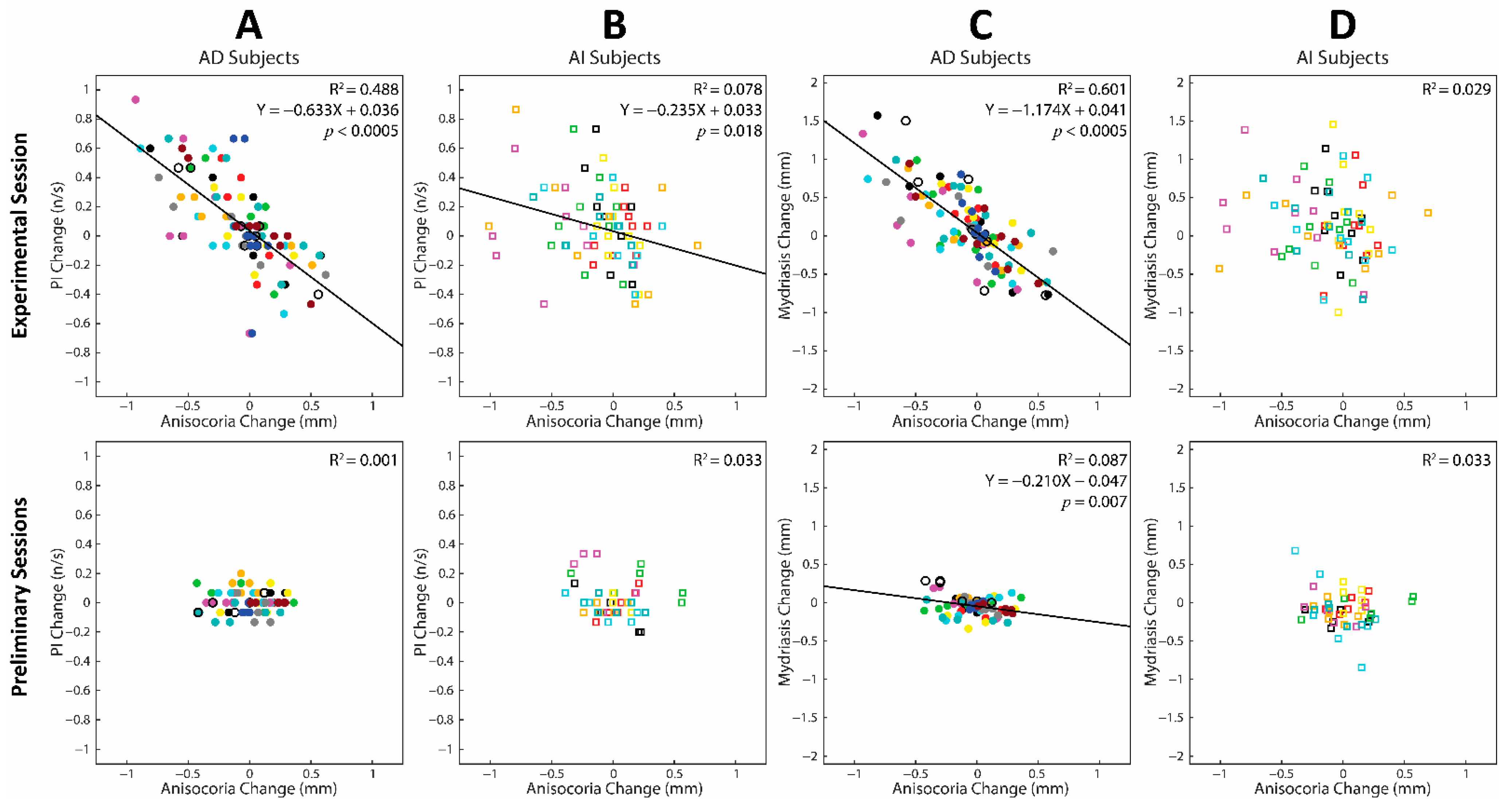
3.2. Correlations between the Changes in Task-Related Parameters and Anisocoria Observed in the Experimental Session: Single Subjects Analysis
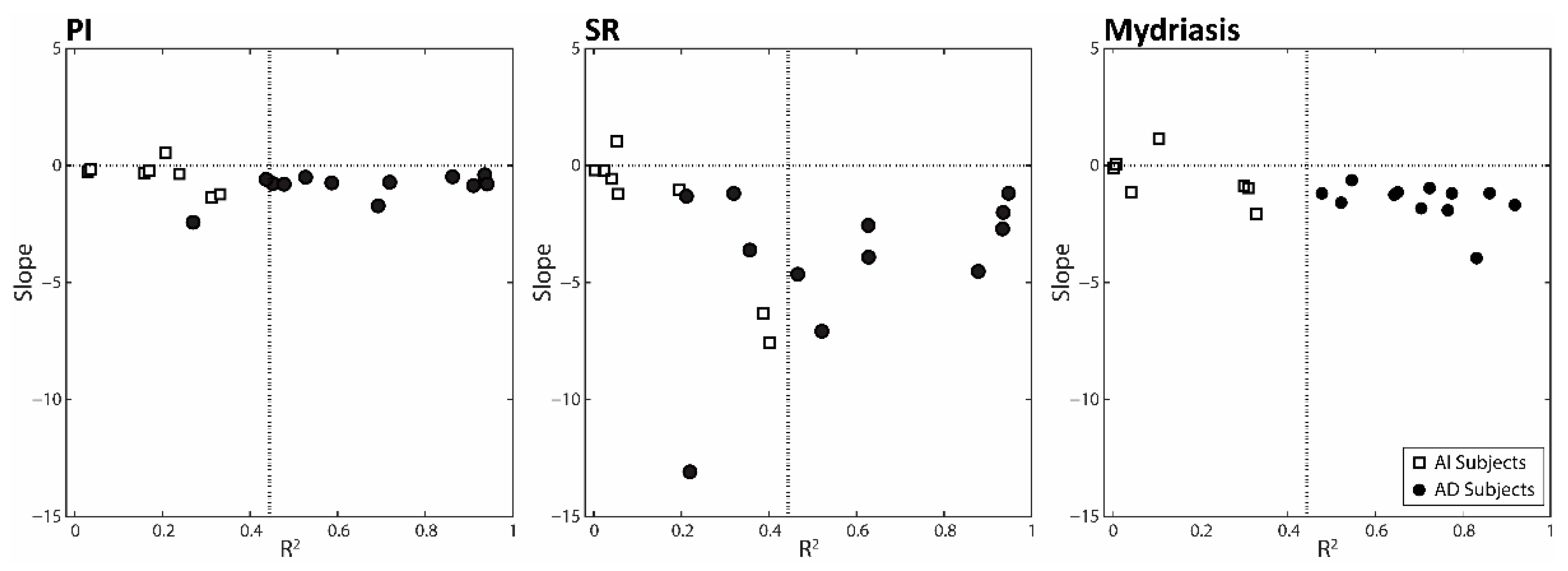
3.3. Changes in Anisocoria and Task-Related Parameters Induced by Changing Arches Position and Occlusal Condition: Differences between AD and AI Subjects
3.4. Correlations between Changes in Task-Related Parameters and Anisocoria Observed during the Experimental and Preliminary Sessions: Cumulative Regression Analysis
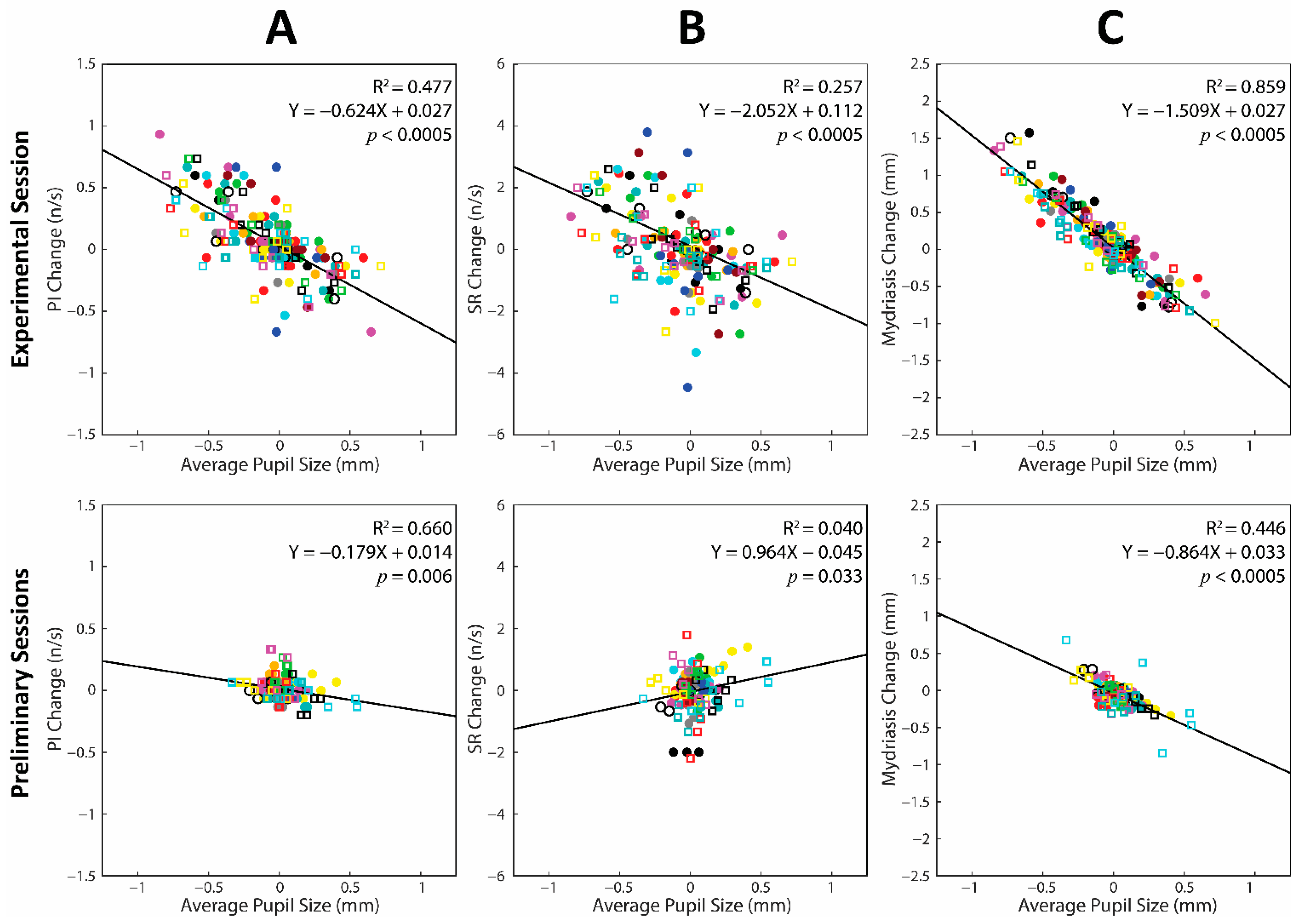
3.5. Relation between Changes in Task-Related Parameters and Average Pupil Size during the Experimental Session: Single Subject Analysis
3.6. Changes in Average Pupil Size and Task-Related Parameters during the Experimental and the Preliminary Sessions: A Cumulative Regression Analysis
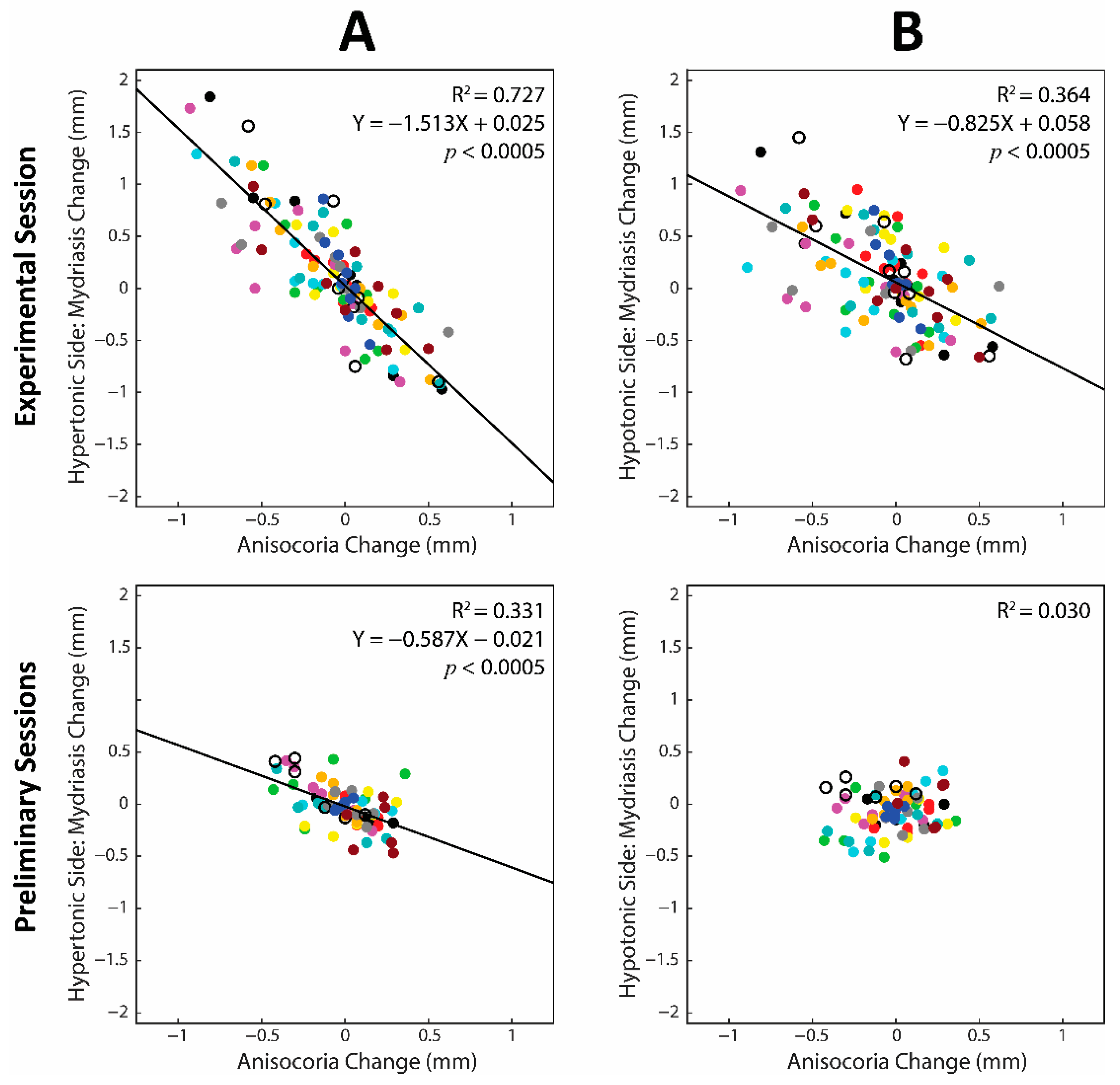
3.7. Contribution of the Hypertonic and Hypotonic Side to Changes in Anisocoria and Average Pupil Size
4. Discussion
4.1. Effects of Imprint/Bite Wearing on EMG Asymmetry and Anisocoria
4.2. Correlation between Changes in Task-Related Parameters and Anisocoria
4.3. Dependence of Changes in Task-Related Parameters upon Pupil Size Changes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lomber, S.G.; Payne, B.R. Removal of two halves restores the whole: Reversal of visual hemineglect during bilateral cortical or collicular inactivation in the cat. Vis. Neurosci. 1996, 13, 1143–1156. [Google Scholar] [CrossRef]
- Koch, G.; Bonni, S.; Giacobbe, V.; Bucchi, G.; Basile, B.; Lupo, F.; Versace, V.; Bozzali, M.; Caltagirone, C. Theta-burst stimulation of the left hemisphere accelerates recovery of hemispatial neglect. Neurology 2012, 78, 24–30. [Google Scholar] [CrossRef]
- Andres, M.; Masson, N.; Larigaldie, N.; Bonato, M.; Vandermeeren, Y.; Dormal, V. Transcranial electric stimulation optimizes the balance of visual attention across space. Clin. Neurophysiol. 2020, 131, 912–920. [Google Scholar] [CrossRef]
- Kerkhoff, G. Spatial hemineglect in humans. Prog. Neurobiol. 2001, 63, 1–27. [Google Scholar] [CrossRef]
- Wackym, P.A.; Balaban, C.D.; Mackay, H.T.; Wood, S.J.; Lundell, C.J.; Carter, D.M.; Siker, D.A. Longitudinal Cognitive and Neurobehavioral Functional Outcomes Before and After Repairing Otic Capsule Dehiscence. Otol. Neurotol. 2016, 37, 70–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayar, D.A.; Kumral, E.; Celebisoy, N. Cognitive functions in acute unilateral vestibular loss. J. Neurol. 2020, 267, 153–159. [Google Scholar] [CrossRef]
- Cho, M.-J.; Shin, H.-E.; Amano, A.; Song, K.-B.; Choi, Y.-H. Effect of Molar Occlusal Balance on Cognitive Function in the Elderly. Int. Dent. J. 2021. [Google Scholar] [CrossRef] [PubMed]
- Saikia, U.P.; Chander, N.G.; Balasubramanian, M. Effect of fixed dental prosthesis on the brain functions of partially edentulous patients—Pilot study with power spectrum density analysis. Eur. Oral Res. 2020, 54, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.-S.; Liang, Z.-L.; Wu, M.-J.; Feng, L.; Liu, L.-L.; Zhang, J.-J. Reduced brain-derived neurotrophic factor expression in cortex and hippocampus involved in the learning and memory deficit in molarless SAMP8 mice. Chin. Med. J. 2011, 124, 1540–1544. [Google Scholar]
- De Cicco, V.; Cataldo, E.; Barresi, M.; Parisi, V.; Manzoni, D. Sensorimotor trigeminal unbalance modulates pupil size. Arch. Ital. Biol. 2014, 152, 1–12. [Google Scholar] [PubMed]
- De Cicco, V.; Barresi, M.; Tramonti Fantozzi, M.P.; Cataldo, E.; Parisi, V.; Manzoni, D. Oral Implant-Prostheses: New Teeth for a Brighter Brain. PLoS ONE 2016, 11, e0148715. [Google Scholar] [CrossRef] [Green Version]
- Tramonti Fantozzi, M.P.; De Cicco, V.; Argento, S.; De Cicco, D.; Barresi, M.; Cataldo, E.; Bruschini, L.; D’Ascanio, P.; Faraguna, U.; Manzoni, D. Trigeminal input, pupil size and cognitive performance: From oral to brain matter. Brain Res. 2021, 1751, 147194. [Google Scholar] [CrossRef] [PubMed]
- Tramonti Fantozzi, M.P.; Lazzarini, G.; De Cicco, V.; Briganti, A.; Argento, S.; De Cicco, D.; Barresi, M.; Cataldo, E.; Bruschini, L.; D’Ascanio, P.; et al. The path from trigeminal asymmetry to cognitive impairment: A behavioral and molecular study. Sci. Rep. 2021, 11, 1–17. [Google Scholar] [CrossRef]
- Silvetti, M.; Seurinck, R.; Van Bochove, M.E.; Verguts, T. The influence of the noradrenergic system on optimal control of neural plasticity. Front. Behav. Neurosci. 2013, 7, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffing, R.C.; Seitz, A.R. Pupillometry as a Glimpse into the Neurochemical Basis of Human Memory Encoding. J. Cogn. Neurosci. 2015, 27, 765–774. [Google Scholar] [CrossRef] [Green Version]
- Kihara, K.; Takeuchi, T.; Yoshimoto, S.; Kondo, H.M.; Kawahara, J.I. Pupillometric evidence for the locus coeruleus-noradrenaline system facilitating attentional processing of action-triggered visual stimuli. Front. Psychol. 2015, 6, 827. [Google Scholar] [CrossRef] [Green Version]
- Joshi, S.; Li, Y.; Kalwani, R.M.; Gold, J.I. Relationships between Pupil Diameter and Neuronal Activity in the Locus Coeruleus, Colliculi, and Cingulate Cortex. Neuron 2016, 89, 221–234. [Google Scholar] [CrossRef] [Green Version]
- Reimer, J.; McGinley, M.J.; Liu, Y.; Rodenkirch, C.; Wang, Q.; McCormick, D.A.; Tolias, A.S. Pupil fluctuations track rapid changes in adrenergic and cholinergic activity in cortex. Nat. Commun. 2016, 7, 13289. [Google Scholar] [CrossRef]
- Einhäuser, W. The Pupil as Marker of Cognitive Processes. In Computational and Cognitive Neuroscience of Vision; Zhao, Q., Ed.; Cognitive Science and Technology; Springer: Singapore, 2017; pp. 141–169. ISBN 978-981-10-0213-7. [Google Scholar]
- Cedarbaum, J.M.; Aghajanian, G.K. Afferent projections to the rat locus coeruleus as determined by a retrograde tracing technique. J. Comp. Neurol. 1978, 178, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.F.; Wang, B.R.; Peng, Z.Z.; Li, J.S. Morphological characteristics and terminating patterns of masseteric neurons of the mesencephalic trigeminal nucleus in the rat: An intracellular horseradish peroxidase labeling study. J. Comp. Neurol. 1991, 303, 286–299. [Google Scholar] [CrossRef]
- Craig, A.D. Spinal and trigeminal lamina I input to the locus coeruleus anterogradely labeled with Phaseolus vulgaris leucoagglutinin (PHA-L) in the cat and the monkey. Brain Res. 1992, 584, 325–328. [Google Scholar] [CrossRef]
- Zerari-Mailly, F.; Buisseret, P.; Buisseret-Delmas, C.; Nosjean, A. Trigemino-solitarii-facial pathway in rats. J. Comp. Neurol. 2005, 487, 176–189. [Google Scholar] [CrossRef]
- Couto, L.B.; Moroni, C.R.; dos Reis Ferreira, C.M.; Elias-Filho, D.H.; Parada, C.A.; Pelá, I.R.; Coimbra, N.C. Descriptive and functional neuroanatomy of locus coeruleus-noradrenaline-containing neurons involvement in bradykinin-induced antinociception on principal sensory trigeminal nucleus. J. Chem. Neuroanat. 2006, 32, 28–45. [Google Scholar] [CrossRef] [PubMed]
- Dauvergne, C.; Smit, A.E.; Valla, J.; Diagne, M.; Buisseret-Delmas, C.; Buisseret, P.; Pinganaud, G.; VanderWerf, F. Are locus coeruleus neurons involved in blinking? Neurosci. Res. 2008, 61, 182–191. [Google Scholar] [CrossRef]
- Fujita, K.; Matsuo, K.; Yuzuriha, S.; Kawagishi, K.; Moriizumi, T. Cell bodies of the trigeminal proprioceptive neurons that transmit reflex contraction of the levator muscle are located in the mesencephalic trigeminal nucleus in rats. J. Plast. Surg. Hand Surg. 2012, 46, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Ban, R.; Hama, Y.; Yuzuriha, S. Eyelid Opening with Trigeminal Proprioceptive Activation Regulates a Brainstem Arousal Mechanism. PLoS ONE 2015, 10, e0134659. [Google Scholar] [CrossRef]
- Schwarz, L.A.; Luo, L. Organization of the Locus Coeruleus-Norepinephrine System. Curr. Biol. 2015, 25, R1051–R1056. [Google Scholar] [CrossRef] [Green Version]
- Mercante, B.; Enrico, P.; Floris, G.; Quartu, M.; Boi, M.; Serra, M.P.; Follesa, P.; Deriu, F. Trigeminal nerve stimulation induces Fos immunoreactivity in selected brain regions, increases hippocampal cell proliferation and reduces seizure severity in rats. Neuroscience 2017, 361, 69–80. [Google Scholar] [CrossRef]
- Berridge, C.W.; Waterhouse, B.D. The locus coeruleus–noradrenergic system: Modulation of behavioral state and state-dependent cognitive processes. Brain Res. Rev. 2003, 42, 33–84. [Google Scholar] [CrossRef]
- Aston-Jones, G.; Cohen, J.D. An Integrative Theory of Locus Coeruleus-Norepinephrine Function: Adaptive Gain and Optimal Performance. Annu. Rev. Neurosci. 2005, 28, 403–450. [Google Scholar] [CrossRef] [Green Version]
- Spinnler, H.; Tognoni, G.; The Italian Group on the Neuropsychological Study of Aging. Italian standardization and classification of Neuropsychological tests. Ital. J. Neurol. Sci. 1987, 6 (Suppl. S8), 1–120. [Google Scholar]
- Nnoaham, K.E.; Kumbang, J. Transcutaneous electrical nerve stimulation (TENS) for chronic pain. Cochrane Database Syst. Rev. 2008, CD003222. [Google Scholar] [CrossRef] [Green Version]
- Dao, T.T.; Lavigne, G.J.; Charbonneau, A.; Feine, J.S.; Lund, J.P. The efficacy of oral splints in the treatment of myofascial pain of the jaw muscles: A controlled clinical trial. Pain 1994, 56, 85–94. [Google Scholar] [CrossRef]
- Steiger, J.H. Tests for comparing elements of a correlation matrix. Psychol. Bull. 1980, 87, 245–251. [Google Scholar] [CrossRef]
- Lenhard, W.; Lenhard, A. Testing the Significance of Correlations; ResearchGate: Belin, Germany, 2014. [Google Scholar]
- Cohen, J.; Cohen, P. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences, 3rd ed.; Lawrence Erlbaum Associates: Mahwah, NJ, USA, 2003. [Google Scholar]
- Soper, D.S. Significance of the Difference between Two Slopes References—Analytics Calculators. Available online: https://www.analyticscalculators.com/references.aspx?id=103 (accessed on 31 March 2021).
- De Cicco, V.; Tramonti Fantozzi, M.P.; Cataldo, E.; Barresi, M.; Bruschini, L.; Faraguna, U.; Manzoni, D. Trigeminal, Visceral and Vestibular Inputs May Improve Cognitive Functions by Acting through the Locus Coeruleus and the Ascending Reticular Activating System: A New Hypothesis. Front. Neuroanat. 2018, 11, 130. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, S.; Yang, Q.; Xu, Z.; Zhang, S.; Fan, S.; Liu, C.; Li, X.; Ma, C. Trigeminal nerve electrical stimulation: An effective arousal treatment for loss of consciousness. Brain Res. Bull. 2021, 169, 81–93. [Google Scholar] [CrossRef]
- Gilzenrat, M.S.; Nieuwenhuis, S.; Jepma, M.; Cohen, J.D. Pupil diameter tracks changes in control state predicted by the adaptive gain theory of locus coeruleus function. Cogn. Affect. Behav. Neurosci. 2010, 10, 252–269. [Google Scholar] [CrossRef]
- Murphy, P.R.; Robertson, I.H.; Balsters, J.H.; O’Connell, R.G. Pupillometry and P3 index the locus coeruleus-noradrenergic arousal function in humans. Psychophysiology 2011, 48, 1532–1543. [Google Scholar] [CrossRef] [PubMed]
- Kucewicz, M.T.; Dolezal, J.; Kremen, V.; Berry, B.M.; Miller, L.R.; Magee, A.L.; Fabian, V.; Worrell, G.A. Pupil size reflects successful encoding and recall of memory in humans. Sci. Rep. 2018, 8, 4949. [Google Scholar] [CrossRef]
- Oliva, M. Pupil size and search performance in low and high perceptual load. Cogn. Affect. Behav. Neurosci. 2019, 19, 366–376. [Google Scholar] [CrossRef] [Green Version]
- Van den Brink, R.L.V.; Murphy, P.R.; Nieuwenhuis, S. Pupil Diameter Tracks Lapses of Attention. PLoS ONE 2016, 11, e0165274. [Google Scholar] [CrossRef]
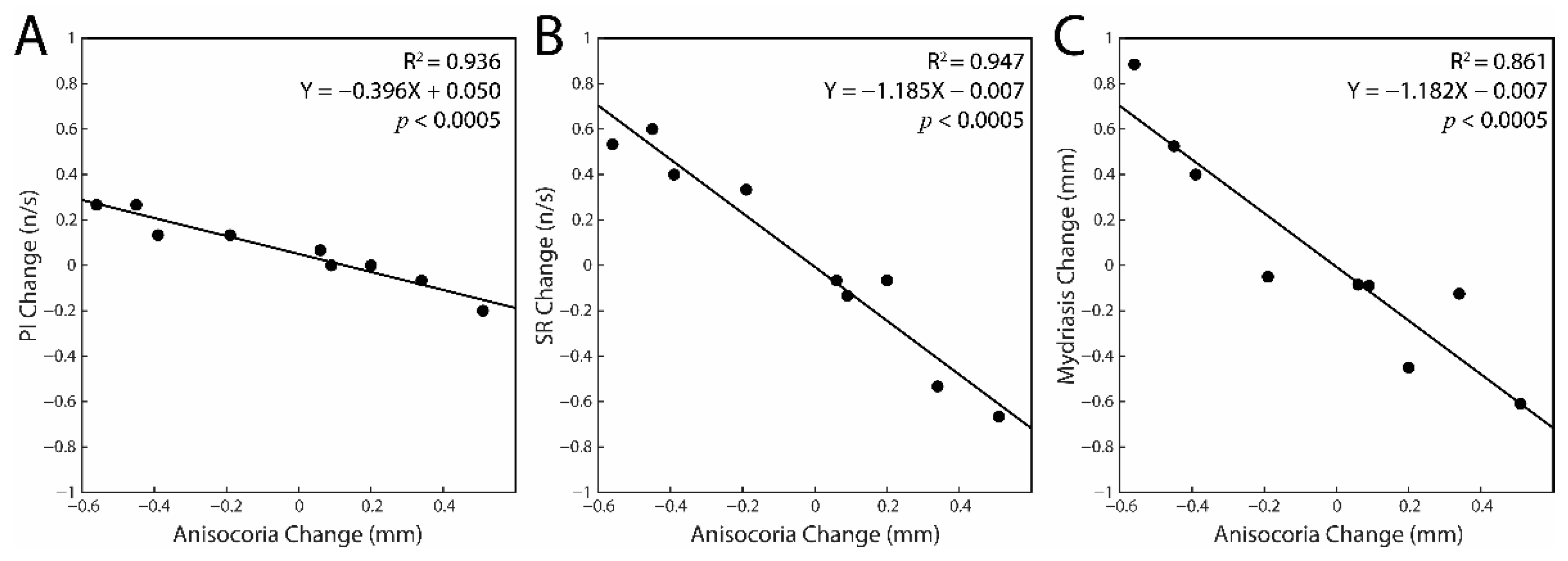
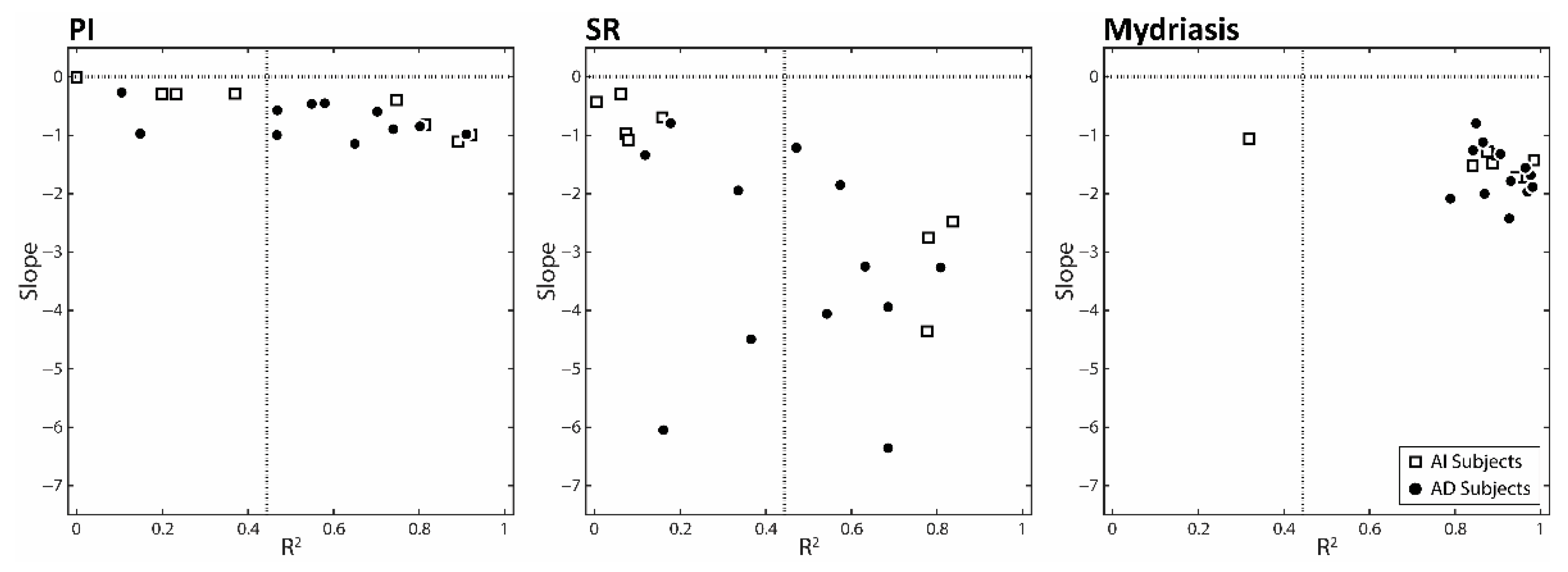
| Group | ID Subject | PI (p) | SR (p) | Mydriasis (p) | PI (R2) | SR (R2) | Mydriasis (R2) | Average (R2) | |
|---|---|---|---|---|---|---|---|---|---|
| AD Subjects | 12 | 0.001 | 0.005 | 0.005 | 0.936 | 0.947 | 0.861 | 0.915 | |
| 18 | 0.001 | 0.005 | 0.002 | 0.942 | 0.934 | 0.765 | 0.880 | ||
| 20 | 0.001 | 0.005 | 0.002 | 0.911 | 0.878 | 0.775 | 0.855 | ||
| 17 | 0.001 | 0.005 | 0.023 | 0.863 | 0.935 | 0.546 | 0.781 | ||
| 15 | 0.004 | 0.011 | 0.009 | 0.719 | 0.627 | 0.651 | 0.666 | ||
| 9 | 0.016 | 0.011 | 0.004 | 0.587 | 0.628 | 0.724 | 0.646 | ||
| 3 | 0.005 | 0.028 | 0.005 | 0.693 | 0.521 | 0.705 | 0.639 | ||
| 2 | 0.027 | 0.213 | 0.005 | 0.527 | 0.211 | 0.919 | 0.552 | ||
| 11 | 0.039 | 0.113 | 0.009 | 0.478 | 0.319 | 0.643 | 0.480 | ||
| 8 | 0.053 | 0.043 | 0.039 | 0.437 | 0.466 | 0.478 | 0.460 | ||
| 4 | 0.047 | 0.090 | 0.028 | 0.452 | 0.356 | 0.522 | 0.443 | ||
| 19 | 0.152 | 0.203 | 0.001 | 0.270 | 0.219 | 0.831 | 0.440 | ||
| Mean ± SD | 0.651 ± 0.227 | 0.587 ± 0.283 | 0.702 ± 0.139 | 0.647 ± 0.177 | |||||
| AI Subjects | 1 | 0.118 | 0.074 | 0.107 | 0.312 | 0.387 | 0.328 | 0.342 | |
| 7 | 0.105 | 0.067 | 0.598 | 0.331 | 0.402 | 0.042 | 0.259 | ||
| 16 | 0.272 | 0.699 | 0.12 | 0.169 | 0.023 | 0.310 | 0.167 | ||
| 14 | 0.183 | 0.234 | 0.822 | 0.238 | 0.195 | 0.008 | 0.147 | ||
| 13 | 0.621 | 0.602 | 0.127 | 0.037 | 0.041 | 0.300 | 0.126 | ||
| 5 | 0.219 | 0.556 | 0.394 | 0.206 | 0.052 | 0.106 | 0.121 | ||
| 10 | 0.288 | 0.542 | 0.888 | 0.159 | 0.056 | 0.003 | 0.073 | ||
| 6 | 0.663 | 0.906 | 0.899 | 0.029 | 0.002 | 0.002 | 0.011 | ||
| Mean ± SD | 0.185 ± 0.112 | 0.145 ± 0.165 | 0.137 ± 0.149 | 0.156 ± 0.104 | |||||
| AD + AI Subjects | Mean ± SD | 0.465 ± 0.299 | 0.410 ± 0.325 | 0.476 ± 0.316 | 0.450 ± 0.288 |
| AD Subjects | AI Subjects | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BITE OFF | BITE ON | BITE OFF | BITE ON | |||||||||
| Open1 | Open1 vs. Contact | Contact | Open1 | Open1 vs. Contact | Contact | Open1 | Open1 vs. Contact | Contact | Open1 | Open1 vs. Contact | Contact | |
| Average Pupil Size | 3.97 ± 0.70 | p < 0.0005 | 4.32 ± 0.67 | 4.01 ± 0.72 | p < 0.0005 | 3.79 ± 0.65 | 4.18 ± 0.71 | p < 0.0005 | 4.62 ± 0.67 | 4.21 ± 0.74 | p = 0.031 | 4.04 ± 0.64 |
| Anisocoria | 0.43 ± 0.32 | p < 0.0005 | 0.71 ± 0.32 | 0.46 ± 0.27 | p < 0.0005 | 0.14 ± 0.10 | 0.42 ± 0.21 | NS | 0.46 ± 0.35 | 0.37 ± 0.23 | p = 0.021 | 0.13 ± 0.06 |
| PI | 1.74 ± 0.51 | p = 0.001 | 1.64 ± 0.45 | 1.73 ± 0.50 | p < 0.0005 | 2.18 ± 0.42 | 1.98 ± 0.66 | p = 0.002 | 1.77 ± 0.57 | 2.03 ± 0.72 | p < 0.0005 | 2.33 ± 0.74 |
| SR | 12.92 ± 2.24 | p = 0.009 | 12.33 ± 2.17 | 12.65 ± 2.09 | p < 0.0005 | 14.32 ± 1.52 | 13.68 ± 2.30 | p < 0.0005 | 12.98 ± 2.13 | 13.73 ± 2.45 | p = 0.013 | 14.73 ± 2.59 |
| Average Mydriasis | 1.41 ± 0.35 | p < 0.0005 | 0.85 ± 0.28 | 1.37 ± 0.34 | p < 0.0005 | 1.83 ± 0.48 | 1.37 ± 0.29 | p < 0.0005 | 0.67 ± 0.32 | 1.41 ± 0.31 | p = 0.001 | 1.71 ± 0.36 |
| ID Subjects | PI (R2) | SR (R2) | Mydriasis (R2) |
|---|---|---|---|
| 1 | 0.891 | 0.777 | 0.965 |
| 2 | 0.909 | 0.632 | 0.926 |
| 3 | 0.105 | 0.118 | 0.849 |
| 4 | 0.739 | 0.543 | 0.969 |
| 5 | 0.747 | 0.159 | 0.875 |
| 6 | 0.922 | 0.837 | 0.887 |
| 7 | 0.198 | 0.073 | 0.841 |
| 8 | 0.549 | 0.686 | 0.865 |
| 9 | 0.467 | 0.366 | 0.976 |
| 10 | 0.232 | 0.078 | 0.984 |
| 11 | 0.910 | 0.178 | 0.906 |
| 12 | 0.579 | 0.471 | 0.930 |
| 13 | 0.814 | 0.781 | 0.944 |
| 14 | 0.000 | 0.004 | 0.319 |
| 15 | 0.802 | 0.808 | 0.964 |
| 16 | 0.369 | 0.061 | 0.900 |
| 17 | 0.468 | 0.336 | 0.842 |
| 18 | 0.702 | 0.574 | 0.981 |
| 19 | 0.148 | 0.161 | 0.789 |
| 20 | 0.650 | 0.686 | 0.869 |
| Mean ± SD | 0.560 ± 0.298 | 0.416 ± 0.294 | 0.879 ± 0.143 |
| AD Subjects | AI Subjects | ||||||
|---|---|---|---|---|---|---|---|
| Dependent Variable (Y) | Independent Variable (X) | R2 | Slope | p | R2 | Slope | p |
| PI | Pupil Size, Hypertonic Side | 0.589 | −0.540 | <0.0005 | 0.382 | −0.448 | <0.0005 |
| SR | 0.361 | −1.933 | <0.0005 | 0.224 | −1.394 | <0.0005 | |
| Mydriasis | 0.882 | −1.102 | <0.0005 | 0.744 | −1.166 | <0.0005 | |
| PI | Pupil Size, Hypotonic Side | 0.189 *** | −0.595 | <0.0005 | 0.168 ** | −0.323 | <0.0005 |
| SR | 0.094 *** | −1.926 | 0.001 | 0.076 * | −0.881 | 0.019 | |
| Mydriasis | 0.428 *** | −1.494 | <0.0005 | 0.603 * | −1.140 | <0.0005 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tramonti Fantozzi, M.P.; Marconi, O.; Simoni, F.; De Cicco, V.; De Cicco, D.; Cataldo, E.; Barresi, M.; Bruschini, L.; d’Ascanio, P.; Faraguna, U.; et al. Coupling between Trigeminal-Induced Asymmetries in Locus Coeruleus Activity and Cognitive Performance. Symmetry 2021, 13, 1676. https://doi.org/10.3390/sym13091676
Tramonti Fantozzi MP, Marconi O, Simoni F, De Cicco V, De Cicco D, Cataldo E, Barresi M, Bruschini L, d’Ascanio P, Faraguna U, et al. Coupling between Trigeminal-Induced Asymmetries in Locus Coeruleus Activity and Cognitive Performance. Symmetry. 2021; 13(9):1676. https://doi.org/10.3390/sym13091676
Chicago/Turabian StyleTramonti Fantozzi, Maria Paola, Ottavia Marconi, Federica Simoni, Vincenzo De Cicco, Davide De Cicco, Enrico Cataldo, Massimo Barresi, Luca Bruschini, Paola d’Ascanio, Ugo Faraguna, and et al. 2021. "Coupling between Trigeminal-Induced Asymmetries in Locus Coeruleus Activity and Cognitive Performance" Symmetry 13, no. 9: 1676. https://doi.org/10.3390/sym13091676
APA StyleTramonti Fantozzi, M. P., Marconi, O., Simoni, F., De Cicco, V., De Cicco, D., Cataldo, E., Barresi, M., Bruschini, L., d’Ascanio, P., Faraguna, U., & Manzoni, D. (2021). Coupling between Trigeminal-Induced Asymmetries in Locus Coeruleus Activity and Cognitive Performance. Symmetry, 13(9), 1676. https://doi.org/10.3390/sym13091676







